Abstract
Mulberry has a good tolerance to cadmium (Cd) and is considered a candidate plant for phytoremediation. The rhizosphere microbial community plays an important role in phytoremediation. Nevertheless, little information on the rhizosphere microbial community mechanisms in mulberry during the phytoremediation of Cd-contaminated soil is available. In this study, the remediation efficiency of mulberry in pots subjected to three simulated Cd pollution levels and their rhizosphere bacterial communities during the remediation process were analyzed. “Yuesang 11” was used as the test mulberry variety, and three simulated Cd pollution levels were set by adding three concentrations of Cd (Cd5, 5 mg kg−1; Cd3, 3 mg kg−1; Cd2, 2 mg kg−1). The results showed that the elimination rates of Cd in the rhizosphere soil were 81.7%, 85.3%, and 57.9% under the stress of the Cd2, Cd3, and Cd5 conditions, respectively. Meanwhile, 3,082,583 high-quality sequence reads and 976 operational taxonomic units were successfully obtained from the mulberry rhizosphere soil by high-throughput absolute quantification sequencing and further assigned to 11 bacterial phyla and 26 families. Of these, decreased abundances of 19 bacteria at the family level and increased abundances of seven bacteria under Cd stress were revealed by comparative analysis. Based on the alpha diversity indices (Chaol, Shannon and Simpson) and principal component analysis, the rhizosphere bacterial diversity of the Cd5 condition was significantly decreased, but that of the Cd2 and Cd3 conditions was not different from that of soil without Cd (CK). Likewise, redundancy analysis showed that the abundances of Acidobacteria Gp2, Acidobacteria Gp13, and Sphingobacteria were significantly positively associated with the elimination rates of Cd. This study suggested that the mulberry rhizosphere contains a relatively stable bacterial community consisting of diverse Cd-resistant bacteria, providing a scientific basis for remediating heavy-metal polluted soils using mulberry.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01090-3.
Keywords: Cd, Mulberry rhizosphere, Bacteria community, Stress response
Introduction
Cadmium (Cd), a heavy metal belonging to group 12 (IIB) [1], is extremely toxic and was first listed among the 12 chemicals that pose global hazards by the United Nations Environment Program in 1984 [2]. A report in China Youth Daily published on 1st February 2012 stated that nearly 20% of arable land was contaminated with heavy metals and that Cd pollution was one of the most serious soil heavy metal pollutants in China [3]. Numerous studies have demonstrated that Cd in the soil not only is toxic to plants but also causes serious health issues for animals and humans by entering the food chain [4]. It is therefore urgent to find ways to effectively remediate Cd-contaminated soils [5].
Phytoremediation is a green technology with good public reception and is a preferable option to clean up heavy metal-contaminated sites [6, 7]. In phytoremediation, most removal of heavy metals occurs because of the activity of microbes in the rhizosphere [8, 9]. Rhizosphere microorganisms have evolved many strategies to counter heavy metal stress, including the active efflux of metals, metal ion sequestration, Cd accumulation, and enzymatic detoxification [10–12]. Therefore, various rhizosphere microbes support different functions, indicating the significance of functional diversity within microbial communities in plant and heavy metal-contaminated soil systems [13, 14]. Therefore, the characterization of rhizosphere microbial diversity and its community is crucial to further understanding microbe-heavy metal interactions in phytoremediation.
Mulberry (Morus alba L.) is a perennial woody plant characterized by large biomass, well-developed roots, and high resistance to drought, salts, and heavy metals [15]. Numerous studies in recent years have focused on the accumulation, mobility, and environmental safety of Cd in the soil-mulberry-silkworm system, indicating that mulberry has an excellent tolerance and enrichment capacity for Cd [16–21]. Although the micro-environmental characteristics of Cd-polluted soil after 3 years of remediation by mulberry using microbiological isolation culture methods have been reported [22], there is still limited information about the interaction of Cd and rhizosphere microbiota in phytoremediation by mulberry because more than 99% of soil bacteria are non-culturable [23]. In this study, the characterization of mulberry rhizosphere bacterial communities using high-throughput sequencing technology and the efficiency of Cd remediation under different simulated Cd pollution conditions were analyzed to further understand the phytoremediation mechanisms of mulberry
Materials and methods
Plant cultivar and soil preparation
This study was conducted using pots. The Yuesang 11 mulberry variety is a Cd-tolerant ecological cultivar originating from Guangdong Province and was used as test material for this study. The test soil was collected from suburban farmland in Nanchang Province, China (116°014′N, 28°375′E). Its physical and chemical properties were as follows: pH 5.02 (soil to water ratio 1:2.5); total nitrogen (TN), 0.85 g kg−1; soil organic matter (SOM), 2.96 g kg−1; total phosphorus (TP), 0.32 g kg−1; total potassium (TK), 68.57 g kg−1; and an undetectable level of total Cd, meeting the third level of soil environmental quality in accordance with GB15618-1995 (Soil Environmental Quality Standards, GB15618-1995, China) [23].
Trial design and index determination
Trial design
Three Cd-contaminated soil levels were prepared by adding soil with 5 mg, 3 mg, or 2 mg of Cd per kg after treatment with CdCl2·2H2O solution [24] to simulate three realistic Cd pollution levels: severe (Cd5), moderate (Cd3), and light (Cd2). Soil without Cd (CK) was used as a blank control. The concentration of CdCl2·2H2O in the stock solution was 1 g L−1. The treated soil was first stabilized for 30 days and then added to one pot; also, the 10g soil per each treatment was collected to analyze the initial concentration of Cd. Finally, one mulberry seedling with consistent growth was planted. All mulberry plants were grown in an illumination incubator at 25±2°C under a 16-h photo-period with a light intensity of 3,600 l×. The soil moisture content was maintained at approximately 60%. The experiment design was a randomized block and each treatment was replicated three times, and each time with 3 pots, for a total of 9 pots per treatment.
Soil sample collection
The rhizosphere soil was collected after 90 days of growth; that mulberry plant was at the vigorous growth period named the eight-leaf stage [25]. Samples were collected as follows: we removed loosely adherent soil from the rhizosphere soil by shaking gently, leaving only a proportion of tightly adherent soil as rhizosphere soil, and three plants per treatment were selected. Approximately 10 g of rhizosphere soil per independent replication for each treatment was mixed, homogenized, and then divided into two parts [26]. One was used to analysis of the final concentration of Cd and its solubility after being air-dried, ground, and sieved at 0.25 mm. The other was transferred to 15-mL sterile centrifuge tubes, stored in a liquid nitrogen tank, and transported to the laboratory for determination of the bacterial community structure.
