Summary
Background
This study aimed to evaluate the efficacy and safety of RAY1216, a novel inhibitor of 3-chymotrypsin-like cysteine protease (3CLpro), in adults with coronavirus disease 2019 (COVID-19).
Methods
This phase 2, single centre, randomised, double-blind, placebo-controlled trial included hospitalised patients between August 14, 2022, and September 26, 2022, in Sanya Central Hospital (The Third People’s Hospital of Hainan Province) in China with no severe symptoms if they had laboratory-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection for not more than 120 h (5 days) and a real-time quantitative polymerase chain reaction (qPCR) cycle threshold (Ct) value of ≤30 for both the open reading frames 1 ab (ORF1ab) and nucleocapsid (N) genes within 72 h before randomisation. Half of the participants (n = 30) were randomly assigned (2:1) to receive either RAY1216 or a matched placebo three times a day (TID) for 5 days (15 doses in total), while the other half received RAY1216 plus ritonavir (RAY1216 plus RTV) or a matched placebo every 12 h for 5 days (10 doses in total). The primary endpoint was the time of viral clearance. Secondary outcomes included the changes of the SARS-CoV-2 RNA viral load, the positivity rate of the nucleic acid test, and the recovery time of clinical symptoms. A safety evaluation was performed to record and analyse all adverse events that occurred during and after drug administration as well as any cases in which dosing was halted because of these events. Clinicaltrials.gov identifier: ChiCTR2200062889.
Findings
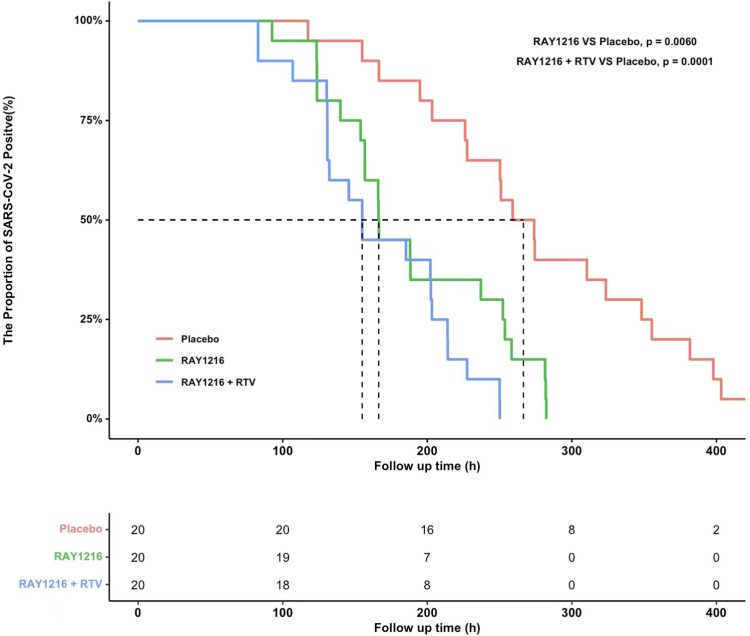
The viral shedding times in the RAY1216 and RAY1216 plus RTV groups were 166 h (95% confidence interval (CI): 140–252) and 155 h (95%CI: 131–203), respectively, which were 100 h (4.2 days) and 112 h (4.6 days) shorter than that of the placebo group, respectively (RAY1216 group vs. Placebo p = 0.0060, RAY1216 plus RTV group vs. Placebo p = 0.0001). At 24 h, 72 h, and 120 h after administration, the viral RNA loads in the RAY1216 and RAY1216 plus RTV groups were significantly less than those of the placebo groups. At 280 h (11.5 days) after administration, the nucleic acid test results in the RAY1216 and RAY1216 plus RTV groups were both negative. The common adverse events related to the investigational drugs were mild and self-limiting laboratory examination abnormalities.
Interpretation
Our findings suggest that RAY1216 monotherapy and RAY1216 plus ritonavir both demonstrated significant antiviral activity and reduced the duration of COVID-19 while maintaining a satisfactory safety profile. Considering the limited clinical application of RTV, it is recommended to use RAY1216 alone to further verify its efficacy and safety.
Funding
This study was sponsored by the Key Research and Development Program of China (2022YFC0868700).
Keywords: SARS-CoV-2, COVID-19, 3-Chymotrypsin-like cysteine protease, RAY1216, Viral shedding time, Efficacy and safety
Research in context.
Evidence before this study
To assess the existing studies on 3-chymotrypsin-like cysteine protease treatment, we searched PubMed, ClinicalTrials.gov, and FDA.gov, using the search terms “SARS-CoV-2”, “COVID-19” or “3-chymotrypsin-like cysteine protease” for any articles published or trials recruited between December 1, 2019 and May 31, 2023. Paxlovid is the first 3CLpro inhibitor of SARS-CoV-2, but it has complex interactions with other medications and is not recommended for those have certain medical conditions. RAY1216 is a broad-spectrum 3CLpro inhibitor of SARS-CoV-2 that was developed by Guangdong Raynovent Biotechnology Co., Ltd. The phase 1 study results demonstrate that RAY1216 has a favourable tolerability, safety, and pharmacokinetic profile and that it can reduce SARS-CoV-2 replication, viral load levels, and infectious virus titres in vivo and in vitro, regardless of whether it is used in combination with ritonavir or not.
