Abstract
Pig pasteurellosis, caused by Pasteurella multocida, is an acute infection that also has economic implications for pig farmers. We report the complete genome sequence of a P. multocida, serovar B:2 ‘Soron’ strain isolated from the blood of a pig that had died of pasteurellosis in India. The isolate was not found to be haemorrhagic septicaemia (HS) specific B:2 by the PCR assay. The genome of ‘Soron’ strain is a single circular chromosome of 2,272,124 base pairs in length and contains 2014 predicted coding regions, 4 ribosomal RNA operons, and 52 tRNAs. It has 1812 protein-coding genes that were also found in reference sequence PmP52Vac. Phylogenetic analysis revealed that Pm_P52VAc and P. multocida ‘Soron’ serovar B:2 were clustered in different clades. Pasteurella multocida ‘Soron’ serovar B:2 was found to cluster with the same ancestor of Pm70, which is of avian origin. The genome was found to contain regions that encode proteins which may confer resistance to various antibiotics including cephalosporin, which is used to treat pasteurellosis. The isolate was also found to harbour a phage region. This strain represents a novel multi-locus sequence type (MLST) that has not been previously identified, as all of the alleles used for MLST were found, but did not match any of the alleles in the database with 100% nucleotide identity. The most closely related ST was ST221. This is the first whole-genome sequence from P. multocida serovar B:2 of pig origin.
Keywords: Pasteurellosis, WGS, Pathogenicity, Serovar, Swine
Introduction
Pasteurella multocida, a Gram-negative, non-spore-forming, and non-motile rod, and is a member of the Pasteurella genus under family Pasteurellaceae [1]. Capsular typing and lipopolysaccharide (LPS) typing are the two key markers that are used for typing of P. multocida. Based on the antigenicity of the capsule, P. multocida species have been classified into five serovars — A, B, D, E, and F and 16 serovars based on LPS antigens [2–4]. Pasteurella multocida serovar A strains are usually involved in respiratory diseases, while P. multocida serovar B typically causes septicaemic pasteurellosis. The toxigenic capsular serovar D strains, and less frequently type A strains, cause progressive atrophic rhinitis (PAR). Acute septicaemic pasteurellosis caused by P. multocida serovar B:2 strains have been reported in India [5, 6]. Pasteurellosis is a result of complex interactions between host factors and the major virulence factors including the capsule, lipopolysaccharide (LPS), adhesions, and the outer membrane [7]. Some additional virulence factors in P. multocida are dermonectrotic toxin, diverse adhesions, iron acquisition mechanisms [8], and a number of virulence associated genes [9].
Endotoxic shock is attributed to the death of some animals with pasteurellosis, as previous treatment of animals with anti-TNFα antibodies before endotoxaemia occurred reduced or abrogated clinical signs [10]. Sixteen isolates of P. multocida cultured from cases diagnosed as acute septicaemic pasteurellosis in Vietnamese pigs demonstrated marked homogeneity among (capsular type-B specific) HSB-PCR positive swine isolates, which showed genotypic identity with P. multocida type B:2 from buffaloes diagnosed with HS [11]. It has been reported that organisms isolated from outbreaks of acute septicaemic pasteurellosis in pigs are pathogenic not only to pigs but also to other species such as rabbits, mice, guinea pigs, buffaloes, and goats [12]. In a previous study, the comparative genomic analysis of P. multocida from different hosts showed absence of host-specific genes of P. multocida, and phylogenetic analysis demonstrated that the same LPS and multi-locus sequence typing (MLST) genotypes often clustered together amongst the rest of the P. multocida genomes [13].
Pig pasteurellosis is an important disease of pigs and has a tremendous economic impact on livestock producers. However, P. multocida isolates are quite variable at the genomic level and there is minimal information on isolates of pig origin circulating in India. The present study was undertaken to complete the whole-genome sequence of the P. multocida ‘Soron’ serovar B:2 strain isolated from a pig that had died of septicaemia.
Material and methods
Isolation, initial characterization, and whole genome assembly
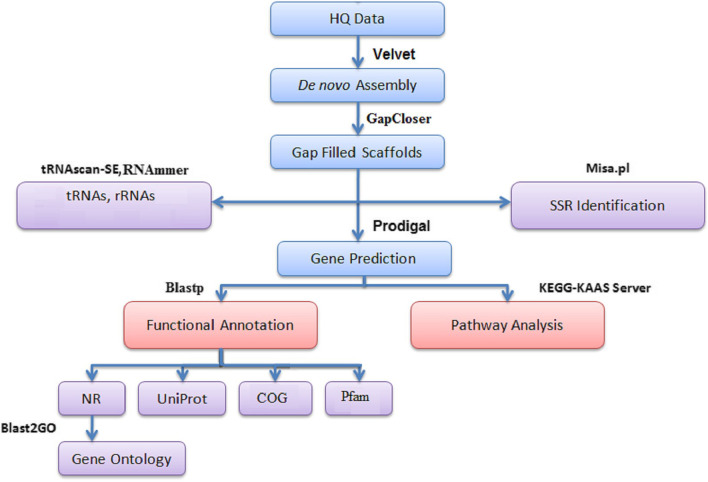
The P. multocida strain ‘Soron’ serovar B:2 was isolated from blood drawn from the heart of an adult female pig that had died of pasteurellosis at Livestock Production Management (Pig) Section at Indian Veterinary Research Institute, Izatnagar (Uttar Pradesh), India. The pig was examined at the post-mortem house by a pathologist for gross pathological lesions. The isolate was identified as P. multocida by PCR [14] and capsule-specific PCR assays [15]; then, conventional tests were also performed [16]. The bacteria prepared from a pure stock culture were grown in brain heart infusion (BHI) broth at 37 °C for 12–16 h. DNA was isolated from the sample by the Xcelgen bacterial gDNA isolation Kit. The AQubit® 2.0 Fluorometer was used to measure the concentration of DNA. The quality of DNA was checked on 0.8% agarose gel (loaded 3 μl) for the single intact band. The gel was run at 110 V for 30 min. The paired-end sequencing library was prepared using the Truseq Nano DNA Library prep kit. DNA (200 ng) was mechanically sheared into smaller fragments by Covaris focused-ultrasonicator followed by a continuous step of end-repair where an A is added to the 3′ ends, making the DNA fragments ready for adapter ligation. The amplified library was analysed in Bioanalyser 2100 (Agilent Technologies) using a high-sensitivity DNA chip as per manufacturer’s instructions. Following measurement of the concentration of input for the library through Qubit and range of size of the reads through Bioanalyser, the library was loaded onto Illumina platform for cluster generation and sequencing. Paired-End sequencing (2 × 150 bp) was carried out. A total of 2.62 Gb of data was generated (Table 1). Denovo assembly of P. multocida ‘Soron’ serovar B:2 strain was performed using Velvet [17] (Fig. 1). The MLST RIRDC scheme was used to determine the sequence type using the PubMLST database [18, 19].
Table 1.
