Abstract
Pseudomonas aeruginosa is a common infectious agent associated with respiratory diseases in boas and pythons, however, the histopathology, resistance and virulence are yet described for this species. In this study, we investigated a dying Burmese python rescued from tropical rainforest in Hainan. Clinical signs were open-mouthed breathing, abnormal shedding and anorexia. Abundant yellow mucopurulent secretions were observed in highly ectatic segmental bronchi by postmortem. Histopathological lesions included systemic pneumonia, enteritis, nephritis and carditis. P. aeruginosa was the only species isolated from heart blood, kidney, trachea and lung. The phenotype analysis demonstrated that the isolates had strong biofilm, and were sensitive to amikacin, spectinomycin, ciprofloxacin, norfloxacin and polymyxin B, moreover, the LD50 of the most virulent isolate was 2.22×105 cfu/mL in a zebrafish model. Molecular epidemiological analysis revealed that the isolates belonged to sequence type 3495, the common gene patterns were toxA + exoSYT + phzIM + plcHN in virulence and catB + blaTEM + ant (3'')-I+ tetA in resistance. This study highlights that P. aeruginosa should be worth more attention in wildlife conservation and raise the public awareness for the cross infection and cross spread between animals and human.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01038-7.
Keywords: Pseudomonas aeruginosa, Burmese python, Histopathology, Virulence, Resistance, Biofilm
Highlights
• Epidermal cells of multiple organs were invaded by Pseudomonas aeruginosa in the wild Burmese python.
• Common gene patterns in the isolates were toxA + exoSYT + phzIM + plcHN for virulence and catB + blaTEM + ant (3'')-I + tetA for resistance.
• Biofilm was necessary for virulence of the isolates infection in a zebrafish model.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01038-7.
Introduction
The Burmese python (Python bivittatus) is one of the longest and heaviest non-venomous pythons in the world. This reptile is native to south and southeast Asia, including the south of China (for example, Hainan province and Hong Kong city) [1]. Currently, the population is largely declining in the wild as a result of illegal capture, overexploitation, diseases and natural habitat loss, and the Burmese python has been listed in CITES Appendix II, of the IUCN red list (https://cites.org/eng/app/appendices.php or https://www.iucnredlist.org/en) as vulnerable and is nationally protected in China [2, 3].
Respiratory tract disease, such as Mycoplasma agassizii infection [4], is common in captive and wild Burmese pythons, although few data are available, According to our research, pneumonia and tracheitis are typically caused by opportunistic Gram-negative bacterial infections, such as Salmonella enterica subsp. houtenae and Pseudomonas aeruginosa [5, 6], but it is noticed only when the disease is advanced and the clinical signs and symptoms are very obvious, which leads to high mortality rates among captive pythons.
P. aeruginosa is a ubiquitous pathogen in natural settings that causes infection not only of the respiratory system, urinary tract, blood and other parts of the body in humans and animals, but also causes rotten root in plants and environmental contaminations, particularly those strains with increased antibiotic resistance [7–10]. The pathogenesis, virulence factors, antibiotic resistance and interactions with host have been intensively studied for this pathogen. P. aeruginosa displays a vast repertoire of virulence factors, antibiotic resistance determinants, regulatory circuits and signaling systems, which confer remarkable plasticity and adaptability. The main virulence factors include cell-associated lipopolysaccharide, biofilm, motility structures, the quorum sensing system, secretion systems, two-component systems, extracellular outer membrane vesicles, cytotoxins, and antioxidant enzymes and their regulation [11]. P. aeruginosa readily acquires high resistance to various antibiotics (for example aminoglycosides, quinolones and β-lactams) through a myriad of intrinsic and acquired mechanisms, including outer membrane permeability, efflux systems, antibiotic-inactivating enzyme and biofilm-mediated adaptive antibiotic resistance [12]. Histologically, severe immune cell infiltration into the bronchioles, severe alveolitis with pulmonary infiltrates and hemorrhagic necrosis are the common signs in the infected lungs of patients, a mouse model and in farmed mink, respectively [13–15]. The infection of tropical snakes (one Python molurus and two Boa constrictors) by P. aeruginosa was also described in 2013 as deposition of fibrinoid in the walls of the myocardial blood vessels, necrotic stomatitis and necrotic exudative pneumonia, hyaline droplet degeneration of the hepatocytes and the kidney epithelium, diffuse fibrinoid degeneration of the connective tissue within all viscera and focal infiltrations of pseudoeosinophile leukocytes in the spleen [16]. However, the pathogenesis of P. aeruginosa infection in the Burmese python is yet to be elucidated, but is necessary for the effective and conservation of this reptile. This study reports a natural-P. aeruginosa-infection case in a wild Burmese python and reveals the histopathology, the genotype/phenotype profiles of virulence and resistance, and the multilocus sequence types of the isolates.
Materials and methods
Burmese python
A wild Burmese python was rescued in Hainan Tropical Rainforest National Park by the Boa Rescue and Breeding Center of Hainan Province (BRBCH) in 2019. However, it was too weak to eat and died in several days later. The body was then sent to Hainan University for etiological analysis.
Necropsy and histopathology
The trachea, lungs, heart, liver and kidney were collected by routine anatomical methods [6]. Histological analysis was carried out by paraffin sectioning and observation at 100× and 400× magnification under a light microscope [17].
