Abstract
Pediococcus pentosaceus is a lactic acid bacterium that has probiotic potential proven by studies. However, its viability can be affected by adverse conditions such as storage, heat stress, and even gastrointestinal passage. Thus, the aim of the present study was to microencapsulate and characterize microcapsules obtained by spray drying and produced only with whey powder (W) or whey powder combined with pectin (WP) or xanthan (WX) in the protection of P. pentosaceus P107. In the storage test at temperatures of − 20 °C and 4 °C, the most viable microcapsule was WP (whey powder and pectin), although WX (whey powder and xanthan) presented better stability at 25 °C. In addition, WX did not show stability to ensure probiotic potential (< 6 Log CFU mL−1) for 110 days and the microcapsule W (whey powder) maintained probiotic viability at the three temperatures (− 20 °C, 4 °C, and 25 °C) for 180 days. In the exposition to simulated gastrointestinal juice, the WX microcapsule showed the best results in all tested conditions, presenting high cellular viability. For the thermal resistance test, WP microcapsule was shown to be efficient in the protection of P. pentosaceus P107 cells. The Fourier transform infrared spectroscopy (FTIR) results showed that there was no chemical interaction between microcapsules of whey powder combined with xanthan or pectin. The three microcapsules produced were able to protect the cell viability of the microorganism, as well as the drying parameters were adequate for the microcapsules produced in this study.
Keywords: Spray drying, Pediococcus pentosaceus, Thermal resistance, Protection
Introduction
In several fermented products and the mammal’s microbiota, Pediococcus pentosaceus — a bacterium belonging to the group of lactic acid bacteria (BAL) — is detected [1]. It is used as a starter culture in fermentation, mainly of vegetable products and canned meats, increasing their conservation as well as having technological properties for the production of metabolites (pediocins) and uses, such as a probiotic in food [1, 2].
Studies demonstrated the probiotic potential of Pediococcus spp., revealing its ability to adhere to epithelial cells, and resistance to both gastric and intestinal passage [2, 3]. Nevertheless, this viability can be compromised during storage, food processing, or even by the passage through the gastrointestinal tract itself [4, 5]. For food products to be effective as probiotics, they depend on the number of viable microorganism cells in the product at the time of consumption, with at least 6–7 log of CFU g−1 or mL−1 recommended [6].
To guarantee workability and offer protection to cells during storage, microencapsulation was sought, being a process that enables “packaging” of the object of protection, thus guarding it against undesirable adverse conditions [7, 8]. The spray drying technique is one of the most used in the microencapsulation of microorganisms, due to its low cost and high efficiency [9].
For this process to take place, matrices containing different materials are used, for instance, polysaccharides (gums and maltodextrins), proteins (caseins, gelatin, and albumins), and lipids (mono and diglycerides) [10]. Among the materials, whey has been in the spotlight in recent years, due to its high nutritional capacity, containing proteins of high value (albumin) and low cost in parallel to other noble materials [11].
Another encapsulating material used is pectin, a homopolysaccharide, abundant in the cell wall of fruits, used by the food industry as an additive, gelling, and thickener, and in the pharmaceutical industry as a coating for medicines. Such characteristics, alongside its insolubility in the digestive tract, can assist in the protection of microorganisms [9, 12]. Beyond that, its fibrous property is appreciated from a dietetic point of view, contributing to consumer welfare [13].
Xanthan gum, on the other hand, was also used as a material, extracellular heteropolysaccharide, produced by strains of Xanthomonas and is considered a biopolymer for industrial use. It is a gum with high thickening power, and viscosity can be relative, according to manipulation parameters, whereby temperature and pH [14]. Generally, it has high viscosity in low concentrations, which is why whey powder is used in the food industry [15]. Its pseudo-plastic capacity alongside resistance to temperature and some enzymes is a positive factor in the microencapsulation [16].
The aim of the present study was to microencapsulate and characterize microcapsules obtained by spray drying and produced only with whey powder or whey powder combined with pectin or xanthan in the protection of P. pentosaceus P107. For this, the efficiency and yield of the drying process, and viability of P. pentosaceus P107 under storage conditions during 180 days at the temperature of − 20 °C, 4 °C, and 25 °C, as well as survival to the gastrointestinal simulated juice, thermal resistance and, morphology of microcapsule were evaluated.
Material and methods
Microorganism and culture conditions
The P. pentosaceus P107 (P. pentosaceus P107) was isolated from the baked sliced ham, identified and cataloged in previous studies, belonging to the Collection of probiotic bacteria and starter cultures of the Food Microbiology Laboratory—Department of Food Science and Technology (DCTA) / Federal University of Pelotas (UFPel). For high-density cell culture, the growth was performed in two stages, with whey powder being used in both, having an average composition of 11% proteins, 1.5% fats, 6% mineral salts, and 3% moisture, according to the manufacturer (Relat®—Estação, RS, Brazil). The reconstitution was carried out in sterile distilled water at 6% (m v−1) followed by heat treatment (65 °C for 30 min) [15].
At the end of the process, the cell suspension was obtained at a concentration of approximately 14 Log CFU/mL−1 of P. pentosaceus P107, kept under refrigeration at 4 °C.
Microencapsulation by spray drying
Whey powder was evaluated alone and associated with either pectin or xanthan gum, as encapsulating materials for the production of the microcapsules. Aerosil 200, an anti-caking agent (Pharma-chemistry; Malaga, Spain), was added to all prepared solutions at a concentration of 1.25%.
The prepared solutions were as follows: (W): whey powder (6%) dissolved in sterile distilled water; (WX): whey powder (6%) and xanthan pruni synthesized by Xanthomonas arboricola pv pruni strain 101 (1.25%), produced at the Bioprocess Technology Laboratory (CDTec / UFPel, Brazil), previously (24 h) dissolved in water sterilized at 100 °C; (WP): whey powder (4%) and commercial pectin (Pharma-chemistry; Malaga, Spain) (2%) dissolved in sterile distilled water at 100 °C. All solutions were subjected to heat treatment for 30 min at 65 °C and kept under agitation in an orbital shaker.
After the cell suspension, P. pentosaceus P107 (14 Log CFU mL−1) was added to the respective encapsulating solutions and kept under agitation in a magnetic stirrer at 150 rpm at room temperature until the moment of the spray drying. All solutions were subjected to the drying process using a spray dryer (LabMaq – MSDi 1.0, São Paulo, SP, Brasil). The parameters were 100 °C outset, and 68 °C ending, the feed flux, 0.25 L h−1, and 3.00 m3 min−1 airflow. The final dry product was collected in sterile flasks and stored at different storage temperatures, − 20 °C, 4 °C, and 25 °C.
Characterization of the microcapsules
The morphology of the microcapsules was determined in 7 days of housing at − 20 °C. For this, samples were fixed with a double-sided carbon tape, spray-coated with gold (Leica, model EM SCD 500, Wetzlar, Germany), and scanned by electron microscopy (SEM) Jeol®, model JSM—6610LV (Jeol®, Tokyo, Japan), in high vacuum mode to examine the morphology of the surface. The average particle size was determined with the aid of the Image J software, starting with 50 measurements [17].
Water activity (aw) was measured by a direct method, at 25 °C, using the Labtouch-aW analyzer (Novasina, Neuheimstrasse, Switzerland) [17].
The moisture present in the microcapsule was determined gravimetrically by oven drying, according to the guidelines of the Association of Official Analytical Chemists (AOAC, 2012), by method No. 990.20 [17].
Color analyses were determined using a colorimeter (Minolta Chroma Meter CR-300, Osaka, Japan), with a CIE ‘L * a * b *’ reading system, proposed by the Commission Internationale de I’Eclairage (CIE) [17].
Microcapsule rupture test
To determine the disruption of the microcapsules, two solutions were used: solution A: containing PBS buffer (100 mM, pH 7.4), and solution B: 0.5% saline solution pH 2.5 containing 3 mg mL−1 of pepsin in times of 30, 60, and 120 min. For all microcapsules, 0.01 g of the powder was suspended again in 1 mL of both solutions (A and B), shaken in a vortex, and maintained in an orbital shaker at 37 °C, 150 rpm [18].