Next-generation sequencing data statistics
| File name | Reads in R1 | Reads in R2 | Total Reads (R1 + R2) | Total Bases (R1 + R2) | Total data (Gb) |
|---|---|---|---|---|---|
| P. multocida, ‘Soron’ serovarB:2 | 9,104,673 | 9,104,673 | 18,209,346 | 2,629,391,375 | 2.62 |
Fig. 1.
Denovo assembly of P. multocida ‘Soron’ serovar B:2 strain
Non-coding RNA prediction and gene prediction, functional annotation, and gene ontology
tRNAscan-SE was used for the identification of probable tRNA genes. A local version of tRNAscan-SE v.2.0 with parameters—omf was used for the detection of tRNAs. Similarly, the RNAmmer software package to identify RNAs in the genome of the Pasteurella multocida serovar B:2 ‘Soron’. The assembled scaffolds from P. multocida, ‘Soron’ serovar B:2 sample obtained were subjected to gene prediction using Prodigal v2.6.3. The predicted proteins from the P. multocida ‘Soron’ serovar B:2 strain were subjected to similarity search against NCBI’s non-redundant (nr) database [20] using the BLASTp algorithm with an e-value threshold of 1e − 5. OMP genes, viz., a structural protein–OmpAl; transporter proteins — MetNIand TolC; binding protein — HasR(3CSL); membrane-associated enzymes — OMPLA (1QD6), NanH (2VW1), and NanB (2KVK5); and the protein assembly machine gene — Omp85 of P. multocida ‘Soron’ serovar B:2 were compared to Pm70 (NC-002663.1) and P52VAC (ALBZ01) strains. Gene Ontology (GO) annotation was obtained for the annotated proteins using blast260 [21] command line v-1.4.1. GO sequence distribution helped in specifying all the annotated nodes comprising GO functional groups. Genes associated with similar functions were assigned to same GO functional group. The GO sequence distribution was analysed for all the three GO domains: biological processes, molecular function, and cellular components. Comparative analysis of gene annotations in different databases — UniProt [22] [https://www.uniprot.org/uniparc/UPI00000002E4], Pfam [23], and COG [24] was also done.
Pathway analysis and mauve genome alignment
Pathway analysis, ortholog assignment, and mapping of genes to the biological pathways were performed using the KEGG automatic annotation server (KAAS). All predicted gene sequences from P. multocida, ‘Soron’ serovar B:2 sample was compared against the KEGG database [25] using BLASTP with a threshold bit score value of 60 (default). Further, Mauve [26] genome alignment was utilized to visualize genomic differences between strains such as inversions, rearrangements, insertions, and deletions through locally collinear blocks (LCBs). Pasteurella multocida P52, a strain from cattle, was used for comparison in this study because this strain is used occasionally for the vaccination of pigs in India, due to the non-availability of fully characterized pig strain(s) of P. multocida. Thus, in the present study, P. multocida ‘Soron’ strain serovar B:2 was investigated for genomic differences with Pm_70 (NC_002663.1), X73 (NZ_CM001580.1), P52VAC (ALBZ01), Pm_36950 (NC_016808.1), Pm_HB01 (NZ_CP06976.1), Pm_3480 (NC_017764.1), and Pm_HN06 (NC_017027.1/NC_017035.1).
Phylogenetic-based analysis using SNPS and comparative analysis using orthoMCL
The phylogenetic relationship between P. multocida strains from different host species was analysed using core-genome single nucleotide polymorphisms (SNPs) with the parSNP (version 1.5.3) software [27]. Within parSNP, muscle was used for aligning sequences, followed by phylogenetic tree construction with RAxML [28]. A total of 93 genomes (chromosome or scaffold level assemblies) of P. multocida strain were retrieved from NCBI isolated different host species including avian, bovine, porcine, and rabbit sources. The SNPs were identified by comparing each of the WGSs against the reference P. multocida ATCC 43137 genome sequence (GenBank accession NO. CP008918, avian host). It was observed that 66% of genomes between all these 95 strains was used for SNP calling and phylogeny. Phylogeny was visualized using iTOL [29].
Orthologs were identified using the OrthoMCLv 2.09 software [30]. OrthoMCL is an algorithm and a set of tools that can be used for the identification of orthologous genes within a set of genomes. Two genomes of P. multocida ‘Soron’ serovar B:2 and Pm_P52VAC were considered for ortholog identification. OrthoMCL performs all-versus-all analysis using BLASTP (blastall v2.2.24) with a default parameter (an “e-value” threshold of 1 × 10−5 and “percentage match cutoff” of 50%).
Identification of horizontal gene pool, antibiotic, and metal resistance genes
The genomic islands were predicted using IslandViewer 4 [31], a computational tool that integrates IslandPath-DIMOB, SIGI-HMM, Islander, and IslandPick. Antimicrobial and metal resistance genes were predicted using a comprehensive antibiotic resistance database (CARD) [32] and BacMet [33], respectively. CARD and BacMet are manually curated databases containing high-quality reference data on the molecular basis of antimicrobial and metal-resistance genes. The phage regions were predicted using PHASTER, which accurately identifies, annotates, and displays prophage sequences within bacterial genomes or plasmids.
Comparative analysis of SORON with NIVEDI strain isolated from Buffalo
The comparative analysis was done to delineate the differences between the strains, viz., ATCC 43137, SORON, NIVEDIPm32, NIVEDIPm34, NIVEDIPm35, and P52VAC [34]. Initially, phylogeny based on SNP variants was evaluated using parSNP to differentiate the clades of these strains. Further, BLAST Ring Image Generator (BRIG) [35] was used to compare the genomes of these strains with ATCC 43137 taken as the reference genome. Some important genes of P. multocida were put in the compared circular plot to know the identity among the strains.
Antimicrobial susceptibility testing
Antimicrobial susceptibility test was carried out using the disc diffusion method as described by Van Driessche [36]. Two replicates were performed for SORON isolate against nine antibiotics, namely, amoxyclav 30 mcg, ampicillin/sulbactam 10/10 mcg, amikacin 30 mcg, ceftriaxone 30 mcg, cefotaxime 30 mcg, erythromycin 15 mcg, gentamycin 120 mcg, norfloxacin 10 mcg, and tetracycline 30 mcg (Hi-media, India). The bacteria suspensions were cultured on Mueller-Hinton agar then the antibiotic where placed on the plates. After 24 h of incubation, the average zone of inhibition was measured and interpreted as per the table provided by the manufacturer.
