Abstract
We evaluated the diversity and enzymatic activities of culturable fungi recovered from cotton baits submerged for 2 years in Hennequin Lake, King George Island, and from benthic biofilms in Kroner Lake, Deception Island, South Shetland Islands, maritime Antarctica. A total of 154 fungal isolates were obtained, representing in rank abundance the phyla Ascomycota, Basidiomycota and Mortierellomycota. Thelebolus globosus, Goffeauzyma sp., Pseudogymnoascus verrucosus and Metschnikowia australis were the most abundant taxa. The fungal community obtained from the biofilm was more diverse and richer than that recovered from the cotton baits. However, diversity indices suggested that the lakes may harbour further fungal diversity. The capabilities of all cultured fungi to produce the extracellular enzymes cellulase, protease, lipase, agarase, carrageenase, invertase, amylase, esterase, pectinase, inulinase and gelatinase at low temperature were evaluated. All enzymes were detected, but the most widely produced were protease and pectinase. The best enzymatic indices were obtained from Holtermanniella wattica (for invertase, esterase), Goffeauzyma sp. (amylase), Metschnikowia australis (protease), Mrakia blollopis (cellulase, pectinase), Pseudogymnoascus verrucosus (agarase, carrageenase) and Leucosporidium fragarium (inulinase). The detection of multiple enzymes reinforces the ecological role of fungi in nutrient cycling in Antarctic lakes, making nutrients available to the complex aquatic food web. Furthermore, such low-temperature-active enzymes may find application in different biotechnological processes, such as in the textile, pharmaceutical, food, detergent and paper industries, as well as environmental application in pollutant bioremediation processes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-022-00834-x.
Keywords: Antarctica, Biotechnology, Freshwater, Fungi, Enzymes
Introduction
Antarctica is known for its extreme environmental characteristics, including low temperatures, high salinity and exposure to UV radiation, low water and nutrient availability, pH variation and freeze–thaw cycles [1, 2]. Less than 0.4% of Antarctica’s area is free of permanent snow and ice, which includes lakes ecosystems [3]. Antarctic lakes differ in characteristics such as depth, trophic status, physicochemical properties and age, offering opportunities to study the diversity, ecology and evolution of microbial life under extreme conditions [2, 4].
Microorganisms are often the dominant life forms in Antarctica, possessing multiple adaptive strategies supporting their survival [5]. In Antarctic lakes, these extreme conditions represent stress factors for the resident microbiota [6]. Amongst the groups of microorganisms present, fungi inhabiting Antarctic lakes play a key role in biogeochemical cycles and the mineralization of organic matter [7–9], which are essential for the balance of micro- and macronutrients in lake systems. However, despite their acknowledged importance, there are a limited number of reports of the fungal communities of lakes in Antarctica, most of which originate from maritime Antarctica [2, 6, 8, 10]. As a result of their ecological role as decomposers, these fungi are also potentially producers of enzymes active at low temperatures, which could be of industrial interest [11–13]. Such enzymes can be up to an order of magnitude more active at low temperatures compared to mesophilic enzymes [14]. The synthesis of psychrotolerant enzymes is a widely studied element of organismal adaptative strategies in response to low temperatures [15–17]. The use of enzymes active at low temperatures in industrial and biotechnological processes can result in considerable reduction in energy costs, with their higher catalytic activities resulting from structural adaptations that give more molecular flexibility than is characteristic of mesophilic and thermophilic enzymes [18, 19].
Our study focused on two lakes in the South Shetland Islands, (i) Hennequin Lake on King George Island and (ii) Kroner Lake on Deception Island, whose surrounding catchments contain different levels of available organic matter. We deployed cotton baits for 2 years in Hennequin Lake and sampled natural benthic biofilm in Kroner Lake. From these samples, we isolated and identified culturable freshwater fungi and assessed their capacity to produce a range of enzymes active at low temperatures.
Materials and methods
Sampling
Polystyrene bags (n = 3), each containing three pieces (~ 50 cm long) of sterile cotton bait, were submerged and fixed at two points (A and B) in Hennequin Lake, Hennequin Point, King George Island during the austral summer of December 2016. They were retrieved 2 years later in 2018 (Fig. 1c). In the austral summer of December 2018, benthic biofilm samples were obtained from 15 different sites around Kroner Lake (Fig. 1d), Whalers Bay, Deception Island. Hennequin Lake is shallow (between 1.5 and 10 m depth) and surrounded by extensive moss carpets, providing abundant organic matter to the lake [20]. Kroner Lake is also a shallow water body (maximum depth 2.5 m) and is a unique geothermally heated lagoon in Antarctica. It is connected to the flooded caldera of the island (Port Foster) and is characterized by the development of dense benthic algal mats [21]. Biofilm samples were collected using a sterilized spatula and placed in sterilized plastic bags (Whirl–pak®, Nasco/USA). After collection, the samples were stored at − 20 °C until fungal isolation. The biofilm samples were processed in the Antarctic Microbiology Laboratory of the Brazilian polar support vessel Almirante Maximiano (H-41). The cotton baits were processed in Laboratory of Polar Microbiology and Tropical Connections, Department of Microbiology, Federal University of Minas Gerais, Brazil.
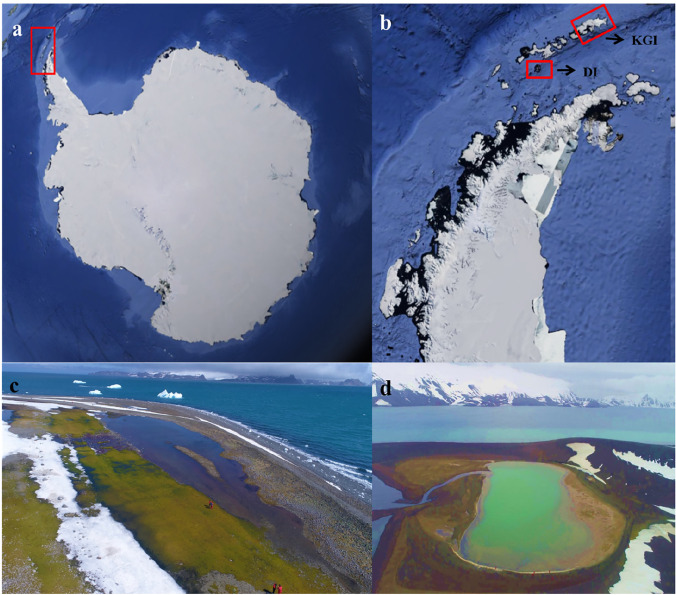
Fig. 1.
Satellite images showing the locations of King George Island and Deception Island in the South Shetland Island archipelago, maritime Antarctica, and photographs of the two lakes sampled in the current study. a Antarctic Continent with Antarctic Peninsula highlighted by red rectangle, b Antarctic Peninsula with King George Island (KGI) and Deception Island (DI) highlighted by red rectangle, c coastal supralittoral Hennequin Lake, Hennequin Point on King George Island and d Kroner Lake on Deception Island.
