Abstract
We report a case of disseminated Scedosporium apiospermum infection in a neutropenic patient with acute myeloid leukemia. Due to progression of the mycosis after 7 days of amphotericin B lipid complex therapy and to high susceptibility of the mold to voriconazole in vitro, the patient was treated with intravenous voriconazole. After a few days of therapy, fever disappeared and skin lesions improved. However, the patient died after 1 month due to intestinal bleeding. Despite a negative outcome, this case seems to indicate a promising role for voriconazole in the treatment of S. apiospermum infections.
Scedosporium apiospermum and its teleomorph Pseudallescheria boydii have been occasionally reported as causes of systemic infections in immunocompromised patients (4, 10–12, 14, 17, 21). Amphotericin B is considered to be ineffective against S. apiospermum infections, and in vitro sensitivity studies have shown that the high levels of the drug that are required are often not clinically achievable (12). Miconazole, ketoconazole, and itraconazole are effective in vitro and have been employed successfully, but severe infections often fail to respond to these azoles (4, 9). New therapeutic approaches are needed for the treatment of P. boydii and S. apiospermum infections.
Voriconazole is a new triazole antifungal agent derived from fluconazole, with one triazole moiety replaced by a fluoropyrimide grouping and a methyl group added to the propanol backbone. Voriconazole is effective in vitro against yeasts and filamentous fungi (1, 13, 16) and has proved to be effective in vivo against aspergillosis in humans and in animal models (2, 3, 8).
We report on the voriconazole treatment of an immunocompromised patient with disseminated S. apiospermum infection. A 25-year-old man was diagnosed with acute myeloid leukemia in January 1996. Remission was not achieved despite various courses of conventional intensive chemotherapy. On 14 October 1996 the patient was readmitted in good general condition for further treatment of his refractory leukemia with fludarabine, cytosine arabinoside, and granulocyte colony-stimulating factor (650 μg/day subcutaneously for 6 days starting on the 1st day of chemotherapy) (7, 19). Antifungal prophylaxis was not given. The patient developed a fever on 21 October while he was profoundly granulocytopenic, and empirical broad-spectrum antibacterial therapy was given. The patient complained of pain in his face and developed multiple papular skin lesions that were large and erythematous with rapidly developing purplish-black, necrotic centers. Due to persistent fever despite 10 days of antibiotic treatment, empirical antifungal therapy with amphotericin B lipid complex (Abelcet; Liposome Company, Princeton, N.J.) (3 mg/kg of body weight/day) was instituted (the patient had previously developed severe intolerance to conventional amphotericin B deoxycholate).
Nasal swabs and aspirates of one of the skin lesions were cultured onto tryptose agar base that contained 10% sheep blood incubated at 37°C and Sabouraud’s 2% dextrose agar with 0.5 mg of chloramphenicol per ml incubated at 30°C. After 3 days, Sabouraud’s culture yielded white, cottony colonies that later turned gray. Microscopic examination showed septate hyaline hyphae with conidia 9 by 5 μm in diameter borne terminally, singly, or in small groups on elongated simple or branched conidiophores or laterally on hyphae. The conidia were ovoid, with the larger end toward the apex, and appeared to be cut off at the base, with a distinct brown wall. The fungus was identified as S. apiospermum, an anamorphic state of P. boydii. Pathological examination of a skin biopsy in a hematoxylin-and-eosin-stained preparation revealed an inflammatory infiltrate with branching septate fungal hyphae in the necrotic tissue. A total body computerized tomograph showed infectious involvement of the paranasal sinuses, lungs, liver, spleen, and pancreas, and thickening