Abstract
Purpose
There remains uncertainty as to which risk factors are important for the development of defaecatory problems as a result of heterogeneity of published evidence. Understanding the impact of risk factors may be important in selecting targets for disease prevention or reversal. The aim of this study was to identify and evaluate risk factors for faecal incontinence and chronic constipation.
Methods
Risk factors for chronic constipation and faecal incontinence were long-listed from scientific literature, then anonymously evaluated (by 50 predominantly colorectal surgical experts from the UK Pelvic Floor Society) using a Delphi technique. Each risk factor was rated as independent, a co-factor, or not a risk factor. Independent risk factors were rated between 1 (not important) and 10 (critically important) with mean (± standard deviation) calculated.
Results
Thirty-eight risk factors for chronic constipation were evaluated. Eighteen were classed as independent and 16 as co-factors. Opioid analgesia (7.87 ± 2.05), eating disorders (7.80 ± 1.72), and history of abuse (7.70 ± 1.89) were scored as most important independent risk factors. Female sex (6.60 ± 2.02) was considered an independent risk factor but increasing age was rated a co-factor. Thirty-three risk factors for faecal incontinence were evaluated. Twenty were classed as independent and eight as co-factors. Third- or fourth-degree tear (8.88 ± 1.57), instrumental delivery (8.47 ± 1.58), and grand multiparity (8.00 ± 1.63) were rated most important. Increasing age (7.41 ± 2.14) and female sex (7.58 ± 2.05) were both considered independent risk factors.
Conclusions
Several risk factors for chronic constipation and faecal incontinence were selected by Delphi approach. These factors will feed forward into Bayesian models of disease prediction that combine data and expert knowledge.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10151-023-02843-w.
Keywords: Risk factors, Faecal incontinence, Constipation, Delphi
Introduction
Faecal incontinence and chronic constipation are common disorders of defaecation, with faecal incontinence affecting around 6% [1], and chronic constipation affecting between 10% and 15% of the population, depending on diagnostic criteria used [2]. Both conditions have impact on quality of life [3], resulting in significant health burden [3, 4]. While advances have been made in the assessment of anorectal function, which has improved the understanding of the pathophysiology underlying disorders of defaecation [5], better understanding of risk factors may reveal further insights into pathophysiology, direct future research, and, most importantly, highlight targets for disease prevention or modification.
Several risk factors for developing faecal incontinence and chronic constipation have been identified, predominantly through cross-sectional studies which vary in the definitions used, methodology, study population, and potential risk factors assessed. These limitations prevent aggregation of results by meta-analysis [6].
The Delphi technique is an effective method for arriving at consensus on broad and complex problems [7, 8]. Using knowledge and experience of experts (primarily colorectal surgeons) in the field of pelvic floor disorders, in combination with contemporary published evidence, this Delphi study aimed to identify and evaluate risk factors for faecal incontinence and chronic constipation as a preliminary step toward building Bayesian models of disease prediction that combine data and expert knowledge.
Methods
Delphi methodology
The Delphi technique is characterised by four methodological features: expert participants, iterative rounds of enquiry, a dependency of the design of subsequent rounds on the basis of the response of the previous round, and anonymous participation [9, 10].
Expert participants
Participants were recruited voluntarily from members of The Pelvic Floor Society (TPFS), a UK subspecialty society affiliated to the Association of Coloproctology of Great Britain & Ireland. Email invitations to take part in the survey in May 2021 were sent to the whole membership (399 members) in April 2021. TPFS membership criteria ensured the selection of current specialists in pelvic floor disorders.
Rounds of enquiry and dependency of the design of subsequent rounds
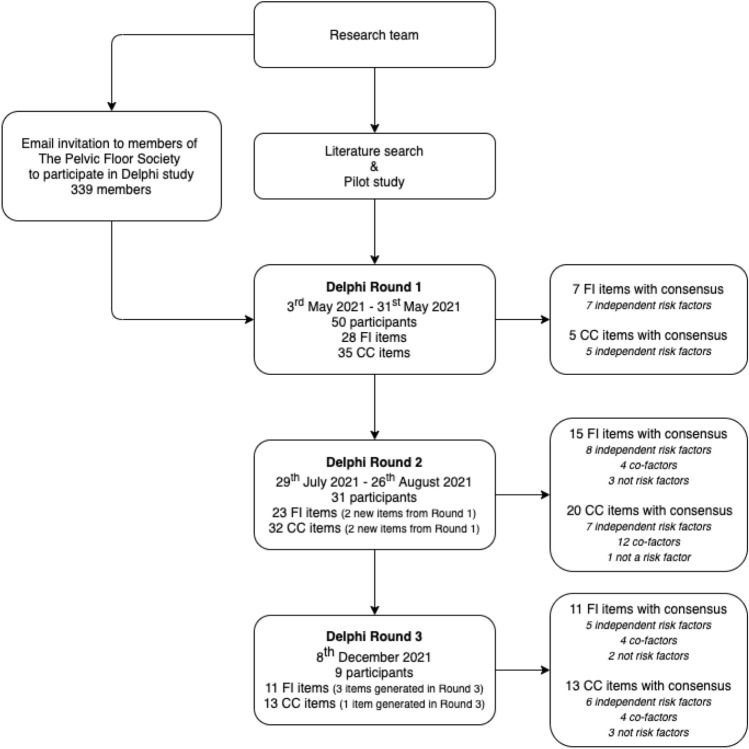
In reviewing recent Delphi studies conducted in the field of coloproctology (Supplement A, Fig. 1), most conducted between two and three rounds of voting (Supplement A, Fig. 2), with modification of survey items between rounds (Supplement A, Fig. 3). This current study included two rounds of anonymous voting by online surveys, followed by a final round of anonymous voting within a consensus meeting (mixed face-to-face and virtual) as illustrated in Fig. 1.