Bacterial examination
Heart blood and the bacterial inocula from trachea exudates and each internal organ including the spleen, lungs, heart, liver and kidney were collected during necropsy as previously described [6, 17]. The samples were then streaked onto Luria-Bertani (LB) agar and incubated at 35 ℃ for 24 h. The bacterial isolates were identified by Gram staining, 16S rRNA gene sequencing (primer set listed in Table S1) and phylogenetic analysis using GenBank BLAST and MEGA-X software [17, 18].
Antimicrobial susceptibility testing
Based on the Kirby-Bauer disk diffusion method and the standard protocol of the Clinical and Laboratory Standards Institute guidelines [19], seven classes of antibiotics including β-lactams, aminoglycosides, quinolones, chloramphenicol, macrolides, sulfonamides and peptides (Table 1) were used in a drug sensitivity test. The susceptibility was determined as sensitive, intermediate or resistant by measuring the size of the inhibitory zone of each test isolates with a quality control strain of Escherichia coli (ATCC25922).
Table 1.
Resistance pattern of the P. aeruginosa isolates
| Group | Antibiotics | Standard(mm) | Isolates (100%) | |||
|---|---|---|---|---|---|---|
| R | I | S | R | |||
| β-lactams | Amoxicillin | ≤13 | 14-16 | ≥17 | ≤13 | R |
| Penicillin | ≤10 | 11-15 | ≥16 | ≤10 | R | |
| Cephalexin | ≤14 | 15-17 | ≥18 | ≤14 | R | |
| Cefalotin | ≤14 | 15-17 | ≥18 | ≤14 | R | |
| Ampicillin | ≤13 | 14-16 | ≥17 | ≤13 | R | |
| Cefoperazone | ≤15 | 16-20 | ≥21 | ≤15 | I | |
| Aminoglycosides | Streptomycin | ≤11 | 12-14 | ≥15 | ≤11 | R |
| Gentamicin | ≤12 | 13-14 | ≥15 | ≤12 | R | |
| Tobramycin | ≤12 | 13-14 | ≥15 | ≤12 | R | |
| Kanamycin | ≤13 | 14-17 | ≥18 | ≤13 | R | |
| Amikacin | ≤14 | 15-16 | ≥17 | ≤14 | S | |
| Spectinomycin | ≤12 | 13-18 | ≥19 | ≤12 | S | |
| Quinolones | Levofloxacin | ≤13 | 14-16 | ≥17 | ≤13 | R |
| Ciprofloxacin | ≤15 | 16-20 | ≥21 | ≤15 | S | |
| Norfloxacin | ≤12 | 13-16 | ≥17 | ≤12 | S | |
| Tetracyclines | Tetracycline | ≤14 | 15-18 | ≥19 | ≤14 | R |
| Doxycycline | ≤12 | 13-15 | ≥16 | ≤12 | R | |
| Chloramphenicol | Chloramphenicol | ≤12 | 13-17 | ≥18 | ≤12 | R |
| Florfenicol | ≤12 | 13-17 | ≥18 | ≤12 | R | |
| Macrolides | Erythromycin | ≤13 | 14-22 | ≥23 | ≤13 | R |
| Roxithromycin | ≤10 | 11-15 | ≥26 | ≤10 | R | |
| Sulfonamides | Compound trimethoprim | ≤12 | 13-16 | ≥17 | ≤12 | R |
| Peptides | Polymyxin B | ≤8 | 9-11 | ≥12 | ≤8 | S |
‘R’ represents resistant, ‘I’ represents moderately resistant and ‘S’ represents sensitive to antibiotic
Assessment of biofilm formation
Biofilm formation was measured using a 96-well microtiter plate and the crystal violet assay [20]. In brief, an overnight bacterial culture was adjusted to an OD595 of 1.0 then diluted 1:40. The dilution was then added to a microtiter plate (200 μL per well), for 24 h at 37 °C to allow for biofilm formation. The wells were washed with sterile PBS three times, fixed with 100 μL of methanol solution for 15 min, stained with 125 μL of 0.1% crystal violet solution for 5 min, rewashed with sterile PBS three times, finally, the dye was resolubilized the dye with 100 μL of 95% ethanol solution for 30 min at 37 °C. The OD595 value of each well was quantified by a microplate reader. Escherichia coli (ATCC 25922) was used as a negative control. Biofilm production was assessed as described before [21]. The average optical density value (ODc) of the negative control (LB broth) was used as the cut-off. Biofilm formation by each isolate was then determined as absent, weak, moderate or strong, as shown in Table S1.
Genotype assay of resistance and virulence
The resistance and virulence genes were screened using PCR amplification. Primers, annealing temperatures and the amplicon length of each gene are listed in Table S2 [22–27]. The size of each PCR product was detected via 1% agarose gel electrophoresis. Genotypic patterns were determined according to the detector complement of genes.
Multilocus sequence typing (MLST)
According to the schemes, loci and primers of the P. aeruginosa typing database (https://pubmlst.org/bigsdb?db=pubmlst_paeruginosa_seqdef), seven endogenous genes: acsA (acetyl coenzyme A synthetase), aroE (shikimate dehydrogenase), guaA (GMP synthase), mutL (DNA mismatch repair protein), nuoD (NADH dehydrogenase I chain C, D subunit), ppsA (phosphoenolpyruvate synthase) and trpE (antioxidant synthetase component I) were amplified and sequenced. The sequence type (ST) was assigned based on the allelic profile of the seven amplicons. The specific ST of each isolate was obtained from the above MLST website.