Source: Google Earth Pro, 2019 (a and b) and Luiz H. Rosa (c and d)
Fungal isolation
In order to isolate the fungi adhering to the cotton baits, the samples were thawed and kept at a temperature of 4 °C until processing. Solid culture media were used for fungal isolation: dichloran rose-bengal agar (Himedia, India), minimal medium (0.025% peptone, 0.5% glucose, 0.698% K2HPO4, 0.544% KH2PO4, 0.1% (NH4)2SO4, 0.11% MgSO4·7H2O, 2% agar), marine agar (Himedia, India) and malt extract agar (5% malt extract, 2% agar). All media were supplemented with 0.01% chloramphenicol (Sigma Aldrich, USA). Isolation was carried out in duplicate and two different techniques were used: (i) direct seeding, in which 1 cm from each of the three cotton baits and from each of the two points of the lake (A1, A2, A3, B1, B2 and B3) was inoculated into separate Petri dishes containing the different culture media, and (ii) serial dilution in 900 µL saline (0.85% NaCl) with 1 cm of cotton baits from each sample (A1, A2, A3, B1, B2 and B3), corresponding to dilutions of 10−1 and 10−2, and 100 µL of each suspension, inoculated in the same culture media as above.
The enrichment method was used for the isolation of cellulolytic fungi present in the cotton bait samples. A 5 cm cotton bait fragment from each sample from the two points of the lake (A1, A2, A3, B1, B2 and B3) was inoculated into 20 mL of CMC liquid culture medium (0.67% yeast nitrogen base, 1% carboxymethylcellulose, 0.01% chloramphenicol). After incubation for 7 days at 10 °C, 100 µL of this suspension were plated on CMC solid medium.
Biofilm sub-samples were processed in duplicate on Sabouraud agar (Himedia, India) and marine agar supplemented with 0.01% chloramphenicol. One hundred microliters of biofilm from each of the 15 collection points was inoculated on each medium. All plates were incubated at 10 °C for 60 days after which fungal colony forming units (CFU L−1) were counted.
All morphologically distinct colonies recovered were sub-cultured onto YM medium (0.3% yeast extract, 0.3% malt extract, 0.5% peptone, 2% glucose, 2% agar) and grouped into different morphotypes based on their macroscopic characteristics (colour, texture and type of colony border). All fungal isolates were deposited in the Collection of Microorganisms and Cells of the Federal University of Minas Gerais under the code UFMGCB.
Fungal identification
DNA extraction was carried out as described by Rosa et al. [22]. For filamentous fungi, the internal transcribed spacer (ITS) region was amplified using the universal primers ITS1 and ITS4 [23]. Amplification of the ITS region was performed as described by Rosa et al. [22]. In addition, amplification of the β-tubulin gene [24], which is commonly used to delineate fungal taxa with low intraspecific variation, was completed using the primers Bt2a/Bt2b, following Gonçalves et al. [25]. Yeasts were grouped and identified following Kurtzman et al. [26]. Yeast molecular identities were confirmed by sequencing the D1-D2 variable domains of the large subunit rRNA gene using the primers NL1 and NL4 [27]. Fungi with query coverage and identity ≥ 99% were considered to represent the same taxon. Representative consensus sequences of the fungal taxa were deposited in the NCBI GenBank database (Tables 1 and 2). To achieve species-rank identification based on ITS and β-tubulin data, the consensus sequence was aligned with all sequences from related species retrieved from the GenBank database using BLAST [28]. Fungal classification followed Kirk et al. [29] and the databases MycoBank (http://www.mycobank.org) and the Index Fungorum (http://www.indexfungorum.org).
Table 1.
Identification of Antarctic fungi recovered from biofilms by targeted rDNA sequencing and analysis using the Basic Local Alignment Search Tool (BLASTn)
| Top BLAST | UFMGCBa code | Density (UFC L−1) | Identity (%) | Query coverage (%) | bpe analysed | Proposed taxa (access in GenBank dabase) |
|---|---|---|---|---|---|---|
| Pseudogymnoascus verrucosus (KJ755525)b | 17059 | > 300 | 100 | 100 | 457 | Pseudogymnoascus verrucosus (MZ556823f) |
| Antarctomyces psychrotrophicus (NR164292)b | 17047 | 42 | 100 | 100 | 444 | Antarctomyces psychrotrophicus (MZ556824f) |
| Mortierella amoeboidea (JX976073)b | 17041 | 60 | 99 | 99 | 443 | Mortierella sp. 1 (MZ556825f) |
| Mortierella elongatula (NR111582)b | 17048 | 1 | 96 | 100 | 428 | Mortierella sp. 2 (MZ556826f) |
| Beauveria amorpha (NR111601)b | 17049 | 2 | 99 | 100 | 408 | Beauveria amorpha (MZ556827f) |
| Arthroderma curreyi (MH858822)b | 17052 | 15 | 87 | 100 | 505 | Arthroderma sp. (MZ556828f) |
| Pseudogymnoascus roseus (NR165894)b | 17051 | 38 | 100 | 97 | 433 | Pseudogymnoascus sp. (MZ556829f) |
|
Penicillium goetzii (MT558933)b Penicillium chrysogenum (AY495981)d |
17078 | > 300 |
100 98 |
100 100 |
458 372 |
Penicillium sp. (MZ556830f) |
| Holtermanniella wattica (NG058307)c | LB2 | 1 | 100 | 100 | 514 | Holtermanniella wattica (MZ557052g) |
| Metschnikowia australis (KY108453)c | LB6 | 6 | 100 | 100 | 374 | Metschnikowia australis (MZ557053g) |
| Leucosporidium fragarium (NG058330)c | LB1 | 32 | 99 | 100 | 569 | Leucosporidium fragarium (MZ557054g) |
| Vishniacozyma victoriae (NG057678)c | LB5 | 1 | 100 | 100 | 569 | Vishniacozyma victoriae (MZ557055g) |
| Rhodotorula mucilaginosa (KY109056)c | LB15 | 1 | 100 | 100 | 420 | Rhodotorula mucilaginosa (MZ557056g) |
|
Debaryomyces hansenii (MK394104)c Debaryomyces fabryi (MK394103)b |
LB7 | 1 |
100 100 |
100 100 |
457 409 |
Debaryomyces sp. (MZ557057g, MZ556834f) |
| Tetracladium globosum (NG059961)c | LB20 | 3 | 97 | 100 | 409 | Tetracladium sp. (MZ557058g) |
| Mrakia blollopis (NG057710)c | LB21 | 3 | 99 | 100 | 523 | Mrakia blollopis (MZ557059g) |
aUFMGCB, collection of microorganisms and cells of the Federal University of Minas Gerais; ebp, base pairs. Taxa subject to BLAST analysis based on bITS, cD1-D2 domain and dβ-tubulin. Sequences of fITS e gD1-D2 deposited in the GenBank database. BLASTn, Basic Local Alignment Search Tool
Table 2.
