Summary
Background
Convincing clinical evidence regarding completely opioid-free postoperative pain management using erector spinae plane block (ESPB) in patients undergoing open major hepatectomy (OMH) is lacking. Herein, we aimed to compare the postoperative analgesic efficacy of the visualised continuous opioid-free ESPB (VC-ESPB) and conventional intravenous opioid-based postoperative pain management in hepatocellular carcinoma (HCC) patients undergoing OMH.
Methods
This open-label, randomised, controlled, non-inferiority trial enrolled patients with HCC undergone open major hepatectomy in Fujian Provincial Hospital and compared the postoperative analgesic efficacy of VC-ESPB (VC-ESPB group) and conventional intravenous opioid-based pain management regimen (conventional group). Patients were randomly assigned (1:1) to VC-ESPB group and conventional group. Patients were not masked to treatment allocation. The VC-ESPB group was treated with intermittent injections of 0.25% ropivacaine (bilateral, 30 mL each side) given every 12 h through catheters placed in the space of erector spinae and an opioid-free intravenous pump (10-mg tropisetron diluted to 100 mL with 0.9% normal saline [NS]) for postoperative pain management. The conventional group did not receive ESPB and was treated with a conventional intravenous opioid-based pump (2.5-μg/kg sufentanil and 10-mg tropisetron diluted to 100 mL with 0.9% NS). Patients in the VC-ESPB group underwent magnetic resonance imaging (MRI) to identify local anaesthetic diffusion after ESPB was performed under ultrasound guidance. The primary outcome was postoperative analgesic efficacy, which was indicated by the cumulative area under the curve (AUC) of the pain visual analogue scale scores (range, 0–10; a higher score indicates more pain) obtained at rest and at movement until 48 h postoperatively after leaving the post-anaesthesia care unit (PACU). Herein, an AUC of 26.5 was set as the noninferiority margin, which needed to be satisfied for both cumulative AUCPACU-48 h at rest and cumulative AUCPACU-48 h at movement. Per protocol participants were included in primary and safety analyses. This trial was registered with ChiCTR.org.cn (ChiCTR1900026583).
Findings
Between October 30, 2019, and May 1, 2023, 106 patients were enrolled and randomly assigned to the VC-ESPB group (n = 53) and the conventional group (n = 53). After the dropout (n = 5), a total of 101 patients (VC-ESPB group, n = 50; conventional group, n = 51) were analysed. Both the level of cumulative AUCPACU-48 h (at rest: 160.08 ± 38.00 vs. 164.94 ± 31.00; difference [90% CI], −4.861 [−16.308, 6.585]) and cumulative AUCPACU-48 h (at movement: 209.64 ± 28.98 vs. 212.59 ± 33.11; difference [90% CI], −2.948 [−13.236, 7.339]) were similar between the VC-ESPB and control groups within the first postoperative 48 h. The upper limit of the 90% CIs for the difference in cumulative ACUPACU-48 h at rest and at movement did not reach the upper inferiority margin (26.5). During the first postoperative 48 h, the rate of nonsteroidal anti-inflammatory drug rescue analgesia was similar between the VC-ESPB group and conventional group (n = 16, 32.0% vs. n = 11, 21.6%; P = 0.236). Treatment-related death was not observed in the VC-ESPB group (n = 0, 0%) and conventional group (n = 0, 0%). In VC-ESPB group, local site paralysis (n = 1, 2.0%) was observed in one patient and rash (n = 1, 2.0%) was observed in another patient. One patient in the conventional group was observed with rash preoperatively (n = 1, 2.0%). The VC-ESPB group had significantly lower rates of postoperative nausea (n = 2, 4.0%, vs. n = 9, 17.6%, P = 0.028), vomiting (n = 1, 2.0% vs. n = 8, 15.7%, P = 0.031) and lower incidence of major complications (n = 4, 8.0% vs. n = 6, 11.8%; P = 0.033).
Interpretation
This study demonstrates the noninferiority of VC-ESPB when compared with the conventional opioid-based approach for postoperative pain management after OMH, suggesting that it is feasible to achieve opioid-free postoperative pain management for OMH.
Funding
The Joint Funds for the Innovation of Science and Technology, Fujian Province, China; the Youth Scientific Research Project of Fujian Provincial Health Commission; the Fujian Research and Training Grants for Young and Middle-aged Leaders in Healthcare; and the Key Clinical Specialty Discipline Construction Program of Fujian, China.
Keywords: Hepatocellular carcinoma, Pain, Open major hepatectomy, Postoperative management, Opioid
Research in context.
Evidence before this study
We searched PubMed, China National Knowledge Infrastructure (CNKI) and UpToDate (available at: https://www.uptodate.com/) for publications about erector spinae plane block in English or Chinese from database inception until June 18, 2023, using the following search terms (“erector spinae plane block”) AND (“opioids” OR “postoperative pain management” OR “hepatectomy”). Reference of relevant articles and reviews were also screened for additional publications. Due to the use of opioid, patients undergoing hepatectomy face a higher risk of opioid-related adverse events and the resulting increase in healthcare costs. A completely opioid-free postoperative pain management would be ideal for them but lacking. A long-lasting erector spinae plane block (ESPB) with explicit successful blockade identification seems promising. However, the existing ESPB trials still have technical shortcomings, such as the lack of sustained analgesic effects and a clear evaluation of the successful blockade of local anaesthetic diffusing into the paravertebral space, which cannot form a unified conclusion. And there are currently no clinical trials of ESPB on open major hepatectomy (OMH) with the goal of opioid-free postoperative pain management.