Analysis of Cd solubility in rhizosphere soils
Cd solubility was analyzed using Tessier extraction [27]. The extracted Cd fractions, including the exchangeable (EXC), carbonate-bound (CAB), Fe-Mn oxide (FMO), organic matter (SOM), and residue (RES) fractions, were extracted and then analyzed by inductively coupled plasma–mass spectrometry (ICP–MS, Thermo 6300) [28]. The elimination rate of Cd was calculated using the following formula: R = (Ci − Cf)/Ci, in this formula, R represented the elimination rate of Cd, Ci represented the initial concentration of Cd, and Cf represented the final concentration of Cd.
DNA extraction and absolute quantification 16S-seq
The DNA extraction of the rhizosphere soil (0.5g) was performed using the MP soil reagent kit (CAT NO. 116560) according to the manufacturer’s instructions. The integrity of the extracted DNA was assessed through agarose gel electrophoresis, and the concentration and purity of extracted DNA were tested using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Inc., USA) and a Qubit 3.0 fluorometer (Invitrogen, Carlsbad, CA, USA ), for assessing the sequence in the library that was between 120 and 200 bp without nonspecific amplification. Profiling was set to 20 ng μL−1, and total content was required to be ≥ 500 ng. To obtain an accurate and reliable absolute abundance of soil bacteria [29], the absolute quantification 16S-seq by MiSeq [30] was conducted in Genesky Biotechnologies Inc., Shanghai, China. The spike-in sequences with identical conserved regions to natural 16S rRNA genes and variable regions replaced by random sequences with approximately 40% GC content were artificially synthesized. Furthermore, nine different spike-in sequences with known gradient copy numbers were added to the sampled DNA pools, functioning as internal standard and allowing the absolution quantification across samples. Thus, the V3-V4 hypervariable regions of microbial 16S rRNA gene and spike-in sequences were amplified with a forward primer (Illumina adapter sequence 1 + CCTACGGGNGGCWGCAG) and reverse primer (Illumina adapter sequence 2 + GACTACHVGGGTATCTAATCC) [31–33].
PCR amplification was achieved on an ABI 2720 thermal cycler (Thermo Fisher Scientific, MA, USA) with a TopTaq DNA polymerase kit (Transgen, Beijing, China). After library quantification, pooling, and quality check, all samples were sequenced using Illumina NovaSeq 6000 (Illumina, USA) sequencing (2×250 bp). Each biological replicate was replicated three times. The detected numbers of spike-in reads can be used for read amount normalization of different samples DNA and further for absolute quantification of microbial taxa.
Illumina MiSeq sequence processing and analysis
The raw read sequences were processed in Quantitative Insights Into Microbial Ecology 2 (QIIME 2™, version 2019.10) [34]. The adaptor and primer sequences were trimmed using the cutadapt plugin. The DADA2 plugin was used for quality control and to identify amplicon sequence variants (ASVs) [35]. The bacteria taxonomic assignments of ASV-representative sequences were performed with a confidence threshold of 0.8 by a pre-trained Naive Bayes classifier in RDP (version 11.5) [36]. Thus, the raw sequencing data which was the bacterial 16S rRNA was deposited in Genome Sequence Archive (GSA) in China National Center for Bioinformation (CNCB) with the accession number CRA009661.
Data analysis
The alpha-diversity (Chaol index, Simpson index, Shannon index) of bacteria was calculated using QIIME2 [34]. Microbiota dissimilarity among the four treatments (Cd2, Cd3, Cd4, and CK) was depicted by principal coordinate analysis (PCoA). Associations between rhizosphere bacteria and environmental parameters (the concentration of different Cd chemical forms and elimination rate of Cd) were performed using the Spearman correlation test. The statistical significance of data was determined using a one-way ANOVA analysis followed by the Duncan’s test (SPSS 12.0, SPSS Inc., USA). The visualization of data and analysis results was all accomplished by R software (v 4.0.5).
Results and analysis
Composition and forms of Cd in mulberry rhizosphere soil subjected to Cd stress
As can be seen in Table 1, there was considerable interspecific variation in the composition and forms of Cd in mulberry rhizosphere soil subjected to Cd stress. Specificity, the total concentrations of Cd in the rhizosphere soil subjected to various treatments differed (0.367 mg/kg for Cd2, 0.440 mg/kg for Cd3, 2.107 mg/kg for Cd5) (Table 1). The speciation of Cd in the rhizosphere soil under different Cd stresses consisted of five forms, including EXC-Cd, FMO-Cd, RES-Cd, CAB-Cd, and SOM-Cd. Among them, the highest relative Cd content was found for EXC-Cd (33.52% for Cd2, 34.78% for Cd3, 44.76% for Cd5), followed by FMO-Cd (28.07% for Cd2, 34.09% for Cd3, 34.15% for Cd5), and the lowest content was found for RES-Cd (3.54% for Cd2, 2.95% for Cd3, 1.9% for Cd5). Additionally, the elimination rate of Cd was highest for the Cd3 condition, 85.3%, followed by the Cd2 condition, 81.7%, and the lowest rate was observed for the Cd5 condition, 57.9%.
Table 1.