Added value of this study
In this phase 2, single centre, randomised, double-blind, placebo-controlled study, we found the viral shedding times in the RAY1216 and RAY1216 plus RTV groups were shorter than those in the placebo group, respectively. Both dosage regimens of RAY1216 tablets both have clinical potential for the treatment of SARS-CoV-2 infection if administrated within 5 days of the initial positive nucleic acid test result, and their antiviral efficacies are comparable.
Implications of all the available evidence
Our findings suggest that RAY1216 monotherapy and RAY1216 plus ritonavir both demonstrated significant antiviral activity and reduced the duration of COVID-19 while maintaining a satisfactory safety profile. The common adverse events related to the investigational drugs were mild and self-limiting laboratory examination abnormalities. Considering the limited clinical application of RTV, it is recommended to use RAY1216 alone to further verify its efficacy and safety.
Introduction
Rapid global dissemination and widespread devastation have resulted from the coronavirus disease 2019 (COVID-19) pandemic. This disease is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The spike protein is the target of the majority of COVID-19 vaccines and therapeutic antibodies. However, the Omicron variant of the spike protein has now become the dominant variant globally, resulting in increased vaccine breakthrough rates and widespread resistance to neutralising antibodies.1,2 As a result, finding effective therapies for COVID-19 patients that do not target the spike protein remains a top priority in order to overcome the upcoming unexpected mutations before the COVID-19 epidemic can be fully controlled.
For a specific viral replication cycle of SARS-CoV-2, 3-chymotrypsin-like cysteine protease (3CLpro) is essential for the processing of viral polyproteins into functional units, making it an attractive antiviral target.3 Therefore, SARS-CoV-2 replication can be inhibited by drugs selectively inhibiting 3CLpro, and there is a low likelihood of off-target activity due to the lack of known human analogues.4 Paxlovid is an oral antiviral drug developed by Pfizer that combines nirmatrelvir and ritonavir. Nirmatrelvir is a 3CLpro inhibitor of SARS-CoV-2, and ritonavir works as a cytochrome P450 3A4 inhibitor to slow the metabolism of nirmatrelvir and raise its serum levels. On December 22, 2021, the United States Food and Drug Administration issued an Emergency Use Authorisation for Paxlovid to treat COVID-19 in adults and children (≥12 years old and weighing ≥40 kg) at high risk of developing severe COVID-19.5 However, Paxlovid has complex interactions with other medications, such as systemic corticosteroids, antiarrhythmic drugs, anticancer drugs, calcium channel blockers, and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Due to the ritonavir component of the combination, Paxlovid is not recommended for those with certain medical conditions.6 Therefore, because of the rising number of cases and fatalities globally as well as the extremely limited treatment options, there is an urgent unmet medical need for new efficient 3CLpro inhibitors with fewer restrictions on combined medication that can control COVID-19.
RAY1216 is a broad-spectrum 3CLpro inhibitor of SARS-CoV-2 that was developed by Guangdong Raynovent Biotechnology Co., Ltd. The phase 1 study results demonstrate that RAY1216 has a favourable tolerability, safety, and pharmacokinetic profile and that it can reduce SARS-CoV-2 replication, viral load levels, and infectious virus titres in vivo and in vitro, regardless of whether it is used in combination with ritonavir or not. In this phase 2, single centre, randomised, double-blind, placebo-controlled study, we evaluated the efficacy and safety of RAY1216 alone or in combination with ritonavir (RAY1216 plus RTV) in participants infected with SARS-CoV-2 when Omicron was the dominant variant.
Methods
Study design
This phase 2, single centre, randomised, double-blind, placebo-controlled trial evaluated the efficacy and safety of RAY1216 in adults with COVID-19 who had asymptomatic, mild, or moderate symptoms. Specifically, Sanya Central Hospital, which has been designated as a centre for the care of COVID-19 patients, was the site of this study. The trial registration number of this study is ChiCTR2200062889, and the local ethics committee approved this study (institutional review board number: LL2208109). All participants provided informed consent prior to enrolment and intervention. Eligible patients were aged between 18 and 70 years old, had a confirmed case of SARS-CoV-2 infection of ≤120 h (5 days) prior to randomisation, and had a cycle threshold (Ct) value ≤ 30 for both the open reading frame 1 ab (ORF1ab) and N gene by real-time quantitative polymerase chain reaction (qPCR) within 72 h of randomisation (The ORF1ab gene and the N gene are the known major structural proteins of SARS-CoV-2 that are involved in viral pathogenicity and infectivity.) This study included patients with only mild or moderate COVID-19. The exclusion criteria were as follows: patients with severe or critical COVID-19, patients receiving antiviral therapy, patients with systemic infections other than COVID-19, and patients with a body mass index of ≥30 kg/m2. Adult patients who met any of the following criteria and could not be explained by reasons other than SARS-CoV-2 infection were defined as severe COVID-19 adult patients: 1. Presence of dyspnea, with a respiratory rate (RR) of ≥30 breaths per minute; 2. Resting oxygen saturation (SpO2) ≤93% while breathing ambient air; 3. Arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mmHg (1 mmHg = 0.133 kPa). In high-altitude areas (altitude exceeding 1000 m), the PaO2/FiO2 ratio was corrected using the following formula: PaO2/FiO2 × [760/ambient pressure (mmHg)]; and 4. Progressive worsening of clinical symptoms and significant progression (>50%) of lung lesions within 24–48 h based on radiographic imaging. Adult patients with critical COVID-19 were defined as those who met one of the following criteria: 1. Presenting with respiratory failure requiring mechanical ventilation; 2. Presenting with shock; and 3. Presenting with multi-organ dysfunction requiring Intensive Care Unit monitoring and treatment.