Added value of this study
We developed a new visualised continuous erector spinae plane block (VC-ESPB) procedure with intuitive local anaesthetic diffusion (successful blockade) assessment through visualised magnetic resonance imaging program. And we designed a new long-lasting administration mode of ESPB. This is the first randomized controlled trial demonstrated the noninferiority of the VC-ESPB in postoperative pain management for OMH, when compared with conventional opioid-based postoperative pain management. It firstly demonstrated that VC-ESPB was feasible to achieve opioid-free postoperative pain management for OMH. Furthermore, this study assessed the safety of VC-ESPB and found that the VC-ESPB showed the encouraging “after-effects” in postoperative pain management. And the VC-ESPB was identified to contribute to reducing complications, promoting postoperative recovery, and improving the textbook outcome rate of OMH.
Implications of all the available evidence
The VC-ESPB remains financially favourable or comparable as it decreases other healthcare costs by shortening length of hospital stay and reducing complications. Together with its low-risk and easy-to-perform features, the VC-ESPB provides a promising clinical application prospect.
Introduction
In China, the majority of patients with hepatocellular carcinoma (HCC) are infected with hepatitis B virus (HBV) and have cirrhosis.1 In patients with resectable HCC, major hepatectomy is needed in cases with large lesions or special tumour locations, and an open approach is still the classic choice.2 Opioids remain the cornerstone for anaesthesia and postoperative pain management in hepatectomy. However, the use of opioids has several adverse effects, including opioid dependency, increased complications, and increased healthcare costs.3,4
Open major hepatectomy (OMH) is a traumatic surgery that requires aggressive incision and large opioid doses, which increase the incidence of delayed awakening, respiratory depression, drowsiness, nausea, vomiting, and other gastrointestinal system complications. These lead to prolonged hospital stays and increased healthcare costs.5 More importantly, OMH reportedly results in impaired metabolism of opioids, leading to elevated serum opioid concentrations. Owing to the relative reduction in the functional volume of the remnant hepatic parenchyma after major hepatectomy in patients with HCC and cirrhosis, the postoperative regular doses of opioids that need to be metabolised by the liver may be “excessive” and associated with a higher risk of opioid-related adverse events.6, 7, 8 Therefore, patients undergoing hepatectomy require close monitoring and dose adjustment to avoid opioid overdose and the abovementioned adverse effects. Thus, it is important to reduce postoperative opioid use as much as possible in such patients. A completely opioid-free postoperative analgesia model would be ideal but remains to be studied.
Multimodal analgesia helps reduce the use of opioids after hepatectomy.9 However, in the specific context of OMH, multimodal analgesia can be challenging and may have deleterious drawbacks, such as it makes the use of epidural block limited by possible perioperative coagulation dysfunction.10, 11, 12 The use of peripheral nerve blockade with relatively low-risk, easy-to-perform techniques, such as erector spinae plane block (ESPB), is currently an emerging approach.13,14
ESPB is among the latest opioid-sparing anaesthesia modalities. Previous studies have reported that ESPB is associated with reduced intraoperative opioid consumption.15, 16, 17, 18, 19 However, there are conflicting reports on its utility for postoperative pain management. A study reported lower postoperative opioid demand after laparoscopic hepatectomy in patients who underwent ESPB.17 In contrast, a recent randomised clinical trial (RCT) on patients having undergone laparoscopic hepatectomy failed to demonstrate the superiority of ESPB.18 Most of the existing studies were carried on the patients undergone laparoscopic approach, which is considered as a minimally invasive approach with insignificant postoperative pain. The difference results may be related to the pain thresholds for laparoscopic approach in different populations. In addition, some studies reported that the effect of single injections of ESPB lasted only 8–12 h, indicating an insufficiently long-lasting effect. And Kang et al. compared the analgesic efficacy of continuous ESPB and intrathecal morphine over 72 h and found that the 48-h cumulative opioid consumption with the former was not lower than that with the latter after laparoscopic hepatectomy.20 Except for the effect of maintenance time, a successful blockade of ESPB should also take into account the quantification of local analgesics infiltration in the paravertebral space, which were not quantitated in most previous studies. Thus, designing a long-lasting ESPB with explicit successful blockade identification seems promising, while information on its performance remains inadequate. Most importantly, to our knowledge, whether successful blockade and long-lasting ESPB can achieve completely opioid-free postoperative pain management in patients having undergone OMH remains to be investigated.
We designed a new visualised continuous ESPB (VC-ESPB) procedure to achieve completely opioid-free postoperative pain management. In this RCT, bilateral catheterised ESPB was performed alongside continuous intermittent injections of a long half-life local anaesthetic (ropivacaine), and we assessed extent of drug diffusion success by B-ultrasound and magnetic resonance imaging (MRI) after the ESPB procedure. This trial was aimed at evaluating the noninferiority of the opioid-free VC-ESPB compared with a conventional opioid-based programme in terms of postoperative pain management after OMH.
