Abstract
The coinfections by some microorganisms have been related to severe diseases in humans and animals, where immunosuppressive agents favor opportunistic behavior of other pathogens. A 4-month-old, female mixed-breed dog with a two-week history of inappetence, prostration, emaciation, and respiratory distress was admitted at a veterinary hospital in Brazil. Tachycardia, pale mucous membranes, severe respiratory distress, and a large number of ticks (Rhipicephalus sanguineus s.l.) in different body regions were observed at clinical examination. Hematological examination of dog showed leukocytosis, neutrophilia, mild anemia, and thrombocytopenia, whereas unremarkable values in biochemical tests. Thoracic radiography revealed a pleural effusion image. Blood and the pleural fluid (purulent aspect) samples were subjected to qPCR (16S rRNA and dsb genes) and sequencing, which identified Ehrlichia canis and Anaplasma platys coinfection. An aggregate of coccoid-to-branching or long filamentous microorganisms, surrounded by pyogranulomatous inflammatory reaction was seen at the cytology of the pleural fluid. Bacteriological culture of pleural effusion showed colonies compatible with the genus Nocardia, which revealed gram-positive filamentous organisms with a tendency of fragmentation and were identified as Nocardia otitidiscaviarum in mass spectrometry (MALDI-TOF MS). Therapy of N. otitidiscaviarum isolate using levofloxacin (supported by a previous in vitro susceptibility testing) and doxycycline for E. canis and A. platys resulted in complete resolution of the clinical picture. Here, we report for the first time a triple coinfection by Nocardia otitidiscaviarum, A. platys, and E. canis in a dog with pleural effusion, where debilitating or immunosuppressive conditions induced by A. platys and E. canis coinfection probably contributed to the opportunistic behavior of N. otitidiscaviarum.
Keywords: Canine cyclic thrombocytopenia, Canine monocytic ehrlichiosis, Canine tick-borne pathogens, Emerging vector-borne pathogens, Nocardiosis
Introduction
Members of the family Anaplasmataceae comprise various species of obligate intracellular bacteria with human and animal relevance, including the genera Anaplasma and Ehrlichia [1]. These small coccoid-to-pleomorphic pathogens are found in cytoplasmatic vacuoles from a diversity of mammalian host cells, particularly leukocytes, platelets, and other bone marrow-derived cells, forming inclusion bodies or morulae [2]. They are characterized by a complexity of life cycles in invertebrate and vertebrate hosts, and some of them have been considered emergent vector-borne pathogens to humans [1].
Ehrlichia canis and Anaplasma platys are the primary causal agents of canine monocytic ehrlichiosis and canine cyclic thrombocytopenia, respectively, with worldwide distribution [3]. They are related to chronic multisystemic signs in dogs, including anemia, hemorrhage, fever, inappetence, lymphadenopathy, splenomegaly, and nonspecific hematological disorders (anemia, thrombo-to-pancytopenia), which can induce a debilitating or immunosuppressive condition with a potential lethal course, although asymptomatic infections may also occur [3, 4].
In humans, A. platys infection in women from Venezuela postulated the potential of this bacterium as a tick-borne zoonotic pathogen [5]. Likewise, E. canis has been proposed as a causal agent of human monocytic ehrlichiosis [6], and isolated from human patients [7], which indicates a putative relevance of these tick-borne microorganisms as human pathogens [8].
Nocardia species are a complex group of facultative intracellular filamentous bacteria related to a set of opportunistic clinical infections in humans, livestock, companion animals, and wildlife [9, 10]. They are ubiquitous saprophytic soil-borne organisms usually found in soil, water, dust, plants, degraded organic matter, and other environmental sources [11].
PCR-based methods and 16S rRNA sequencing have allowed taxonomic reclassification of Nocardia species [12]. At present, 119 species are known in the “List of Prokaryotic names with Standing Nomenclature” [13], and it is estimated that approximately 50 species are able to infect humans and/or animals [10], including N. otitidiscaviarum [14, 15].
Cutaneous-subcutaneous disorders [16], pneumonia [17], and systemic dissemination, e.g., osteomyelitis [18] and organ abscesses [19], represent the most common clinical manifestations of canine nocardiosis.
Nocardia-induced infections in humans have been considered a neglected [20] or misdiagnosed disease [12]. The pathogen affects particularly immunosuppressed patients, and an increasing number of cases have been reported around the world [15].
Concomitant infections by bacterial, viral, and protozoal agents have been increasingly reported in companion animals [3, 17, 21, 22], where debilitating or immunosuppressive agents favor coinfections by some opportunistic pathogens, which may result in difficulties on therapy approaches and poor prognosis. In this scenario, we report the first case of a triple coinfection by Nocardia otitidiscaviarum, A. platys, and E. canis in a dog with pleural effusion signs.
Material and methods (Case report)
In March 2019, a 4-month-old, female mixed-breed dog with a two-week history of inappetence, prostration, emaciation, and respiratory distress was admitted to a veterinary hospital in the central region of the State of São Paulo, Brazil. According to the owner, the dog lived with other puppies in a house in the urban area. The animal had been recently adopted, and there was no available history of vaccination status. Previously, the animal had been treated with ceftriaxone (30 mg/kg/12 h, for 5 days), and due to treatment failure, this antimicrobial was subsequently replaced by amoxicillin/clavulanic acid (20 mg/kg/12h, for 7 days), also ineffective.
