Abstract
Antimicrobial resistance among bacteria present in ready-to-eat foods is an emerging concern. Hence, this study investigated the presence of extended-spectrum and AmpC β-lactamases (ESBL/AmpC)-producing Enterobacterales (ESBL-E) and the dissemination of mcr-1 in ESBL-E from ready-to-eat food samples (RTE) in Algeria. RTE food samples (n = 204) were aseptically collected and selectively cultured using MacConkey agar. The isolates were screened for ESBL production using the DDST test, confirmed ESBL-E isolates were identified using different conventional methods and MALDI-TOF MS, antibiotic susceptibility was determined using the disc diffusion and broth microdilution assay, ESBL-E isolates were analyzed for colistin and ESBL/AmpC encoding genes by PCR, and food samples were analyzed by univariate and multiple logistic regression. Overall, 48 (17.4%) of the 276 Enterobacterales were confirmed as ESBL producers, with a high prevalence in soups (40%), salads (25%), and cream-filled pastries (23.8%). Antibiotic susceptibility testing revealed that all the ESBL-E isolates were found multi-drug resistant. PCR revealed that blaTEM, blaCTX-M, blaCMY-2, blaOXA-1, and blaSHV were the most frequently detected. blaCTX-M-9 and blaCTX-M-1 were the predominant CTX-M types. Furthermore, four isolates were positive for mcr-1; three of them harbored the colistin resistance gene and ESBL/AmpC genes (2 E. cloacae and 1 S. enterica). To the best of our knowledge, this is the first report that detects the presence of the mcr-1 gene in ESBL-E strains isolated from RTE foods in Algeria. These findings suggest an urgent need for strict policies that prevent the spread and transmission of ESBL-E in food.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01082-3.
Keywords: ESBL, Colistin, Ready-to-eat food, Enterobacterales, Antibiotic susceptibility, Algeria
Introduction
Foodborne diseases are one of the most common health problems in the world, and they are a major cause of low economic productivity and morbidity [1, 2]. Ready-to-eat foods (RTE) are responsible for causing many foodborne diseases because they are eaten without further cooking, washing, or additional preparation before consumption. And they could be contaminated with different pathogens during the preparation process, commercial distribution, or marketing [3–5]. According to the World Health Organization (WHO), foodborne infections kill 420,000 people each year [6].
The severity of foodborne infections can be partly caused by the presence of multi-drug-resistant bacteria, and one of the reasons for the rise of antimicrobial resistance is the excessive use of antibiotics in humans, in veterinary medicine, and, more importantly, in food production chains [7–9]. Hence, resistant bacteria use food as an ideal medium for growth and thus causing foodborne illnesses following the consumption of contaminated food [10].
Enterobacterales are the most prevalent bacteria incriminated in foodborne infections [11, 12]. They are responsible for causing community- and nosocomial-acquired infections in humans [13]. Their resistance to beta-lactams, which are the primary therapeutic choices used to treat infections, has become a public health concern [14–16]. This is due to their ability to produce a variety of β-lactamases, such as cephalosporinases (AmpC) and extended-spectrum β-lactamases (ESBLs) [17, 18]. ESBLs can dismantle first-, second-, and third-generation cephalosporins and penicillins. And some of them can also break down fourth-generation cephalosporins [19–21]. Therefore, the dissemination of extended-spectrum β-lactamases-producing Enterobacterales (ESBL-E) in the community outside the hospital could affect the future use of expanded-spectrum beta-lactam antibiotics to treat some severe infections, such as bacteraemia and urinary tract infections. Hence, healthcare professionals could be obligated to use last-resort antibiotics such as colistin. However, the effectiveness of colistin has been questioned due to the emergence of the colistin resistance gene, mcr-1 [22]. This gene has been found recently in food samples of different origins [12, 23, 24].
Information is scarce regarding the presence and characteristics of ESBL-E in RTE foods in Algeria. Earlier, Yaici et al. [8] reported the presence of ESBL/AmpC genes in Escherichia coli and Klebsiella pneumoniae in sandwiches. Hence, to the best of our knowledge, this study is the first to screen AmpC/ESBL and colistin resistance on a large variety of RTE foods. Thus, we evaluated the presence of ESBL/AmpC genes and the dissemination of mcr-1 in extended-spectrum β-lactamases-producing Enterobacterales isolated from ready-to-eat foods in Algeria.
Material and methods
Study area and the sampling
The study was carried out from November 2021 to March 2022, in which a total of 204 samples of RTE foods were collected from four provinces in Algeria: Batna (n =160), Algiers (n = 20), Setif (n = 14), and Biskra province (n = 10). Food samples were randomly collected from restaurants (n = 30), bakeries (n =13), and supermarkets (n = 5), and each vendor was visited only once. Ten categories of the high-circulating RTE food categories were chosen: cream-filled pastries (n = 42), rice dishes (n = 31), chicken dishes (n = 29), salads (n = 25), cooked potato (n = 22), meat dishes (n = 14), cheese (n =11), fish dishes (n = 8), soups (n = 7), and 15 mixed dishes (they usually contain a gratin with pasta, potatoes, and chicken/meat). The samples were stored in sterile bags and immediately transported to the laboratory in a cooler box for analysis within 4 h after the collection.
Isolation and phenotypic identification of ESBL-producing Enterobacterales isolates (ESBL-E)
Food samples (25 g) were homogenized with a mixer in 225 mL of trypticase soy broth (TSB, Sigma, Germany), followed by incubation at 37 °C for 24 h. The samples were then streaked on MacConkey agar plates and incubated for 24 h at 37 °C. MacConkey agar is a selective and differential medium used to isolate non-fastidious Gram-negative rods, presumptive Enterobacterales colonies per morphology/color were picked from each sample, and they were identified using Gram staining, biochemical characterization (TSI test), the API 20E Galeries, and MALDI-TOF MS.
Enterobacterales isolates were then used for the initial screening of ESBL production; they were streaked on MacConkey agar (Sigma, Germany) supplemented with 2 mg/L cefotaxime as previously described by Costa et al. [25]. Isolates showing growth on MacConkey agar were then screened for susceptibility to aztreonam (AZT; 30 μg), cefotaxime (CTX; 30 μg), and ceftazidime (CAZ; 30 μg) by the disc diffusion method. E. coli ATCC 25922 was used as a control strain. The isolates showing reduced susceptibility to one or all antibiotics used were confirmed using the double disc synergy test [26].
The double disc synergy test (DDST)
The double-disc synergy test was used to confirm the ESBL-resistance phenotype. Discs containing cefotaxime, cefoxitin, aztreonam, and ceftazidime were used with and without amoxicillin–clavulanic acid (AMC, 20/10 μg) on the same plate containing Muller-Hinton agar (Oxoid, UK). A positive test result was determined when a 5 mm increase in the zone diameter was observed compared to that of a disc without clavulanic acid [26].
Phenotypic antimicrobial resistance characterization of ESBL-E isolates
Disc diffusion method
The antimicrobial susceptibility of ESBL-E isolates was tested using the Kirby-Bauer disc diffusion method according to the European Committee on Antimicrobial Susceptibility Testing guidelines [27]. Thirteen antibiotics (Oxoid, UK) were used in this study, and they included cefotaxime (CTX; 30 μg), aztreonam (AZT; 30 μg), ceftazidime (CAZ; 30 μg), amoxicillin-clavulanic acid (AMC; 20/10 μg), tetracycline (TET; 30 μg), gentamicin (GN; 10 μg), cefazolin (CZ; 30 μg), amikacin (AK; 30 μg), kanamycin (KAN; 30μg), chloramphenicol (C; 30 μg), trimethoprim-sulfamethoxazole (SXT; 1.25/23.75 μg), imipenem (IMI; 10 μg), and ciprofloxacin (CIP; 5 μg). Plates were incubated for 18 h at 35 ± 1 °C. The inhibition zone was measured, and the breakpoints were interpreted according to EUCAST (2022). The E. coli strain ATCC 25922 was used as a control strain.