Diversity indices of fungal communities and physicochemical characteristics of the two Antarctic lakes sampled
| Index | Cotton baits (Hennequin Lake) | Biofilm (Kroner Lake) | Total |
|---|---|---|---|
| Number of taxa | 15 | 16 | 31 |
| Number of isolates | 94 | 60 | 154 |
| Fisher-α | 5.04 | 7.14 | 8.97 |
| Margalef | 3.08 | 3.66 | 4.96 |
| Simpson | 0.85 | 0.76 | 0.91 |
| Temperature (°C) | 6.5 | 6.5 | - |
| Conductivity (mS cm−1) | 105 | 323 | - |
| pH | 9 | 7.86 | - |
All results of diversity indices were obtained with 95% confidence and bootstrap values were calculated from 1000 replicates using the PAST computer program 1.90
Fungal diversity
To quantify species diversity, richness and dominance, Fisher’s ɑ, Margalef’s and Simpson’s indices were used, respectively. Species accumulation curves were obtained using the Mao Tao index. For the construction of these curves, the density of each taxon obtained was used. All results were obtained with 95% confidence, and bootstrap values were calculated from 1000 replicates using the PAST computer program 1.90 [30]. A Venn diagram was prepared as described by Bardou et al. [31] to illustrate the similarities in fungal community composition obtained from the cotton baits and biofilm samples.
Enzyme production
Filamentous fungi and yeasts were initially grown on YM agar at 15 °C for 7 and 2 days, respectively. Then, for filamentous fungi, one plug (0.5 mm in diameter) of agar culture medium including fungal growth was transferred to solid culture media containing the specific substrate for each enzyme. For yeasts, a needle loop from each colony was inoculated directly into the solid medium containing the specific substrate for each enzyme. The isolates were incubated for 7 days in Petri dishes at 15 °C. All assays were performed in duplicate. Cellulase, protease, lipase, agarase, carrageenase, invertase, amylase, esterase, pectinase, inulinase and gelatinase enzymes were evaluated as described below. Enzymatic activity was determined as described by Hankin and Anagnostakis [32], through assessing the relationship between the diameter of the degradation halo and the diameter of the colony, expressed as enzymatic index (EI), using the formula enzymatic index (EI) = diameter of the degradation halo/colony diameter.
Cellulase: YM (1:10) plus carboxymethylcellulose (5 g L−1) replacing glucose. Petri dishes were flooded with a solution of Congo red (2.5 g L−1 in 0.1 M Tris HCl buffer, pH 8) for 15 min, which was then discarded, and the plates were flooded with 1 M NaCl for 15 min, which was also discarded. Positive cellulase activity was defined as a clear halo around the colony on a red background [33]. Protease: Sabouraud dextrose agar (65 g L−1) plus skimmed milk (10 g L−1). A positive reaction was detected as a clear halo (produced by casein degradation) around the colony in the opaque medium [34, 35]. Lipase: 1 g L−1 peptone, 0.5 g L−1 yeast extract, 15 g L−1 agar, 31.25 mL L−1 olive oil as carbon source and rhodamine B solution 0.01% v/v (10 mL L−1) in absolute ethanol. Positive activity was detected by the presence of an orange fluorescent halo under UV light at 350 nm (interaction of rhodamine B with fatty acids released by the enzymatic hydrolysis of olive oil) [35]. Agarase: YM supplemented with 1.5% agar, the enzyme activity being determined by the addition of lugol [36]. Carrageenase: YM supplemented with 1.5% carrageenan (Sigma Aldrich, USA). Enzyme activity was determined by the addition of lugol [36]. Amylase: starch agar [6.7 g L−1 YNB (Difco), 2 g L−1 soluble starch and 20 g L−1 agar]. Enzyme activity was determined by the addition of lugol [34, 37]. For agarase, carragenase and amylase activities, lugol was used to the reveal the enzymatic halo formation. Invertase: YM with 2% sucrose and 0.003% bromocresol green. The appearance of a yellow halo was indicative of positive activity [38]. Esterase: bacteriological peptone (10 g L−1), NaCl (5 g L−1), CaCl2 2H2O (4 g L−1) and Tween 80 (10 g L−1). Positive activity indicated by a white precipitate around the colony [33]. Pectinase: YM (1:10), pH 7.0, 10 g L−1 pectin. The plates were flooded with 10 g L−1 of CTAB (cetyltrimethylammonium bromide) and positive activity was indicated by a clear halo [33, 39]. Inulinase: inulin agar (10 g L−1 inulin, 2 g L−1 yeast extract, 5 g L−1 peptone, 0.5 g L−1 MgSO4, NaCl 0.5 g L−1, CaCl2 0.15 g L−1 and 20 g L−1 agar [pH 6.0]). Enzyme activity was determined by the addition of lugol [40]. Gelatinase: YM prepared with 160 g L−1 of gelatin as a gelling agent in place of agar. Liquefaction of the medium around the colony indicated positive activity [37].
Results
Fungal identification and diversity
A total of 154 fungal isolates were obtained from different culture media (Fig. S1). Of these, 89 (58%) were filamentous fungi and 65 (42%) were yeasts. Ninety-four fungal isolates were obtained from cotton baits (45 filamentous fungi and 49 yeasts) and 60 isolates were obtained from biofilm samples (44 filamentous fungi and 16 yeasts).
The fungi were identified using molecular biological approaches (Tables 1 and 2) as belonging to the phyla Ascomycota, Basidiomycota and Mortierellomycota. Ascomycota was the most abundant, represented by 89 (58%) isolates, followed by 58 Basidiomycota isolates (38%) and seven Mortierellomycota isolates (4%). From the cotton baits, Thelebolus globosus was the most abundant filamentous fungus (24 isolates) and Goffeauzyma sp. the most abundant yeast (19 isolates). From the biofilm samples, Pseudogymnoascus verrucosus (27 isolates) was the most abundant filamentous fungus and Metschnikowia australis (five isolates) the most abundant yeast. In addition, single isolates of the genus Penicillium were obtained from the cotton bait samples, and of the genera Holtermanniella, Vishniacozyma, Rhodotorula, Debaryomyces and Mortierella from the biofilm samples.