Methods
Study design
This open-label, randomised, controlled, non-inferiority trial was approved by the institutional review board of Fujian Provincial Hospital (#K2019-09-027) and conducted in compliance with the Declaration of Helsinki. This study followed the Consolidated Standards of Reporting Trials guidelines. Written informed consent was obtained from all patients.
Participants
This study was performed from October 30, 2019 to May 1, 2023 at Fujian Provincial Hospital, which is the largest liver cancer centre in Southeast China. In this study, major hepatectomy was defined as removal of ≥3 segments. Adult patients with HCC scheduled to undergo OMH (≥3 segments) were eligible for inclusion, including extended left hepatectomy (left hemihepatectomy and left trisectionectomy), extended right hepatectomy (right hemihepatectomy and right trisectionectomy), and central hepatectomy (S4∖5∖8). Patients with any contraindication for ESPB, such as a history of allergies to local anaesthetics, were excluded. Detailed selection criteria are shown in eAppendix 1 (Supplemental Digital Content). Per protocol participants were included in primary and safety analyses.
Randomisation and masking
Patients were randomly assigned to the VC-ESPB or the conventional group in a 1:1 ratio. Randomisation was performed by third-party professional medical staff by generating a random number for each patient, which was then entered into a computer interactive response system to obtain the patient’s randomised group allocation. Similar-looking drug packages were prepared by third-party staff for both groups. The participants and treating clinicians were not masked to allocation.
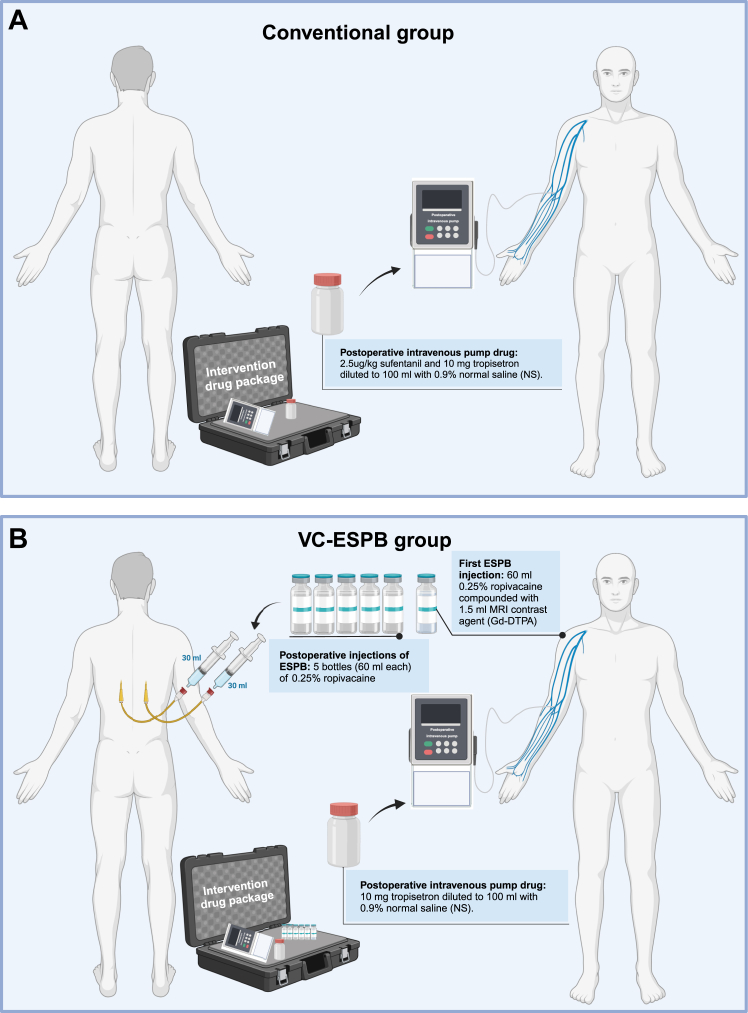
The corresponding drug packages were prepared and distributed by third-party staff as follows. (1) Control drug package (Fig. 1A): A conventional opioid-based postoperative intravenous pump (2.5-μg/kg sufentanil and 10 mg of tropisetron diluted to 100 mL with 0.9% normal saline (NS) was used. (2) Intervention drug package (Fig. 1B): Herein, 60 mL of 0.25% ropivacaine compounded with 1.5 mL of MRI contrast agent (Gd-DTPA) was prepared for the first preoperative injection of ESPB (30 mL on each side). Five bottles (60 mL each) of 0.25% ropivacaine were used in postoperative injections of ESPB (every 12 h, 30 mL each side), and a postoperative opioid-free intravenous pump [10 mg of tropisetron diluted to 100 mL with 0.9% NS].18, 19, 21, 22, 23, 24 The third-party staff performed patient randomisation and recorded information about patient allocation and the corresponding intervention. The participants and treating clinicians were not masked to allocation due to ethical considerations.
Fig. 1.
Trial intervention diagram. (A) The intervention of the VC-ESPB group. (B) The intervention of the conventional group. Abbreviations: VC-ESPB, visualised continuous erector spinae plane block.
