Abstract
A 63-year-old man with advanced pancreatic cancer and pyloric obstruction underwent surgical gastrojejunostomy. Malignant biliary obstruction appeared eight months after surgery and was managed with endoscopic ultrasound (EUS)-guided hepaticogastrostomy (HGS). Subsequently, afferent limb obstruction caused by cancer invasion occurred. Although an intestinal metal stent could not be placed, a biliary metal stent was deployed via the HGS route, which successfully decompressed the afferent limb; the abdominal symptoms subsequently disappeared. In future similar cases, decompression of the dilated intestine through the HGS and biliary stent might be a viable treatment option.
Keywords: afferent limb obstruction, pancreatic cancer
Introduction
Gastric outlet obstruction (GOO) caused by mechanical obstruction of the pylorus or duodenum in patients with advanced pancreatic cancer results in oral intake becoming impossible, vomiting and severe malnutrition (1). Current palliative procedures are based on surgical gastrojejunostomy (GJJ) and endoscopic duodenal stenting with a self-expanding metal stent (SEMS) (2). These patients are also often complicated with biliary obstruction, but a papillary approach to biliary drainage is difficult in those with GOO (3). Recently, endoscopic ultrasound (EUS)-guided hepaticogastrostomy (HGS) has been developed as an alternative method of managing these patients (4).
We herein report a difficult case of pancreatic cancer complicated with obstructive jaundice in addition to duodenal stenosis. Duodenal stenosis caused by the invasion of pancreatic cancer resulted in afferent limb obstruction, known as afferent limb syndrome (ALS). In the present case, after initial EUS-HGS for obstructive jaundice, drainage from the bile duct route formed by this technique was successful in improving duodenal expansion due to afferent limb obstruction.
Case Report
A 63-year-old Japanese man was admitted to our hospital because of continuous abdominal symptoms of nausea, vomiting and epigastric pain. Several close examinations revealed pancreatic body cancer with pyloric stenosis of the stomach. He was diagnosed with unresectable locally advanced pancreatic cancer, but his general condition was good, and a long-term survival was expected. Therefore, he decided to undergo surgical GJJ and was able to take food orally. Subsequently, he received treatment with anti-cancer drugs, and modified FOLFIRINOX (oxaliplatin 85 mg/m2, irinotecan 180 mg/m2, leucovorin 400 mg/m2 Day 1, 5-FU 2,400 mg/m2 ×48 hour intravenous injection, every 14 days) was administered for 6 months without any adverse effects.
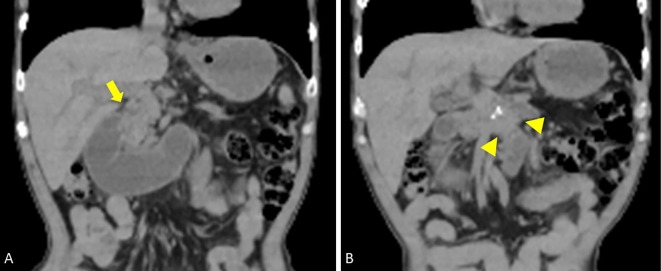
However, obstructive jaundice appeared eight months after GJJ. Magnetic resonance imaging cholangiography revealed stenosis of the distal bile duct due to invasion from pancreatic cancer. Since it was difficult to perform endoscopic drainage via a trans-papillary approach, he underwent EUS-HGS, in which a SEMS (Niti-S S-type Stent Spring Stopper; Century Medical, Tokyo, Japan) was deployed between the left intrahepatic bile duct and stomach for reduction of jaundice (Fig. 1A-C). After EUS-HGS, the serum bilirubin levels were successfully decreased, but postprandial vomiting appeared again one month after the procedure. Computed tomography (CT) showed no evidence of SEMS dislocation but did reveal duodenal dilation as well as pancreatic cancer invasion around the Treitz ligament (Fig. 2A, B). In particular, the primary lesion was likely to have directly invaded the third part of the duodenum (Fig. 2B; see arrowheads). Comprehensively, the abdominal symptoms were deemed most likely to be due to ALS caused by tumor invasion-related intestinal stricture.
