Abstract
The current research as aimed (i) to isolate and select the purple nonsulfur bacteria (PNSB) possessing the potassium-solubilizing ability from acid paddy fields and (ii) to evaluate the ability to release the plant growth-promoting substances (PGPS) of selected PNSB. A total of 35 acid sulfate (AS) soil samples were collected in An Giang province, Vietnam. Then, 70 PNSB strains were isolated from the AS soil samples. In the current study, the isolated strains were screened and selected according to their tolerability to acidic conditions, ability to solubilize potassium, and characteristics of a plant growth promoter on basic isolation media with various incubation conditions. Therein, three strains, TT07.4, AN05.1, and AC04.1, presented the highest potassium solubilization under the microaerobic light (11.8–17.7 mg L−1) and aerobic dark (16.4–24.7 mg L−1) conditions and stresses from Al3+, Fe2+, and Mn2+ toxicity. The selected strains were identified as Rhodopseudomonas pentothenatexigens by the 16S rDNA sequence, with 99% similarity. The selected acidic-resistant strains possessed the traits of biofertilizers under both microaerobic light and aerobic dark conditions, with abilities to fix nitrogen (0.17–6.24; 7.93–11.2 mg L−1); solubilize phosphorus from insoluble compounds with 3.22–49.9 and 9.49–11.2 mg L−1 for Al-P, 21.9–25.8 and 20.2–25.1 mg L−1 for Ca-P, and 10.1–29.8 and 18.9–23.2 mg L−1 for Fe-P; produce 5-aminolevulinic acid (0.63–3.01; 1.19–6.39 mg L−1), exopolymeric substances (0.14–0.76; 0.21–0.86 mg L−1), indole-3-acetic acid (12.9–32.6; 13.6–17.8 mg L−1), and siderophores (28.4–30.3; 6.15–10.3%). The selected potassium-solubilizing strains have a great potential to apply in liquid form into rice seed and solid form in AS soils to supply nutrients and PGPS for enhancing rice growth and grain yield.
Keywords: Acid sulfate soil, Biofertilizer, Metal toxicity, Plant growth-promoting substance, Potassium solubilization
Introduction
Acid sulfate (AS) soils have an area of 1.6 million ha, occupying 40% of soils in the Mekong Delta out of a total of 1.8 million ha of AS soils in Vietnam [1]. This negatively affected rice cultivation in the Mekong Delta area as AS soils are correlated with several serious constraints, including ultralow pH value, high levels of aluminum, iron, manganese toxicity, and undersupply of phosphorus (P) [2, 3]. Potassium (K) insufficiency is correlated with the formation of the oxidation product of sulfide mineral, jarosite, which acts as an infinite sink for K in the upper sulfuric horizon and decreases the amount of K that is willingly provided for plant growth [4]. Following macronutrients like nitrogen (N) and P, K is the most important nutrient that plays a crucial role in the metabolism and development of plants, improves grain quality, and decreases diseases to contribute to crop yield [5]. In normal soils for agriculture, soluble K content fluctuates from 2 to 5 mg L–1 [6]. K presents in several forms of AS soils, consisting of the mineral K, nonexchangeable K, and exchangeable K [7]. However, the exchangeable K content in AS soils is so little [8]. For example, roughly 72 % of soils require immediate K fertilizer application for good crop yield in India [9]. The majority of soil K contents was immobilized and unavailable for plants, imbalanced fertilization was applied, plant productivity went up, and K content in the soil system was inadequate; the deficiency of K contents in crops has been widely reported [10, 11].
Supplementing exchangeable K for soil is necessary to optimize the nutrition for cultivation. Commonly, to obviate this problem, chemical techniques, such as chemical fertilizers, have been widely provided in farming to increase nutrient availability and plant yield, even though this method is not ideal for sustainable agricultural systems and leads to degradation of soil structures and reduction in nutrients available in the soil [12]. As a result, a long-term solution to the serious problems associated with rice cultivation on AS soils is needed. The N and P constraints have been overcome by biological methods using purple nonsulfur bacteria (PNSB) [13, 14]. Potassium is widely recognized as one of the most essential nutrients for rice. Most rice-growing countries are becoming more aware of a balanced nutrient supply, including adequate K nutrition for rice and rice-based cropping systems, to maintain high yield through higher yields and increase in cropping intensification. The application of P fertilizer led to fixing K in soil [8, 15]. The bacteria can release K from inorganic and insoluble forms of soil K via K solubilization [16]. It has been reported that inoculation with K-solubilizing bacteria positively affected different plants’ growth [8, 11, 17]. Thus, plants might not be able to absorb a part of the K pool. Several bacteria, namely, Bacillus cereus, Paenibacillus mucilaginosus, and Burkholderia sp., can solubilize K minerals in soils [10, 18–20] by various mechanisms.
Bacteria produce different organic acids, and inorganic acids (H+ production), which release K from the minerals and become available to the plants. It can be named as acidolysis mechanism [21], chelation, exchange reactions, and complexolysis mechanism [22]. Mechanisms may rely on various organic acids, including oxalic acid, tartaric acids, and gluconic acid, which have been reviewed by Etesami et al. [23] and found in bacteria that can release soluble K from K-fixed minerals. Moreover, applying selected K-solubilizing bacteria could dissolve K forms, enhancing growth and yield [5, 24]. The promotion of plant growth and yield by K-solubilizing bacteria has been widely concerned in many studies. For instance, an inoculation of seeds and seedlings of different plants with strains of K-solubilizing bacteria significantly increased the germination rate, seedling vigor, growth and yield of crops, and K uptake by plants under greenhouse and field conditions [23].
Purple nonsulfur bacteria are usually found in various habitats, including AS soils [25]. They possess a variety of functions as biofertilizers and plant growth promoters by fixing N2, solubilizing P, and producing plant growth-promoting substances (PGPS); exopolymeric substances (EPS), siderophores, 5-aminolevulinic acid (ALA), and indole-3-acetic acid (IAA) [13, 14, 26, 27]. These compounds supported the enhancements in rice growth under stress conditions [13, 28–30]. For example, ALA-producing PNSB improved rice growth under saline conditions [30]. However, studies on K nutrients production by PNSB are limited. Some previous studies showed that PNSB, R. palustris G5, and R. palustris RP1n1, could solubilize K, but its quantity has not been reported [31, 32]. Moreover, the efficacy of PNSB on mineral K dissolving in AS soils has not been studied. Moreover, Pandey et al. [5] suggested that future research should concentrate on isolating and applying indigenous K solubilizers to plants from various environmental conditions, such as nutrient-deficient soils and acidic stress, to improve the availability of K for plants. In particular, their ability to produce PGPS under conditions of Al3+, Fe2+, and Mn2+ in AS soils. This was due to the metal ions can adversely affect the soil microbes in many ways, including a reduction in total microbial biomass [33] and in numbers of specific populations [34] and changes in the microbial population structure [35]. Mechanisms via which those beneficial bacteria survive under harsh conditions may include keeping metal ions away from the sites, pushing out metal ions from cells, forming complexes with metal ions by metal-binding proteins or other cell components, reducing toxicities of metal ions, and methylating and demethylating metal ions [36].
Ultimately, the current research was conducted aiming to isolate and select the K-solubilizing PNSB from acid paddy fields based on their abilities to provide crops with nutrients (N, P, and K) and PGPS (ALA, EPS, IAA, and siderophores) for an ultimate goal of the sustainable agricultural system.
Materials and methods
Study areas and sample collecting
The soil samples collected from acidic paddy fields in Tri Ton and Tinh Bien districts, An Giang province, Vietnam, were used to isolate PNSB to possibly obtain strains that resist acidic conditions. The soil samples, near rice rhizosphere, were collected in the summer-autumn season 45–50 days after sowing. Each soil sample was taken from 13 different locations at a depth of 10 cm on top of the horizontal soil surface in each field to obtain a representative sample. Samples were pooled to obtain at least 500 g of soil. Polyethylene bags/bottles with low oxygen diffusion rates and no headspace were used to preserve shifts in soil redox potential. Prior to use for the research, all samples were held in a refrigerator at 4 °C. In this research, the nonexchangeable K source is potassium aluminum silicate (AlKO6Si2).
Analysis of soil physicochemical properties
The collected soil samples were analyzed for the soil physicochemical properties as follows. Soil samples were determined separately by using 1.0 M KCl (pHKCl) or deionized water (pHH2O) at a ratio of 1:5 for soil:solvent for pH and electrical conductivity (EC) measurements. Particularly, dried soil samples (1 g) were mixed vigorously in 5 mL deionized water/5 mL 1.0 M KCl. The pH of the supernatant was measured by a pH meter and an EC meter (Thermo Fisher Scientific Inc., Franklin, MA, USA). In general, the pHH2O value is used to determine actual acidity. In contrast, pHKCl is used to evaluate AS soils’ exchangeable acidity since pyritic minerals in the soils were oxidized to form H2SO4 to change from an Al-soil to the H-saturated soil and dissolve clay minerals by exchangeable H+ ion [37]. Soil texture was analyzed by Robinson’s pipette method to determine granulometric fraction [38]. The dichromate oxidation procedure by thermal conductivity technique of sulfuric acid was used to convert organic carbon compounds to inorganic carbon before titrate with ferrous sulfate heptahydrate for total carbon determination, which was originally proposed by Walkley and Black [39].
Isolation of PNSB from soil samples
The PNSB isolation was performed using a basic isolation medium (BIM) [40]. The medium contains 1.0 g (NH4)2SO4, 0.5 g K2HPO4, 0.2 g MgSO4, 2.0 g NaCl, 5.0 g NaHCO3, 1.5 g yeast extract, 1.5 g glycerol, and 0.03 g l-cysteine in 1 L of distilled water; and the solid medium was made with the addition of 1.5% agar. The pH of the liquid medium was adjusted with sterile 1 M HCl via 0.45-μm filter (Durapore® PVDF membrane, Merck Millipore Ltd.) to reach the pH value at 5.0, which is equivalent to the actual AS conditions [25]. However, the agar and solution were autoclaved separately before mixing with sterilized 1 M HCl solution.
To isolate PNSB, 1.0 g of soil from each sample was added to the 15-mL test tubes containing 10-mL BIM broth. Then, sterilized liquid paraffin was added to the medium at 1 cm in height to achieve anaerobic conditions for 1 week. All sample test tubes were statically incubated under continuous light using tungsten bulbs with light intensities ranging from 3000 to 4000 lux (E27, 60W, 220 V~50 Hz). Then, any test tubes whose colors turned red, pink, or brown were used to isolate PNSB by streaking on BIM agar plates; normally, re-streaking was required to obtain pure cultures. All plates were incubated in anaerobic conditions using an anaerobic jar and gas-pak. After Gram staining, each colony was examined under a light microscope to determine its purity. The pure colonies were then maintained at 4 °C until used, preserved in a 20% glycerol solution, and stored at − 80 °C.
Selection of PNSB strains in acidic conditions
Each culture was subcultured twice in BIM broth, pH 7.0, to obtain active culture, and the OD660 of each culture broth was measured using a spectrophotometer at 660 nm. The optical density of each culture was modified to 0.5 by diluting with BIM broth before doing experiments; i.e., the bacteria density was roughly 105 CFU mL−1, at which the bacteria could propagate well [41]. The isolated PNSB strains were tested under both microaerobic light and aerobic dark conditions. Then, 10% of each sample was inoculated into test tubes (15 × 150 mm) containing 9.0 mL BIM broth, pH 7.0. Each culture tube with a small amount of headspace for microaerobic light conditions rapidly became anaerobic with light intensities ranging from 3,000 to 4,000 lux (E27, 60 W, 220V ~ 50Hz). On the other hand, 10% of each sample incubated in aerobic dark conditions was performed in the screw cap test tube (25 × 150 mm in size, 50 mL) containing 18 mL of BIM broth and shaken in a shaking incubator (model: WIS-10RL, WiseCube), which can control the rate and temperature at 150 rpm, 30 °C. The bacterial growth of all culture tubes was measured via a spectrophotometer at the wavelength of 660 nm after 72 h of incubation. Any PNSB isolates that grew faster than 1.0 optical density under both incubation conditions were investigated for further screening to obtain acid-resistant strains.