Broth microdilution assay (BMD)
ESBL-E isolates (n = 48) were also screened for their other antibiotics using the broth microdilution assay (BMD). The EURGNCOL sensititre (Thermo Fisher, Vantaa, Finland) plates were used for the BMD assay, and it consisted of a panel of five antibiotics [colistin (0.25–8 μL; COL); piperacillin/tazobactam (1/4–32/4 μL; P/T4); ceftolozane/tazobactam (0.25/4–8/4 μL; C/T; ceftazidime/avibactam (1/4–16/4 μL; CZA); Meropenem (0.12–16 μL; MER)]. The sensititre plates were automatically read using the Sensititre Vizion Digital MIC Viewing System (Thermo Scientific, Vantaa, Finland). All interpretations were based on the epidemiological cut-off (ECOFF) values established by the EUCAST (2022).
DNA extraction
All the phenotypically confirmed ESBL-E isolates were genotyped using multiplex polymerase chain reaction (mPCR) to identify the ESBL/AmpC genes that confer resistance to beta-lactam antibiotics and mcr-1 gene that confers resistance to colistin. Genomic DNA was extracted using the QIACUBE connect system (Qiagen GmBH, Qiagen Strasse 1, Hilden, Germany) using the Qiagen blood and tissue kit, following the manufacturer’s instructions. The extracted genomic DNA was quantified with a Qubit fluorometer 4.0 (Invitrogen, Singapore).
Detection of ESBL/AmpC genes and the colistin resistance gene mcr-1
Each mPCR was conducted using a 25 μL reaction mix. The reaction mix was made up of 17 μL of the Hyclone PCR grade water (Fischer Scientific, USA), 5 μL of the 10× Dream Taq buffer (including 20mM of MgCl2), 1 μL of the dNTP mix (10mM), 0.5 μL of the Taq DNA polymerase (VWR Life Science, Helsinki, Finland), 1 μL of primers (Metabion, München, Germany), and 0.5 μL of the DNA template [28]. The mPCR was performed in a Maxygene II thermocycler (Corning, USA) using the primer sequences, and the amplification parameters are shown in Table 1. The genetic targets were: multiplex I (blaTEM, blaSHV, and blaOXA-1), multiplex II (blaCTX-M groups 1, 2, and 9), and blaCTX-M groups 8/25 as well as simplex PCR to screen for the mobile colistin resistance gene (mcr-1). The PCR amplicons were loaded onto 2% Tris-acetate-EDTA (TAE) gel, and electrophoresis was performed at 100 V for 1 h. The separated bands were viewed on a gel imager (Alpha Innotech, California, USA).
Table 1.
The primer sequences used in the detection of ESBL, AmpC, and colistin genes
| Gene targeted | Sequence | Amplification parameters | Expected band size (pb) | Reference |
|---|---|---|---|---|
| TEM-1 and 2 and its variants |
CATTTCCGTGTCGCCCTTATTC CGTTCATCCATAGTTGCCTGAC |
98 °C, 2 min; 32 cycles of 98 °C, 10 s, 56 °C, 30 s, 72 °C, 75 s, 72 °C, 6 min |
800 | [29] |
| SHV-1 and its variants |
AGCCGCTTGAGCAAATTAAAC ATCCCGCAGATAAATCACCAC |
713 | ||
| OXA-1, OXA-4, and OXA-30 |
GGCACCAGATTCAACTTTCAAG GACCCCAAGTTTCCTGTAAGTG |
564 | ||
| CTX-M-1 including 3 and 15 | TTAGGAARTGTGCCGCTGYAa |
94 °C, 10 min; 32 cycles of 94 °C, 40 s, 60 °C, 40 s, 72 °C, 75 s, 72 °C, 6 min |
688 | |
| CGATATCGTTGGTGGTRCCATa | ||||
| CTX-M-2 and its variants |
CGTTAACGGCACGATGAC CGATATCGTTGGTGGTRCCATa |
404 | ||
| CTX-M-9 and its variants | TCAAGCCTGCCGATCTGGT | 561 | ||
| TGATTCTCGCCGCTGAAG | ||||
| CTX-M-8, 25, 26, and 39 |
AACRCRCAGACGCTCTACa TCGAGCCGGAASGTGTYATa |
326 | ||
| CMY-2 to CMY-7 |
CGAAGAGGCAATGACCAGAC ACGGACAGGGTTAGGATAGYa |
538 | ||
| MCR-1 |
AGTCCGTTTGTTCTTGTGGC AGATCCTTGGTCTCGGCTTG |
94 °C, 15 min; 25 cycles of 94 °C, 30 s, 58 °C, 90 s, 72 °C, 60 s, 72 °C, 10 min | 320 | [30] |
aY = T or C, R = A or G, S = G or C, D = A or G or T
Statistical analysis
Data were analyzed using IBM SPSS version 20.0. Descriptive analysis was done to analyze the prevalence of ESBL-E isolated from different food samples. Chi-square tests with Bonferroni-adjusted p values were performed to compare differences. Spearman’s rank correlation test was used to study the correlation between phenotypic and genotypic resistance profiles. Descriptive statistics are presented as a comparison of ESBL-Enterobacterales carriers and non-carriers. Potential statistical associations between the study variables and the acquisition of ESBL-E were explored in univariate analyses performed with logistic regression, and the following variables were used: vendor types, the city, cream-filled pastries, cooked chicken, salads, cooked potato, mixed dishes, meat dishes, cheese, fish dishes, and soups. Factors that were statistically significant in univariate analysis (p < 0.05) were included in multiple logistic regression analysis to determine variables associated with the ESBL-E carriage. p value < 0.05 was considered significant unless otherwise specified.
Results
Prevalence and identification of ESBL-E
Of the two hundred and four (204) samples screened, a total of 276 Enterobacterales were isolated (Table S1). ESBL-producing Enterobacterales were detected in 17.4% (n = 48/276) of the samples from 30 shops out of the 48 visited (n = 62.5%).
The highest percentage of ESBL-E was found in soups (n = 4/48, 40%), and the highest number of ESBL-E was found in cream-filled pastries (n = 20/48, 23.8%) (Table 2). Moreover, according to the univariate analysis, three variables (vendor types, city, and soups) were associated with ESBL-Enterobacterales carriage (Table 3). All statistically significant variables from the univariate analysis were included in the multiple regression analysis to exclude possible cofounders. The multiple logistic regression model showed that the only independent predictor of colonization with ESBL-Enterobacterales was the variable “soups” (OR: 0.58; 95%CI 0.05–0.73, p = 0.02) (Table S2).
Table 2.
Distribution of ESBL-E in ready-to-eat food samples
| Category |
N° samples |
N° Enterobacterales |
N° ESBL-E isolates |
ESBL isolates (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E. cloacae | E. sakazakii | C. freundii | E. cancerogenus | S. enterica | E. aerogenes | C. youngae | H. alvei | ||||
| Cream-filled pastries | 42 | 84 | 20 | 14 (70.0) | 2 (10.0) | 2 (10.0) | 2 (10) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rice dishes | 31 | 55 | 4 | 0 (0.0) | 2 (50.0) | 1 (25.0) | 0 (0) | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) |
| Cooked chicken | 29 | 30 | 7 | 4 (57.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | 1 (14.3) | 0 (0.0) | 1 (14.3) |
| Salads | 25 | 12 | 3 | 2 (66.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cooked potato | 22 | 22 | 4 | 2 (50.0) | 0 (0.0) | 2 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mixed dishes | 15 | 24 | 1 | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Meat dishes | 14 | 21 | 3 | 2 (66.6) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cheese | 11 | 9 | 1 | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Fish dishes | 8 | 9 | 1 | 0 (0.0) | 1 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Soups | 7 | 10 | 4 | 4 (100) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 204 | 276 | 48 | 29 (60.4) | 6 (12.5) | 6 (12.5) | 2 (4.1) | 2 (4.1) | 1 (2.1) | 1 (4.8) | 1 (4.8) |
Table 3.