Fig. 1.
Delphi study schema. FI, faecal incontinence; CC, chronic constipation
The first-round questionnaire (Supplement B) was developed by PC, MH, and CK on the basis of a pilot survey carried out at TPFS annual meeting in November 2019 and a review of current literature. Potential risk factors were long-listed from textbooks [11–14], the most recent systematic reviews on the prevalence of constipation [15] and faecal incontinence [1, 6] (including an evaluation of the original studies included in these reviews), and a literature search for new studies published since the systematic reviews up to the 1 May 2021. The final selection of potential risk factors for inclusion in this study was determined by the senior author to produce a feasible questionnaire. The questionnaire was divided into three sections: participants’ characteristics, potential risk factors for faecal incontinence, and potential risk factors for chronic constipation. The first section captured the participant’s clinical role, years of experience in that role, and the volume of patients seen with disorders of defaecation in an average month.
For potential risk factors, each item could be rated by the participant as an independent risk factor, a co-factor, or ‘not a risk factor’. An independent risk factor was defined as a risk factor which can increase the risk of faecal incontinence or chronic constipation in an individual even if there are no other risk factors present. For a co-factor to increase the risk, an individual must also have at least one other risk factor. Classification to ‘not a risk factor’ was based on the belief that it neither increased the risk of faecal incontinence or chronic constipation alone or in combination with other factors. All risk factors classified as independent were then rated by importance using a score between 1 (not important) to 10 (critically important) on a Likert scale.
The second and third rounds were dependent on the results of the first and second rounds, respectively. Any risk factor which reached consensus was not carried forward into the subsequent round; any rating option with 10% of the votes or fewer were removed in the subsequent round; new items from participants’ suggestions were reviewed and added to the subsequent round as appropriate (see Supplementary A, Table 1). At the third-round consensus meeting, the results from the previous rounds were presented along with a summary of evidence. Participants voted on the basis of real-time presentation of the results. If consensus was not reached, the participants were invited to have a discussion followed by a re-vote, which was then accepted as final.
Anonymous process
Anonymous online tools were used to conduct the surveys (www.onlinesurveys.ac.uk) and the live voting (www.mentimeter.com). The study was co-ordinated by non-voting members of the research team for impartiality.
Definition of consensus
This study used an agreement level of 70% or greater to define consensus. Percentage agreement is a recognised approach to define consensus [8] and 70% agreement or greater is a commonly used parameter in recent Delphi studies within the field of coloproctology (see Supplement A, Fig. 4).
Statistical analysis
Classification outcomes were presented as counts and percentages. Importance ratings were presented as mean and standard deviation. Statistical analysis was performed using Microsoft Excel, version 16.58 (Microsoft Corporation, Redmond, WA, USA).
Ethical approval
The study was approved by the Queen Mary Ethics of Research Committee (QMERC20.228).
Results
Participant characteristics
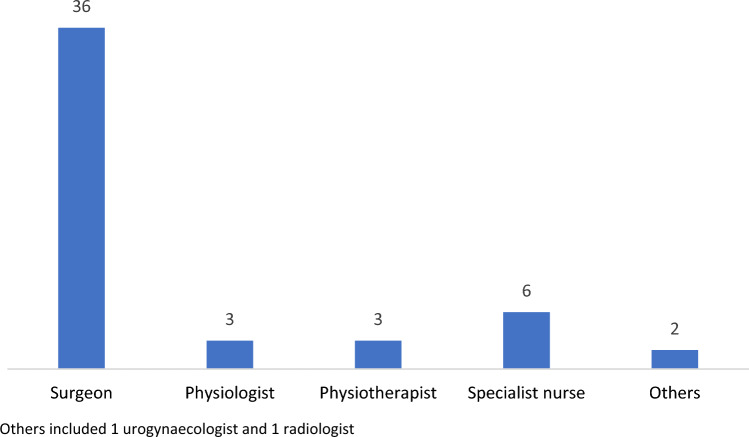
Fifty members of TPFS participated in the first round of the study (response rate of 14.7%). Participant clinical roles are summarised in Fig. 2 with the majority being colorectal surgeons (n = 36; 72%). Median experience was 15 (8–20) years and the number of patients with faecal incontinence and chronic constipation seen in an average month by participants was 10 (5–20) and 15 (10–20), respectively. Thirty-one members participated in the second round of the study and nine members participated in all three rounds.
Fig. 2.