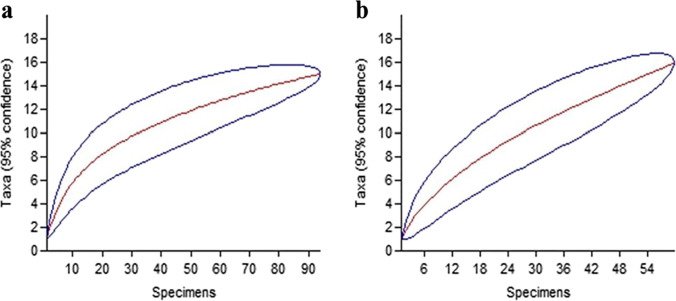
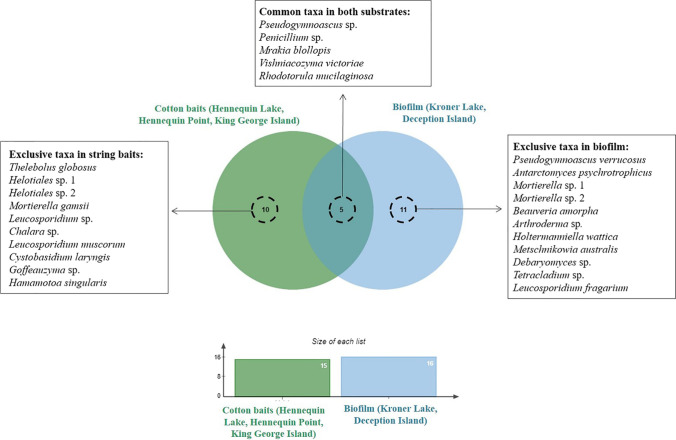
The Mao Tao rarefaction curves for both lakes (Fig. 2) did not reach asymptote, suggesting that the lakes may harbour further diversity, likely a result of the low overall number of samples obtained. The diversity, richness and dominance indices for the fungal communities associated with the cotton baits and biofilms, and the physicochemical characteristics of the lake water, are shown in Table 2. The fungal community obtained from the biofilm samples was more diverse (Fisher’s-α) and richer (Margalef) compared with that from the cotton baits. However, the dominance (Simpson) of some taxa was higher within the cotton bait samples than in the biofilms. Of the 26 taxa identified, 10 were exclusive to the cotton bait samples and 11 exclusive to biofilm samples, with only five shared between the two substrates (Fig. 3).
Fig. 2.
Mao Tao rarefaction curves, with 95% confidence limits, of fungal communities isolated from samples of a cotton baits and b biofilm from Antarctic lakes; blue lines indicate 95% confidence limits
Fig. 3.
Venn diagram illustrating the similarities in fungal community composition obtained from cotton baits and biofilm samples from Antarctic lakes
Kroner Lake (Deception Island) is a lake that experiences marine water intrusion and is characterized by considerable environmental and biological heterogeneity [21]. This lake occupies a shallow circular depression in an ash plain, where geothermal activity provides a source of thermal energy [41]. In contrast, lakes located on King George Island are usually small and shallow, located in depressions formed by glacial scour and subsequent deglaciation, although some larger and deeper lakes are also present [42]. The cotton baits and biofiolm substrates examined in the current study clearly differed, as well has having different histories with the cotton baits being deployed in Lake Hennequin for two full years, while the natural biofilms were sampled from different benthic substrates in Kroner Lake. Our data indicated higher diversity and richness indices in the fungal community of the biofilms than of the cotton baits (Table 2). The marine water intrusion in Kroner Lake, which introduces macroalgal biomass and likely other marine biological components, may be linked with the fungal community detected in the biofilm samples displaying higher diversity and richness indices.
Enzymatic activities
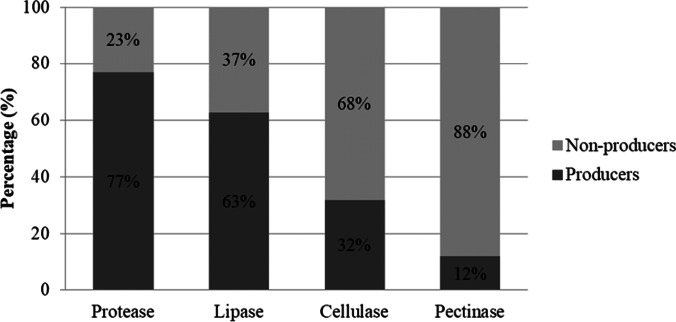
We examined the ability of the isolated fungi to produce 11 enzymes, all potentially involved in the degradation of organic matter. In assessing whether a microorganism is a good producer of extracellular enzymes in a solid medium, Lealem and Gashe [43] established that the EI must be greater than or equal to 2 [44]. Positive confirmation of activity was obtained for all 11 enzymes evaluated (Fig. S2). Amongst the fungi assayed, only four (3%) isolates of Mortierella amoeboidea failed to produce detectable activity of any of the enzymes tested. The most commonly detected enzymes were protease (77%) and lipase (63%) and the least detected were cellulase (32%) and pectinase (12%) (Fig. 4). The best enzymatic indices were obtained from Holtermanniella wattica UFMGCB LB2 for invertase and esterase, Goffeauzyma sp. UFMGCB L68 for amylase, Metschnikowia australis UFMGCB LB13 for protease, Mrakia blollopis UFMGCB L76 for cellulase, Mrakia blollopis UFMGCB LB21 for pectinase, Pseudogymnoascus verrucosus UFMGCB 17076 for agarase and carrageenase and Leucosporidium fragarium UFMGCB LB4 for inulinase (Table S1). Arthroderma sp. UFMGCB 17052 was able to produce gelatinase, which is the first report of this enzyme from this genus.
Fig. 4.
Enzymes detected with higher and lower frequency amongst all isolates. Protease and lipase were the most frequently observed, while cellulase and pectinase were the least frequent
Discussion
Fungal identification and diversity
In our study, T. globosus and Goffeauzyma sp. were the most abundant fungi isolated from the cotton baits and P. verrucosus and M. australis from the biofilm samples. Species of Pseudogymnoascus, isolated from both cotton baits and biofilm, are widely reported in polar regions and are abundant in Antarctica [45]. Pseudogymnoascus includes species able to produce bioactive secondary metabolites and enzymes with potential biotechnological applications [46]. The genus Metschnikowia was detected in the biofilm samples. According to Batista et al. [47], M. australis is an endemic Antarctic species that displays unique metabolic characteristics that assist it to survive the continent’s stressful conditions. Members of the genus Thelebolus, a genus detected in the cotton baits, are known to combine psychrophilic features and an association with guano, suggesting that the isolates obtained here may be associated with avian vectors [48]. Thelebolus globosus is endemic to Antarctica and has a more limited distribution [48]. Amongst yeast species isolated from cold habitats, members of the genus Goffeauzyma, detected in cotton baits, are amongst the most frequently recorded in the cryosphere, although the genus has also been reported from boreal and temperate habitats [49].
Some taxa identified in the present study have previously been reported from Antarctic lakes in the Larsemann Hills, Vestfold Hills and McMurdo Dry Valleys. These include the psychrophilic genera Thelebolus and Pseudogymnoascus and the cold-adapted cosmopolitan genera Beauveria, Debaromyces and Penicillium [10]. In a study evaluating the fungal communities of five lakes in maritime Antarctica (King George Island and Deception Island), Gonçalves et al. [2] isolated both endemic and cosmopolitan genera (Pseudogymnoascus, Antarctomyces, Mortierella, Penicillium and Thelebolus). Rojas-Jimenez et al. [50] reported Mrakia taxa from lake basins permanently covered with ice in a study using an independent cultivation technique.