Viable cell counts were determined by means of serial dilution in 0.15% peptone water, playting by drop plate technique on MRS agar, incubated at 37 °C for 48 h under anaerobic conditions and the concentration of viable cells was expressed in Log CFU g−1[18].
Encapsulation efficiency (EE%) and process yield
To determine the encapsulation efficiency (EE%), Eq. 1 was used and presented as a percentage [16]:
| 1 |
where N is the number of viable cells after the process and N0 is the number of viable cells before the process.
The yield was expressed as a percentage, calculated according to Eq. 2 [19]:
| 2 |
Viability of P. pentosaceus P107 microencapsulated cells during storage at different temperatures
The viability of microcapsules containing P. pentosaceus P107 was carried out during 180 days of storage, at three different temperatures, − 20 °C ± 1 °C (freezing), 4 °C ± 1 °C (refrigeration), and 25 °C ± 1 °C (ambient). To disrupt the WP and WX microcapsules, 0.01 g of each microcapsule was dispersed in 1 mL of 0.5% saline solution (mv−1) pH 2.5, containing 3 mg mL−1 of pepsin, and kept in an orbital shaker at 150 rpm at 37° C for 30 min (Rosolen et al. 2019). For microcapsule W, 0.01 g was suspended in 1 mL of PBS buffer (100 mM, pH 7.4) and kept in an orbital shaker at the 150 rpm at 37 °C for 30 min [11]. The viable cell count was determined according to “Microcapsule rupture test.”
Survival of microencapsulated cells exposed to in vitro simulated gastrointestinal conditions
Prior to 7 days of storage at − 20 °C, microcapsules were subjected to 60 min of exposure to gastric juice and 240 min to simulated intestinal juice [15]. For this, 0.1 g of the microcapsules was exposed to 1 mL of simulated gastric juice, containing 3.0 mg mL−1 of pepsin and 0.5% saline solution (m v−1), at pH 2.0, 2.5, and 3.0. And separately for the simulated intestinal juice, the solution was prepared with 1.0 mg mL−1 of pancreatin and 0.5% saline (m v−1), at pH 8.0, with or without 0.5% bovine bile. Both were sterilized by filtration through 0.22 μm membranes (Sartorius Stedim Biotech, GmbH, Goettingen, Germany). The viable cell count was performed according to “Microcapsule rupture test.”
Thermal resistance
The thermal resistance was performed using 1 g of microcapsules in 9 mL of 0.1% peptone water (mv−1) put in test tubes and subjected to conditions of 65 °C for 30 min and 72 °C for 15 s in a thermostatic bath (QUIMIS, Brazil), followed by an immersion ice bath for 10 min [20]. After that, the viable cell count was performed, as described in “Microcapsule rupture test.”
Fourier transform infrared spectroscopy (FTIR)
The FTIR analysis was carried out using a Shimadzu IR Prestige 21 FTIR-ATR spectrometer, at the Biomaterials Development and Control Center (CDC-Bio), College of Dentistry of the UFPel. The samples were finely ground and the mixture was then compressed into pellets. Reading was performed between wavelengths 600 and 4000 cm−1[21].
Statistical analysis
The data were submitted to variance analysis (ANOVA) using the Graph pad Prism 7 program followed by Tukey’s test to compare the averages at a significance level of 95% (p < 0.05). The analyses were performed in the triple, and the results of the microencapsulation were calculated with the averages of three independent experiments.
Results and discussion
Characterization of microcapsules of P. pentosaceus P107
In order for probiotic cultures to maintain their stability during long-term storage, the moisture content must be constant and less than 5% [20, 22]. In the present study, the microcapsules showed moisture values of 0.86% (± 0.01), 1.93% (± 0.02), and 3.40% (± 0.01) for W, WX, and WP, respectively, which were considered appropriate, in accordance with the mentioned above. The different values can be attributed to differences in the wall materials of each microcapsule and to the total content of encapsulating material since it facilitates the formation of more compact walls, which may restrict the diffusion of water vapor from the interior of the microcapsule to the surface during the spray drying [21]. Due to its hydrophilic character, pectin can retain more water in the encapsulating matrix, by its tendency to form hydrogen-water bonds [12], which may explain the higher values for WP microcapsule in terms of moisture and water activity. The degree of complex formation between these two materials (whey powder and pectin) results in a variation of the values of water activity and moisture, since stronger complexes, depending on pH, for example, increase the binding of water within the complex making it less available for evaporation in capillary tubes during the drying process [23]. Ghasemi et al. [23] found a value of 3.96% moisture using whey and pectin complex in the encapsulation of orange peel oil, while Eckert et al. [11] found even higher values (4.12%) for L. plantarum ATCC 8014 microencapsulated only with whey. However, Khorshidi et al. [24] microencapsulated L. acidophilus with alginate and used whey and xanthan gum to coat the microcapsules by the extrusion technique, finding moisture values of 7.74%. The differences in the moisture values reported in the literature demonstrate that the moisture contents of microcapsules are not determined by just one factor, being related to technique and its parameters and the choice of encapsulating materials [25, 26].
The Aw values of the microcapsules obtained in this study, 0.233 ± 0.01, 0.244 ± 0.01, and 0.265 ± 0.02, respectively, are within the normal range (less than 0.6) for atomized product and the minimum required to maintain the cell viability [27]. The literature reports that the Aw values of less than 0.3 increase the stability of the dried products, due to the lower amount of free water available for biochemical reactions, ie, longer shelf life [11, 17, 27]. Whey powder, for its high protein content, decreases the Aw values, since proteins bind water leaving less water available for chemical and/or biological processes. On the other hand, when adding carbohydrates, such as pectin and xanthan gum, the unbound water values increase; however, the interaction with the protein causes the Aw values to remain within the maximum recommended levels. It is worth mentioning that concerning moisture and water activity, the drying conditions, especially inlet and outlet temperatures, are very important for the formation of powders within the appropriate parameters [28]. Eckert et al. [11] when microencapsulating L. plantarum ATCC 8014 with whey by spray drying obtained 0.251 water activity, while Ghasemi et al. [23] showed values less than 0.2 when combining whey and pectin to encapsulate orange peel oil.
As for the color attribute, all microcapsules showed high values for L *, 95.65, 94.24, and 96.12, respectively, for W, WX, and WP, indicating light colors; negative values for a *, − 1.67, − 0.56, and − 0.24, respectively, for W, WX, and WP, indicating shades of green; and finally, b * positive values, 5.68, 7.19, and 4.82, also respectively, for W, WX and WP, indicating trend in yellow. These parameters can be assigned to the predominant use of whey powder for the microcapsules produced with the three solutions, since it has a light yellow color, explaining the tendency of microcapsules to yellow. However, drying conditions such as temperature and flow rate also influence its final color [11, 29, 30]. The final color may impact the food matrix to be applied; thus, white pattern microcapsules have convenient features for applications in different formulations [31].
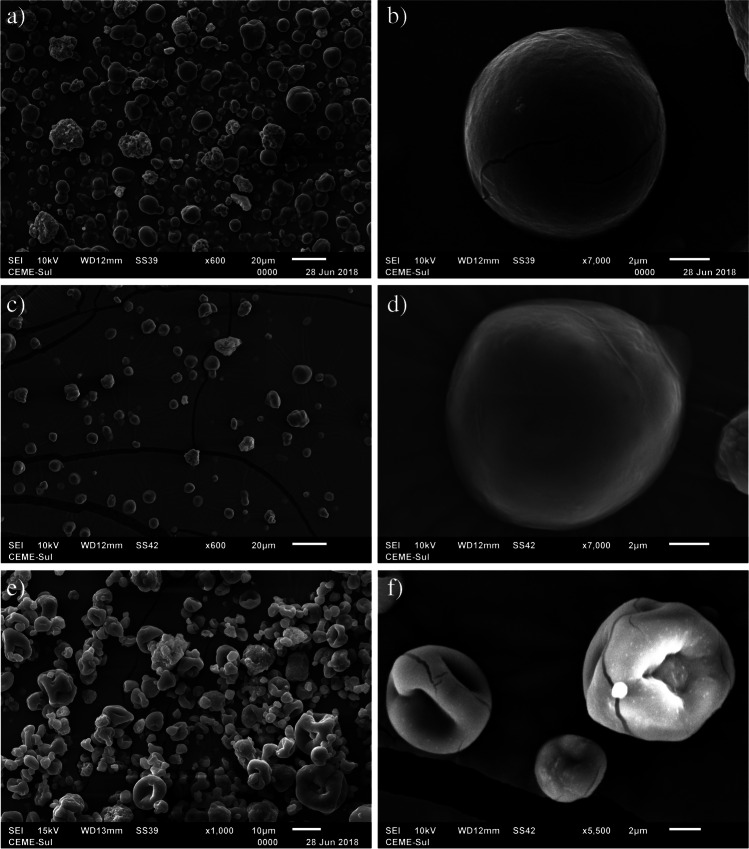
Figure 1 shows scanning electron microscopy (SEM) images of the microcapsules W, containing whey powder (ab); WX, containing xanthan and whey powder (bc); and WP, containing whey powder and pectin (a). The microcapsules containing whey powder (W), and whey powder with xanthan gum (WX) are symmetrical and rounded with average sizes of 12.32 µm ± 0.80, and 9.87 ± 0.76, respectively. In contrast, microcapsules with whey powder and pectin (WP) had an average size of 6.99 ± 0.67 µm and some deformations on their surface, which may be due to the interaction of the material with the high pressure exerted by spraying and shrinkage during the process and cooling [32].