Procedures
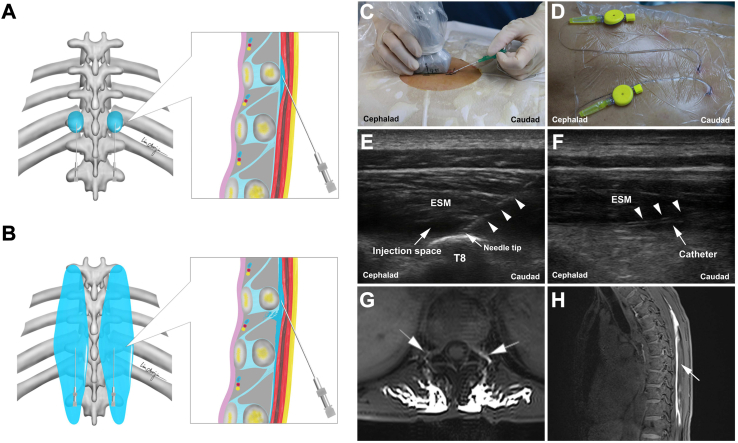
An experienced anesthesiologist performed the VC-ESPB procedure in an anaesthesia preparation room with all aseptic precautions. The procedure was performed was performed preoperatively under all aseptic precautions in the prone position by an experienced anesthesiologist in the anaesthesia preparation room after achieving venous access and monitoring of vital signs. The bilateral transverse process of T8 and the erector spinae were marked using ultrasound and the overlying skin was anaesthetised with 3 mL of 2% lidocaine. A 20-G trocar was inserted from the caudal end toward the cephalic end in the space between the erector spinae muscle and the transverse process of T8 under ultrasound guidance. Five millilitres of 0.9% NS was injected to enlarge the space. Subsequently, a catheter was inserted through the sheath tube at a depth of 4–5 cm to reach the enlarged space, and the tip of the catheter was positioned between the transverse processes of T7 and T8, which was based on the distribution of nerves innervating the liver, the incision, and the symmetric diffusion of the injected liquid. After confirming the appropriate position of the catheter, the first dose of the given drug was injected [60 mL of 0.25% ropivacaine compounded with 1.5 mL of MRI contrast agent (Gd-DTPA), 30 mL each side]. Liquid diffusion of the injection space was observed using the ultrasound. The same procedure was repeated on the contralateral side (Fig. 2A–F). The pain visual analogue scale (VAS, scores: 0–10; a higher score indicates more pain) was used by the anesthesiologist to evaluate pain 30 min before and after catheterisation. The patient was kept in the prone position for 15 min with monitoring, and any adverse events or complaints were recorded by the anesthesiologist who performed the VC-ESPB procedure. After observation, the patient was transferred to undergo fast enhanced T1-sequence MRI scanning of the thoracic spine to confirm the bilateral diffusion of the injected fluid in the paravertebral space (Fig. 2G and H). Diffusion was evaluated by a professional radiologist. Considering the symmetric diffusion of the injected liquid and the distribution of nerves innervating the liver and incision, any contrast observed in the paravertebral space of any segment from T5 to T10 was considered as the successful blockade of VC-ESPB.
Fig. 2.
Visualised continuous ESPB procedure. (A) Diagram of ESPB. (B) Diagram of ESPB. (C) ESPB puncture under ultrasonic guidance. (D) Bilateral fixed catheters. (E) Ultrasonic image of the enlarged injection space. The triangles indicate the puncture needle. (F) Ultrasonic image of the catheter. The triangles indicate the catheter. (G) MRI positive: diffusion of local anaesthetic in the paravertebral space. (H) Longitudinal diffusion of the local anaesthetic in the thoracic spine. Abbreviations: ESPB, erector spinae plane block; ESM: erector spinae muscle; T8: transverse processes of the T8 vertebrae; MRI, magnetic resonance imaging.
General anaesthesia was performed by another experienced anesthesiologist and administered by total intravenous technique using propofol, sufentanil, remifentanil, and cis-atricurium (details are provided in Supplemental Digital Content). All operations were performed by experienced surgeons who had specialised in hepatobiliary surgery for more than 20 years. A reverse L-shaped incision was made in the right upper abdomen of all patients, and the length of the incision was recorded as the value adjusted by height (eAppendix 2 in Supplemental Digital Content). An ultrasonic scalpel (Ethicon, Cincinnati, OH) was used to free the ligaments and resect the hepatic parenchyma. The Pringle manoeuvre was routinely used. Skin staplers were used for skin incision suture in all patients. Fluid management [maintenance of low central venous pressure below 5 cmH2O) with close monitoring hepatectomy] was performed as per the enhanced recovery after surgery (ERAS) guidelines, and intraoperative hypothermia was prevented using forced air warming systems according to the ERAS guidelines.25,26
Intravenous pumps of the drugs to be given were started after extubation. In the conventional group, patients received the conventional opioid-based intravenous pump (2.5-μg/kg sufentanil and 10 mg of tropisetron diluted to 100 mL with 0.9% NS; Fig. 1A). In the VC-ESPB group, postoperative injection of the given drugs was administered via fixed catheters every 12 h until 48 h postoperatively at the post-anaesthesia care unit (PACU) (30 mL on each side, five postoperative injections in total) with the patient in prone position. Patients in VC-ESPB group also received the intravenous pump (10 mg of tropisetron diluted to 100 mL with 0.9% NS; Fig. 1B).
