Abstract
Secondary fungal infections are frequently observed in COVID-19 patients. However, the occurrence of candiduria in these patients and its risk factors are underexplored. We evaluated the risk factors of candiduria in COVID-19 patients, including inflammatory mediators that could be used as prognostic markers. Clinical information, laboratory test results, and outcomes were collected from severely ill COVID-19 patients with and without candiduria. Candida species identification, antifungal susceptibility, and plasma inflammatory mediators’ measurements were performed. Regression logistic and Cox regression model were used to evaluate the risk factors. A higher risk of longer hospitalization and mortality were observed in patients with candiduria compared to those with COVID-19 only. Candiduria was caused by Candida albicans, C. glabrata, and C. tropicalis. Isolates with intermediate susceptibility to voriconazole and resistant to caspofungin were identified. Classic factors such as the use of corticosteroids and antibacterials, the worsening of renal function, and hematological parameters (hemoglobin and platelets) were found to predispose to candiduria. The mediators IL-1β, IL-1ra, IL-2, CXCL-8, IL-17, IFN-γ, basic FGF, and MIP-1β were significantly increased in patients with COVID-19 and candiduria. Furthermore, IFN-γ, IL-1ra, and CXCL-8 were associated with the occurrence of candiduria in COVID-19 patients, whereas basic FGF, IL-1β, and CXCL-8 were associated with the risk of death in these patients. Classical and immunological factors were associated with worse prognosis among patients with COVID-19 and candiduria. Some mediators, especially CXCL-8, can be a reliable biomarker of fungal coinfection and may guide the diagnostic and the treatment of these patients.
Keywords: COVID-19, Candiduria, Pro-inflammatory cytokines, CXCL-8, Mortality
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infects the lungs and invade epithelial cells and type II pneumocytes. It occurs through the binding of its surface spike protein to the angiotensin-converting enzyme 2 (ACE2) receptor [1]. This interaction, followed by viral replication, damages not only the lungs, but also the heart, kidneys, and intestine. Furthermore, coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 triggers an inflammatory cascade that may be harmful to the host [2].
The severe tissue damage, the immunologic derangement resulting from SARS-CoV-2 infection, and the invasive procedures in the severe cases frequently predispose to secondary fungal infections [3, 4]. Previous studies have demonstrated a higher number of cases of COVID-19-associated candidiasis (CAC), aspergillosis (CAPA), mucormycosis (CAM), and histoplasmosis in the different countries [5].
Candida species are major constituents of the human mycobiome and the leading cause of invasive fungal infections. Studies from Spain [6], India [7], Italy [4], UK [8], and China [9] reported occurrence rates of CAC of 0.7% (7/989), 2.5% (15/ 596), 8% (3/43), 12.6% (17/135), and 23.5% (4/17), respectively. Overall, due to neglected approach towards fungal infections, morbidity and mortality has been showed to worsen during the COVID-19 pandemic. For example, a recent systematic review showed that CAC doubled the mortality of critically ill COVID-19 patients [10]. In addition, a previous study from our group revealed that hospitalized patients with severe COVID-19 and candiduria have a 5-times higher risk of death when compared to patients without the coinfections [11].
Classical clinical factors such as prolonged intensive care unit (ICU) stays, central venous catheters, and broad-spectrum antibiotic use, may be key factors associated with candidiasis in COVID-19 patients. However, some reports suggest that the occurrence of candidiasis and the consequent increased mortality rate in COVID-19 patients can be related to impaired immune responses. It should further increase the awareness of clinicians for more effective diagnosis and treatment [12–14]. In this aspect, a study in Italy reported three cases of candidemia among patients with severe symptoms of COVID-19 and after treatment with tocilizumab, an IL-6 receptor blocker, suggesting that this interleukin may be related to fungal coinfection [15].
Although some studies have addressed the immune response in COVID-19 patients with candidemia, the immunological mechanisms involved in candiduria are poorly understood. Altogether, in this study, we aimed to verify the characteristics and the risk factors of candiduria in COVID-19 patients, including the evaluation of the plasma inflammatory mediators in these patients.
Methods
Patients’ enrollment, clinical evaluation, and ethical aspects
Between May and November 2020, we enrolled patients with COVID-19 and candiduria coinfection (n=18) and patients with COVID-19 alone as a control group (n=31), admitted in the ICU of the Hospital Eduardo de Menezes, Belo Horizonte, Brazil. The control group was determined by randomly selecting patients with a diagnosis of COVID-19, which had no reported candidemia/candiduria during their ICU stay. However, they could have bacterial infections. COVID-19 diagnosis was confirmed by a positive real-time polymerase chain reaction (RT-PCR) for SARS-CoV-2 in the nasopharyngeal swab, associated with suggestive signs, symptoms, and radiological findings. Candiduria was diagnosed by urine culture in CHROMagar® Candida differential medium. Plasma samples for the analysis of hematological, biochemical, and immunological parameters were collected on the same day of candiduria identification. Informed consent was obtained from all participants. This study was approved by the National Ethics Committee (Comissão Nacional de Ética em Pesquisa- CONEP) and the hospital's Ethics Committee (CAAE: 30627320.6.0000.0008).
The following information was obtained from the medical records of patients: (1) patient characteristics: age, gender, use of antibiotics (number), and use of corticosteroids; (2) co-morbidities: diabetes, hypertension; (3) biochemical and hematological parameters: urea, creatinine, hemoglobin, platelet; (4) inflammatory parameters: leukocytes, C-reactive protein, lactate dehydrogenase; (5) outcome: length of hospital stay and mortality.