Zebrafish experiment
As previously described [6], three-month-old healthy zebrafish (Danio rerio) were raised in a laboratory for at least 1 week. The fish were then fasted for 24 h and infected with 10 µl of bacterial suspension by intraperitoneal injection. Table 3 shows the doses of each isolate used in this experiment. Reactions and cumulative mortalities (%) were recorded daily for 96 h. The half-maximal lethal dose (LD50) was calculated via the Bliss method using SPSS software. Bacterial examination was repeated from the seroperitoneum of the diseased fish to confirm whether the infection was successful or not. The Ethical Committee for Animal Laboratories, at Hainan University permitted all protocols.
Table 3.
Genotypic profiles of resistance and virulence of P. aeruginosa isolates
| Category | Function | Genes | Coding protein | Isolates(100%) |
|---|---|---|---|---|
| Resistance | Chloramphenicol | catB | Type B chloramphenicol O-acetyltransferase | + |
| β-lactams | blaTEM | Class A extended-spectrum beta-lactamase | + | |
| blaPER | Class A extended-spectrum beta-lactamase | - | ||
| Aminoglycosides | ant (3’)-I | Nucleotidyltransferase | + | |
| ac (6’)-II | N-acetyltransferase | - | ||
| Tetracyclines | tetA | Tetracycline efflux MFS transporter | + | |
| Macrolides | ermA | 23S rRNA (adenine(2058)-N(6))-methyltransferase | - | |
| ermB | 23S rRNA (adenine(2058)-N(6))-methyltransferase | - | ||
| Quinolone | gyrA | DNA gyrase subunit A | - | |
| gyrB | DNA topoisomerase (ATP-hydrolyzing) subunit B | - | ||
| Virulence | Type III secretion system | exoU | Succinoglycan biosynthesis glycosyltransferae | - |
| exoS | Exoenzyme S | + | ||
| exoY | Adenylate cyclase | + | ||
| exoT | Exoenzyme T | + | ||
| Type II secretion system | toxA | Exotoxin A | + | |
| Oxidative stress | phzM | Phenazine-specific methyltransferase | + | |
| phzI | Autoinducer synthase | |||
| Phospholipase C | plcH | Hemolytic phospholipase C | + | |
| plcN | Non-hemolytic phospholipase C | + | ||
| Type IV pili | pilB | ATPase | - |
‘+’ indicates positive and ‘–’ indicates negative
Results
Gross examination and histopathology
The wild Burmese python was approximately 2 meters long and had dysecdysis, with patches of dry and peeling skin remaining (Fig. 1). According to the BRBCH the snake was lethargic and weak, displayed dyspnea and open-mouth breathing, and was not eating when rescued. After the death of the snake a few days later, a postmortem revealed a blocked airway with excess yellow mucus (Fig. 2). Histopathology showed that the exudate was composed of heterophilic cells, necrotic debris and tissue fluid in the lungs (Fig. 3B), hemorrhagic foci in the heart (Fig. 3E), and epithelial cell abscission in the trachea (Fig. 3C), intestine (Fig. 3A) and kidneys (Fig. 3D).
Fig. 1.

Burmese python. Arrows indicate the pieces remaining after abnormal ecdysis
Fig. 2.
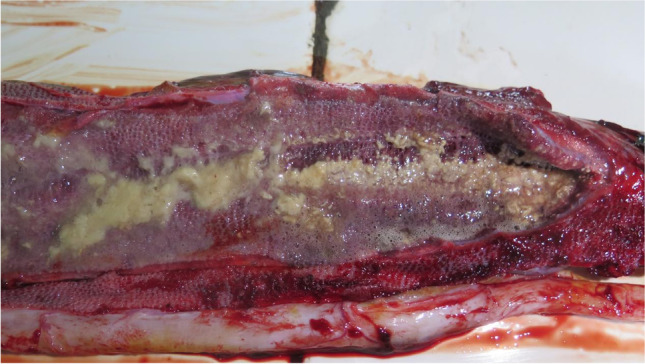
Anatomical analysis of the lung of the Burmese python. The lung was blocked with excess yellow mucus
Fig. 3.
Histopathology of various tissues of the Burmese python. A Intestine. Shed intestinal columnar epithelial cells (black arrow), bar = 500 µm. B Lung. Exudates in the air chambers (black arrow), bar = 500 µm. C Trachea. The mucosal epithelium of the trachea is sloughed off (white arrow), bar = 500 µm. D. Kidney. Dropped renal tubular epithelial cells (Black arrow); steatosis (blue arrow); cell pyknosis and necrosis (red circle), bar = 50 µm. E Heart. Hemorrhagic foci (white arrow), bar = 500 µm
Bacterial examination
Rough blue-green colonies were isolated from the samples of blood, trachea, lung and kidney on LB agar. Gram staining revealed that the cells were slender rod-shaped and Gram-negative. Four isolates, designated MDM1, MDM2, MDM3 and MDM4 (per isolate per sample), were randomly selected for 16S rRNA gene sequencing and were identified as P. aeruginosa via BLAST and phylogenetic analysis (Fig. 4).