For pilot studies with moderate-to-large effect sizes, a pretrial including 15–20 participants per group is sufficient to correct for bias in the estimates obtained from the pretrial. Additionally, considering the implementation of centralised control measures during the local outbreak of COVID-19 in China, where the local epidemic cycle could be controlled within approximately 1–2 months, the challenges of implementing the trial during the disease outbreak (such as closed-loop management and difficulty in obtaining informed consent) were taken into account. Considering this study as exploratory clinical research and to ensure completion of the study during the pandemic period, a sample size of 60 participants with 20 participants per group was determined.
The primary endpoint of this trial was to compare the time of viral clearance achieved by RAY1216 to that of placebo. The secondary outcomes were to measure changes in the SARS-CoV-2 RNA viral load, the proportion of patients with positive qPCR test results of SARS-CoV-2 after treatment, and the recovery time of clinical symptoms in SARS-CoV-2-infected patients treated with RAY1216 or placebo for 21 consecutive days, including the 5-day treatment period and the 16-day follow-up period. The purpose of the safety evaluation was to record and analyse all adverse events that occurred during and after drug administration as well as any cases in which dosing was halted because of these events.
Randomisation and masking
The blinded statistician employed the block randomisation method to generate the random allocation tables for cohort 1 and cohort 2 by investigational drug and placebo in a 2:1 ratio, with a block length of 6. The blinded statistician blind the drugs according to the table of generated random allocation, with the drug number corresponding to the random number. The eligible patients were assigned matching numbers in the order of successful screening, followed by randomisation and administration.
The study adopted a double-blind design, with participants, researchers, and the trial statistician all being masked to the treatment allocation until the final analysis.
Intervention and follow-up procedures
The optimal antiviral effect of protease inhibitors and other antiviral drugs is usually achieved when the unbound plasma concentration is sustained above the concentration at which 90% inhibition of viral replication (EC90) occurs.7 A population pharmacokinetic model of RAY1216 was built during the dose escalation based on the preliminary single ascending dose/multiple ascending dose data from healthy adults in a phase 1 clinical trial to simulate RAY1216 concentrations at different doses and regimens. With the population pharmacokinetic model, the RAY1216 (300 mg)/ritonavir (100 mg) two times a day (BID) and RAY1216 (400 mg) three times a day (TID) simulation showed that most participants would achieve the target minimum concentration above the in-vitro EC90 after the first dose.
After obtaining informed consent, the baseline demographic information, medical history, vaccination status, vital signs, disease severity, and Ct values from all participants were collected. As shown in Fig. 1, this study employed a clinical trial design in which the included patients were randomised into two major cohorts. Cohort 1 consisted of 30 participants, with a ratio of 2:1 between the RAY1216 group and the placebo group. The participants received either 400-mg RAY1216 tablets or their corresponding placebos, administered TID with approximately 6 h between each dose, for a total duration of 5 days (15 doses). Cohort 2 also comprised 30 participants, with a ratio of 2:1 between the treatment group and the control group. The participants were administered a combination therapy of RAY1216 and ritonavir. This involved taking one tablet of 300 mg of RAY1216 and 100 mg of ritonavir or their respective placebos, BID with approximately 12 h between each dose, for a total duration of 5 days (10 doses). After enrolling 30 patients in Cohort 1, the screening of patients in Cohort 2 began.
Fig. 1.
Randomisation, treatment assignments, and follow-up. The numbers of patients in each group are provided.
The prospectively collected clinical data included 14 clinical symptoms (stuffy or runny nose, sore throat, shortness of breath, cough, fever, low energy or tiredness, muscle or body aches, chills or shivering, fever, nausea, vomiting, diarrhoea, and loss of taste or smell), laboratory tests (qPCR test for SARS-CoV-2, blood cell count, and blood chemistry), severity, treatment process, and prognosis. On days 1, 2, 4 ± 1, 6 ± 1, 10 ± 2, 15 ± 2, and 21 ± 3 after hospitalisation, nasopharyngeal swabs of the participants were taken to measure the SARS-CoV-2 viral load by qPCR (Applied Biosystems, ABI 7500). Methodological validation for SARS-CoV-2 quantitative detection was performed prior to sample testing, with a lower limit of quantification of 200 copies/mL. All sampling workers had to undergo rigorous training to ensure the consistency and reliability of the nasopharyngeal swab results. In addition, nucleic acid testing was performed every day if the Ct value was >30 for either the ORF1ab or N gene to ensure that the viral shedding time could be acquired as soon as possible. A Ct value of >35 for both the ORF1ab and N genes was considered a negative test, and two consecutive negative tests were regarded as negative conversion. The interval between the first dose administration of the investigative product and the first negative test in two consecutive tests was used to define the viral shedding time.
Clinicians monitored the vital signs and disease conditions of the patients on a daily basis, and the treatment regimens were retrospectively reviewed after the follow-up. Throughout the study, researchers took note of any serious or unusual side effects that emerged. The clinical and laboratory adverse events were evaluated for severity.
Statistical analysis
Continuous variables were presented as the mean and standard deviation (SD), and categorical variables were presented as the count and proportion. The viral shedding time was compared between the two groups in the primary and subgroup analyses. Cox regression was used to calculate the hazard ratio and 95% confidence interval (CI). Two-sided p < 0.05 was regarded as statistically significant.
Role of the funding source
The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. All authors had full access to all the data in this study, and the corresponding authors had the final responsibility to submit this study for publication.