Fig. 1.
SEM images of microcapsules of Pediococcus pentosaceus P107 obtained by spray drying whey powder W (a) 600 × and (b) 7000 × ; whey powder and xanthan WX (c) 600 × and (d) 7000 × ; and whey powder and pectin WP (e) 1000 × and (f) 5000 ×
Considering the size of the microcapsules obtained, they are adequate (up to 80 µm) to be inserted in food because small particles ensure a homogeneous and high-quality product without affecting the sensory properties [9]. Rosolen et al. [15] found average values of 12.73 µm for Lactococcus lactis subsp. lactis R7 microcapsules with whey powder and inulin. Otherwise, Nunes et al. [4] obtained microcapsules of L. acidophilus with inulin, trehalose, and Hi-maize by spray drying, with sizes ranging from 6.68 and 7.30 µm. Regarding the morphology, the W and WX microcapsules were mostly spherical in shape and had smooth surfaces. Cracks, as seen in Fig. 1b, were formed during the microscopy zoom process; this can occur due to the interaction of electrons within the sample, especially in materials sensitive to the electron beam directed by the microscopy equipment [33]. The WP capsule was spherical but with a rough surface, which can be explained by the fast formation of the microcapsule wall before the beginning of the expansion period due to the high drying rates of the droplets [34]. Uscategui et al. [28] verified that the microcapsules that had a low concentration of whey had a spherical but wrinkled morphology, unlike the combinations with a higher content of whey, which, in addition to being spherical, remained smooth, as the proteins present in whey are capable of forming a film on the particles when adequate total quantity. This suggests that in the present study, the greatest influence on WP morphology is not the combination with pectin, but the lower volume of whey (4%), when compared to the other microcapsules evaluated (6%). Rosolen et al. [15] used whey and inulin to microencapsulate L. lactis R7 by spray drying and the microcapsules presented morphology similar to W and WX of the present study.
Microcapsule rupture test, viability, encapsulation efficiency (EE%) and process yield
Table 1 shows the plug effect of 100 mM PBS (pH 7.4) and the saline solution (0.5% pH 2.5 with 3 pepsin mg mL−1), hereinafter referred to as PBS buffer and gastric solution, respectively, on the disruption of the studied microcapsules.
Table 1.
Release of bacterial cells from microcapsules when immersed into the PBS buffer or simulated gastric fluids with various exposure times at 37 °C
| Time (min) | PBS buffer 100 mM pH 7.4 | Gastric solution (saline solution 0.5% pH 2.5 and pepsin 3 mg mL−1) | ||||
|---|---|---|---|---|---|---|
| Log CFU g−1 | ||||||
| W | WX | WP | W | WX | WP | |
| 30 | 12.85 ± 0.34a,A | 7.09 ± 0.13c,B | 0.00 ± 0.00a,B | 8.92 ± 0.07a,B | 12.99 ± 0.20a,A | 13.63 ± 0.15a,A |
| 60 | 12.69 ± 0.10a,A | 8.15 ± 0.15b,B | 0.00 ± 0.00a,B | 8.76 ± 0.26a,B | 11.58 ± 0.51b,A | 9.15 ± 0.15b,A |
| 120 | 11.87 ± 0.67a,A | 9.15 ± 0.15a,B | 0.00 ± 0.00a,B | 8.44 ± 0.39a,B | 11.15 ± 0.15b,A | 9.00 ± 0.10b,A |
W, microcapsule whey powder; WX, microcapsule whey powder and xanthan; WP, microcapsule whey powder and pectin
Results represent the mean (standard deviations), n = 3
a–cDifferent superscript lowercase letters in the same column represent statistical difference (p < 0.05)
Means with different superscript capital letters represent a defined difference (p < 0.05) between the solutions tested for the same microcapsule
The PBS plug at a concentration of 100 mM has been described in the literature as a microcapsule tear solution [4, 11, 17, 35]. According to the results obtained, the whey powder (W) microcapsule of the present study, when exposed to the PBS plug for over 30 min time, showed a viable cell concentration of 12.85 Log CFU g−1. In contrast, the same plug promoted partial disruption of WX microcapsules and was not able to rupture the WP microcapsules and provoke the total exposure of the microorganism.
When the gastric solution was evaluated, the higher cell concentrations were obtained at 30 min for WX (12.99 Log CFU g−1) and WP (13.63 Log CFU g−1), reducing significantly (p < 0.05) the cell count depending on exposure time. It is noteworthy that both pectin and xanthan thickeners have properties that are strongly influenced by acidic pH and by the temperature of 37 °C, compromising the structure of the microcapsule [36, 37].
Understanding the tearing process of microcapsules and the delivery of their content is complex, as it involves several factors. There are few reports in the literature that explore the tearing properties of encapsulated bacteria; some of the mechanisms are described as diffusion, erosion, and fragmentation. In addition, factors such as changes in pH, the activity of proteolytic enzymes, osmotic stress, and time staying in a tearing solution, contribute to the process [38].
Whey proteins when bound to pectin polysaccharides form complexes through non-covalent interactions, such as electrostatic forces in both solution and interfaces, hydrogen bonding, and hydrophobic interactions under various conditions. Xanthan, when in acid solution (pH 3.0), is negatively charged while whey proteins are positively charged, and when this repulsive electrostatic force cancels out, the particles coated with both materials quickly separate, causing ruptures of the microcapsule and exposing the microorganism [39].
The initial viable cell count for W, WX, and WP microcapsules was 13.50 Log CFU g−1, 13.67 Log CFU g−1, and 13.76 Log CFU g−1, respectively. After the drying process, the microcapsules W and WX were reduced by 1.05 Log CFU g−1 and 1.28 Log CFU g−1, respectively, showing no significant difference. The WP microcapsules obtained the lowest cell reduction (0.13 Log CFU g−1) showing the highest encapsulation efficiency (99.05%) in relation to the others, as shown in Table 2.
Table 2.
Cell viability, encapsulation efficiency, and yield of the process of Pediococcus pentosaceus P107 microencapsulated using different materials before and after spray drying
| Number of viable cells (Log CFU g−1) | Reduction (Log CFU g−1) | Encapsulation efficiency (EE%) | Yield (%) | ||
|---|---|---|---|---|---|
| Before spray drying | After spray drying | ||||
| W | 13.50 ± 0.20 | 12.45 ± 0.71 | 1.05 ± 0.21a | 92.22 ± 0.50b | 17.37 ± 1.05a |
| WX | 13.67 ± 0.50 | 12.39 ± 0.80 | 1.28 ± 0.30a | 90.63 ± 0.25c | 9.58 ± 0.98b |
| WP | 13.76 ± 0.22 | 13.63 ± 0.50 | 0.13 ± 0.28b | 99.05 ± 0.20a | 15.23 ± 0.67a |
W, microcapsule whey powder; WX, microcapsule whey powder and xanthan; WP, microcapsule whey powder and pectin
Results represent the mean (standard deviations), n = 3
a–cMeans with different superscript lowercase letters in the same column represent statistical difference (p < 0.05)
The combination of whey powder and pectin demonstrated the best encapsulation efficiency, which can be explained by the fact that this polysaccharide is a nano-porous polymer, (2–50 nm) allowing only water and smaller particles to diffuse into the produced microcapsules. Pediococcus pentosaceus P107 has an average size ranging from 2 to 8 µm, making it comparatively larger than the pores of the complex used, allowing the retention of a large number of bacteria inside the microcapsules, when compared to other encapsulating materials [40].