Fig. 4.
16S rRNA phylogenetic tree of the isolates. Phylogenetic analysis of P. aeruginosa isolated from the wild Burmese pythons. The evolutionary history was inferred using the neighbor-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The evolutionary distances were computed using the maximum composite likelihood method. The phylogenetic tree was constructed using MEGA-X software
Antimicrobial susceptibility testing
The four isolates showed the same antimicrobial susceptibility profile (Table 1) with multiple antibiotic resistance to seven types of antibiotics, including 17 drugs in total seven β-lactams (with the exception of moderate sensitivity to cefoperazone), four aminoglycosides, one quinolone (levofloxacin), two tetracyclines, two chloramphenicol, two macrolides and one sulfonamide (compound trimethoprim). The four isolates were susceptible to two aminoglycosides (amikacin and spectinomycin), two quinolones (ciprofloxacin and norfloxacin), and polypeptide polymyxin B.
Assessment of biofilm formation
The results of the crystal violet assay showed that all four isolates formed biofilm and the values of OD595 were seven to nine folds higher than that of the negative control (Table 2), which is more than the threshold (four folds) and revealed that all isolates were strong biofilms producers (Table S1).
Table 2.
Biofilm production capability of the P. aeruginosa isolates
| Isolates | OD595 | ratio | Biofilm |
|---|---|---|---|
| MDM1 | 0.5900±0.008718 | 7.2544 | Strong |
| MDM2 | 0.6470±0.009539 | 7.9552 | Strong |
| MDM3 | 0.4490±0.009000 | 5.5207 | Strong |
| MDM4 | 0.7387±0.01007 | 9.0827 | Strong |
| Negative control | 0.08133±0.001528 | 1 | 0 |
Genotype patterns of resistance and virulence
In total, 10 resistance genes and 10 virulence genes were detected by PCR and sequencing. The results were identical for the four isolates (Table 3). The typical genotype pattern of resistance was catB (chloramphenicol resistance) + blaTEM (β-lactam resistance) + ant (3'')-I (aminoglycoside resistance) + tetA (tetracycline resistance), while the typical genotype pattern of virulence included eight genes, namely toxA (T2SS secretion system), exoS/exoY/exoT (T3SS secretion system), phzI/phzM (oxidative stress-related genes) and plcH/plcN (phospholipase genes). Notably, the gyrA and gyrB genes encoding quinolone resistance were not detected by PCR.
MLST
According to the analysis of the sequences and the allele spectrum in the MLST network database of P. aeruginosa, all four strains belonged to ST3495, which has been registered in database.
Zebrafish infection model
As shown in Table 3, all of the isolates were virulent to zebrafish and death occurred among the groups injected with high doses of isolates, with LD50 values ranging from 2.22×105 cfu/mL to 4.0×107 cfu/mL. Using zebrafish as an animal model, the obvious signs of P. aeruginosa infection was bleeding under the gills and abdomen (Fig 5A and B). Infection was confirmed by recovering isolates from the diseased fish and identifying them by PCR with the primers listed in Table S2 [28].
Fig. 5.
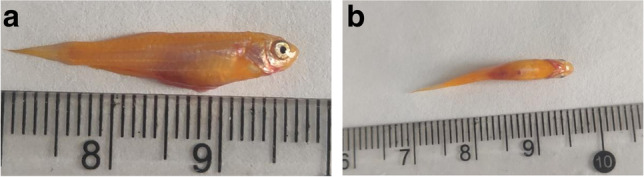
Zebrafish infected with the P. aeruginosa isolates. Hemorrhagic foci in the gills and abdomen are indicated by arrows in (A) and (B), respectively
Discussions
P. aeruginosa is an opportunistic pathogen causing acute or chronic infection in its hosts, which include humans and various domestic and wild animals [7]. In this study, we describe a case of P. aeruginosa infection in a wild Burmese python. According to the gross examination, histopathology and bacterial examination, the death was resulted from asphyxia caused by a blockage in the respiratory tract of excess yellow mucus, sepsis caused by the spread of P. aeruginosa among the organs of the heart, kidney and lung via the blood circulation.
Histopathology showed that the infection of P. aeruginosa caused the death of epithelial cells, including intestinal columnar cells, renal tubular epithelial cells and respiratory epithelial cells and detachment from the main tissues (Fig 3A-C), with some cells showing fatty degeneration and necrosis (Fig 3D). Previous studies have shown that P. aeruginosa infection involves the adherence, invasion, and remodeling of host epithelial cells and finally resulting in cell death by inducing the mitochondrial dysfunction or other mechanisms [29–31]. Hence, the invasion and destruction of epithelial cells appears to be a common and crucial mechanism of host infection by P. aeruginosa including the case of the Burmese python. The pathology of disease in the python therefore confirmed that death was caused by the P. aeruginosa isolates. Moreover, disease was caused by a single virulent strain rather than a mixture of different strains, because the four isolates from blood and from three different organs were identical in terms of their genotypic/phenotypic profiles of resistance and their sequence type (ST3495).