Results
Baseline characteristics
Overall, this study enrolled 60 eligible patients between August 14, 2022, and September 26, 2022, and they were randomly assigned to the RAY1216, RAY1216 plus RTV, or placebo group, with 20 cases each (Fig. 1). The average age of all enrolled participants was 38.9 years old (SD: 11.4), and 26 (43%) patients were male. Except for one participant in the placebo group, most of the participants received the vaccine, with 13 (22%) receiving two doses and 46 (77%) receiving a third booster dose. The mean duration of disease for all participants at baseline was 79.8 (SD: 20.3) h, and the mean viral load was 8.1 (SD: 0.7) log10 copies/mL. In this trial, 33 (55%) of the patients had a history of previous illnesses or surgeries, including various surgical and medical procedures [6 (10%)], as well as various musculoskeletal and connective tissue disorders [4 (7%)]. The incidence rates of liver and biliary system diseases, neurological disorders, and gastrointestinal system diseases were all 2 (3%). There were no important differences observed between the study groups. Within 48 h prior to randomisation, the patients did not receive antiviral drugs, oxygen support, monoclonal antibodies, or steroid treatment (Table 1).
Table 1.
Baseline characteristics of the study participants.
| Placebo group (n = 20) | RAY1216 (n = 20) | RAY1216 plus RTV (n = 20) | |
|---|---|---|---|
| Age, Mean (SD) | 39.9 (9.6) | 39.4 (13.6) | 37.4 (11.0) |
| Sex | |||
| Male, n (%) | 7 (35%) | 10 (50%) | 9 (45%) |
| Female, n (%) | 13 (65%) | 10 (50%) | 11 (55%) |
| Body mass index (kg/m2), Mean (SD) | 22.1 (2.7) | 21.9 (3.2) | 23.0 (2.6) |
| Vaccination, n (%) | 19 (95%) | 20 (100%) | 20 (100%) |
| Symptoms, n (%) | 18 (90%) | 19 (95%) | 18 (90%) |
| Initial virus copy number (log10 copies/mL), Mean (SD) | 8.4 (0.9) | 7.9 (0.6) | 8.0 (0.5) |
| Course of disease (h), Median (IQR) | 82.6 (68.6, 91.8) | 88.5 (77.0, 101.5) | 73.7 (60.0, 82.7) |
| N gene (cycle threshold values), Median (IQR) | 14.5 (13.9, 16.1) | 16.2 (15.4, 18.1) | 17.0 (16.3, 18.4) |
| ORF1ab gene (cycle threshold values), Median (IQR) | 16.5 (15.4, 18.1) | 17.7 (16.9, 19.1) | 19.9 (18.9, 21.0) |
| Standard-of-care | |||
| Antiviral drugsa | 0 (0%) | 0 (0%) | 0 (0%) |
| O2 support | 0 (0%) | 0 (0%) | 0 (0%) |
| Monoclonal antibodyb | 0 (0%) | 0 (0%) | 0 (0%) |
| Steroidsc | 0 (0%) | 0 (0%) | 0 (0%) |
IQR = interquartile range, SD = standard deviation.
Antiviral drugs: Paxlovid, Azvudine, Molnupiravir.
Monoclonal antibody: amubarvimab and romlusevimab.
Steroids: dexamethasone, methylprednisolone.
Primary and secondary efficacy objectives
The viral load shedding times were 166 h (95%CI: 140–252) and 155 h (95%CI: 131–203) in the RAY1216 and RAY1216 plus RTV groups, respectively, which were 100 h (4.2 days) (p = 0.0060) and 112 h (4.6 days) (p = 0.0001) shorter than that in the placebo group. At 24 h after administration, the viral RNA load in the RAY1216 group was 0.8 log10 copies/mL less than that in the placebo group (p = 0.031). Furthermore, compared to the baseline values, the RAY1216 group and the RAY1216 plus RTV group had a 1.09 and 1.14 log10 copies/mL greater reduction in the viral RNA load at 72 h than the placebo group (RAY1216 group vs. placebo, p = 0.0009; RAY1216 plus RTV group vs. placebo, p = 0.0004) (Table 2 and Fig. 2). The patients in the RAY1216 and RAY1216 plus RTV groups both had negative qPCR results at 280 h (11.5 days) after drug administration (Fig. 3). In addition, Cox regression analysis showed that the hazard ratios (HRs) in the RAY1216 and RAY1216 plus RTV groups were 3.3 (95% CI: 1.6, 6.9; p = 0.0013) and 6.1 (95% CI: 2.7, 13.6; p < 0.0001), respectively, compared with the placebo group.
Table 2.
Analysis of the differences in SARS-CoV-2 viral shedding time, the changes in viral RNA loading, and clinical symptom recovery time in the three groups.