Fungal identification
The presumptive identification of Candida spp. was performed by culturing the collected urine in CHROMagar® Candida differential medium. For the definitive identification, yeast isolates were identified by polymerase chain reaction (PCR). Species-specific primers were used for Candida albicans (5'-TGTTGCTCTCTCGGGGGCGGCCG-3'), Candida glabrata (5'-TGGGCTTGGGACTCTCGCAGCTC-3'), Candida tropicalis (5'-TGGGCGGTAGGAGAATTGCGTTA-3'), and Candida parapsilosis (5'-GCATCAGTTTGAGCGGTAGGATAAGC -3'). The universal reverse primer used was the NL4 (5'-CGTCCGTGTTTCAAGACGG-3'). Yeast isolates that were not identified by PCR, were then submitted to sequencing reactions using the Big Dye kit version 3.1 (Applied Biosystems, USA) in combination with the ABI 3730 automated system. The amplicons for sequencing reactions were obtained by PCR products using primers ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and NL4 (5'-CGTCCGTGTTTCAAGACGG-3'). The DNA sequences obtained from the yeasts were compared with the sequences of type or reference species deposited in GenBank and belonging to international culture collections, using the BLASTn program (Basic Local Alignment Serch Tool - version 2.215 of BLAST 2.0), available on the NCBI portal (http://www.ncbi.nlm.nih.gov/blast/).
Antifungal susceptibility
The susceptibility of Candida spp. isolates was evaluated according to the yeast broth microdilution method recommended by the Clinical and Laboratory Standards Institute (CLSI) — document M27 A3 [16]. Itraconazole, voriconazole, and amphotericin B were tested at concentrations from 0.03 to 16 mg/L; fluconazole and caspofungin were used at concentrations from 0.125 to 64 and 0.015 to 8.0 mg/L, respectively. Plates were incubated at 37 °C for 24 h, and the reading to determine the minimum inhibitory concentration (MIC) was performed visually by observing the turbidity. Candida albicans ATCC 18804, Candida tropicalis ATCC 750, and Candida glabrata ATCC 2001 were used as quality controls as recommended by the CLSI. From the MIC values, the MIC50 and MIC90 (MIC values that inhibited 50 and 90% of the isolates, respectively) for each Candida spp. were calculated.
Analysis of the patients’ immunological mediators
The quantification of 27 mediators, including cytokines, chemokines, and growth factors, was performed using the screening panel kit Bio-Plex Pro™ Human Cytokine 27-plex Assay (Bio-Rad, Hercules – CA, USA). Samples were read using the Bio-Plex 200 equipment, and the analyses were performed using the Manager software (Instituto René Rachou - Fiocruz Minas). The results were analyzed as the average of the fluorescence intensities and expressed in pg/dl.
Statistical analysis
To compare the patients’ characteristics and outcomes between groups, categorical variables were summarized as numbers (percentages) and analyzed using the Fisher’s exact test. Continuous variables were presented as mean ± SD and analyzed using Mann–Whitney U or Student’s t test; p < 0.05 was considered statistically significant. Mean levels of cytokines, chemokines, and growth factors were compared using Student’s t test.
Conditional logistic regression was used to investigate associations of factors with candiduria in COVID-19 patients. Variables with a P value of < 0.1 in the univariate analysis were included in the multivariate model. The odds ratio (OR) was calculated to determine the strength of association. Correlations between the concentration of the immunological, hematological, and biochemical parameters were assessed by Spearman tests; and the heatmap was created using the GraphPad Prism Program, version 9.00 (GraphPad Software, San Diego, CA, USA).
Kaplan–Meier curves were built relatively to group status (death or discharged) in patients with COVID-19 and candiduria. Patients were categorized according to the median value, considering the (i) high levels of cytokines, chemokines, and growth factor (> median) and (ii) low levels of biomarkers (≤ median). Cox model was first applied for comparative analysis of Kaplan–Meier curves for each cytokine, chemokine, and growth factor; and second with all biomarkers together and adjusted for age and sex as covariates. Hazard ratios (HR) were estimated via the Cox regression model.
Statistical analyzes were performed using the GraphPad Prism Program, version 9.00 (GraphPad Software, San Diego, CA, USA), and the Epi Info 3.5.3 program (Centers for Disease Control and Prevention, Atlanta, USA, available at http://www.openepi.com). For all tests, a p value < 0.05 was considered statistically significant.
Results
Clinical characteristics of patients with COVID-19 and candiduria
Mean age and gender distribution were similar between patients with COVID-19 alone and those with COVID-19 with candiduria (Table 1). There was no difference regarding the use of corticosteroids and antibacterials between the groups. However, the number of prescribed antibacterial agents was statistically higher in patients with COVID-19 and candiduria compared to those with COVID-19 only (5.28 versus 2.24; p < 0.0001). The percentage of patients with diabetes did not differ between the groups. On the other hand, the percentage of patients with hypertension was higher in COVID-19 compared to COVID-19 and candiduria group (64.51 versus 22.22%, p = 0.007). Among the hematological, biochemical, and inflammatory parameters, the mean values of serum creatinine and urea, and C-reactive protein, were significantly higher in patients with candiduria compared to the group with COVID-19 alone (2.37 versus 1.27 mg/dL, p = 0.002; 125.00 versus 75.77 mg/dL, p = 0.006; 162.90 versus 94.48 mg/L, p = 0.023, respectively). Mean hemoglobin and platelet levels were significantly lower in patients with candiduria (10.02 versus 12.39 g/dL, p = 0.001; 215333 versus 273533/mm3, p = 0.041, respectively). Furthermore, there was no statistical difference of leukocytes counting between the analyzed groups. Interestingly, patients with COVID-19 and candiduria had a significantly longer length of hospital stay and mortality compared to those with COVID-19 only (34.44 versus 12.48 days, p < 0.001; 77.78 versus 29.03% of patients, p = 0.001, respectively).
Table 1.