Concentration and forms of Cd in mulberry rhizosphere soil subjected to different Cd stress
| Treatment | EXC-Cd | CAB-Cd | FMO-Cd | SOM-Cd | RES-Cd | Total-Cd (mg kg−1) | Elimination rate of Cd | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg kg−1) | Relative content rate(%) | Concentration(mg kg−1) | Relative content rate (%) | Concentration(mg kg−1) | Relative content rate (%) | Concentration(mg Kg−1) | Relative content rate (%) | Concentration(mg kg−1) | Relative content rate (%) | |||
| Cd2 | 0.123±0.001b | 33.52±2.126b | 0.110±0.001b | 29.97±1.854a | 0.103±0.001b | 28.07±3.407ab | 0.017±0.001b | 4.63±0.016a | 0.013±0.001a | 3.54±0.016a | 0.367±0.016b | 0.817±0.026ab |
| Cd3 | 0.153±0.001b | 34.78±2.846b | 0.110±0.001b | 25.00±1.864a | 0.150±0.001b | 34.09±2.564a | 0.013±0.001b | 2.95±0.018bc | 0.013±0.001a | 2.95±0.016ab | 0.440±0.018b | 0.853±0.024a |
| Cd5 | 0.943±0.002a | 44.76±3.715a | 0.350±0.002a | 16.61±1.281b | 0.707±0.003a | 34.15±2.491a | 0.067±0.001a | 3.18±0.014b | 0.040±0.002b | 1.90±0.008b | 2.107±0.013a | 0.579±0.019b |
| CK | ND | ND | ND | ND | ND | ND | ND | |||||
EXC-Cd, exchangeable cadmium; CAB-Cd, carbonate-bound cadmium; FMO-Cd, Fe-Mn-oxide-associated cadmium; SOM-Cd, stands for organic matter-bound cadmium; RES-Cd, residual cadmium; relative content rate = (the concentration of a certain form Cd/the concentration of total Cd)×100%; ND indicates the Cd was not detected; the value represents mean ± standard deviations, and columns with different small letters indicate a significant difference (P=0.05)
Rhizosphere bacterial community in mulberry subjected to different Cd stress levels
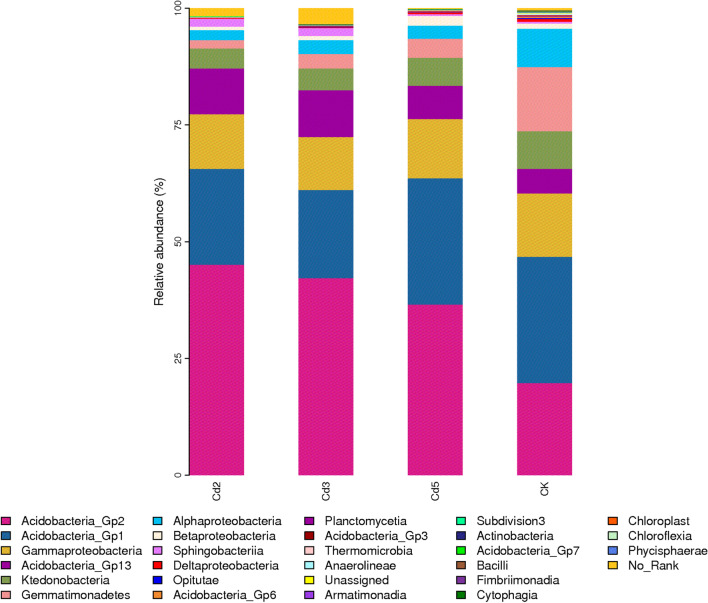
From all rhizosphere soil samples, sequencing analysis yielded a total of 3,082,583 high-quality sequence reads with an average length of approximately 400 bp of effective sequence, 976 OTUs, and a 97% sequence identity threshold. The obtained soil OTUs were assigned to 12 bacterial phyla and 27 classes (Fig. 1, Table 2). However, the rhizosphere bacteria responded differently to Cd stress. The relative abundance of most rhizosphere bacteria at the family level after Cd stress treatment was significantly lower than that in the control (CK), including Acidobacteria Gp1, Gammaproteobacteria, Betaproteobacteria, and Gemmatimonadetes. Nevertheless, seven classes, including Acidobacteria Gp2, Acidobacteria Gp13, Alphaproteobacteria, Sphingobacteriia, Chloroflexia, Anaerolineae, and Fimbriimonadia, exhibited an increased relative abundance in the Cd stress condition compared to the CK condition, and a negative correlation with the concentrations was observed in the Cd stress condition.
Fig. 1.
Comparisons of the absolute abundance of rhizosphere bacterial (at the family level) of mulberry subjected to different Cd stress
Table 2.
The absolute abundance of the rhizosphere bacteria in mulberry subjected to different Cd stress
| Taxon | Treatment | ||||
|---|---|---|---|---|---|
| phylum | family | Cd2 | Cd3 | Cd5 | CK |
| Acidobacteria | Acidobacteria Gp2 | 2409a | 2253ab | 1953b | 1054c |
| Acidobacteria Gp1 | 1097b | 1008b | 1443a | 1443a | |
| Acidobacteria Gp13 | 523a | 533a | 379b | 282c | |
| Acidobacteria Gp6 | 1b | 0b | 4b | 17a | |
| Acidobacteria Gp3 | 5b | 3b | 4b | 27a | |
| Acidobacteria Gp7 | 2b | 0b | 3b | 13a | |
| Proteobacteria | Gammaproteobacteria | 622b | 608b | 679ab | 725a |
| Alphaproteobacteria | 117b | 161b | 150b | 436a | |
| Betaproteobacteria | 38b | 47b | 59b | 109a | |
| Deltaproteobacteria | 5b | 10b | 17b | 30a | |
| Bacteroidetes | Sphingobacteriia | 93a | 91a | 22b | 22b |
| Cytophagia | 3a | 1b | 1b | 1b | |
| Actinobacteria | Actinobacteria | 73a | 43b | 21c | 15cd |
| Chloroflexi | Ktedonobacteria | 229c | 250c | 322b | 430a |
| Thermomicrobia | 4b | 3b | 4b | 46a | |
| Unassigned | 2b | 3b | 2b | 28a | |
| Chloroflexia | 0b | 0b | 0b | 65a | |
| Anaerolineae | 1b | 0b | 1b | 13a | |
| Gemmatimonadetes | Gemmatimonadetes | 96bc | 163b | 217b | 735a |
| Armatimonadetes | Fimbriimonadia | 0b | 2b | 1b | 14a |
| Armatimonadia | 2b | 3b | 4b | 34a | |
| Verrucomicrobia | Opitutae | 0b | 4b | 5b | 64a |
| Subdivision3 | 2b | 3b | 4a | 3b | |
| Planctomycetes | Planctomycetia | 2b | 6b | 4b | 28a |
| Firmicutes | Bacilli | 0b | 1b | 0b | 36a |
| Chloroplast | Chloroplast | 1b | 0b | 1b | 21a |
| Others | No rank | 90b | 181a | 5c | 22b |
Different lowercase letters in the same line indicate a significant difference (P < 0.05) between different treatments
Alpha diversity of the rhizosphere bacterial community in mulberry subjected to different Cd stress levels
The alpha diversities of rhizosphere soil bacteria in each treatment were analyzed based on the Chao1 and Simpson and Shannon diversity indices and are shown in Table 3. Compared with the control values, the Shannon diversity index and the Chaol index were decreased, while the Simpson diversity index was increased in Cd-stressed soil. Interestingly, for the Shannon, Chaol, and Simpson indices, there was a significant difference only between the Cd5 treatment and the control (p=0.023, 0.047, 0.042).