According to a recent molecular epidemiological study on P. aeruginosa, two isolates of ST3495 were also recovered from the feces of residents at long-term care facilities in France [32]. By comparing of the resistance and virulence profiles of these two human isolates with the four isolates from the Burmese python, all possessed the exoS gene and lacked the exoU gene, while the two human isolates were sensitive to tobramycin and had moderate resistance to ciprofloxacin, whereas the four isolates were resistant to both tobramycin and ciprofloxacin (Table 1). It is contradictory that tobramycin is recommended for the clinical management of moderate to severe bacterial infections, especially in cystic fibrosis patients with P. aeruginosa infection, but is seldomly used as a veterinary drug in domestic animals, such as dogs and cats, while ciprofloxacin is a broad-spectrum antibiotic widely used to treat infections both in animals and nosocomial infections in humans. Resistance to both tobramycin and ciprofloxacin is acquired by mutations and by adaptions involving biofilm-mediated resistance rather than being acquired via horizontal transfer of resistance genes from microbial communities or soil/water environments [12]. We speculate that such uncorrelated evolution of resistance to antibiotic exposition would be generated via the accumulation of random mutations. The four isolates in this study were surprisingly consistent with the nosocomial clinical isolates from Panama hospital [33] in terms of their sensitivity to aminoglycosides (amikacin and spectinomycin), quinolones (ciprofloxacin and norfloxacin), and polypeptide polymyxin B. This highlights the challenges facing antibiotic therapy for P. aeruginosa infection in humans and animals, and the risk of cross-transmission and cross-infection at the interface of wild environments and human communities.
Inconsistencies were observed between the resistance phenotypes to macrolides (resistance to erythromycin and roxithromycin) and quinolone (resistance to levofloxacin, but sensitivity to ciprofloxacin and norfloxacin) and the genetic profiles showing a lack of the ermAB genes and intact gyrAB genes in the isolates ; although there was a partial correlation between resistance to chloramphenicol, β-lactam, aminoglycoside and tetracycline and the presence of the catB, blaTEM, ant (3'')-I and tetA genes. Many factors may contribute to those discrepancies such as undetected resistance genes and undiscovered resistance mechanisms. In fact, macrolide resistance can be generated via many mechanisms, including ribosomal protection protein genes (ermA, ermB and ermD), efflux pump genes (msrA, mefA and mefE), enzymatic modification genes (mphB, ereA and ereB), and integron genes (intI2 and intI3) [34, 35]. In the same way, the mechanisms of quinolone resistance include two categories of mutations and the acquisition of resistance-conferring genes. Resistance mutations generally refer to the genes encoding DNA topoisomerase IV, DNA gyrase, and efflux pumps, whereas acquired resistance mainly refer to plasmid-encoded Qnr proteins [36]. Future studies involving whole genome sequencing [37] may aid our understanding of the resistance mechanisms in P. aeruginosa isolates
Our findings showed that the isolates possessed 8 of the 10 tested virulence genes, including exoA, which encodes ExoA, an exotoxin secreted by the type II secretion system that inhibits protein synthesis in host cells [38], and exoY, which encodes cytotoxin ExoY, an effector secreted through the type III secretion system to regulate host cytoskeletal organization and signal transduction [39]. Our results also revealed that the presence of exoS and exoU differs significantly between isolates and appears to be mutually exclusive [40], because exoS was detected but not exoU in the four isolates. A hypothesis for this exclusivity is that ExoS and ExoU confer enhanced fitness in distinct ecological niches.
In addition, the pilB gene which encodes the ATPase PilB that promotes polar type IV pili assembly in P. aeruginosa, was not detected in the four isolates in this study. It has previously been reported that pilB mutants show no reduction in virulence [41], but were unable to assemble surface pili [42], showed decreased motility [43] and disrupted levels of intracellular cAMP [44]. Considering that type IV pili promote early biofilm formation [45], a lack of pili would be expected to weaken the development of biofilm, yet in fact all of the isolates produced strong biofilm. This may indicate that type IV pili are not necessary for biofilm formation, but are reportedly involved in shaping the cellular architecture of P. aeruginosa [46].
Biofilms plays an important role in the generation of antimicrobial resistance, evasion of host immune defenses, and persistent infections. Recent studies have shown that bacterial biofilms are involved in 65% of infectious diseases and more than 80% of chronic infections [47]. P. aeruginosa is the main pathogen associated with this type of medical infection [48]. Our data displayed a negative correlation between the amount of biofilm and the LD50 value (Tables 3 and 4), which implied that the virulence was positively associated with the ability to form biofilm for the four isolates, as the profiles of resistance and virulence genes were consistent among the isolates.
Table 4.