Upon arrival at the hospital, the dog showed inappetence and severe respiratory distress. On clinical examination, tachycardia, pale mucous membranes, and a large number of ticks Rhipicephalus sanguineus sensu lato (s.l.) were observed in different body regions of the dog. The animal was submitted to complete blood cell count (CBC) [23] and selected biochemical serum exams (alanine transaminase-ALT, aspartate transferase-AST, urea, creatinine, albumin, total protein) [24], thoracic imaging examination [25], aseptic pleural puncture for cytology and bacteriological culture [9], and blood collection for molecular diagnosis of Anaplasmataceae species.
Bacteriological and mycological culture
Material collected from the pleural puncture was submitted to conventional bacteriological culture on bovine blood agar (5%) and MacConkey agar (Oxoid™, São Paulo, Brazil), simultaneously under aerobic and microaerophilic conditions (5% CO2), incubated for 5 days at 37°C. Pleural material was also cultured on Sabouraud agar (Oxoid™, São Paulo, Brazil), and incubated aerobically for 15 days at 37°C. In addition, a clinical sample of pleural puncture was aerobically cultured in Lowenstein-Jensen (Oxoid™, São Paulo, Brazil) for 90 days at 37°C. The microorganisms were previously identified based on morphological features of colonies and Gram staining [9].
Mass spectrometry and PCR
Diagnosis of bacteria in the species-level was carried out using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry-MALDI-TOF MS (Bruker and Daltonics™, Bremen, Germany). The spectra of microorganisms were analyzed between 2,000 and 20,000 m/z by FlexControl 3.3 software. Identification at genus and species levels was considered >1.7 and >2.0, respectively [26].
Genomic DNA was obtained from the Nocardia isolate using the phenol-chloroform method [27]. The extracted DNA was subjected to PCR of the 16S rRNA gene with modifications [28, 29] using the primers 27F (5'AGAGTTTGATCCTGGCTCAG3') and 1492R (5'GGTTACCTTGTTACGACTT3'), which amplify a 1,512 bp fragment.
The fragments generated by PCR were purified using a GFX PCR DNA and Gel Band Purification Kit (GE Healthcare Bio-Sciences™). Subsequently, the fragment was sequenced using a Big Dye Kit (Applied Biosystems, Perkin-Elmer™) in an ABI PRISM-310 Genetic Analyzer, following the manufacturer's instructions. The resulting sequences were edited using BioEdit software (Ibis Biosciences™) and analyzed using the basic local alignment search tool (BLAST) to compare their identity with other sequences available in GenBank.
Phylogenetic analysis of Nocardia
The sequence generated in the PCR was aligned through the MUSCLE program (Geneious Prime™ software), with 14 sequences of different isolates of Nocardia species from various countries around the world, available at GenBank: N. africana (NR_117311), N. asiatica (NR_028644), N. asteroides (AF430019), N. brasiliensis (NR115828), N. brevicatena (NR041862), N. carnea (NR118200), N. concava (AB126880 and NR040996), N. farcinica (NR115831), N. higoensis (AB108778), N. nova (NR115835 and NR117343), N. otitidiscaviarum (DQ659912), and N. pseudobrasiliensis (NR119184). Then, 1,063 characters were aligned. The phylogenetic tree for the isolate was inferred by the neighbour-joining method, using a software (Geneious Prime™) with the Jukes-Cantor substitution model. The values observed here represent the percentage of 1,000 resampling bootstraps. The homologous sequence of the HSP gene of Mycobacterium fortuitum (AJ536039) was used as an outgroup pathogen.
In vitro antimicrobial susceptibility test
Isolates were submitted to in vitro antimicrobial disk diffusion testing based on Clinical and Laboratory Standards Institute (CLSI) guidelines [30, 31] with some modifications. Briefly, isolates were cultured aerobically on sheep blood agar (5%) at 37°C to ensure purity. After 48 h of incubation, isolates were inoculated in brain heart infusion broth and incubated aerobically at 37°C for 48 h. Then, sterile glass beads were added to decrease the typical clump formation of actinomycetes and gently vortexed until an appropriate optical density (OD) of 0.5 McFarland scale, to inoculate an adequate number of colony-forming units (cfu). Inhibition zones and classification of susceptibility were interpreted after 48-72 h [32]. Nine antimicrobials from three different classes were used: aminoglycosides (amikacin 30 μg, gentamicin 10 μg), beta-lactams (amoxicillin/clavulanic acid 30 μg, cefuroxime 30 μg, ceftiofur 30 μg, ceftriaxone 30 μg, imipenem 10 μg), and fluoroquinolones (levofloxacin 5 μg, marbofloxacin 5 μg).
Hematological and cytological examination
Blood and serum of the dog were subjected, respectively, to hematological examination [23] in an automated hematology analyzer (Nihon Kohden™, Celltac alfa, MEK 6550J/K, São Caetano do Sul, SP, Brazil) and biochemical tests (protein, albumin, urea, creatinine, AST, ALT) using an automated benchtop chemistry analyzer (BS 200E, Mindray™; Shenzhen, China) with commercially available reagents (Bioclin, Belo Horizonte, Brazil) [24].
The material collected from the pleural puncture was submitted to cytological examination (Gram and Diff-Quick staining) [9].