Whey proteins have the ability to interact with glycoproteins present on the bacterial surface, making them a biocompatible material in the protection of the microorganism, due to their adhesive potential. When mixed solutions of whey proteins and xanthan polysaccharides are heated, there is competition between the gelling and phases separation processes. Once gelling occurs the basic structure of the gel is established and therefore the separation of phases is delayed, which contributes to the formation of a continuous and stable microcapsule in the drying process [41, 42].
Considering the yield of the process, it is influenced by drying parameters, in addition to environmental and physical–chemical properties of the encapsulating material. The yield obtained in the present study for W microcapsule was 17.37%, while was 9.58% and 15.23% for WX and for WP microcapsules, respectively, with W and WP being significantly higher. The influencers mentioned above can result in greater deposit of the material in the drying chamber of the equipment [43], justifying the lower yield in relation to the literature. In addition, much of the work brings the yield factor as process yield, i.e., the efficiency of the same. There are few comparative studies, however; Arslan-Tontul and Erbas [44] found yields ranging from 39.33 g to 54.65 g per 100 g when microencapsulating Saccharomyces cerevisiae var: Boulardii with different wall materials by spray drying.
Feasibility of storage of microencapsulated cells of P. pentosaceus P107 at different temperatures
In this study, the microcapsules were evaluated by their ability to maintain the viability of P. pentosaceus P107 during storage at different temperatures (− 20 °C, 4 °C, and 25 °C) (Table 3).
Table 3.
Viability of Pediococcus pentosaceus P107 microencapsulated for 180 days
| *Temperature | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (days) | − 20 °C | 4 °C | 25 °C | ||||||||
| W | WX | WP | W | WX | WP | W | WX | WP | |||
|
0 7 14 21 30 45 60 75 110 140 180 |
12.45 ± 0.71a 10.95 ± 0.04b 9.15 ± 0.15c 8.52 ± 0.60c 8.00 ± 0.00c 7.15 ± 0.15b 7.77 ± 0.01b 7.44 ± 0.39a 7.84 ± 0.01a 7.84 ± 0.06a 7.58 ± 0.21a |
12.39 ± 1.00a 10.40 ± 0.80b 10.58 ± 0.51b 10.00 ± 0.00b 9.88 ± 0.51b 10.00 ± 0.01a 9.58 ± 0.51a 8.12 ± 0.11b 7.85 ± 0.15a 7.54 ± 0.06b 7.27 ± 0.02a |
13.63 ± 0.50a 13.54 ± 0.06a 13.00 ± 0.00a 12.69 ± 0.35a 12.58 ± 0.51a 8.88 ± 0.78a 8.15 ± 0.15b 7.95 ± 0.04a,b 7.77 ± 0.68a 7.74 ± 0.17a,b 7.59 ± 0.11a |
12.45 ± 0.71a 10.00 ± 0.00b 8.80 ± 0.06b 8.58 ± 0.51c 8.58 ± 0.55b 7.47 ± 0.02c 7.54 ± 0.06b 7.44 ± 0.39a 7.59 ± 0.27a 7.46 ± 0.15a 7.15 ± 0.15a |
12.39 ± 1.00a 9.95 ± 0.42b 9.58 ± 0.51b 9.99 ± 0.61b 9.89 ± 0.78b 9.00 ± 0.22b 8.72 ± 0.12a 8.00 ± 0.00a 7.58 ± 0.51a 7.52 ± 0.04a 7.27 ± 0.02a |
13.63 ± 0.50a 13.54 ± 0.06a 13.51 ± 0.20a 12.00 ± 0.00a 12.00 ± 0.03a 11.59 ± 0.11a 7.69 ± 0.35b 7.88 ± 0.78a 7.85 ± 0.15a 7.52 ± 0.04a 7.15 ± 0.15a |
12.45 ± 0.71a 11.25 ± 0.24b 7.90 ± 0.05b 7.62 ± 0.15b 7.60 ± 0.00b 7.56 ± 0.24b 7.54 ± 0.06a 7.47 ± 0.00a 7.47 ± 0.02a 7.18 ± 0.14a 6.13 ± 0.39a |
12.39 ± 1.00a 8.16 ± 0.15c 7.16 ± 0.15c VC VC VC VC VC VC VC VC |
13.63 ± 0.50a 13.39 ± 0.08a 13.25 ± 0.24a 13.15 ± 0.15a 13.00 ± 0.12a 10.69 ± 0.35a 6.44 ± 0.39b VC VC VC VC |
||
*Storage at freezing: − 20 °C; refrigeration: 4 °C, and ambient temperatures: 25 °C
a–cMeans ± standard deviation with different superscript capital letters indicates significant difference (p < 0.05) between microcapsules in the same period and storage temperature
W, microcapsule whey powder; WX, microcapsule whey powder and xanthan; WP, microcapsule whey powder and pectin; VC, viable cells < 6 log CFU g−1
A crucial point during the storage of probiotic-based products is that probiotic microorganisms must be kept at high counts during the shelf life of the products. Specific storage methods can help maintain these microorganisms, expanding their use in foods, reducing storage costs, and increasing shelf life [45]. The cell viability presented was > 6 log CFU g−1 in W microcapsules, at the end of 180 days for the three storage temperatures. Similar results were shown by the WX microcapsules (7.27, 7.27 log CFU g−1) and WP microcapsules (7.59, 7.15 log CFU g−1), although at temperatures of − 20 °C and 4 °C, respectively. Likewise, Liao et al. (2017) evaluated the feasibility of storage at temperatures of − 20 °C, 4 °C, 25 °C, and 37 °C Lactobacillus casei K1, microencapsulated by spray drying. The authors reported high viability when stored at − 20 °C over the other temperatures. According to De Castro-Cislaghi et al., [35] the stability of the microencapsulated probiotic is increased in low temperatures, such as refrigeration and freezing temperature.
A survey by Yao et al. [46] evaluated the storage survival at 4 °C of L. salivarius Li01 in lyophilization-dried alginate or alginate-gelatin microgels, which showed a 5-week reduction of only 2.4 log 10 and 17 log 10; however, this represents combined values of > 6 log CFU g−1 for alginate and close to this value for alginate to gelatin, lower values compared with the present study in which the maintenance of viability was observed above 180 days (6 months) for all three microcapsules presented below. During storage at 25 °C, there was a loss of cell viability from the 21st day for WX and 75th days for WP, showing that the temperature has negative effects on the wall of the microcapsule and, thus, on the micro-organism protection, corroborating with the encapsulation efficiency data, where WP presented the highest value (99%) compared to WX (90%). The whey powder used alone plays an important role as an encapsulating agent to maintain the viability of P. pentosaceus P107 during the storage, as it maintained the minimum probiotic viability for 180 days at the three temperatures tested, with its EE% results being intermediate compared to the other microcapsules (92%). Moreover, as many LAB are indigenous in milk, the whey is considered a suitable matrix, making the microcapsules an environment with chemical-physical and biological characteristics adequate to maintain these microorganisms [42].
Oliveira et al. [26] found higher viability losses at 37 °C when compared to 7 °C for microencapsulated Bifidobacterium lactis by coacervation with casein and pectin, followed by atomization. Similarly, Vega-Sagardía et al. [47] encapsulated L. fermentum UCO-979C with alginate and xanthan, added or not oil, and found high viability at 4 °C when compared to 25 °C. Adaptations to the environment, such as heat stress and moisture content, are important conditions for probiotics to survive at high temperatures [48]. A greater reduction in the number of encapsulated microorganisms was observed in the storage at 25 °C because at this temperature the metabolic activity is higher and nutrients are consumed quickly. Similarly, other studies have shown that microencapsulation using thermoprotective agents increases the survival of microorganisms during storage because mechanical, oxidative, and osmotic stress is minimized [49].