| Placebo (n = 20) | RAY1216 (n = 20) | RAY1216 plus RTV (n = 20) | p-value | |
|---|---|---|---|---|
| Viral load shedding time | ||||
| (h) (95% CI) | 267 (203, 348) | 166 (140, 252)c | 155 (131, 203)d | 0.0060c, 0.0001d |
| Hazard ratio (95% CI) | 3.3 (1.6, 7.0)c | 6.1 (2.7, 13.6)d | 0.0013c, 0.0001d | |
| Hazard ratio (95% CI)b | 2.1 (1.0, 4.6)c | 5.0 (2.2, 11.2)d | 0.054c, 0.0001d | |
| Viral RNA loading change from baseline [LSmean (SE)] | ||||
| 24 h | −0.5 (0.3) | −1.3 (0.2)c | −1.0 (0.2)d | 0.031c, 0.15d |
| 72 h | −1.7 (0.2) | −2.7 (0.2)c | −2.8 (0.2)d | 0.0009c, 0.0004d |
| 120 h | −3.1 (0.2) | −3.9 (0.1)c | −3.5 (0.2)c | 0.010c, 0.20d |
| Recovery of clinical symptoms | ||||
| Number with complete symptom records (%) | 10/18 (56%)a | 17/19 (90%)a | 8/18 (44%)a | |
| Median recovery time (h), (95%CI) | 382.9 (156.0, −) | 247.0 (69.5, 335.7)c | − (180.0, −)d | 0.035c, 0.70d |
There were 2, 1, and 2 asymptomatic patients in the placebo, RAY1216, and RAY1216 plus RTV groups.
Adjusted baseline viral load.
Compared with the Placebo group.
Compared with the Placebo group.
Fig. 2.
The change of SARS-CoV-2 viral load [LSmean (SE)] at different time points for each treatment group. Black dots, placebo group; Blue dots, RAY1216 group; Triangle, RTV group. Points represent the least-square means, and bars indicate the standard error. The changes from baseline, derived from an analysis of covariance model, are shown.
Fig. 3.
The Kaplan–Meier estimate of the proportion of patients with positive nucleic acid test results after treatment. Red line, placebo group; Green line, RAY1216 group; Blue line, RAY1216 + RTV group.
In order to investigate the recovery time of clinical symptoms, 14 clinical symptoms of the patients were assessed. In the placebo, RAY1216, and RAY1216 plus RTV groups, there were 2, 1, and 2 asymptomatic patients, respectively (Table 2). Patients whose symptoms did not improve due to early study termination or by the end of the follow-up period (21 days after administration) were deemed censored. As a result, the recovery time of clinical symptoms was found to not be statistically significant (p = 0.035 in the RAY1216 group, p = 0.70 in the RAY1216 plus RTV group). However, a trend toward a shorter time to sustained clinical recovery was observed in the RAY1216 group compared to the placebo group (247.0 h vs. 382.9 h). In this study, there were no patients with severe symptoms who required other antiviral drugs, corticosteroids, or monoclonal antibodies.
Adverse events
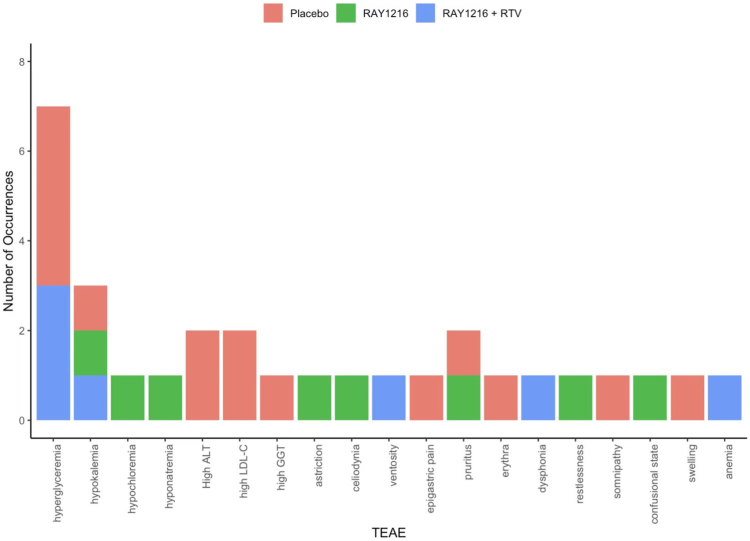
All of the treatment-related adverse events (TEAEs) are shown in Fig. 4, and there was no serious adverse event (SAE) reported among the three groups (Table 3). The incidences of TEAEs were 20% (4/20), 0%, and 5% (1/20) in the placebo, RAY1216, and RAY1216 plus RTV groups, respectively (Table 3). The only adverse reaction related to the study drug occurred in the RAY1216 plus RTV group, and it was grade-2 hypertriglyceridemia, according to the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, which resolved without further intervention. One participant in the RAY1216 group experienced a grade-4 adverse event, hyponatremia, which might not have been related to the study drug and was thought to be caused by dietary factors during the epidemic; this participant improved after the oral administration of rehydration salts.
Fig. 4.
The treatment-related adverse events (TEAEs). Red, placebo group; Green, RAY1216 group; Blue, RAY1216 + RTV group.
Table 3.
The incidence of adverse events in the three groups.
| Placebo Group (n = 20) | RAY1216 (n = 20) | RAY1216 plus RTV (n = 20) | |
|---|---|---|---|
| All adverse events, n (%) | 8 (40%) | 3 (15%) | 5 (25%) |
| Treatment-related adverse events, n (%) | 4 (20%) | 0 (0%) | 1 (5%) |
| Severe adverse events, n (%) | 0 (0%) | 0 (0%) | 0 (0%) |
Discussion
Hainan, China, reported 6592 confirmed COVID-19 cases and 9055 asymptomatic carriers between August 1, 2022, and September 16, 2022, with 32 (0.2%) being severe or critical cases and 1 (0.006%) dying. These severity and mortality rates were significantly less than the 50,354 confirmed cases in Wuhan, China, with a severity rate of 63% and a death rate of 7.7% in 2020,8 and the 42,379 confirmed cases in Shanghai, China, with a severity rate of 0.4% and a death rate of 0.1% in 2022.9 Previous research and all of the data presented above suggest that the Omicron variant caused more infections but fewer severe cases or deaths, despite the fact that continuous outbreaks and a large infectious population overburdened and exhausted the medical system.1 The number of infected people is likely to rise dramatically as epidemic prevention and control become more liberalised.