As mentioned previously, a 10% inoculum size of the selected PNSB from the primary screening was inoculated to the medium with a pH value of 4.5 and incubated for 72 h under both incubating conditions. Any isolates that could grow in BIM broth, pH 4.5, with OD660 over 1.0 under microaerobic light and over 0.5 under aerobic dark conditions were selected for further tests.
Screening of acting as biofertilizer and plant growth promoter
Ability to fix N2
The BIM broth, pH 4.5, that excluded (NH4)2SO4 was used for testing the nitrogen-fixing ability of PNSB. They were grown under both incubating conditions. After 3 days of incubation, culture broths were centrifuged at 8000 rpm for 15 min. The culture supernatants were used to detect NH4+ concentration by the blue phenol colorimetric method using a spectrophotometer at 640 nm [42]. The uninoculated medium served as a control.
Ability to solubilize phosphorus
Inoculums of PNSB were prepared in BIM broth, pH 4.5, under microaerobic light conditions as in previous experiments. Modified liquid BIM without K2HPO4, pH 5.0, and the supplementation of individual insoluble P forms, 0.5 g AlPO4°2H2O L−1, 0.5 g FePO4°2H2O L−1, or 0.5 g Ca3(PO4)2 L−1, were used to test P solubilization. A 10% inoculant of each strain was inoculated into 9 mL of modified BIM and incubated under both incubates for 3 days. The released P concentration from insoluble P compounds by tested PNSB was measured by the ascorbic acid method [43]. Briefly, culture supernatants were centrifuged at 10,000 rpm for 15 min. The absorbance of the solution was detected at the wavelength of 880 nm by the color development of molybdenum blue via the reaction of the reagents of ammonium molybdate and antimony potassium tartrate with orthophosphate to form phosphomolybdic acid. The uninoculated PNSB tube was used as the control treatment.
Ability to produce ALA, EPS, IAA, and siderophores
As in previous experiments, each PNSB inoculum was prepared in BIM broth, pH 4.5, under microaerobic light conditions. Determining the ability to release IAA followed the protocol for growing PNSB and culture supernatant collection, similar to the procedure for measuring NH4+. However, in this case, the modified BIM broth, included with 100 mg L−1 tryptophan, was used as a precursor for IAA production. On day 2 of incubation, culture supernatants were used to determine IAA content using Salkowski’s colorimetric technique. Shortly, after mixing a 0.75 mL aliquot of culture supernatant and 3 mL of Salkowski’s reagent (4.5 g of FeCl3 per liter in 10.8 M H2SO4), the mixture was incubated for 20 min at room temperature and the IAA concentration was quantified at the wavelength of 535 nm [44]. The control treatment was BIM broth.
Determination of ALA production by PNSB strains followed a similar procedure to the IAA production measurement, but the BIM broth contained precursors of 0.563 g glycine L−1 and 5.44 g sodium acetate L−1. After 2-day incubation, the ALA concentration was analyzed using the spectrophotometric method described by Burnham [45]. Each culture broth was centrifuged at 8000 rpm for 15 min, and a mixture of 2 mL sodium acetate (1 M, pH 4.70) and 0.05 mL acetylacetone was decanted into test tubes containing 1 mL of centrifuged broth. The mixture was heated in a boiling water bath for 15 min, and after cooling, 3.5 mL of modified Ehrlich’s reagent was added to each test tube. After 20 min, ALA concentration was measured at a wavelength of 553 nm.
The extraction of soluble EPS was modified from the protocol by Eboigbodin and Biggs [46]. In short, to precipitate soluble EPS, culture supernatant and cold ethanol (4 °C) were mixed at a ratio of 1: 2.2 and incubated at – 20 °C for 24 h. The suspension was centrifuged at 8000 rpm for 15 min at 4 °C to separate EPS. The dry weight of EPS was calculated as described by Ferreira et al. [47].
Siderophores production was determined by the universal method [48]. Each PNSB was grown under both investigated conditions, including microaerobic light and aerobic dark conditions, and then centrifuged at 8000 rpm for 15 min to manufacture culture supernatants, and then, siderophores were measured at 630 nm using a mixture of 1.0 mL culture supernatant and 1.0 mL chrome azurol S (CAS) assay solution. After 3 days of incubation, the quantity of released siderophores was determined. In brief, 1.0 mL of supernatant was mixed with 1.0 mL chrome azurol S (CAS) assay solution, and siderophore concentration was detected at a wavelength of 630 nm. Uninoculated medium broth with CAS solution was used as the control treatment. The generation of siderophores was measured according to the reduction in blue color, expressed as percent siderophore units (SU): SU (percent) = (Ar − As)/Ar × 100, where Ar is the reference absorbance at 630 nm and As is the sample absorbance at 630 nm.
Selection of PNSB strains for potassium solubilization and toxicity tolerance
Selection of Al3+-, Mn2+-, and Fe2+-resistant PNSB in acidic conditions
Under microaerobic light and aerobic dark conditions, acid-resistant PNSB strains were further selected for their resistance to Al3+, Fe2+, and Mn2+. According to Attanandana and Vacharotayan [49], the toxicity threshold of aluminum to cause rice plant death was 68 mg Al3+ L−1 in soil solution, and rice was stressed at iron and manganese concentrations at 250 mg L−1 [49] and 1000 mg L−1 [50], respectively. The threshold concentrations of those metal ions for rice were used to design screening steps according to their frequencies found in AS soils [51] and applied to BIM broth with a pH value of 4.5. Chemical compounds used were AlCl3°6H2O, FeSO4°7H2O, and MnCl2°2H2O, as sources of Al3+, Fe2+, and Mn2+, respectively. They were sterilized separately by passing through 0.45-μm pore size membrane filters and added to the sterile BIM broth at the above concentrations. An inoculum size of 10% of each PNSB was inoculated into the medium and incubated for 3 days under both microaerobic light and aerobic dark conditions. Bacterial growth was measured using a spectrophotometer at a wavelength of 660 nm. The growth inhibition was calculated in percent by comparing the differences in growth between toxicants BIM broth and a nontoxicant medium with no supplementation of Al, Fe, and Mn toxicants.
Selection of PNSB strains for potassium solubilization
The BIM broth excluding K2HPO4, at pH 4.5, with supplementation of 0.5 g AlKO6Si2 L−1 was suggested to determine the K solubilization ability of isolated strains. Each inoculum was prepared in BIM broth, pH 4.5, and incubated under both incubating conditions for 72 h. Each inoculum was adjusted to 0.50 at a wavelength of 660 nm for investigating the K solubilization. Moreover, 10% of each inoculum was taken into 9 mL of the modified BIM broth in test tubes as previously described for conditions of microaerobic light or aerobic dark. The uninoculated modified BIM broth served as the control treatment. After 72-h incubation, 2 mL of each sample was centrifuged at 8000 rpm for 5 min, and culture supernatant was used to measure K concentration by the atomic absorption spectrophotometry at a wavelength of 766.5 nm [52]. The results in the tables were derived from differences between the control and other treatments. Therefore, the concentrations of K produced in the control treatment were zero compared to the results of the other treatments presented in the current study.
Identification of selected PNSB
The PNSB strains, which were chosen based on their acid, Al, Fe, and Mn resistances, and can act as plant growth promoters, were identified using the 16S rDNA sequence analysis. They were grown in BIM broth, pH 7.0, for 48 h under microaerobic light conditions. Moreover, 2 mL of each culture was centrifuged for 5 min at 10,000 rpm to obtain a cell pellet and used for DNA extraction (Genomic DNA Prep Kit, BioFACTTM). The genomic DNA samples were resolved using 1.0% w/v agarose gel electrophoresis and checked under the UV transilluminator to test the concentration and purity. To amplify the observed 16S rDNA sequence, a polymerase chain reaction (PCR) was performed using a pair of primers: 16S Forward Primer, 8F (5′-AGA GTT TGA TCC TGG CTC AG-3′); 16S reverse primer, 1492R (5′-GGT TAC CTT GTT ACG ACT T-3′) [53]. The process was described in iProof™ High-Fidelity PCR Kit (BioRad, Hercules, CA) via a T100TM thermocycler (BioRad). The thermal-cycling process of the PCR technique was adjusted based on the following protocol: the DNA was denatured at 95 °C, annealed at 55 °C, and elongated at 72 °C. The initial DNA denaturation lasted 5 min and the final extension lasted 10 min. There were 30 cycles of denaturation, 30 s long; annealing, 30 s long; and extension, 2 min long. After being amplified, PCR products were confirmed by DNA markers through the electrophoresis method using 1.0% w/v agarose gel and 1× TAE buffer and later examined under the UV transilluminator. They were then purified using a TIANquick Midi Purification Kit (Tiangen Biotech Ltd., Beijing, China) as described in the manufacturer’s guide.
After the purification step, PCR products were analyzed by an automated DNA sequencer at Macrogen DNA Sequencing Service (Macrogen, Seoul, Korea). The sequencing results and chromatograms were imported BioEdit, version 7.0.5.3 [54], and ChromasPro, version 1.7, for further investigation. A comparison was performed to determine the most similar sequence with the observed one using the available sequences in the GenBank database of the National Center for Biotechnology Information (NCBI). The neighbor-joining phylogenetic tree was rebuilt by using MEGA software, version 6.06 [55], after the multiple sequence alignments were performed through the CLUSTALW program, wherein the evolutionary distance matrix was estimated by using the Jukes–Cantor model and topologies of the neighbor-joining trees were calculated by using the bootstrap resampling method based on 1000 replicates.
Statistical analysis
The data shown in this article are mean values of three replications unless otherwise stated. The data were used to perform a one-way analysis of variance by using SPSS software, version 13.0. Means were separated by analysis of variance (ANOVA), and the significant differences were verified using Duncan’s post hoc test with p ≤ 0.05.
Results
Physicochemical properties of soil samples for PNSB isolation
The range of soil pHH2O values fluctuated from 4.01 to 7.25, while the soil pHKCl showed lower values, 3.55–6.25, in all surveyed sites. The electrical conductivity (EC) value in soil samples ranged from 196 to 1703 μS cm−1 (Table 1). Particularly, the pH of soil samples in the Tri Ton district was 4.01–6.69, while in the Tinh Bien district was 4.71–7.25. Similarly, the potential acidity of the soil extracted with 1.0 M KCl solution also had low average values in the Tri Ton and Tinh Bien areas, approximately 3.55–5.60, assessed at low-to-moderate acidity. The average EC conductivity in soil was determined from 120 to 1703 mS cm−1; the highest value was recorded in Tan Tuyen commune, in Tri Ton district, and the lowest was 120 mS cm−1 in two An Hao communes, Tinh Bien district. All soils from the five locations shared equivalent organic matter values, ranging from 2.99 to 7.21% C. Other parameters, such as NH4+ content, available P content, K+, and soil texture (clay, silt, and sand), also appeared with a similar pattern and were recorded in Table 1.
Table 1.