Table 3.
Alpha diversity of rhizosphere bacteria community in mulberry subjected to different Cd stress
| Treatment | Chaol | Simpson | Shannon |
|---|---|---|---|
| Cd2 | 250.01±21.63ab | 0.0549±0.0017b | 3.19±0.19a |
| Cd3 | 256.36±19.27ab | 0.0533±0.0018b | 3.27±0.27a |
| Cd5 | 225.20±18.46b | 0.0573±0.0013a | 3.02±0.24b |
| CK | 260.62±19.34a | 0.0531±0.0021b | 3.33±0.25a |
Columns with different small letters indicate significant differences (P=0.05)
Changes in the rhizosphere bacterial community in mulberry under different Cd stress levels
To further understand the rhizosphere bacterial community response to Cd stress, changes in the mulberry rhizosphere bacterial community under Cd stress were detected using PCoA. The PCoA biplot with scores and loading in Fig. 2 displays the variance between the samples. The first principal component (PC1) accounts for 63.49% of the variance between samples, and the second principal component (PC2) accounts for 13.36% of the variance between samples. The bacterial communities in the Cd2 and Cd3 conditions were the most similar but were significantly different from those in the Cd5 and CK conditions, indicating that low-concentration Cd stress promoted the richness of rhizosphere soil bacteria, while it inhibited the richness and diversity.
Fig. 2.
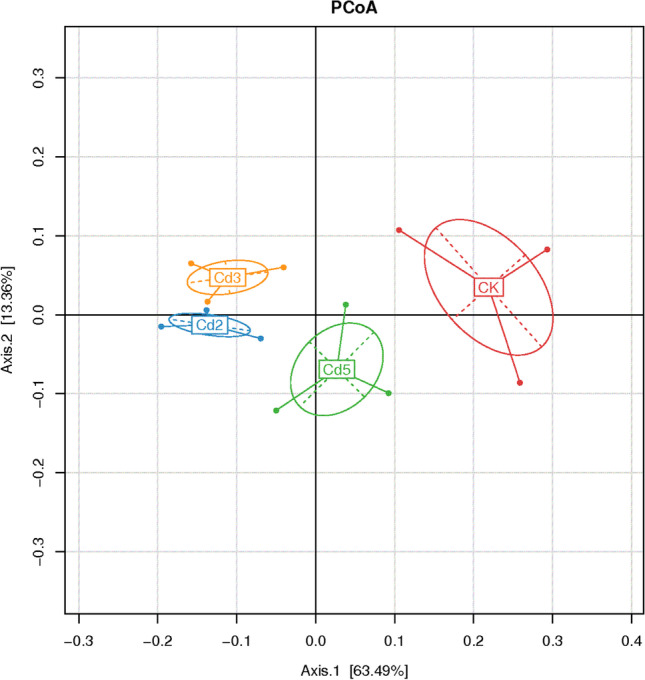
PCoA analysis of the mulberry rhizosphere bacterial community under different Cd stress. This PCoA plot was generated using the matrix of unweighted Unifrac distances. A point represents a sample in the plot. Axis 1 represents 63.49% of the variation in the bacteria community genetic distance and axis 2 represents 13.36% of the variation in the bacteria community genetic distance among samples
Correlation between rhizosphere bacteria and the forms and elimination rate of Cd
To further reveal the intrinsic connection of the rhizosphere soil bacteria and Cd forms as well as the elimination rate of Cd, the relative abundance of 26 classes of bacteria in the mulberry rhizosphere, Cd occurrence concentration, and the elimination rate of Cd were examined using the Spearman correlation analysis. The correlation coefficients (Table 4), indicated by the heatmap shown in Fig. 3, showed that the Cd concentration (TOTAL-Cd) was significantly negatively correlated with the relative abundance of Alphaproteobacteria, Fimbriimonadia, and Chloroflexia (p<0.05), with correlation coefficients of −0.676, −0.595, and −0.646, respectively. Acidobacteria Gp3 was significantly negatively correlated with EXC-Cd (p<0.05), with a correlation coefficient of −0.579. Anaerolineae was significantly negatively correlated with EXC-Cd, CAB-Cd, and RES-Cd (p<0.05), with correlation coefficients of −0.636, −0.603, and −0.656, respectively. Acidobacteria Gp2, Acidobacteria Gp13, and Sphingobacteriia were significantly positively correlated with the Cd elimination rate in soil, with correlation coefficients of 0.774, 0.589, and 0.746, respectively. However, Acidobacteria Gp1, Ktedonobacteria, Gemmatimonadetes, Deltaproteobacteria, Acidobacteria Gp6, and Anaerolineae were significantly negatively correlated with the Cd elimination rate in soil (p<0.05), with correlation coefficients of −0.634, −0.704, −0.676, −0.606, −0.749, and −0.669, respectively.
Table 4.
Correlation between rhizosphere bacteria and the forms and elimination rate of Cd
| Rhizosphere bacteria | Factor | Correlation coefficients | P value |
|---|---|---|---|
| Alphaproteobacteria | TOTAL-Cd | −0.676 | 0.015* |
| Fimbriimonadia | TOTAL-Cd | −0.595 | 0.041* |
| Chloroflexia | TOTAL-Cd | −0.645 | 0.023* |
| Acidobacteria_Gp3 | EXC-Cd | −0.578 | 0.048* |
| Anaerolineae | EXC-Cd | −0.636 | 0.026* |
| Anaerolineae | CAB-Cd | −0.602 | 0.037* |
| Anaerolineae | RES-Cd | −0.655 | 0.020* |
| Acidobacteria_Gp2 | Elimination rate of Cd | 0.774 | 0.003** |
| Acidobacteria_Gp1 | Elimination rate of Cd | −0.633 | 0.026* |
| Acidobacteria_Gp13 | Elimination rate of Cd | 0.589 | 0.043* |
| Ktedonobacteria | Elimination rate of Cd | −0.704 | 0.010* |
| Gemmatimonadetes | Elimination rate of Cd | −0.676 | 0.015* |
| Sphingobacteriia | Elimination rate of Cd | 0.746 | 0.005** |
| Deltaproteobacteria | Elimination rate of Cd | −0.606 | 0.036* |
| Acidobacteria_Gp6 | Elimination rate of Cd | −0.749 | 0.004** |
| Anaerolineae | Elimination rate of Cd | −0.669 | 0.017* |
Asterisk (*) and (**) indicates significant difference between rhizosphere bacteria and the forms and elimination rate of Cd at P<0.05 and P<0.01
Fig. 3.
