Abstract
Acute respiratory infections are a constant public health problem causing childhood morbidity and mortality worldwide. Reported cases of major respiratory infections decreased in 2020 after restrictive measures were adopted to contain the COVID-19 pandemic, but there is little data on the impact after these measures were relaxed in the subsequent years. This study conducted molecular analysis to identify rhinovirus, respiratory syncytial virus, influenza A virus, and adenovirus in SARS-CoV-2-negative samples taken from symptomatic pediatric patients during 2021 and 2022 to ascertain the impact of pandemic response measures within the broader epidemiological scenario. The positivity rates found were 28.3% and 50.8%, in 2021 and 2022, respectively, representing a significant increase (1.8 times) in the circulation of non-SARS-CoV-2 viruses after the reduction of non-pharmacological measures to contain the COVID-19 pandemic. Within the positive samples, rhinovirus and respiratory syncytial virus were most frequent (44.4 and 18% in 2021; 44.5 and 22.5% in 2022), whereas influenza A and adenovirus were found in lower frequency (12.5 and 5.5% in 2021; 13.4 and 4.9% in 2022, respectively). Because these different respiratory virus diseases produce similar symptoms, diagnosis based on clinical condition alone can be inaccurate, and more reliable testing is required to select the best therapeutic approach for each case. The loosening of restrictive measures to contain the COVID-19 pandemic led to higher numbers of other respiratory infections in pediatric patients. Ongoing surveillance and differential diagnosis of respiratory viruses are required to better understand their seasonal patterns after the COVID-19 pandemic to guide prevention and control strategies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01087-y.
Keywords: Acute respiratory infections, Respiratory syncytial virus, Rhinovirus, Influenza virus, Adenovirus, SARS-CoV-2
Introduction
Respiratory infections are a common cause of childhood morbidity and mortality worldwide [1–3] and consequently represent an important and ongoing public health problem [4]. Acute respiratory syndromes are often restricted to the upper respiratory tract, where they may cause rhinitis, pharyngitis, and laryngitis, but the lower respiratory tract can be affected in more severe manifestations, leading to bronchiolitis and pneumonia [5, 6]. Children may be affected by several episodes of respiratory infections ranging from mild to severe, which may involve hospitalization or even mechanical ventilation [7]. Besides high susceptibility to these infections, high rates of co-infections are also reported, mainly in children under 5 years old [8]. These infections are generally associated with respiratory viruses which exhibit different seasonality patterns [9], with respiratory syncytial virus (RSV), rhinovirus (RV), influenza virus (IV), adenovirus (AdV), and coronaviruses (CoVs) the most prevalent [4, 10].
With the emergence of a new coronavirus (SARS-CoV-2) in December 2019 and the declaration of the resulting COVID-19 pandemic by the World Health Organization (WHO) in early 2020 [11–13], respiratory infections in children became more worrying, since the transmission routes and symptoms of COVID-19 and other respiratory virus infections overlap (fever, cough, dyspnea, and pneumonia), increasing the chances of misdiagnosis [14]. Thus, the COVID-19 pandemic, combined with seasonal peaks of other respiratory viruses, consequently, represents a major risk for co-infections with unknown consequences, especially in pediatric patients.
Brazil’s first cases of COVID-19 were detected in February 2020, just before autumn and winter seasons, which in the southern hemisphere occur from March to September [15]. With vaccines and specific treatments for COVID-19 not yet available, in 2020, WHO recommended non-pharmacological interventions to contain transmission of the virus such as social isolation, masking, closing schools, hand hygiene, and travel restrictions [13]. These measures effectively reduced the circulation of SARS-CoV-2 and other respiratory viruses like influenza A (IAV) and RSV in both hemispheres, as well as hospitalizations for bronchiolitis in 2020 [16, 17]. Although these actions effectively contain virus spread [18], the Brazilian population adopted them to varying degrees throughout the country, began to abandon them during 2021, and by early 2022 were no longer utilizing them. This scenario allowed us to investigate respiratory viruses in SARS-CoV-2-negative samples taken during the course of the COVID-19 pandemic and also examine how loosening preventive measures impacted the transmission of these viruses in 2021 and 2022.