The values of LD50 of P. aeruginosa isolates
| Isolate | Dose(cfu/mL) | Death number of /tails | Mortality rate/% | LD50(cfu/mL) |
|---|---|---|---|---|
| MDM1 | 108 | 4/6 | 66.7 | 1.33×107 |
| 107 | 3/6 | 50 | ||
| 106 | 2/6 | 33.3 | ||
| 105 | 0 | 0 | ||
| MDM2 | 108 | 6/6 | 100 | 2.98×106 |
| 107 | 4/6 | 66.7 | ||
| 106 | 2/6 | 33.3 | ||
| 105 | 1/6 | 16.7 | ||
| MDM3 | 108 | 4/6 | 66.7 | 4.0×107 |
| 107 | 2/6 | 33.3 | ||
| 106 | 1/6 | 16.7 | ||
| 105 | 0 | 0 | ||
| MDM4 | 108 | 6 | 100 | 2.22×105 |
| 107 | 5 | 83.3 | ||
| 106 | 4 | 66.7 | ||
| 105 | 3 | 50 |
In summary, this study primarily described the case of a wild Burmese python with suppurative pneumonia and sepsis caused by P. aeruginosa infection. Histopathology highlighted that the invasion of epidermal cells was a common mechanism by which this pathogen infects the host including the Burmese python. Bacteria were isolated from the blood and multiple organs. Our findings highlight the risk of potential cross- infection between animals and humans because all isolates belonged to the originally detected nosocomial ST3495, as determined by molecular epidemiology. Our findings reconfirmed the discrepancies between phenotype and genotype regarding the intricate mechanisms of resistance in this pathogen and emphasize that susceptibility testing is irreplaceable by molecular methods and plays an important role in clinical practice. Finally, the zebrafish infection model revealed that biofilm was important for the virulence of P. aeruginosa as PCR analysis confirmed that the isolates had the same genotypic profiles of resistance and virulence. In conclusion, this study highlights that attention should be paid to P. aeruginosa as an emerging opportunistic pathogen in wild animals.
Supplementary Information
Additional file 1. Table S1. Standard of Biofilmformation capability. Table S2. Primer sets used in the study.
Acknowledgments
We thank the Boa Rescue and Breeding Center of Hainan Province (BRBCH) for providing the python for this study. We thank Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Authors’ contributions
Jiping Zheng, Jifeng Zeng conceived the experiments. Roushan Li, Bo Ling, Xin Wang, Nuo Yang, Lixia Fan performed the experiments. Guiying Guo, Xuesong Li and Fei Yan analyzed the results. Roushan Li and Jiping Zheng wrote the paper. All authors read and approved the final manuscript.
Funding
This study was supported by the Hainan Provincial Natural Science Foundation of China (Grant 321MS007 to G. Guo, 821MS029 to N. Yang, 821RC1052 to L. Fan and 2019RC084 to J. Zheng) and the Natural Science Foundation of China (32060131 to J. Zheng and 32060788 to J. Zeng).
Availability of data and material
GenBank accession numbers for the 16S rRNA gene of Pseudomonas aeruginosa isolated from Burmese python are MDM1 (trachea) (MZ618953), MDM2 (lung) (MZ618954), MDM3 (heart) (MZ618955) and MDM4 (liver) (MZ618956).
Declarations
Ethics approval
This study was conducted as per the recommendations for the care and use of laboratory animals. The Hainan University Application for Animal Welfare and Ethical Review approved the experimental protocol under approval number HNUAUCC-2020-00183.
Conflicts of interest
The authors confirm that there are no conflicts of interest regarding the publication of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Roushan Li, Bo Ling and Jifeng Zeng contributed equally to this work.
References
- 1.Barker DG, Barker TM. The distribution of the Burmese python, Python bivittatus in China. Bull Chicago Herp Soc. 2010;45(5):86–88. [Google Scholar]
- 2.Stuart B, Nguyen TQ, Thy N, Grismer L, Chan-Ard T, Iskandar D, Golynsky E, Lau MWN (2012) Python bivittatus (errata version published in 2019). The IUCN Red List of Threatened Species 2012: e.T193451A151341916. 10.2305/IUCN.UK.2012-1.RLTS.T193451A151341916.en
- 3.Wang S, Zhao EM. China red data book of endangered animals (Amphibia and Reptilia) Beijing: Science press; 1998. [Google Scholar]
- 4.Penner JD, Jacobson ER, Brown DR, et al. A novel Mycoplasma sp. associated with proliferative tracheitis and pneumonia in a Burmese python (Python molurus bivittatus) J Comp Pathol. 1997;1173:283–288. doi: 10.1016/s0021-9975(97)80024-2. [DOI] [PubMed] [Google Scholar]
- 5.Zeng J, Lin J, Li X, et al. Identification of a strain of Pseudomonas Aeruginosa isolated from python and its tolerance to antibiotics. J Trop Biol. 2013;402:173–176. doi: 10.15886/j.cnki.rdswxb.2013.02.005. [DOI] [Google Scholar]
- 6.Pu W, Guo G, Yang N, et al. Three species of Aeromonas (A. dhakensis, A. hydrophila and A. jandaei) isolated from freshwater crocodiles (Crocodylus siamensis) with pneumonia and septicemia. Lett Appl Microbiol. 2019;683:212–218. doi: 10.1111/lam.13112. [DOI] [PubMed] [Google Scholar]
- 7.Bergan T (1981) Human- and Animal-Pathogenic Members of the Genus Pseudomonas. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The Prokaryotes. Springer, Berlin, Heidelberg, pp. 666–700. 10.1007/978-3-662-13187-9_59