Clinical characteristics, hematological and biochemical parameters of COVID-19 patients with or without candiduria
| COVID-19 (n=31) | COVID-19 and candiduria (n=18) | P value | |
|---|---|---|---|
| Patient characteristics | |||
| Age (years) | 62.87 ± 14.23 | 64.83 ± 13.03 | 0.634 |
| Female gender | 15 (48.38%) | 10 (55.56%) | 0.769 |
| Use of corticosteroids | 14 (45.16%) | 13 (72.22%) | 0.082 |
| Use of antibacterials | 27 (87.10%) | 17 (94.44%) | 0.639 |
| Number of antibacterials | 2.24 ± 1.28 | 5.28 ± 2.02 | <0.0001 |
| Co-morbidities | |||
| Diabetes | 13 (41.94%) | 10 (55.56%) | 0.390 |
| Hypertension | 20 (64.51%) | 4 (22.22%) | 0.007 |
| Biochemical/hematological data | |||
| Serum creatinine (mg/dL) | 1.27 ± 0.99 | 2.37 ± 1.46 | 0.002 |
| Urea (mg/dL) | 75.77 ± 49.88 | 125.00 ± 51.34 | 0.006 |
| Hemoglobin (g/dL) | 12.39 ± 2.08 | 10.02 ± 2.73 | 0.001 |
| Platelet count (/mm3) | 273533 ± 98859 | 215333 ± 132144 | 0.041 |
| Inflammatory parameters | |||
| Leukocytes count (/mm3) | 12803 ± 12419 | 13889 ± 9838 | 0.406 |
| C-reactive protein (mg/L) | 94.48 ± 90.19 | 162.90 ± 111.10 | 0.023 |
| Lactate dehydrogenase (mM/L) | 1.46 ± 0.45 | 1.55 ± 0.85 | 0.821 |
| Outcome | |||
| Length of hospital stay (days) | 12.48 ± 5.82 | 34.44 ± 22.99 | <0.001 |
| In-hospital mortality | 9 (29.03%) | 14 (77.78%) | 0.001 |
In bold, significantly different parameters between the two groups of patients
Candida albicans and non-albicans presenting different antifungal susceptibility profiles caused candiduria in COVID-19 patients
Among the 18 patients with candiduria, eight was caused by C. albicans, five by C. tropicalis and five by C. glabrata. Regarding drug susceptibility, 50% and 80% of C. albicans and C. tropicalis isolates, respectively, presented intermediate susceptibility to voriconazole and 40% of C. glabrata isolates were resistant to caspofungin (Table 2).
Table 2.
Number of isolates and minimal inhibitory concentration (MIC) of antifungal agents for Candida spp. from patients with COVID-19 and candiduria
| Species (number of isolates) | Antifungal | Range MIC | MIC50 | MIC90 | S (%) | I (%) | R (%) |
|---|---|---|---|---|---|---|---|
| C. albicans (n = 8) | Fluconazole | 0.06–0.25 | 0.125 | 0.25 | 100 | NA | 0 |
| Itraconazole | <0.03–0.25 | 0.125 | 0.20 | 100* | NA | 0 | |
| Voriconazole | 0.06–0.5 | 0.25 | 0.50 | 50 | 50 | 0 | |
| Caspofungin | <0.015–0.125 | 0.06 | 0.10 | 100 | 0 | 0 | |
| Amphotericin B | 0.5–1.0 | 1.0 | 1.0 | 100* | NA | 0 | |
| C. tropicalis (n = 5) | Fluconazole | 0.25–0.50 | 0.5 | 0.5 | 100 | 0 | 0 |
| Itraconazole | <0.03–0.125 | 0.093 | 0.125 | 100* | NA | 0 | |
| Voriconazole | 0.125–0.25 | 0.25 | 0.25 | 20 | 80 | 0 | |
| Caspofungin | 0.06–0.125 | 0.093 | 0.125 | 100 | 0 | 0 | |
| Amphotericin B | 0.25–2.0 | 1.0 | 2.0 | 100* | NA | 0 | |
| C. glabrata (n = 5) | Fluconazole | 0.50–8.0 | 2.0 | 5.6 | 100 | NA | 0 |
| Itraconazole | 0.06–1.0 | 0.25 | 0.70 | 100* | NA | 0 | |
| Voriconazole | 0.125–4.0 | 0.5 | 2.8 | NA | NA | NA | |
| Caspofungin | 0.03–0.5 | 0.5 | 0.5 | 60 | 0 | 40 | |
| Amphotericin B | 0.5–2.0 | 0.5 | 1.60 | 100* | NA | 0 |
*Epidemiological cutoff (Pfaller et al., 2012). MIC Minimal inhibitory concentration; S susceptible; I intermediate; R resistant; NA not available. MIC is represented by μg/mL. Antifungal susceptibility (S, I, or R) is represented by the percentage of isolates for each agent
Level of immunological meditators in patients with COVID-19 and candiduria
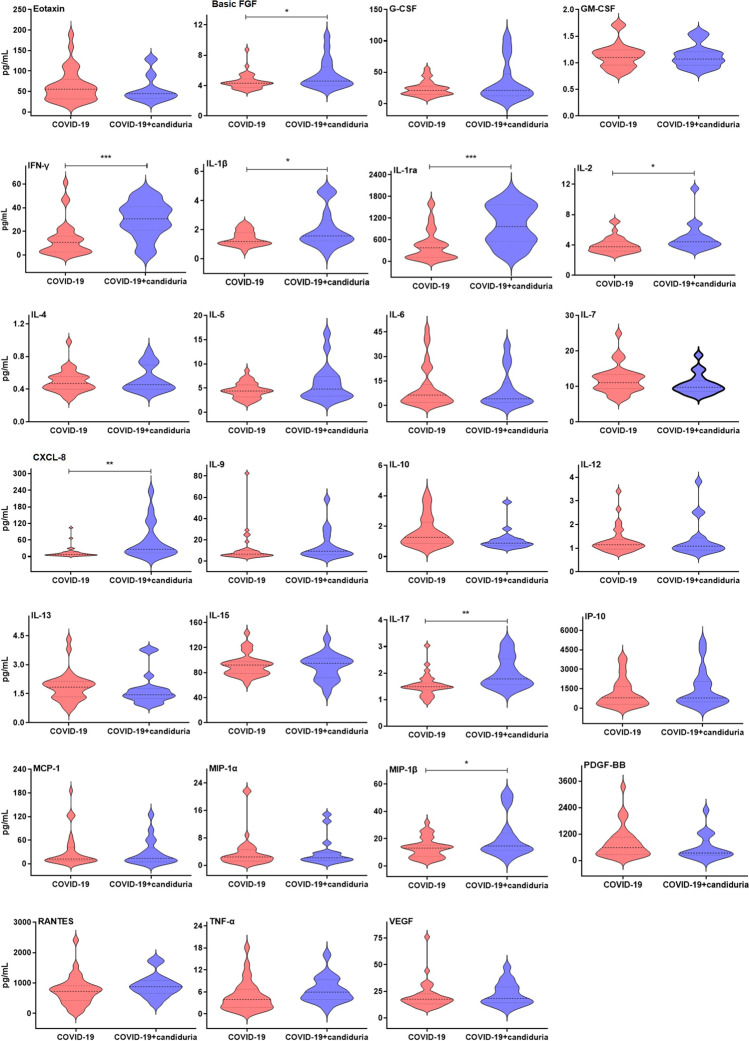
Subsequently, we analyzed and compared the serological immune response of COVID-19 patients with and without candiduria. The levels of IL-1β (p=0.0176), IL-1ra (p=0.0002), IL-2 (p=0.0241), CXCL-8 (p=0.0014), IL-17 (p=0.0023), interferon γ (IFN-γ, p= 0.0005), basic fibroblast growth factor (basic FGF, p=0.0277), and macrophage inflammatory protein 1β (MIP-1β; p=0.0251) were significantly increased in plasma from COVID-19 and candiduria patients compared to those with COVID-19 alone (Fig. 1).