Univariate logistic regression analyses of determinants for ESBL/pAmpC-producing Enterobacterales carriage isolated from ready-to-eat food samples
| Variables | ESBL-Enterobacterales carriage | Univariate analysis | ||
|---|---|---|---|---|
| Negative (n = 226) | Positive (n = 48) | ORa (95%CIb) | p value | |
| Vendor | ||||
| Restaurents | 140 | 25 | - | - |
| Bakeries | 66 | 21 | 0.56 (0.32–0.97) | 0.040* |
| Supermarkets | 20 | 2 | 3.18 (0.68–14.7) | 0.139 |
| City | ||||
| Batna | 195 | 36 | - | - |
| Algiers | 15 | 0 | 3.32 (1.23–5.43) | 0.453 |
| Biskra | 5 | 6 | 1.23 (0.56–8.09) | 0.830 |
| Setif | 11 | 6 | 0.33 (0.21–0.50) | 0.008* |
| Source of food | ||||
| Cream-filled pastries | 67 | 20 | 0.59 (0.31–1.12) | 0.107 |
| Cooked chicken | 36 | 7 | 1.10 (0.46–2.66) | 0.816 |
| Meat dishes | 15 | 3 | 1.06 (0.29–3.83) | 0.922 |
| Fish dishes | 5 | 1 | 1.06 (0.12–9.31) | 0.956 |
| Rice dishes | 38 | 4 | 2.22 (0.75–6.55) | 0.148 |
| Cheese | 10 | 1 | 2.17 (0.27–17.4) | 0.464 |
| Mixed dishes | 6 | 1 | 1.28 (0.15–10.8) | 0.820 |
| Salads | 16 | 3 | 1.14 (0.32–4.08) | 0.837 |
| Soups | 4 | 1 | 0.04 (0.005–0.44) | 0.008* |
| Cooked potato | 32 | 4 | 1.81 (0.61–5.39) | 0.284 |
aOdds ratio (OR)
bConfidence interval of odds ratio
Among the ESBL-E isolates, Enterobacter cloacae were the most isolated species (n = 29/48, 60.4%), followed by Enterobacter sakazakii (n = 6/48, 12.5%), Citrobacter freundii (n = 6/48, 12.5%), Enterobacter cancerogenus (n = 2/48, 4.1%), Salmonella enterica (n = 2/48, 4.1%), Enterobacter aerogenes (n = 2/48, 4.1%), and single isolates of Citrobacter youngae (n = 1/48, 2.9%) and Hafnia alvei (n = 1/48, 2.9%).
Antibiotic susceptibility patterns
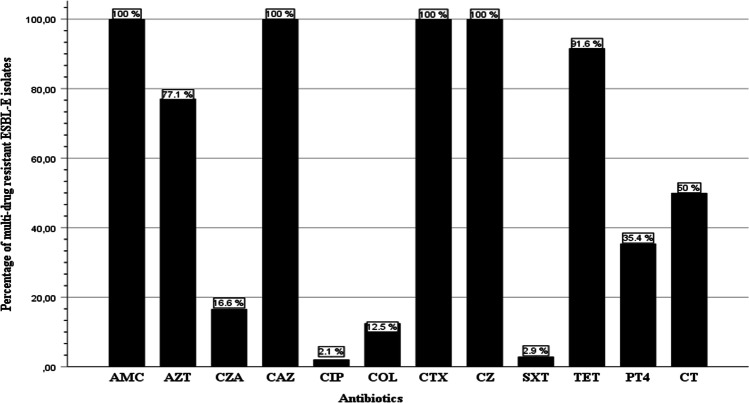
The frequency of antimicrobial resistance of ESBL-E isolates in general and based on the food samples is presented in Fig. 1 and Table 4, respectively. All the ESBL isolates (n = 48) were found resistant to ceftazidime (100%), cefazolin (100%), cefotaxime (100%), and amoxicillin-clavulanic acid (100%), followed by tetracycline (46/48, 95.8%) and aztreonam (37/48, 77.1%), while resistance to ciprofloxacin and trimethoprim-sulfamethoxazole was 2.1% each (1/48). However, no resistance was observed against the other antibiotics used in the disc diffusion method (amikacin, kanamycin, chloramphenicol, and imipenem).
Fig. 1.
Resistance phenotypes of ESBL-E isolated from ready-to-eat food in Algeria. CTX: cefotaxime, AZT: aztreonam, CAZ: ceftazidime, AMC: amoxicillin–clavulanic acid, TET: tetracycline, CZ: cefazolin, SXT: trimethoprim sulfamethoxazole, CIP: ciprofloxacin, P/T4: piperacillin/tazobactam, COL: colistin, CZA: ceftazidime/avibactam, C/T: ceftolozane/tazobactam
Table 4.
Frequency of antimicrobial resistance of ESBL-E isolates depending on the food origin
| Antimicrobial class | Antibiotic | Cream-filled pastries | Rice dishes | Cooked chicken | Meat dishes | Cooked potato | Fish dishes | Salads | Cheese | Mixed dishes | Soups |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cephalosporins | CAZ | 20 (100) | 4 (100) | 7 (100) | 3 (100) | 4 (100) | 1 (100) | 3 (100) | 1 (100) | 1 (100) | 4 (100) |
| CXT | 19 (95.0) | 4 (100) | 6 (85.7) | 3 (100) | 4 (100) | 1 (100) | 3 (100) | 1 (100) | 1 (100) | 4 (100) | |
| CZ | 20 (100) | 4 (100) | 7 (100) | 3 (100) | 4 (100) | 1 (100) | 3 (100) | 1 (100) | 1 (100) | 4 (100) | |
| Quinolones | CIP | 0 (0) | 0 (0) | 1 (14.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Monobactams | AZT | 17 (85.0) | 3 (75.0) | 3 (42.8) | 2 (66.6) | 4 (100) | 0 (0) | 1 (33.3) | 0 (0) | 1 (100) | 4 (100) |
| Aminoglycosides | AKA | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| KAN | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| GEN | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| β-Lactamase inhibitors | AMC | 20 (100) | 4 (100) | 7 (100) | 3 (100) | 4 (100) | 1 (100) | 3 (100) | 1 (100) | 1 (100) | 4 (100) |
| P/T4 | 9 (45.0) | 2 (50.0) | 1 (14.9) | 1 (33.3) | 1 (25.0) | 0 (0) | 1 (33.3) | 0 (0) | 1 (100) | 1 (25.0) | |
| CZA | 2 (10.0) | 1 (25.0) | 1 (14.9) | 1 (33.3) | 1 (25.0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 1 (25.0) | |
| C/T | 12 (60.0) | 3 (75.0) | 2 (28.5) | 1 (33.3) | 1 (25.0) | 0 (0) | 2 (66.6) | 0 (0) | 1 (100) | 3 (75.0) | |
| Carbapenems | MER | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| IPI | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Tetracyclines | TET | 19 (95.0) | 3 (75.0) | 7 (100) | 3 (100) | 4 (100) | 1 (100) | 3 (100) | 1 (100) | 1 (100) | 4 (100) |
| Others | COL | 2 (10.0) | 0 (0) | 1 (14.3) | 1 (33.3) | 1 (25.0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) |
| SXT | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| CHL | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Extensive MDR | 2 (10.0) | 0 (0) | 2 (28.5) | 1 (33.3) | 1 (25.0) | 0 (0) | 1 (33.3) | 0 (0) | 0 (0) | 0 (0) | |
Furthermore, results from the BMD assay showed that 50% of the isolates were resistant to the fifth-generation Cephalosporin, the ceftolozane-tazobactam combination (n = 24/48), followed by the piperacillin-tazobactam combination (n = 17/48, 35.4%), and ceftazidime-avibactam (n = 8/48, 16.6%), and interestingly, 12.5% of the isolates were resistant to colistin (n = 6/48). This polymyxin E antibiotic is considered a last resort (reserve) antibiotic. Moreover, all the ESBL-E isolates were found multi-drug resistant (MDR) [31], and 7 (14.5%) isolates were resistant to ≥ 5 classes of antimicrobials, indicating extensive multi-drug resistant phenotype (Table S3).