Participants’ clinical role (n = 50). Others included 1 urogynaecologist and 1 radiologist
Classification of risk factors
Faecal incontinence
In the first round, 28 potential risk factors for faecal incontinence based on long-listing were evaluated (Table 1; Supplement A, Table 2). Seven were considered independent risk factors, including third- or fourth-degree tear (96% agreement), instrumental delivery (92% agreement), parity (70% agreement), grand multiparity (72% agreement), congenital conditions such as Hirschsprung disease or anorectal agenesis (80% agreement), peripheral nerve injury such as cauda equina syndrome (84% agreement), and spinal conditions such as spinal trauma (80% agreement). Two additional items (anal trauma, medications which may cause diarrhoea) were added by participant suggestion.
Table 1.
Evaluation of potential risk factors for faecal incontinence
| Potential risk factor | Agreement (%) | ||
|---|---|---|---|
| Not a risk factor | Co-factor | Independent | |
| Socio-demographic and lifestyle | |||
| Increasing age | – | 16 | 84 |
| Intensive exercise | 22 | – | 78 |
| Female sex | – | 29 | 71 |
| Institutional living | – | 90 | 10 |
| Dietary factors | – | 87 | 13 |
| Obesity | 0 | 86 | 14 |
| Excessive alcohol consumption | 10 | 74 | 16 |
| Ethnicity | 97 | 3 | – |
| Unemployment | 90 | 10 | – |
| Low socioeconomic status | 71 | 29 | 0 |
| Obstetric | |||
| Third- or fourth-degree tear | 0 | 4 | 96 |
| Instrumental delivery | 2 | 6 | 92 |
| Prolonged second stage of labour | 13 | 87 | |
| Grand multiparity | 2 | 26 | 72 |
| Parity (versus nulliparity) | 4 | 26 | 70 |
| High birth weight babies | – | 100 | 0 |
| Episiotomy, first- or second-degree tear | 100 | 0 | 0 |
| Sphincteric | |||
| Surgical trauma such as haemorrhoidectomy, internal sphincterotomy | – | 7 | 94 |
| Anal trauma/rape (not consensual anal intercourse)* | 0 | 13 | 87 |
| Atraumatic conditions such as scleroderma or idiopathic internal sphincter atrophy | – | 19 | 81 |
| Reconstructive surgery for congenital malformations such as Hirschsprung or anorectal agenesis | 0 | 20 | 80 |
| Extra-sphincteric | |||
| Pelvic radiotherapy | – | 0 | 100 |
| Inflammatory bowel disease | 0 | 0 | 100 |
| Previous rectal resection | 0 | 0 | 100 |
| Evacuation disorders (obstructed defaecation) | – | 11 | 89 |
| Peripheral nerve injuries such as cauda equina syndrome | 0 | 16 | 84 |
| Chronic diarrhoea | – | 19 | 81 |
| Spinal conditions such as spinal trauma | 0 | 20 | 80 |
| Central nervous system conditions such as stroke or multiple sclerosis | – | 26 | 74 |
| Medications which may cause diarrhoea* | 0 | 100 | 0 |
| Neurodiverse conditions such as autism** | 0 | 100 | 0 |
| Diabetes mellitus | – | 74 | 26 |
| Depressive disorders | 100 | 0 | 0 |
*Added following round 1
**Added following round 3
In the second round, 23 potential risk factors for faecal incontinence were evaluated. Eight were categorised as independent risk factors including increasing age (84% agreement), female sex (71% agreement), prolonged second stage of labour (87% agreement), surgical trauma such as haemorrhoidectomy or lateral sphincterotomy (94% agreement), anal trauma or rape (87% agreement), atraumatic conditions such as scleroderma or idiopathic internal sphincter atrophy (81% agreement), chronic diarrhoea (81% agreement), and central nervous system conditions such as stroke or multiple sclerosis (74% agreement). Four were classified as co-factors, and three were considered ‘not risk factors’ for faecal incontinence, including ethnicity (97% agreement), unemployment (90% agreement), and low socioeconomic status (71% agreement).
In the third round, 11 potential risk factors were evaluated (including 3 new items generated in the consensus meeting). Five were considered independent risk factors including extreme exercise (78% agreement), pelvic radiotherapy (100%), inflammatory bowel disease (100% agreement), previous rectal resection (100% agreement), and evacuation disorders (89% agreement). Four were considered co-factors, and episiotomy, first- or second-degree tear (100% agreement), and depressive disorders (100% agreement) were considered not risk factors for faecal incontinence.
Chronic constipation
In the first round, 35 long-listed risk factors for chronic constipation were evaluated (Table 2; Supplement A, Table 3). Five were classified as independent risk factors, including degenerative central nervous conditions such as Parkinson’s disease (76% agreement), peripheral nerve injuries such as cauda equina syndrome (76% agreement), spinal cord disorders such as spinal trauma (74% agreement), previous reconstructive surgery for Hirschsprung disease or congenital anorectal malformation (74% agreement), and opioid analgesia (88% agreement). Two additional items (history of childhood constipation, family history of constipation) were added from participants’ suggestions.
Table 2.