Diagnosis of Anaplasmataceae species
The blood and pleural puncture samples were subjected to DNA extraction using Wizard Genomic DNA Purification Kit (Promega™, Madison WI, USA). Genomic DNA was processed by real-time PCR to detect E. canis and A. platys rickettsia. Primers Dsb-330F (5'GATGTCGATTATGAAACATGAAGAAAT3') [2] and Dsb-481R (5'TGCTTGTAATGTAGTGCTGCAT3') were used to amplify a 147-bp fragment of the Ehrlichia dsb gene. The specificity for E. canis was guaranteed by a fluorogenic probe (5’ABI-AGCTAGTGCTGCTTGGGCAACTTTGAGTGAA-QSY3’) (Life Technology™, Austin, TX, USA) used in the real-time reaction [33]. To detect A. platys, the primers A.pla-F (5’CGGATTTTTGTCGTAGCTTGCTAT3’) and A.pla-R (5’CCATTTCTAGTGGCTATCCCATACTACT3’) were used to amplify a 147-bp fragment of the A. platys 16S gene. The specificity for A. platys was guaranteed by a fluorogenic probe (5’6FAM–TGGCAGACGGGTGAGTAATGCATAGGA-QSY3’) (Life Technology™, Austin, TX, USA) used in the real-time reaction. The reactions were performed using Path-ID™ qPCR Master Mix (Life Technology™, Austin, TX, USA) according to the manufacturer’s protocol. Cycle threshold (Ct) values below 40 were considered positive.
Subsequently, to obtain an amplicon of 409-bp of the dsb gene for sequencing analysis, the positive sample was subjected to a conventional PCR assay, according to a protocol previously described [2, 34], using DSB-330F and DSB-728R primers (5’CTGCTCTATGTCACTTTCTCTTAAAGT3’). The products of amplification were purified using Illustra GFX PCR DNA and Gel Band Purification Kit (GE Healthcare™) commercial kits, according to the manufacturers’ recommendations, and their sequences were determined on an automated DNA sequencer (Applied Biosystems 3500/3500xL Genetic Analyzer™), following the manufacturer’s instructions. The sequences obtained were compared by Blast Analysis with other Ehrlichia spp. sequences available on GenBank.
Results
The hematological examination revealed leukocytosis (33.9 x 109 leukocytes/L), neutrophilia (30.3 x 109 neutrophils/L), mild anemia (4.5 x 109 erythrocytes/L), and thrombocytopenia (67 x 103 platelets/μL), while biochemical tests showed unremarkable values. Thoracic radiography revealed a pleural effusion image (Fig. 1). Pleural effusion material collected revealed a purulent aspect.
Fig. 1.
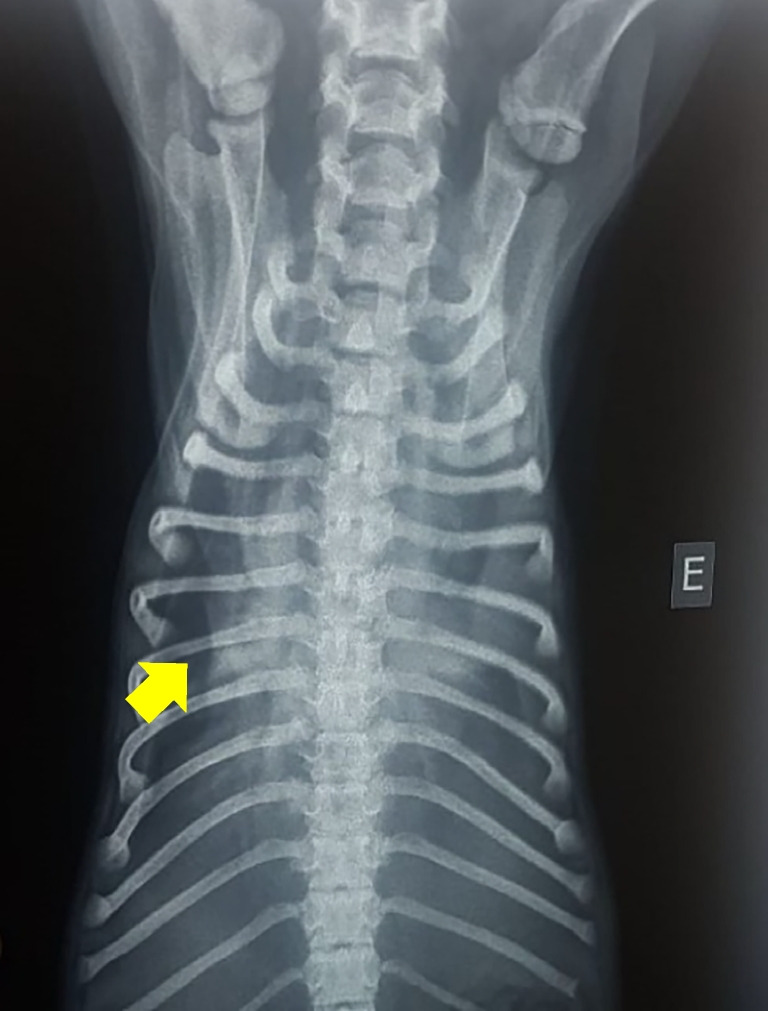
Radiographic image of pleural effusion (arrow) in a dog caused by Nocardia otitidiscaviarum
Real-time PCR (Ct <40) identified E. canis and A. platys DNA in the blood sample and the pleural fluid. Partial DNA sequences of 250-nucleotide PCR-positive blood samples were generated and were 100% identical to multiple corresponding E. canis sequences available on GenBank (AF403710, CP000107, DQ460715, DQ460716). It was not possible to obtain the nucleotide sequence of the A. platys product due to the quality of the DNA template.