Material and methods
Samples and molecular investigation
Nasopharyngeal swab samples were obtained from pediatric patients (0–14 years old) residing in São José do Rio Preto and nearby cities with symptoms of respiratory tract infection (fever, nasal congestion, cough, and/or dyspnea) from February to July of 2021 and 2022 (Fig. 1). The total RNA was extracted from 100 μL of the nasopharyngeal swab samples according to Possebon et al. [19] and stored at −80 °C. Samples were tested for SARS-CoV-2 RNA using one-step real-time polymerase chain reaction (RT-qPCR) with primers and probes targeting the envelope (E), the nucleocapsid (N) region of the SARS-CoV-2 genome, and the human RNase P, according to the GeneFinder COVID-19 Plus RealAmp Kit (OSANG Healthcare, South Korea). All samples that tested positive for COVID-19 were excluded. Negative samples then underwent additional testing with the GoTaq Probe 1-Step RT-qPCR System (Promega, Brazil) for four different respiratory viruses (RSV, RV, AdV, and IAV), with primers and probes from the US Centers for Disease Control and Prevention (CDC)’s real-time RT-PCR assays for non-influenza respiratory viruses (Supplementary Table 1). The RT-qPCR was conducted in the QuantStudio 3 Real-Time PCR System (Thermo Fisher Scientific, USA); results were analyzed using QuantStudio 3 software v. 1.5.1 (Thermo Fisher Scientific, USA) and interpreted based on cycle quantification value (Cq), considering positive samples those presenting Cq ≤ 40.
Fig. 1.
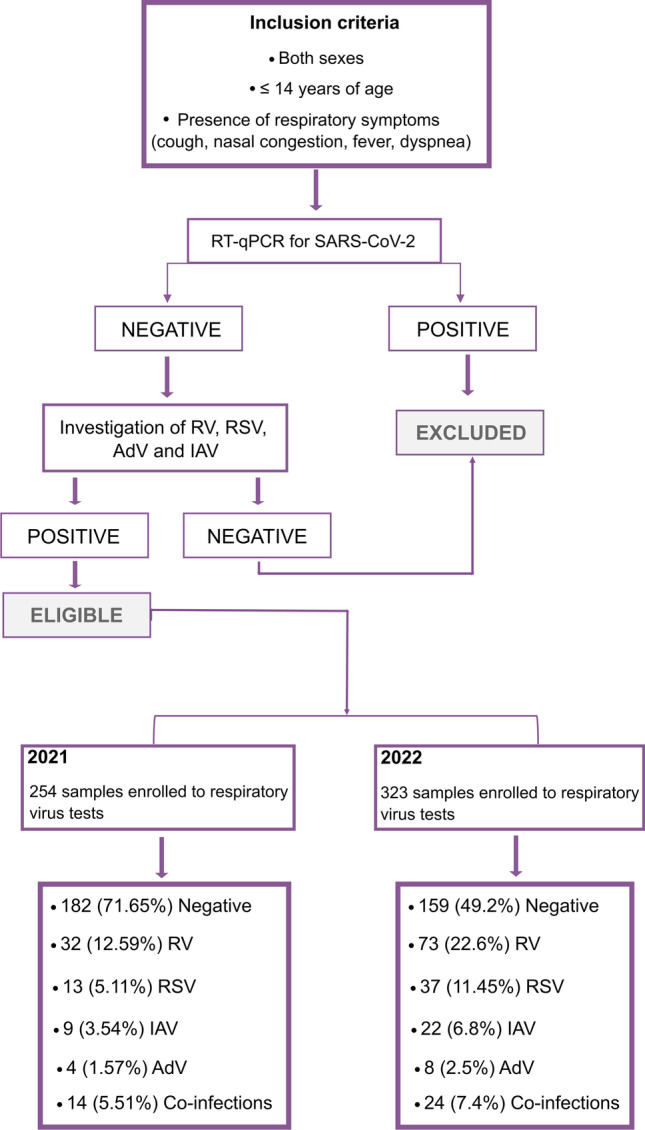
Study design. Patients included and respiratory virus testing results
Statistical analysis
Data exhibiting continuous variables were subjected to the Kolmogorov-Smirnov normality test, while data with non-normal distributions were subjected to the Mann-Whitney test. Pearson’s chi-squared test and Spearman’s rank correlation coefficient test were used to compare categorical variables; p < 0.05 was considered statistically significant. Statistical analyses were performed using RStudio v. 3.6.3 [20] and SPSS Statistics v. 25 [21] software.
Results
During February–July of 2021 and 2022, a total of 254 and 323 SARS-CoV-2-negative samples, respectively, were obtained from pediatric patients (age range: 0–14 years) exhibiting respiratory symptoms (Fig. 1). The numbers of female and male patients tested in 2021 and 2022 were similar (Supplementary Table 2). Average patient ages were 5.14 years in 2021 and 4.25 in 2022.