Evaluation of potential risk factors for chronic constipation
| Potential risk factor | Agreement (%) | ||
|---|---|---|---|
| Not a risk factor | Co-factor | Independent | |
| Socio-demographic and lifestyle | |||
| Poor diet | – | 10 | 90 |
| Female sex | 0 | 13 | 88 |
| Increasing age | – | 100 | 0 |
| Shift work | 10 | 87 | 3 |
| Obesity | – | 84 | 16 |
| Lack of exercise | – | 81 | 19 |
| Low socioeconomic status | 19 | 81 | 0 |
| Institutional living | – | 78 | 22 |
| Ethnicity | 87 | 13 | – |
| Unemployment | 71 | 29 | – |
| Medical history | |||
| Metabolic conditions such as hypercalcaemia | – | 0 | 100 |
| Connective tissue diseases such as scleroderma | – | 11 | 89 |
| Ehlers-Danlos syndrome or joint hypermobility syndrome | – | 16 | 84 |
| History of childhood constipation* | 0 | 23 | 77 |
| Hypothyroidism | – | 29 | 71 |
| Diabetes mellitus | 16 | 84 | – |
| Pregnancy | – | 71 | 29 |
| Family history of constipation* | 100 | – | – |
| Neurological conditions | |||
| Multiple sclerosis | – | 7 | 94 |
| Degenerative central nervous system conditions such as Parkinson’s disease | 2 | 22 | 76 |
| Peripheral nerve injuries such as cauda equina syndrome | 0 | 24 | 76 |
| Spinal cord disorders such as spinal trauma | 0 | 26 | 74 |
| Cognitive impairment (any cause) | – | 78 | 22 |
| Previous stroke | – | 77 | 23 |
| Mental health | |||
| Eating disorders | – | 7 | 94 |
| History of abuse (sexual, physical, or neglect) | – | 10 | 90 |
| Neurodiverse conditions such as autism** | – | 22 | 78 |
| Severe endogenous depression | – | 87 | 13 |
| Surgical history | |||
| Previous rectal resection | 0 | 0 | 100 |
| Reconstructive surgery for congenital malformations such as Hirschsprung or anorectal agenesis | 4 | 22 | 74 |
| Previous gynaecological surgery | – | 78 | 22 |
| Previous abdominal surgery such as appendicectomy or cholecystectomy | 89 | 11 | – |
| Medications | |||
| Anticholinergic agents | – | 0 | 100 |
| Opioid analgesia | 0 | 12 | 88 |
| Calcium channel blockers | 16 | 81 | 3 |
| 5-HT3 receptor antagonist | 19 | 74 | 7 |
| Bile acid sequestrants | 23 | 71 | 7 |
| Cation-containing agents | 13 | 71 | 16 |
*Added following round 1
**Added following round 3
In the second round, 32 potential risk factors for chronic constipation were evaluated. Seven were categorised as independent risk factors, including poor diet (90% agreement), Ehlers-Danlos syndrome or joint hypermobility syndrome (84% agreement), hypothyroidism (71% agreement), history of childhood constipation (77% agreement), multiple sclerosis (94% agreement), eating disorders (94% agreement), and history of abuse (90% agreement). Twelve were considered co-factors, and ethnicity (87% agreement) was considered not a risk factor for chronic constipation.
In the third round, 13 potential risk factors remained without consensus (including 1 new item generated in the consensus meeting). Six were considered independent risk factors, including female sex (88% agreement), metabolic conditions such as hypercalcaemia (100% agreement), connective tissue diseases such as scleroderma (89% agreement), neurodiverse conditions such as autism (78% agreement), previous rectal resection (100% agreement), and anticholinergic agents (100% agreement). Four were considered co-factors and three were considered not risk factors for chronic constipation, including unemployment (71% agreement), family history of constipation (100% agreement), and previous abdominal surgery such as appendicectomy or cholecystectomy (89% agreement).
Importance rating of risk factors
Faecal incontinence
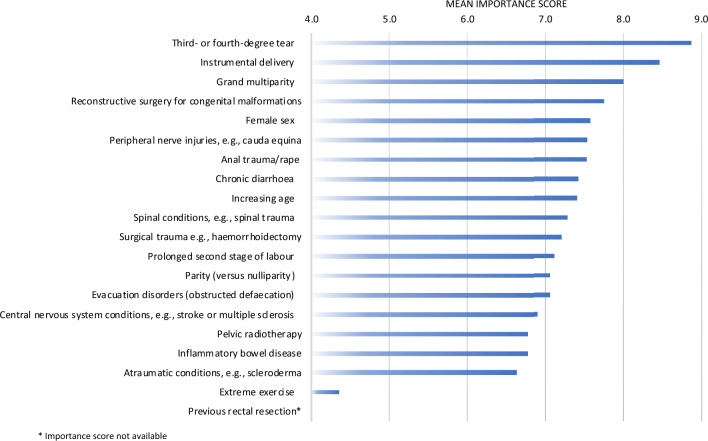
The independent risk factors considered most important for faecal incontinence were third- and fourth-degree tear (mean importance score of 8.88 ± 1.57), instrumental delivery (mean importance score of 8.47 ± 15.8), and grand multiparity (mean importance score of 8.00 ± 1.63) (Fig. 3). The independent risk factor considered least important was extreme exercise (mean importance score of 4.35 ± 2.26). Detailed results including the mean importance scores (± SD) are provided in Supplement A, Table 4.
Fig. 3.