Molecular characterization of colistin resistance and beta-lactamase genes
The details of the phenotypic and genotypic antimicrobial resistance of ESBL-E isolates are described in Table 5. Among the 48 ESBL-producing Enterobacterales, the mobile colistin resistance gene (mcr-1) was detected in 8.3% of the isolates (4/48), two isolates were recovered from cream-filled pastries (2 E. cloacae isolates), one from chicken dishes (1 S. enterica isolate), and one from cooked potato (1 E. cloacae isolate). Moreover, a statistically significant positive correlation was found between the colistin resistance phenotype and the mcr-1 gene (r = 0.798, p < 0.01).
Table 5.
Phenotypic and genotypic characteristics of ESBL-E isolated from different food
| Strain ID | Food | Type of vendora | City | Isolated strain | Antibiotic resistance profileb | Extended-spectrum beta-lactamases genes | AmpC | Colistin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTXM1 | CTXM2 | CTXM8 | CTXM9 | SHV | TEM | OXA1 | CMY-2 | mcr-1 | ||||||
| E75 | Cream-filled pastries | BK | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ | - | - | - | + | - | - | - | - | - |
| E80 | BK | Batna | E. cancerogenus | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T | - | - | - | - | - | + | - | - | - | |
| E81 | BK | Batna | E. cancerogenus | CAZ, TET, AMC, AZT, FOX, CZ, C/T, PIP-TAZ | + | - | - | - | - | - | - | + | - | |
| E90 | BK | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, CZA, C/T, PIP TAZ | - | - | - | - | - | + | - | + | - | |
| E119 | BK | Batna | E. sakazakii | CAZ, TET, AMC, CXT, FOX, CZ, C/T, PIP-TAZ | - | - | - | + | - | - | - | + | - | |
| E120 | BK | Batna | C. freundii | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T, PIP-TAZ | - | - | - | - | - | + | - | + | - | |
| E122 | BK | Batna | C. freundii | CAZ, TET, AMC, AZT, CXT, FOX, CZ | - | - | - | - | - | - | - | - | - | |
| E129 | BK | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ | + | - | - | - | - | + | - | + | - | |
| E132 | BK | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ | + | - | - | - | - | + | - | - | - | |
| E144 | BK | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T | - | - | - | - | - | - | - | + | - | |
| E145 | BK | Batna | E. sakazakii | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T, PIP-TAZ | - | - | - | + | - | - | - | + | - | |
| E164 | BK | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ | - | + | - | + | - | - | - | - | - | |
| E168 | BK | Batna | E. cloacae | CAZ, TET, AMC, CXT, FOX, CZ, C/T, PIP-TAZ | - | + | - | - | - | - | - | - | - | |
| E178 | BK | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, CZA, C/T, PIP-TAZ | - | - | - | + | - | - | - | - | - | |
| E173 | BK | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T, PIP-TAZ | - | - | - | - | - | - | - | - | - | |
| E174 | BK | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ | - | - | - | - | - | - | - | + | - | |
| E184 | BK | Batna | E. cloacae | CAZ, AMC, AZT, CXT, FOX, CZ | - | + | - | - | - | - | - | + | - | |
| E204 | BK | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ | - | - | - | + | - | - | - | - | - | |
| E221 | BK | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T, COL, PIP-TAZ | + | - | - | + | - | - | - | - | + | |
| E216 | BK | Batna | E. cloacae | CAZ, TET, AMC, CXT, FOX, CZ, C/T, COL, PIP-TAZ | - | - | - | - | - | - | - | - | + | |
| E229 | Meat dishes | R | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T, PIP-TAZ | - | - | - | - | - | - | - | - | - |
| E269 | R | Batna | E. cloacae | CAZ, TET, SXT, AMC, AZT, CXT, FOX, CZ, CZA, COL | - | - | - | + | - | - | - | - | - | |
| E385 | R | Biskra | E. sakazakii | CAZ, TET, AMC, CXT, FOX, CZ | - | - | - | + | - | - | - | + | - | |
| E278 | Cooked potato | R | Setif | C. freundii | CAZ, TET, AMC, AZT, CXT, FOX, CZ, CZA, C/T, PIP-TAZ | - | - | - | - | - | - | - | - | - |
| E290 | R | Setif | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, COL | - | - | - | - | - | - | + | - | + | |
| E305 | R | Setif | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ | - | + | - | + | - | - | - | - | - | |
| E375 | R | Biskra | C. freundii | CAZ, TET, AMC, AZT, CXT, FOX, CZ | - | - | - | + | - | - | - | - | - | |
| E106 | Rice dishes | R | Batna | E. sakazakii | CAZ, TET, AMC, AZT, CXT, FOX, CZ | - | - | - | - | - | + | + | + | - |
| E160 | R | Setif | E. sakazakii | CAZ, TET, AMC, CXT, FOX, CZ, CZA, C/T | - | - | - | - | - | + | - | + | - | |
| E351 | R | Batna | C. freundii | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T, PIP-TAZ | - | - | - | - | - | + | - | + | - | |
| E382 | R | Setif | C. youngae | CAZ, AMC, AZT, CXT, FOX, CZ, C/T, PIP-TAZ | - | - | - | - | - | + | - | + | - | |
| E319 | Soups | R | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, CZA, C/T | - | - | - | - | - | + | - | - | - |
| E329 | R | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ | - | - | - | - | - | - | - | - | - | |
| E330 | R | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T, PIP-TAZ | - | - | - | - | - | + | - | - | - | |
| E334 | R | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T | + | - | - | - | - | - | - | - | - | |
| E12 | Salads | R | Batna | E. cloacae | CAZ, TET, AMC, CXT, FOX, CZ, CZA, C/T, COL | - | - | - | + | - | + | - | + | - |
| E111 | R | Setif | S. enterica | CAZ, TET, AMC, AZT, CXT, FOX, CZ | - | - | + | - | - | + | - | + | - | |
| E311 | R | Batna | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T, PIP-TAZ | - | - | - | - | - | + | - | - | - | |
| E09 | Cooked chicken | R | Batna | H. alvei | CAZ, TET, AMC, CZ | + | - | - | - | + | - | + | + | - |
| E152 | R | Batna | E. cloacae | CAZ, TET, AMC, CXT, FOX, CZ | - | - | - | + | - | - | - | - | - | |
| E256 | SM | Batna | S. enterica | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T, COL, PIP-TAZ | - | - | + | - | - | - | - | + | + | |
| E310 | R | Batna | E. aerogenes | CAZ, TET, AMC, CXT, FOX, CZ, CZA | - | - | - | - | - | + | - | + | - | |
| E389 | R | Biskra | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, CIP | + | - | - | - | - | + | - | + | - | |
| E364 | R | Biskra | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T | + | - | - | - | - | - | + | + | - | |
| E368 | R | Biskra | E. cloacae | CAZ, TET, AMC, AZT, CXT, FOX, CZ | + | - | - | - | - | - | - | - | - | |
| E356 | Fish dishes | SM | Batna | E. sakazakii | CAZ, TET, AMC, CXT, FOX, CZ | - | - | - | - | - | - | - | - | - |
| E369 | Cheese | R | Biskra | E. cloacae | CAZ, TET, AMC, CXT, FOX, CZ | - | - | - | - | - | + | - | - | - |
| E374 | Mixed dishes | R | Biskra | C. freundii | CAZ, TET, AMC, AZT, CXT, FOX, CZ, C/T, PIP-TAZ | - | - | - | - | - | + | - | - | - |
aBK, bakery; R, restaurant; SM, supermarket
bCTX, cefotaxime; FOX, cefoxitine; AZT, aztreonam; CAZ, ceftazidime; AMC, amoxicillin–clavulanic acid; TET, tetracycline; CZ, cefazolin; SXT, trimethoprim sulfamethoxazole; CIP, ciprofloxacin; P/T4, piperacillin/tazobactam; COL, colistin; CZA, ceftazidime/avibactam; C/T, ceftolozane/tazobactam
Regarding the ESBL/AmpC genes, the blaCTX-M gene was the most abundant, as it was detected in 58.3% (28/48) of the isolates, followed by blaCMY-2 (43.8%, 21/48), blaTEM (37.5%, 18/48), blaOXA-1 (6.3%, 3/48), and blaSHV (2.1%, 1/48). Moreover, 19 isolates (39.6%) produced both an ESBL and a plasmid-mediated AmpC beta-lactamase, and 7 isolates (14.6%) did not harbor any ESBL/AmpC genes (Fig. 2).