Figure 1.
Endoscopic ultrasound (EUS)-guided hepaticogastrostomy (HGS) with spring-stoppered partially covered self-expanding metal stents (SEMSs). A: EUS image of puncturing the dilated left intrahepatic bile duct. B: Endoscopic image of the stomach after implantation of the SEMS. C: Fluoroscopy image after undergoing EUS-HGS. Arrows indicate the SEMS placed between the left intrahepatic bile duct and the stomach.
Figure 2.
Computed tomography (CT) image at the time of worsening of abdominal symptoms one month after EUS-HGS. A: CT image of the expanded duodenum. The arrow indicates the stenosis of the duodenal bulbs. B: CT image of the primary lesion of pancreatic cancer (arrowhead). The tumor directly invaded the third part of the duodenum.
Because he did not consent to a second surgical operation for drainage, we planned to place an intestinal SEMS at the duodenal obstruction via the GJJ route. However, it was difficult to insert the guidewire through the duodenal obstruction, although the duodenoscope was able to reach the site. We also failed to place an intestinal SEMS through the pyloric ring route in the antegrade fashion. Therefore, to improve afferent limb obstruction, we next attempted the following: under duodenoscopy, we first inserted the guidewire into the extended duodenum through the stenosis of distal bile duct via the EUS-HGS route and then dilated the stenosis with balloon catheter, and the catheter tube was replaced (5-Fr endoscopic naso-biliary drainage tube pigtail type; Gadelius Medical, Tokyo, Japan) along the guidewire (Fig. 3A-C).
Figure 3.
Endoscopic antegrade naso-duodenum tube placement via the EUS-HGS route. A: Endoscopic image during the procedure of the guidewire placement into the expanded duodenum through the metallic stent. B: Fluoroscopy image of bile duct dilation with a balloon catheter. C: Fluoroscopy image after the placement of the naso-duodenum tube. The arrowhead indicates the tip of the tube. D: CT image of the duodenum with improved expansion.
Intestinal fluid with a high concentration of pancreatic amylase (45,772 IU/mL) was drained from the tube at 150-650 mL per day. After we confirmed the disappearance of the symptoms and the shrinkage of the duodenum for one week (Fig. 3D), we eventually replaced the biliary SEMS (Zeostent V; Zeon Medical, Tokyo, Japan) at the stenosis of the distal bile duct via the EUS-HGS route (Fig. 4A) in the antegrade stenting fashion and then confirmed that contrast media in the lumen of the duodenum smoothly flowed back to the stomach cavity (Fig. 4B). One week later, the patient was able to resume oral intake and restart chemotherapy. He is currently alive without any adverse events, including cholangitis, five months after the procedure.
Figure 4.
Endoscopic antegrade stenting via the EUS-HGS route. A: Fluoroscopy image during the placement the self-expandable metallic stent into the bile duct. B: Fluoroscopy image showing that contrast media in the lumen of the duodenum smoothly flowed back into the stomach cavity.
Discussion
Pancreatic cancer is a refractory cancer with a poor prognosis that shows strong invasion and metastasis (5). We experienced a case of ALS due to duodenal invasions of pancreatic cancer after GJJ surgery, which resulted in obstruction and marked dilatation of the afferent limb. Since ALS can cause severe abdominal symptoms and worsen the patient's general condition (6), appropriate treatments should be conducted to improve the quality of life. These treatments include drainage and reoperation of the dilated intestinal tract (7), but surgery can be highly invasive, and effective ways of treating such patients have not yet been established. However, the EUS-guided gastroenterostomy technique with a lumen-apposing metal stent was recently developed and has shown promise (8).
In the present case, we were able to drain the extended duodenum in a relatively noninvasive manner using the bile duct route formed after EUS-HGS. EUS-HGS is a practical method of bile duct drainage for patients in whom a trans-papillary approach is difficult. Furthermore, recent improvements in several devices for EUS-HGS have resulted in an increase in the technical success rate and a reduction in adverse events (9). The present experience suggests that the EUS-HGS route is stable and that procedures using this route can be performed safely. We show the scheme of the present case before and after procedure in Fig. 5.