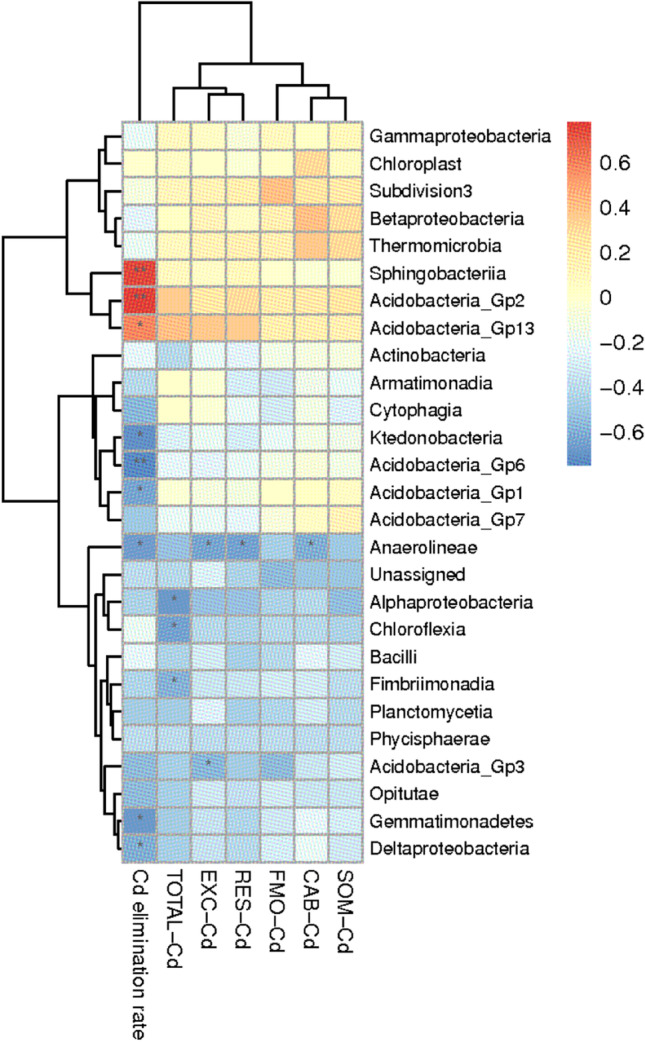
Heatmap of correlation between rhizosphere bacteria and the forms and elimination rate of Cd. The values in the scale bar mean the correlation coefficient between individual bacteria at the level of class and the forms and elimination rate of Cd, and the asterisks indicate statistically significant differences according to Kruskal-Wallis tests (*P < 0.05 and **P < 0.01) in the plot
Discussion
In our study, A great difference in the elimination rate of Cd in mulberry rhizosphere soil was observed, with Cd elimination rates of 85.3% in the Cd3, 81.7% in the Cd2, and 57.9% in the Cd5 conditions, demonstrating that the Cd elimination capacity of mulberry rhizosphere soil initially increased and then decreased with increasing Cd stress level, which might be related to the strong accumulating and translocating heavy metals capacity of mulberry [21, 37–39]. Additionally, a similar phenomenon in which low concentrations of heavy metals may promote the accumulation capacity of heavy metals and higher concentrations inhibit accumulation capacity has also been observed in other hyperaccumulator plants, such as Alfalfa [40] and Amaranthus caudatus [41].
Generally, when faced with heavy metal stress, the rhizosphere soil microbial system responds rapidly and sensitively, leading to rapid iteration and complex shifts in bacterial community structures [42–44]. Studies have found that the abundance of some low-resistance microorganisms, such as Proteobacteria and Bacillus, is reduced by heavy metal toxicity [45, 46], but the abundance of metal-resistant bacteria, such as Bacteroidetes and Acidobacteria, is increased [47, 48]. Similar results were found in our study. Most bacteria in mulberry rhizosphere soil displayed a decreased abundance, while seven classes, Acidobacteria Gp2, Acidobacteria Gp13, Alphaproteobacteria, Sphingobacteriia, Chloroflexia, Anaerolineae, and Fimbriimonadia, showed an increased abundance.
Several studies have shown that chronic exposure to heavy metals reduces the biomass of microorganisms and their activity as well as the diversity of their communities [49, 50]. Normally, microbial community diversity is positively correlated with heavy metal pollutant concentrations [42]. However, as shown in Table 4, the Shannon, Chaol, and Simpson indices, as comprehensive indicators of species richness and evenness [51], showed a significant difference under Cd stress at concentrations less than 3 mg kg−1, while these indices significantly decreased under Cd stress at 5 mg kg−1 compared with the control condition. In addition, PCoA (Fig. 2) showed that the bacterial community structures of mulberry rhizosphere soil in the Cd2 and Cd3 conditions were similar and were significantly different from those in the Cd5 and CK conditions. This result indicated that the mulberry rhizosphere bacterial diversity could tolerate elevated Cd concentrations, except for excessively high Cd concentrations. A similar finding was reported by Wang et al. [52], in which low mercury concentration had accelerated the growth of pakchoi and increased the abundance of nitrogen-fixing microorganism and the richness of the community, whereas the medium and high mercury concentration had represented inhibiting effect.
Furthermore, in this study, the correlation heatmap showed that Acidobacteria Gp2, Acidobacteria Gp13, and Sphingobacteriia were significantly positively correlated with the rate of Cd decline in soil, indicating that these bacteria have the competitive advantages of material circulation and soil energy transformation, thus becoming the dominant bacteria participating in heavy metal accumulation and compartmentalization.