Mean importance score for independent risk factors for faecal incontinence. *Importance score not available
Chronic constipation
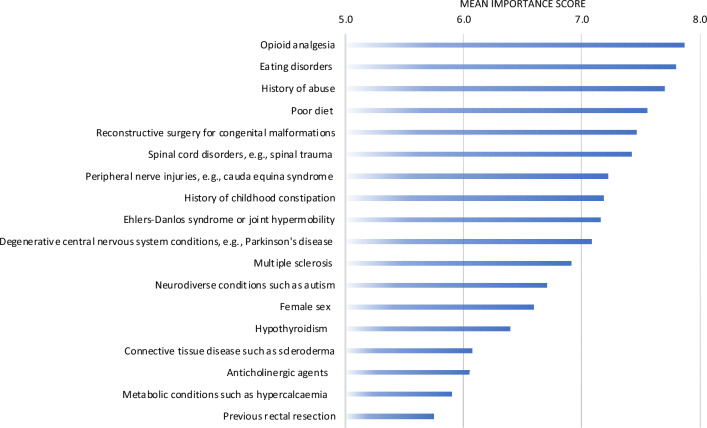
The independent risk factors considered most important for chronic constipation were opioid analgesia (mean importance score of 7.87 ± 2.05), eating disorders (mean importance score of 7.80 ± 1.72), and history of abuse (mean importance score of 7.70 ± 1.89) (Fig. 4). The independent risk factor considered least important was previous rectal resection (mean importance score of 5.75 ± 2.47). Detailed results including the mean importance scores (± SD) are provided in Supplement A, Table 5.
Fig. 4.
Mean importance score for independent risk factors for chronic constipation
Discussion
To our knowledge, this is the first study to comprehensively evaluate the importance of potential risk factors for benign disorders of defaecation using Delphi methodology. Consensus was achieved for classification (independent risk factor, co-factor, not a risk factor) of all potential risk factors evaluated (33 for faecal incontinence and 38 for chronic constipation). Mean importance scores were also produced for 19 of the 20 independent risk factors for faecal incontinence and all the 18 independent risk factors for chronic constipation.
Age and sex were the most evaluated risk factors for faecal incontinence and chronic constipation in the literature. While female sex was considered an independent risk factor for faecal incontinence and chronic constipation, increasing age was classified as an independent risk factor for faecal incontinence but a co-factor for chronic constipation. Meta-analyses of studies reporting the prevalence of chronic constipation in the general population have reported a significant association with female sex [4, 16, 17]. Barberio et al. [2] reported higher pooled prevalence of functional constipation in women compared with men, irrespective of the Rome definition used. The evidence for increasing age as a risk factor for chronic constipation is less consistent. While Suares and Ford [15] reported a modest increase in the pooled prevalence (17% in the ≥ 60 years compared with 12% in the < 29 years) and risk of chronic constipation in the higher age group (OR of 1.41 in the ≥ 60 years compared with < 29 years as baseline), Barberio et al. [2] found no statistical differences in the prevalence of functional constipation between the different age groups. A recent population survey even showed the highest prevalence of Rome IV functional constipation in the youngest age group (9.9% in those aged 18–29 years) [17]. Evidence from population studies largely supports the association between faecal incontinence and age and female sex [6, 18, 19].
The independent risk factors considered most important through Delphi approach for faecal incontinence were obstetric factors, including third- or fourth-degree tears (i.e. obstetric anal sphincter injury), instrumental delivery, and grand multiparity. Several systematic reviews have concluded that obstetric anal sphincter injury is significantly associated with an increased risk of anal [20–22] and faecal incontinence [22]. A meta-analysis by Cattani et al. [22] demonstrated a significant risk of anal incontinence associated with forceps delivery (OR 1.35 [CI 1.12–1.63]) and vacuum extraction delivery (OR 1.17 [CI 1.04–1.31]). The evidence of association between multiparity and anal or faecal incontinence is equivocal, with several studies reporting a significant association [23–25] and others suggesting the contrary [19, 26].
The current study did not consider episiotomy and first- or second-degree tears to be a risk factor for faecal incontinence. Several systematic reviews and meta-analyses have been performed to assess the risk of anal or faecal incontinence associated with episiotomy. LaCross et al. [20] suggested an increased risk of anal incontinence (OR 1.74 [CI 1.28–2.38]) in women who had an episiotomy; however, Bols et al. [21] did not find any significant association between first- or second-degree tear with faecal incontinence. Cattani et al. [22] described an increased risk of anal (OR 1.51 [CI 1.16–1.96], p = 0.002) but not faecal (OR 1.11 [CI 0.36–3.41], P = 0.85) incontinence when an episiotomy is performed. Interpretation of these findings is challenging because of heterogeneity in episiotomy practice (routine vs. selective) and type of episiotomy performed (median vs. mediolateral).
One of the most important independent risk factors for chronic constipation was history of abuse, but no large population study has examined this risk factor, most likely because of its sensitive nature. Several small observational studies have consistently reported a significant association between history of abuse and constipation [27], functional evacuation disorder [28, 29], symptoms of incomplete evacuation [30], or multiple pelvic floor complaints [31].