Considering the previous opioid poisoning events and strict bias control, all intravenous pumps were locked to administer only the basic dose (2 mL/h, dosage as described as above), of which patient-controlled additional use was invalid. The decision to provide rescue analgesia was made by the surgeons and was considered only when the patient requested it and the VAS at rest was ≥4. Flurbiprofen (50 mg) was the only nonsteroidal anti-inflammatory drug (NSAID) used for rescue analgesia. The VC-ESPB catheters and all intravenous pumps were removed 48 h postoperatively. The ERAS protocol was followed in all patients.13 All patients received postoperative glycemic control (<8.3 mmol/L) and encouragement for early mobilisation according to the ERAS guideline.25,26
Outcomes
The primary outcome was to compare the overall postoperative 48-h analgesic efficacy as indicated by the cumulative area under the curve (AUC) of the pain rating according to the VAS scores and postoperative time; the co-primary outcome comprised cumulative AUCPACU–48 h at rest and at movement (eAppendix 3 in Supplemental Digital Content). The time points were set at PACU (0 h), 12 h, 24 h, and 48 h after leaving the PACU. The formula for AUC calculation was as follows:
| AUC = ∑(VASI + VASj) × (tj − ti) ÷ 2 |
| Cumulative AUCPACU-48 h = [(VAS0 h + VAS12 h) × 12 ÷ 2] + [(VAS12 h + VAS24 h) × 12 ÷ 2] + [(VAS24 h + VAS48 h) × 24 ÷ 2] |
VASi and VASj are the VAS scores corresponding to two adjacent observation postoperative time points tj and ti (j > i), respectively. VAS0 h was the VAS at PACU, and VAS was scored on a scale of 0–10, with higher scores indicating more severe pain. The postoperative VAS score [PACU (0 h), 12 h, 24 h, 48 h, 72 h, and 96 h] at rest and at movement was calculated and collected by third-party staff. The co-primary outcomes were both cumulative AUCPACU-48 h at rest and cumulative AUCPACU-48 h at movement. Noninferiority margin was satisfied for both cumulative AUCPACU-48 h at rest and cumulative AUCPACU-48 h at movement.
The secondary outcomes included the rate of postoperative rescue analgesia, postoperative anaesthesia events, and recovery outcomes and ESPB-related events. The textbook outcome was used to evaluate the quality of postoperative outcomes (eAppendix 2 in Supplemental Digital Content). The textbook outcome was defined as the absence of ESPB events, positive margins, anaesthesia events, complications, prolonged postoperative length of hospital stay (≥10 d), readmission, and mortality.27, 28, 29, 30
Statistical analysis
PASS 11.0 (NCSS, LLC, Utah, United States) was used to estimate the sample size based on our previous attempts in OMH and clinical considerations due to the lack of appropriate references in existing literature. The noninferiority was set according to 1/10–1/5 of the mean of conventional group as recommended.31,32
The cumulative AUCPACU-48 h at rest of our previous ten attempts of VC-ESPB procedures (mean 170.40, SD 38.59) and conventional procedures (mean 166.80, SD 34.15), and the cumulative AUCPACU-48 h at movement of the ten attempts of VC-ESPB procedures (mean 216.60, SD 32.05) and conventional procedures (mean 211.80, SD 34.76) were calculated. Based on that, we set the threshold for noninferiority of the VC-ESPB group for the primary outcome no higher than 26.5 higher from the conventional group in the mean cumulative AUCPACU-48 h, which is within the range of 1/10–1/5 both at rest and at movement. Based on that, a total of 90 patients were calculated (1:1 ratio) to be needed (45 each group) for the primary analysis at rest, with a significance level one side α = 0.05 and a power of (1—β) = 0.90. Similarly, a total of 84 patients were calculated (1:1 ratio) to be needed (42 each group) for the primary analysis at movement. According to the larger sample size required for the two primary outcomes calculated respectively, and to allowing for patient drop out or inadequate data collection we added a 10% contingency factor and chose to enrol 50 patients in each group or 100 patients in total at least.
SPSS 26.0 (IBM Corporation, NY, United States) and JASP software (version 0.16.3) were used to analyse data. Continuous variables were presented as mean (SD) or median [interquartile range (IQR)] and were compared using the Student’s t test or Mann–Whitney U test as appropriate. Categorical variables were expressed as absolute frequencies (percentage) and compared using the Pearson χ2 test or Fisher’s exact test as appropriate. For primary outcomes, difference with 90% confidence interval [CI] were calculated to estimate the non-inferiority, with the upper limit of 90% CI lower than 26.5. The margin was satisfied for both cumulative AUCPACU-48 h at rest and cumulative AUCPACU-48 h at movement so that a P value of <0.05 based on one-sided testing was still considered as the significance level in noninferiority analysis. Comparisons of VAS scores between the two groups at each time point were analysed by using the Student's t test. Logistic regression analysis was used to analyse the factors for the textbook outcome. A P value of <0.05 was considered statistically significant. This trial is registered with ChiCTR.org.cn, ChiCTR1900026583.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. XC Z and DF W had access to dataset and had final responsibility for the decision to submit for publication.