Bacteria with similar functions included Actinobacteria, Firmicutes, Actinomycetes, Acidobacteria, and Proteobacteria [42, 53]. Additionally, Alphaproteobacteria, Fimbriimonadia, and Chloroflexia were significantly negatively correlated with the Cd concentration (TOTAL-Cd), Acidobacteria Gp3 was significantly negatively correlated with EXC-Cd, and Anaerolineae was significantly negatively correlated with EXC-Cd, CAB-Cd, and RES-Cd. These results indicate that the functional bacteria targeting the detoxification and tolerance of Cd pollution in the mulberry rhizosphere soil represent adaptive differentiation, which in turn affected the rhizoremediation rate and performance [54, 55], consistent with previous reports [56–58]. The results also further confirm that rhizosphere microorganisms contribute to metal speciation in soil, which may be a key factor for effective phytoremediation [59]. Therefore, a new method for bioremediation using the synergistic relationships between plants and microbes in the rhizosphere has been created and applied in the remediation of heavy metal contamination in soil, which will be the main focus of further study.
Supplementary information
(DOCX 80 kb)
Author contributions
Experimental design and writing—original draft preparation, Guiping Hu; the determination of Cd solubility, Hongmei Cao; writing—review and editing, Chuan Ye; sequencing data analysis, Feng Wang. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Youth Foundation of Jiangxi Province (20202BABL215022), the High-level and high-skilled leading personnel training project of Jiangxi Province (2022) and the Modern Agricultural Industry Technology System of Jiangxi Province (JARS-23-3).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rapisarda V, Miozzi E, Loreto C, Matera S, Fenga C, Avola R, Ledda C. Cadmium exposure and prostate cancer: insights, mechanisms and perspectives. Front Biosci (Landmark Ed) 2018;23:1687–1700. doi: 10.2741/4667. [DOI] [PubMed] [Google Scholar]
- 2.Xu YN, Niu ML, Zhang XY. Review of heavy metals pollution in China: source and status. Resour Econ Environ Prot. 2013;2:55. [Google Scholar]
- 3.Liu XD, Liu F, Huang WH, Peng JY, Shen TT, He Y. Quantitative determination of Cd in soil using laser-induced breakdown spectroscopy in air and air conditions. Molecules. 2018;23:2492. doi: 10.3390/molecules23102492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanika K, Sukhmeen KK, Puja O, Renu B, Asma AA, Manzer HS, Ghada SA, Parvaiz A. Microbial fortification improved photosynthetic efficiency and secondary metabolism in lycopersicon esculentum plants under Cd stress. Biomolecules. 2019;9(10):581. doi: 10.3390/biom9100581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng TY, He XL, Zhou RY, Qiao GR, Han XJ, Qiu WM, Chi LF, Zhang DY, Liu MY. Identification and functional characterization of ABCC transporters for Cd tolerance and accumulation in Sedum alfredii hance. Sci Rep. 2020;10:20928. doi: 10.1038/s41598-020-78018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo Y, Liu F, Ren J. Research progress on evaluation methods of phytoremediation effectiveness of soil contaminated with heavy metal. Appl Chem Ind. 2020;49(3):755–760. [Google Scholar]
- 7.Wang PY, Chao DY. Phytoremediation of heavy metal contamination and related molecular mechanisms in plants. Chin J Biotechnol. 2020;36(3):426–435. doi: 10.13345/j.cjb.190332. [DOI] [PubMed] [Google Scholar]
- 8.Ding QB, Chao YQ, Wang SZ, Chen Y, Qiu R. Research on function of rhizosphere microbial diversity in phytoremediation of heavy metal polluted soils. J South China Nor Univer. 2016;48(2):1–12. [Google Scholar]
- 9.Lian TX, Huang YY, Xie XN, Huo XH, Muhammad QS, Tian L, Lan T, Jin J. Rice SST variation shapes the rhizosphere bacterial community, conferring tolerance to salt stress through regulating soil metabolites. mSystems. 2020;5(6):e00721-20. doi: 10.1128/mSystems.00721-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Ouyang LM, Kong PJ, Yang ZY, Wu W, Zhu DL, Zhang LL. Rhizospheria bacteria of Poplus euphratica improve resistance of wood plants to heavy metals. Chin J Appl Ecol. 2015;26(9):2811–2816. [PubMed] [Google Scholar]
- 11.Luo CG, Deng YW, Liang J, Zhu SP, Wei ZY, Guo XB, Luo XP. Exogenous rare earth element-yttrium deteriorated soil microbial community structure. J Rare Earths. 2018;36(4):430–439. [Google Scholar]
- 12.Ma Y, Luo YM, Teng Y. Plant growth promoting rhizobacteria and their role in phytoremediation of heavy metal contaminated soils. Acta Pedol Sin. 2013;50(5):1021–1031. [Google Scholar]
- 13.Li J, Jin Z, Gu Q. Effect of plant species on the function and structure of the bacterial community in the rhizosphere of leadzinc mine tailings in Zhejiang China. Can J Microbiol. 2011;57(7):569–577. doi: 10.1139/w11-054. [DOI] [PubMed] [Google Scholar]
- 14.Wagg C, Schlaeppi K, Banerjee S, Kuramae EE, van der Heijden MGA. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat Commun. 2019;10:4841. doi: 10.1038/s41467-019-12798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Huang R, Jiang S, Qin Z, Yan X. Adsorption of Cd(II) by rhizosphere and non-rhizosphere soil originating from mulberry field under laboratory condition. Int J Phytoremediat. 2018;20:378–383. doi: 10.1080/15226514.2017.1393396. [DOI] [PubMed] [Google Scholar]
- 16.Li XY, Chen TB, Tan YB, Fu BT, Yang J, Song B, Yang SC, Xie YF. Concentrations and risk of heavy metals in grain of wheat grown in Beijing. Geogr Res. 2008;6:1340–1346. [Google Scholar]