Potentially modifiable risk factors for faecal incontinence and chronic constipation included dietary factors and obesity. Dietary factors were considered a co-factor for faecal incontinence and an independent risk factor for chronic constipation. A systematic review by Colavita and Andy [32] found very limited data on the role of diet in the pathogenesis of faecal incontinence. Only one out of four studies found an association between low dietary fibre and faecal incontinence, but all five studies which assessed the effectiveness of diet as a treatment for faecal incontinence showed that fibre supplement improved faecal incontinence symptoms. Studies that have evaluated the dietary differences between individuals with and without chronic constipation have found a significant association with fluid intake [16, 33, 34], but evidence of association between low dietary fibre and chronic constipation is equivocal [35]; however, several systematic reviews have surmised that fibre supplementation is an effective treatment for chronic constipation [36, 37]. Obesity was considered a co-factor for both faecal incontinence and chronic constipation. Evidence from several observational studies have found a significant association between obesity and faecal [19, 26, 38–40] or flatus incontinence [38, 41], which may be due to an increased risk of loose stools [39] or the use of medications for weight loss [42] or diabetes [43]. Though some studies have reported no significant relationship [44] or an inverse relationship [16] between obesity and chronic constipation when defined as hard or infrequent stools, several studies have suggested an association between obesity and difficulty in rectal evacuation [39, 41]. Other modifiable risk factors for faecal incontinence included diarrhoea, evacuation disorder, diabetes, medications, and excessive alcohol consumption. Other modifiable risk factors for chronic constipation included eating disorders, diabetes, hypothyroidism, lack of exercise, and medications.
Diarrhoea (or loose stools) was considered one of the most important risk factors for faecal incontinence by several observational studies [18, 19, 26] but our experts assigned less importance to this risk factor. This discrepancy likely reflects the clinical practice of our experts, who were predominantly British colorectal surgeons, and may differ from opinions of patients and other specialists (general practitioner and gastroenterologists). This is a significant limitation on the generalisability of our findings. Further, although our results may be relevant to other developed countries with similar populations and risk factors, they may not be applicable to countries with less economic and healthcare resources. Acknowledging that the Delphi technique has been criticised for the quality of scientific evidence, the validity of the results, and the inconsistency of the study design [9, 10], we generated the questionnaire from scientific evidence published in peer-reviewed journals and were rigorous in pre-defining and adhering to the core elements of the Delphi process. Though the first round of this study was a structured round, which is a deviation from the ‘classical’ Delphi approach [10], this is common practice within clinical Delphi studies [9] and is consistent with recent Delphi publications within the field of coloproctology (Supplement A). The opportunity for ‘experts’ to freely express their opinion was maintained by the provision of free-text suggestions. The method enabled the importance of risk factors with low volume of evidence due to sensitive nature, e.g. history of abuse, or low prevalence within the general population, e.g. anal trauma, to be evaluated in the same manner as well-established risk factors such as age. There was an attrition rate between rounds (41% from round 1 to round 2, and 71% from round 2 to round 3), which is not unexpected in a Delphi study [45]. Despite this, we were able to maintain a multidisciplinary representation of experienced practitioners, which included colorectal surgeons, clinical nurse specialists, gastrointestinal physiologists, and a radiologist, from the first to the final round. Finally, this study did not include patients in the participants as it would be not have been possible to ensure unbiased and up to date clinical knowledge across all participants.
Conclusion
This study has highlighted some risk factors that may be modifiable in terms of prevention or treatment. The results will be used to inform a Bayesian risk prediction tool to assist clinical assessment of a patient’s risk of developing disorders of defaecation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge collaborators for the Disorders of Defaecation Delphi Group for their dedicated participation in this study: Miss E V Carrington, Dr C Chew, Mrs A Curry, Miss K Gorissen, Mrs S Morris, Mr S Siddiqi, and Mr A Williams. We would like to thank Miss Karen Telford, Mr Wesley Lai, and members of The Pelvic Floor Society for supporting this research.
Data availability
The data that support the findings of this study are available from the corresponding author, PC, upon reasonable request.
Declarations
Conflict of interest
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. PC, MH, and NF have no conflict of interest. CHK has received financial remuneration from Medtronic Inc. as speaker fees and for expert advisory committees, and research support from Saluda Medical. SMS has received honoraria for teaching from MMS/Laborie.
Ethical approval
The study was approved by the Queen Mary Ethics of Research Committee (QMERC20.228).