As a member of the Coronaviridae family of enveloped, single-strand, positive-sense RNA viruses, the SARS-CoV-2 viral genome is translated into approximately 30 proteins upon entry into the host cell cytoplasm.10,11 3CLpro, the main viral protease of Coronaviridae, is an appealing target for antiviral drugs because it is required for the proteolytic maturation of viral polyproteins and is highly conserved among coronaviruses.12 Paxlovid is the first oral 3CLpro inhibitor targeting the SARS-CoV-2 virus13,14 that consists of the combination of nirmatrelvir, a second-generation protease inhibitor, and ritonavir, a pharmacological enhancer; a low dose of ritonavir helps slow down the metabolism or breakdown of nirmatrelvir so that it remains active in the body for longer at higher concentrations and helps to fight the virus.15 Paxlovid remains the recommended and preferred treatment for SARS-CoV-2 viral infection. According to a study of Paxlovid, by day 28 following randomisation, 1% (6/607) of Paxlovid recipients who received treatment within 5 days of the onset of symptoms were hospitalised up to day 28 without dying, compared to 7% (41/612) of the placebo group who experienced 10 fatalities (2%).16 Paxlovid also has been shown to be effective against the Omicron variant, and a phase 2–3 double-blind, randomised, controlled trial demonstrated that it was 89% effective in patients at risk of serious illness, lowering the hospitalisation rate by 6% and accelerating the viral clearance and viral load.4
RAY1216 is an oral broad-spectrum 3CLpro inhibitor with strong anti-SARS-CoV-2 efficacy in preclinical studies. In addition, it has demonstrated favourable safety and pharmacokinetic profiles in healthy patients. The phase 1 clinical study including 72 patients showed that RAY1216 tablets containing 200–1600 mg administered at a single time, RAY1216 tablets (300–700 mg) in combination with ritonavir (100 mg BID) for 5 consecutive days, RAY1216 (200 mg) in combination with ritonavir (100 mg) at a single time, and RAY1216 (400 mg TID) for 5 consecutive days were tolerated and safe for all the patients, with no SAEs. In the current randomised, double-blind, clinical trial among adults with COVID-19, both RAY1216 alone and RAY1216 plus RTV inhibited SARS-CoV-2 replication in vivo potently. A total of 60 participants were enrolled in this study, and the baseline characteristics and the SARS-CoV-2 RNA viral load in the three groups with 20 patients each were comparable. Within 5 days of the first positive nucleic acid results, RAY1216 alone and RAY1216 plus RTV significantly accelerated the viral shedding by 4.2 days (6.9 vs. 11.1) (p = 0.0060) and 4.6 days (6.5 vs. 11.1) (p = 0.0001), respectively. According to the results of the Cox regression analysis, compared with the placebo group, the HRs in the RAY1216 and RAY1216 plus RTV groups were 3.3 (95% CI: 1.6, 6.9) and 6.1 (95% CI: 2.7, 13.6), respectively, indicating that shortening the disease duration with RAY1216 could be an additional strategy for combating this disease. In addition, the recovery times of clinical symptoms in the RAY1216 and RAY1216 plus RTV groups were shorter than those of the placebo group; however, there was no significant difference among the groups, which may be related to the small sample size. Notably, this study was conducted when Omicron was the dominant variant, and the results show the high effectiveness of RAY1216 against infection with the Omicron variant.
Two of the most important factors to determine when and how oral antiviral agents are used include the indications and the intervention time. Our findings suggest that RAY1216 could benefit patients who are treated within 120 h (5 days) of the initial positive nucleic acid test result, with a single dose of RAY1216 outperforming RAY1216 plus RTV in terms of clinical outcomes, particularly at 72 h after administration. Furthermore, most of the patients in this study had received the primary vaccination series or a booster vaccination, implying that RAY1216 may still benefit immunised patients. There was also a trend showing that RAY1216 was superior to placebo in terms of the clinical efficacy measure of time to sustained clinical recovery, but the difference was not statistically significant. This finding could be due to some missing data owing to incomplete records, causing some of the patient data to be omitted from the analysis, or the fact that the symptoms did not recover by the end of the follow-up period (21 days after administration).