Results
Isolation, initial characterization, and whole genome assembly
Pasteurella multocida ‘Soron’ serovar B:2 strain was characterized by biochemical tests, PCR assay, and its pathogenicity in mice and in pigs reported elsewhere [16]. Cardiac blood is the best source of sample for the isolation of Pasteurella. Only macroscopic pathological examination was done which showed ventral parts of the body, ear, fore, and hind limbs had cyanosis. Abdominal muscles and subcutaneous tissues showed congestion and haemorrhages. The lungs, kidney, and intestine were congested. Parietal surface of intestine and lungs showed fibrinous exudation. Heart blood smear revealed bipolar organisms and indistinguishable from Pasteurella spp. suggested septicaemia. Pasteurella multocida ‘Soron’ B:2 was found not to be specific to HS B:2 by PCR. The NGS data on velvet analysis yielded 33 scaffolds and 35 contigs. The average scaffold size was 68,852 bp with an N50 of 429,512 bp. The max scaffold size was 542,219. The total genome length was 2,271,124.
Non-coding RNA prediction and gene prediction, functional annotation, and gene ontology
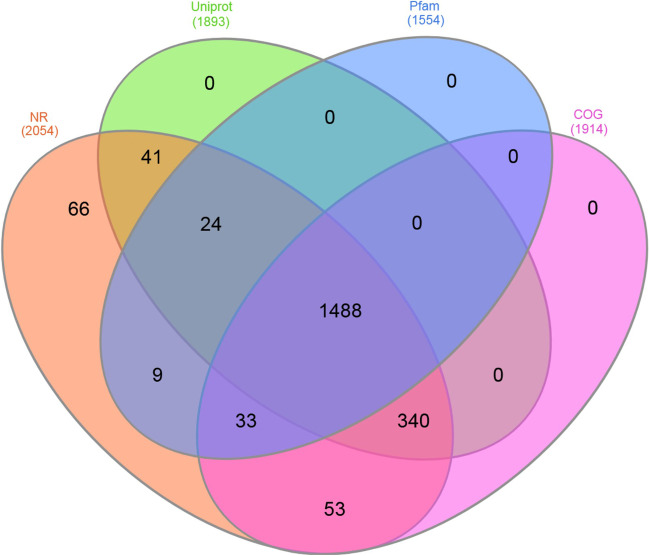
The genome had 53 tRNAs and 5 rRNAs, out of which four were 5S_rRNA, and one was 16S_rRNA. In the genome, 2057 genes have been predicted in Prodigal. The blast search yielded top-hits primarily against P. multocida. On comparing the gene annotation in different databases, 1488 genes were commonly predicted across BlastP, UniProt, Pfam, and COG. A total number of 340 genes were predicted commonly only by COG, Uniprot, and NR; 33 by only COG, Pfam, and NR; 9 by only NR and Pfam; 41 only by Uniport and NR; 53 by only COG and NR; and 66 only by NR (Fig. 2).
Fig. 2.
Prediction of genes by COG, Uniprot, NR, and Pfam
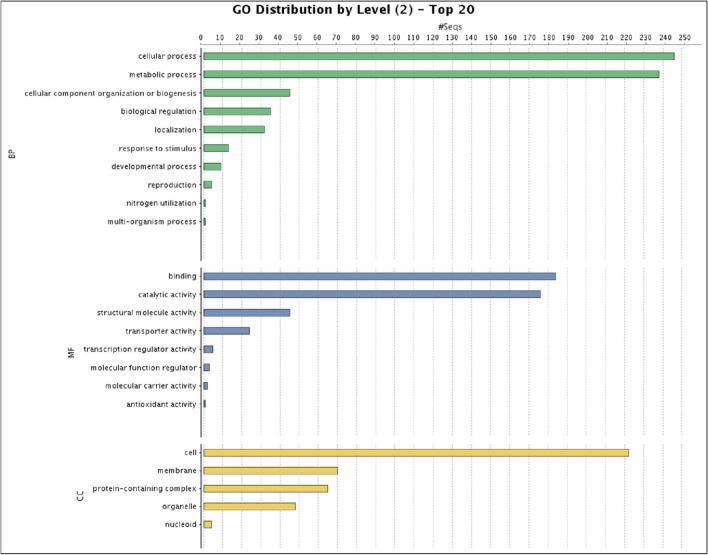
A total of 305 genes were assigned GO terms in the P. multocida ‘Soron’ serovar B:2 genome. A single gene can be placed in one or more GO terms or GO category. The gene distribution in different categories was 279, 223, and 273 in biological process, cellular component, and molecular function categories, respectively (Fig. 3).
Fig. 3.
The gene distribution in different categories
The outer membrane protein sequences (OMPs) of P. multocida ‘Soron’ serovar B:2- OmpA, TolC, MetNI, HasR, OMPLA, NanH, NanB, Omp85, and FhaC were compared with the OMPs of the strains Pm70 and P52VAC. Most of them had an amino acid identity greater than 95% (Table 2), except for OmpA, which did not have a hit in P52VAC. The possible absence of the hit in P52VAC for OmpA may be due to the contig-level assembly of the P52VAC genome.
Table 2.
Comparison of the OMP proteins of ‘Soron’ strain with Pm70 and P52VAC
| Outer Membrane Proteins | Pm70 | P52VAC |
|---|---|---|
| OmpA (1BXW) | 1010/1062 (95%) | No hit |
| TolC (1EK9) | 1363/1368 (99%) | 1355/1368 (99%) |
| MetNI (3DHW) | 1023/1035 (99%) | 1026/1035 (99%) |
| HasR (3CSL) | 2509/2547 (99%) | 2514/2547 (99%) |
| OMPLA (1QD6) | 866/873 (99%) | 859/873 (98%) |
| NanH (2VW1) | 2167/2249 (96%) | 2168/2251 (96%) |
| NanB (2KVK5) | 1933/2068 (93%) | 1175/1182 (99%) |
| Omp85 | 2360/2376 (99%) | 2363/2376 (99%) |
Pathway analysis and mauve genome alignment
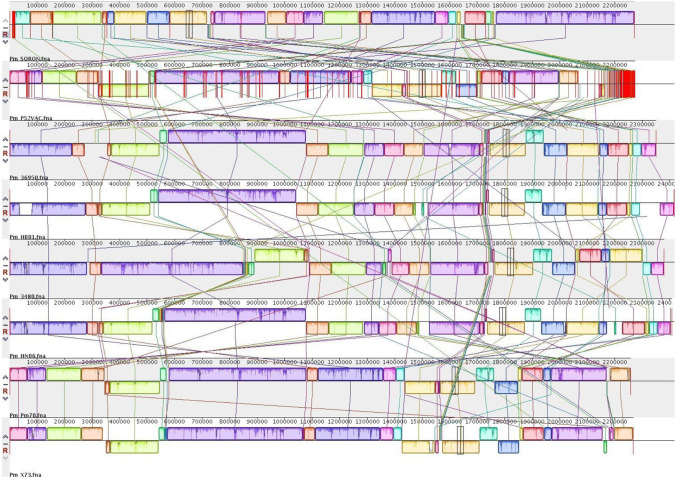
A 2057 predicted gene sequences from P. multocida type B:2 ‘Soron’ genome were compared against the KEGG database, and it was observed that 1506 genes were mapped and represented in metabolic pathways of major biomolecules such as carbohydrates, lipids, nucleotides, amino acids, glycans, cofactors, vitamins, terpenoids, and polyketides. Some proteins also represented involvement in genetic information processing, environmental information processing, and cellular processes (Table 3). The local linear blocks generated from Mauve across P. multocida genomes on multiple sequence alignment are given in Fig. 4. All the genomes demonstrated rearrangements using the ‘Soron’ genome as the reference. PmP52Vac had several rearrangements in comparison to the other genomes considered in the study. Most of the sections of the genome in PmP52VAC, Pm36950, PHB01, and Pm3480 were found to be inverted in comparison to P. multocida ‘Soron’ serovarB:2.