Physicochemical properties of soil samples collected from acidic paddy fields in Tri Ton and Tinh Bien districts, An Giang province, Vietnam
| Code sample | pHH2O | pHKCl | EC (μS cm−1) | NH4+ (mg kg−1) | P available (mg kg−1) | K+ (meq 1000 g−1) | OM (%C) | Soil texture (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Clay | Silt | Sand | ||||||||
| Tan Tuyen commune in Tri Ton district | ||||||||||
| TT01 | 6.39 ± 0.12 | 5.35 ± 0.07 | 468 ± 5.26 | 33.6 ± 1.13 | 15.6 ± 0.32 | 0.56 ± 0.14 | 4.21 ± 0.16 | 48.9 ± 0.56 | 49.9 ± 1.25 | 1.21 ± 0.09 |
| TT02 | 5.80 ± 0.11 | 4.57 ± 0.01 | 629 ± 7.20 | 31.2 ± 1.21 | 7.56 ± 0.27 | 0.47 ± 0.06 | 7.21 ± 0.12 | 49.2 ± 0.44 | 49.5 ± 1.22 | 1.33 ± 0.01 |
| TT03 | 6.29 ± 0.08 | 4.76 ± 0.10 | 392 ± 3.16 | 35.1 ± 1.65 | 12.8 ± 0.94 | 0.83 ± 0.08 | 3.16 ± 0.14 | 47.8 ± 0.33 | 51.1 ± 0.87 | 1.09 ± 0.03 |
| TT04 | 4.87 ± 0.14 | 4.32 ± 0.06 | 1444 ± 8.22 | 23.5 ± 0.67 | 5.98 ± 1.67 | 0.46 ± 0.14 | 4.17 ± 0.11 | 50.1 ± 0.62 | 48.7 ± 0.65 | 1.16 ± 0.08 |
| TT05 | 6.69 ± 0.22 | 5.33 ± 0.17 | 334 ± 5.66 | 36.9 ± 1.24 | 16.3 ± 1.25 | 0.44 ± 0.22 | 5.26 ± 0.13 | 49.6 ± 0.37 | 49.1 ± 0.24 | 1.32 ± 0.06 |
| TT06 | 5.30 ± 0.14 | 4.33 ± 0.08 | 548 ± 3.88 | 28.6 ± 1.41 | 5.22 ± 1.46 | 0.39 ± 0.19 | 3.48 ± 0.15 | 51.2 ± 0.11 | 47.6 ± 0.37 | 1.24 ± 0.11 |
| TT07 | 4.60 ± 0.09 | 4.30 ± 0.11 | 1188 ± 5.66 | 22.3 ± 0.09 | 4.65 ± 1.74 | 0.44 ± 0.16 | 4.65 ± 0.14 | 48.9 ± 1.23 | 49.7 ± 0.88 | 1.41 ± 0.07 |
| TT08 | 5.07 ± 0.17 | 4.33 ± 0.14 | 1703 ± 1.88 | 25.7 ± 0.17 | 8.97 ± 1.62 | 0.56 ± 0.11 | 3.54 ± 0.13 | 52.6 ± 0.34 | 46.2 ± 0.79 | 1.16 ± 0.08 |
| TT09 | 6.01 ± 0.06 | 5.60 ± 0.22 | 390 ± 2.56 | 28.3 ± 0.26 | 6.98 ± 1.23 | 0.43 ± 0.12 | 5.26 ± 0.17 | 47.9 ± 0.89 | 50.8 ± 0.69 | 1.28 ± 0.13 |
| TT10 | 5.43 ± 0.04 | 4.44 ± 0.16 | 860 ± 7.20 | 24.6 ± 0.33 | 4.15 ± 0.99 | 0.48 ± 0.17 | 4.22 ± 0.16 | 46.8 ± 0.32 | 51.9 ± 3.12 | 1.27 ± 0.14 |
| Ta Danh commune in Tri Ton district | ||||||||||
| TD01 | 5.40 ± 0.11 | 4.36 ± 0.14 | 196 ± 3.69 | 23.6 ± 0.24 | 6.32 ± 0.14 | 0.27 ± 0.24 | 5.62 ± 0.17 | 50.1 ± 0.21 | 48.3 ± 0.22 | 1.56 ± 0.12 |
| TD02 | 5.34 ± 0.07 | 4.34 ± 0.08 | 390 ± 2.58 | 22.7 ± 0.16 | 7.11 ± 0.61 | 0.35 ± 0.23 | 5.14 ± 0.46 | 47.8 ± 0.56 | 50.9 ± 2.98 | 1.33 ± 0.23 |
| TD03 | 4.01 ± 0.05 | 3.55 ± 0.04 | 504 ± 5.22 | 31.4 ± 0.18 | 8.20 ± 1.23 | 0.46 ± 0.15 | 5.28 ± 0.18 | 50.6 ± 0.47 | 48.1 ± 1.76 | 1.28 ± 0.21 |
| TD04 | 4.62 ± 0.05 | 4.66 ± 0.26 | 406 ± 5.09 | 25.9 ± 0.21 | 6.44 ± 0.14 | 0.35 ± 0.08 | 6.33 ± 0.29 | 52.8 ± 0.21 | 45.8 ± 1.86 | 1.42 ± 0.25 |
| TD05 | 4.59 ± 0.20 | 3.88 ± 0.17 | 392 ± 4.58 | 26.4 ± 0.17 | 5.66 ± 0.22 | 0.39 ± 0.14 | 4.35 ± 0.16 | 54.6 ± 1.13 | 44.0 ± 0.73 | 1.36 ± 0.17 |
| TD06 | 4.75 ± 0.16 | 3.84 ± 0.32 | 504 ± 5.20 | 23.4 ± 0.98 | 5.81 ± 0.17 | 0.44 ± 0.03 | 6.22 ± 0.22 | 50.7 ± 0.20 | 48.1 ± 0.47 | 1.24 ± 0.06 |
| TD07 | 4.54 ± 0.18 | 4.06 ± 0.14 | 680 ± 5.69 | 22.5 ± 2.46 | 8.96 ± 0.19 | 0.41 ± 0.07 | 5.24 ± 0.17 | 49.6 ± 0.17 | 48.8 ± 0.99 | 1.56 ± 0.07 |
| TD08 | 4.28 ± 0.12 | 4.10 ± 0.24 | 758 ± 4.58 | 29.7 ± 2.98 | 6.35 ± 0.11 | 0.36 ± 0.25 | 3.89 ± 0.26 | 47.8 ± 0.21 | 51.0 ± 1.54 | 1.24 ± 0.11 |
| TD09 | 4.91 ± 0.07 | 4.19 ± 0.20 | 662 ± 5.22 | 28.1 ± 1.21 | 6.22 ± 0.07 | 0.38 ± 0.11 | 2.99 ± 0.17 | 49.6 ± 0.17 | 49.0 ± 1.12 | 1.37 ± 0.09 |
| TD10 | 5.22 ± 0.08 | 4.56 ± 0.16 | 520 ± 2.11 | 22.6 ± 0.46 | 7.14 ± 0.06 | 0.51 ± 0.26 | 3.14 ± 0.11 | 56.4 ± 0.30 | 42.2 ± 0.78 | 1.42 ± 0.09 |
| An Cu commune in Tinh Bien district | ||||||||||
| AC01 | 6.45 ± 0.16 | 6.25 ± 0.09 | 616 ± 4.22 | 34.6 ± 3.63 | 10.2 ± 1.13 | 0.51 ± 0.01 | 3.22 ± 0.08 | 50.6 ± 0.27 | 47.8 ± 0.65 | 1.63 ± 0.14 |
| AC02 | 7.22 ± 0.09 | 5.93 ± 0.11 | 288 ± 10.8 | 38.7 ± 2.45 | 9.88 ± 0.88 | 0.42 ± 0.06 | 5.46 ± 0.14 | 48.5 ± 0.21 | 49.9 ± 0.21 | 1.58 ± 0.08 |
| AC03 | 6.18 ± 0.12 | 5.52 ± 0.22 | 446 ± 5.60 | 34.1 ± 1.74 | 12.6 ± 0.34 | 0.43 ± 0.11 | 5.27 ± 0.12 | 49.1 ± 0.28 | 49.3 ± 0.87 | 1.64 ± 0.06 |
| AC04 | 6.28 ± 0.28 | 4.75 ± 0.17 | 273 ± 1.88 | 36.2 ± 2.14 | 13.4 ± 0.36 | 0.43 ± 0.11 | 5.28 ± 0.08 | 46.9 ± 0.23 | 51.5 ± 4.12 | 1.64 ± 0.05 |
| AC05 | 7.24 ± 0.08 | 5.25 ± 0.19 | 168 ± 1.58 | 38.5 ± 2.56 | 11.4 ± 0.47 | 0.38 ± 0.10 | 4.56 ± 0.16 | 52.3 ± 0.33 | 46.2 ± 2.16 | 1.47 ± 0.11 |
| An Hao commune in Tinh Bien district | ||||||||||
| AH01 | 4.71 ± 0.16 | 4.56 ± 0.17 | 174 ± 5.94 | 15.6 ± 1.78 | 5.64 ± 0.14 | 0.45 ± 0.08 | 3.97 ± 0.21 | 51.6 ± 1.56 | 47.1 ± 0.66 | 1.32 ± 0.03 |
| AH02 | 6.10 ± 0.27 | 5.06 ± 0.12 | 120 ± 3.65 | 30.2 ± 1.36 | 7.68 ± 0.12 | 0.52 ± 0.14 | 3.92 ± 0.16 | 54.2 ± 1.54 | 44.4 ± 0.17 | 1.44 ± 0.07 |
| AH03 | 6.71 ± 0.23 | 4.86 ± 0.16 | 136 ± 3.22 | 36.0 ± 1.54 | 8.95 ± 0.35 | 0.53 ± 0.16 | 5.08 ± 0.13 | 53.9 ± 2.13 | 44.5 ± 3.11 | 1.62 ± 0.06 |
| AH04 | 6.30 ± 0.10 | 5.52 ± 0.14 | 280 ± 2.80 | 33.4 ± 1.26 | 9.24 ± 0.42 | 0.44 ± 0.14 | 4.87 ± 0.17 | 54.6 ± 0.76 | 44.2 ± 2.82 | 1.25 ± 0.18 |
| AH05 | 6.78 ± 0.15 | 4.72 ± 0.08 | 252 ± 2.56 | 39.2 ± 1.41 | 8.15 ± 0.12 | 0.58 ± 0.12 | 4.09 ± 0.14 | 54.1 ± 0.51 | 44.3 ± 5.14 | 1.56 ± 0.06 |
| An Nghiep commune in Tinh Bien district | ||||||||||
| AN01 | 6.44 ± 0.14 | 4.88 ± 0.20 | 156 ± 2.54 | 28.7 ± 1.32 | 11.3 ± 0.17 | 0.38 ± 0.17 | 6.05 ± 0.23 | 48.7 ± 2.69 | 49.9 ± 1.78 | 1.42 ± 0.14 |
| AN02 | 6.35 ± 0.02 | 5.46 ± 0.14 | 488 ± 5.11 | 27.2 ± 1.75 | 12.7 ± 0.32 | 0.39 ± 0.21 | 4.56 ± 0.20 | 46.3 ± 1.54 | 52.3 ± 2.14 | 1.38 ± 0.12 |
| AN03 | 5.01 ± 0.06 | 4.95 ± 0.15 | 128 ± 1.42 | 20.8 ± 1.65 | 9.21 ± 0.21 | 0.37 ± 0.27 | 4.33 ± 0.16 | 48.2 ± 0.88 | 50.5 ± 3.16 | 1.29 ± 0.14 |
| AN04 | 7.25 ± 0.18 | 5.75 ± 0.23 | 156 ± 2.56 | 25.4 ± 2.56 | 16.8 ± 0.16 | 0.41 ± 0.17 | 5.33 ± 0.09 | 47.2 ± 0.65 | 51.3 ± 0.78 | 1.55 ± 0.16 |
| AN05 | 5.18 ± 0.17 | 4.81 ± 0.14 | 154 ± 5.24 | 21.2 ± 2.41 | 8.67 ± 0.17 | 0.44 ± 0.21 | 6.07 ± 0.16 | 47.3 ± 0.47 | 51.2 ± 0.99 | 1.48 ± 0.11 |
Isolation and selection of PNSB from soil samples
There were 70 PNSB strains isolated from soil samples in 35 acidic paddy fields in Tri Ton and Tinh Bien districts, An Giang Province, Vietnam. They were able to grow in BIM, pH 7.0, under both microaerobic light and aerobic dark conditions. They are Gram-negative bacteria with a diameter of colonies ranging from 0.5 to 2.0 mm, rod-shaped cells, and motility. Among them, 88.6% had red colonies and 11.4% had brown colonies. All 70 PNSB strains were used to determine the acidic-resistant ability.