Sub-samples of the same cotton baits from Hennequin Lake analysed in the current study were also subjected to metabarcoding in a separate study [20]. Their analyses revealed a total of 119,148 DNA reads representing 27 taxa of fungi from the phyla Ascomycota, Basidiomycota, Mortierellomycota, Chytridiomycota and Rozellomycota; amongst them, the taxa Thelebolus globosus, Helotiales sp., Mortierella gamsii, Chalara sp., Penicillium sp., Mrakia sp., Goffeauzyma sp. and Tetracladium sp. were also detected by culturing methods in our study.
Enzymatic activities
The demand for new or better biocatalysts with different substrate selectivity, chiral selectivity, stability and activity at various pH and temperatures can be addressed through studies of microbiota, which represent a potentially vast natural reservoir of biological activities [51]. Due to their structural and functional complexity, the application of natural enzymes in biotechnological processes still remains limited, and these enzymes often require modification before industrial use (e.g. improvements in catalytic efficiency, stereoselectivity and stability through combinatorial engineering strategies); protein engineering technologies allow the development of enzymes with high activity against non-natural targets, in non-natural conditions such as the presence of organic solvents, and even for chemical transformations not found in nature [51].
The results obtained in our study are consistent with those of Krishnan et al. [52, 53], Carrasco et al. [37, 54], Duarte et al. [35], Martinez et al. [40], Martorell et al. [33, 55], Troncoso et al. [38], Furbino et al. [36], Poveda et al. [39] and Tsuji [56]. These studies reported that fungi from different Antarctic environments including soil, macroalgae, marine sponges and terrestrial/marine waters also produced some of the enzymes confirmed in the current study. The production of all enzymes detected here has been reported by fungi isolated from cold environments. Extracellular enzymes produced by Antarctic fungi may represent an important element of their survival strategies in different Antarctic soils [53]. Our study showed that fungi living in Antarctic lakes also produce different extracellular enzymes, which can display the same role as those produced by their terrestrial counterparts. These enzymes catalyse the transformation of different substrates and can play an important role in the decomposition of organic matter in Antarctic ecosystems. Furthermore, enzymes produced by Antarctic microorganisms may be useful in the bioremediation of hydrocarbons and associated pollutants resulting from increasing contamination by fuel release in parts of Antarctica [57]. We detected large variation in the enzymatic index between the different fungal isolates, particularly referring to T. globosus, A. psychrotrophicus, P. verrucosus, L. muscorum, Goffeauzyma sp., M. blollopis and M. australis, which may indicate intraspecific variation.
Thelebolus species are frequent in lakes and are also found in association with skuas, petrels and other birds, and lichen thalli, in Antarctica [2, 10, 48, 58]. Amongst Thelebolus species, T. microsporus isolated from Antarctica showed α-amylase activity, suggesting its potential application as a detergent additive, in textile processing and in the food industry [59]. Thelebolus sp. recovered from Antarctic soil was characterized as producer of protease [52].
Antarctomyces psychrotrophicus was the species with the highest number of isolates able to produce different enzymes. Isolates of this Antarctic endemic fungus were able to produce the enzymes protease, esterase, cellulase and lipase. Antarctomyces (Thelebolales) is an endemic Antarctic genus that includes only two known species, A. psychrotrophicus isolated from soils of maritime and continental Antarctica [60] and A. pellizariae isolated from Antarctic snow [61]. These endemic species can produce enzymes at low temperature, such as cellulase, as described by [52].
Pseudogymnoascus includes various cold-adapted species commonly found in Antarctic environments/substrates [41], which have been reported as promising producers of enzymes active at low temperatures. Loperena et al. [62] recovered strains of Geomyces pannorum (now Pseudogymnoascus pannorum) from Antarctic macroalgal thalli, which showed high gelatinase activities at low temperature (4 °C). In addition, Poveda et al. [39] isolated Geomyces sp. from marine Antarctic sponges able to produce high levels of cold-active pectinases (at 10 °C), while also being capable of producing the enzyme in mesophilic conditions (at 30 °C). Furbino et al. [36] detected Pseudogymnoascus sp. from the Antarctic macroalga Iridaea cordata capable of producing carrageenase and agarase.
Leucosporidium muscorum, recovered from both cotton baits and biofilms, produced a broad range of enzymes. Leucosporidium is a basidiomycetous yeast that belongs to the order Leucosporidiales, which includes species known to produce various enzymes [36]. Leucosporidium taxa recovered from Antarctic soils and sediments were reported to produce lipase, invertase, esterase, pectinase, cellulase and amylase [33, 37, 38]. Leucosporidium muscorum isolated from Antarctic marine sediments was reported to produce ASNase [63].
Different unidentified Goffeauzyma sp. isolates recovered in our study had good enzymatic indices for several of the enzymes assayed. Goffeauzyma (synonym Cryptococcus) is abundant in Antarctica [64] and some species, such as Goffeauzyma gilvescens, are known to produce laccase [65]. Tsuji [56] isolated Goffeauzyma strains from Antarctic soils able to produce lipase, protease and cellulase.
In our study, Mrakia blollopis produced the widest enzymatic spectrum. Mrakia includes species recognized as cold-adapted in Arctic [66, 67], alpine [68] and Patagonian [69] ecosystems. In Antarctica, representatives of Mrakia are reported from soils, lake sediments [56, 70–72] and lichen thalli [58, 73]. Mrakia species from Antarctic soils and lakes have been reported to produce cellulase, protease, lipase, invertase, amylase, esterase, pectinase and gelatinase [33, 37, 38, 52, 54, 56]. da Silva et al. [73] recovered M. gelida, M. niccombsii and Mrakia sp. from Antarctic lichens thalli, capable of producing a broad range of extracellular hydrolases including protease, amylase, cellulase, esterase, lipase and pectinase.
Metschnikowia australis is a yeast species abundant in association with Antarctic macroalgae [74] but, despite this association, is apparently unable to display agarolytic or carragenolytic activities [36]. However, we detected some M. australis isolates from biofilms sampled in Kroner Lake that were able to produce protease, carragenase and inulinase. Amongst the enzymes assayed, protease and lipase were the most commonly detected, while cellulase and pectinase were the least detected. This may reflect the presence of different organic components in the sampled lake environments but further studies would be required to confirm this. Our data are consistent with those of Duarte et al. [35], Carrasco et al. [37] and Martinez et al. [40], who reported that M. australis recovered from soil, marine sediments, freshwater lakes and in association with Salpa sp. in Antarctica were able to produce lipase, pectinase, inulinase and protease.