Fig. 1.
Cytokines, chemokines and growth factors in plasma samples from patients with COVID-19 and candiduria (blue, n = 18), and COVID-19 only (red, n = 31). The horizontal lines in the inner box plot represent the median and interquartile range. Pairwise comparisons T-test show significantly higher levels of basic FGF, IL-1β, IL-1ra, IL-2, CXCL-8, IL-17, IFN-γ, and MIP-1β in patients with COVID-19 and candiduria compared to COVID-19 group (***: p<0.001, **: p<0.01, *: p<0.05)
Risk factors for candiduria in COVID-19 patients
Then, we investigated which patient’s characteristics would represent risk factors for candiduria in patients with COVID-19. Furthermore, we evaluated whether the increased levels of immunological mediators in the plasma of patients with COVID-19 and candiduria could be correlated with the coinfection and used as predictive biomarkers.
Several variables such as hypertension, number of antibacterial agents, serum creatinine, urea, hemoglobin level, cytokines IFN-γ, IL-1ra, and CXCL-8 were significantly associated with candiduria in COVID-19 patients in the univariate regression analysis (Table 3). However, in the multivariate conditional regression model, only the use of corticosteroids and number of administered antibacterials were risk factors independently associated with candiduria in COVID-19 patients.
Table 3.
Conditional logistic regression for candiduria in COVID-19 patients
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P value | OR | 95% CI | P value |
| Age (< 65; ≥ 65 years) | 0.933 | 0.288–3.027 | 0.909 | |||
| Gender | 1.328 | 0.419–4.209 | 0.630 | |||
| Diabetes | 1.731 | 0.536–5.587 | 0.359 | |||
| Hypertension | 0.157 | 0.042–0.596 | 0.007 | 0.026 | 0.000–1.624 | 0.084 |
| Use of corticosteroids | 3.152 | 0.903–11.006 | 0.072 | 71.300 | 1.011–5028.037 | 0.049 |
| Use of antibacterials | 2.508 | 0.259–24.303 | 0.428 | |||
| Number of antibacterials (< 3; ≥ 3) | 39.020 | 4.557–334.084 | 0.001 | 149.768 | 1.482–15136.982 | 0.033 |
| Serum creatinine (mg/dL) (low/high) | 0.082 | 0.018–0.362 | 0.001 | 0.011 | 0.000–5.917 | 0.160 |
| Urea (mg/dL) (low/high) | 0.104 | 0.024–0.457 | 0.003 | 66.978 | 0.056–80272.155 | 0.245 |
| Hemoglobin (g/dL) (low/high) | 4.491 | 1.260–16.006 | 0.021 | |||
| Platelet count (/mm3) (low/high) | 2.998 | 0.883–10.176 | 0.708 | 0.097 | 0.001–8.613 | 0.308 |
| Leukocytes count (/mm3) (low/high) | 0.700 | 0.216–2.265 | 0.552 | |||
| C-reactive protein (mg/L) (low/high) | 0.353 | 0.101–1.233 | 0.103 | 0.108 | 0.002–4.917 | 0.253 |
| Lactate dehydrogenase (mM/L) (low/high) | 0.700 | 0.177–2.771 | 0.611 | |||
| Basic FGF (low/high) | 0.632 | 0.200–1.995 | 0.434 | 0.417 | 0.011–16.516 | 0.641 |
| IFN-γ (low/high) | 0.091 | 0.021–0.387 | 0.001 | |||
| IL-1β (low/high) | 0.502 | 0.157–1.610 | 0.247 | |||
| IL-1ra (low/high) | 0.156 | 0.042–0.581 | 0.006 | 0.028 | 0.001–1.690 | 0.743 |
| IL-2 (low/high) | 0.347 | 0.107–1.130 | 0.079 | 0.087 | 0.001–10.849 | 0.087 |
| CXCL-8 (low/high) | 0.096 | 0.023–0.401 | 0.001 | 1.089 | 0.035–34.301 | 0.322 |
| IL-17 (low/high) | 0.310 | 0.093–1.028 | 0.056 | 1.858 | 0.046–75.843 | 0.962 |
| MIP-1β (low/high) | 1.000 | 0.319–3.135 | 1.000 | |||
| Length of hospital stay (< 20; ≥ 20 days) | 8.423 | 2.072–54.234 | 0.003 | 10.887 | 0.357–332.411 | 0.171 |
| In hospital mortality | 8.556 | 2.207–33.170 | 0.002 | |||
OR Odds ratio; CI confidence interval. In bold, significantly different parameters between the two groups of patients
We also evaluated the relationship between the occurrence of candiduria and the prognosis of patients with COVID-19. The analysis revealed an association between candiduria in COVID-19 patients with longer length of hospitalization and higher mortality rate (Table 3).