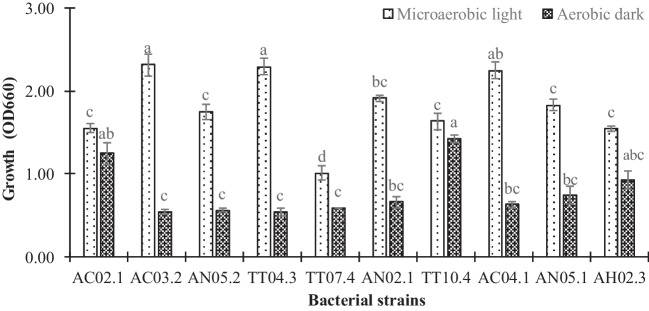
Acidic resistance of isolated PNSB based on their growth in BIM, pH 4.5, after 72-h incubation (OD660 > 1.0 in microaerobic light conditions and > 0.5 in aerobic dark conditions) found only 10 strains that could grow well. Only these, including AC02.1, AC03.2, AC04.1, AN02.1, AN05.1, AN05.2, AH02.3, TT04.3, TT07.4, and TT10.4, resulted in OD660 values over 1.0, which indicated excellent growth under acidic condition. This condition was similar to acidic soil properties, which increases their efficiency in field applications. The average value at OD660 was 1.81 in microaerobic light conditions and 0.78 in aerobic dark conditions. Those 10 of 70 PNSB isolates were selected for further tests (Fig. 1).
Fig. 1.
Growth of selected purple nonsulfur bacteria in basic isolation medium (BIM), pH 4.5 under conditions of microaerobic light and aerobic dark. Different lowercase letters in each bar indicate significant differences among strains under both incubating conditions
Evaluation of isolated PNSB based on producing plant growth-promoting substances under acidic condition
Acidic-resistant PNSB for acting as biofertilizers by fixing N2
A total of 10 strains were capable of growing under high acidity at pH 4.5 and toxic conditions of Al3+, Fe2+, and Mn2+. They had the ability to fix N2 in BIM broth, pH 4.5, and excluded a chemical compound containing N (Table 2). Under microaerobic light conditions, the released NH4+ content by tested PNSB strains ranged from 0.17 to 6.24 mg L−1. Strain TT07.4 performed the highest N2 fixation capacity. For aerobic dark conditions, the N2 fixation capacity of tested strains ranged from 6.15 to 13.6 mg L−1. Strains AC02.1 and AC04.1 achieved the highest content with NH4+ values of 13.6 mg L−1 and 11.2 mg L−1, respectively (Table 2).
Table 2.
Concentrations of ammonium from N2 fixation and soluble phosphorus from various P-sources by selected purple nonsulfur bacteria in basic isolation medium broth, pH 4.5 under microaerobic light and aerobic dark conditions
| Strain | NH4+ (mg L−1) | Al-P (mg L−1) | Ca-P (mg L−1) | Fe-P (mg L−1) | ||||
|---|---|---|---|---|---|---|---|---|
| ML | AD | ML | AD | ML | AD | ML | AD | |
| AC02.1 | 4.54b ± 0.48 | 13.6a ± 0.34 | 2.89e ± 0.10 | 11.1ab ± 0.00 | 28.1abc ± 1.53 | 15.6ef ± 0.19 | 20.7d ± 1.42 | 14.9e ± 3.29 |
| AC03.2 | 3.22c ± 0.65 | 9.27bc ± 0.62 | 19.9c ± 5.07 | 10.7ab ± 0.33 | 29.9a ± 0.45 | 16.5e ± 0.64 | 1.53jh ± 1.10 | 14.9e ± 1.31 |
| AN05.2 | 3.48c ± 0.00 | 10.6b ± 0.22 | 3.37e ± 0.10 | 12.5a ± 0.33 | 28.9ab ± 0.83 | 11.6g ± 1.40 | 33.0a ± 0.99 | 37.4a ± 2.93 |
| TT04.3 | 3.00c ± 0.29 | 6.24cd ± 0.14 | 7.77de ± 4.30 | 10.7ab ± 1.67 | 21.9e ± 0.83 | 24.0ab ± 0.64 | 26.0c ± 2.01 | 17.8cd ± 1.39 |
| TT07.4 | 6.24a ± 0.26 | 7.93bcd ± 0.10 | 3.22e ± 0.05 | 9.49b ± 0.29 | 23.4de ± 2.93 | 25.1a ± 1.98 | 18.8e ± 0.37 | 23.2b ± 0.95 |
| AN02.1 | 1.27d ± 0.48 | 8.86bcd ± 0.50 | 3.03e ± 0.05 | 7.67c ± 1.53 | 28.8ab ± 1.72 | 19.8d ± 0.51 | 3.02j ± 0.36 | 17.7cd ± 1.31 |
| TT10.4 | 0.31d ± 0.10 | 6.15d ± 0.43 | 26.3b ± 0.62 | 6.91c ± 1.72 | 21.9e ± 0.00 | 22.7bc ± 0.32 | 10.1f ± 0.62 | 18.9c ± 0.07 |
| AC04.1 | 0.31d ± 0.10 | 11.2a ± 0.76 | 27.0b ± 6.07 | 11.2ab ± 1.10 | 25.8cd ± 0.51 | 20.2d ± 0.26 | 29.8b ± 0.55 | 22.4b ± 0.29 |
| AN05.1 | 0.17d ± 0.00 | 8.98bcd ± 0.05 | 49.9a ± 5.50 | 9.82b ± 0.14 | 24.1de ± 1.34 | 21.5cd ± 1.98 | 0.88h ± 0.51 | 15.9de ± 0.66 |
| AH02.3 | 0.50d ± 0.10 | 7.11cd ± 0.82 | 12.0d ± 1.77 | 10.4b ± 0.62 | 27.0bc ± 0.57 | 13.6fg ± 2.36 | 3.43j ± 1.61 | 18.3cd ± 0.44 |
| Significant differences | * | * | * | * | * | * | * | * |
Values are means ± their standard deviations (n = 3). Different lowercase letters in each column indicate significant differences at P < 0.05 (*)
PNSB for acting as biofertilizers by solubilizing phosphorus
Ability to solubilize Al-P
The potential to dissolve P from the Al-P compound under microaerobic light conditions ranged from 2.89 to 49.9 mg L−1, and strain AN05.1 was the most effective to dissolve P concentration (Table 2). While under aerobic dark conditions, it ranged from 6.91 to 12.5 mg L−1, with the highest concentration found in strain AN05.2 (Table 2). Under both incubating conditions, strains AN05.2 and AN05.1 obtained the highest soluble concentration from the Al-P compound.
Ability to solubilize Ca-P
The dissolved P concentration from the Ca-P compound ranged from 9.50 to 17.5 mg L−1 under microaerobic light conditions, while under aerobic dark conditions was in a range of 11.6–25.1 mg L−1, with highest P producing PNSB strains in AC03.2 and TT07.4, respectively (Table 2).
Ability to solubilize Fe-P
The results showed that the P solubilization from Fe-P by tested isolates ranged from 0.88 to 33.0 mg L−1 under microaerobic light conditions, and it ranged from 14.9 to 37.4 mg L−1 under aerobic dark conditions (Table 2). Strains AN05.2 and AC04.1 ranked first and second in dissolved Fe-P content in both microaerobic light and aerobic dark conditions, respectively.
Ability to release plant growth-promoting substances by PNSB
Exopolymeric substances (EPS)
There was a large range in the amount of EPS produced by the tested PNSB (Table 3). In detail, PNSB produced EPS ranging from 0.19 to 0.76 mg L−1 and from 0.21 to 0.92 mg L−1 under microaerobic light and aerobic dark conditions, respectively. Strains AN05.2 and AC04.1 secreted the highest amount of EPS, significantly different from other strains under both incubating conditions: 0.66 mg L−1 and AC04.1 0.76 mg L−1 under microaerobic light conditions and 0.92 and 0.86 mg L−1 under aerobic dark conditions.
Table 3.
Productions of 5-aminolevulinic acid (ALA), exopolymeric substances (EPS), indole-3-acetic acid (IAA), and siderophores by selected purple nonsulfur bacteria in basic isolation medium broth, pH 4.5 under microaerobic light, and aerobic dark conditions
| Strain | ALA (mg L−1) | EPS (mg L−1) | IAA (mg L−1) | Siderophores (%) | ||||
|---|---|---|---|---|---|---|---|---|
| ML | AD | ML | AD | ML | A D | ML | AD | |
| AC02.1 | 0.96d ± 0.06 | 3.73c ± 0.21 | 0.19de ± 0.02 | 0.39cd ± 0.05 | 21.3c ± 1.18 | 7.70e ± 0.59 | 30.3b ± 1.70 | 7.87de ± 0.86 |
| AC03.2 | 1.77b ± 0.06 | 2.49e ± 0.12 | 0.26cde ± 0.02 | 0.47c ± 0.03 | 9.48e ± 1.18 | 12.4d ± 0.04 | 28.2bc ± 0.68 | 9.30bc ± 0.57 |
| AN05.2 | 1.05d ± 0.09 | 4.59b ± 0.23 | 0.66ab ± 0.09 | 0.92a ± 0.03 | 21.9c ± 1.78 | 16.0bc ± 0.59 | 34.1a ± 1.59 | 7.01ef ± 0.72 |
| TT04.3 | 0.77e ± 0.02 | 2.70e ± 0.16 | 0.28cd ± 0.07 | 0.35d ± 0.03 | 9.12e ± 1.54 | 11.3d ± 1.78 | 26.7c ± 0.80 | 3.43g ± 1.00 |
| TT07.4 | 0.63e ± 0.11 | 3.92c ± 0.02 | 0.14e ± 0.03 | 0.21e ± 0.04 | 12.9d ± 1.36 | 16.0bc ± 0.59 | 30.3b ± 1.93 | 8.73cd ± 0.43 |
| AN02.1 | 1.70b ± 0.02 | 3.38d ± 0.07 | 0.37c ± 0.04 | 0.50c ± 0.11 | 14.2d ± 0.45 | 26.7a ± 1.78 | 28.5bc ± 1.25 | 9.16bcd ± 0.43 |
| TT10.4 | 1.35c ± 0.07 | 2.24f ± 0.01 | 0.23de ± 0.04 | 0.31de ± 0.02 | 9.72e ± 0.95 | 18.4b ± 1.78 | 29.8b ± 0.23 | 11.2a ± 0.71 |
| AC04.1 | 1.63b ± 0.12 | 1.19g ± 0.04 | 0.76a ± 0.08 | 0.86a ± 0.01 | 32.6a ± 0.59 | 13.6cd ± 1.78 | 28.4bc ± 0.45 | 10.3ab ± 0.72 |
| AN05.1 | 3.01a ± 0.21 | 6.39a ± 0.10 | 0.62b ± 0.15 | 0.72b ± 0.15 | 27.3b ± 1.18 | 17.8b ± 1.18 | 30.0b ± 1.36 | 6.15f ± 0.07 |
| AH02.3 | 1.07d ± 0.05 | 4.76b ± 0.10 | 0.27cde ± 0.06 | 0.30de ± 0.05 | 27.8b ± 1.78 | 16.0bc ± 1.78 | 34.2a ± 0.39 | 1.57h ± 1.14 |
| Significant differences | * | * | * | * | * | * | * | * |
Values are means ± their standard deviations (n = 3). Different lowercase letters in each column indicate significant differences at P < 0.05 (*)
δ-Aminolevulinic acid (ALA)
ALA production by PNSB ranged from 0.96 to 3.01 mg L−1 under microaerobic light conditions, while it was recorded from 1.19 to 6.39 mg L−1 under aerobic dark conditions. Strain AC04.1 was the best to release ALA under both incubating conditions (Table 3).