Survival of microencapsulated P. pentosaceus P107 exposed to gastrointestinal tract simulated
One of the main barriers to oral administration of probiotic bacteria is the low pH of the stomach associated with the high concentration of hydrochloric acid. Therefore, the encapsulation technique is a useful tool to increase the protection of such micro-organisms when exposed to these conditions.
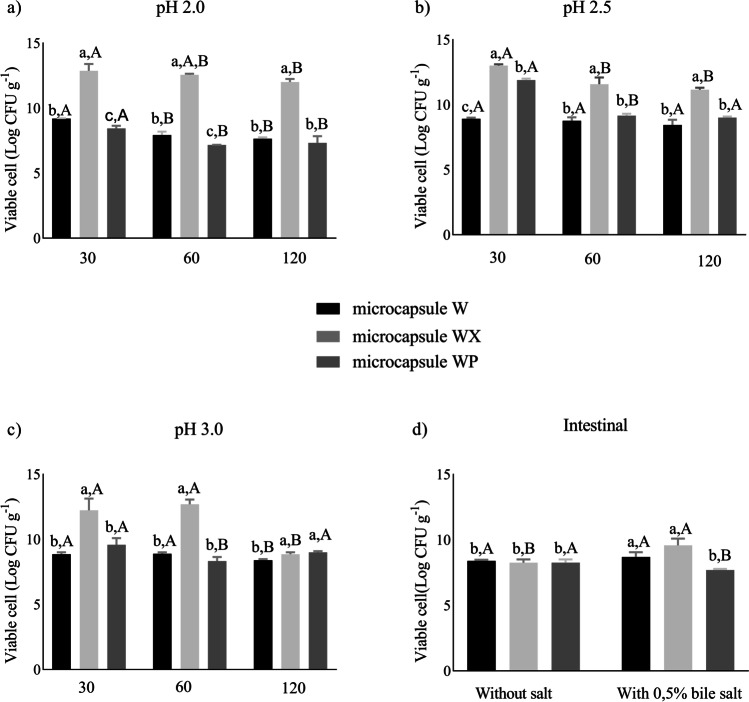
In the present study, there was significant protection by the WX microcapsules, demonstrated by cell viability higher than 10 Log CFU g−1 of P. pentosaceus P107, 120 min after the exposure to simulated gastric juice at different pH (2.0, 2.5) (Fig. 2a, b) and greater than 9 Log CFU g−1 at pH 3.0 (Fig. 2c). This can be explained by the fact that the very low pH has an effect on the electrostatic bonds between xanthan and whey, which begin to disappear once amine groups deionize, while carboxyl groups retain a negative charge. The network formed by the encapsulant material is capable of expanding and absorbing water to buffer the acid compounds present in the gastric fluid, as they penetrate the microcapsules [47].
Fig. 2.
Pediococcus pentosaceus P107 microencapsulated viability up to 7 days storage at − 20 °C during exposure to the simulated gastrointestinal tract. a Gastric fluid pH 2.0; b gastric fluid pH 2.5; c gastric fluid pH 3.0, both at times of 30, 60, and 120 min; d intestinal fluid pH 8.0 with 0.5% with bile salts and without, both by 240 min. a–cMeans ± standard deviation with different superscript letters indicates a lower significant difference between the microcapsules for the same time and pH (p < 0.05). A–CMeans ± standard deviation with different superscript capital letters denotes significant differences between the same microcapsule in the different analysis times for the same pH (p < 0.05). W, whey powder microcapsule; WX, whey powder with xanthan microcapsule; WP, whey powder with pectin microcapsule
In contrast, both W and WP microcapsules, after the same period (120 min), had significantly lower values (< 9 Log CFU g−1) demonstrating that the enzyme action time and acid concentration are crucial for the integrity of the microcapsule and protection of the micro-organism. It has been reported that the dense network of hydrogel formed by combining whey to xanthan or pectin reduces the diffusion rate from the microcapsule, thereby reducing the exposure of the microorganism to the acidic medium which it is exposed [49, 50].
The presence or absence of bile salts in the simulated intestinal juice did not affect the viability of P. pentosaceus P107 (Fig. 2d). In the absence of bile salts, the microcapsules showed no significant difference. However, the presence of bile salts promoted the microcapsule rupture and exposure of the microorganism, showing a reduction of 4.16, 3.41, and 5.94 Log CFU g−1 to W, WX, and WP microcapsules, respectively, being inversely proportional to the values of EE%, where WP microcapsule had the best rate, followed by W and WX microcapsules. These results demonstrated that results of survival to tests such as gastric and intestinal simulated juices are not directly proportional to EE%; however, a good general EE% of all microcapsules (> 90%) results in good test survival values.
Chen et al. [51] demonstrated that xanthan microcapsules linked with chitosan, when exposed to 1% bile salts for 120 min, presented a reduction in 2.06 Log CFU g−1. On the other hand, Rosolen et al. [15] found high cell viability in cells of L. lactis R7 microencapsulated, after 240-min exposure to simulated intestinal tract both in the presence (reduction of 2.3 Log CFU g−1) and in the absence of bile salts (2.88 Log CFU g−1).
It is noteworthy that the results may be affected by the encapsulating material used, by the metabolic interaction of the microorganism, or by the natural resistance of these bacteria to different pH and digestive enzymes [52].
Evaluation of heat resistance
The survival of microencapsulated P. pentosaceus P107 exposed to temperatures of slow pasteurization (65 °C for 30 min) and fast pasteurization (72 °C for 15 s), is shown in Table 4. Such temperatures were chosen to simulate the thermal processes that probiotic formulations are submitted to in the food industry, aiming to inactivate pathogenic microorganisms, which can lead to a significant loss of cellular viability of the probiotics. This fact justifies the search for methods such as microencapsulation that are able to protect the viability of these microorganisms by creating a protective barrier against heat stress [7].
Table 4.
Viability and percentage of survival of Pediococcus pentosaceus P107 microencapsulated and subjected to heat treatment at different temperatures
| Microcapsule | Survival rate (%) | |
|---|---|---|
| 65 °C by 30 min | 72 °C by 15 s | |
| W | 84.37 ± 1.12c | 90.75 ± 0.25b |
| WX | 87.91 ± 0.18b | 99.18 ± 0.18a |
| WP | 92.46 ± 0.78a | 99.31 ± 0.69a |
W, whey powder microcapsule; WX, whey powder and xanthan microcapsule; WP, whey powder and pectin microcapsule
Results represent the mean (standard deviations), n = 3
a–cMeans with different superscript lowercase letters in the same column represent statistical difference (p < 0.05)
The results showed that the microencapsulated cells with whey powder (W) were significantly more sensitive to heat treatment (p < 0.05), however still obtained counts that demonstrate probiotic potential (> 6 Log CFU g−1) in both tests. For the heat treatment of 65 °C for 30 min, the best results were shown by WP microcapsules with a survival rate of 92.46%, followed by WX microcapsules with 87.91% survival. It can be observed that there was no change in the survival of P. pentosaceus P107 (WP and WX) when subjected to 72 °C for 15 s.
In the study of Ilha et al. [20], the Lactobacillus paracasei FNU cells microencapsulated with skim milk and whey by spray drying were subjected to thermal testing at 65 °C for 30 min and showed a percentage of survival of approximately 70%. Yao et al. [46] demonstrated that alginate and gelatin microgels were more effective in protecting the probiotic from heat stress at 65 °C for 15 and 30 min when compared to microgels using only alginate as a coating material, corroborating the results of the present study, where the microcapsule with only whey (W) was the one with the lowest protection value when compared to WX and WP at both temperatures. However, the general values of cell viability obtained by Ilha et al. [20] were significantly lower than in the present study. Etchepare et al. [53] evaluated the viability of L. acidophillus microencapsulated in multiple layers by ion gelling using calcium alginate and whey. When the particle-containing alginate is submitted to the thermal resistance test (72 °C for 15 s), 77% survival was observed. When the particle receives a layer of whey, the survival increases to 82%, demonstrating the effect of the coatings used in the protection of the microorganism. However, the values are lower than those obtained in the present study.