of the loop wall was observed. The in vitro activities of various antifungal drugs against the isolate (Table 1) were examined by a macrobroth dilution test based upon National Committee for Clinical Laboratory Standards tentative standards against several opportunistic molds (5, 6). The inoculum was prepared by diluting a stock conidial suspension to obtain the final inoculum size of approximately 0.5 × 104 to 5 × 104 CFU/ml. Susceptibility to voriconazole (Pfizer Central Research, Sandwich, United Kingdom) was tested by diluting a 10 mg/ml solution in RPMI 1640 (Sigma Chemical Co., St. Louis, Mo.). The amphotericin B MIC was defined as the lowest concentration which prevented visible growth after 48 h of incubation. The MICs of the other drugs were defined as the concentrations which reduced growth by 70% after 48 h of incubation. Itraconazole and voriconazole showed the highest in vitro activities against the isolate. In light of persistent fever, an increase in the number and the size of skin lesions after 7 days of Abelcet treatment, in vitro susceptibility data, difficulty obtaining intravenous miconazole in time (the intravenous formulation is not commercially available), and the unpredictable absorption of oral itraconazole in the presence of severe mucositis and enterocolitis as well as the lack of an intravenous formulation, it was decided on 8 November that the patient would be treated with intravenous voriconazole, which was supplied by Pfizer Central Research for compassionate use. The first two doses of the drug were administered at 400 mg/12 h, and then the patient received maintenance doses of 200 mg/12 h (6 mg/kg/day). Despite persisting profound neutropenia, significant clinical improvement was observed within a few days. Fever disappeared, the skin lesions progressively decreased in size (Fig. 1), and no further lesions appeared. On 12 November, due to delayed recovery from neutropenia, treatment with granulocyte colony-stimulating factor (300 μg/day subcutaneously) was started.
TABLE 1.
In vitro susceptibility of an S. apiospermum isolate to seven antifungal drugs
| Drug | MIC (μg/ml) |
|---|---|
| Amphotericin B | 4 |
| Flucytosine | >64 |
| Miconazole | 2 |
| Ketoconazole | 1 |
| Itraconazole | 0.25 |
| Fluconazole | 32 |
| Voriconazole | 0.25 |
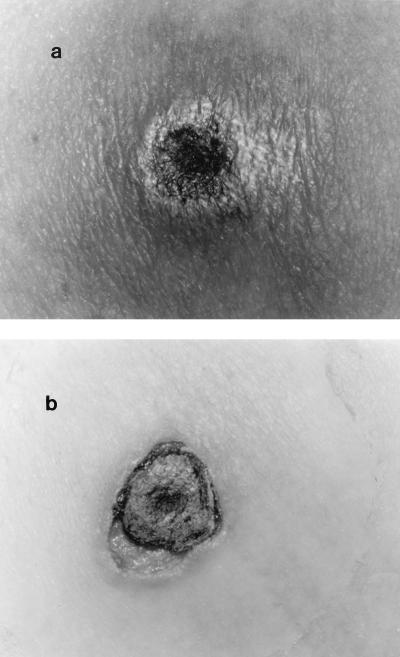
FIG. 1.
(a) S. apiospermum abdominal skin lesion with purplish-black necrotic center surrounded by large erythema. The fungal lesion worsened despite 7 days of therapy with amphotericin B lipid complex. (b) After 10 days of voriconazole therapy, the erythema disappeared. Only a scab was left.
After 21 November, the patient developed abdominal pain and had several episodes of hematemesis and melena. Signs of necrotizing colitis (12-mm thickening of the wall and pneumatosis intestinalis) were documented in an ultrasound examination. Due to severe neutropenia and piastrinopenia, the patient was considered ineligible for surgical intervention. Bone marrow examination showed severe aplasia and persistence of leukemic cells. Despite severe neutropenia that was unresponsive to growth factor treatment, the patient was persistently afebrile and skin lesions continued to improve with voriconazole therapy. On 7 December, the patient died, with massive intestinal bleeding that was probably due to intestinal perforation. No autopsy was performed.
The prognosis of systemic fungal infections in immunocompromised patients, particularly in those with profound and persistent neutropenia, may be dependent upon the ability of an antimicrobial compound to prevent the progression of infection until recovery from an impaired immune status occurs. Given the low activity of amphotericin B, an important problem in the management of S. apiospermum invasive infections, particularly in severely immunocompromised patients, is the lack of an effective intravenous antifungal drug (intravenous miconazole is no longer commercially available).