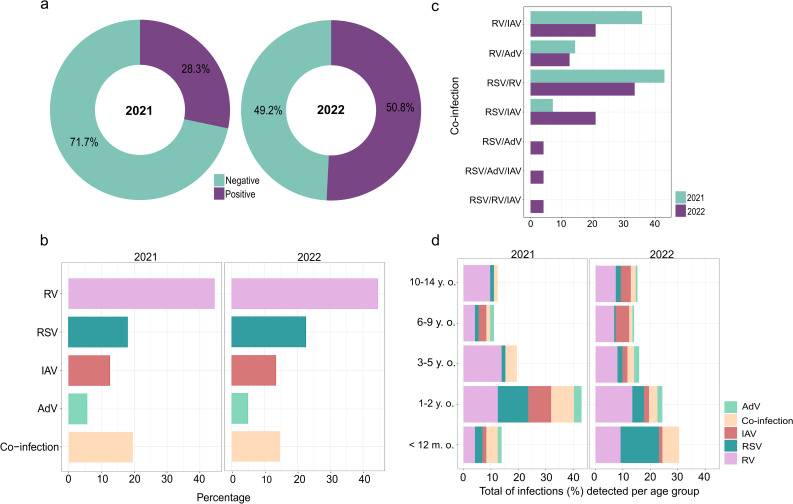
We tested the samples from both years for RV, RSV, IAV, and AdV and found that 28.3% (n = 72) and 50.8% (n = 164) of the samples were positive for at least one of the viruses tested (Fig. 2a). Between 2021 and 2022, a significant increase was observed in the sample positivity rate (p < 0.001), mainly driven by growth in RSV infections and co-infections (Fig. 2b, Supplementary Table 2). The distribution of infections by different pathogens detected during the study period showed a significant difference in virus frequency (Table 1), with RV most prevalent, followed by RSV, IAV, and AdV in 2021 and 2022 (Fig. 2b, Supplementary Table 2). We also found total co-infection rates of 19.4% in 2021 and 14.6% in 2022 (Table 1); the most prevalent co-infections were RSV/RV (the most frequent viruses), followed by RV/IAV and RSV/IAV and RV/AdV. Co-infections between RSV/AdV, RSV/RV/IAV, and RSV/AdV/IAV were only detected in 2022 (Fig. 2c).
Fig. 2.
Circulating respiratory viruses in pediatric patients in 2021 and 2022. a Percentage of samples testing negative and positive for RV, RSV, IAV, AdV, and co-infections. b Percentage of positive samples per year for each virus tested. c Percentage of co-infections detected in 2021 and 2022. d Distribution of infections caused by the different viruses according to age group. RV, rhinovirus; IAV, influenza A virus; RSV, respiratory syncytial virus; AdV, adenovirus; y.o., years old; m.o.; months old
Table 1.
Number of respiratory virus infections in 2021 and 2022
| Virus/year | 2021 | 2022 | ||||
|---|---|---|---|---|---|---|
| Number of infected patients | % | Pearson’s chi-squared test | Number of infected patients | % | Pearson’s chi-squared test | |
| AdV | 4 | 5.5 |
X2 = 31.194 df = 4 p = 2.794e−06 |
8 | 4.9 |
X2 = 74.476 df = 4 p = 2.572e−15 |
| IAV | 9 | 12.5 | 22 | 13.4 | ||
| RSV | 13 | 18 | 37 | 22.5 | ||
| RV | 32 | 44.4 | 73 | 44.5 | ||
|
Co-infection Total |
14 72 |
19.4 − |
24 164 |
14.6 − |
||
AdV adenovirus, IAV influenza A virus, RSV respiratory syncytial virus, RV rhinovirus
The epidemiological profile of the infected patients did not indicate any relation between sex, median age, and infection, but a significant difference was observed (p = 0.01) when the distribution of the different viruses was examined according to age group because of the higher incidence of respiratory infections in children aged 0 to 2 years old compared to other age groups (Supplementary Table 2). Additionally, the pattern of respiratory infections by age group shifted in 2022 compared to 2021; in 2021, the 1-to-2-year-old group was the most affected by respiratory viruses, but children under 12 months were the most affected the following year due to the increase in RSV prevalence (Fig. 2d).