- 8.Argudín MA, Deplano A, Meghraoui A, et al. Bacteria from animals as a pool of antimicrobial resistance genes. Antibiotics (Basel) 2017;6(2):12. doi: 10.3390/antibiotics6020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroth MN, Cho JJ, Green SK, et al. Epidemiology of Pseudomonas aeruginosa in agricultural areas. J Med Microbiol. 2018;67(8):1191–1201. doi: 10.1099/jmm.0.000758. [DOI] [PubMed] [Google Scholar]
- 10.Crone S, Vives-Flórez M, Kvich L, et al. The environmental occurrence of Pseudomonas aeruginosa. APMIS. 2020;128(3):220–231. doi: 10.1111/apm.13010. [DOI] [PubMed] [Google Scholar]
- 11.Qin S, Xiao W, Zhou C, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther. 2022;7(1):199. doi: 10.1038/s41392-022-01056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pang Z, Raudonis R, Glick BR, et al. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37(1):177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Kuang Z, Hao Y, Walling BE, et al. Pseudomonas aeruginosa elastase provides an escape from phagocytosis by degrading the pulmonary surfactant protein-A. PLoS One. 2011;6(11):e27091. doi: 10.1371/journal.pone.0027091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brao KJ, Wille BP, Lieberman J, et al. Scnn1b-transgenic BALB/c mice as a model of pseudomonas aeruginosa infections of the cystic fibrosis lung. Infect Immun. 2020;88(9):e00237–00220. doi: 10.1128/IAI.00237-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salomonsen CM, Boye M, Høiby N, et al. Comparison of histological lesions in mink with acute hemorrhagic pneumonia associated with Pseudomonas aeruginosa or Escherichia coli. Can J Vet Res. 2013;77(3):199–204. [PMC free article] [PubMed] [Google Scholar]
- 16.Aleksandrov M, Petkov A. Case of Pseudomonas aeruginosa infection in tropical snakes. Vet Med Nauki. 1985;22(7):53–61. [PubMed] [Google Scholar]
- 17.Di Z, Zheng J, Zhang H, et al. Natural outbreaks and molecular characteristics of Streptococcus agalactiae infection in farmed American bullfrog (Rana catesbeiana) Aquaculture. 2022;551:737885. doi: 10.1016/j.aquaculture.2021.737885. [DOI] [Google Scholar]
- 18.Messick JB, Berent LM, Cooper SK. Development and evaluation of a PCR-based assay for detection of Haemobartonella felis in cats and differentiation of H. felis from related bacteria by restriction fragment length polymorphism analysis. J Clin Microbiol. 1998;36(2):462–466. doi: 10.1128/JCM.36.2.462-466.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CLSI. (2021) Performance standards for antimicrobial susceptibility testing. M100. 31st Edition. Clinical and Laboratory Standards Institute, Wayne. http://em100.edaptivedocs.net/Login.aspx [DOI] [PMC free article] [PubMed]
- 20.Rehman MNU, Wang Y, Pan J, et al. Histological and molecular characterization of Edwardsiella tarda infection in Siamese crocodile (Crocodylus siamensis) hatchlings. Aquaculture. 2021;535:736367. doi: 10.1016/j.aquaculture.2021.736367. [DOI] [Google Scholar]
- 21.Kaczorek E, Małaczewska J, Wójcik R, et al. Biofilm production and other virulence factors in Streptococcus spp. isolated from clinical cases of bovine mastitis in Poland. BMC Vet Res. 2017;13(1):398. doi: 10.1186/s12917-017-1322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma Z, Zou Y, Wang H, et al. Detection of related resistance genes of chloramphenicol and tetracycline in multidrug-resistant Pseudomonas aeruginosa. Chin J Nosocomiol. 2009;19(02):135–137. [Google Scholar]
- 23.Mavroidi A, Tzelepi E, Tsakris A, et al. An integron-associated beta-lactamase (IBC-2) from Pseudomonas aeruginosa is a variant of the extended-spectrum beta-lactamase IBC-1. J Antimicrob Chemother. 2001;48(5):627–630. doi: 10.1093/jac/48.5.627. [DOI] [PubMed] [Google Scholar]
- 24.Shigemura K, Osawa K, Miura M, et al. Azithromycin resistance and its mechanism in Neisseria gonorrhoeae strains in Hyogo Japan. Antimicrob Agents Chemother. 2015;59(5):2695–2699. doi: 10.1128/AAC.04320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao L, Wang S, Li X, et al. Development of in vitro resistance to fluoroquinolones in Pseudomonas aeruginosa. Antimicrob Resist Infect Control. 2020;9(1):124. doi: 10.1186/s13756-020-00793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Zhang X, Wang Z, et al. Distribution and expression of T3SS virulence genes in Pseudomonas aeruginosa and its correlation with drug resistance. Chin J Clin Lab Sci. 2019;37(01):14–18. doi: 10.13602/j.cnki.jcls.2019.01.04. [DOI] [Google Scholar]
- 27.Haghi F, Zeighami H, Monazami A, et al. Diversity of virulence genes in multidrug resistant Pseudomonas aeruginosa isolated from burn wound infections. Microb Pathog. 2018;115:251–256. doi: 10.1016/j.micpath.2017.12.052. [DOI] [PubMed] [Google Scholar]
- 28.Deng Y, Wu Y, Tan A, et al. Analysis of antimicrobial resistance genes in Aeromonas spp. isolated from cultured freshwater animals in China. Microb Drug Resist. 2014;20(4):350–356. doi: 10.1089/mdr.2013.0068. [DOI] [PubMed] [Google Scholar]