Results
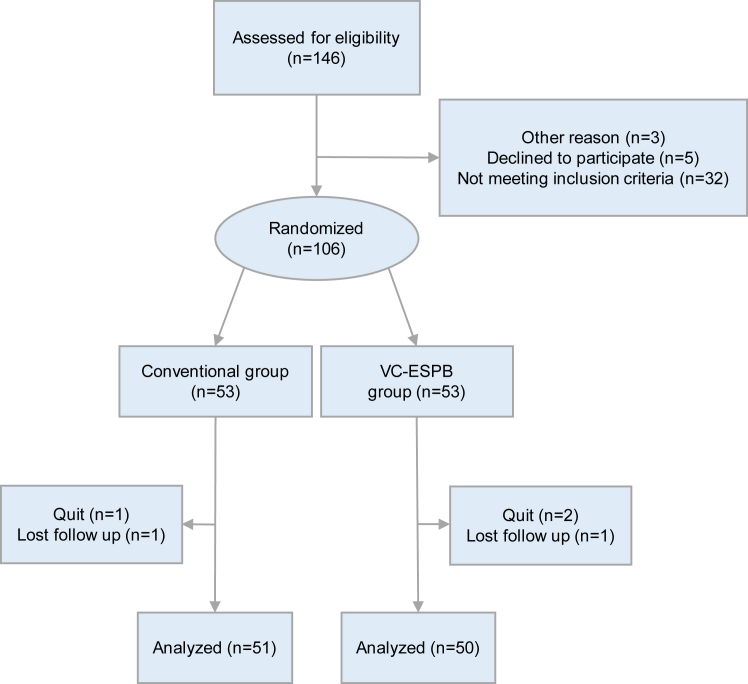
Among the 146 patients screened during the study period, 106 were finally enrolled and randomly assigned to groups (VC-ESPB group–53, conventional group–53). After dropout, a total of 101 patients (95.3%; 69 men, 32 women; VC-ESPB group–50, conventional group–51) completed the follow-up (Fig. 3, Table 1) without missing data, indicating a larger sample size than the calculated minimum requirement. A flowchart of the participants is shown in Fig. 3. The comparisons of the VAS scores before and after catheterisation and the ESPB-related events suggested that the modified procedure did not increase the clinical risk (All P > 0.05, Table 2). In VC-ESPB group, one patient complaint a local site paralysis at the left puncture site (n = 1, 2.0%) and one patient was observed with several small rashes next to the adhesive catheter fixation dressing (n = 1, 2.0%). For the comparison, these adverse symptoms were also recorded in the conventional group preoperatively even they did not receive ESPB procedures (Table 2). Incidentally, one patient in the conventional group was observed with a very localised small rash on the left waist preoperatively (n = 1, 2.0%). No treatment-related death was observed in the VC-ESPB group (n = 0, 0%) and the conventional group (n = 0, 0%). In addition, no catheter dislodgment occurred in the VC-ESPB group. Forty-six patients in the VC-ESPB group (92.0%) showed diffusion in the paravertebral space on MRI, which confirmed successful drug diffusion (Table 2).
Fig. 3.
CONSORT flow diagram.
Table 1.
Characteristic.
| Characteristic | Study group |
|
|---|---|---|
| VC-ESPB group (n = 50) | Conventional group (n = 51) | |
| Age, mean (SD), y | 55.00 (9.76) | 55.29 (11.68) |
| Sex, No. (%) | ||
| Female | 17 (34.0) | 15 (29.4) |
| Male | 33 (66.0) | 36 (70.6) |
| BMI, mean (SD), kg/m2 | 21.74 (2.53) | 21.80 (2.30) |
| Height, mean (SD), cm | 164.30 (7.42) | 165.61 (7.39) |
| Weight, mean (SD), kg | 58.77 (8.63) | 60.02 (9.05) |
| ASA grade, No. (%) | ||
| I | 8 (16.0) | 10 (19.6) |
| II | 31 (62.0) | 29 (56.9) |
| III | 11 (22.0) | 12 (23.5) |
| ECOG performance status, No. (%) | ||
| 0 | 46 (92.0) | 44 (86.3) |
| 1 | 4 (8.0) | 7 (13.7) |
| Cardiovascular comorbidities, No. (%) | 7 (14.0) | 9 (17.6) |
| Diabetes, No. (%) | 4 (8.0) | 2 (3.9) |
| Previous liver resection, No. (%) | 5 (10.0) | 6 (11.8) |
| Albumin level, mean (SD), g/dL | 41.62 (6.34) | 40.67 (7.16) |
| Bilirubin level, mean (SD), mmol/L | 20.50 (8.72) | 21.60 (9.97) |
| ALT level, mean (SD), U/L | 54.38 (33.70) | 53.80 (40.02) |
| AST level, mean (SD), U/L | 46.02 (31.13) | 45.61 (33.63) |
| Platelet level, mean (SD), 109/L | 237.00 (58.67) | 235.94 (67.32) |
| Creatinine level, mean (SD), mmol/L | 67.62 (13.06) | 65.76 (15.36) |
| INR, mean (SD), | 1.06 (0.06) | 1.06 (0.06) |
| Lactate, mean (SD), mmol/L | 0.90 (0.32) | 0.88 (0.26) |
| AFP level, median (IQR), ng/mL | 80.3 (5.3–9798.7) | 127.1 (5.4–6348.5) |
| Etiology, No. (%) | ||
| Hepatitis B | 43 (86.0) | 43 (84.3) |
| Hepatitis C | 1 (2.0) | 1 (2.0) |
| Other | 6 (12.0) | 7 (13.7) |
| ICG-15 min, mean (SD), % | 8.71 (2.83) | 8.36 (2.70) |
| Child-Pugh class, No. (%) | ||
| A | 48 (96.0) | 46 (90.2) |
| B | 2 (4.0) | 5 (9.8) |
| Radiological cirrhosis present, No. (%) | 36 (72.0) | 34 (66.7) |