An aggregate of coccoid-to-branching or long filamentous microorganisms, surrounded by an infiltrate of neutrophils, macrophages, lymphocytes, plasma, and multinucleated cells was seen in the cytology of the pleural puncture material, characterized as a pyogranulomatous inflammatory reaction.
Bacteriological culture of pleural effusion revealed circular, convex, rough, odorless, nonhemolytic, firmly adherent colonies, with white pigmentation, on bovine blood and Sabouraud agar, after 48 h of incubation, compatible with Nocardia species (Fig. 2). Gram staining of the colonies revealed gram-positive filamentous organisms, with coccoid-to-branching aspect (Fig. 3), and a tendency of fragmentation, presumably identified as the genus Nocardia. No growth was seen on the MacConkey and Loewenstein-Jensen media. Mass spectrometry (MALDI-TOF MS) spectra of the isolate was >2.0, and the microorganism was identified as Nocardia otitidiscaviarum.
Fig. 2.
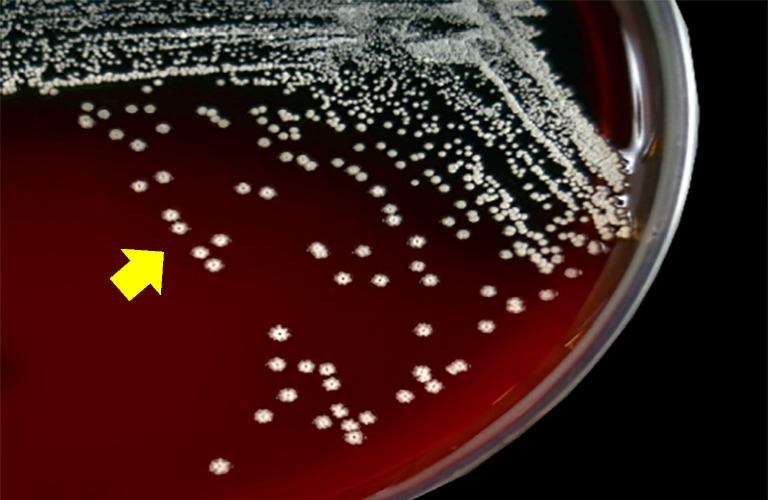
Circular, rough, nonhemolytic, firmly adherent white colonies of Nocardia otitidiscaviarum (arrow) in bovine blood agar, after 48 h incubation, isolated from a dog with pleural effusion
Fig. 3.
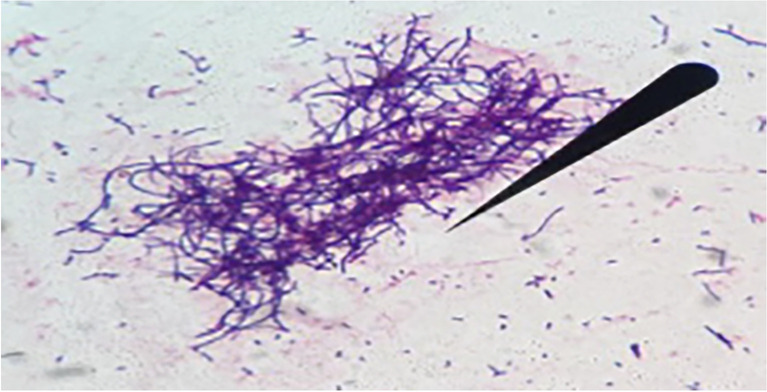
An aggregate of gram-positive coccoid-to-branching filamentous aspect (arrow) of Nocardia otitidiscaviarum isolated from a dog with pleural effusion (Gram 1000x)
The interspecies similarities of N. otitidiscaviarum and other Nocardia species identified in humans and domestic animals are shown in the phylogenetic tree (Fig. 4). The nucleotide sequences of the 16S rRNA genes of N. otitidiscaviarum were deposited in GenBank under accession number ON157238.1.
Fig. 4.
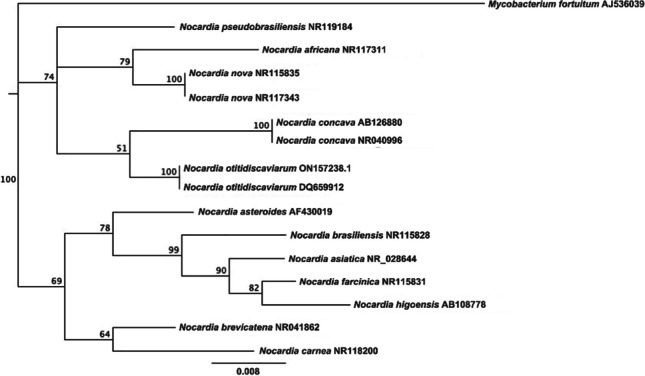
Phylogenetic tree of Nocardia otitidiscaviarum showing species similarities with other Nocardia identified in domestic animals and humans, using 16S rRNA gene sequences and neighbor-joining method. Bootstrap values greater than 50% significance are indicated. The tree was rooted with Mycobacterium fortuitum (AJS36)
In vitro antimicrobial susceptibility testing showed that the N. otitidiscaviarum isolate was susceptible to amikacin, gentamicin, imipenem, and levofloxacin. This finding supported the treatment of the dog with levofloxacin (15 mg/kg/24h/40 days, IV), while the molecular diagnosis of E. canis and A. platys coinfection supported the therapy with doxycycline (10 mg/kg/24h/30 days, PO).