Searching for immune, hematological, and biochemical biomarkers for COVID-19 and candiduria
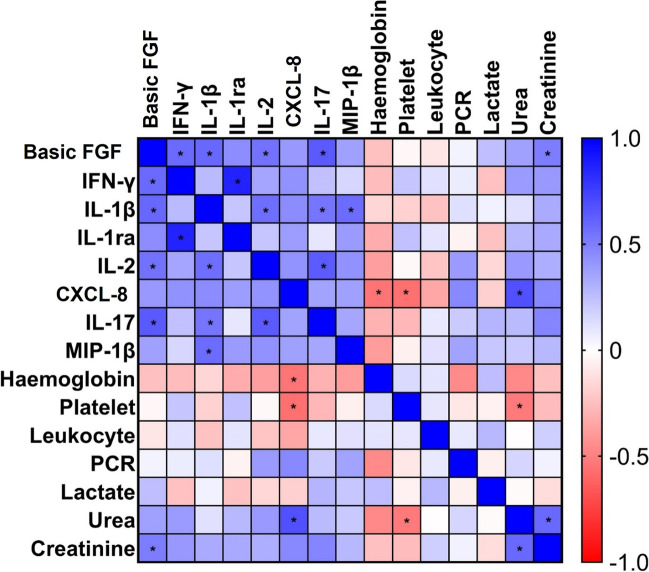
Next, we asked whether the levels of immunological mediators correlate with each other and with hematological and biochemical parameters. The heatmap presented in Fig. 2 illustrates the significantly positive correlations among the pro-inflammatory cytokines, chemokine, and growth factor, except for CXLC-8, in patients with COVID-19 and candiduria. Basic FGF and CXLC-8 also demonstrated positive correlation with creatinine and urea, respectively. Negative correlations were observed for CXLC-8 with hemoglobin and platelet. The leukocyte, PCR, and lactate dehydrogense did not show any significant correlations with the other biomarkers.
Fig. 2.
Spearman correlation matrix among immunological, hematological, and biochemical biomarkers in patients with COVID-19 and candiduria. Positive correlation coefficients are shown in shadows of blue, while negative correlations in shadows of red. The * represents significant correlations (p < 0.05)
Immune mediators as biomarkers of the risk of death in COVID-19 and candiduria group
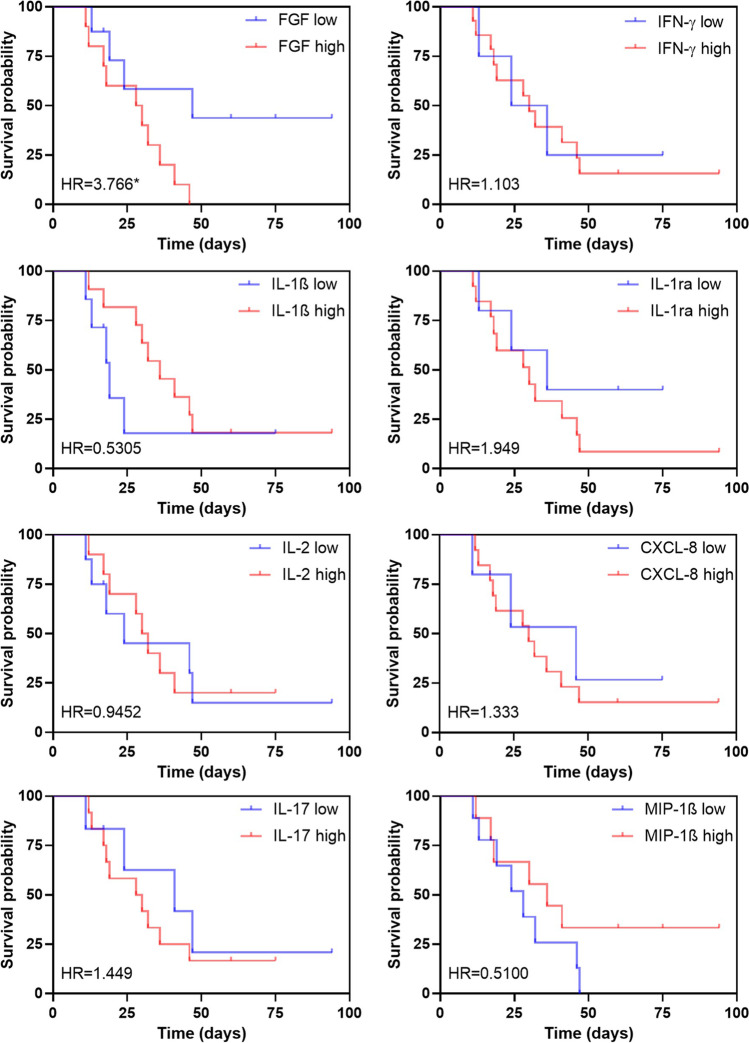
We also analyzed if the increased production of cytokines, chemokine, and growth factor influenced the patient’s survival. We then stratified the patients according to the levels of immunological mediators, considering high versus low levels by using the median values of all analyzed patients in this study as cutoffs. We first evaluated how each mediator could predict the survival of the patients. The results from Cox regression showed that basic FGF was significantly associated with increased risk of death from patients with COVID-19 and candiduria (Fig. 3). Subsequently, we considered all mediators together in the model (data not shown), for their relative independence, and adjusted for sex and age. Interestingly, the high levels of basic FGF (HR=37.150) and CXLC-8 (HR=16.020), and the low levels of IL-1β (HR=0.021) were significantly associated with reduced survival (p=0.008, p=0.003, and p=0.013, respectively).
Fig. 3.
Survival curves based on the increased levels of cytokines, chemokine, and growth factor in patients with COVID-19 and candiduria. Cox regression model showing overall survival with hazard ratio (HR) for each mediator if high (red) or low (blue). The * represents the significant result (p < 0.05)
Discussion
In this study, we evaluated the causes and consequences of candiduria in severely ill COVID-19 patients. Candida spp. urinary tract infections can be caused either by the ascending (via the urethra) or the hematogenous routes. In this aspect, candiduria can lead to candidemia, which is associated with high morbidity and mortality [17]. However, since most urinary tract infections are caused by bacteria, the diagnostic of yeast is often ignored and definition of candiduria is not standardized.