- 29.Bentzmann S, Roger P, Puchelle E. Pseudomonas aeruginosa adherence to remodelling respiratory epithelium. Eur Respir J. 1996;9(10):2145–2150. doi: 10.1183/09031936.96.09102145. [DOI] [PubMed] [Google Scholar]
- 30.Maurice NM, Bedi B, Yuan Z, et al. Pseudomonas aeruginosa induced host epithelial cell mitochondrial dysfunction. Sci Rep. 2019;9(1):11929. doi: 10.1038/s41598-019-47457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deshpande R, Zou C. Pseudomonas aeruginosa induced cell death in acute lung injury and acute respiratory distress syndrome. Int J Mol Sci. 2020;21(15):5356. doi: 10.3390/ijms21155356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martak D, Gbaguidi-Haore H, Meunier A, et al. High prevalence of Pseudomonas aeruginosa carriage in residents of French and German long-term care facilities. Clin Microbiol Infect. 2022;28(10):1353–1358. doi: 10.1016/j.cmi.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Sambrano H, Castillo JC, Ramos CW, et al. Prevalence of antibiotic resistance and virulent factors in nosocomial clinical isolates of Pseudomonas aeruginosa from Panamá. Braz J Infect Dis. 2021;25(1):101038. doi: 10.1016/j.bjid.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szczepanowski R, Linke B, Krahn I, et al. Detection of 140 clinically relevant antibiotic-resistance genes in the plasmid metagenome of wastewater treatment plant bacteria showing reduced susceptibility to selected antibiotics. Microbiology. 2009;155(Pt7):2306–2319. doi: 10.1099/mic.0.028233-0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S, Ding J, Razanajatovo RM, et al. Interactive effects of polystyrene microplastics and roxithromycin on bioaccumulation and biochemical status in the freshwater fish red tilapia (Oreochromis niloticus) Sci Total Environ. 2019;648:1431–1439. doi: 10.1016/j.scitotenv.2018.08.266. [DOI] [PubMed] [Google Scholar]
- 36.Hooper DC, Jacoby GA. Mechanisms of drug resistance: quinolone resistance. Ann N Y Acad Sci. 2015;1354(1):12–31. doi: 10.1111/nyas.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piddock LJV. Assess drug-resistance phenotypes, not just genotypes. Nat Microbiol. 2016;1(8):16120. doi: 10.1038/nmicrobiol.2016.120. [DOI] [PubMed] [Google Scholar]
- 38.Algammal AM, Enany ME, El-Tarabili RM, et al. Prevalence, antimicrobial resistance profiles, virulence and enterotoxins-determinant genes of MRSA isolated from subclinical bovine mastitis in Egypt. Pathogens. 2020;9(5):362. doi: 10.3390/pathogens9050362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cowell BA, Evans DJ, Fleiszig SM. Actin cytoskeleton disruption by ExoY and its effects on Pseudomonas aeruginosa invasion. FEMS Microbiol Lett. 2005;250(1):71–76. doi: 10.1016/j.femsle.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 40.Feltman H, Schulert G, Khan S, et al. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147(Pt 10):2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 41.Siryaporn A, Kuchma SL, O’Toole GA, et al. Surface attachment induces Pseudomonas aeruginosa virulence. Proc Natl Acad Sci U S A. 2014;111(47):16860–16865. doi: 10.1073/pnas.1415712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunn D, Bergman S, Lory S. Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili. J Bacteriol. 1990;172(6):2911–2919. doi: 10.1128/jb.172.6.2911-2919.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koch MD, Black ME, Han E, et al. Pseudomonas aeruginosa distinguishes surfaces by stiffness using retraction of type IV pili. Proc Natl Acad Sci U S A. 2022;119(20):e2119434119. doi: 10.1073/pnas.2119434119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fulcher NB, Holliday PM, Klem E, et al. The Pseudomonas aeruginosa Chp chemosensory system regulates intracellular cAMP levels by modulating adenylate cyclase activity. Mol Microbiol. 2010;76(4):889–904. doi: 10.1111/j.1365-2958.2010.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maldarelli GA, Piepenbrink KH, Scott AJ, et al. Type IV pili promote early biofilm formation by Clostridium difficile. Pathog Dis. 2016;74(6):ftw061. doi: 10.1093/femspd/ftw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klausen M, Heydorn A, Ragas P, et al. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol. 2003;48(6):1511–1524. doi: 10.1046/j.1365-2958.2003.03525.x. [DOI] [PubMed] [Google Scholar]
- 47.Jamal M, Ahmad W, Andleeb S, et al. Bacterial biofilm and associated infections. J Chin Med Assoc. 2018;81(1):7–11. doi: 10.1016/j.jcma.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Bisht K, Baishya J, Wakeman CA. Pseudomonas aeruginosa polymicrobial interactions during lung infection. Curr Opin Microbiol. 2020;53:1–8. doi: 10.1016/j.mib.2020.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Standard of Biofilmformation capability. Table S2. Primer sets used in the study.
Data Availability Statement
GenBank accession numbers for the 16S rRNA gene of Pseudomonas aeruginosa isolated from Burmese python are MDM1 (trachea) (MZ618953), MDM2 (lung) (MZ618954), MDM3 (heart) (MZ618955) and MDM4 (liver) (MZ618956).