The animal was reevaluated weekly throughout treatment period, including clinical examination, supported by hematological and selected aforementioned serum biochemical tests. After ~10 days of antimicrobial therapy, the animal showed interest by food, gradual recovery of pulmonary function, while complete resolution of the clinical picture and normal (unremarkable) hematological parameters were observed after the treatment period.
Discussion
In this study, we described pleural effusion caused by N. otitidiscaviarum, where concomitant infection by tick-borne pathogens E. canis and A. platys probably induced a debilitating or immunosuppressive condition that favored the opportunistic behavior of N. otitidiscaviarum.
Infection by Anaplasmataceae species trigger autoimmune processes that impair the host's immune response, and E. canis infection even promotes the multiplication and exacerbation of clinical signs resulting from A. platys infection [8]. In the present report, the E. canis and A. platys coinfection likely contributed to the invasion and pleural infection of N. otitidiscaviarum.
Ehrlichia canis is the primary agent of canine monocytic ehrlichiosis. This tick-borne disease remains a problem for veterinarians and owners from many countries [3, 4], including Brazil [35] due to high mortality rates and economic costs related to treatment. Most clinically infected dogs show chronic multisystemic signs that include anemia, hemorrhage, fever, lymphadenopathy, and splenomegaly, in addition to nonspecific hematological disorders, e.g., mild-to-severe anemia, and thrombo-to-pancytopenia [3, 4, 36], which are consistent with the pale mucous membranes, mild anemia, and severe thrombocytopenia found in the dog of the present report.
Anaplasma platys is another vector-borne pathogen that exhibits similar clinical signs to canine ehrlichiosis. Nonetheless, A. platys is a platelet-specific microorganism that causes mild-to-severe thrombocytopenia, but also asymptomatic infections [8, 37]. The dog reported here revealed severe thrombocytopenia and mild anemia that is compatible with A. platys infections. Nonetheless, due to similar clinical signs and hematological findings of both canine ehrlichiosis and anaplasmosis, it is not possible to predict in the dog reported which clinical features and erythrogram abnormalities were caused by coinfection of these tick-borne pathogens. In this report, the dog revealed leukocytosis and neutrophilia that were probably caused by Nocardia-induced infection [38].
Both E. canis and A. platys are tick-borne pathogens transmitted mainly by Rhipicephalus sanguineus ticks [39]. In fact, upon arrival at the hospital, a large number of R. sanguineus were observed in different body regions of the dog, reinforcing the importance of this tick in the transmission of Anaplasmataceae species to dogs.
Antimicrobials from the tetracycline group, i.e., doxycycline and minocycline, are considered of choice for E. canis and A. platys infections given their therapeutic intracellular concentrations in blood, tissues, and cells, with better efficacy in acute infections. However, poor prognosis is observed in the chronic phase, or when there are coinfections by these tick-borne pathogens [8, 37], probably due to the development of multisystemic signs [3, 4]. In the current report, the success of therapy could be credited to the susceptibility of both E. canis and A. platys to doxycycline, and to the fact that the animal was treated in acute phase of infection [8].
Cutaneous-subcutaneous lesions [38], pneumonia [17], and systemic dissemination [19] are seen as the most frequent clinical forms of canine nocardiosis. The current report describes pleural effusion in a dog, which can be considered an uncommon clinical picture of Nocardia infections in dogs [38].
Traumatic inoculation through puncture wounds or inhalation represents the main route of transmission of Nocardia species to dogs [18, 38]. In a retrospective study involving 28 cases of nocardiosis in cattle and dogs from Brazil, N. otitidiscaviarum had a higher prevalence among diseased dogs (7/9=77.8%) [40], although the diagnosis at the species-level of the pathogen was based on classical phenotypic methods. In fact, our dog presented a pleural effusion caused by N. otitidiscaviarum, whose transmission could be related to inhalation of the pathogen from the environment, since Nocardia species are ubiquitous saprophytic microorganisms, widely distributed in soil, dust, degraded organic matter, water, and other environmental sources [11].
In humans, nocardiosis have also been seen as an opportunistic disease [12, 15]. N. otitidiscaviarum identified in the dog reported here has also been described in human patients [15]. Besides no clear evidence of the transmission of pathogenic Nocardia from pets-to-humans, the current report describes pleural effusion-related N. otitisdiscaviarum that has been described in both people and dogs, a finding that represents implications in human health due to the close contact of dogs with their owners.