- 17.Lei M, Pan Y, Chen C, Du H, Tie B, Yan X, Huang R. Application of economic plant for remediation of cadmium contaminated soils: three mulberry (Moms alba L.) varieties cultivated in two polluted fields. Chemosphere. 2019;236:124379. doi: 10.1016/j.chemosphere.2019.124379. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Jiang S, Yan X, Qin Z, Jia C, Li Z, Zhang J, Huang R. The mobility of cadmium and lead in the soil-mulberry-silkworm system. Chemosphere. 2020;242:125179. doi: 10.1016/j.chemosphere.2019.125179. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Jiang S, Li Z, Yan X, Qin Z, Huang R. Field scale remediation of Cd and Pb contaminated paddy soil using three mulberry (Morus alba L.) cultivars. Ecol Eng. 2019;129:38–44. [Google Scholar]
- 20.Jiang YB, Yan XP, Huang RZ, Jiang SM, Zhang J, Qing ZX, Li ZB. Ecological application of mulberry. Chi Sericul. 2018;39(3):45–51. [Google Scholar]
- 21.Huang RZ, Li YP, Jiang YB, Jia CH, Jiang SM, Yan XP, Qin ZX, Luo J. Effect of cadmium and lead combined stress on growth of mulberry saplings and contents of heavy metal in mulberry leaf. Sci Sericul. 2018;44(5):665–671. [Google Scholar]
- 22.Xu N, Hu GP, Shi XP, Wang LX, Cao HM, Wang JW, Du XM, Ye WG. Micro-environmental characteristics of Cd and Pb polluted soil after remediation by mulberry. Chin Agricul Sci Bulletin. 2019;35(24):66–72. [Google Scholar]
- 23.Pham Van HT, Kim J. Cultivation of unculturable soil bacteria. Trends Biotechnol. 2012;30(9):475–484. doi: 10.1016/j.tibtech.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Fedyaeva ОА, Poshelyuzhnaya EG, Trenikhin MV, Zakharov VA, Kuleshov DA, Fisenko TE, Lutayeva IA. Optical properties research of cadmium sulphide nanoparticles received by the interaction of the reverse emulsions based on sodium bis(2-ethilhexyl) sulfosuccinate. Proce Eng. 2016;152:40–44. [Google Scholar]
- 25.Ye JJ, Yin H, Sun B, Shi XQ, Duan ZA, Cui WZ. An investigation to the contents of 1-deoxynojimycin in mulberry tree. Sci Sericul. 2009;35(4):722–727. [Google Scholar]
- 26.Bu WS, Chen FS, Wang FC, Fang XM, Mao R, Wang HM. The species specific responses of nutrient resorption and carbohydrate accumulation in leaves and roots to nitrogen addition in a subtropical mixed plantation. Can J For Res. 2019;49:826–835. [Google Scholar]
- 27.Tessier A, Campbell PGC, Bisson M. Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem. 1979;51:844–851. [Google Scholar]
- 28.Cheng S, Liu G, Zhou C, Sun R. Chemical speciation and risk assessment of cadmium in soils around a typical coal mining area of China. Ecotox Environ Safe. 2018;160:67–74. doi: 10.1016/j.ecoenv.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Stokell JR, Hamp TJ, Steck TR. Examining changes in bacterial abundance in complex communities using next-generation sequencing is enhanced with quantitative PCR. Anton Leeuw. 2016;109(8):1161–1166. doi: 10.1007/s10482-016-0707-4. [DOI] [PubMed] [Google Scholar]
- 30.Tkacz A, Hortala M, Poole PS. Absolute quantitation of microbiota abundance in environmental samples. Microbiome. 2018;6(1):110. doi: 10.1186/s40168-018-0491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotthauwe JH, Witzel KP, Liesack W. The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol. 1997;63(12):4704–4712. doi: 10.1128/aem.63.12.4704-4712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang S, Yu Y, Gao R, Wang H, Zhang J, Li R, Long X, Shen Q, Chen W, Cai F. High-throughput absolute quantification sequencing reveals the effect of different fertilizer applications on bacterial community in a tomato cultivated coastal saline soil. Sci Total Environ. 2019;687:601–609. doi: 10.1016/j.scitotenv.2019.06.105. [DOI] [PubMed] [Google Scholar]
- 33.Munyaka PM, Eissa N, Bernstein CN, Khafipour E, Ghia JE. Antepartum antibiotic treatment increases offspring susceptibility to experimental colitis: a role of the Gut Microbiota. PloS One. 2015;10:e0142536. doi: 10.1371/journal.pone.0142536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolyen E, Rideout J, Dillon M, Bokulich N, Abnet C, Al-Ghalith G, Alexander H, Alm E, Arumugam M, Asnicar F, Bai Y, Bisanz J, Bittinger K, Brejnrod A, Brislawn C, Brown C, Callahan B, Caraballo-Rodríguez A, Chase J, Cope E, Da Silva R, Diener C, Dorrestein P, Douglas G, Durall D, Duvallet C, Edwardson C, Ernst M, Estaki M, Fouquier J, Gauglitz J, Gibbons S, Gibson D, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley G, Janssen S, Jarmusch A, Jiang L, Kaehler B, Kang K, Keefe C, Keim P, Kelley S, Knights D, Koester I, Kosciolek T, Kreps J, Langille M, Lee J, Ley R, Liu Y, Loftfeld E, Lozupone C, Maher M, Marotz C, Martin B, McDonald D, McIver L, Melnik A, Metcalf J, Morgan S, Morton J, Naimey A, Navas-Molina J, Nothias L, Orchanian S, Pearson T, Peoples S, Petras D, Preuss M, Pruesse E, Rasmussen L, Rivers A, Robeson M, Rosenthal P, Segata N, Shafer M, Shifer A, Sinha R, Song S, Spear J, Swaford A, Thompson L, Torres P, Trinh P, Tripathi A, Turnbaugh P, Ul-Hasan S, van der Hooft J, Vargas F, VázquezBaeza Y, Vogtmann E, von Hippel M, Walters W, Wan Y, Wang M, Warren J, Weber K, Williamson C, Willis A, Xu Z, Zaneveld J, Zhang Y, Zhu Q, Knight R, Caporaso J. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiao Y, Zhou JX, Wang XP. Effects of lead and cadmium combined stress on seed germination and seedling growth of mulberry. J Beijing Fores Univer. 2020;42(4):32–40. [Google Scholar]
- 38.Jiang YB, Jiang SM, Huang RZ, Wang M, Cao H, Li ZB. Accumulation of Cd by three forage mulberry (Morus atropurpurea Roxb.) cultivars in heavy metal-polluted farmland: a field experiment. Environ Sci Pollut Res. 2021;28:3354–3360. doi: 10.1007/s11356-020-10744-w. [DOI] [PubMed] [Google Scholar]
- 39.Wang KR, Gong H, Wang Y, Seatm Van DZ. Toxic effects of cadmium on Morus alba L. and Bombyx moril L. Plant Soil. 2004;261:171–180. [Google Scholar]