New therapeutic approaches to infections due to this fungus have been recently proposed. The successful use of itraconazole in two patients (14, 15) and that of oral terbinafine in a pulmonary infection refractory to itraconazole therapy (18) have been reported.
The combination of amphotericin B and antifungal azoles has been proposed based on evidence for in vitro synergism and the absence of antagonism between the drugs (20). However, to our knowledge, there are no reports on the use of this combination therapy in humans or in animal models. Recently, the in vitro activity of voriconazole against several opportunistic molds has been compared to those of amphotericin B, fluconazole, and itraconazole (13). The voriconazole MICs for 23 isolates of P. boydii were lower than those of the other drugs in all instances.
Voriconazole’s in vitro antimicrobial characteristics and availability in an intravenous formulation indicate a promising role for the drug in the treatment of S. apiospermum infections.
In our patient, voriconazole therapy seemed to show good activity in the control of systemic mycosis, as fever disappeared and skin lesions improved despite persisting profound neutropenia. We admit that, considering the outcome of the patient, this single experience is of limited value; however, persistent hematologic malignancy that prevented bone marrow recovery and hemorrhagic complications influenced the negative outcome.
Because of the low incidence of this emerging mycosis and the difficulty in performing a large clinical trial with sufficient predictive power for assessing new therapeutic approaches against P. boydii and S. apiospermum infections, in vivo studies in appropriate animal models are needed.
Acknowledgments
This work was supported by grants from ACRO Project CNR Italy Applicazioni cliniche nella ricerca oncologica (no. 920221939).
We thank Antonio Cassone (Department of Bacteriology and Medical Micology, Istituto Superiore di Sanità, Rome, Italy) for confirming the identity of S. apiospermum.
REFERENCES
- 1.Barry A L, Brown S D. In vitro studies of two triazole antifungal agents (voriconazole [UK-109,496] and fluconazole) against Candida species. Antimicrob Agents Chemother. 1996;40:1948–1949. doi: 10.1128/aac.40.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denning D, Del Favero A, Gluckman E, Norfolk D, Ruhnke M, Yonren S, Troke P, Sarantis N. Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. UK-109,496, a novel, wide-spectrum triazole derivate for the treatment of fungal infections: clinical efficacy in acute invasive aspergillosis, abstr. F80; p. 126. [Google Scholar]
- 3.Dupont B, Denning D, Lode H, Yonren S, Troke P, Sarantis N. Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. UK-109,496, a novel, wide-spectrum triazole derivate for the treatment of fungal infections: clinical efficacy in chronic invasive aspergillosis, abstr. F81; p. 127. [Google Scholar]
- 4.Dworzack D L, Clark R B, Borkowski W J, Jr, Smith D L, Dykstra M, Pugsley M P, Horowitz E A, Connolly T L, McKinney D L, Hostetler M K, Fitzgibbons J F, Galant M. Pseudallescheria boydii brain abscess: association with near-drowning and efficacy of high-dose, prolonged miconazole therapy in patients with multiple abscesses. Medicine. 1989;68:218–224. [PubMed] [Google Scholar]
- 5.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menezes P, Messer S A, Nelson P W, Odds F C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estey E, Thall P, Andreef M, Beran M, Kantarjian H, O’Brien S, Escudier S, Robertson L E, Koller C, Kornblau S. Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or granulocyte colony-stimulating factor. J Clin Oncol. 1994;12:671–678. doi: 10.1200/JCO.1994.12.4.671. [DOI] [PubMed] [Google Scholar]