Figure 5.
Schematic representation of the present case. The red circle indicates the primary lesion. The red mist field indicates the part that was obstructed due to tumor invasion. The yellow curved line indicates the naso-duodenum drainage tube. The ocher area is the expanded duodenum. A: Illustration after gastric-jejunum bypass surgery. B: Illustration of the duodenal expansion that occurred after EUS-HGS. C: Illustration when the naso-duodenum drainage tube was placed via the EUS-HGS route. D: Illustration after self-expandable metallic stent deployment into the bile duct via the EUS-HGS route.
No serious short-term adverse events were observed as a result of this series of procedures, but caution should be exercised regarding long-term complications. Future adverse events may include, for example, severe cholangitis due to the reflux of duodenal fluid into the bile duct or early recurrence of bile duct obstruction. However, it was recently reported that EUS antegrade stenting combined with EUS-HGS may obtain long-term stent patency and prevent adverse events, such as cholangitis (10). In addition to bile duct obstruction and inflammation, regurgitation of pancreatic enzyme has also been reported to cause histological changes and tumorigenesis of the biliary epithelium (11). Therefore, it is necessary to carefully monitor how persistent regurgitation of pancreatic enzymes induced by the present procedure affects bile duct stenosis and inflammation as well as stent patency.
In conclusion, we experienced a case in which duodenal extension was successfully improved by performing drainage using the trans-gastric-bile duct route formed after EUS-HGS. Our experience might be useful for the treatment of ALS complicated with obstructive jaundice caused by pancreatic cancer.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Schantz SP, Evans TK, Coffey RJ, Schickler W. Palliative gastroenterostomy for pancreatic cancer. Am J Surg 147: 793-796, 1984. [DOI] [PubMed] [Google Scholar]
- 2. Uemura S, Iwashita T, Iwata K, et al. Endoscopic duodenal stent versus surgical gastrojejunostomy for gastric outlet obstruction in patients with advanced pancreatic cancer. Pancreatology 18: 601-607, 2018. [DOI] [PubMed] [Google Scholar]
- 3. Iwashita T, Uemura S, Tezuka R, Senju A, Yasuda I, Shimizu M. Current status of EUS-guided antegrade intervention for biliary diseases in patients with surgically altered anatomy. Dig Endosc 35: 2022. [DOI] [PubMed] [Google Scholar]
- 4. Iwashita T, Doi S, Yasuda I. Endoscopic ultrasound-guided biliary drainage: a review. Clin J Gastroenterol 7: 94-102, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 72: 7-33, 2022. [DOI] [PubMed] [Google Scholar]
- 6. Pannala R, Brandabur JJ, Gan S-I, et al. Afferent limb syndrome and delayed GI problems after pancreaticoduodenectomy for pancreatic cancer: single-center, 14-year experience. Gastrointest Endosc 74: 295-302, 2011. [DOI] [PubMed] [Google Scholar]
- 7. Termsinsuk P, Chantarojanasiri T, Pausawasdi N. Diagnosis and treatment of the afferent loop syndrome. Clin J Gastroenterol 13: 660-668, 2020. [DOI] [PubMed] [Google Scholar]
- 8. Irani S. Placing a lumen-apposing metal stent despite ascites: feasibility and safety. VideoGIE 5: 586-590, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ogura T, Higuchi K. Technical review of developments in endoscopic ultrasound-guided hepaticogastrostomy. Clin Endosc 54: 651-659, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogura T, Kitano M, Takenaka M, et al. Multicenter prospective evaluation study of endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting (with video). Dig Endosc 30: 252-259, 2017. [DOI] [PubMed] [Google Scholar]
- 11. Fujii H, Yang Y, Tang R, et al. Epithelial cell proliferation activity of the biliary ductal system with congenital biliary malformations. J Hepatobiliary Pancreat Surg 6: 294-302, 1999. [DOI] [PubMed] [Google Scholar]