Nocardiosis in dogs are commonly unresponsive to conventional antimicrobials, particularly among animals with systemic dissemination, resulting in a poor prognosis, except for skin lesions [19, 38]. In turn, a prolonged antimicrobial therapy using levofloxacin (a broad-spectrum fluoroquinolone), and weekly clinical revaluation (including hematological and biochemical tests) of the dog reported here with pleural effusion resulted in complete recovery of the animal. Likewise, cellulitis infection in a cat harboring a virulent Rhodococcus equi (another actinomycetes as Nocardia species) revealed an effective resolution of cutaneous lesion using also a prolonged therapy protocol (40 days) with the fluroquinolone marbofloxacin [40]. Therefore, the effectiveness of therapy in our dog may be attributed, in part, to the intracellular action of levofloxacin, and its widespread distribution in blood and tissues, including pulmonary tract [41]. In addition, the in vitro antimicrobial susceptibility profile of the N. otitidiscaviarum isolate revealed sensitivity to levofloxacin, highlighting the importance of in vitro susceptibility testing previous the therapeutic approaches.
Concomitant infections by bacterial, viral, and protozoal pathogens in dogs have increasingly been reported around the world [3, 21, 22, 42], and has emphasized that agents that induce debilitating or immunosuppressive conditions may favor coinfections by pathogens with opportunistic nature [22].
Missing epidemiological or/no information data of the dog that had been recently adopted, and no sequencing of A. platys may be considered limitations of the present report.
Overall, clinical and epidemiological aspects, hematological and imaging examination, bacteriological and mycological culture, and different molecular approaches were assessed to diagnose purposes, and enabled report the first case of a triple coinfection by N. otitidiscaviarum, A. platys, and E. canis in a dog with pleural effusion, where debilitate or immunosuppressive conditions induced by A. platys and E. canis coinfection probably favored the opportunistic behavior of N. otitidiscaviarum.
Acknowledgements
We appreciate the support of the National Council for Scientific and Technological Development (CNPq), Brazil, for research productivity fellowships given to Márcio Garcia Ribeiro (#310345/2020-0), Daniel Moura de Aguiar (#303677/2018-0), Valeria Dutra (#308651/2019-7), and Luciano Nakazato (#314068/2020-1).
Author contributions
Conceptualization, methodology, and investigation: M.G. Ribeiro, C.P.C. da Silva, L.M. Pchevuzinske; Sampling, hematological, and serum biochemical tests: C.P.C. da Silva; Bacteriological and cytological diagnosis, and mass spectrometry: M.G. Ribeiro, F.V.R. Portilho, N.R. Paschoal, B.O. de Almeida; Molecular identification of Anaplasmataceae species: D.M. de Aguiar, V. Dutra, L. Nakazato, N.A. Pereira; Data analysis and writing: R.K. Takahira, A.K. Siqueira, A.A.L. de Souza, C.A. Rodrigues, T.S. Bello, M.F. Arabe Filho; P.J.L. Paz; Review and editing: all the authors.
Declarations
Ethics approval
This study was conducted under the Ethics Committee on Animal Use (CEUA) guidelines of the School of Veterinary Medicine and Animal Sciences, São Paulo State University-UNESP, Botucatu, SP, Brazil (protocol number 169/2014).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Márcio Garcia Ribeiro, Email: marcio.ribeiro@unesp.br.
Carolina Polo Camargo da Silva, Email: carolpolo89@gmail.com.
Larissa Marry Pchevuzinske, Email: pche.lary@gmail.com.
Fábio Vinícius Ramos Portilho, Email: fv_portilho@hotmail.com.
Amanda Keller Siqueira, Email: kellersiqueira@gmail.com.
Regina Kiomi Takahira, Email: regina.takahira@unesp.br.
Natália Rodrigues Paschoal, Email: nrpaschoal@gmail.com.
Adriana Aparecida Lopes de Souza, Email: adriana.souza@unesp.br.
Carolina Aparecida Rodrigues, Email: carolina.rodrigues@unesp.br.
Beatriz Oliveira de Almeida, Email: bia_oa@hotmail.com.
Thaís Spessotto Bello, Email: thaisspessotto@gmail.com.
Marcelo Fagali Árabe Filho, Email: fagali.arabe@unesp.br.
Patrik Júnior de Lima Paz, Email: patrik.paz@unesp.br.
Valéria Dutra, Email: valdutra@ufmt.br.
Luciano Nakazato, Email: lucnak@ufmt.br.
Nathalia Assis Pereira, Email: nathaliaassis89@gmail.com.
Daniel Moura de Aguiar, Email: daniel.aguiar@ufmt.br.