Table 3.
KEGG pathway distribution
| #Level 1 | Level 2 | No. of genes |
|---|---|---|
| 09100 Metabolism | 09101 Carbohydrate metabolism | 247 |
| 09102 Energy metabolism | 103 | |
| 09103 Lipid metabolism | 54 | |
| 09104 Nucleotide metabolism | 77 | |
| 09105 Amino acid metabolism | 159 | |
| 09106 Metabolism of other amino acids | 29 | |
| 09107 Glycan biosynthesis and metabolism | 57 | |
| 09108 Metabolism of cofactors and vitamins | 126 | |
| 09109 Metabolism of terpenoids and polyketides | 15 | |
| 09110 Biosynthesis of other secondary metabolites | 28 | |
| 09111 Xenobiotics biodegradation and metabolism | 18 | |
| 09120 Genetic information processing | 09121 Transcription | 4 |
| 09122 Translation | 82 | |
| 09123 Folding, sorting, and degradation | 45 | |
| 09124 Replication and repair | 75 | |
| 09130 Environmental information processing | 09131 Membrane transport | 152 |
| 09132 Signal transduction | 68 | |
| 09140 Cellular processes | 09141 Transport and catabolism | 9 |
| 09142 Cell motility | 8 | |
| 09143 Cell growth and death | 18 | |
| 09145 Cellular community — prokaryotes | 67 | |
| 09150 Organismal systems | 09149 Aging | 12 |
| 09151 Immune system | 6 | |
| 09152 Endocrine system | 18 | |
| 09154 Digestive system | 2 | |
| 09156 Nervous system | 2 | |
| 09158 Development and regeneration | 1 | |
| 09159 Environmental adaptation | 6 | |
| 09160Human diseases | 09161 Cancer: overview | 13 |
| 09162 Cancer: specific types | 4 | |
| 09163 Immune disease | 1 | |
| 09164 Neurodegenerative disease | 9 | |
| 09166 Cardiovascular disease | 5 | |
| 09167 Endocrine and metabolic disease | 5 | |
| 09171 Infectious disease: bacterial | 14 | |
| 09172 Infectious disease: viral | 1 | |
| 09174 Infectious disease: parasitic | 1 | |
| 09175 Drug resistance: antimicrobial | 32 | |
| 09176 Drug resistance: antineoplastic | 7 | |
| 09180 Brite hierarchies | 09181 Protein families: metabolism | 189 |
| 09182 Protein families: genetic information processing | 526 | |
| 09183 Protein families: signalling and cellular Processes | 416 | |
| 09190 Not included in pathway or brite | 09191 Unclassified: metabolism | 91 |
| 09192 Unclassified: genetic information processing | 19 | |
| 09193 Unclassified: signalling and cellular processes | 52 | |
| 09194 Poorly characterized | 89 | |
| 09143 Cell growth and death | 18 | |
| 09145 Cellular community — prokaryotes | 67 | |
| 09150 Organismal systems | 09149 Aging | 12 |
| 09151 Immune system | 6 | |
| 09152 Endocrine system | 18 | |
| 09154 Digestive system | 2 | |
| 09156 Nervous system | 2 | |
| 09158 Development and regeneration | 1 | |
| 09159 Environmental adaptation | 6 | |
| 09160 Human diseases | 09161 Cancer: overview | 13 |
| 09162 Cancer: specific types | 4 | |
| 09163 Immune disease | 1 | |
| 09164 Neurodegenerative disease | 9 | |
| 09166 Cardiovascular disease | 5 | |
| 09167 Endocrine and metabolic disease | 5 | |
| 09171 Infectious disease: bacterial | 14 | |
| 09172 Infectious disease: viral | 1 | |
| 09174 Infectious disease: parasitic | 1 | |
| 09175 Drug resistance: antimicrobial | 32 | |
| 09176 Drug resistance: antineoplastic | 7 | |
| 09180 Brite hierarchies | 09181 Protein families: metabolism | 189 |
| 09182 Protein families: genetic information processing | 526 | |
| 09183 Protein families: signalling and cellular Processes | 416 | |
| 09190 Not included in pathway or brite | 09191 Unclassified: metabolism | 91 |
| 09192 Unclassified: genetic information processing | 19 | |
| 09193 Unclassified: signalling and cellular processes | 52 | |
| 09194 Poorly characterized | 89 | |
| 09143 Cell growth and death | 18 | |
| 09145 Cellular community — prokaryotes | 67 | |
| 09150 Organismal systems | 09149 Aging | 12 |
| 09151 Immune system | 6 | |
| 09152 Endocrine system | 18 | |
| 09154 Digestive system | 2 | |
| 09156 Nervous system | 2 | |
| 09158 Development and regeneration | 1 | |
| 09159 Environmental adaptation | 6 | |
| 09160 Human diseases | 09161 Cancer: overview | 13 |
| 09162 Cancer: specific types | 4 | |
| 09163 Immune disease | 1 | |
| 09164 Neurodegenerative disease | 9 | |
| 09166 Cardiovascular disease | 5 | |
| 09167 Endocrine and metabolic disease | 5 | |
| 09171 Infectious disease: bacterial | 14 | |
| 09172 Infectious disease: viral | 1 | |
| 09174 Infectious disease: parasitic | 1 | |
| 09175 Drug resistance: antimicrobial | 32 | |
| 09176 Drug resistance: antineoplastic | 7 | |
| 09180 Brite hierarchies | 09182 Protein families: genetic information Processing | 526 |
| 09183 Protein families: signalling and cellular processes | 416 | |
| 09190 Not included in pathway or brite | 09191 Unclassified: metabolism | 91 |
| 09192 Unclassified: genetic information processing | 19 | |
| 09193 Unclassified: signalling and cellular processes | 52 | |
| 09194 Poorly characterized | 89 | |
| 09143 Cell growth and death | 18 | |
| 09145 Cellular community — prokaryotes | 67 | |
| 09150 Organismal systems | 09149 Aging | 12 |
| 09151 Immune system | 6 | |
| 09152 Endocrine system | 18 | |
| 09154 Digestive system | 2 | |
| 09156 Nervous system | 2 | |
| 09158 Development and regeneration | 1 | |
| 09159 Environmental adaptation | 6 | |
| 09160 Human diseases | 09161 Cancer: overview | 13 |
| 09162 Cancer: specific types | 4 | |
| 09163 Immune disease | 1 | |
| 09164 Neurodegenerative disease | 9 | |
| 09166 Cardiovascular disease | 5 | |
| 09167 Endocrine and metabolic disease | 5 | |
| 09171 Infectious disease: bacterial | 14 | |
| 09172 Infectious disease: viral | 1 | |
| 09174 Infectious disease: parasitic | 1 | |
| 09175 Drug resistance: antimicrobial | 32 | |
| 09176 Drug resistance: antineoplastic | 7 | |
| 09180 Brite hierarchies | 09181 Protein families: metabolism | 189 |
| 09182 Protein families: genetic information processing | 526 | |
| 09183 Protein families: signalling and cellular processes | 416 | |
| 09190 Not included in pathway or brite | 09191 Unclassified: metabolism | 91 |
| 09192 Unclassified: genetic information processing | 19 | |
| 09193 Unclassified: signalling and cellular processes | 52 | |
| 09194 Poorly characterized | 89 |
Fig. 4.