Few studies evaluate the protection that the microcapsule can provide to the microorganism when exposed to lethal temperatures. The high temperature and short time (72 °C for 15 s) are preferred for products containing encapsulated probiotics because the microcapsule is capable of protecting from the generated damage, such as protein denaturation and destruction of nucleic acids, which would lead to cellular apoptosis [54]. The viscoelastic properties of polysaccharides associated with the colloidal protein system improve electrostatic interactions between wall materials determining their level of thermotolerance [52, 55].
The microencapsulation process or heat treatment during food processing induces in matrices such as whey the release of sulfur amino acids, reducing redox potential, thus aiding probiotic survival [56]. It is noteworthy that there is a long way to go in research relating wall materials to the thermal resistance of microencapsulated probiotics.
FTIR analysis
Figure 3 presents the FTIR spectra of whey powder (W), whey powder with xanthan (WX), whey with pectin (WP) microcapsules, and P. pentosaceus P107 (cell free). Regarding the spectrum W, a peak between 3600 and 3000 cm−1 was observed related to the free hydroxyl group (O − H). The peak at 2927 cm−1 refers to the C − H stretching bond [57]. The peak at 1632 cm−1 is related to vibratory stretching bonds of groups C = O (amide type I). The peaks at 1537 cm−1 and 1367 cm−1 correspond to the bending groups of N − H of amide type II and the C − N stretching of amide I structures, respectively [58]. The functional group represented by the peak at 1039 cm−1 is related to the bending of the C − N bond [59]. The microcapsule WX showed a small decrease in the peaks at 1632 cm−1 and 1537 cm−1.
Fig. 3.
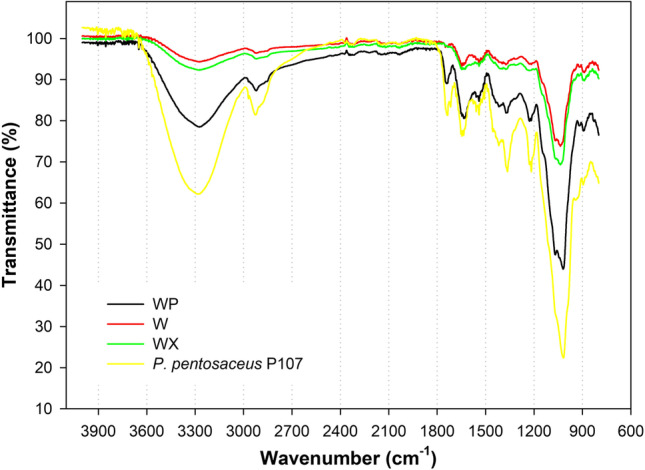
Fourier transform infrared spectroscopy analysis of microcapsules of Pediococcus pentosaceus P107 obtained by spray drying whey powder (W), whey powder and xanthan (WX), whey powder and pectin (WP), and P. pentosaceus P107 (cell free)
Similar results were observed by Xiao et al. [42] after the whey protein isolate hydrogel was coated with xanthan gum, also reporting an increase in the peak up to 3564 cm−1, and attributing this behavior to the introduction of polysaccharide molecules. The FTIR spectrum of P. pentosaceus (cell free) showed peaks in the region of 3100–2800 cm−1, which correspond to stretching vibrations of the functional groups of fatty acids present in cell membranes. Between 1800 and 1500 cm−1 are found the peaks of amide I and amide II of proteins and peptides, while the region of 1200–900 cm−1 provides information regarding the carbohydrates present in the cell wall [60].
The spectrum of the microcapsule of whey powder combined with pectin (WP) showed characteristic peaks observed at 1754 cm−1 and 1643 cm−1, related to the esterified and non-esterified carboxyl groups, respectively [58]. These are characteristic peaks of pectin, indicating that there was no new specific peak found in WP and WX, which suggests that there was no new chemical bond between the materials. This type of physical incorporation can help preserve the intrinsic nature of the encapsulated compound. Similar results were obtained by Hosseinnia et al. [57], who studied the microencapsulation of Ziziphora clinopodioides essential oil by whey protein isolate and pectin.
Conclusions
The present study demonstrated that whey powder and its association with pectin and xanthan as encapsulating materials were able to protect P. pentosaceus P107 during microencapsulation by spray drying. During storage the microcapsules WP (whey powder and pectin) and WX (whey powder and xanthan) remained stable at temperatures of − 20 °C and 4 °C for 180 days; however, when then subjected to a temperature of 25 °C, there was a loss of cell viability from the 21st day for WX and 75th days for WP, showing that the temperature has negative effects on the wall of the microcapsule and, thus, on the micro-organism protection. The WX microcapsule was the best in protecting cell viability when submitted to the simulated gastrointestinal juice. WP microcapsule, on the other hand, showed the greatest survival when subjected to heat treatment and W showed better storage stability at the three temperatures studied. The identification of the functional groups and the structural changes resulting from the encapsulation process were analyzed by FTIR, and the results showed that there was no chemical interaction between microcapsules of whey powder combined with xanthan or pectin.
In summary, the combination of proteins and polymers in the composition of probiotic microcapsules promotes an improvement in survival and maintenance of cell viability. It can be suggested that the microcapsules produced presented specific morphology, thermal resistance, and resistance to simulated gastrointestinal, in addition to low solubility in water, demonstrating great potential for application in food.
The results obtained in this study provided important information regarding the microencapsulation of probiotics by spray drying, contributing to future research in the area of food or medicine, given the satisfactory and positive path in relation to the long-term storage of this microorganism in its viable state, capable of promoting consumer health.
Author contribution
FB, WS, and SP conceived and designed this research. RP, AF, PO, and FC contributed new reagents or analytical tools. FB, GL, and MR performed experiments. All authors analyzed data. FB and MD wrote the manuscript. All authors read and approved the final version of the manuscript.