- 8.George D, Miniter P, Andriole V T. Efficacy of UK-109,496, a new azole antifungal agent, in an experimental model of invasive aspergillosis. Antimicrob Agents Chemother. 1996;40:86–91. doi: 10.1128/aac.40.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg S L, Geha D J, Marshall W F, Inwards D J, Hoagland H C. Successful treatment of simultaneous pulmonary Pseudallescheria boydii and Aspergillus terreus infection with oral itraconazole. Clin Infect Dis. 1993;16:803–805. doi: 10.1093/clind/16.6.803. [DOI] [PubMed] [Google Scholar]
- 10.Gumbart C H. Pseudallescheria boydii infection after bone marrow transplantation. Ann Intern Med. 1983;99:193–194. doi: 10.7326/0003-4819-99-2-193. [DOI] [PubMed] [Google Scholar]
- 11.Guyotat D, Piens M A, Bouvier R, Fiere D. A case of disseminated Scedosporium apiospermum infection after bone marrow transplantation. Mykosen. 1987;30:151–154. doi: 10.1111/j.1439-0507.1987.tb03961.x. [DOI] [PubMed] [Google Scholar]
- 12.Lutwick L I, Galgiani J N, Johnson R H, Stevens D A. Visceral fungal infections due to Petriellidium boydii (Allescheria boydii). In vitro drug sensitivity studies. Am J Med. 1976;61:632–640. doi: 10.1016/0002-9343(76)90141-8. [DOI] [PubMed] [Google Scholar]
- 13.McGinnis M R, Pasarell L, Sutton D A, Fothergill A W, Cooper C R, Jr, Rinaldi M G. In vitro evaluation of voriconazole against some clinically important fungi. Antimicrob Agents Chemother. 1997;41:1832–1834. doi: 10.1128/aac.41.8.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nomdedeu J, Brunet S, Martino R, Altes A, Ausina V, Domingo Albos A. Successful treatment of pneumonia due to Scedosporium apiospermum with itraconazole: case report. Clin Infect Dis. 1993;16:731–733. doi: 10.1093/clind/16.5.731. [DOI] [PubMed] [Google Scholar]
- 15.Piper J P, Golden J, Brown D, Broestler J. Successful treatment of Scedosporium apiospermum suppurative arthritis with itraconazole. Pediatr Infect Dis J. 1990;9:674–675. [PubMed] [Google Scholar]
- 16.Radford S A, Johnson E M, Warnock D W. In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogens. Antimicrob Agents Chemother. 1997;41:841–843. doi: 10.1128/aac.41.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Travis L B, Roberts G D, Wilson W R. Clinical significance of Pseudallescheria boydii: a review of 10 years’ experience. Mayo Clin Proc. 1985;60:531–537. doi: 10.1016/s0025-6196(12)60571-0. [DOI] [PubMed] [Google Scholar]
- 18.Verweij P E, Cox N J M, Meis J F G. Oral terbinafine for treatment of pulmonary Pseudallescheria boydii infection refractory to itraconazole therapy. Eur J Clin Microbiol Infect Dis. 1997;15:26–28. doi: 10.1007/BF01575117. [DOI] [PubMed] [Google Scholar]
- 19.Visani G, Tosi P, Zinzani P L, Manfroi S, Ottaviani E, Testoni N, Clavio M, Cenacchi A, Gamberi B, Carrara P. FLAG (fludarabine + high-dose cytarabine + G-CSF): an effective and tolerable protocol for the treatment of ‘poor risk’ acute myeloid leukemias. Leukemia. 1994;8:1842–1846. [PubMed] [Google Scholar]
- 20.Walsh T J, Peter J, McGough D A, Fothergill A W, Rinaldi M G, Pizzo P A. Activities of amphotericin B and antifungal azoles alone and in combination against Pseudallescheria boydii. Antimicrob Agents Chemother. 1995;39:1361–1364. doi: 10.1128/aac.39.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winston D J, Jordan M C, Rhodes J. Allescheria boydii infections in the immunocompromised host. Am J Med. 1977;63:830–835. doi: 10.1016/0002-9343(77)90170-x. [DOI] [PubMed] [Google Scholar]