- 40.María LF, Sabrine H, Sarra HHB, Mohammed B, Luis EH. Specific mechanisms of tolerance to copper and cadmium are compromised by a limited concentration of glutathione in alfalfa plants. Plant Sci. 2015;233:165–173. doi: 10.1016/j.plantsci.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Anita S, Sheo MP. Effect of agro-industrial waste amendment on Cd uptake in amaranthus caudatus grown under contaminated soil: an oxidative biomarker response. Ecotox Environ Safe. 2014;100:105–113. doi: 10.1016/j.ecoenv.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Cavalca L, Corsini A, Canzi E, Zanchi R. Rhizobacterial communities associated with spontaneous plant species in long-term arsenic contaminated soils. World J Microbiol Biotechnol. 2015;31(5):735–746. doi: 10.1007/s11274-015-1826-1. [DOI] [PubMed] [Google Scholar]
- 43.Rastogi G, Sbodio A, Tech JJ, Trevor VS, Gitta LC, Leveau HJJ. Leaf microbiota in an agroecosystem: spatiotemporal variation in bacterial community composition on field-grown lettuce. Int Soc Microb Ecol J. 2012;6(10):1816–1822. doi: 10.1038/ismej.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu ZY, Tang M, Chen H, Ban YH, Zhang HH. Microbial community structure in the rhizosphere of sophora viciifolia grown at a lead and zinc mine of northwest China. Sci Total Environ. 2012;435-436:453–5464. doi: 10.1016/j.scitotenv.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 45.Du M, Zheng MG, Liu AF, Wang L, Pan X, Liu J, Ran XB. Effects of emerging contaminants and heavy metals on variation in bacterial communities in estuarine sediments. Sci Total Environ. 2022;832:155118. doi: 10.1016/j.scitotenv.2022.155118. [DOI] [PubMed] [Google Scholar]
- 46.Geng N, Xia YF, Lu DB, Bai Y, Zhao YF, Wang H, Ren LX, Xu CD, Hu ET, Sun GJ, Chen XY. The bacterial community structure in epiphytic biofilm on submerged Macrophyte Potamogetom crispus L. and its contribution to heavy metal accumulation in an urban industrial area in Hangzhou. J Hazard Mater. 2022;430:128455. doi: 10.1016/j.jhazmat.2022.128455. [DOI] [PubMed] [Google Scholar]
- 47.Cui H, Ou Y, Wang LX, Yan BX, Li YX, Ding DW. Phosphate rock reduces the bio-availability of heavy metals by influencing the bacterial communities during aerobic composting. J Integr Agr. 2021;5(20):1137–1146. [Google Scholar]
- 48.Feng XY, Wang QL, Sun YH, Zhang SW, Wang FY. Micro-plastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J Hazard Mater. 2022;424:127364. doi: 10.1016/j.jhazmat.2021.127364. [DOI] [PubMed] [Google Scholar]
- 49.Baćmaga M, Borowik A, Kucharski J, Tomkiel M, Wyszkowska J. Microbial and enzymatic activity of soil contaminated with a mixture of diflufenican + mesosulfuron - methyl + iodosulfuron - methyl - sodium. Environ Sci Pollut Res Int. 2015;22:643–656. doi: 10.1007/s11356-014-3395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Fang L, Lin L, Luan T, Tam NF. Effects of low molecular-weight organic acids and dehydrogenase activity in rhizosphere sediments of mangrove plants on phytoremediation of polycyclic aromatic hydrocarbons. Chemosphere. 2014;99:152–159. doi: 10.1016/j.chemosphere.2013.10.054. [DOI] [PubMed] [Google Scholar]
- 51.Wu B, Luo H, Wang X, Liu H, Peng H, Sheng M, Xu F, Xu H. Effects of environmental factors on soil bacterial community structure and diversity in different contaminated districts of southwest China mine tailings. Sci Total Environ. 2022;802:149899. doi: 10.1016/j.scitotenv.2021.149899. [DOI] [PubMed] [Google Scholar]
- 52.Wang XY, Zhao H, Tan ZY, Lu ZH, Song XJ. Effect of mercury stress on pakchoi growth, soil nitrogen-fixing microorganism community structure and abundance. Chi Vegeta. 2022;8:63–72. [Google Scholar]
- 53.Ge Y, Lou YH, Xu MM, Wu C, Jun M, Lei S, Fang X, Yan X. Spatial distribution and influencing factors on the variation of bacterial communities in an urban river sediment. Environ Pollut. 2021;272(1):115984. doi: 10.1016/j.envpol.2020.115984. [DOI] [PubMed] [Google Scholar]
- 54.Li YP, Sun MJ, He W, Wang H, Pan H, Yang QG, Lou YH, Zhang YP. Effect of phosphorus supplementation on growth, nutrient uptake, physiological responses, and cadmium absorption by tall fescue (festuca arundinacea schreb.) exposed to cadmium. Ecotoxicol Environ Saf. 2021;213:112021. doi: 10.1016/j.ecoenv.2021.112021. [DOI] [PubMed] [Google Scholar]
- 55.Zhang C, Nie S, Liang J, Zeng GG, Wu HP, Hua SS, Liu JY, Yuan YJ, Xiao HB, Deng LJ, Xiang HY. Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci Total Environ. 2016;557-558:785–790. doi: 10.1016/j.scitotenv.2016.01.170. [DOI] [PubMed] [Google Scholar]
- 56.Hemme CL, Green SJ, Rishishwar L, Prakash O, Pettenato A, Chakraborty R, Deutschbauer AM, Van Nostrand JD, Wu LY, He ZL, Jordan IK, Hazen TC, Arkin AP, Kostka JE, Zhou JZ. Lateral gene transfer in a heavy metal contaminated groundwater microbial community. MBio. 2016;7(2):e02234–e02215. doi: 10.1128/mBio.02234-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martínez-Bussenius C, Navarro CA, Jerez CA. Microbial copper resistance: importance in biohydrometallurgy. Microb Biotechnol. 2017;10(2):279–295. doi: 10.1111/1751-7915.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ijaz A, Imran A, Anwarul Haq M, Khan QM, Afzal M. Phytoremediation: recent advances in plant-endophytic synergistic interactions. Plant Soil. 2016;405:179–195. [Google Scholar]
- 59.Meena VS, Meena SK, Verma JP, Kumar A, Aeron A, Mishra PK, Bisht JK, Pattanayak A, Naveed M, Dotaniya M. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: a review. Ecol Eng. 2017;107:8–32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 80 kb)