No statistically significant differences were observed between 2021 and 2022 in the most frequent symptoms reported (cough, nasal congestion, and fever) (Fig. 3a), but dyspnea and loss of appetite differed significantly (p = 0.001 and p = 0.027, respectively) according to the viral infection. Dyspnea is often associated with more severe manifestations and was reported in 39% of co-infections and 30% of RSV infections (Fig. 3a, Supplementary Table 2). Of the 577 patients tested for respiratory viruses in both years, we were able to recover hospitalization data from 285 (49.4%) and found that 24.2% of the hospitalized patients tested positive for at least one of the viruses. Different hospitalization rates were observed for these viruses (p = 0.006): RSV infections were the leading cause of hospitalization (69%), followed by co-infections (62.5%) (Fig. 3b, Supplementary Table 2). Despite the considerable hospitalization rate, few patients required mechanical ventilation (Supplementary Table 2).
Fig. 3.
Clinical profile of tested patients. a Main symptoms reported according to the detected respiratory viruses: adenovirus (AdV), influenza A virus (IAV), syncytial respiratory virus (RSV), rhinovirus (RV), and co-infections in 2021 and 2022; the size and color scale of each point correspond to the frequency of each symptom. b Hospitalization rate for 285 patients with complete chart data by infection with adenovirus (AdV), influenza A virus (IAV), syncytial respiratory virus (RSV), rhinovirus (RV), co-infections, and negative samples in 2021 and 2022
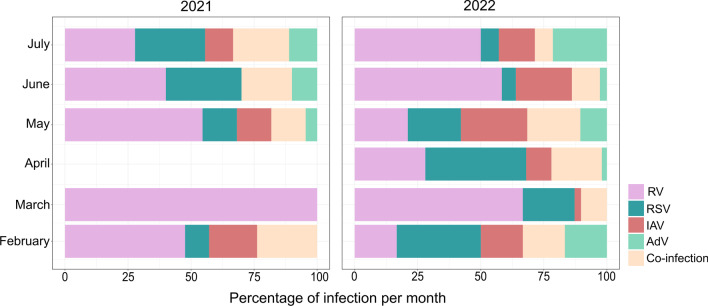
Respiratory infections caused by the different viruses tested were found throughout the sampled periods; however, differences in the peaks of infection for all viruses were observed in 2022. In 2021, peaks of RV were detected in March and May, while the prominent peaks in 2022 were seen in March and June (Fig. 4), and peaks of RSV, the second most frequent virus detected, appeared in June 2021 and April 2022 (Fig. 4). Infections by IAV and AdV were less frequent during both years, but IAV cases peaked in May 2021 and 2022. In general, co-infections were mainly detected in June 2021 and May 2022, the months presenting simultaneous infections for all viruses (Fig. 4). It is important to note that no samples were available from April 2021, since high numbers of COVID-19 infections and deaths led officials to impose a lockdown in the city; thus, this is one limitation of our study.
Fig. 4.
Seasonality patterns for adenovirus (AdV), influenza A (IAV), syncytial respiratory virus (RSV), rhinovirus (RV), and co-infections from February to July of 2021 and 2022
Discussion
This study demonstrated co-circulation of four respiratory viruses in children during 2021 and 2022 amid the COVID-19 pandemic. While 2021 was marked by lockdowns, severe restrictions, and school closings, these measures were relaxed significantly in 2022 with the reopening of schools and flexibilization of masking rules and social isolation protocols. Our findings corroborate this situation by showing a significant difference in the circulation of respiratory viruses alongside SARS-CoV-2 and a 1.8-fold increase in the positivity rate for RSV, RV, AdV, and IAV among symptomatic patients between 2021 and 2022.
In the samples from patients who tested negative for COVID-19, we found that RV and RSV were the most common of the four tested pathogens found in viral infections and co-infections in children presenting flu syndromes, while IAV and AdV were less frequent. Because most health services do not routinely test for RV and RSV, the numbers for these infections are underestimated. Our findings reflect WHO reports noting the lack of data on cases of influenza-like illness or acute respiratory infections caused by other pathogens, probably due to scarce testing [22]. In most health systems in Brazil, epidemiological surveillance for respiratory viruses is limited to influenza strains [23], and infections caused by other respiratory pathogens and co-infections are subsequently underestimated since these viruses are often associated with the common cold in children and adults [24]. In our study, however, we documented a significant increase in cases of dyspnea, a more severe manifestation, in patients infected with RSV. Indeed, several studies have reported that RSV infections are the leading cause of childhood bronchiolitis worldwide and accounted for a total of 3,000,000 hospitalizations and 120,000 deaths each year in children under 5 years of age prior to the COVID-19 pandemic [24, 25]. Furthermore, some studies have found an association between RV infections and the exacerbation of respiratory diseases such as asthma, mainly in patients under 2 years old, the age group where the most severe cases occur [26]. We also showed that RSV/RV were the most frequent co-infections, with 50% and 44.4% of these cases in 2021 and 2022, respectively. Co-infection with RSV was associated with a greater risk of severe RV infection, leading to a significant increase in illness severity [27]. In fact, we found dyspnea was a frequent symptom in co-infections (as it is in RSV infections), which may indicate a more severe manifestation. Similarly, hospitalization rates for RSV infection and co-infections were higher than for the other infections we tested for, indicating that both conditions may increase the risk of acute respiratory syndromes. Moreover, RSV/IAV and RV/IAV co-infections were diagnosed frequently in 2021 and 2022, indicating the need to reinforce flu vaccination programs to avoid co-infections and the risk of severe symptoms.