The occurrence of candiduria, as well as the causative species, is underexplored in COVID-19 patients. In our study, C. albicans was the most isolated species from candiduria in COVID-19 patients, followed by C. glabrata and C. tropicalis. Similarly, previous study demonstrated that of the Candida species in patients with COVID-19 and candiduria, the most prevalent species were C. albicans, C. parapsilosis, C. tropicalis, and C. glabrata [18]. Furthermore, most of C. albicans and C. tropicalis from our study presented intermediate susceptibility to voriconazole and 40% of C. glabrata isolates were resistant to caspofungin. The first study [11] carried out in this regard was performed by our group with patients with COVID-19 and revealed that Candida spp. and Aspergillus spp. represented 98.2% (54) and 5.5% (3) of the fungal isolates, respectively. These isolates were obtained from different sites such as tracheal aspirate (n = 33), urine (n = 25), blood (n = 4), catheter tip (n = 4), sputum (n = 3), and mini-BAL (n = 1). Subsequently [19], we identified the following prevalence of fungal species in these patients: C. albicans (54%), C. tropicalis (23%), C. glabrata (14%), C. parapsilosis (3%), C. kefyr (3%), C. lusitaniae (1%), A. nomius (1%), and A. flavus (1%). Regarding susceptibility profile, two isolates (5%) of C. albicans and two isolates (20%) of C. glabrata were resistant to caspofungin. In addition, one isolate (5.88%) of C. tropicalis and seven isolates (70%) of C. glabrata were resistant to voriconazole, and two isolates (20%) of C. glabrata resistant to fluconazole. These observations reinforce the importance of identification at the species level to provide an adequate selection of the antifungal therapy. It is of great significance, especially when considering that candiduria may be a sentinel of a disseminated infection, and that echinocandins are a first-line antifungal for the treatment of invasive candidiasis [20, 21].
The number of antibacterial agents administered was statistically higher in patients with COVID-19 and candiduria compared to those with COVID-19 alone. Furthermore, the multivariate conditional regression model showed the use of corticosteroids and the number of administered antibacterial agents as the only risk factors independently associated with candiduria in COVID-19 patients. The use of corticosteroids is recommended by the World Health Organization (WHO) for the treatment of patients with severe symptoms of COVID-19, and although they attenuate the inflammatory response generated by the virus, they cause immunosuppression and might therefore increase the risk for candidiasis [22]. COVID-19 patients in the ICU usually receive broad-spectrum antibiotics as empirical therapy, particularly during the first pandemic wave [19]. These drugs suppress the bacterial microbiota of the gastrointestinal and genital system, which facilitate colonization by Candida, predisposing to candiduria [23]. In this aspect, a previous study demonstrated that ICU patients, who receive four different antibiotics and have colonization of clinical specimens such as urine, presented 80% increased risk of developing candidemia [24].
Patients with COVID-19 and candiduria had a significantly longer mean hospital length of stay and mortality compared to those with COVID-19 only. The univariate regression analysis also revealed an association between candiduria in COVID-19 patients and longer hospitalization period and higher mortality rate. In a study from Spain, 22% of the patients who stayed more than 7 days in the ICU developed candiduria and approximately one-third of ICU patients with a positive Candida culture had a urinary catheter, which are common characteristics in severely ill COVID-19 patients in the first pandemic wave [24].
The immunological response in candidemia has been previously studied. In CAC, immunological factors have been recently added to all well-known risk factors such as mechanical ventilation, parenteral nutrition, broad spectrum anti-bacterial treatment, indwelling central venous or bladder catheters, older age, comorbidities, lymphopenia, and corticosteroids [15]. However, the mechanisms involved in the immunological defense related to candiduria are not completely understood [25]. More specifically, there is a knowledge gap about how an immune response contributes to the pathologies in candiduria in COVID-19 patients. A previous study performed an ex vivo experiment, in which blood samples from COVID-19 patients were exposed to the C. albicans lysate, resulting in a lower production of IL-6, TNF, IL-1α, IL-1β, IL-10, and IFN-γ compared to control — blood from a healthy donor exposed to the fungus [26]. However, we pioneered in describing the cytokine profile in blood samples from patients with COVID-19 and candiduria. Our results demonstrated that COVID-19 and candiduria induced an increase in the level of cytokines IL-1β, IL-1ra, IL-2, CXLC-8, IL-17, basic FGF, IFN-γ; and of chemokine CCL4/MIP-1β in the plasma of patients. We also observed significantly positive correlations among most of the eight cytokines, chemokine, and growth factor analyzed in patients with COVID-19 and candiduria. These cytokines (except IL-1ra) primarily play a pro-inflammatory role and are part of the cytokines release syndrome (CRS) or macrophage activation syndrome (MAS) in severely ill COVID-19 patients [2, 27].
Next, we evaluated whether these elevated cytokines were related to the occurrence of candiduria and increased risk of death in patients with COVID-19. IFN-γ and IL-1ra were significantly associated with the occurrence of candiduria in COVID-19 patients, whereas basic FGF and IL-1β were significantly associated with the risk of death in the SARS-CoV-2 and candiduria cases. The role of the cytokines evaluated in our study has been described for both SARS-CoV-2 and Candida infections. For example, the IL-1 family members, the pro-inflammatory IL-1β and the IL-1 receptor antagonist (IL-1ra, anti-inflammatory), are associated with the “cytokine storm” seen in patients with severe COVID-19 [28, 29]. In this context, the use of IL-1β signaling antagonists, such as the anti-IL-1b monoclonal antibody canakinumab or the IL-1 receptor antagonist anakinra were able to reduce the need for supplemental oxygen therapy, fever, time in the ICU, and mortality in COVID-19 patients [30, 31]. Likewise, IL-1β is activated by neutrophils during the early stages and of Candida spp. infection and by the inflammasome in the later stages. In addition, fungal cell wall β-glucan induces the IL-1ra expression [32]. Furthermore, pro-inflammatory cytokines and chemokines induce several pathological factors including hypercoagulation and pathological angiogenesis. Endotheliopathy could give rise to hypercoagulation by altering the level of different factors such as the von Willebrand factor. Regarding the pathogenesis of angiogenesis, proangiogenic factors such as the vascular endothelial growth factor (VEGF), basic FGF or placental growth factors (PlGF) were upregulated in the plasma or lung biopsies of COVID-19 patients [33, 34].