The local linear blocks generated from Mauve across P. multocida genomes
Phylogenetic analysis using SNPS and comparative analysis using orthoMCL
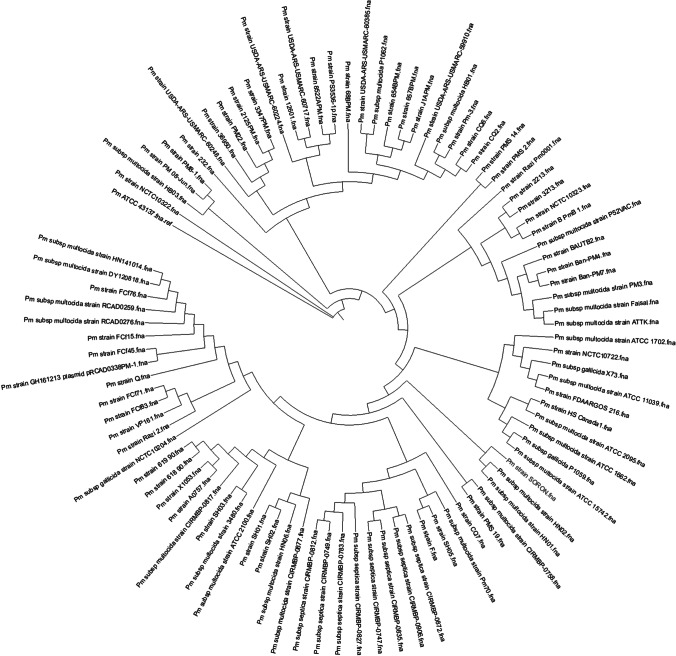
The phylogenetic analysis of the 93 sequences revealed that Pm_P52VAC and P. multocida ‘Soron’ serovar B:2 clustered in different clades. Pasteurella multocida ‘Soron’ serovar B:2 was found clustered with HN01 and HN02 strains, whereas Razi_Pm0001 and BAUTB2 were immediate neighbours of Pm_52VAC (Fig. 5). Pasteurella multocida ATCC 43137 and 4 strains of porcine origin were found clustered in one clade under the same node that contains P. multocida strains of bovine origin. Most of the serovar A strains of bovine origin clustered into one clade. Also, strains from rabbits clustered into one subclade as they belonged to serovar A. Serovar D strains originated from rabbit and pig were found clustered in one group. The vaccine strain P52VAC, a buffalo isolate, was found clustered with the isolates of bovine belonging to serovar B. Pasteurella multocida ‘Soron’ serovar B:2 was found to be clustered to the same ancestor of Pm70, which is of avian origin. This Pm70 strain was also found closely associated with strains of sheep and goats. SH05, which is Serovar F, is the strain of pig origin that was found closely associated with the ‘Soron’ strain. Most of the avian origin strains were found clustered under the same node. Some bovine origin strains of serovar A were clustered along with the strains of turkey and rabbit origin that belonged to serovar A. Furthermore, the PmSoron and PmP52Vac sequences were compared using orthoMCL. These sequences were found to have 1812 shared genes. A total of 237 and 193 genes were unique to PmSoron and PmP52Vac, respectively (Fig. 6).
Fig. 5.
The phylogenetic analysis of the 93 sequences showing Pm_P52VAC and P. multocida ‘Soron’ serovar B:2 clustered in different clades
Fig. 6.
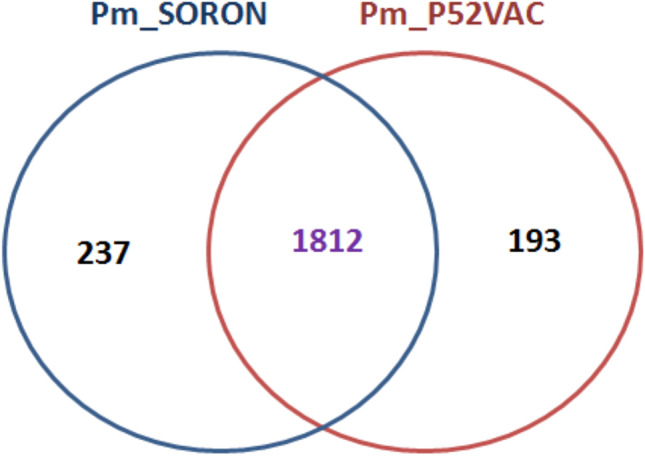
Comparison of a total of 237 and 193 genes using orthoMCL
Identification of horizontal gene pool, antibiotic, and metal resistance genes
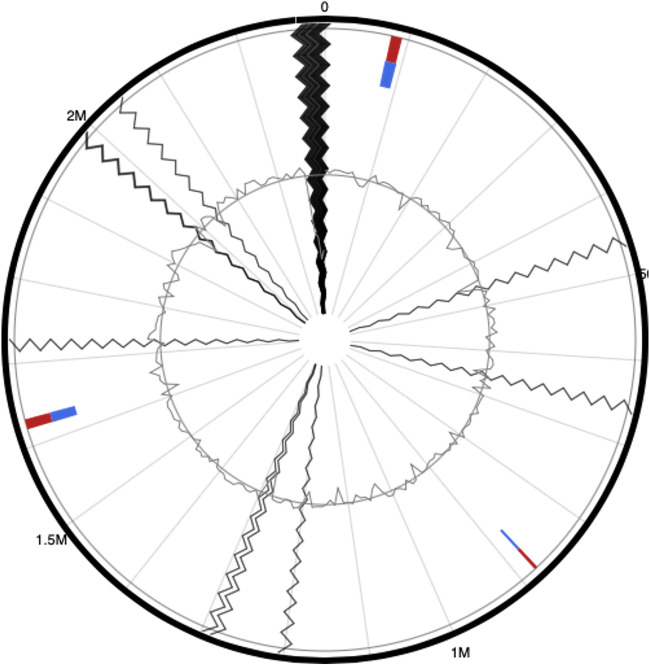
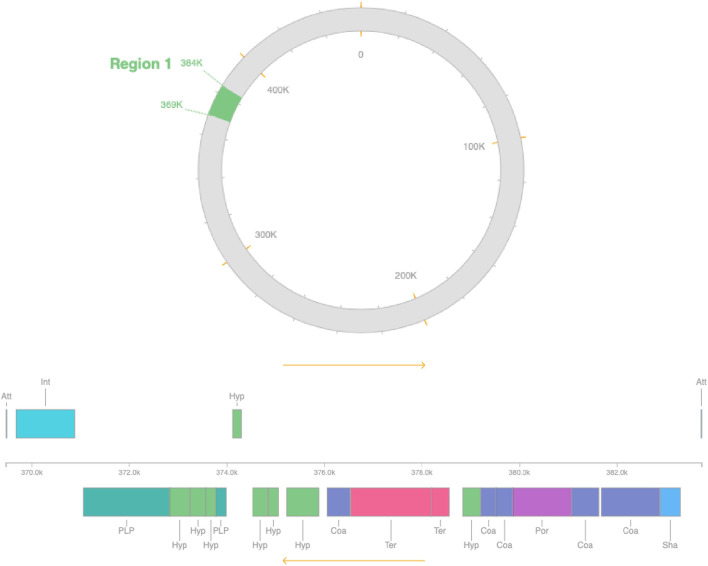
The genome was found to contain proteins that may confer resistance to elfamycin, cephalosporin, cephamycin, and carbapenams. Using Islandviewer 4, the genome was found to contain 34 GIs of which 21 were found encoding hypothetical proteins and 13 functional proteins (Fig. 7). Furthermore, BacMet analysis revealed the presence of an ABC transporter, as well as proteins associated with resistance to bicyclomycin and tolerance to molybdenum and nickel. Using PHASTER, it was observed that the genome contains only one phage region that contains various phage proteins (Fig. 8).