Considering that the COVID-19 pandemic is still ongoing, this scenario of co-infections is even more concerning. Although we have not evaluated co-infections between SARS-CoV-2 and other viruses, we can surmise that greater circulation of these viruses after the loosening of preventive measures would favor co-infections, with unknown clinical consequences for pediatric patients. In general, prior to the COVID-19 pandemic, the co-infection rates for RSV and other coronaviruses in children with bronchiolitis were 69% in endemic areas [28]. Hashemi et al. [29] reported that in patients diagnosed with SARS-CoV-2 who subsequently died, co-infection rates with other respiratory viruses such as metapneumovirus (MPV), AdV, RSV, bocavirus (HBoV), and parainfluenza (PIV) ranged from 1.9 to 22.3%. These findings emphasize the need for adequate diagnosis and ongoing monitoring of seasonal respiratory viruses during the current pandemic to avoid the spread of viruses which could be responsible for more severe clinical outcomes when associated with SARS-CoV-2. This study presented important results related to the circulation of non-SARS-CoV-2 respiratory viruses in children. However, one limitation is that we did not test for influenza B or other common respiratory viruses such as HBoV, PIV, and MPV. One of the reasons we did not test for the influenza B virus is its low circulation in Brazil, according to the WHO [30]. Considering the number of negative samples from 2021 and 2022, adding other viruses to the screening panel may be an interesting avenue for further investigation.
In this study, we reported that children 1–2 years old were more frequently affected by acute respiratory infections during the study period. This finding may be related to the immaturity of the immune system during the early years of life, making children more susceptible to these pathogens; adaptive immune response is known to still be maturing at this stage, and is only complete after the first decade of life [31]. For this reason, even though newborns may receive maternal protection through IgG antibodies during their first months, they remain immunologically vulnerable for a relatively long period [31]. Of the viruses we tested for, we demonstrated that RSV was most prevalent in younger children. Previous studies have estimated that most children are infected with RSV before 2 years of age and can present more severe symptoms [32]. Persistent immunity to RSV is known to be lacking, and consequently, re-infections are common but tend to be restricted to the upper respiratory tract [32].
Interestingly, separate analysis of infection patterns for the different respiratory viruses in 2021 and 2022 showed a shift in the age group most affected by respiratory infections: in 2021, this group was children aged 1 to 2 years old, while in 2022, children under 12 months old were most affected, mainly due to the increase in RSV cases. The reasons for this finding are unclear; with schools reopening in 2022, we would expect older children to be more affected by respiratory infections. One hypothesis that could explain this phenomenon would be the maternal anti-RSV antibodies inherited via transplacental immunity and/or immunity conferred by breastfeeding could help protect neonates during the first months of life [33]. Furthermore, in 2020, RSV circulation was severely lower or even absent in Brazil and other countries [16–18, 34]. This may have contributed to less exposure of pregnant women to this virus and consequently reduced transplacental anti-RSV IgG transport [31]; this, in turn, may have led to decreased protection in newborns and an increase in RSV cases among children under 12 months of age, especially infants less than 6 months old in 2022, when protective measures were loosened and circulation of other respiratory viruses rebounded, as shown in this study. Still, the presence of IgG maternal antibodies in neonates is considered controversial [35]. Another hypothesis could be higher exposure of children under 12 months old to infected individuals, due to the reduction of social isolation measures to contain the COVID-19 pandemic.
Because respiratory viruses such as influenza, RSV, RV, AdV, and SARS-CoV-2 share similar transmission routes, the restrictive measures taken during the pandemic efficiently contain viral spread [34, 36]. But once these infections are established, diagnosis is only based on clinical symptoms. We found in this study that the most frequent symptoms were cough, nasal congestion, and fever for all the viruses we studied, including co-infections, while dyspnea was more commonly observed in cases of RSV and co-infections. Within the pandemic scenario, accurate diagnosis of disease etiology becomes even more complicated since the most common symptoms of COVID-19 in pediatric patients are also fever and cough [37]. Therefore, an accurate diagnosis and the speed with which intensive care wards can fill up reinforce the need for continuous monitoring of the most common circulating viruses to predict new outbreaks, especially those peaks of infections outside regular seasonality patterns.