Conclusions
Our study demonstrated that Antarctic lakes harbour both cosmopolitan and endemic cold-adapted fungi capable of producing various extracellular enzymes at low temperatures. These enzymes likely contribute to the degradation of organic matter in freshwater ecosystems, emphasizing the important ecological role of fungi in nutrient cycling and release. Furthermore, these low-temperature-active enzymes may have potential for application in different biotechnological processes, such as in the textile, pharmaceutical, food, detergent and paper industries, in addition to pollutant bioremediation processes in the environment.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This study received financial support from CNPq, PROANTAR, FAPEMIG, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). P. Convey is supported by NERC core funding to the British Antarctic Survey’s ‘Biodiversity, Evolution and Adaptation’ Team.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gocheva YG, Krumova ET, Slokoska LS, et al. Cell response of Antarctic and temperate strains of Penicillium spp. to different growth temperature. Mycol Res. 2006;110:1347–1354. doi: 10.1016/j.mycres.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Gonçalves VN, Vaz AB, Rosa CA, Rosa LH. Diversity and distribution of fungal communities in lakes of Antarctica. FEMS Microbiol Ecol. 2012;82:459–471. doi: 10.1111/j.1574-6941.2012.01424.x. [DOI] [PubMed] [Google Scholar]
- 3.Rosa LH, Ogaki MB, Lirio JM, Vieira R, Coria SH, Pinto OHB, Carvalho-Silva M, Convey P, Rosa CA, Câmara PEAS. Fungal diversity in a sediment core from climate change impacted Boeckella Lake, Hope Bay, north-eastern Antarctic Peninsula assessed using metabarcoding. Extremophiles. 2022;26:16. doi: 10.1007/s00792-022-01264-1. [DOI] [PubMed] [Google Scholar]
- 4.Vincent WF. Evolutionary origins of Antarctic microbiota: invasion, selection and endemism. Antarct Sci. 2000;12:374–385. doi: 10.1017/S0954102000000420. [DOI] [Google Scholar]
- 5.Kappen L. Lichens in the Antarctic region. In: Friedmann EI, editor. Antarctic microbiology. New York, NY: Wiley-Liss Inc; 1993. pp. 433–490. [Google Scholar]
- 6.Ellis-Evans JC. Microbial diversity and function in Antarctic freshwater ecosystems. Biodivers Conserv. 1996;5:1395–1431. doi: 10.1007/BF00051985. [DOI] [Google Scholar]
- 7.Kuehn KA. Lentic and lotic habitats as templets for fungal communities: traits, adaptations, and their significance to litter decomposition within freshwater ecosystems. Fungal Ecol. 2016;19:135–154. doi: 10.1016/j.funeco.2015.09.009. [DOI] [Google Scholar]
- 8.Ogaki MB, Vieira R, Lírio JM, et al. Diversity and ecology of fungal assemblages present in lakes of Antarctica. In: Rosa LH, et al., editors. Fungi of Antarctica: diversity, ecology and biotechnological applications. Switzerland: Springer; 2019. pp. 69–97. [Google Scholar]
- 9.Ogaki MB, Vieira R, Muniz MC, et al. Diversity, ecology, and bioprospecting of culturable fungi in lakes impacted by anthropogenic activities in Maritime Antarctica. Extremophiles. 2020;24:637–655. doi: 10.1007/s00792-020-01183-z. [DOI] [PubMed] [Google Scholar]
- 10.Brunati M, Rojas JL, Sponga F, et al. Diversity and pharmaceutical screening of fungi from benthic mats of Antarctic lakes. Mar Genomics. 2009;2:43–50. doi: 10.1016/j.margen.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Cavicchioli R, Charlton T, Ertan H, et al. Biotechnological uses of enzymes from psychrophiles. Microb Biotechnol. 2011;4:449–460. doi: 10.1111/j.1751-7915.2011.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martorell MM, Ruberto LAM, de Figueroa LICD, Mac Cormack WP. Antarctic yeasts as a source of enzymes for biotechnological applications. In: Rosa LH, editor. Fungi of Antarctica: diversity, ecology and biotechnological applications. Switzerland: Springer; 2019. pp. 285–304. [Google Scholar]
- 13.Zucconi L, Canini F, Temporiti ME, Tosi S. Extracellular enzymes and bioactive compounds from antarctic terrestrial fungi for bioprospecting. Int J Environ Res Public Health. 2020;17:6459. doi: 10.3390/ijerph17186459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margesin R, Feller G. Biotechnological applications of psychrophiles. Environ Technol. 2010;31:835–844. doi: 10.1080/09593331003663328. [DOI] [PubMed] [Google Scholar]
- 15.Gerday C, Aittaleb M, Arpigny JL, et al. Psychrophilic enzymes: a thermodynamic challenge. Biochim Biophys Acta Protein Struct Mol Enzymol. 1997;1342:119–131. doi: 10.1016/S0167-4838(97)00093-9. [DOI] [PubMed] [Google Scholar]
- 16.Feller G, Gerday C. Psychrophilic enzymes: hot topics in cold adaptation. Nat Rev Microbiol. 2003;1:200–208. doi: 10.1038/nrmicro773. [DOI] [PubMed] [Google Scholar]
- 17.Buzzini P, Branda E, Goretti M, Turchetti B. Psychrophilic yeasts from worldwide glacial habitats: diversity, adaptation strategies and biotechnological potential. FEMS Microbiol Ecol. 2012;82:217–241. doi: 10.1111/j.1574-6941.2012.01348.x. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui KS, Cavicchioli R. Cold-adapted enzymes. Annu Rev Biochem. 2006;75:403–433. doi: 10.1146/annurev.biochem.75.103004.142723. [DOI] [PubMed] [Google Scholar]
- 19.Javed A, Qazi JI. Psychrophilic microbial enzymes implications in coming biotechnological processes. Am Sci Res J Eng Technol Sci (ASRJETS) 2016;23:103–120. [Google Scholar]
- 20.de Souza LM, Ogaki MB, Câmara PE, et al. Assessment of fungal diversity present in lakes of Maritime Antarctica using DNA metabarcoding: a temporal microcosm experiment. Extremophiles. 2021;25:77–84. doi: 10.1007/s00792-020-01212-x. [DOI] [PubMed] [Google Scholar]
- 21.Izaguirre I, Allende L, Tell G. Algal communities of a geothermally heated lagoon on Deception Island (South Shetland Islands) Polar Biol. 2006;29:364–371. doi: 10.1007/s00300-005-0065-6. [DOI] [Google Scholar]
- 22.Rosa LH, Vaz AB, Caligiorne RB, et al. Endophytic fungi associated with the Antarctic grass Deschampsia antarctica Desv. (Poaceae) Polar Biol. 2009;32:161–167. doi: 10.1007/s00300-008-0515-z. [DOI] [Google Scholar]