Importantly, we found that CXLC-8 was one of the most robust biomarkers of candiduria in COVID-19 patients, since it was associated with the diagnosis of fungal infection or colonization, and with the risk of death of the patients. CXLC-8 is a potent pro-inflammatory cytokine that plays a key role in the recruitment and activation of neutrophils during inflammation and is commonly increased in patients with COVID-19. Given the frequent neutrophilia seen in patients infected with SARS-CoV-2, it is possible that CXLC-8 contributes to the pathophysiology of COVID-19 [35, 36]. CXLC-8 is also the main mediator released at sites of Candida spp. infection, where it exhibits potent anti-Candida activity by recruiting and activating neutrophils and monocytes that recognize and eliminate fungal elements [32].
In conclusion, our results suggest that immunomodulation induced or increased by SARS-COV-2 and Candida spp. is not trivial. Thus, the early measurements of inflammatory mediators can be reliable predictors of fungal coinfection and may help in the diagnostic and in the treatment of COVID-19-associated candidiasis.
The relatively small sample size and the conduction of a single-center study could lead a limitation of our analyses and further investigations on SARS-CoV-2-Candida co-infected patients are needed. However, this study also has the strength to provide insights into the immune pathogenesis in patients with COVID-19 and candiduria and could be of value for the rational development of therapeutic strategies for the effective treatment of these coinfected patients.
Author contributions
All authors contributed to the study conception and design. Vanessa C.R. Magalhães, Rachel B. Caligiorne, Alexandre S. Moura, Ana Raquel O. Santos, Tatiani Fereguetti, Juliana C. Martins, Lívia F. Rabelo, and Ana C. Lyon contributed to the patient enrollment, sample and data collection. Junya L. Singulani, Danielle L. Silva, Caroline M. Lima, and Vanessa C.R. Magalhães were responsible for the microbiological identification and antimicrobial susceptibility tests. Junya L. Singulani, Olindo A. Martins-Filho, and Jordana G. A. Coelho dos Reis were responsible for the analysis of inflammatory mediators. Daniel A. Santos performed conceptualization, funding acquisition, project administration, resources and supervision. The first draft of the manuscript was written by Junya L. Singulani, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Fundação de Amparo à Pesquisa do Estado de Minas Gerais — FAPEMIG (Grant number APQ-00267–20); and Brazilian Ministry of Health and Conselho Nacional de Desenvolvimento Científico e Tecnológico — CNPq (440010/2018–7; 402200/2021–7). D. A. S. (303762/2020–9) and J. L. S. (163516/2020–0) are research fellows of the CNPq.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available to protect our participants’ sensitive data but are available from the corresponding author on reasonable request.
Declarations
Ethics approval
This study was approved by the National Ethics Committee (Comissão Nacional de Ética em Pesquisa- CONEP) and the hospital’s Ethics Committee (CAAE: 30627320.6.0000.0008).
Consent to participate
Each participant signed written informed participatory consent.
Competing interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrati C, Carsetti R, Bordoni V, Sacchi A, Quintarelli C, Locatelli F, Ippolito G, Capobianchi MR. The immune response as a double-edged sword: the lesson learnt during the COVID-19 pandemic. Immunology. 2022;167(3):287–302. doi: 10.1111/imm.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26(10):1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antinori S, Galimberti L, Milazzo L, Ridolfo AL. Bacterial and fungal infections among patients with SARS-CoV-2 pneumonia. Infez Med. 2020;28(suppl 1):29–36. [PubMed] [Google Scholar]
- 5.Roudbary M, Kumar S, Kumar A, Černáková L, Nikoomanesh F, Rodrigues CF. Overview on the prevalence of fungal infections, immune response, and microbiome role in COVID-19 patients. J Fungi (Basel) 2021;7(9):720. doi: 10.3390/jof7090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Vidal C, Sanjuan G, Moreno-Garcia E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, Fernandez-Pittol M, Pitart C, Inciarte A, Bodro M, Morata L, Ambrosioni J, Grafia I, Meira F, Macaya I, Cardozo C, Casals C, Tellez A, Castro P, Marco F, Garcia F, Mensa J, Martinez JA, Soriano A. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2020;27(1):83. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhary A, Tarai B, Singh A, Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April-July 2020. Emerg Infect Dis. 2020;26(11):2694–2696. doi: 10.3201/eid2611.203504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, Pandey M, Whitaker H, May A, Morgan M, Wise MP, Healy B, Blyth I, Price JS, Vale L, Posso R, Kronda J, Blackwood A, Rafferty H, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2021;73(7):e1634–e1644. doi: 10.1093/cid/ciaa1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krzych Ł, Putowski Z, Gruca K, Pluta MP. Mortality in critically ill COVID-19 patients with fungal infections: a comprehensive systematic review and meta-analysis. Pol Arch Intern Med. 2022;132(5):16221. doi: 10.20452/pamw.16221. [DOI] [PubMed] [Google Scholar]
- 11.Silva DL, Lima CM, Magalhães VCR, Baltazar LM, Peres NTA, Caligiorne RB, Moura AS, Fereguetti T, Martins JC, Rabelo LF, Abrahão JS, Lyon AC, Johann S, Santos DA. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. J Hosp Infect. 2021;113:145–154. doi: 10.1016/j.jhin.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seagle EE, Jackson BR, Lockhart SR, Georgacopoulos O, Nunnally NS, Roland J, Barter DM, Johnston HL, Czaja CA, Kayalioglu H, Clogher P, Revis A, Farley MM, Harrison LH, Davis SS, Phipps EC, Tesini BL, Schaffner W, Markus TM, Lyman MM. The landscape of Candidemia during the coronavirus disease 2019 (COVID-19) pandemic. Clin Infect Dis. 2022;74(5):802–811. doi: 10.1093/cid/ciab562. [DOI] [PubMed] [Google Scholar]