The seasonality of respiratory viruses can vary according to the weather and the region. Studies prior to the COVID-19 pandemic reported that the seasonality of the influenza virus varies, but to a lesser extent in tropical regions, while peaks of infection can be observed in the winter immediately after RSV outbreaks [1, 9]. Rhinovirus and adenovirus infections fluctuate throughout the year [9], and in Brazil, the RSV season starts in late February, with peaks of infection in April and May [38]. Our results did not demonstrate clear seasonality patterns for the respiratory viruses we investigated since they were found in all the sampled months. We detected peaks of infection that differed in 2022 and 2021, which may indicate that the loosening of restrictive measures to control the COVID-19 pandemic may have impacted the circulation pattern of these respiratory viruses, in turn making it difficult to predict new outbreaks and yet again reinforcing the need for molecular surveillance throughout the year.
Conclusion
Our findings demonstrated an increase of 1.8 times in acute respiratory infection cases by non-SARS-CoV-2 viruses in 2022 compared to 2021. Moreover, as the peaks of infections caused by the different respiratory viruses changed in the sampled period, the continuous monitoring of these viruses is essential to early detect new outbreaks and, thus, adopt effective measures to control infections and reduce hospitalization rates.
Supplementary information
(DOCX 11.1 kb)
(XLSX 14 kb)
Author contribution
A.K.L.S., C.A.B., and M.L.N. conceived and designed the study. A.K.L.S., C.A.B., M.G.S., and M.R.R. collected the samples. A.K.L.S., C.A.B., L.S., and B.C.M. carried out the respiratory infection diagnostics. C.A.B. and C.F.S. performed statistical analyses. A.K.S.L., C.A.B., F.A.G., T.P.S., and C.F.S. conducted the data analyses and interpretation. A.K.S.L. and C.A.B. wrote the first draft of the manuscript. C.A.B., C.F.S., L.S., and M.L.N. edited and revised the manuscript. M.L.N. provided the resources for the survey. All the authors approved the final version of the manuscript. All the authors had full access to all the data used in this study and had final responsibility for the decision to submit the text for publication.
Funding
This work was supported by the Rede Corona-Ômica BR MCTI/FINEP, which is part of Rede Vírus/MCTI (FINEP 01.20.0029.000462/20, CNPq 404096/2020-4, CNPq fellowship 382032/2020-9 to C.A.B.). Funding support was also received from the FAPESP-COVID Program (Grant 2020/04836-0 to M.L.N.), from CNPq via the PIBIC fellowship (122597/2021-4) to A.K.S.L., and the Centers for Research in Emerging Infectious Diseases (CREID), “The Coordinating Research on Emerging Arboviral Threats Encompassing the Neotropics (CREATE-NEO)” grant 1U01AI151807 awarded to M.L.N. by the National Institutes of Health (NIH/USA). M.L.N. is a CNPq Research Fellow.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Faculdade de Medicina de São José do Rio Preto (FAMERP) (protocol number: CAAE 31588920.0.0000.5415, on November 29, 2021). Informed consent was not required, since all samples were collected for routine diagnosis and data were analyzed anonymously, ensuring total confidentiality for all participants.
Competing interests
The authors declare no competing interests.
Disclaimer
The funders had no role in the design of the study, collection, analyses, or interpretation of data, writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ana Karoline Sepedro Lima and Cecília Artico Banho contributed equally to this work.
Contributor Information
Ana Karoline Sepedro Lima, Email: ana.lima@edu.famerp.br.
Cecília Artico Banho, Email: ceci.abanho@gmail.com.
Lívia Sacchetto, Email: liviasacchetto@gmail.com.
Beatriz de Carvalho Marques, Email: bbiacarvalhomarques@gmail.com.
Mariana Guedes dos Santos, Email: mariana.guedes@edu.famerp.br.
Milene Rocha Ribeiro, Email: mrocharibeiro@yahoo.com.br.
Flora A. Gandolfi, Email: florafef04@gmail.com
Tatiana Pissolati Sakomura, Email: tatisakomura@gmail.com.
Cássia Fernanda Estofolete, Email: cassiafestofolete@gmail.com.
Maurício Lacerda Nogueira, Email: mauricio.nogueira@edu.famerp.br.