Funding
This study was funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Código Financeiro 001. The authors also thank the Brazilian agencies for their financial support: CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico, and FAPERGS—Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul – Brasil.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Responsible Editor: Elaine Cristina Pereira de Martinis
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jiang S, Cai L, Lv L, Li L. Pediococcus pentosaceus, a future additive or probiotic candidate. Microb Cell Fact. 2021;20(1):1–14. doi: 10.1186/S12934-021-01537-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porto MCW, Kuniyoshi TM, Azevedo POS, Vitolo M, Oliveira RPS. Pediococcus spp.: an important genus of lactic acid bacteria and pediocin producers. Biotechnol Adv. 2017;35(3):361–374. doi: 10.1016/j.biotechadv.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Dubey V, Mishra AK, Ghosh AR. Cell adherence efficacy of probiotic Pediococcus pentosaceus GS4 (MTCC 12683) and demonstrable role of its surface layer protein (Slp) J Proteomics. 2020;226:103894. doi: 10.1016/J.JPROT.2020.103894. [DOI] [PubMed] [Google Scholar]
- 4.Nunes GL, Etchepare M de A, Cichoski AJ, et al (2018) Inulin, hi-maize, and trehalose as thermal protectants for increasing viability of Lactobacillus acidophilus encapsulated by spray drying. LWT - Food Sci Technol 89(October 2017):128–133. 10.1016/j.lwt.2017.10.032
- 5.Rathore S, Desai PM, Liew CV, Chan LW, Heng PWS. Microencapsulation of microbial cells. J Food Eng. 2013;116(2):369–381. doi: 10.1016/j.jfoodeng.2012.12.022. [DOI] [Google Scholar]
- 6.Food and Agriculture Organisation of the United Nations and WHO Working Group (2002) Guidelines for the evaluation of probiotics in food. Published online 1–11. http://www.fao.org/es/ESN/Probio/probio.htm. Accessed 26 Oct 2021
- 7.Yao M, Xie J, Du H, McClements DJ, Xiao H, Li L. Progress in microencapsulation of probiotics: a review. Compr Rev Food Sci Food Saf. 2020;19(2):857–874. doi: 10.1111/1541-4337.12532. [DOI] [PubMed] [Google Scholar]
- 8.Ye Q, Georges N, Selomulya C. Microencapsulation of active ingredients in functional foods: from research stage to commercial food products. Trends Food Sci Technol. 2018;78:167–179. doi: 10.1016/j.tifs.2018.05.025. [DOI] [Google Scholar]
- 9.Martín MJ, Lara-Villoslada F, Ruiz MA, Morales ME. Microencapsulation of bacteria: a review of different technologies and their impact on the probiotic effects. Innov Food Sci Emerg Technol. 2015;27:15–25. doi: 10.1016/j.ifset.2014.09.010. [DOI] [Google Scholar]
- 10.Kavitake D, Kandasamy S, Devi PB, Shetty PH. Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods – a review. Food Biosci. 2017;2018(21):34–44. doi: 10.1016/j.fbio.2017.11.003. [DOI] [Google Scholar]
- 11.Eckert C, Serpa VG, Felipe dos Santos AC, et al. Microencapsulation of Lactobacillus plantarum ATCC 8014 through spray drying and using dairy whey as wall materials. LWT - Food Sci Technol. 2017;82:176–183. doi: 10.1016/j.lwt.2017.04.045. [DOI] [Google Scholar]
- 12.Khodaei D, Hamidi-Esfahani Z, Lacroix M. Gelatin and low methoxyl pectin films containing probiotics: film characterization and cell viability. Food Biosci. 2020;36:100660. doi: 10.1016/J.FBIO.2020.100660. [DOI] [Google Scholar]
- 13.Sansone F, Mencherini T, Picerno P, Amore M, Aquino RP, Lauro MR. Maltodextrin / pectin microparticles by spray drying as carrier for nutraceutical extracts. J Food Eng. 2011;105(3):468–476. doi: 10.1016/j.jfoodeng.2011.03.004. [DOI] [Google Scholar]
- 14.Kang Y, Li P, Zeng X, et al. Biosynthesis, structure and antioxidant activities of xanthan gum from Xanthomonas campestris with additional furfural. Carbohydr Polym. 2019;216:369–375. doi: 10.1016/J.CARBPOL.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Rosolen MD, Bordini FW, de Oliveira PD, et al (2019) Symbiotic microencapsulation of Lactococcus lactis subsp. lactis R7 using whey and inulin by spray drying. LWT 115. 10.1016/j.lwt.2019.108411
- 16.Annan NT, Borza AD, Hansen LT. Encapsulation in alginate-coated gelatin microspheres improves survival of the probiotic Bifidobacterium adolescentis 15703T during exposure to simulated gastro-intestinal conditions. Food Res Int. 2008;41(2):184–193. doi: 10.1016/j.foodres.2007.11.001. [DOI] [Google Scholar]
- 17.Fritzen-Freire CB, Prudêncio ES, Amboni RDMC, Pinto SS, Negrão-Murakami AN, Murakami FS. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res Int. 2012;45(1):306–312. doi: 10.1016/j.foodres.2011.09.020. [DOI] [Google Scholar]
- 18.Rosolen MD, Bordini FW, de Oliveira PD, et al. Symbiotic microencapsulation of Lactococcus lactis subsp. lactis R7 using whey and inulin by spray drying. Lwt. 2019;115(January):108411. doi: 10.1016/j.lwt.2019.108411. [DOI] [Google Scholar]
- 19.Gonsalves JKMC, Costa AMB, Sousa DP De, Cavalcanti SCH (2009) Osbeck pelo método da coacervação simples. Sci Plena 5; n 11(L):1–8
- 20.Ilha EC, da Silva T, Lorenz JG, de Oliveira Rocha G, Sant’Anna ES (2015) Lactobacillus paracasei isolated from grape sourdough: acid, bile, salt, and heat tolerance after spray drying with skim milk and cheese whey. Eur Food Res Technol 240(5):977–984. 10.1007/s00217-014-2402-x
- 21.Rajam R, Anandharamakrishnan C. Microencapsulation of Lactobacillus plantarum (MTCC 5422) with fructooligosaccharide as wall material by spray drying. LWT - Food Sci Technol. 2015;60(2):773–780. doi: 10.1016/j.lwt.2014.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peighambardoust SH, Golshan Tafti A, Hesari J. Application of spray drying for preservation of lactic acid starter cultures: a review. Trends Food Sci Technol. 2011;22(5):215–224. doi: 10.1016/j.tifs.2011.01.009. [DOI] [Google Scholar]
- 23.Ghasemi S, Jafari SM, Assadpour E, Khomeiri M. Production of pectin-whey protein nano-complexes as carriers of orange peel oil. Carbohydr Polym. 2017;177(August):369–377. doi: 10.1016/j.carbpol.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Khorshidi M, Heshmati A, Taheri M, Karami M, Mahjub R. Effect of whey protein- and xanthan-based coating on the viability of microencapsulated Lactobacillus acidophilus and physiochemical, textural, and sensorial properties of yogurt. Food Sci Nutr. 2021;9(7):3942–3953. doi: 10.1002/fsn3.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardiner GE, Sullivan EO, Kelly J, et al. Comparative survival rates of human-derived probiotic Lactobacillus paracasei and L. salivarius strains during heat treatment and spray drying. Appl Environ Microbiol. 2000;66(6):2605–2612. doi: 10.1128/AEM.66.6.2605-2612.2000.Updated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira AC, Moretti TS, Boschini C, Baliero JCC, Freitas O, Favaro-Trindade CS. Stability of microencapsulated B. lactis (BI 01) and L. acidophilus (LAC 4) by complex coacervation followed by spray drying. J Microencapsul. 2007;24(7):685–693. doi: 10.1080/02652040701532908. [DOI] [PubMed] [Google Scholar]
- 27.Favaro-Trindade CS, Santana AS, Monterrey-Quintero ES, Trindade MA, Netto FM. The use of spray drying technology to reduce bitter taste of casein hydrolysate. Food Hydrocoll. 2010;24(4):336–340. doi: 10.1016/j.foodhyd.2009.10.012. [DOI] [Google Scholar]
- 28.Ruano Uscategui DC, Ciro Velásquez HJ, Sepúlveda Valencia JU. Concentrates of sugarcane juice and whey protein: study of a new powder product obtained by spray drying of their combinations. Powder Technol. 2018;333:429–438. doi: 10.1016/j.powtec.2018.04.025. [DOI] [Google Scholar]
- 29.Aryana KJ, McGrew P. Quality attributes of yogurt with Lactobacillus casei and various prebiotics. LWT - Food Sci Technol. 2007;40(10):1808–1814. doi: 10.1016/j.lwt.2007.01.008. [DOI] [Google Scholar]
- 30.Jiang N, Dev Kumar G, Chen J, Mishra A, Mis Solval K. Comparison of concurrent and mixed-flow spray drying on viability, growth kinetics and biofilm formation of Lactobacillus rhamnosus GG microencapsulated with fish gelatin and maltodextrin. LWT. 2020;124:109200. doi: 10.1016/J.LWT.2020.109200. [DOI] [Google Scholar]