Fig. 2.
Percentage of ESBL/AmpC genes in ESBL-E isolates from ready-to-eat food
In the 28 blaCTX-M isolates, 13 were clustered as blaCTX-M-9 group (27.0%); 9 were clustered as blaCTX-M-1 (18.8%), which is comprised of blaCTX-M-1, blaCTX-M-3, and blaCTX-M 15; 4 were clustered as blaCTX-M-2 (8.3%); and 2 were clustered as blaCTX-M-8 (4.2%). Moreover, the co-carriage of at least two beta-lactamase genes was detected in 22.9% (11/48) of the isolates, and three E. cloacae isolates presented the same combination (blaCTX-M1/blaTEM/blaCMY-2).
The correlation between antibiotic resistance phenotypes and ESBL/AmpC genes showed a positive correlation between the presence of blaCTX-M1 and resistance to ciprofloxacin (r = 0.304, p = 0.036). However, a negative correlation was observed between the presence of blaCTX-M1 and resistance to cefoxitin (r = − 0.304, p = 0.036).
Of the E. cloacae isolates, 58.6% (n = 17), 31.0% (n = 9), and 27.6% (n = 8) were positive for blaCTX-M, blaTEM, and blaCMY-2, respectively. Among the E. sakazakii isolates, 83.3% (n = 5), 50% (n = 3), and 33.3% (n = 2) harbored blaCMY-2, blaCTX-M, and blaTEM, respectively. While among C. freundii isolates, 50% (n = 3), 33.3% (n = 2), and 16.7% (n = 1) harbored blaTEM, blaCMY-2, and blaCTX-M, respectively (Table S4). Moreover, based on the food samples, 55.0% (n = 11), 45.0% (n = 9), and 25.0% (n = 5) of cream-filled pastries samples harbored blaCTX-M, blaCMY-2, and blaTEM genes, respectively; 85.7% (n = 6), 71.4% (n = 5), and 28.6% (n = 2) of cooked chicken samples harbored blaCTX-M, blaCMY-2, and blaTEM, respectively.
Furthermore, it was found that there is a significant positive association between the presence of S. enterica and blaCTX-M8 (p ≤ 0.001), H. alvei and blaSHV (p ≤ 0.001), and a significant negative association between the presence of E. cloacae and blaCMY-2 (p ≤ 0.001). And interestingly, it was found that there is a significant positive association between rice and the presence of blaCMY-2 and blaTEM in ESBL-E isolates (p = 0.006).
Discussion
Beta-lactamase-producing Enterobacterales are no longer solely associated with the healthcare system. Thus, it is essential to investigate their presence in other possible reservoirs, such as food. A total of 204 ready-to-eat foods were analyzed, and 48 isolates were found to be ESBL-producing Enterobacterales with a prevalence of 17.4%. These results are similar to the 19.7% prevalence reported in India [32], lower than the 42.4% that was reported in Saudi Arabia [33], but higher than the 5% and 1.9% reported in Switzerland [34] and China [12], respectively. The different prevalence rates observed in these studies may be due to differences in the methodology, sample size, food origin, and ESBL isolation techniques.
Cream-filled pastries were the most analyzed samples in this study, and they harbored a high number of ESBL-E (n = 20); these products are rich in milk, eggs, and raw constituents, making them rich nutrient media for microbial growth [35]. We could not find any study that reported the presence of ESBL-E in cream-filled pastries. Hence, this is the first study to the best of our knowledge that analyzes the presence of ESBL-E isolates in cream-filled pastries. Moreover, based on the multiple logistic regression analysis, the variable “soups” was the only independent predictor of colonization with ESBL-Enterobacterales; soups are usually cooked in large quantities, and kept unrefrigerated for some time, and then processed again; this way of cooking and dealing with soups can facilitate their contamination by ESBL-E isolates.
While several studies had reported the occurrence of ESBL in E. coli as the most prevalent ESBL-E in food [8, 36–38], we detected the highest prevalence of ESBL in E. cloacae. These bacteria are capable of causing a wide variety of infections, such as septicemia and pneumonia [39], and they have been found in some studies on RTE food [32, 38, 40]. The large diversity of ESBL-E in food may be related to the enormous diversity of RTE food and the ease by which food items can be contaminated during processing, packaging, or storing [41].
The present study shows that all the isolates were resistant to ceftazidime, cefazolin, cefotaxime, and amoxicillin-clavulanic acid, as well as high resistance to ceftolozane (a fifth-generation cephalosporin). Moreover, all the ESBL-E isolates were found multi-drug resistant [31], and 14.6% were found to be extensively multi-drug resistant [31]. Many factors could explain the rise of antibiotic resistance in the food industry, such as the use of antibiotics in animal feeds as growth promoters to improve the performance of food animals [42], the irrigation of plants and crops through sewage water contaminated with fecal material from the effluent of surrounding farms [43, 44], and the use of preservatives and disinfectants for food protection, that can create pressure on the microbial populations, which can trigger them to adapt by developing transient resistances [45, 46].
Interestingly, 95.8% of the isolates were found resistant to tetracycline; this antibiotic is widely used against Gram-negative bacteria, especially in livestock production, because of its few side effects and low cost [47]. However, its extended use in the food and agriculture industry appears to be the reason behind the observed high resistance in our study.
Furthermore, 12.5% of the isolates were resistant to colistin (a reserve antibiotic). This is close to the 19.5% colistin resistance rate reported in RTE foods in Brazil [48], but higher than those found in China (3.2%, [12]) and Spain (2.0%, [49]), respectively. The prevalence of mcr-1 was 8.3%, which is higher than those found in Germany (3.8%, [50]) but lower than those found in Brazil (19.5%) [48]. The presence of ESBL-E harboring the mcr-1 gene in RTE food is a cause for public health concern since this could contribute to the acceleration of the spread of the mcr-1 gene in the environment. In this study, we report for the first time, to our knowledge, the identification of colistin-resistant ESBL-producing Enterobacterales strains carrying the mcr-1 gene in ready-to-eat foods in Algeria.
Screening for the presence of ESBL/AmpC genes revealed the presence of blaCMY-2, blaCTX-M, blaSHV, blaTEM, and blaOXA-1 genes. The presence of the plasmid-mediated AmpC cephalosporinase gene blaCMY-2 has been previously reported across the globe [8, 51–53]; its high prevalence in this study is because it is considered the most common type of plasmid AmpC β-lactamase in the world [54]. The predominance of blaCTX-M in RTE foods (52.0%) is in accordance with a previous study analyzing sandwiches in Algeria [8] and studies from other countries [32, 55–58]. Moreover, 14.6% of the ESBL isolates that were positive in the double disc synergy test did not harbor any ESBL/AmpC genes. These isolates could carry other ESBL/AmpC genes not investigated in the present study, such as blaACT/MIR, blaDHA, and blaMOX. Hence, these findings highlight a new avenue that could be explored in future studies.