References
- 1.Li Y, Reeves RM, Wang X et al (2019) Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Health 7(8). 10.1016/S2214-109X(19)30264-5 [DOI] [PubMed]
- 2.Kengne-Nde C, Kenmoe S, Modiyinji AF, Njouom R (2020) Prevalence of respiratory viruses using polymerase chain reaction in children with wheezing, a systematic review and meta-analysis. PLoS One 15. 10.1371/journal.pone.0243735 [DOI] [PMC free article] [PubMed]
- 3.WHO MORTALITY DATABASE (2021) Respiratory infections. https://platform.who.int/mortality/themes/theme-details/topics/topic-details/MDB/respiratory-infections. Accessed 17 Jan 2023
- 4.Schuster JE, Williams J, v. (2018) Emerging respiratory viruses in children. Infect Dis Clin North Am 32(1). 10.1016/j.idc.2017.10.001 [DOI] [PMC free article] [PubMed]
- 5.Bicer S, Giray T, Çöl D et al (2013) Virological and clinical characterizations of respiratory infections in hospitalized children. Ital J Pediatr 39(1). 10.1186/1824-7288-39-22 [DOI] [PMC free article] [PubMed]
- 6.Tregoning JS, Schwarze J (2010) Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 23(1). 10.1128/CMR.00032-09 [DOI] [PMC free article] [PubMed]
- 7.Backman K, Ollikainen H, Piippo-Savolainen E, Nuolivirta K, Korppi M (2018) Asthma and lung function in adulthood after a viral wheezing episode in early childhood. Clin Exp Allergy 48(2). 10.1111/cea.13062 [DOI] [PubMed]
- 8.Nascimento MS, de Souza AV, de Ferreira AVS, Rodrigues JC, Abramovici S, da Filho LVFS (2010) High rate of viral identification and coinfections in infants with acute bronchiolitis. Clinics 65(11). 10.1590/S1807-59322010001100014 [DOI] [PMC free article] [PubMed]
- 9.Moriyama M, Hugentobler WJ, Iwasaki A (2020) Annual review of virology seasonality of respiratory viral infections. Annu Rev Virol:7. 10.1146/annurev-virology-012420-022445 [DOI] [PubMed]
- 10.García-García ML, Calvo C, Falcón A et al (2010) Role of emerging respiratory viruses in children with severe acute wheezing. Pediatr Pulmonol 45(6). 10.1002/ppul.21225 [DOI] [PMC free article] [PubMed]
- 11.Wu P, Hao X, Lau EHY et al (2020) Real-time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Eurosurveillance 25(3). 10.2807/1560-7917.ES.2020.25.3.2000044 [DOI] [PMC free article] [PubMed]
- 12.WHO. WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020 - World Health Organization. World Health Organization.
- 13.Burrel S, Hausfater P, Dres M et al (2021) Co-infection of SARS-CoV-2 with other respiratory viruses and performance of lower respiratory tract samples for the diagnosis of COVID-19. Int J Infect Dis:102. 10.1016/j.ijid.2020.10.040 [DOI] [PMC free article] [PubMed]
- 14.Giovanetti M, Slavov SN, Fonseca V et al (2021) Genomic epidemiology reveals how restriction measures shaped the SARS-CoV-2 epidemic in Brazil. medRxiv. 10.1101/2021.10.07.21264644
- 15.Mao S, Huang T, Yuan H et al (2020) Epidemiological analysis of 67 local COVID-19 clusters in Sichuan Province, China. BMC Public Health 20(1). 10.1186/s12889-020-09606-4 [DOI] [PMC free article] [PubMed]
- 16.Friedrich F, Ongaratto R, Scotta MC et al (2021) Early impact of social distancing in response to coronavirus disease 2019 on hospitalizations for acute bronchiolitis in infants in Brazil. Clin Infect Dis 72(12). 10.1093/cid/ciaa1458 [DOI] [PMC free article] [PubMed]
- 17.Kuitunen I, Artama M, Mäkelä L, Backman K, Heiskanen-Kosma T, Renko M (2020) Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J. 10.1097/INF.0000000000002845 [DOI] [PubMed]
- 18.van Brusselen D, de Troeyer K, ter Haar E et al (2021) Bronchiolitis in COVID-19 times: a nearly absent disease? Eur J Pediatr 180(6). 10.1007/s00431-021-03968-6 [DOI] [PMC free article] [PubMed]
- 19.Possebon FS, Ullmann LS, Malossi CD et al (2022) A fast and cheap in-house magnetic bead RNA extraction method for COVID-19 diagnosis. J Virol Methods:300. 10.1016/j.jviromet.2021.114414 [DOI] [PMC free article] [PubMed]
- 20.R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available at: https://www.R-project.org. Accessed 7 Aug 2023
- 21.IBM Corp (2019) IBM SPSS Statistics for Macintosh, Version 26.0. Armonk, NY: IBM Corp. Available at https://www.ibm.com/mysupport/s/topic/0TO500000001yjtGAA/spss-statistics?language=en_US. Accessed 7 Aug 2023
- 22.World Health Organization. Influenza surveillance report. Available at https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/influenza-surveillance-outputs. Accessed 7 Aug 2023
- 23.Ministério da Saúde. PORTARIA No 2.693, DE 17 DE NOVEMBRO DE 2011. https://bvsms.saude.gov.br/bvs/saudelegis/gm/2011/prt2693_17_11_2011.html.