- 23.White TJ, Bruns T, Lee SJWT, Taylor J. Amplifcation and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: a guide to methods and applications. Cambridge: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 24.Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl Environ Microbiol. 1995;61:1323–1330. doi: 10.1128/aem.61.4.1323-1330.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonçalves VN, Carvalho CR, Johann S, et al. Antibacterial, antifungal and antiprotozoal activities of fungal communities present in different substrates from Antarctica. Polar Biol. 2015;38:1143–1152. doi: 10.1007/s00300-015-1672-5. [DOI] [Google Scholar]
- 26.Kurtzman CP, Fell JW, Boekhout T. The yeasts: a taxonomic study. 5. Elsevier; 2011. [Google Scholar]
- 27.Lachance MA, Bowles JM, Starmer WT, Barker JSF. Kodamaea kakaduensis and Candida tolerans, two new ascomycetous yeast species from Australian Hibiscus flowers. Can J Microbiol. 1999;45:172–177. doi: 10.1139/w98-225. [DOI] [PubMed] [Google Scholar]
- 28.Altschul SF, Madden TL, Schäffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirk PM, Cannon PF, Minter DW, Stalpers JA. Dictionary of the fungi. 10. Wallingford: CAB International; 2008. [Google Scholar]
- 30.Hammer Ø, Harper DA, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4:9. [Google Scholar]
- 31.Bardou P, Mariette J, Escudié F, et al. Jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15:293. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hankin L, Anagnostakis SL. The use of solid media for detection of enzyme production by fungi. Mycologia. 1975;67:597–607. doi: 10.1080/00275514.1975.12019782. [DOI] [Google Scholar]
- 33.Martorell MM, Ruberto LAM, Fernández PM, et al. Bioprospection of cold-adapted yeasts with biotechnological potential from Antarctica. J Basic Microbiol. 2017;57:504–516. doi: 10.1002/jobm.201700021. [DOI] [PubMed] [Google Scholar]
- 34.Brizzio S, Turchetti B, de Garcia V, et al. Extracellular enzymatic activities of basidiomycetous yeasts isolated from glacial and subglacial waters of northwest Patagonia (Argentina) Can J Microbiol. 2007;53:519–525. doi: 10.1139/W07-010. [DOI] [PubMed] [Google Scholar]
- 35.Duarte AWF, Dayo-Owoyemi I, Nobre FS, et al. Taxonomic assessment and enzymes production by yeasts isolated from marine and terrestrial Antarctic samples. Extremophiles. 2013;17:1023–1035. doi: 10.1007/s00792-013-0584-y. [DOI] [PubMed] [Google Scholar]
- 36.Furbino LE, Pellizzari FM, Neto PC, et al. Isolation of fungi associated with macroalgae from maritime Antarctica and their production of agarolytic and carrageenolytic activities. Polar Biol. 2018;41:527–535. doi: 10.1007/s00300-017-2213-1. [DOI] [Google Scholar]
- 37.Carrasco M, Rozas JM, Barahona S, et al. Diversity and extracellular enzymatic activities of yeasts isolated from King George Island, the sub-Antarctic region. BMC Microbiol. 2012;12:1–9. doi: 10.1186/1471-2180-12-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Troncoso E, Barahona S, Carrasco M, et al. Identification and characterization of yeasts isolated from the South Shetland Islands and the Antarctic Peninsula. Polar Biol. 2017;40:649–658. doi: 10.1007/s00300-016-1988-9. [DOI] [Google Scholar]
- 39.Poveda G, Gil-Durán C, Vaca I et al (2018) Cold-active pectinolytic activity produced by filamentous fungi associated with Antarctic marine sponges. Biol Res 51. 10.1186/s40659-018-0177-4 [DOI] [PMC free article] [PubMed]
- 40.Martinez A, Cavello I, Garmendia G, et al. Yeasts from sub-Antarctic region: biodiversity, enzymatic activities and their potential as oleaginous microorganisms. Extremophiles. 2016;20:759–769. doi: 10.1007/s00792-016-0865-3. [DOI] [PubMed] [Google Scholar]
- 41.Priddle J, Heywood RB. Evolution of Antarctic lake ecosystems. Biol J Lin Soc. 1980;14:51–66. doi: 10.1111/j.1095-8312.1980.tb00097.x. [DOI] [Google Scholar]
- 42.Shevnina E, Kourzeneva E. Thermal regime and components of water balance of lakes in Antarctica at the Fildes peninsula and the Larsemann Hills. Tellus A: Dyn Meteorol Oceanogr. 2017;69:1317202. doi: 10.1080/16000870.2017.1317202. [DOI] [Google Scholar]
- 43.Lealem F, Gashe BA. Amylase production by a Gram-positive bacterium isolated from fermenting tef (Eragrostis tef) J Appl Bacteriol. 1994;77:348–352. doi: 10.1111/j.1365-2672.1994.tb03084.x. [DOI] [Google Scholar]
- 44.Soares IA, Flores AC, Zanettin L, et al. Identificação do potencial amilolítico de linhagens mutantes do fungo filamentoso Aspergillus nidulans. Food Sci Technol. 2010;30:700–705. doi: 10.1590/S0101-20612010000300021. [DOI] [Google Scholar]
- 45.Rosa LH, Zani CL, Cantrell CL, et al. Fungi in Antarctica: diversity, ecology, efects of climate change, and bioprospection for bioactive compounds. In: Rosa LH, et al., editors. Fungi of Antarctica: diversity, ecology and biotechnological applications. Switzerland: Springer; 2019. pp. 1–18. [Google Scholar]
- 46.Díaz A, Villanueva P, Oliva V, et al. Genetic transformation of the filamentous fungus Pseudogymnoascus verrucosus of Antarctic origin. Front Microbiol. 2019;10:2675. doi: 10.3389/fmicb.2019.02675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Batista TM, Hilário HO, Moreira RG, et al. Draft genome sequence of Metschnikowia australis strain UFMG-CM-Y6158, an extremophile marine yeast endemic to Antarctica. Genome Announc. 2017;5:e00328–e417. doi: 10.1128/genomeA.00328-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Hoog GS, Göttlich E, Platas G, et al. Evolution, taxonomy and ecology of the genus Thelebolus in Antarctica. Stud Mycol. 2005;51:33–76. [Google Scholar]
- 49.Buzzini P, Turchetti B, Yurkov A. Extremophilic yeasts: the toughest yeasts around? Yeast. 2018;35:487–497. doi: 10.1002/yea.3314. [DOI] [PubMed] [Google Scholar]