Based on food samples, cream-filled pastries were predominately contaminated with ESBL-E isolates harboring blaCTX-M9, blaTEM, and blaCTX-M-1. Interestingly, blaCTX-M-1 is regularly found in animal sources [59], and its presence in pastries proves that the origin of ESBL-E contaminating RTE food is highly diverse. In cooked chicken samples, the presence of most of the ESBL genes was detected (blaCTX-M1, blaCTX-M8, blaCTX-M9, blaTEM, blaOXA-1, blaSHV), with the predominance of blaCTX-M-1, which is in accordance with previous studies on RTE food of animal origin, who found that blaCTX-M-1 was the most prevalent subtype [32, 40, 49, 60].
The combination blaCTX-M-1/blaTEM/blaCMY-2 was the most prevalent in this study, which is in line with previous studies [37, 61, 62], and interestingly, the co-carriage of ESBL genes and colistin resistance gene, mcr-1, was observed in three isolates (E221, E256, E305). Zhang et al. (2021) [12] also reported ESBL and mcr-1 gene co-carriage in RTE foods in China. Rhouma and Letellier [63] reported that there is a possible historical link between the presence of the mcr-1 gene with ESBL genes. However, more studies are needed to confirm this hypothesis.
Moreover, in this study, analyzing the phenotype–genotype correlations showed that a positive correlation between blaCTX-M1 and ciprofloxacin was observed. Colodner [64] suggested that ESBL-producing bacteria often show cross-resistance with other groups of antibiotics, such as fluoroquinolones, which may explain in part these findings. Furthermore, a negative correlation between blaCTX-M1 and cefoxitin was observed. This may indicate that the presence of blaCTX-M1 gene is a bad biomarker for resistance to cefoxitin which is not in accordance with the study of Ramadan et al. [65], who found that the presence of a blaCTX-M gene might be leveraged as a good indicator for resistance to beta-lactam antibiotics. Long et al. [66] found that the blaCTX-M gene has a substantial correlation with the phenotypic resistance of ceftriaxone and cefepime.
In conclusion, our findings showed that RTE foods such as filled cream pastries, salads, and soups are important ESBL/AmpC reservoirs, and we report, for the first time, the presence of colistin-resistant ESBL-E strains carrying the mcr-1 gene in ready-to-eat foods in Algeria. Moreover, we report the co-carriage of an ESBL- and an mcr-1 gene with zoonotic implications. Hence, strict policies and active surveillance of RTE foods should be implemented to control the spread of these resistant genes and to ensure food safety.
Supplementary Information
Acknowledgements
This study did not receive any funding. However, AIA received salaries from an Academy of Finland-funded project (WASTPAN-grant number 1339417).
Author contributions
ZN and AIA conducted laboratory analysis of samples, ZN wrote the manuscript, BA and OR edited the paper, and AH revised the article. All authors read and approved the final manuscript.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vincenti S, Raponi M, Sezzatini R, Giubbini G, Laurenti P. Enterobacteriaceae antibiotic resistance in ready-to-eat foods collected from hospital and community canteens: analysis of prevalence. J Food Prot. 2018;81(3):424–429. doi: 10.4315/0362-028X.JFP-17-317. [DOI] [PubMed] [Google Scholar]
- 2.Beshiru A, Okoh AI, Igbinosa EO. Processed ready-to-eat (RTE) foods sold in Yenagoa Nigeria were colonized by diarrheagenic Escherichia coli which constitute a probable hazard to human health. PLoS One. 2022;17(4):e0266059. doi: 10.1371/journal.pone.0266059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabedo L, Picart Barrot L, Teixidó Canelles A. Prevalence of Listeria monocytogenes and Salmonella in ready-to-eat food in Catalonia, Spain. J Food Prot. 2008;71(4):855–859. doi: 10.4315/0362-028x-71.4.855. [DOI] [PubMed] [Google Scholar]
- 4.Arslan S, Eyi A. Antimicrobial resistance and ESBL prevalence in Escherichia Coli from retail meats. J Food Saf. 2011;31:262–267. [Google Scholar]
- 5.Karikari KB, Kpordze SW, Yamik DY, Saba CKS. Ready-to-eat food as sources of extended-spectrum b-lactamase producing Salmonella and E. coli in Tamale, Ghana. Front trop Dis. 2022;3:834048. [Google Scholar]
- 6.WHO (2015) https://www.who.int/news/item/03-12-2015-who-s-first-ever-global-estimates-of-foodborne-diseases-find-children-under-5-account-for-almost-one-third-of-deaths. Accessed 20 Jan 2023
- 7.Chantziaras I, Boyen F, Callens B, Dewulf J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J Antimicrob Chemother. 2014;69(3):827–834. doi: 10.1093/jac/dkt443. [DOI] [PubMed] [Google Scholar]
- 8.Yaici L, Haenni M, Métayer V, Saras E, Mesbah Zekar F, Ayad M, Touati A, Madec JY. Spread of ESBL/AmpC-producing Escherichia coli and Klebsiella pneumoniae in the community through ready-to-eat sandwiches in Algeria. Int J Food Microbiol. 2017;245:66–72. doi: 10.1016/j.ijfoodmicro.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Zaatout N, Bouras S, Slimani N. Prevalence of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae in wastewater: a systematic review and meta-analysis. J Water Health. 2021;19(5):705–723. doi: 10.2166/wh.2021.112. [DOI] [PubMed] [Google Scholar]
- 10.Zaatout N, Ayachi A, Kecha M. Epidemiological investigation of subclinical bovine mastitis in Algeria and molecular characterization of biofilm-forming Staphylococcus aureus. Trop Anim Health Prod. 2020;52(1):283–292. doi: 10.1007/s11250-019-02015-9. [DOI] [PubMed] [Google Scholar]
- 11.Geser N, Stephan R, Hächler H. Occurrence and characteristics of extended-spectrum β-lactamase (ESBL) producing Enterobacteriaceae in food producing animals, minced meat and raw milk. BMC Vet Res. 2012;8:21. doi: 10.1186/1746-6148-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Huang Y, Yang G, Lei T, Chen M, Ye Q, Wang J, Gu Q, Wei X, Zhang J, Wu Q. High prevalence of multidrug-resistant Escherichia coli and first detection of IncHI2/IncX4-plasmid carrying mcr-1 E. coli in retail ready-to-eat foods in China. Int J Food Microbiol. 2021;355:109349. doi: 10.1016/j.ijfoodmicro.2021.109349. [DOI] [PubMed] [Google Scholar]
- 13.Jarzab A, Gorska-Fraczek S, Rybka J, Witkowaka D. Enterobacteriaceae infection-diagnosis, antibitioc resistance and prevention. Postepy Hig Med Dosw. 2011;65:55–72. doi: 10.5604/17322693.933273. [DOI] [PubMed] [Google Scholar]
- 14.Bassetti M, Pecori D, Sibani M, Corcione S, Rosa FG. Epidemiology and treatment of MDR Enterobacteriaceae. Curr Treat Options Infect Dis. 2015;7:291–316. [Google Scholar]
- 15.Ye Q, Wu Q, Zhang S, Zhang J, Yang G, Wang J. Characterization of extended-spectrum b-lactamase-producing Enterobacteriaceae from retail food in China. Front Microbiol. 2018;9(4):1709. doi: 10.3389/fmicb.2018.01709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xylia P, Botsaris G, Chrysargyris A, Skandamis P, Tzortzakis N. Variation of microbial load and biochemical activity of ready-to-eat salads in Cyprus as affected by vegetable type, season, and producer. Food Microbiol. 2019;83:200–210. doi: 10.1016/j.fm.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 17.Pitout JD. Extraintestinal pathogenic Escherichia coli: an update on antimicrobial resistance, laboratory diagnosis and treatment. Expert Rev Anti Infect Ther. 2012;10(10):1165–1176. doi: 10.1586/eri.12.110. [DOI] [PubMed] [Google Scholar]