In this study, no virological rebound was observed in either the placebo group or the RAY1216 plus RTV group. In the RAY1216 group, one patient met the definition of virological rebound (1/20, 5%), which was like the nirmatrelvir-ritonavir group (2.3%).17 The patient had a viral load of 3.9 log10 copies/mL on day 10 and 3.1 log10 copies/mL on day 6, with an increase of more than 0.5 log10 copies/mL from day 6 to day 10. However, the patient’s COVID-19 symptoms had disappeared after day 6, and until day 10 upon discharge, the patient remained free of any COVID-19 symptoms. This finding suggests that virological rebound may be a characteristic of certain SARS-CoV-2 viral infections.18
Previous studies have shown that Paxlovid has an overall satisfactory safety profile, with the most common adverse events being dysgeusia (5.6%) and diarrhoea (3.1%) as well as elevated fibrin D-dimer and liver enzyme levels, headache, and nausea. Few people reported SAEs like low renal creatinine or COVID-19 pneumonia, and most were mild, self-limiting grade 1 or 2. Compared to other studies, the incidence of liver injury was about the same.19,20 However, evidence emerging from a real-life study suggests an overall higher frequency of adverse events with Paxlovid than that found in clinical trials. The different prevalence rates of adverse events may be attributed to differences between the real-life population and that included in clinical trials, especially regarding age.21 In addition, dysgeusia (metallic taste) was much more frequently (12.4% vs. 5.6%) observed in those taking Paxlovid in this real-life study than that reported in the literature.21 This finding is likely related to ritonavir as a real-world pharmacovigilance study showed that the incidence of dysgeusia was out of proportion to the background effects of COVID-19 infection and was specific to ritonavir use.22 As a strong cytochrome P450 3A4 inhibitor, the use of ritonavir is associated with a high drug interaction risk. Hopkins and colleagues found that dosing of ritonavir resulted in a >2-fold increase in the steady-state area under the plasma concentration–time curve and maximal concentration for six of the ten kinase inhibitors studied.23 When the kinase inhibitor was co-administered with ritonavir, dose reductions to 10–75% of the original dose were required to achieve an area under the plasma concentration–time curve within 1.25-fold of the value in the absence of ritonavir.23 So, before prescribing this Paxlovid regimen, physicians should thoroughly evaluate the concomitant medications, such as recreational drugs and herbal supplements, since clinically significant drug–drug interactions might be caused by ritonavir.24,25
In this study, the adverse events were comparable to those of other studies and the phase 1 clinical study, with the exception of an adverse event of grade-4 hyponatremia in one participant in the RAY1216 group that may be irrelevant to the study drug. Despite the absence of definitive data on the prevalence of hyponatremia in SARS-CoV-2-infected patients, Aggarwal et al. found that half of COVID-19 patients had low sodium levels,26 and Ho et al. reported COVID-19 patients presenting with hyponatremia and seizures.27 Fluid overload as well as salt and fluid loss through the kidney, gastrointestinal system, and excessive sweating are all common causes of hyponatremia. In the current study, a 67-year-old woman had a poor appetite, but the adverse events were thought to be caused by dietary factors during the epidemic, and she improved after the oral administration of rehydration salts. There were no serious adverse reactions reported among the three groups in this study, and the only adverse reaction related to the study drug occurred in the RAY1216 plus RTV group, which was hypertriglyceridemia that resolved without further intervention.
This study has several limitations that must be addressed. First, the sample size and single-centre study limited the clinical value of RAY1216 for COVID-19, and future studies are needed to be performed in a multicentre setting. Second, although the results of this study indicate that RAY1216 tablets significantly shorten the time to viral clearance, statistical analysis of the participants’ prior infection status was not conducted, and severe/critical patients were not included. Third, this study was conducted in Sanya, China, where, according to the prevailing Chinese policy at the time, all patients diagnosed with COVID-19 were required to receive hospitalisation or centralised isolation treatment. However, in other countries, COVID-19 patients were managed clinically on an outpatient basis. Therefore, it remains unclear whether the virological benefits reported in this study can be consistently maintained in different environments. The trial was conducted in a COVID population of not primary clinical research interest, as they are healthy, young, vaccinated participants. In a real-word setting, this population is not usually treated due to extremely low risk of clinical progression. Finally, we did not perform examinations for the mutation and sub-lineages of SARS-CoV-2 as our study was conducted between August 14, 2022, and September 26, 2022, during the peak period of Omicron variant prevalence, and the Centres for Disease Control and Prevention of Sanya, China, have shown that the Omicron variant BA. 5.1.3, which was officially considered, was the strain of this epidemic in Sanya.
In conclusion, the two dosage regimens of RAY1216 tablets both have clinical potential in the treatment of SARS-CoV-2 infection if applied within 5 days of the initial positive nucleic acid test result, and their antiviral efficacies are comparable. Due to the limited study of multiple drug combinations of RTV and RAY1216 and the fact that RAY1216 monotherapy outperformed RAY1216 plus RTV in terms of clinical outcomes, particularly at 72 h after administration, RAY1216 monotherapy at 400 mg TID should be given priority as the recommended dosing regimen.
Contributors
Ling Lin was the chief investigator and led the clinical team. Ling Lin, Hai-jun Li, Zi-feng Yang, and Nan-shan Zhong contributed to the trial design and the safety assessments, monitoring, and oversight of drug interactions. Bei Wang contributed to produce the first draft of this article. Hai-jun Li contributed to statistical analysis and ran the economic assessments. Bei Wang, Mi-mi Cai, Zhao-xin Lin, Ying-na Wei, Fei Yang, Ya-min Zhu, and Ling Lin were responsible for study implementation and data acquisition. Xia-fei Ou and Rui-huan Cai were responsible for blood specimen collection. Shu-hua Wu contributed to drug management and distribution. Ling Lin, Hai-jun Li, Zi-feng Yang, and Nan-shan Zhong have verified the underlying data and have final responsibility for the decision to submit this article for publication. All authors had full access to all the data in this study, and the corresponding authors had the final responsibility to submit this study for publication.
Data sharing statement
The study protocol is published on the Chinese Clinical Trial Registry: ChiCTR2200062889. All extracted data and analytical codes are available from the corresponding author upon request.
Declaration of interests
No potential conflicts of interest were reported by the authors.
Acknowledgements
The study was sponsored by Key Research and Development Program of China (2022YFC0868700). We thank all of the patients who volunteered for this trial and the study site personnel for their contributions.
Footnotes
Translation: For the Chinese translation of the abstract see Supplementary Materials section.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102189.