Indole-3-acetic acid (IAA)
The PNSB strains synthesized IAA ranged from 9.12 to 32.6 mg L−1 under microaerobic light conditions, with the highest content in strain AC04.1 (Table 3). The second rank was recorded by strains AN05.1 and AH02.3, ranging from 27.3 to 27.8 mg L−1. For aerobic dark conditions, strain AN02.1 possessed the maximum IAA content, followed by strains TT10.4 and AN05.1 with 26.7 and 17.8 mg L−1, respectively.
Siderophores
The ability to secrete siderophores of PNSB ranged from 26.7 to 34.2%. In particular, strains AN05.2 and AH02.3 were the highest synthesized siderophores, with 34.1–34.2% under microaerobic light conditions (Table 3). This was followed by strains AC02.1, TT07.4, TT10.4, and AN05.1, with concentrations ranging from 29.8 to 30.3%. For aerobic dark conditions, the siderophores synthesis of PNSB ranged from 1.57 to 11.2%. Particularly, strain TT10.4 was measured with the highest siderophores content. Moreover, strain AN05.1 produced an equal concentration of 10.3%.
Selection of the plant growth promoting and acidic tolerant PNSB for resisting toxicity and solubilizing potassium
Screening PNSB for Al3+, Fe2+, and Mn2+ resistances
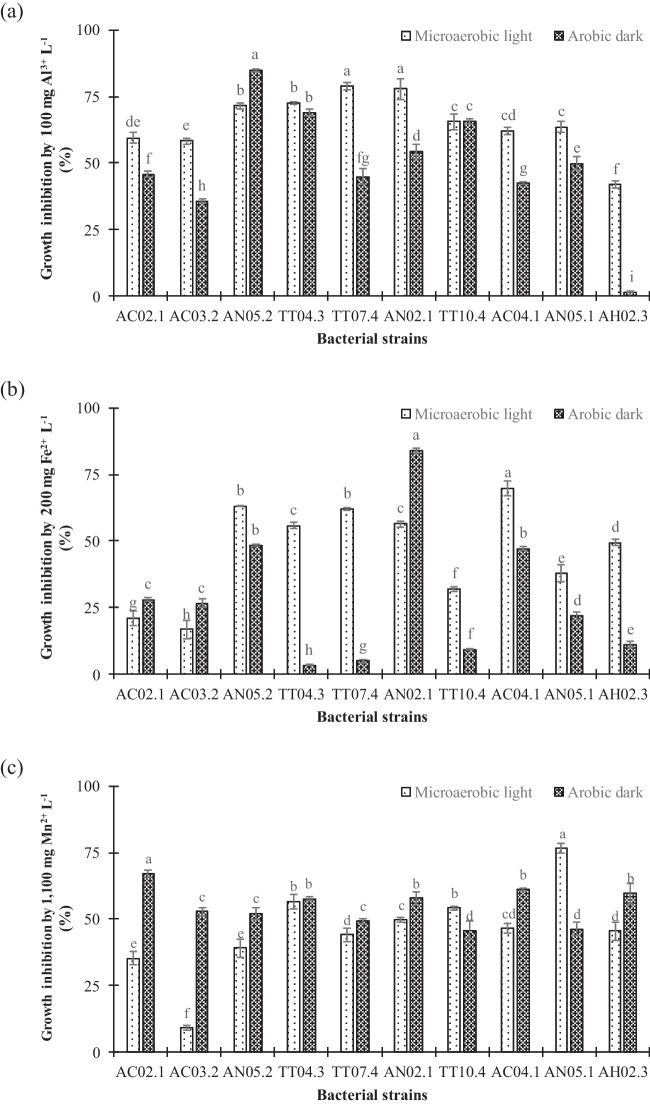
Resistance to Al3+ toxicity
Under Al3+ toxicity with the critical dose of 100 mg Al3+ L−1, the result found that the growth of the 10 PNSB strains was inhibited by less than 80% under microaerobic light conditions and less than 85% in aerobic dark conditions. The PNSB strain with the minimum growth inhibition by Al3+ toxicity was strain AH02.3 at 41.8% under microaerobic light conditions and 1.52% under aerobic dark conditions. In comparison, the growth inhibitions of the AC02.1 and AC03.2 strains by Al3+ toxicity were, respectively, 58.3% and 59.5% under microaerobic light conditions. Under aerobic dark conditions, the growth of five PNSB strains, including AC02.1, AC03.2, AN05.1, TT07.4, and AC04.1, was inhibited at a level lower than 50%. As can be seen, the AH02.3 strain had the ability to tolerate the Al3+ toxicity best (Fig. 2a).
Fig. 2.
Growth inhibition of selected purple nonsulfur bacteria under acidic condition by a Al3+, b Fe2+, and c Mn2+ under microaerobic light and aerobic dark conditions. Different lowercase letters in each bar indicate significant differences among strains under both incubating conditions
Resistance to Fe2+ toxicity
All 10 selected PNSB strains had the ability to resist Fe2+ toxicity at the critical dose of 250 mg Fe2+ L−1 as their growth inhibition was lower than 70% and 85% under microaerobic light and aerobic dark, respectively. Particularly, under microaerobic light conditions, the inhibition of Fe2+ toxicity on bacterial growth ranged from 16.6 to 69.8%. Lower inhibition was found in five PNSB strains: AC02.1 (21.1%), AC03.2 (16.6%), TT10.4 (31.9%), AN05.1 (37.8%), and AH02.3 (49.4%). Under aerobic dark conditions, Fe2+ inhibited bacterial growth from 3.33 to 83.9% on tested PNSB strains. Five PNSB strains (TT04.3, TT07.4, TT10.4, AN05.1, and AH02.3) were least inhibited by Fe2+ as the growth limitation was less than 25% (3.33–21.8%). Only strain AN02.1 was the most susceptible to Fe2+, with a growth limitation of 83.9% under aerobic dark conditions (Fig. 2b).
Resistance to Mn2+ toxicity
At a critical dose of 1.100 mg Mn2+ L−1, most of the PNSB strains possessed growth inhibition higher than 50% by the Mn2+ toxicity under both incubating conditions, except for the TT04.3, TT10.4, and AN05.1 strains, with growth inhibition up to 54.1–76.5%. On the other hand, under aerobic dark conditions, only three PNSB strains (TT07.4, TT10.4, and AN05.1) showed growth inhibition lower than 50% (45.7–49.2%) (Fig. 2c).
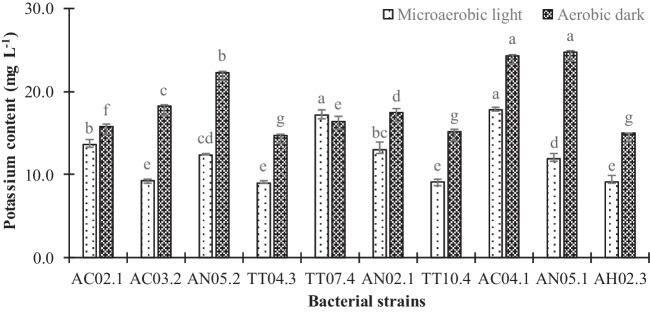
Selection of PNSB strains for potassium solubilization
After 72 h of cultivation under acidic conditions, all 10 selected PNSB strains were able to dissolve K from mineral K, AlKO6Si2 (Fig. 3). Experimental results found the dissolved K content in a range of 9.00–17.7 mg K+ L−1 under microaerobic light conditions and 14.8–24.7 mg K+ L−1 under aerobic dark conditions. Under microaerobic light conditions, two strains, AC04.1 (17.7 mg K+ L−1) and TT07.4 (17.2 mg K+ L−1), showed higher K concentration than other strains in the same conditions. For aerobic dark conditions, three strains AN05.1 (24.7 mg K+ L−1), AC04.1 (24.3 mg K+ L−1), and AN05.2 (22.3 mg K+ L−1), produced the highest dissolved K content. Overall, the following PNSB strains, AC04.1, AN05.1, and TT07.4, showed the highest K solubilization capacity under both incubating conditions.
Fig. 3.
Potassium content of selected purple nonsulfur bacteria strains in basic isolation medium broth, pH 4.5 under conditions of microaerobic light and aerobic dark. Different lowercase letters in each bar indicate significant differences among strains under both incubating conditions
Based on the ability to solubilize K, then to tolerate metal ions, the three PNSB TT07.4, AC04.1, and AN05.1 strains were selected for the further test, even though their ability to tolerate metal ions was moderate, they produced the highest dissolved K content.
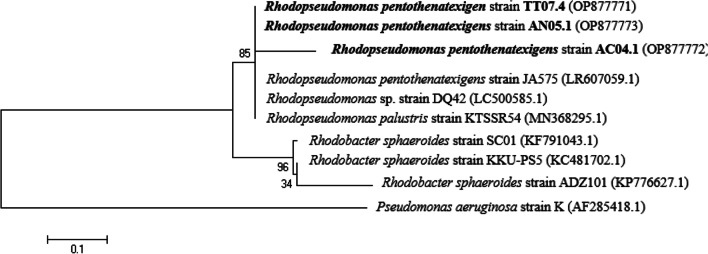
Identification of potent PNSB
Based on 16S rRNA gene sequences and available gene sequences in the GenBank database, the TT07.4, AN05.1, and AC04.1 strains were identified as Rhodopseudomonas pentothenatexigen. (Fig. 4).
Fig. 4.
Neighbor-joining phylogenetic trees based on 16S rDNA sequences of three selected purple nonsulfur bacteria strains compared to the closely related strains in the GenBank database. The percentage levels of bootstrap analysis of 1000 replicates are indicated at each node. Bar, 0.1 substitutions per nucleotide position. Pseudomonas aeruginosa strain K was used as the outgroup strain. Access numbers of GenBank sequences are implied in brackets
Discussion
Adaption of potassium-solubilizing bacteria in the acidic environment
The soil pHKCl of collected samples from all sites was less than 6.25. Particularly, the values of soil pHKCl and pHH2O collected in Tri Ton district, An Giang province, Vietnam, fluctuated from 4.01 to 7.25 and 3.55 to 6.25, respectively. Based on the results, the soil pH was classified as moderately acidic [56]. Generally, in all surveyed sites, the soil pHH2O was higher than pHKCl. This was because H+ was released from the K+ extractant [25]. EC values in soil samples ranged from 120 to 1444 μS cm−1 (Table 1), indicating that the soil should be classified as potential AS soil, according to Anjos et al. [44]. EC values suggest that soil in all studied areas produced no effect on crop yield [56]. However, in AS soils, Al3+, Fe2+, and Mn2+ were found in high concentrations, which are considered the main constraints for rice growth [25, 28, 57]. Those metals might also be toxic to PNSB growth, so isolated PNSB were screened based on their resistance to the critical concentrations of toxicants found in AS soils. All 10 selected PNSB strains from 70 isolates had the pattern to resist in order Fe2+ > Mn2+ > Al3+ toxicity at their critical threshold for rice. Simultaneously, this research also investigated the biofertilizers and plant growth promotors functions of selected PNSB for obtaining potent PNSB for application in AS soils.