Footnotes
Collaborators of the Disorders of Defaecation Delphi Group are listed in the Acknowledgements.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
P. Chaichanavichkij, Email: p.chaichanavichkij@qmul.ac.uk
Disorders of Defaecation Delphi Group:
E. V. Carrington, C. Chew, A. Curry, K. Gorissen, S. Morris, S. Siddiqi, and A. Williams
References
- 1.Sharma A, Yuan L, Marshall RJ, et al. Systematic review of the prevalence of faecal incontinence. Br J Surg. 2016;103:1589–1597. doi: 10.1002/bjs.10298. [DOI] [PubMed] [Google Scholar]
- 2.Barberio B, Judge C, Savarino EV, Ford AC. Global prevalence of functional constipation according to the Rome criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:638–648. doi: 10.1016/S2468-1253(21)00111-4. [DOI] [PubMed] [Google Scholar]
- 3.Irvine EJ, Ferrazzi S, Pare P, et al. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol. 2002;97:1986–1993. doi: 10.1016/S0002-9270(02)04199-0. [DOI] [PubMed] [Google Scholar]
- 4.Shafe ACE, Lee S, Dalrymple JSO, Whorwell PJ. The LUCK study: laxative usage in patients with GP-diagnosed constipation in the UK, within the general population and in pregnancy. An epidemiological study using the General Practice Research Database (GPRD) Ther Adv Gastroenterol. 2011;4:343–363. doi: 10.1177/1756283X11417483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heitmann PT, Vollebregt PF, Knowles CH, et al. Understanding the physiology of human defaecation and disorders of continence and evacuation. Nat Rev Gastroenterol Hepatol. 2021;18:751–769. doi: 10.1038/s41575-021-00487-5. [DOI] [PubMed] [Google Scholar]
- 6.Ng K-SS, Sivakumaran Y, Nassar N, Gladman MA. Fecal incontinence: community prevalence and associated factors - a systematic review. Dis Colon Rectum. 2015;58:1194–1209. doi: 10.1097/DCR.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 7.Jones J, Hunter D. Qualitative research: consensus methods for medical and health services research. BMJ. 1995;311:376. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niederberger M, Spranger J. Delphi technique in health sciences: a map. Front Public Health. 2020;8:1–10. doi: 10.3389/fpubh.2020.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jünger S, Payne SA, Brine J, et al. Guidance on conducting and reporting Delphi studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med. 2017;31:684–706. doi: 10.1177/0269216317690685. [DOI] [PubMed] [Google Scholar]
- 10.Rowe G, Wright G, Bolger F. Delphi: a reevaluation of research and theory. Technol Forecast Soc Change. 1991;39:235–251. doi: 10.1016/0040-1625(91)90039-I. [DOI] [Google Scholar]
- 11.Knowles CH, Bharucha AE. Chronic constipation. In: Keighley MRB, Williams NS, editors. Keighley & Williams’ surgery of the anus, rectum, and colon. 4. Boca Raton: CRC; 2018. pp. 305–345. [Google Scholar]
- 12.Abrams P, Cardozo L, Wagg A, Wein AJ (2017) Incontinence, 6th ed. International Continence Society, Bristol
- 13.Bharucha AE, Rao SSC, Felt-Bersma R, et al. et al. Anorectal disorders. In: Drossman DA, Chang L, Chey WD, et al.et al., editors. Rome IV functional gastrointestinal disorders: disorders of gut-brain interaction. 4. Raleigh: The Rome Foundation; 2016. pp. 1182–1191. [Google Scholar]
- 14.Mearin F, Lacy BE, Chang L, et al. et al. Bowel disorders. In: Drossman DA, Chang L, Chey WD, et al.et al., editors. Rome IV functional gastrointestinal disorders: disorders of gut-brain interaction. 4. Raleigh: Elsevier; 2016. pp. 1002–1015. [Google Scholar]
- 15.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:1582–1591. doi: 10.1038/ajg.2011.164. [DOI] [PubMed] [Google Scholar]
- 16.Markland AD, Palsson O, Goode PS, et al. Association of low dietary intake of fiber and liquids with constipation: evidence from the national health and nutrition examination survey. Am J Gastroenterol. 2013;108:796–803. doi: 10.1038/ajg.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palsson OS, Whitehead W, Törnblom H, Sperber AD. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology. 2020 doi: 10.1053/j.gastro.2019.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Rey E, Choung RS, Schleck CD, et al. Onset and risk factors for fecal incontinence in a US community. Am J Gastroenterol. 2010;105:412–419. doi: 10.1038/ajg.2009.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ditah I, Devaki P, Luma HN, et al. Prevalence, trends, and risk factors for fecal incontinence in United States adults, 2005–2010. Clin Gastroenterol Hepatol. 2014;12:636–643.e2. doi: 10.1016/j.cgh.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 20.LaCross A, Groff M, Smaldone A. Obstetric anal sphincter injury and anal incontinence following vaginal birth: a systematic review and meta-analysis. J Midwifery Womens Health. 2015;60:37–47. doi: 10.1111/jmwh.12283. [DOI] [PubMed] [Google Scholar]
- 21.Bols EMJ, Hendriks EJM, Berghmans BCM, et al. A systematic review of etiological factors for postpartum fecal incontinence. Acta Obstet Gynecol Scand. 2010;89:302–314. doi: 10.3109/00016340903576004. [DOI] [PubMed] [Google Scholar]