Contributor Information
Zi-feng Yang, Email: jeffyah@163.com.
Nan-shan Zhong, Email: nanshan@vip.163.com.
Ling Lin, Email: linling0606@163.com.
Appendix ASupplementary data
References
- 1.Christensen P.A., Olsen R.J., Long S.W., et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with Coronavirus disease 2019 caused by the omicron variant of severe acute Respiratory syndrome Coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192:642–652. doi: 10.1016/j.ajpath.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dejnirattisai W., Huo J., Zhou D., et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185:467–484.e15. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilgenfeld R. From SARS to MERS:crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS J. 2014;281:4085–4096. doi: 10.1111/febs.12936. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond J., Leister-Tebbe H., Gardner A., et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–1408. doi: 10.1056/NEJMoa2118542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration . 2021. Emergency use authorization 105.https://www.fda.gov/media/155049/download Available at: [Google Scholar]
- 6.Najjar-Debbiny R., Gronich N., Weber G., et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients [published correction appears in clin Infect Dis. 2023 mar 01:] Clin Infect Dis. 2023;76(3):e342–e349. doi: 10.1093/cid/ciac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy M.B., Morcos P.N., Le Pogam S., et al. Pharmacokinetic/Pharmacodynamic predictors of clinical potency for hepatitis C virus nonnucleoside polymerase and protease inhibitors. Antimicrob Agents Chemother. 2012;56(6):3144–3156. doi: 10.1128/AAC.06283-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.COVID-19 map in China. https://voice.baidu.com/act/newpneumonia/newpneumonia/?from=osari_aladin_banner&city=%E4%B8%8A%E6%B5%B7-%E4%B8%8A%E6%B5%B7
- 10.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drayman N., DeMarco J.K., Jones K.A., et al. Masitinib is a broad coronavirus 3CL inhibitor that blocks replication of SARS-CoV-2. Science. 2021;373(6557):931–936. doi: 10.1126/science.abg5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elmezayen A.D., Al-Obaidi A., Şahin A.T., Yelekçi K. Drug repurposing for coronavirus (COVID-19): in silico screening of known drugs against coronavirus 3CL hydrolase and protease enzymes. J Biomol Struct Dyn. 2020;39(8):2980–2992. doi: 10.1080/07391102.2020.1758791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drożdżal S., Rosik J., Lechowicz K., et al. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist Updat. 2021;59 doi: 10.1016/j.drup.2021.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad B., Batool M., Ain Q.U., Kim M.S., Choi S. Exploring the binding mechanism of PF-07321332 SARS-CoV-2 protease inhibitor through molecular dynamics and binding free energy simulations. Int J Mol Sci. 2021;22(17):9124. doi: 10.3390/ijms22179124. Published 2021 Aug 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfizer’s novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC-HR study. 2021. https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate Available from:
- 16.Mahase E. COVID-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. Br Med J. 2021;375:n2713. doi: 10.1136/bmj.n2713. [DOI] [PubMed] [Google Scholar]
- 17.Anderson A.S., Caubel P., Rusnak J.M., et al. Nirmatrelvir-ritonavir and viral load rebound in Covid-19. N Engl J Med. 2022;387(11):1047–1049. doi: 10.1056/NEJMc2205944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson A.S., Caubel P., Rusnak J.M. EPIC-HR trial investigators. Nirmatrelvir-ritonavir and viral load rebound in Covid-19. N Engl J Med. 2022;387(11):1047–1049. doi: 10.1056/NEJMc2205944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekpanyapong S., Bunchorntavakul C., Reddy K.R. COVID-19 and the liver: lessons learnt from the EAST and the WEST, A year later. J Viral Hepat. 2022;29:4–20. doi: 10.1111/jvh.13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzitelli M., Mengato D., Sasset L., et al. Molnupiravir and nirmatrelvir/ritonavir: tolerability, safety, and adherence in a retrospective cohort study. Viruses. 2023;15(2):384. doi: 10.3390/v15020384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cvancara D.J., Baertsch H.C., Lehmann A.E., et al. Postmarketing reporting of paxlovid-related dysgeusia: a real-world pharmacovigilance study [published online ahead of print, 2023 Feb 5] Otolaryngol Head Neck Surg. 2023;169(1):55–61. doi: 10.1002/ohn.278. [DOI] [PubMed] [Google Scholar]
- 23.Hopkins A.M., Sorich M.J., McLachlan A.J., et al. Understanding the risk of drug interactions between ritonavir-containing COVID-19 therapies and small-molecule kinase inhibitors in patients with cancer. JCO Precis Oncol. 2023;7 doi: 10.1200/PO.22.00538. [DOI] [PubMed] [Google Scholar]
- 24.Paxlovid drug-drug interactions | COVID-19 treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/ritonavir-boosted-nirmatrelvir--paxlovid-/paxlovid-drug-drug-interactions/
- 25.Imran L., Zubair R., Mughal S., Shakeel R. Ritonavir-boosted Nirmatrelvir and COVID-19 outcomes in the age of Omicron variant. Ann Med Surg (Lond) 2023;85(2):313–315. doi: 10.1097/MS9.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal S., Garcia-Telles N., Aggarwal G., et al. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis (Berl) 2020;7:91–96. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- 27.Ho K.S., Narasimhan B., Kumar A., et al. Syndrome of inappropriate antidiuretic hormone as the initial presentation of COVID-19: a novel case report. Nefrologia. 2021;41:219–220. doi: 10.1016/j.nefro.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.