- 50.Rojas-Jimenez K, Wurzbacher C, Bourne EC, et al. Early diverging lineages within Cryptomycota and Chytridiomycota dominate the fungal communities in ice-covered lakes of the McMurdo Dry Valleys. Antarct Sci Rep. 2017;7:15348. doi: 10.1038/s41598-017-15598-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruno S, Coppola D, di Prisco G, et al. Enzymes from marine polar regions and their biotechnological applications. Mar Drugs. 2019;17:544. doi: 10.3390/md17100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krishnan A, Alias SA, Wong CMVL, et al. Extracellular hydrolase enzyme production by soil fungi from King George Island, Antarctica. Polar Biol. 2011;34:1535–1542. doi: 10.1007/s00300-011-1012-3. [DOI] [Google Scholar]
- 53.Krishnan A, Convey P, Gonzalez-Rocha G, Alias SA. Production of extracellular hydrolase enzymes by fungi from King George Island. Polar Biol. 2016;39:65–76. doi: 10.1007/s00300-014-1606-7. [DOI] [Google Scholar]
- 54.Carrasco M, Villarreal P, Barahona S, et al. Screening and characterization of amylase and cellulase activities in psychrotolerant yeasts. BMC Microbiol. 2016;16:1–9. doi: 10.1186/s12866-016-0640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martorell MM, Ruberto LAM, Fernandez PM, et al. Biodiversity and enzymes bioprospection of antarctic filamentous fungi. Antarct Sci. 2019;31:3–12. doi: 10.1017/S0954102018000421. [DOI] [Google Scholar]
- 56.Tsuji M. Genetic diversity of yeasts from East Ongul Island, East Antarctica and their extracellular enzymes secretion. Polar Biol. 2018;41:249–258. doi: 10.1007/s00300-017-2185-1. [DOI] [Google Scholar]
- 57.Zakaria NN, Convey P, Gomez-Fuentes C, et al. Oil bioremediation in the marine environment of Antarctica: a review and bibliometric keyword cluster analysis. Microorganisms. 2021;9:419. doi: 10.3390/microorganisms9020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Santiago IF, Soares MA, Rosa CA, Rosa LH. Lichensphere: a protected natural microhabitat of the non-lichenised fungal communities living in extreme environments of Antarctica. Extremophiles. 2015;19(6):1087–1097. doi: 10.1007/s00792-015-0781-y. [DOI] [PubMed] [Google Scholar]
- 59.Singh S, Singh P, Singh S, Sharma P. Pigment, fatty acid and extracellular enzyme analysis of a fungal strain Thelebolus microsporus from Larsemann Hills. Antarctica Polar Record. 2014;50(1):31–36. doi: 10.1017/S0032247412000563. [DOI] [Google Scholar]
- 60.Stchigel AM, Cano J, MacCormack CW. Antarctomyces psychrotrophicus gen. et sp. nov., a new ascomycete from Antarctica. Mycol Res. 2001;105:377–382. doi: 10.1017/S0953756201003379. [DOI] [Google Scholar]
- 61.de Menezes GC, Godinho VM, Porto BA, et al. Antarctomyces pellizariae sp. nov., a new, endemic, blue, snow resident psychrophilic ascomycete fungus from Antarctica. Extremophiles. 2017;21:259–269. doi: 10.1007/s00792-016-0895-x. [DOI] [PubMed] [Google Scholar]
- 62.Loperena L, Soria V, Varela H, et al. Extracellular enzymes produced by microorganisms isolated from maritime Antarctica. World J Microbiol Biotechnol. 2012;28:2249–2256. doi: 10.1007/s11274-012-1032-3. [DOI] [PubMed] [Google Scholar]
- 63.Freire RKB, Mendonça CMN, Ferraro RB, et al. Glutaminase-free L-asparaginase production by Leucosporidium muscorum isolated from Antarctic marine-sediment. Prep Biochem Biotechnol. 2021;51(3):277–288. doi: 10.1080/10826068.2020.1815053. [DOI] [PubMed] [Google Scholar]
- 64.Zhang T, Zhang YQ, Liu HY, et al. Cryptococcus fildesensis sp. nov., a psychrophilic basidiomycetous yeast isolated from Antarctic moss. Int J Syst Evol Microbiol. 2014;64(2):675–679. doi: 10.1099/ijs.0.054981-0. [DOI] [PubMed] [Google Scholar]
- 65.Rovati JI, Pajot HF, Ruberto L, et al. Polyphenolic substrates and dyes degradation by yeasts from 25 de Mayo/King George Island (Antarctica) Yeast. 2013;30:459–470. doi: 10.1002/yea.2982. [DOI] [PubMed] [Google Scholar]
- 66.Pathan AAK, Bhadra B, Begum Z, Shivaji S. Diversity of yeasts from puddles in the vicinity of Midre Lovénbreen glacier, Arctic and bioprospecting for enzymes and fatty acids. Curr Microbiol. 2010;60:307–314. doi: 10.1007/s00284-009-9543-3. [DOI] [PubMed] [Google Scholar]
- 67.Singh P, Singh SM. Characterization of yeast and flamentous fungi isolated from cryoconite holes of Svalbard, Arctic. Polar Biol. 2012;35:575–583. doi: 10.1007/s00300-011-1103-1. [DOI] [Google Scholar]
- 68.Thomas-Hall SR, Turchetti B, Buzzini P, et al. Cold-adapted yeasts from Antarctica and Italian Alps-description of three novel species: Mrakia robertii sp. nov., Mrakia blollopis sp.nov. and Mrakiella niccombsii sp. nov. Extremophiles. 2010;14:47–59. doi: 10.1007/s00792-009-0286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de García V, Brizzio S, Libkind D, et al. Biodiversity of cold-adapted easts from glacial meltwater rivers in Patagonia Argentina. FEMS Microbiol Ecol. 2007;59:331–341. doi: 10.1111/j.1574-6941.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- 70.di Menna ME. Yeasts in Antarctic soils. Antonie Van Leeuwenhoek. 1966;32:29–38. doi: 10.1007/BF02097443. [DOI] [PubMed] [Google Scholar]
- 71.Suji M, Fujiu S, Xiao N, et al. Cold adaptation of fungi obtained from soil and lake sediment in the Skarvsnes ice-free area, Antarctica. FEMS Microbiol Lett. 2013;346:121–130. doi: 10.1111/1574-6968.12217. [DOI] [PubMed] [Google Scholar]
- 72.Gomes ECQ, Godinho VM, Silva DAS, et al. Cultivable fungi present in Antarctic soils: taxonomy, phylogeny, diversity, and bioprospecting of antiparasitic and herbicidal metabolites. Extremophiles. 2018;22:381–393. doi: 10.1007/s00792-018-1003-1. [DOI] [PubMed] [Google Scholar]
- 73.da Silva MK, da Silva AV, Fernandez PM et al (2022) Extracellular hydrolytic enzymes produced by yeasts from Antarctic lichens. Anais da Academia Brasileira de Ciências94. 10.1590/0001-3765202220210540 [DOI] [PubMed]
- 74.Loque CP, Medeiros AO, Pellizzari FM, et al. Fungal community associated with marine macroalgae from Antarctica. Polar Biol. 2010;33(5):641–648. doi: 10.1007/s00300-009-0740-0. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.