Fig. 7.
Assessment of the genome Pasteurella multocida which found encoding hypothetical proteins and 13 functional proteins
Fig. 8.
PHASTER shows that the genome contains only one phage region that contains various phage proteins
The genome was further assessed for virulence genes. The pfHB2 toxin, the cysteine protease, was found spanning from 1,082,594 to 1,083,200 bp. The haemagglutinin gene was found from1,082,594 to 1,083,325 bp, the ptfA gene-subunit of type IV fimbriae from 1,116,609 to 1,117,004 bp, and the nanH-sialidase gene from 1,967,754 to 1,966,872 bp.
MLST analysis
The specific sequence type of the ‘Soron’ strain used in this study was unable to be determined. The analysis suggested that the most closely related sequence type was ST221, but mutations in the gdh and pmi genes resulted in nucleotide identities of 99.6% and 99.8%, respectively. Since all of the genes used for MLST were identified, but a specific sequence type was not identified, this likely represents a novel combination of alleles that does not yet exist in the MLST database.
Analysis of SORON with NIVEDI strain isolated from buffalo
Upon phylogenetic analysis of these genomes, it was observed that the Soron was clustering in a different clade compared to the clades of other isolates. All three NIVEDI strains along with P52VAC were found clustering together (Fig. 9). Clustering of these strains may be due to of their bovine origin. Analysis revealed that some genomic regions in NIVEDI and SORON strains were either less or not identical with ATCC 43137 strain. However, most of these regions are currently not annotated in the NCBI database. These are represented as faded region in Fig. 10. Some genes, viz., ompa, NanB, hyaD, and trep, had less identity in these strains compared to ATCC 43137 (Table 4).
Fig. 9.
All three NIVEDI strains were found clustering together
Fig. 10.
Some genomic regions in NIVEDI and SORON strains were either less or not identical with ATCC 43137 strain
Table 4.
Comparative analysis of genes of SORON and NIVEDI strain
| Gene | Function |
|---|---|
| omp85 | OMP85_target: outer membrane insertion C-terminal signal domain protein |
| METN | ABC_MetN: D-methionine ABC transporter, ATP-binding protein |
| NanH | Sialidase |
| NanB | Sialidase |
| OMPLA | Membrane associated enzyme |
| hasR | Heme acquisition system receptor |
| ToIC | Transporter protein |
| ompa | Porin |
| torY | Cytochrome c-type protein TorY |
| hyaD | Hyaluronan synthase |
| idnD | L-idonate 5-dehydrogenase |
| gno | Gluconate 5-dehydrogenase |
| treC | Alpha,alpha-phosphotrehalase |
| treP | PTS system, trehalose-specific IIBC component |
| treR | Trehalose operon repressor |
| rimO | Ribosomal protein S12 methylthiotransferase |
| udp | Uridine phosphorylase |
| tkt | Transketolase |
| lex1 | Lipooligosaccharide biosynthesis protein lex-1 |
| rpmE | Ribosomal protein L31 |
Antimicrobial susceptibility testing
The Soron isolate was tested against nine antibiotics (Table 5). There was no evidence of any resistance to amoxyclav, amikacin, ampicillin/sulbactam, cefotaxime, ceftriaxone, erythromycin, gentamicin, norfloxacin, and tetracycline in Soron pig isolate, while amoxyclav showed intermediate zone of inhibition against Soron isolate.
Table 5.
Antibiotic susceptibility testing against Soron isolate
| S. no | Antibiotics | Soron | |
|---|---|---|---|
| Zone of inhibition (mm) | |||
| 1 | Amoxyclav (AMC) 30 mcg | 14 | I |
| 2 | AmpicillinSulbactam (A/S) 10/10 mcg | 27 | S |
| 3 | Amikacin (AK) 30 mcg | 19 | S |
| 4 | Cefotaxime (CTX) 30 mcg | 26 | S |
| 5 | Ceftriaxone (CTR) 30 mcg | 32 | S |
| 6 | Erythromycin (E) 15 mcg | 25 | S |
| 7 | Gentamicin (HLG) 120 mcg | 34 | S |
| 8 | Norfloxacin (NX) 10 mcg | 34 | S |
| 9 | Tetracycline (TE) 30 mcg | 29 | S |
R resistant, I intermediate, S susceptible
Discussion
Global trends in infectious diseases of swine demonstrate that the implications of P. multocida outbreaks are a major vulnerability of the global swine industry [37]. Furthermore, current genomic information on isolates of pig origin from India is lacking, which emphasizes the importance of completely characterizing P. multocida ‘Soron’ serovar B:2 at a genomic level. The P. multocida ‘Soron’ B:2 was not specific to cattle B:2. MLST analysis demonstrated that this was a novel MLST that has not been previously identified, but was related to ST221, which has not been extensively characterized. PmP52VAC demonstrated several rearrangements in comparison to P. multocida serovar B:2 ‘Soron’, indicating structural differences at the genomic level despite them both belonging to serovar B:2. Furthermore, genomic inversions of Pm36950, PHB01, and Pm3480 in comparison to P. multocida ‘Soron’ serovar B:2 ratifies the ‘Soron’ strain to be an isolate with structural rearrangements.