References
- 1.Llanes A, Rajeev S. First whole genome sequence of Anaplasma platys, and obligate intracellular rickettsial pathogen of dogs. Pathog. 2020;9:e277. doi: 10.3390/pathogens9040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguiar DM, Hagiwara MK, Labruna MB. In vitro isolation and molecular characterization of an Ehrlichia canis strain from São Paulo, Brazil. Braz J Microbiol. 2008;39:489–493. doi: 10.1590/S1517-83822008000300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pesapane R, Foley J, Thomas R, Castro LR. Molecular detection and characterization of Anaplasma platys and Ehrlichia canis in dogs from northern Colombia. Vet Microbiol. 2019;233:184–189. doi: 10.1016/j.vetmic.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Dubie T, Mohammed Y, Terefe G, Juhar T. An insight review on canine ehrlichiosis with emphasis on its epidemiology and pathogenicity importance. Global J Vet Med Res. 2014;2:59–60. [Google Scholar]
- 5.Arraga-Alvarado CM, Qurollo BA, Parra OC, Berrueta MA, Hegarty BC, Breitschwerdt EB. Molecular evidence of Anaplasma platys infection in two women from Venezuela. Am J Trop Med Hyg. 2014;91:1161–1165. doi: 10.4269/ajtmh.14-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez M, Bodor M, Zhang C, Xiong Q, Rikihisa Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann New York Acad Sci. 2006;1078:110–117. doi: 10.1196/annals.1374.016. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson WL, Allen KE, McQuiston JH, Breitschwerdt EB, Little SE. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol. 2010;26:205–212. doi: 10.1016/j.pt.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Diniz PP, Aguiar DM. Ehrlichiosis and Anaplasmosis: An update. Vet Clin Small Anim. 2022;52:1225–1266. doi: 10.1016/j.cvsm.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Quinn PJ, Markey BK, Leonard FC, Fitzpatrick ES, Fanning S, Hartigan PJ. Veterinary microbiology and microbial disease. 2. Chichester, West Sussex, UK: Wiley-Blackwell; 2011. p. 1231. [Google Scholar]
- 10.Conville PS, Brown-Elliot BA, Smith T, Zelazny AM. The complexities of Nocardia taxonomy and identification. J Clin Microbiol. 2018;56(17):1–10. doi: 10.1128/JCM.01419-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaman BL, Beaman L. Nocardia species: Host-parasite relationships. Clin Microbiol Rev. 1994;7:213–264. doi: 10.1128/CMR.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta HH, Shamoo Y. Pathogenic Nocardia: A diverse genus of emerging pathogens or just poorly recognized? Plos Pathog. 2020;16(e1008280):1–7. doi: 10.1371/journal.ppat.1008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genus Nocardia (2021). https://lpsn.dsmz.de/genus/nocardia (Access: September 24, 2021).
- 14.Ribeiro MG, Salerno T, Mattos-Guaraldi ALD, Camello TCF, Langoni H, Siqueira AK, Paes AC, Fernandes MC, Lara GHB. Nocardiosis: an overview and additional report of 28 cases in cattle and dogs. Rev Inst Med Trop São Paulo. 2008;50:177–185. doi: 10.1590/S0036-46652008005000004. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Barricarte R. Isolated nocardiosis, and unrecognized primary immunodeficiency? Front Microbiol. 2020;11:1–17. doi: 10.3389/fimmu.2020.590239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farias MR, Werner J, Ribeiro MG, Rodigheri SM, Cavalcante CZ, Chi KD, Condas LAZ, Gonoi T, Matsuzama T, Yazama K. Uncommon mandibular osteomyelitis in a cat caused by Nocardia africana. BMC Vet Res. 2012;8:239. doi: 10.1186/1746-6148-8-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribeiro AIT, Burema MC, Borges APS, Bruno VCM, Néspoli PEB, Colodel EM, Gouvêa FHF, Dutra V, Nakazato L, Ribeiro MG, Aguiar DM. Pyogranulomatous pleuropneumonia caused by Nocardia asiatica in a dog coinfected with canine morbillivirus (canine distemper virus) Vet Med Sci. 2020;6:25–31. doi: 10.1002/vms3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilligas J, Wie EV, Barr J, Russell KE, Perry AL, Weeks BR, Zhang S. Vertebral osteomyelitis and multiple cutaneous lesions in a dog caused by Nocardia pseudobrasiliensis. J Vet Int Med. 2014;28:1621–1625. doi: 10.1111/jvim.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eroksuz Y, Gursoy NF, Karapinar T, Karabulut B, Incili CA, Yerlikaya Z, Toraman ZA, Timurkan MO, Eroksuz H. Systemic nocardiosis in a dog caused by Nocardia cyriacigeorgica. BMC Vet Res. 2017;13:30. doi: 10.1186/s12917-017-0945-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duggal DS, Chug TD. Nocardiosis: A neglected disease. Med Princ Pract. 2020;29:514–523. doi: 10.1159/000508717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Headley SA, Oliveira TES, Pereira AHT, Moreira JM, Michelazzo MMZ, Pires BG, Marutani VHB, Xavier AAC, Di Santis GW, Garcia JL, Alfieri AA. Canine morbillivirus (canine distemper virus) with concomitant canine adenovirus, canine parvovirus-2, and Neospora caninum in puppies: a retrospective immunohistochemical study. Sci Rep. 2018;8:e13477. doi: 10.1038/s41598-018-31540-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portilho FVR, Paes AC, Megid J, Hataka A, Neto RT, Headley SA, Oliveira TES, Colhado BS, de Paula CL, Guerra ST, Mota AR, Listoni FJP, Takai S, Ribeiro MG. Rhodococcus equi pVAPN type causing pneumonia in a dog coinfected with canine morbillivirus (distemper virus) and Toxoplasma gondii. Microb Pathog. 2019;129:112–117. doi: 10.1016/j.micpath.2019.01.048. [DOI] [PubMed] [Google Scholar]