| SLV, mean (SD), mL | 1158.13 (98.02) | 1175.33 (106.15) |
| Calculated-SRLV, mean (SD), mL | 687.89 (115.11) | 685.60 (123.53) |
| Surgical procedure, No. (%) | ||
| Extended left hepatectomy | 16 (32.0) | 13 (21.6) |
| Left hemihepatectomy | 9 (56.3) | 9 (69.2) |
| Left trisectionectomy | 7 (43.8) | 4 (30.8) |
| Extended right hepatectomy | 24 (48.0) | 30 (58.8) |
| Right hemihepatectomy | 21 (87.5) | 26 (86.6) |
| Right trisectionectomy | 3 (12.5) | 4 (13.3) |
| Central hepatectomy (S4/5/8) | 10 (20.0) | 8 (15.7) |
| Liver resection difficulty level, No. (%) | ||
| Grade 1 | 0 (0) | 0 (0) |
| Grade 2 | 20 (40.0) | 22 (43.1) |
| Grade 3 | 30 (60.0) | 29 (56.9) |
| Lesion, No. (%) | ||
| Unifocal | 30 (60.0) | 36 (74.5) |
| Multifocal | 20 (40.0) | 15 (25.5) |
| Maximum tumour size, median (IQR), cm | 8.00 (5.00–13.00) | 7.50 (4.20–11.00) |
| Macrovascular invasion, No. (%) | 12 (24.0) | 15 (29.4) |
| Microvascular invasion, No. (%) | 23 (46.0) | 27 (52.9) |
| Satellite lesions, No. (%) | 3 (6.0) | 2 (3.9) |
| METAVIR grade of liver fibrosis, No. (%) | ||
| F0 (no) | 5 (10.0) | 7 (13.7) |
| F1 (minimal) | 11 (22.0) | 9 (17.6) |
| F2 (mild) | 22 (44.0) | 22 (43.1) |
| F3 (moderate) | 11 (22.0) | 12 (23.5) |
| F4 (severe) | 1 (2.0) | 1 (2.0) |
| AJCC stage, No. (%) | ||
| I | 12 (24.0) | 13 (25.5) |
| II | 10 (20.0) | 12 (23.5) |
| III | 25 (50.0) | 23 (45.1) |
| IV | 3 (6.0) | 3 (5.9) |
| BCLC stage, No. (%) | ||
| 0 | 0 (0) | 0 (0) |
| A | 0 (0) | 1 (2.0) |
| B | 25 (50.0) | 21 (41.2) |
| C | 25 (50.0) | 29 (56.9) |
VC-ESPB, visualised continuous erector spinae plane block; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ASA, American Society of Anesthesiologists; ECOG, Eastern Cooperative Oncology; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; INR, International Normalized Ratio; AFP, alpha-fetoprotein; ICG-15 min, 15 min indocyanine green retention test; SLV, standard liver volume; SRLV, standard remnant liver volume; AJCC, American Joint Committee on Cancer (8th); BCLC, Barcelona Clinic Liver Cancer.
Table 2.
VC-ESPB catheterization outcomes.
| Characteristic | Study group |
P value | |
|---|---|---|---|
| VC-ESPB group (n = 50) | Conventional group (n = 51) | ||
| MRI positive | 46 (92.0) | – | |
| T7/T8 | 33 (71.7) | ||
| T6/T9 | 9 (19.6) | ||
| T5/T10 | 4 (8.7) | ||
| MRI negative | 4 (8.0) | – | |
| Preoperative abdominal pain complaint | 14 (28.0) | 14 (27.5) | 0.95 |
| VAS before catheterization | 0.30 (0.51) | 0.31 (0.55) | 1.00 |
| VAS after catheterization | 0.32 (0.55)a | – | |
| Depth of catheterization | 4.88 (0.44) | – | |
| VC-ESPB events | |||
| Preoperative decannulation | 0 (0) | – | |
| Suspected allergy | 0 (0) | 0 (0) | 1.00 |
| Local site paresthesia | 1 (2.0) | – | |
| Local site hemorrhage | 0 (0) | – | |
| Local site infection | 0 (0) | – | |
| Pneumothorax | 0 (0) | – | |
| Rash | 1 (2.0) | 1 (2.0) | 1.00 |
| Cardiopalmus | 0 (0) | 0 (0) | 1.00 |
| Dyspnea | 0 (0) | 0 (0) | 1.00 |
| Nausea | 0 (0) | 0 (0) | 1.00 |
| Vomiting | 0 (0) | 0 (0) | 1.00 |
| Fever | 0 (0) | 0 (0) | 1.00 |
| Shock | 0 (0) | 0 (0) | 1.00 |
| Death | 0 (0) | 0 (0) | 1.00 |
Abbreviations: VC-ESPB, visualised continous erector spinae plane block; MRI, magnetic resonance imaging; VAS, visual analog scale.