- 24.Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. The Lancet. 2017;390(10098):946–958. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair H, Nokes DJ, Gessner BD et al (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. The Lancet 375(9725). 10.1016/S0140-6736(10)60206-1 [DOI] [PMC free article] [PubMed]
- 26.Costa LF, Oliveira Queiróz DA, Lopes Da Silveira H et al (2014) Human rhinovirus and disease severity in children. Pediatrics 133(2). 10.1542/peds.2013-2216 [DOI] [PubMed]
- 27.Comte A, Bour JB, Darniot M, Pitoiset C, Aho-Glélé LS, Manoha C (2020) Epidemiological characteristics and clinical outcomes of human rhinovirus infections in a hospitalized population. Severity is independently linked to RSV coinfection and comorbidities. J Clin Virol:125. 10.1016/j.jcv.2020.104290 [DOI] [PubMed]
- 28.Mansbach JM, Hasegawa K, Piedra PA, Sullivan AF, Camargo CA (2020) Severe coronavirus bronchiolitis in the pre-COVID-19 era. Pediatrics 146(3). 10.1542/PEDS.2020-1267 [DOI] [PMC free article] [PubMed]
- 29.Hashemi SA, Safamanesh S, Ghasemzadeh-moghaddam H, Ghafouri M, Azimian A (2021) High prevalence of SARS-CoV-2 and influenza A virus (H1N1) coinfection in dead patients in northeastern Iran. J Med Virol 93(2). 10.1002/jmv.26364 [DOI] [PubMed]
- 30.World Health Organization. Influenza laboratory surveillance information - virus detection by subtype reported to FluNet. Available at https://www.who.int/tools/flunet/flunet-summary. Accessed 7 Aug 2023
- 31.de Moraes-Pinto MI, Suano-Souza F, Aranda CS (2021) Immune system: development and acquisition of immunological competence. J Pediatr (Rio J):97. 10.1016/j.jped.2020.10.006 [DOI] [PMC free article] [PubMed]
- 32.Piedimonte G, Perez MK (2014) Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 35(12). 10.1542/pir.35-12-519 [DOI] [PMC free article] [PubMed]
- 33.Yildiz M, Kara M, Sutcu M et al (2020) Evaluation of respiratory syncytial virus IgG antibody dynamics in mother-infant pairs cohort. Eur J Clin Microbiol Infect Dis 39(7). 10.1007/s10096-020-03841-8 [DOI] [PMC free article] [PubMed]
- 34.Varela FH, Scotta MC, Polese-Bonatto M et al (2021) Absence of detection of RSV and influenza during the COVID-19 pandemic in a Brazilian cohort: likely role of lower transmission in the community. J Glob Health 11. 10.7189/jogh.11.05007 [DOI] [PMC free article] [PubMed]
- 35.di Mattia G, Nenna R, Mancino E et al (2021) During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr Pulmonol 56(10). 10.1002/ppul.25582 [DOI] [PMC free article] [PubMed]
- 36.Wiese AD, Everson J, Grijalva CG (2021) Social distancing measures: evidence of interruption of seasonal influenza activity and early lessons of the SARS-CoV-2 pandemic. Clin Infect Dis 73(1). 10.1093/cid/ciaa834 [DOI] [PMC free article] [PubMed]
- 37.Zimmermann P, Curtis N (2020) COVID-19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr Infect Dis J. 10.1097/INF.0000000000002700 [DOI] [PMC free article] [PubMed]
- 38.Obando-Pacheco P, Justicia-Grande AJ, Rivero-Calle I et al (2018) Respiratory syncytial virus seasonality: a global overview. J Infect Dis 217(9). 10.1093/infdis/jiy056 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 11.1 kb)
(XLSX 14 kb)