- 23.Meyer D, Harvey JW. Veterinary Laboratory Medicine: Interpretation and Diagnosis. 3. St. Louis, Missouri: WB Sanders; 2004. p. 351. [Google Scholar]
- 24.Kaneko JJ, Harvey JW, Bruss ML. Clinical Biochemistry of Domestic Animals. 6. San Diego: Academic Press; 2008. p. 936. [Google Scholar]
- 25.Thrall DE. Textbook of Veterinary Diagnostic Radiology. 7. St. Louis, Missouri: W.B. Saunders and Company; 2018. p. 1000. [Google Scholar]
- 26.Gonçalves JL, Tomazi T, Barreiro JR, Braga PAC, Ferreira CR, Araújo Junior JP, Eberlin MN, Santos MV. Identification of Corynebacterium spp. isolated from bovine intramammary infections by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Vet Microbiol. 2014;173:147–151. doi: 10.1016/j.vetmic.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Russel DW. Molecular cloning: A laboratory manual. 4. New York, NY, USA: Cold Spring Harbor Laboratory Press; 2012. [Google Scholar]
- 28.Lane DJ. 16S/23S rRNA Sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic Acid Techniques in Bacterial Systematic. New York: John Wiley and Sons; 1991. pp. 115–175. [Google Scholar]
- 29.Turner S, Pryer KM, VPW M, Palmer JD. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Euk Microbiol. 1999;46:327–338. doi: 10.1111/j.1550-7408.1999.tb04612.x. [DOI] [PubMed] [Google Scholar]
- 30.Clinical and Laboratory Standards Institute-CLSI . Performance Standards for Antimicrobial Disk and dilution Susceptibility Test for Bacteria isolated from Animals (CLSI VET 015) 5. PA: Wayne; 2020. p. 250. [Google Scholar]
- 31.Clinical and Laboratory Standards Institute-CLSI . Performance standards of Antimicrobial Susceptibility Testing. 30. PA: Wayne; 2020. p. 332. [Google Scholar]
- 32.Condas LAZ, Ribeiro MG, Vargas AC, Yazawa K, Gonoi T, Matsuzama T, Langoni H, Melville PA, Kastelic JP, Barkema HW. Molecular identification and antimicrobial susceptibility of Nocardia spp. isolated from bovine mastitis in Brazil. Vet Microbiol. 2013;167:708–712. doi: 10.1016/j.vetmic.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Doyle CK, Labruna MB, Breitschwerdt EB, Tang Y, Corstvet RE, Hegarty BC, Bloch KC, Li P, Walker DH, McBride JW. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time polymerase chain reaction of the dsb gene. J Mol Diagn. 2005;7:504–510. doi: 10.1016/S1525-1578(10)60581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costa JS, Melo ALT, Witter R, Pacheco TA, Chitarra CS, Carvalho ITS, Nakazato L, Dutra V, Pacheco RC, Aguiar DM. Molecular detection of Ehrlichia canis in Rhipicephalus sanguineus (s.l.) ticks in dogs and their domestic environment in Cuiaba, MT, Brazil. Braz J Vet Res Anim Sci. 2019;56:e153661. doi: 10.11606/issn.1678-4456.bjvras.2019.153661. [DOI] [Google Scholar]
- 35.Aguiar DM, Rodrigues FP, Ribeiro MG, dos Santos B, Muraro LS, Taques IIGG, Campos ANS, Dutra V, Nakazato L, Vieira RFC, Takahira RK. Uncommon Ehrlichia canis infection associated with morulae in neutrophils from naturally infected dogs in Brazil. Transb Emerg Dis. 2019;67:135–141. doi: 10.1111/tbed.13390. [DOI] [PubMed] [Google Scholar]
- 36.Bulla C, Takahira RK, Araújo JP, Jr, Trinca LA, Lopes RS, Wiedmeyer CE. The relationship between the degree of thrombocytopenia and infection with Ehrlichia canis in an endemic area. Vet Res. 2004;35:141–146. doi: 10.1051/vetres:2003038. [DOI] [PubMed] [Google Scholar]
- 37.Eiras DF, Craviotto MB, Vezzani D, Eyal O, Baneth G. First description of natural Ehrlichia canis and Anaplasma platys infections in dogs from Argentina. Comp Immun Microbiol Infect Dis. 2013;36:169–173. doi: 10.1016/j.cimid.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 38.Ribeiro MG (2021) Nocardiosis in Animals. The Merck Veterinary Manual, 12th Rahway, New Jersey, USA: Merck Publishing Group (online version: https://www.merckvetmanual.com/generalized-conditions/nocardiosis/nocardiosisin-animals?autoredirectid=16649). Accessed 1 June 2023
- 39.Stich RW, Schaefer JJ, Bremer WG, Needham GR, Jittapalapong S. Host surveys, ixodid tick biology and transmission scenarios as related to the tick-borne pathogen, Ehrlichia canis. Vet Parasitol. 2008;158:256–273. doi: 10.1016/j.vetpar.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocha BZLL, Portilho FVRP, Garino Junior F, Monti FS, Almeida BA, Souza AAL, Morizane Y, Sakaizawa N, Suzuki Y, Kakuda T, Takai S, Farias MR, Ribeiro MG. Cellulitis-related Rhodococcus equi in a cat harboring VAPA-type plasmid pattern. Microb Pathog. 2021;160:e105186. doi: 10.1016/j.micpath.2021.105186. [DOI] [PubMed] [Google Scholar]
- 41.Giguère S, Prescott JF, Dowling PM. Antimicrobial Therapy in Veterinary Medicine. 5. John Wiley & Sons; 2013. p. 704. [Google Scholar]
- 42.Attipa C, Solano-Gallego L, Papasouliotis K, Soutter F, Morris D, Helps C, Carver S, Tasker S. Association between canine leishmaniosis and Ehrlichia canis co-infection: a prospective case-control study. Paras Vect. 2018;11:184. doi: 10.1186/s13071-018-2717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