- 18.Iseppi R, de Niederhäusern S, Bondi M, Messi P, Sabia C. Extended-spectrum β-lactamase, AmpC, and MBL-producing Gram-negative bacteria on fresh vegetables and ready-to-eat salads sold in local markets. Microb Drug Resist. 2018;24(8):1156–1164. doi: 10.1089/mdr.2017.0198. [DOI] [PubMed] [Google Scholar]
- 19.Egea P, López-Cerero L, Navarro MD, Rodríguez-Baño J, Pascual A. Assessment of the presence of extended-spectrum beta-lactamase-producing Escherichia coli in eggshells and ready-to-eat products. Eur J Clin Microbiol Infect Dis. 2011;30(9):1045–1047. doi: 10.1007/s10096-011-1168-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Bae IK, Lee SH. New definitions of extended-spectrum β-lactamase conferring worldwide emerging antibiotic resistance. Med Res Rev. 2012;32(1):216–232. doi: 10.1002/med.20210. [DOI] [PubMed] [Google Scholar]
- 21.Zurfluh K, Nüesch-Inderbinen M, Morach M, Zihler Berner A, Hächler H, Stephan R. Extended-spectrum-b-lactamase-producing Enterobacteriaceae isolated from vegetables imported from the Dominican Republic, India, Thailand, and Vietnam. App. Environ Microbiol. 2015;81(9):3115–3120. doi: 10.1128/AEM.00258-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu BT, Li X, Zhang Q, Shan H, Zou M, Song FJ. Colistin-resistant mcr-positive Enterobacteriaceae in fresh vegetables, an increasing infectious threat in China. Int J Antimicrob Agents. 2019;54(1):89–94. doi: 10.1016/j.ijantimicag.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Yasir M, Qureshi AK, Kensarah EA, Bibi F, Al-Zahrani IA, Abd El Ghany M, Azhar EI. Draft genome sequence of colistin-resistant and extended-spectrum β-lactamase (ESBL)-producing multidrug-resistant Escherichia coli isolated from poultry meat. J Glob Antimicrob Resist. 2021;27:112–114. doi: 10.1016/j.jgar.2021.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Aklilu E, Harun A, Singh KKB. Molecular characterization of blaNDM, blaOXA-48, mcr-1 and blaTEM-52 positive and concurrently carbapenem and colistin resistant and extended spectrum beta-lactamase producing Escherichia coli in chicken in Malaysia. BMC Vet Res. 2022;18(1):190. doi: 10.1186/s12917-022-03292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa D, Vinue L, Poeta P, Coelho AC, Matos M, Saenz Y, Torres C. Prevalence of extended spectrum beta-lactamase producing Escherichia coli isolates in faecal samples of broilers. Vet Microbiol. 2009;138(3-4):339–344. doi: 10.1016/j.vetmic.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 26.EUCAST (2013) European Committee on Antimicrobial Susceptibility Testing, EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. V.1.0. http://www.amcli.it/wp-content/uploads/2015/10/EUCAST_detection_resistance_mechanisms_V1.pdf. Accessed 20 Jan 2023
- 27.EUCAST (2022) European Committee on Antimicrobial Susceptibility Testing, Breakpoint tables for interpretation of MICs and zone diameters. V.12.0. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf. Accessed 20 Jan 2023
- 28.Päivärinta M, Latvio S, Fredriksson-Ahomaa M, Heikinheimo A. Whole genome sequence analysis of antimicrobial resistance genes, multilocus sequence types and plasmid sequences in ESBL/AmpC Escherichia coli isolated from broiler caecum and meat. Int J Food Microbiol. 2020;315:108361. doi: 10.1016/j.ijfoodmicro.2019.108361. [DOI] [PubMed] [Google Scholar]
- 29.Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–495. doi: 10.1093/jac/dkp498. [DOI] [PubMed] [Google Scholar]
- 30.Rebelo AR, Bortolaia V, Kjeldgaard JS, Pedersen SK, Leekitcharoenphon P, Hansen IM, Guerra B, Malorny B, Borowiak M, Hammerl JA, Battisti A, Franco A, Alba P, Perrin-Guyomard A, Granier SA, De Frutos EC, Malhotra-Kumar S, Villa L, Carattoli A, Hendriksen RS. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. 2018;23(6):17–00672. doi: 10.2807/1560-7917.ES.2018.23.6.17-00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hidler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 32.Sivakumar M, Abass G, Vivekanandhan R, Anukampa SDK, Bhilegaonkar K, Kumar S, Grace MR, Dubal Z. Extended-spectrum beta-lactamase (ESBL) producing and multidrug-resistant Escherichia coli in street foods: a public health concern. J Food Sci Technol. 2021;58(4):1247–1261. doi: 10.1007/s13197-020-04634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Junaid K, Ejaz H, Asim I, Younas S, Yasmeen H, Abdalla AE, Abosalif KOA, Alameen AAM, Ahmad N, Bukhari SNA, Rehman A. Heavy metal tolerance trend in extended-spectrum β-lactamase encoding strains recovered from food samples. Int J Environ Res Public Health. 2021;18(9):4718. doi: 10.3390/ijerph18094718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nüesch-Inderbinen M, Zurfluh K, Peterhans S, Hächler H, Stephan R. Assessment of the prevalence of extended-spectrum β-lactamase-producing Enterobacteriaceae in ready-to-eat salads, fresh-cut fruit, and sprouts from the Swiss market. J Food Prot. 2015;78(6):1178–1181. doi: 10.4315/0362-028X.JFP-15-018. [DOI] [PubMed] [Google Scholar]
- 35.Sharifzadeh A, Hajsharifi-Shahreza M, Ghasemi-Dehkordi P. Evaluation of microbial contamination and chemical qualities of cream-filled pastries in confectioneries of Chaharmahal Va Bakhtiari Province (Southwestern Iran) Osong Public Health Res Perspect. 2016;7(6):346–350. doi: 10.1016/j.phrp.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorado-Garcıa A, Smid J, van Pelt W, Bonten M, Fluit A, Van den Bunt G, Wagenaar J, Hordijk J, Dierikx C, Veldman K. Molecular relatedness of ESBL/AmpC-producing Escherichia coli from humans, animals, food and the environment: A pooled analysis. J Antimicrob. Chemother. 2018;73(2):339–347. doi: 10.1093/jac/dkx397. [DOI] [PubMed] [Google Scholar]
- 37.Freitag C, Michael GB, Li J, Kadlec K, Wang Y, Hassel M, Schwarz S. Occurrence and characterisation of ESBL-encoding plasmids among Escherichia coli isolates from fresh vegetables. Vet Microbiol. 2018;219:63–69. doi: 10.1016/j.vetmic.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 38.Maina J, Ndung'u P, Muigai A, Kiiru J. Antimicrobial resistance profiles and genetic basis of resistance among non-fastidious Gram-negative bacteria recovered from ready-to-eat foods in Kibera informal housing in Nairobi, Kenya. Access Microbiol. 2021;3(6):000236. doi: 10.1099/acmi.0.000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Annavajhala MK, Gomez-Simmonds A, Uhlemann AC. Multidrug-resistant Enterobacter cloacae complex emerging as a global, diversifying threat. Front Microbiol. 2019;31:44. doi: 10.3389/fmicb.2019.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tekiner İH, Özpınar H. Occurrence and characteristics of extended spectrum beta-lactamases-producing Enterobacteriaceae from foods of animal origin. Braz J Microbiol. 2016;47(2):444–451. doi: 10.1016/j.bjm.2015.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giri S, Kudva V, Shetty K, Shetty V. Prevalence and characterization of extended-spectrum β-Lactamase-producing antibiotic-resistant Escherichia coli and Klebsiella pneumoniae in ready-to-eat street foods. Antibiotics (Basel) 2021;10(7):850. doi: 10.3390/antibiotics10070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrijver R, Stijntjes M, Rodríguez-Baño J, Tacconelli E, Babu Rajendran N, Voss A. Review of antimicrobial resistance surveillance programmes in livestock and meat in EU with focus on humans. Clin Microbiol Infect. 