The outer membrane of the bacterium serves as a selective barrier by preventing toxic molecules from entering into the cell, thereby aiding in the survival of the bacterium in diverse environments [38]. OmpA is a slow porin that is related to the physical linkage between the outer membrane and peptidoglycan, and as a structural protein, this would be expected to be highly similar across strains. However, results from this study demonstrated 95% amino acid identity for OmpA between the ‘Soron’ strain and Pm70, and there was no alignment to OmpA of P52VAC. TolC proteins are associated with systems that are responsible for multidrug resistance in bacteria, and TolC of the ‘Soron’ strain had 99% identity with those of Pm70 and P52VAC [39]. Similar results were seen in percent identity comparisons of Has R, which is an OM receptor that binds excreted haem binding proteins [40]. NanH and NanB are two sialidase proteins, which are associated with the outer membrane of P. multocida [41] and contribute to the virulence of some pathogenic organisms [42]. The ‘Soron’ strain demonstrated 96% identity with NanH of Pm70 and P52VAC. For NanB, the ‘Soron’ strain had only 93% identity with Pm70, but 99% with P52VAC. Further investigation is needed to determine whether the diversity within sialidase proteins contributes to differences in virulence. Omp85 is known to contribute to the assembly of proteins and lipids in the bacterial membrane [43] and with 99% identity of Omp85 across both compared strains, indicates a similar role of these proteins in these strains.
The P. multocida ‘Soron’ serovar B:2 strain was found more closely related to the reference genome Pm70 and to other non-HS strains including X73, Pm70, and P1059, whereas P52VAC strain was found to be closely related to HS strains [44]. Pm70 strain, an avirulent avian strain of capsular type F, has more than 100 identified genes involved in virulence [45]. The ptf4 and pfhb2 genes were identified in P. multocida ‘Soron’ serovar B:2 strain, similar to Pm70, X73, and P1059 [46]. Clustering of P. multocida ‘Soron’ type B:2 with nonvirulent strain may be due to the same LPS genotype, as in the previous study, which showed that the SNP-based WGS phylogeny clustering is due to the same LPS genotype [13]. Comparative genomics revealed that there were no genes or genes identified in P. multocida as virulence factors (VFGs) specific to any isolates for any specific type of host [47–49].
Cephalosporins are potent drug for treating pasteurellosis in most cases [50]. The growing concern about the increase in antimicrobial resistances has meant that national and international actions has been taken to monitor, harmonize, and prudently use antibiotics [51, 52]. The presence of a gene potentially conferring resistance to cephalosporin drugs in P. multocida ‘Soron’ type B:2 is somewhat concerning and may be a challenge in treating the disease. Ultimately, the availability of the genome sequence provides a foundation for future research into the epidemiology, evolution, and mechanisms of pathogenesis and host specificity of P. multocida ‘Soron’ serovar B:2.
Abbreviations
- HS
Haemorrhagic septicaemia
- HSB
Capsular type-B specific
Author contribution
Rishendra Verma and Mayank Rawat designed the experiment. Harshit Verma conducted the bacteriological experiments. Ravi Gandham, A. K. Tiwari, Raja Ishaq Nabi Khan, and Manas Ranjan Praharaj were involved in the analysis of the bioinformatic data. Emily Smith supported for the final analysis of the raw data as well as help for the graphical representation of the data. The manuscript was written by Rishendra Verma, Emily Smith, Ravi Gandham, and A. K. Tiwari.
Funding
This work was supported by the Indian Council of Agricultural Research-Indian Veterinary Research Institute, India.
Data availability
Data transparency is supporting our work.
Declarations
Ethics approval
The study did not involve animal experimentation either in animals or humans.
Consent to participate
Not applicable.
Consent for publication
Consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Nucleotide sequence accession number
This Whole Genome Shotgun project has been deposited in GenBank under the BioProject accession number PRJNA698765.
Footnotes
Responsible Editor: Luis Augusto Nero
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peng Z, Wang X, Zhou R, Chen H, Wilson BA, Wu B. Pasteurella multocida: genotypes and genomics. Microbiol Mol Biol Rev. 2019;83(4):e00014–19. doi: 10.1128/MMBR.00014-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rimler RB, Rhoades KR. Serogroup F, a new capsule serogroup of Pasteurella multocida. J Clin Microbiol. 1987;25(4):615–618. doi: 10.1128/jcm.25.4.615-618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter GR. Studies on Pasteurella multocida. I. A hemagglutination test for the identification of serological types. Am J Vet Res. 1955;16(60):481–484. [PubMed] [Google Scholar]
- 4.Heddleston KL, Gallagher JE, Rebers PA. Fowl cholera: gel diffusion precipitin test for serotyping Pasteruella multocida from avian species. Avian Dis. 1972;16(4):925–936. doi: 10.2307/1588773. [DOI] [PubMed] [Google Scholar]
- 5.Pillai AGR, Katiyar AK, Awadhiya RP, Vegad JL. An outbreak of pasteurollosis in swine. Indian Vet J. 1986;63(7):527–529. [Google Scholar]
- 6.Verma ND, Saxena SC. An outbreak of swine pasteurollosis on an organized farm of north-eastern hills regions. Indian J Anim Sci. 1987;57(6):528–532. [Google Scholar]
- 7.Boyce JD, Harper M, Wilkie I, Adler B (2010) Pasteurella. In: Pathogenesis of bacterial infections in animals, 4th Ed. Prescott J. (Ed), Blackwell Publishing, Iowa
- 8.Ewers C, Lübke-Becker A, Bethe A, Kiebling S, Filter M, Wieler LH. Virulence genotype of Pasteurella multocida strains isolated from different hosts with various disease status. Vet Microbiol. 2006;114(3–4):304–317. doi: 10.1016/j.vetmic.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Furian TQ, Borges KA, Laviniki V, et al. Virulence genes and antimicrobial resistance of Pasteurella multocida isolated from poultry and swine. Braz J Microbiol. 2016;47(1):210–216. doi: 10.1016/j.bjm.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cargile JL, MacKay RJ, Dankert JR, Skelley L. Effects of tumor necrosis factor blockade on interleukin 6, lactate, thromboxane, and prostacyclin responses in miniature horses given endotoxin. Am J Vet Res. 1995;56(11):1445–1450. [PubMed] [Google Scholar]
- 11.Townsend KM, O'Boyle D, Phan TT, et al. Acute septicaemic pasteurellosis in Vietnamese pigs. Vet Microbiol. 1998;63(2–4):205–215. doi: 10.1016/s0378-1135(98)00233-8. [DOI] [PubMed] [Google Scholar]
- 12.Murty DK, Kaushik RK. Studies on an outbreak of acute swine pasteurellosis due to Pasteurella multocida Type B (Carter, 1955) Vet Rec. 1965;77:411–416. [PubMed] [Google Scholar]