- 31.Castel V, Rubiolo AC, Carrara CR. Brea gum as wall material in the microencapsulation of corn oil by spray drying: effect of inulin addition. Food Res Int. 2017;2018(103):76–83. doi: 10.1016/j.foodres.2017.10.036. [DOI] [PubMed] [Google Scholar]
- 32.Singh J, Kaur K. Optimizing microencapsulation of a -tocopherol with pectin and sodium alginate. J Food Sci Technol. 2018;55(9):3625–3631. doi: 10.1007/s13197-018-3288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scanning Electron Microscope | Electron Beam Damage - BR. https://www.thermofisher.com/br/en/home/materials-science/learning-center/applications/sample-degradation-scanning-electron-microscope-sem.html Accessed May 23, 2023
- 34.Diệp Huy Vũ P, Rodklongtan A, Chitprasert P (2021) Whey protein isolate-lignin complexes as encapsulating agents for enhanced survival during spray drying, storage, and in vitro gastrointestinal passage of Lactobacillus reuteri KUB-AC5. Lwt 148(May). 10.1016/j.lwt.2021.111725
- 35.De Castro-Cislaghi FP, Silva CDRE, Fritzen-Freire CB, Lorenz JG, Sant’Anna ES (2012) Bifidobacterium Bb-12 microencapsulated by spray drying with whey: Survival under simulated gastrointestinal conditions, tolerance to NaCl, and viability during storage. J Food Eng 113(2):186–193. 10.1016/j.jfoodeng.2012.06.006
- 36.Argin S, Ko P, Lo YM. Food Hydrocolloids The cell release kinetics and the swelling behavior of physically crosslinked xanthan e chitosan hydrogels in simulated gastrointestinal conditions. 2014;40:138–144. doi: 10.1016/j.foodhyd.2014.02.018. [DOI] [Google Scholar]
- 37.Menezes C De, Fernanda M, Rodrigues Z, Cavalheiro P, Etchepare A, Menezes R De. Microencapsulação de probióticos por gelificação iônica externa utilizando pectina Microencapsulation of probiotics by using external ionic gelling pectin. Published online 2015. 10.5902/2179-460X19712
- 38.Matalanis A, Grif O, Mcclements DJ. Food Hydrocolloids Structured biopolymer-based delivery systems for encapsulation , protection , and release of lipophilic compounds. 2011;25. 10.1016/j.foodhyd.2011.04.014
- 39.Owens C, Griffin K, Khouryieh H, Williams K. Creaming and oxidative stability of fish oil-in-water emulsions stabilized by whey protein-xanthan-locust bean complexes: impact of pH. Food Chem. 2018;239:314–322. doi: 10.1016/j.foodchem.2017.06.096. [DOI] [PubMed] [Google Scholar]
- 40.Yasmin I, Saeed M, Pasha I, Zia MA. Development of whey protein concentrate-pectin-alginate based delivery system to improve survival of B. longum BL-05 in simulated gastrointestinal conditions. Probiotics Antimicrob Proteins. 2019;11(2):413–426. doi: 10.1007/s12602-018-9407-x. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Ould Eleya MM, Gunasekaran S. Gelation of whey protein and xanthan mixture: effect of heating rate on rheological properties. Food Hydrocoll. 2006;20(5):678–686. doi: 10.1016/j.foodhyd.2005.07.001. [DOI] [Google Scholar]
- 42.Xiao Y, Han C, Yang H, Liu M, Meng X, Liu B. Layer (whey protein isolate) -by-layer (xanthan gum) microencapsulation enhances survivability of L. bulgaricus and L. paracasei under simulated gastrointestinal juice and thermal conditions. Int J Biol Macromol. 2020;148:238–247. doi: 10.1016/j.ijbiomac.2020.01.113. [DOI] [PubMed] [Google Scholar]
- 43.Johansen P, Merkle HP, Gander B. Technological considerations related to the up-scaling of protein microencapsulation by spray-drying. Eur J Pharm Biopharm. 2000;50(3):413–417. doi: 10.1016/S0939-6411(00)00123-5. [DOI] [PubMed] [Google Scholar]
- 44.Arslan-Tontul S, Erbas M. Single and double layered microencapsulation of probiotics by spray drying and spray chilling. LWT - Food Sci Technol. 2017;81:160–169. doi: 10.1016/j.lwt.2017.03.060. [DOI] [Google Scholar]
- 45.Liao LK, Wei XY, Gong X, Li JH, Huang T, Xiong T. Microencapsulation of Lactobacillus casei LK-1 by spray drying related to its stability and in vitro digestion. LWT - Food Sci Technol. 2017;82:82–89. doi: 10.1016/j.lwt.2017.03.065. [DOI] [Google Scholar]
- 46.Yao M, Wu J, Li B, Xiao H, Julian D, Li L. Food Hydrocolloids Microencapsulation of Lactobacillus salivarious Li01 for enhanced storage viability and targeted delivery to gut microbiota. 2017;72:228–236. 10.1016/j.foodhyd.2017.05.033
- 47.Vega-Sagardía M, Rocha J, Sáez K, Smith CT, Gutierrez-Zamorano C, García-Cancino A. Encapsulation, with and without oil, of biofilm forming Lactobacillus fermentum UCO-979C strain in alginate-xanthan gum and its anti-Helicobacter pylori effect. J Funct Foods. 2017;2018(46):504–513. doi: 10.1016/j.jff.2018.04.067. [DOI] [Google Scholar]
- 48.Zhang Y, Lin J, Zhong Q. The increased viability of probiotic Lactobacillus salivarius NRRL B-30514 encapsulated in emulsions with multiple lipid-protein-pectin layers. Food Res Int. 2015;71:9–15. doi: 10.1016/j.foodres.2015.02.017. [DOI] [Google Scholar]
- 49.Eckert C, Agnol WD, Dallé D, et al. Development of alginate-pectin microparticles with dairy whey using vibration technology: effects of matrix composition on the protection of Lactobacillus spp. from adverse conditions. Food Res Int. 2018;113(June):65–73. doi: 10.1016/j.foodres.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Chew SC, Tan CP, Long K, Nyam KL. In-vitro evaluation of kenaf seed oil in chitosan coated-high methoxyl pectin-alginate microcapsules. Ind Crop Prod. 2015;76:230–236. doi: 10.1016/j.indcrop.2015.06.055. [DOI] [Google Scholar]
- 51.Chen H, Song Y, Liu N, Wan H, Shu G, Liao N. Effect of complexation conditions on microcapsulation of Lactobacillus acidophilus in xanthan-chitosan polyelectrolyte complex gels. Acta Sci Pol Technol Aliment. 2015;14(3):207–213. doi: 10.17306/J.AFS.2015.3.22. [DOI] [PubMed] [Google Scholar]
- 52.de Araújo Etchepare M, Nunes GL, Nicoloso BR, et al. Improvement of the viability of encapsulated probiotics using whey proteins. Lwt. 2019;2020(117):108601. doi: 10.1016/j.lwt.2019.108601. [DOI] [Google Scholar]
- 53.de Araújo Etchepare M, Nunes GL, Nicoloso BR, et al. Improvement of the viability of encapsulated probiotics using whey proteins. LWT. 2020;117:108601. doi: 10.1016/J.LWT.2019.108601. [DOI] [Google Scholar]
- 54.Huang S, Vignolles ML, Chen XD, et al. Spray drying of probiotics and other food-grade bacteria: a review. Trends Food Sci Technol. 2017;63:1–17. doi: 10.1016/j.tifs.2017.02.007. [DOI] [Google Scholar]
- 55.Corona-Hernandez RI, Álvarez-Parrilla E, Lizardi-Mendoza J, Islas-Rubio AR, de la Rosa LA, Wall-Medrano A. Structural stability and viability of microencapsulated probiotic bacteria: a review. Compr Rev Food Sci Food Saf. 2013;12(6):614–628. doi: 10.1111/1541-4337.12030. [DOI] [PubMed] [Google Scholar]
- 56.Pereira KC, Ferreira DCM, Alvarenga GF, Pereira MSS, Barcelos MCS, Costa JMG. Microencapsulação e liberação controlada por difusão de ingredientes alimentícios produzidos através da secagem por atomização: revisão. Brazilian J Food Technol. 2018;21:e2017083. doi: 10.1590/1981-6723.08317. [DOI] [Google Scholar]
- 57.Hosseinnia M, Khaledabad MA, Almasi H. Optimization of Ziziphora clinopodiodes essential oil microencapsulation by whey protein isolate and pectin: a comparative study. Int J Biol Macromol. 2017;101:958–966. doi: 10.1016/J.IJBIOMAC.2017.03.190. [DOI] [PubMed] [Google Scholar]
- 58.Gazme B, Madadlou A. Fabrication of whey protein–pectin conjugate particles through laccase-induced gelation of microemulsified nanodroplets. Food Hydrocoll. 2014;40:189–195. doi: 10.1016/J.FOODHYD.2014.02.017. [DOI] [Google Scholar]
- 59.Assadpour E, Jafari SM, Maghsoudlou Y. Evaluation of folic acid release from spray dried powder particles of pectin-whey protein nano-capsules. Int J Biol Macromol. 2017;95:238–247. doi: 10.1016/J.IJBIOMAC.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 60.Dziuba B, Babuchowski A, Nałecz D, Niklewicz M. Identification of lactic acid bacteria using FTIR spectroscopy and cluster analysis. Int Dairy J. 2007;17(3):183–189. doi: 10.1016/j.idairyj.2006.02.013. [DOI] [Google Scholar]