- 22.Cattani L, Neefs L, Verbakel JY, et al. Obstetric risk factors for anorectal dysfunction after delivery: a systematic review and meta-analysis. Int Urogynecol J. 2021 doi: 10.1007/s00192-021-04723-z. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence JM, Lukacz ES, Nager CW, et al. Prevalence and co-occurrence of pelvic floor disorders in community-dwelling women. Obstet Gynecol. 2008;111:678–685. doi: 10.1097/AOG.0b013e3181660c1b. [DOI] [PubMed] [Google Scholar]
- 24.Kepenekci I, Keskinkilic B, Akinsu F, et al. Prevalence of pelvic floor disorders in the female population and the impact of age, mode of delivery, and parity. Dis Colon Rectum. 2011;54:85–94. doi: 10.1007/DCR.0b013e3181fd2356. [DOI] [PubMed] [Google Scholar]
- 25.de Santos CR, Santos VLCG. Prevalence of fecal incontinence in the urban population of Puso Alegre-Minas Gerais-Brazil. Rev Esc Enferm USP. 2011;45:180–186. doi: 10.1590/S0080-62342011000100025. [DOI] [PubMed] [Google Scholar]
- 26.Rømmen K, Schei B, Rydning A, et al. Prevalence of anal incontinence among Norwegian women: a cross-sectional study. BMJ Open. 2012;2:1–9. doi: 10.1136/bmjopen-2012-001257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leserman J, Drossman DA. Relationship of abuse history to functional gastrointestinal disorders and symptoms: some possible mediating mechanisms. Trauma Violence Abuse. 2007;8:331–343. doi: 10.1177/1524838007303240. [DOI] [PubMed] [Google Scholar]
- 28.Devroede G (2000) Chapter 10 - Early life abuses in the past history of patients with gastrointestinal tract and pelvic floor dysfunctions. In: Mayer EA, Saper CB (eds) Progress in brain research, vol 122. Elsevier Science, Amsterdam, pp 131–155 [DOI] [PubMed]
- 29.Leroi AM, Bernier C, Watier A, et al. Prevalence of sexual abuse among patients with functional disorders of the lower gastrointestinal tract. Int J Colorectal Dis. 1995;10:200–206. doi: 10.1007/BF00346219. [DOI] [PubMed] [Google Scholar]
- 30.Rao SSC, Tuteja AK, Vellema T, et al. Dyssynergic defecation: demographics, symptoms, stool patterns, and quality of life. J Clin Gastroenterol. 2004;38:680–685. doi: 10.1097/01.mcg.0000135929.78074.8c. [DOI] [PubMed] [Google Scholar]
- 31.Beck JJHH, Elzevier HW, Pelger RCMM, et al. Multiple pelvic floor complaints are correlated with sexual abuse history. J Sexual Med. 2009;6:193–198. doi: 10.1111/j.1743-6109.2008.01045.x. [DOI] [PubMed] [Google Scholar]
- 32.Colavita K, Andy UU. Role of diet in fecal incontinence: a systematic review of the literature. Int Urogynecol J. 2016;27:1805–1810. doi: 10.1007/s00192-016-2979-7. [DOI] [PubMed] [Google Scholar]
- 33.Robson KM, Kiely DK, Lembo T. Development of constipation in nursing home residents. Dis Colon Rectum. 2000;43:940–943. doi: 10.1007/BF02237354. [DOI] [PubMed] [Google Scholar]
- 34.Murakami K, Sasaki S, Okubo H, et al. Association between dietary fiber, water and magnesium intake and functional constipation among young Japanese women. Eur J Clin Nutr. 2007;61:616–622. doi: 10.1038/sj.ejcn.1602573. [DOI] [PubMed] [Google Scholar]
- 35.Muller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol. 2005;100:232–242. doi: 10.1111/j.1572-0241.2004.40885.x. [DOI] [PubMed] [Google Scholar]
- 36.Rao SSC, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41:1256–1270. doi: 10.1111/apt.13167. [DOI] [PubMed] [Google Scholar]
- 37.Suares NC, Ford AC. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment Pharmacol Ther. 2011;33:895–901. doi: 10.1111/j.1365-2036.2011.04602.x. [DOI] [PubMed] [Google Scholar]
- 38.Gyhagen M, Bullarbo M, Nielsen TF, Milsom I. Faecal incontinence 20 years after one birth: a comparison between vaginal delivery and caesarean section. Int Urogynecol J. 2014;25:1411–1418. doi: 10.1007/s00192-014-2390-1. [DOI] [PubMed] [Google Scholar]
- 39.Eslick GD, Talley NJ. Prevalence and relationship between gastrointestinal symptoms among individuals of different body mass index: a population-based study. Obes Res Clin Pract. 2016;10:143–150. doi: 10.1016/j.orcp.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 40.Al-Mukhtar Othman J, Åkervall S, Nilsson IEK, et al. Fecal incontinence in nonpregnant nulliparous women aged 25 to 64 years-a randomly selected national cohort prevalence study. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.11.032. [DOI] [PubMed] [Google Scholar]
- 41.Erekson EA, Sung VW, Myers DL. Effect of body mass index on the risk of anal incontinence and defecatory dysfunction in women. Am J Obstet Gynecol. 2008;198:596.e1–596.e4. doi: 10.1016/j.ajog.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Padwal R, Li SK, Lau DCW. Long-term pharmacotherapy for overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Int J Obes. 2003;27:1437–1446. doi: 10.1038/sj.ijo.0802475. [DOI] [PubMed] [Google Scholar]
- 43.Bytzer P, Talley NJ, Jones MP, Horowitz M. Oral hypoglycaemic drugs and gastrointestinal symptoms in diabetes mellitus. Aliment Pharmacol Ther. 2001;15:137–142. doi: 10.1046/j.1365-2036.2001.00896.x. [DOI] [PubMed] [Google Scholar]
- 44.Ohlsson B, Manjer J. Physical inactivity during leisure time and irregular meals are associated with functional gastrointestinal complaints in middle-aged and elder subjects. Scand J Gastroenterol. 2016;51:1299–1307. doi: 10.1080/00365521.2016.1209786. [DOI] [PubMed] [Google Scholar]
- 45.Catherine P. The Delphi technique: myths and realities. J Adv Nurs. 2003;41:376–382. doi: 10.1046/j.1365-2648.2003.02537.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, PC, upon reasonable request.