Potassium-solubilizing PNSB as plant growth promoters
However, PNSB released more siderophores under microaerobic light conditions (26.7–34.2%) compared with under aerobic dark conditions (1.57–11.2%) (Table 3). This might be why the selected PNSB grew better under conditions of microaerobic light than aerobic dark (Fig. 1). On the other hand, EPS should be used to dissolve K rather than siderophores as more released EPS under aerobic dark conditions (Table 3) based on its ability to bind metal ions, including H+ of EPS, in the culture medium and soil to reduce stresses of the acidic and metal toxicity [58, 59]. Hence, the current research suggests that PNSB used at least two mechanisms, particularly EPS, for dissolving K and also ameliorating toxicities of H+, Al3+, Fe2+, and Mn2+ found in AS soils [25, 57]. This is in accordance with Khuong et al. [27] proved that PNSB strains contribute to improving soil quality by reducing toxicity of Al3+, Fe2+, and Mn2+ commonly found in AS soils.
For N2 fixation, strain AC04.1 reached the highest ability to release NH4+ under aerobic dark conditions (11.2 mg L−1), while strain TT07.4 achieved the maximum NH4+ production under the microaerobic light condition, with 6.24 mg L−1 (Table 2). This is consistent with Sakpirom et al. [60] reported that PNSB such as R. palustris and Rubrivivax gelatinosus have ability to fix N2 to provide an N source for plants [14]. However, strains AC04.1 and TT07.4 show their greatest ability under acidic conditions, while strains R. palustris TN110 and R. gelatinosus TN414 under neutral conditions. In addition, selected PNSB strains are able to solubilize different inorganic phosphorus sources with different trends (Table 2). Besides, ALA production was performed by selected PNSB strains that reached up to 3.01 mg L−1 under microaerobic light and 6.39 mg L−1 under aerobic dark conditions (Table 3). The PNSB belongs to the group of bacteria with the ability to provide ALA. This metabolite plays an important role in agriculture for combating a biotic stress such as salinity and drought [61, 62]. Recently, ALA is also considered one possible mechanism to combat the high acidity of PNSB by increasing pH while producing ALA [27]. IAA is a phytohormone that counters adverse effects on plant cells under stress conditions and promotes growth by increasing mineral uptake and elongation of root cells [63]. The PNSB in this present research synthesized IAA in a range of 7.70 to 32.6 mg L−1 under acidic conditions (Table 3). In a previous study, Hsu et al. [64] reported that IAA-producing R. palustris strain PS3 increased the yield of the edible leafy part of vegetables.
The results indicate that selected PNSB in the current research are strong biofertilizers by fixing N2, solubilizing phosphorus, and releasing PGPS under acidic conditions.
Mechanism of potassium-solubilizing PNSB
All 10 tested PNSB strains were capable of dissolving K (Fig. 3), and this is in accordance with the previous works [65, 66]. Among the 10 PNSB isolates, potent strains TT07.4, AN05.1, and AC04.1 strongly dissolved K in the form of the mineral K, AlKO6Si2. Although there have not been any statistical comparisons between the two incubating conditions, visually the microaerobic light conditions (11.8–17.7 mg L−1) performed lower K solubilization than that under the aerobic dark conditions (16.4 to 24.7 mg L−1), except for the case of the TT07.4 isolate (Fig. 3). The exchangeable K content is relatively low in AS soils [67]. However, in the current research, the K-containing mineral AlKO6Si2 was solubilized by the activity of PNSB under acidic conditions like AS soils. Therefore, it might have a great potential to convert K into available form for plants suggesting that the potent PNSB could contribute to reducing the applied K application for rice.
The mechanism of K solubilization has been reported as a combination ability of microorganisms to produce protons, organic acids, siderophores, capsular polysaccharides or EPS, and certain organic ligands [68]. In the current research, PNSB strains were able to release siderophores and EPS (Table 3), which can be explained why they possessed the K-solubilizing function from AlKO6Si2 (Fig. 3). However, the K solubilization has been proved that it does not dependent on pH because the pH of the cultures did not significantly change during the biological K solubilization as reported by Bahadur et al. [69]. At the same time, many mechanisms of K solubilization relate to polysaccharide production, indicating that acids were not excreted in significant quantities. The polysaccharides containing free carboxylic groups are able to proceed with chelation, which solubilizes the insoluble K [69]. Many strains of K-solubilizing bacteria have been investigated in recent studies, which the current study was in accordance with. For instance, Bacillus pseudomycoides strains could solubilize K in soil and can be applied as a biofertilizer, increasing the K uptake in tea plants [70]. In addition, the B. pseudomycoides O–5 strain in the study by Pramanik et al. [70] increased soil K availability by 47.0 ± 7.1 mg kg−1 after 105 days. Many K-solubilizing bacteria have been applied as biofertilizers that promote plant growth. Kaur et al. [71] have found that the Pseudomonas gessardii EU-LWNA-25 strain can solubilize K and promote plant growth, which made it a suitable bioinoculant for Rabi and overcoming the challenges of sustainable agriculture in K-deficient soils. In gallic, an application of the Bacillus mucilaginosus HQ013329 strain not only enhanced plant length, leaf number per plant, bulb diameter, and plant dry weight but also limited weight loss rate and maintained gallic bulbs during storage time [62]. Moreover, the Bacillus aryabhattai SK1-7 strain has been proven to be able to solubilize insoluble K and stimulate the growth of poplar after being applied to soil [72].
PNSB for sustainable agriculture
Potent PNSB strains can not only act as biofertilizers (fixing N2, solubilizing insoluble P fractions and producing PGPS such as ALA, EPS, IAA, and siderophores) (Fig. 3, Tables 2 and 3), but also solubilize K well in this current research. Therefore, it could be possible to apply potent strains AC04.1, AN05.1, and TT07.4 for promoting rice growth in soil with low K-like AS soils for sustainable agriculture. Macronutrients N, P, and K are key to enhancing crop yield, particularly rice; however, farmers need to reduce chemical fertilizers’ use to reduce production costs. Hence, biofertilizers, especially PNSB, provide a chance to achieve the target of farmers as the results in this present research suggest that PNSB could fulfill not only all plant nutrients but also PGPS (Tables 2 and 3 and Fig. 3). Previous studies showed successful PNSB application under normal and stress conditions in both soil pot experiments and paddy fields. Especially, Kantachote et al. [73] proved that PNSB biofertilizers produced a remarkable increase in rice grain yields in both organic and saline paddy fields, along with dramatically decreased CH4 emissions. Rhodopseudomonas palustris TN110 and Rubrivivax gelatinosus TN414 produced NH4+, ALA, and IAA to enhance rice growth in paddy soil [14, 60]. Furthermore, strains Luteovulum sphaeroides W03 and W11 increased rice grain yield by 10% in saline AS soils and 18% in saline soils to reduce 50% of the recommended P dose [29]. This result matches the one by Khuong et al. [30], where a mixture of plant growth promoters, L. sphaeroides strains W01, W14, W22, and W32, increased grain yields up to 86.8% in saline soil. Moreover, strains R. palustris TLS06, VNW02, VNW64, and VNS89 dissolved the insoluble P compounds to increase rice yield in AS soils [13]. Besides, R. palustris TLS12, VNS19, VNS32, VNS62, and VNW95 and R. harwoodiae TLW42 increased NH4+ and available P and reduced Mn2+ in liquid and solid forms to reduce 25% N and P, but the rice grain yield is maintained under AS soils [74]. This is also supported by the evidence that PNSB strains TLS06, VNW02, VNW64, and VNS89 in the acidic medium can reduce toxicities caused by high concentrations of H+, Al3+, Fe2+, and Mn2+ [25, 57, 74]. Furthermore, R. palustris strains TLS12, VNS19, VNS32, VNS62, and VNW95, and R. harwoodiae strain TLW42 are promising PNSB to reduce the toxicity of Mn2+ by releasing PGPS (ALA, EPS, IAA, and siderophores) and plant nutrients (NH4+ and Pavail) [28, 75]. The above results indicated that PNSB act not only as biofertilizers but also as bioremediators to promote rice growth and grain yield under varying stress conditions. Recently, acid-resistant R. palustris KTSSR54 acted as a biocontrol agent by releasing antifungal compounds, particularly EPS, to combat rice fungal pathogens; Bipolaris oryzae NPT0508, Curvularia lunata SPB0627, and Magnaporthe oryzae PTRC63 for promoting rice growth and grain yield [26, 76].
Overall results demonstrated that PNSB, either an individual or a mixed culture of potent strains, have great potential to apply for enhancing rice growth and grain yield in various soils, including AS soils, to meet the goal of sustainable agriculture. This is also how to support organic agriculture by using only biofertilizers and/or integrated plant nutrient management by combining PNSB biofertilizers and chemical fertilizers. Nevertheless, the potent PNSB strains obtained in this research should be first investigated in soil pot experiment before applying them in acidic paddy fields in the future studies.
Conclusions
Among 70 PNSB strains isolated from paddy fields of AS soils in Vietnam, only 10 acid-resistant strains were found. However, only AC04.1, AN05.1, and TT07.4 were promising strains to release soluble K under stress conditions of H+, Al3+, Fe2+, and Mn2+. The potent PNSB are strong biofertilizers to provide plant nutrients (N, P, and K) and PGPS (ALA, EPS, IAA, and siderophores). Due to their adaptability to acidic conditions and their outstanding abilities, the bacterial strains are promising in being applied in AS soil in Vietnam to solubilize K and promote crop cultivation in a wetland. Moreover, they were identified as Rhodopseudomonas pentothenatexigens. In addition, siderophores are considered a mechanism to solubilize K by PNSB, but other principles should be considered in further research. From the current study, the selected PNSB strains would be applied as a biofertilizer in various forms (solid, liquid, and foliar application) on AS fields in Vietnam to measure the biofertilizer’s efficiency on yield and soil fertility of AS soils.
Acknowledgements
We thank Prof. Dr. Duangporn Kantachote for her assistance with the English language.
Author contribution
Conceptualization: Nguyen Quoc Khuong, Jakkapan Sakpirom; methodology: Nguyen Quoc Khuong, Jakkapan Sakpirom; formal analysis and investigation: Truong Oanh Oanh, Le Vinh Thuc, Le Thi My Thu, Do Thi Xuan, Le Thanh Quang, Ly Ngoc Thanh Xuan; writing—original draft preparation: Nguyen Quoc Khuong; writing—review and editing: Nguyen Quoc Khuong, Jakkapan Sakpirom; funding acquisition: Nguyen Quoc Khuong; resources: Nguyen Quoc Khuong, Jakkapan Sakpirom; supervision: Jakkapan Sakpirom.
Funding
The research leading to these results received funding from Can Tho University under Grant Agreement No T2021-94.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Informed consent
Informed consent was obtained from all individual participants included in the study. The participant has consented to the submission of the case report to the journal.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nguyen Quoc Khuong, Email: nqkhuong@ctu.edu.vn.