- 13.Kayaaslan B, Eser F, Kaya Kalem A, Bilgic Z, Asilturk D, Hasanoglu I, Ayhan M, Tezer Tekce Y, Erdem D, Turan S, Mumcuoglu I, Guner R. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses. 2021;64(9):1083–1091. doi: 10.1111/myc.13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arastehfar A, Carvalho A, Nguyen MH, Hedayati MT, Netea MG, Perlin DS, Hoenigl M. COVID-19-associated candidiasis (CAC): an underestimated complication in the absence of immunological predispositions? J Fungi (Basel) 2020;6(4):211. doi: 10.3390/jof6040211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antinori S, Bonazzetti C, Gubertini G, Capetti A, Pagani C, Morena V, Rimoldi S, Galimberti L, Sarzi-Puttini P, Ridolfo AL. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun Rev. 2020;19(7):102564. doi: 10.1016/j.autrev.2020.102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CLSI . CLSI document M27-A3 (ISBN-1-56238-666-2) 3. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. [Google Scholar]
- 17.Odabasi Z, Mert A. Candida urinary tract infections in adults. World J Urol. 2020;38(11):2699–2707. doi: 10.1007/s00345-019-02991-5. [DOI] [PubMed] [Google Scholar]
- 18.Altinkaya Çavuş M, Sav H. Opportunistic. Pol. J Microbiol. 2022;71(3):411–419. doi: 10.33073/pjm-2022-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singulani JL, Silva DL, Lima CM, Magalhães VCR, Baltazar LM, Peres NTA, Caligiorne RB, Moura AS, Santos ARO, Fereguetti T, Martins JC, Rabelo LF, Lyon AC, Johann S, Falcão JP, Santos DA. The impact of COVID-19 on antimicrobial prescription and drug resistance in fungi and bacteria. Braz J Microbiol. 2022;53(4):1925–1935. doi: 10.1007/s42770-022-00818-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- 21.Dias V. species in the urinary tract: is it a fungal infection or not? Future Microbiol. 2020;15:81–83. doi: 10.2217/fmb-2019-0262. [DOI] [PubMed] [Google Scholar]
- 22.Kosaka M, Yamazaki Y, Maruno T, Sakaguchi K, Sawaki S. Corticosteroids as adjunctive therapy in the treatment of coronavirus disease 2019: a report of two cases and literature review. J Infect Chemother. 2021;27(1):94–98. doi: 10.1016/j.jiac.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher JF, Kavanagh K, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infection: pathogenesis. Clin Infect Dis. 2011;52(Suppl 6):S437–S451. doi: 10.1093/cid/cir110. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller MA, Castanheira M. Nosocomial candidiasis: antifungal stewardship and the importance of rapid diagnosis. Med Mycol. 2016;54(1):1–22. doi: 10.1093/mmy/myv076. [DOI] [PubMed] [Google Scholar]
- 25.Poloni JAT, Rotta LN. Urine sediment findings and the immune response to pathologies in fungal urinary tract infections caused by. J Fungi (Basel) 2020;6(4):245. doi: 10.3390/jof6040245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moser D, Biere K, Han B, Hoerl M, Schelling G, Choukér A, Woehrle T. COVID-19 impairs immune response to. Front Immunol. 2021;12:640644. doi: 10.3389/fimmu.2021.640644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasrija R, Naime M. The deregulated immune reaction and cytokines release storm (CRS) in COVID-19 disease. Int Immunopharmacol. 2021;90:107225. doi: 10.1016/j.intimp.2020.107225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson JG, Simpson LJ, Ferreira AM, Rustagi A, Roque J, Asuni A, Ranganath T, Grant PM, Subramanian A, Rosenberg-Hasson Y, Maecker HT, Holmes SP, Levitt JE, Blish CA, Rogers AJ (2020) Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight 5(17):e140289 [DOI] [PMC free article] [PubMed]
- 29.Lucas C, Wong P, Klein J, TBR C, Silva J, Sundaram M, Ellingson MK, Mao T, Oh JE, Israelow B, Takahashi T, Tokuyama M, Lu P, Venkataraman A, Park A, Mohanty S, Wang H, Wyllie AL, CBF V, Earnest R, Lapidus S, Ott IM, Moore AJ, Muenker MC, Fournier JB, Campbell M, Odio CD, Casanovas-Massana A, Herbst R, Shaw AC, Medzhitov R, Schulz WL, Grubaugh ND, Dela Cruz C, Farhadian S, Ko AI, Omer SB, Iwasaki A, Team YI Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavalli G, Larcher A, Tomelleri A, Campochiaro C, Della-Torre E, De Luca G, Farina N, Boffini N, Ruggeri A, Poli A, Scarpellini P, Rovere-Querini P, Tresoldi M, Salonia A, Montorsi F, Landoni G, Castagna A, Ciceri F, Zangrillo A, Dagna L. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study. Lancet Rheumatol. 2021;3(4):e253–e261. doi: 10.1016/S2665-9913(21)00012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Della-Torre E, Della-Torre F, Kusanovic M, Scotti R, Ramirez GA, Dagna L, Tresoldi M. Treating COVID-19 with colchicine in community healthcare setting. Clin Immunol. 2020;217:108490. doi: 10.1016/j.clim.2020.108490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Netea MG, Joosten LA, van der Meer JW, Kullberg BJ, van de Veerdonk FL. Immune defence against Candida fungal infections. Nat Rev Immunol. 2015;15(10):630–642. doi: 10.1038/nri3897. [DOI] [PubMed] [Google Scholar]
- 33.Smadja DM, Mentzer SJ, Fontenay M, Laffan MA, Ackermann M, Helms J, Jonigk D, Chocron R, Pier GB, Gendron N, Pons S, Diehl JL, Margadant C, Guerin C, Huijbers EJM, Philippe A, Chapuis N, Nowak-Sliwinska P, Karagiannidis C, Sanchez O, Kümpers P, Skurnik D, Randi AM, Griffioen AW. COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis. 2021;24(4):755–788. doi: 10.1007/s10456-021-09805-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu ZS, Shu T, Kang L, Wu D, Zhou X, Liao BW, Sun XL, Wang YY. Temporal profiling of plasma cytokines, chemokines and growth factors from mild, severe and fatal COVID-19 patients. Signal Transduct Target Ther. 2020;5(1):100. doi: 10.1038/s41392-020-0211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Li S, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available to protect our participants’ sensitive data but are available from the corresponding author on reasonable request.