- 13.Peng Z, Liang W, Wang F, et al. Genetic and phylogenetic characteristics of Pasteurella multocida isolates from different host species. Front Microbiol. 2018;9:1408. doi: 10.3389/fmicb.2018.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Townsend KM, Frost AJ, Lee CW, Papadimitriou JM, Dawkins HJ. Development of PCR assays for species- and type-specific identification of Pasteurella multocida isolates. J Clin Microbiol. 1998;36(4):1096–1100. doi: 10.1128/JCM.36.4.1096-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Townsend KM, Boyce JD, Chung JY, Frost AJ, Adler B. Genetic organization of Pasteurella multocida cap Loci and development of a multiplex capsular PCR typing system [published correction appears in J Clin Microbiol 2001 Jun; 39(6):2378] J Clin Microbiol. 2001;39(3):924–929. doi: 10.1128/JCM.39.3.924-929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verma H, Rawat M, Samal A, Upamanyu V. Conventional and molecular characterization of Pasteurella multocida isolated from a case of swine septicaemic pasteuerllosis. Indian J Anim Res. 2014;48(6):605–608. doi: 10.5958/0976-0555.2014.00040.5. [DOI] [Google Scholar]
- 17.Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18(5):821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subaaharan S, Blackall LL, Blackall PJ. Development of a multi-locus sequence typing scheme for avian isolates of Pasteurella multocida. Vet Microbiol. 2010;141(3–4):354–361. doi: 10.1016/j.vetmic.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications [version 1; peer review: 2 approved] Wellcome Open Res. 2018;3:124. doi: 10.12688/wellcomeopenres.14826.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 21.Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18):3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- 22.UniProt: the universal protein knowledgebase in 2021. n.d. https://www.uniprot.org/uniparc/UPI00000002E4 [DOI] [PMC free article] [PubMed]
- 23.El-Gebali S, Mistry J, Bateman A, et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019;47(D1):D427–D432. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tatusov RL, Galperin MY, Natale DA, Koonin EV. The COG database: a tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000;28(1):33–36. doi: 10.1093/nar/28.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darling AC, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14(7):1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Treangen TJ, Ondov BD, Koren S, Phillippy AM. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014;15(11):524. doi: 10.1186/s13059-014-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30(9):1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Letunic I, Bork P. Interactive Tree of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Stoeckert CJ, Jr, Roos DS. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13(9):2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertelli C, Laird MR, Williams KP, et al. IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017;45(W1):W30–W35. doi: 10.1093/nar/gkx343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McArthur AG, Waglechner N, Nizam F, et al. The comprehensive antibiotic resistance database. Antimicrob Agents Chemother. 2013;57(7):3348–3357. doi: 10.1128/AAC.00419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pal C, Bengtsson-Palme J, Rensing C, Kristiansson E, Larsson DG. BacMet: antibacterial biocide and metal resistance genes database. Nucleic Acids Res. 2014;42(Database issue):D737–D743. doi: 10.1093/nar/gkt1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prajapati A, Yogisharadhya R, Mohanty NN, Mendem SK, Nizamuddin A, Chanda MM, Shivachandra SB. Comparative genome analysis of Pasteurella multocida serogroup B:2 strains causing haemorrhagic septicaemia (HS) in bovines. Gene. 2022;826:146452. doi: 10.1016/j.gene.2022.146452. [DOI] [PubMed] [Google Scholar]
- 35.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Driessche L, Bokma J, Gille L, et al. Rapid detection of tetracycline resistance in bovine Pasteurella multocida isolates by MALDI Biotyper antibiotic susceptibility test rapid assay (MBT-ASTRA) Sci Rep. 2018;8(1):13599. doi: 10.1038/s41598-018-31562-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanderWaal K, Deen J. Global trends in infectious diseases of swine. Proc Natl Acad Sci U S A. 2018;115(45):11495–11500. doi: 10.1073/pnas.1806068115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hatfaludi T, Al-Hasani K, Boyce JD, Adler B. Outer membrane proteins of Pasteurella multocida. Vet Microbiol. 2010;144(1–2):1–17. doi: 10.1016/j.vetmic.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 39.Piddock LJ. Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol. 2006;4(8):629–636. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 40.Krieg S, Huché F, Diederichs K, et al. Heme uptake across the outer membrane as revealed by crystal structures of the receptor-hemophore complex. Proc Natl Acad Sci U S A. 2009;106(4):1045–1050. doi: 10.1073/pnas.0809406106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizan S, Henk A, Stallings A, Maier M, Lee MD. Cloning and characterization of sialidases with 2–6' and 2–3′ sialyl lactose specificity from Pasteurella multocida. J Bacteriol. 2000;182(24):6874–6883. doi: 10.1128/JB.182.24.6874-6883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19(5):507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knowles TJ, Scott-Tucker A, Overduin M, Henderson IR. Membrane protein architects: the role of the BAM complex in outer membrane protein assembly. Nat Rev Microbiol. 2009;7(3):206–214. doi: 10.1038/nrmicro2069. [DOI] [PubMed] [Google Scholar]
- 44.Moustafa AM, Seemann T, Gladman S, et al. Comparative genomic analysis of Asian haemorrhagic septicaemia-associated strains of Pasteurella multocida identifies more than 90 haemorrhagic Septicaemia-specific genes. PLoS One. 2015;10(7):e0130296. doi: 10.1371/journal.pone.0130296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.May BJ, Zhang Q, Li LL, Paustian ML, Whittam TS, Kapur V. Complete genomic sequence of Pasteurella multocida, Pm70. Proc Natl Acad Sci U S A. 2001;98(6):3460–3465. doi: 10.1073/pnas.051634598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson TJ, Abrahante JE, Hunter SS, et al. Comparative genome analysis of an avirulent and two virulent strains of avian Pasteurella multocida reveals candidate genes involved in fitness and pathogenicity. BMC Microbiol. 2013;13:106. doi: 10.1186/1471-2180-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubatzky KF. Pasteurella multocida and immune cells. Curr Top Microbiol Immunol. 2012;361:53–72. doi: 10.1007/82_2012_204. [DOI] [PubMed] [Google Scholar]
- 48.Wilkie IW, Harper M, Boyce JD, Adler B. Pasteurella multocida: diseases and pathogenesis. Curr Top Microbiol Immunol. 2012;361:1–22. doi: 10.1007/82_2012_216. [DOI] [PubMed] [Google Scholar]
- 49.Wilson BA, Ho M. Pasteurella multocida: from zoonosis to cellular microbiology. Clin Microbiol Rev. 2013;26(3):631–655. doi: 10.1128/CMR.00024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang X, Zhao Z, Hu J, et al. Isolation, antimicrobial resistance, and virulence genes of Pasteurella multocida strains from swine in China. J Clin Microbiol. 2009;47(4):951–958. doi: 10.1128/JCM.02029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO (2015) Global action plan on antimicrobial resistance. http://www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf
- 52.Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA-SPAIN) Report (2018) First joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance. Spain. http://www.resistenciaantibioticos.es/es/publicaciones/informe-jiacra-espana [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data transparency is supporting our work.