Jakkapan Sakpirom, Email: jakkapan.sak@mail.pbru.ac.th.
Truong Oanh Oanh, Email: oanhtruong1801@gmail.com.
Le Vinh Thuc, Email: lvthuc@ctu.edu.vn.
Le Thi My Thu, Email: thule@ctu.edu.vn.
Le Thanh Quang, Email: quahgm@gmail.com.
Ly Ngoc Thanh Xuan, Email: lntxuan@agu.edu.vn.
References
- 1.Minh LQ, Tuong TP, Van Mensvoort MEF, Bouma J. Contamination of surface water as affected by land use in acid sulfate soils in the Mekong River Delta, Vietnam. Agric Ecosyst Environ. 1997;61(1):19–27. doi: 10.1016/S0167-8809(96)01084-5. [DOI] [Google Scholar]
- 2.Nystrand MI, Österholm P, Yu C, Åström M. Distribution and speciation of metals, phosphorus, sulfate and organic material in brackish estuary water affected by acid sulfate soils. Appl Geochem. 2016;66:264–274. doi: 10.1016/j.apgeochem.2016.01.003. [DOI] [Google Scholar]
- 3.Mayakaduwage S, Mosley LM, Marschner P. Threshold for labile phosphate in a sandy acid sulfate soil. Geoderma. 2020;371:114359. doi: 10.1016/j.geoderma.2020.114359. [DOI] [Google Scholar]
- 4.Keene A, Melville MD, BCT M. Super Soil 2004: Proceedings of the Third Australian and New Zealand Soils Conference [CD-ROM] Sydney, Australia: Regional Institute Ltd.; 2004. Using potassium potentials to examine nutrient availability in an acid sulfate soil landscape, northern Australia. [Google Scholar]
- 5.Pandey D, Kehri HK, Zoomi I, Singh U, Chaudhri KL, Akhtar O (2020) Potassium solubilizing microbes: diversity, ecological significances and biotechnological applications. In: Yadav A, Singh J, Rastegari A, Yadav N (ed) Plant microbiomes for sustainable agriculture. Sustainable development and biodiversity. Springer, Cham, 25:263-286. 10.1007/978-3-030-38453-1_9
- 6.Sparks DL, Huang PM. Physical chemistry of soil potassium. In: Munson RD, editor. Potassium in agriculture. ASA-CSSA-SSS; 1985. pp. 201–276. [Google Scholar]
- 7.Li T, Liang J, Chen X, Wang H, Zhang S, Pu Y, Xu X, Li H, Xu J, Wu X, Liu X. The interacting roles and relative importance of climate, topography, soil properties and mineralogical composition on soil potassium variations at a national scale in China. Catena. 2021;196:104875. doi: 10.1016/j.catena.2020.104875. [DOI] [Google Scholar]
- 8.Ahmad M, Nadeem SM, Naveed M, Zahir ZA. Potassium-solubilizing bacteria and their application in agriculture. In: Meena V, Maurya B, Verma J, Meena R, editors. Potassium solubilizing microorganisms for sustainable agriculture. New Delhi: Springer; 2016. pp. 293–313. [Google Scholar]
- 9.Yadav BK, Sidhu AS. Dynamics of potassium and their bioavailability for plant nutrition. In: Meena V, Maurya B, Verma J, Meena R, editors. Potassium solubilizing microorganisms for sustainable agriculture. New Delhi: Springer; 2016. pp. 187–201. [Google Scholar]
- 10.Meena VS, Maurya BR, Verma JP. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol Res. 2014;169(5-6):337–347. doi: 10.1016/j.micres.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Xiao Y, Wang X, Chen W, Huang Q. Isolation and identification of three potassium-solubilizing bacteria from rape rhizospheric soil and their effects on ryegrass. Geomicrobiol J. 2017;34(10):873–880. doi: 10.1080/01490451.2017.1286416. [DOI] [Google Scholar]
- 12.Liu Z, Rong Q, Zhou W, Liang G. Effects of inorganic and organic amendment on soil chemical properties, enzyme activities, microbial community and soil quality in yellow clayey soil. PLoS One. 2017;12(3):e0172767. doi: 10.1371/journal.pone.0172767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khuong NQ, Kantachote D, Onthong J, Xuan LNT, Sukhoom A. Enhancement of rice growth and yield in actual acid sulfate soils by potent acid-resistant Rhodopseudomonas palustris strains for producing safe rice. Plant Soil. 2018;429(1):483–501. doi: 10.1007/s11104-018-3705-7. [DOI] [Google Scholar]
- 14.Sakpirom J, Nunkaew T, Khan E, Kantachote D. Optimization of carriers and packaging for effective biofertilizers to enhance Oryza sativa L. growth in paddy soil. Rhizosphere. 2021;19:100383. doi: 10.1016/j.rhisph.2021.100383. [DOI] [Google Scholar]
- 15.Saha M, Maurya BR, Meena VS, Bahadur I, Kumar A. Identification and characterization of potassium solubilizing bacteria (KSB) from Indo-Gangetic plains of India. Biocatal Agric Biotechnol. 2016;7:202–209. doi: 10.1016/j.bcab.2016.06.007. [DOI] [Google Scholar]
- 16.Bakhshandeh E, Pirdashti H, Lendeh KS. Phosphate and potassium-solubilizing bacteria effect on the growth of rice. Ecol Eng. 2017;103:164–169. doi: 10.1016/j.ecoleng.2017.03.008. [DOI] [Google Scholar]
- 17.Khati P, Mishra PK, Parihar M, Kumari A, Joshi S, Bisht JK, Pattanayak A. Potassium solubilization and mobilization: functional impact on plant growth for sustainable agriculture. In: Yadav A, Rastegari A, Yadav N, Kour D, editors. Advances in plant microbiome and sustainable agriculture: functional annotation and future challenges. Singapore: Springer; 2020. pp. 21–40. [Google Scholar]
- 18.Sun F, Ou Q, Wang N, Xuan Guo Z, Ou Y, Li N, Peng C. Isolation and identification of potassium-solubilizing bacteria from Mikania micrantha rhizospheric soil and their effect on M. micrantha plants. Glob Ecol Conserv. 2020;23:e01141. doi: 10.1016/j.gecco.2020.e01141. [DOI] [Google Scholar]
- 19.Ali AM, Awad MY, Hegab SA, Gawad AMAE, Eissa MA. Effect of potassium solubilizing bacteria (Bacillus cereus) on growth and yield of potato. J Plant Nutr. 2021;44(3):411–420. doi: 10.1080/01904167.2020.1822399. [DOI] [Google Scholar]
- 20.Chen YH, Yang XZ, Zhuang LI, An XH, Li YQ, Cheng CG. Efficiency of potassium-solubilizing Paenibacillus mucilaginosus for the growth of apple seedling. J Integr Agric. 2020;19(10):2458–2469. doi: 10.1016/S2095-3119(20)63303-2. [DOI] [Google Scholar]
- 21.Maurya BR, Meena VS, Meena OP. Influence of Inceptisol and Alfisol’s potassium solubilizing bacteria (KSB) isolates on release of K from waste mica. Vegetos. 2014;27(1):181–187. doi: 10.5958/j.2229-4473.27.1.028. [DOI] [Google Scholar]
- 22.Dotaniya ML, Meena VD, Basak BB, Meena RS. Potassium uptake by crops as well as microorganisms. In: Meena V, Maurya B, Verma J, Meena R, editors. Potassium solubilizing microorganisms for sustainable agriculture. New Delhi: Springer; 2016. pp. 267–280. [Google Scholar]
- 23.Etesami H, Emami S, Alikhani HA. Potassium solubilizing bacteria (KSB): mechanisms, promotion of plant growth, and future prospects: a review. J Soil Sci Plant Nutr. 2017;17(4):897–911. doi: 10.4067/S0718-95162017000400005. [DOI] [Google Scholar]
- 24.Rashwan BR, Shaheen AEA. The impact of potassium sources and bio fertilizer on corn plant and potassium availability in calcareous soil. Asian J Soil Sci. 2021;5(1):27–37. doi: 10.9734/asrj/2021/v5i130099. [DOI] [Google Scholar]
- 25.Khuong NQ, Kantachote D, Onthong J, Sukhoom A. The potential of acid-resistant purple nonsulfur bacteria isolated from acid sulfate soils for reducing toxicity of Al3+ and Fe2+ using biosorption for agricultural application. Biocatal Agric Biotechnol. 2017;12:329–340. doi: 10.1016/j.bcab.2017.10.022. [DOI] [Google Scholar]
- 26.Nookongbut P, Kantachote D, Khuong NQ, Sukhoom A, Tantirungkij M, Limtong S. Selection of acid-resistant purple nonsulfur bacteria from peat swamp forests to apply as biofertilizers and biocontrol agents. J Soil Sci Plant Nutr. 2019;19(3):488–500. doi: 10.1007/s42729-019-00044-9. [DOI] [Google Scholar]
- 27.Khuong NQ, Kantachote D, Nookongbut P, Onthong J, Xuan LNT, Sukhoom A. Mechanisms of acid-resistant Rhodopseudomonas palustris strains to ameliorate acidic stress and promote plant growth. Biocatal Agric Biotechnol. 2020;24:101520. doi: 10.1016/j.bcab.2020.101520. [DOI] [Google Scholar]
- 28.Khuong NQ, Kantachote D, Nookongbut P, Xuan LNT, Nhan TC, Xuan NTT, Tantirungkij M. Potential of Mn2+-resistant purple nonsulfur bacteria isolated from acid sulfate soils to act as bioremediators and plant growth promoters via mechanisms of resistance. J Soil Sci Plant Nutr. 2020;20(4):2364–2378. doi: 10.1007/s42729-020-00303-0. [DOI] [Google Scholar]
- 29.Khuong NQ, Huu TN, Thuc LV, Thu LTM, Xuan DT, Quang LT, Nhan TC, Tran HN, Tien PD, Xuan LNT, Kantachote D. Two strains of Luteovulum sphaeroides (purple nonsulfur bacteria) promote rice cultivation in saline soils by increasing available phosphorus. Rhizosphere. 2021;20:100456. doi: 10.1016/j.rhisph.2021.100456. [DOI] [Google Scholar]
- 30.Khuong NQ, Kantachote D, Dung NTT, Huu TN, Thuc LV, Thu LTM, Quang LT, Xuan DT, Nhan TC, Tien PD, Xuan LNT. Potential of potent purple nonsulfur bacteria isolated from rice-shrimp systems to ameliorate rice (Oryza sativa L.) growth and yield in saline acid sulfate soil. J Plant Nutr. 2023;46(3):473–494. doi: 10.1080/01904167.2022.2087089. [DOI] [Google Scholar]
- 31.Ge H, Zhang F. Growth - promoting ability of Rhodopseudomonas palustris G5 and its effect on induced resistance in cucumber against salt stress. J Plant Growth Regul. 2019;8:180–188. doi: 10.1007/s00344-018-9825-8. [DOI] [Google Scholar]
- 32.Ge H, Liu Z. Alleviation of tetrabromobisphenol A toxicity in soybean seedlings by Rhodopseudomonas palustris RP1n1. Arch Microbiol. 2020;202(4):895–903. doi: 10.1007/s00203-019-01797-8. [DOI] [PubMed] [Google Scholar]
- 33.Giller KE, Witter E, McGrath SP. Toxicity of heavy metals to microorganisms and microbial process in agricultural soils: a review. Soil Biol Biochem. 1998;30:1389–1414. doi: 10.1016/S0038-0717(97)00270-8. [DOI] [Google Scholar]
- 34.Chaudri AM, McGrath SP, Giller KE, Rietz E, Sauerbeck DR. Enumeration of indigenous Rhizobium leguminosarum biovar trifolii in soils previously treated with metal-contaminated sewage sludge. Soil Biol Biochem. 1993;25:301–309. doi: 10.1016/0038-0717(93)90128-X. [DOI] [Google Scholar]
- 35.Gray EJ, Smith DL. Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol Biochem. 2005;37:395–412. doi: 10.1016/j.soilbio.2004.08.030. [DOI] [Google Scholar]