2018;24:577–590. doi: 10.1016/j.cmi.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Pruden A, Larsson DG, Amézquita A, Collignon P, Brandt KK. Management options for reducing the release of antibiotics and antibiotic resistance genes to the environment. Environ Health Perspect. 2013;121(8):878–885. doi: 10.1289/ehp.1206446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Durso LM, Cook KL. Impacts of antibiotic use in agriculture: what are the benefits and risks? Curr Opin Microbiol. 2014;19:37–44. doi: 10.1016/j.mib.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Händel N, Schuurmans JM, Brul S, ter Kuile BH. Compensation of the metabolic costs of antibiotic resistance by physiological adaptation in Escherichia coli. Antimicrob Agents Chemother. 2013;57(8):3752–3762. doi: 10.1128/AAC.02096-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pérez-Rodríguez F, Mercanoglu TB. A State-of-Art review on multi-drug resistant pathogens in foods of animal origin: risk factors and mitigation strategies. Front Microbiol. 2091;6:10. doi: 10.3389/fmicb.2019.02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jahantigh M, Samadi K, Dizaji RE, Salari S. Antimicrobial resistance and prevalence of tetracycline resistance genes in Escherichia coli isolated from lesions of colibacillosis in broiler chickens in Sistan, Iran. BMC Vet Res. 2020;16(1):267. doi: 10.1186/s12917-020-02488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monte DF, Mem A, Fernandes MR, Cerdeira L, Esposito F, Galvão JA, Franco BDGM, Lincopan N, Landgraf M. Chicken meat as a reservoir of colistin-resistant Escherichia coli strains carrying mcr-1 genes in South America. Antimicrob Agents Chemother. 2017;61(5):e02718–e02716. doi: 10.1128/AAC.02718-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vitas AI, Naik D, Pérez-Etayo L, González D. Increased exposure to extended-spectrum β-lactamase-producing multidrug-resistant Enterobacteriaceae through the consumption of chicken and sushi products. Int J Food Microbiol. 2018;269:80–86. doi: 10.1016/j.ijfoodmicro.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 50.Irrgang A, Roschanski N, Tenhagen BA, Grobbel M, Skladnikiewicz-Ziemer T, Thomas K, Roesler U, Käsbohrer A. Prevalence of mcr-1 in E. coli from livestock and food in Germany, 2010-2015. PLoS One. 2016;11(7):e0159863. doi: 10.1371/journal.pone.0159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liebana E, Carattoli A, Coque TM, Hasman H, Magiorakos AP, Mevius D, Peixe L, Poirel L, Schuepbach-Regula G, Torneke K. Public health risks of enterobacterial isolates producing extended-spectrum β-lactamases or AmpC β-lactamases in food and food-producing animals: an EU perspective of epidemiology, analytical methods, risk factors, and control options. Clin Infect Dis. 2013;56(7):1030–1037. doi: 10.1093/cid/cis1043. [DOI] [PubMed] [Google Scholar]
- 52.Kaesbohrer A, Bakran-Lebl K, Irrgang A, Fischer J, Kämpf P, Schiffmann A, Werckenthin C, Busch M, Kreienbrock L, Hille K. Diversity in prevalence and characteristics of ESBL/pAmpC producing E. coli in food in Germany. Vet Microbiol. 2019;233:52–60. doi: 10.1016/j.vetmic.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 53.Aslanta SÖ. High occurrence of CMY-2-type beta-lactamase-producing Escherichia coli among broiler flocks in Turkey. Trop Anim Health Prod. 2020;52(4):1681–1689. doi: 10.1007/s11250-019-02167-8. [DOI] [PubMed] [Google Scholar]
- 54.Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewardson AJ, Renzi G, Maury N, Vaudaux C, Brossier C, Fritsch E, Pittet D, Heck M, van der Zwaluw K, Reuland EA, van de Laar T, Snelders E, Vandenbroucke-Grauls C, Kluytmans J, Edder P, Schrenzel J, Harbarth S. Extended-spectrum β-lactamase-producing Enterobacteriaceae in hospital food: a risk assessment. Infect Control Hosp Epidemiol. 2014;35(4):375–383. doi: 10.1086/675609. [DOI] [PubMed] [Google Scholar]
- 56.Richter L, Du Plessis EM, Duvenage S, Korsten L. Occurrence, identification, and antimicrobial resistance profiles of extended-spectrum and AmpC β-lactamase-producing Enterobacteriaceae from fresh vegetables retailed in Gauteng Province, South Africa. Foodborne Pathog Dis. 2019;16(6):421–427. doi: 10.1089/fpd.2018.2558. [DOI] [PubMed] [Google Scholar]
- 57.Mikhayel M, Leclercq SO, Sarkis DK, Doublet B. Occurrence of the colistin resistance gene mcr-1 and additional antibiotic resistance genes in ESBL/AmpC-producing Escherichia coli from poultry in Lebanon: a nationwide survey. Microbiol Spectr. 2021;9(2):e0002521. doi: 10.1128/Spectrum.00025-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uyanik T, Gülel GT, Alişarli M. Characterization of extended-spectrum beta-lactamase-producing Enterobacterales from organic and conventional chicken meats. Lett Appl Microbiol. 2021;72(6):783–790. doi: 10.1111/lam.13472. [DOI] [PubMed] [Google Scholar]
- 59.Ewers C, Bethe A, Semmler T, Guenther S, Wieler LH. Extended-spectrum betalactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: a global perspective. Clin Microbiol Infect. 2012;18(7):646–655. doi: 10.1111/j.1469-0691.2012.03850.x. [DOI] [PubMed] [Google Scholar]
- 60.Kurittu P, Khakipoor B, Aarnio M, Nykäsenoja S, Brouwer M, Myllyniemi AL, Vatunen E, Heikinheimo A. Plasmid-borne and chromosomal ESBL/AmpC genes in Escherichia coli and Klebsiella pneumoniae in global food products. Front Microbiol. 2021;12:592291. doi: 10.3389/fmicb.2021.592291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HS, Chon JW, Kim YJ, Kim DH, Kim MS, Seo KH. Prevalence and characterization of extended-spectrum-β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in ready-to-eat vegetables. Int J Food Microbiol. 2015;207:83–86. doi: 10.1016/j.ijfoodmicro.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 62.Chishimba K, Hang'ombe BM, Muzandu K, Mshana SE, Matee MI, Nakajima C, Suzuki Y. Detection of extended-spectrum beta-lactamase-producing Escherichia coli in market-ready chickens in Zambia. Int J Microbiol. 2016;2016:5275724. doi: 10.1155/2016/5275724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhouma M, Letellier A. Extended-spectrum β-lactamases, carbapenemases and the mcr-1 gene: is there a historical link? Int J Antimicrob Agents. 2017;49(3):269–271. doi: 10.1016/j.ijantimicag.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 64.Colodner R. Extended-spectrum beta-lactamases: a challenge for clinical microbiologists and infection control specialists. Am J Infect Control. 2005;33(2):104–107. doi: 10.1016/j.ajic.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 65.Ramadan AA, Abdelaziz NA, Amin MA, Aziz RK. Novel blaCTX-M variants and genotype-phenotype correlations among clinical isolates of extended spectrum beta lactamase-producing Escherichia coli. Sci Rep. 2019;9(1):4224. doi: 10.1038/s41598-019-39730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Long L, You L, Wang D, Wang M, Wang J, Bai G, Li J, Wei X, Li S. Highly prevalent MDR, frequently carrying virulence genes and antimicrobial resistance genes in Salmonella enterica serovar 4,[5],12: i:- isolates from Guizhou Province, China. PLoS One. 2022;17(5):e0266443. doi: 10.1371/journal.pone.0266443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.