- 36.Khan MS, Zaidi A, Wani PA, Oves M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ Chem Lett. 2009;7:1–19. doi: 10.1007/s10311-008-0155-0. [DOI] [Google Scholar]
- 37.Coleman NT, Craig D. The spontaneous alteration of hydrogen clay. Soil Sci. 1961;91(1):14–18. doi: 10.1097/00010694-196101000-00004. [DOI] [Google Scholar]
- 38.Robinson GW. A new method for the mechanical analysis of soils and other dispersions. J Agric Sci. 1922;12(3):306–321. doi: 10.1017/S0021859600005360. [DOI] [Google Scholar]
- 39.Walkley A, Black IA. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37(1):29–38. doi: 10.1097/00010694-193401000-00003. [DOI] [Google Scholar]
- 40.Brown JW (2013) Enrichment and isolation of purple non-sulfur bacteria. Department of Biological Sciences. College of Sciences, North Carolina State University. http://www.mbio.ncsu.edu/mb452/purplenonsulfurs/purples.html. Accessed 15 Nov 2022
- 41.Kantachote D, Torpee S, Umsakul K (2005) The potential use of anoxygenic phototrophic bacteria for treating latex rubber sheet wastewater. Electron. J Biotechnol 8(3). 10.2225/vol8-issue3-fulltext-8
- 42.Nelson DW. Determination of ammonium in KCl extracts of soils by the salicylate method. Commun Soil Sci Plant Anal. 1983;14(11):1051–1062. doi: 10.1080/00103628309367431. [DOI] [Google Scholar]
- 43.Murphy J, Riley J. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31–36. doi: 10.1016/S0003-2670(00)88444-5. [DOI] [Google Scholar]
- 44.Glickman E, Dessaux Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61(12):793–796. doi: 10.1128/aem.61.2.793-796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burnham BF. δ-Aminolevulinic acid synthase (from Rhodopseudomonas sphaeroides) Methods Enzymol. 1970;17:195–204. doi: 10.1016/0076-6879(71)17179-0. [DOI] [Google Scholar]
- 46.Eboigbodin KE, Biggs CA. Characterization of the extracellular polymeric substances produced by Escherichia coli using infrared spectroscopic, proteomic, and aggregation studies. Biomacromolecules. 2008;9(2):686–695. doi: 10.1021/bm701043c. [DOI] [PubMed] [Google Scholar]
- 47.Ferreira ML, Casabuono AC, Stacchiotti ST, Couto AS, Ramirez SA, Vullo DL. Chemical characterization of Pseudomonas veronii 2E soluble exopolymer as Cd (II) ligand for the biotreatment of electroplating wastes. Int Biodeterior Biodegrad. 2017;119:605–613. doi: 10.1016/j.ibiod.2016.10.013. [DOI] [Google Scholar]
- 48.Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;28(8):751–759. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 49.Attanandana T, Vacharotayan S. Acid sulfate soils: their characteristics. genesis, amelioration and utilization. Jpn J Southeast Asian Stud. 1986;24(2):154–180. doi: 10.20495/tak.24.2_154. [DOI] [Google Scholar]
- 50.Upjohn B, Fenton G, Conyers M. Soil acidity and liming. Agfact AC.19, 3rd edition. New South Wales Department of Primary Industries; 2005. [Google Scholar]
- 51.Anjos L, Gaistardo C, Deckers J, Dondeyne S, Eberhardt E, Gerasimova M, Harms B, Jones A, Krasilnikov P, Reinsch T, Vargas R, Zhang G, authors. Schad P, Van Huyssteen C, Micheli E, editors (2015) World reference base for soil resources 2014 International soil classification system for naming soils and creating legends for soil maps. Rome (Italy): FAO; 2015. JRC91947
- 52.Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour, PN, Tabatabai MA, Johnston CT, Sumner ME (ed) (1996) Methods of soil analysis. Part 3-Chemical Methods. SSSA Book Ser. 5.3. SSSA, ASA, Madison, W.I. 10.2136/sssabookser5.3
- 53.Suzuki Y, Kelly SD, Kemner KM, Banfield JF. Microbial populations stimulated for hexavalent uranium reduction in uranium mine sediment. Appl Environ Microbiol. 2003;69:1337–1346. doi: 10.1128/AEM.69.3.1337-1346.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 55.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA 6: molecular evolutionary genetics analysis Version 6.0. Mol Biol Evol 30(12):2725-2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed]
- 56.Horneck DA, Sullivan DM, Owen JS, Hart JM. Soil test interpretation guide. Extension Service: Technical Report, Oregon State University; 2011. [Google Scholar]
- 57.Khuong NQ, Kantachote D, Thuc LV, Huu TN, Nhan TC, Nguyen PC, Thu LTM, Van TTB, Xuan NTT, Xuan LNT, Xuan DT. Use of potent acid resistant strains of Rhodopseudomonas spp. in Mn-contaminated acidic paddies to produce safer rice and improve soil fertility. Soil Tillage Res. 2022;221:105393. doi: 10.1016/j.still.2022.105393. [DOI] [Google Scholar]
- 58.Nookongbut P, Kantachote D, Megharaj M, Naidu R. Reduction in arsenic toxicity and uptake in rice (Oryza sativa L.) by As-resistant purple nonsulfur bacteria. Environ Sci Pollut Res. 2018;25(36):36530–36544. doi: 10.1007/s11356-018-3568-8. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen KQ, Kantachote D, Onthong J, Sukhoom A. Al3+ and Fe2+ toxicity reduction potential by acid-resistant strains of Rhodopseudomonas palustris isolated from acid sulfate soils under acidic conditions. Ann Microbiol. 2018;68(4):217–228. doi: 10.1007/s13213-018-1332-4. [DOI] [Google Scholar]
- 60.Sakpirom J, Kantachote D, Nunkaew T, Khan E. Characterizations of purple non-sulfur bacteria isolated from paddy fields, and identification of strains with potential for plant growth-promotion, greenhouse gas mitigation and heavy metal bioremediation. Res Microbiol. 2017;168(3):266–275. doi: 10.1016/j.resmic.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Kang Z, Ding W, Gong X, Liu Q, Du G, Chen J. Recent advances in production of 5-aminolevulinic acid using biological strategies. World J Microbiol Biotechnol. 2017;33(11):200. doi: 10.1007/s11274-017-2366-7. [DOI] [PubMed] [Google Scholar]
- 62.Mounir AM, Osman YM, Khalil OA. Impact of potassium solubilizing bacteria on growth and yield of garlic. Plant Arch. 2020;20(2):8374–8388. [Google Scholar]
- 63.Batista BD, Dourado MN, Figueredo EF, Hortencio RO, Marques JPR, Piotto FA, Bonatelli ML, Settles ML, Azevedo JL, Quecine MC. The auxin-producing Bacillus thuringiensis RZ2MS9 promotes the growth and modifies the root architecture of tomato (Solanum lycopersicum cv. Micro-Tom) Arch Microbiol. 2021;203(7):3869–3882. doi: 10.1007/s00203-021-02361-z. [DOI] [PubMed] [Google Scholar]
- 64.Hsu SH, Shen MW, Chen JC, Lur HS, Liu CT. The photosynthetic bacterium Rhodopseudomonas palustris strain PS3 exerts plant growth-promoting effects by stimulating nitrogen uptake and elevating auxin levels in expanding leaves. Front Plant Sci. 2021;12:573634. doi: 10.3389/fpls.2021.573634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng K, Cai Z, Ding T, Yan H, Liu X, Zhang Z. Effects of potassium-solubulizing and photosynthetic bacteria on tolerance to salt stress in maize. J Appl Microbiol. 2019;126(5):1530–1540. doi: 10.1111/jam.14220. [DOI] [PubMed] [Google Scholar]
- 66.Zhang C, Kong F. Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl Soil Ecol. 2014;82:18–25. doi: 10.1016/j.apsoil.2014.05.002. [DOI] [Google Scholar]
- 67.Trueman AM, McLaughlin MJ, Mosley LM, Fitzpatrick RW (2020) Composition and dissolution kinetics of jarosite-rich segregations extracted from an acid sulfate soil with sulfuric material. Chem Geol 543:119606. 10.1016/j.chemgeo.2020.119606
- 68.Das I, Pradhan M. Potassium-solubilizing microorganisms and their role in enhancing soil fertility and health. In: Meena V, Maurya B, Verma J, Meena R, editors. Potassium solubilizing microorganisms for sustainable agriculture. New Delhi: Springer; 2016. pp. 281–291. [Google Scholar]
- 69.Bahadur I, Maurya R, Roy P, Kumar A (2019) Potassium-solubilizing bacteria (KSB): a microbial tool for K-solubility, cycling, and availability to plants. In: Kumar A, Meena V (ed) Plant growth promoting rhizobacteria for agricultural sustainability: From theory to practices, Springer, Singapore, pp 257-265. 10.1007/978-981-13-7553-8_13. In:
- 70.Pramanik P, Goswami AJ, Ghosh S, Kalita C. An indigenous strain of potassium-solubilizing bacteria Bacillus pseudomycoides enhanced potassium uptake in tea plants by increasing potassium availability in the mica waste-treated soil of North-east India. J Appl Microbiol. 2019;126(1):215–222. doi: 10.1111/jam.14130. [DOI] [PubMed] [Google Scholar]
- 71.Kaur T, Devi R, Kour D, Yadav A, Yadav AN (2021) Plant growth promotion of barley (Hordeum vulgare L.) by potassium solubilizing bacteria with multifarious plant growth promoting attributes. Plant Sci Today 8(sp1):17-24. 10.14719/pst.1377
- 72.Chen Y, Ye J, Kong Q. Potassium-solubilizing activity of Bacillus aryabhattai SK1-7 and its growth-promoting effect on Populus alba L. Forests. 2020;11(12):1348. doi: 10.3390/f11121348. [DOI] [Google Scholar]
- 73.Kantachote D, Nunkaew T, Kantha T, Chaiprapat S. Biofertilizers from Rhodopseudomonas palustris strains to enhance rice yields and reduce methane emissions. Appl Soil Ecol. 2016;100:154–161. doi: 10.1016/j.apsoil.2015.12.015. [DOI] [Google Scholar]
- 74.Khuong NQ, Kantachote D, Thuc LV, Huu TN, Nhan TC, Nguyen PC, Thu LTM, Van TTB, Xuan NTT, Xuan LNT, Xuan DT. Use of potent acid resistant strains of Rhodopseudomonas spp. in Mn-contaminated acidic paddies to produce safer rice and improve soil fertility. Soil Tillage Res. 2022;221:105393. doi: 10.1016/j.still.2022.105393. [DOI] [Google Scholar]
- 75.Nookongbut P, Jingjit N, Kantachote D, Sukhoom A, Tantirungkij M. Selection of acid tolerant purple nonsulfur bacteria for application in agriculture. Chiang Mai Univ J Nat Sci. 2020;19(4):774–790. doi: 10.12982/CMUJNS.2020.0049. [DOI] [Google Scholar]
- 76.Nunkaew T, Kantachote D, Nitoda T, Kanzaki H, Ritchie RJ. Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr Polym. 2015;115:334–341. doi: 10.1016/j.carbpol.2014.08.099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.