P > 0.05, VAS after catheterization (30 min before operation) versus the VAS before catheterization (30 min before catheterization). ESPB events were evaluated preoperatively and recorded. For the comparison, these adverse symptoms were also recorded in the conventional group preoperatively even they did not receive ESPB procedure.
Primary outcome
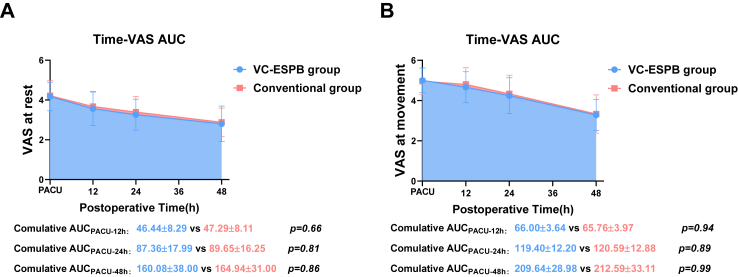
We found no significant differences in the cumulative AUCPACU-48 h between the VC-ESPB group and conventional group at rest (difference, −4.861; 90% CI, −16.308 to 6.585, P < 0.001 for noninferiority) and at movement (difference, −2.948; 90% CI, −13.236 to 7.339; P < 0.001 for noninferiority), with both the bound of the 90% CI upper limits for difference at rest and at movement below the prespecified noninferiority margin (26.5), indicating noninferiority of the postoperative analgesic efficacy in the VC-ESPB group (Table 3, Fig. 4A and 4B).
Table 3.
Primary Outcome and the corresponding details.
| AUC | Study group |
Difference (90% CI) | P valuea | |
|---|---|---|---|---|
| VC-ESPB group (n = 50) | Conventional group (n = 51) | |||
| Comulative AUC at rest | ||||
| AUC-PACU-48 h | 160.08 (38.00) | 164.94 (31.00) | −4.861 (−16.308, 6.585) | <0.001a |
| Comulative AUC at movement | ||||
| AUC-PACU-48 h | 209.64 (28.98) | 212.59 (33.11) | −2.948 (−13.236, 7.339) | <0.001a |
| Details for each time point | VC-ESPB group | Conventional group | Difference (95% CI) | P valueb |
|---|---|---|---|---|
| RestVAS-PACU | 4.18 (0.72) | 4.22 (0.76) | −0.036 (−0.327, 0.256) | 0.81b |
| RestVAS-12 h | 3.56 (0.84) | 3.67 (0.77) | −0.107 (−0.423, 0.210) | 0.51b |
| RestVAS-24 h | 3.26 (0.78) | 3.39 (0.78) | −0.132 (−0.468, 0.204) | 0.44b |
| RestVAS-48 h | 2.80 (0.90) | 2.88 (0.71) | −0.082 (−0.403, 0.238) | 0.61b |
| RestVAS-72 h | 2.02 (1.00) | 2.61 (1.10) | −0.588 (−1.002, −0.173) | 0.006b |
| RestVAS-96 h | 1.32 (0.59) | 1.69 (0.71) | −0.366 (−0.623, 0.109) | 0.006b |
| MoveVAS-PACU | 5.00 (0.61) | 4.96 (0.66) | 0.039 (−0.212, 0.290) | 0.76b |
| MoveVAS-12 h | 4.66 (0.77) | 4.80 (0.83) | −0.144 (−0.460, 0.172) | 0.37b |
| MoveVAS-24 h | 4.24 (0.89) | 4.33 (0.93) | −0.093 (−0.454, 0.267) | 0.61b |
| MoveVAS-48 h | 3.28 (0.78) | 3.33 (0.95) | −0.053 (−0.398, 0.291) | 0.76b |
| MoveVAS-72 h | 2.66 (1.02) | 3.24 (0.97) | −0.575 (−0.969, −0.182) | 0.005b |
| MoveVAS-96 h | 1.60 (0.64) | 2.10 (0.83) | −0.498 (−0.791, −0.205) | 0.001b |
AUC, area under the curve; PACU, post-anaesthesia care unit; VC-ESPB, visualised continuous erector spinae plane block; RestVAS, visual analog scale at rest; MoveVAS, visual analog scale at movement; All variables here were continuous variables that represented as mean (SD).
P value for noninferiority test.
P value, Student’s t tests for difference (VC-ESPB-Convention group) between VC-ESPB group and Conventional group.
Fig. 4.
Comparisons of the overall postoperative analgesic efficacy as indicated by the cumulative AUC of the VAS scores and postoperative time. (A–B) The AUC of the time-VAS curve between the VC-ESPB group and conventional group. Abbreviations: VC-ESPB, visualised continuous erector spinae plane block; AUC, area under the curve; VAS, visual analogue scale.
Details of VAS at each time point
No significant differences of specific VAS score at each time points including the 0 h, 12 h, 24 h, 48 h between the VC-ESPB group and the conventional group were observed respectively (Table 3, Fig. 4A and 4B), which further supported its similar postoperative analgesic efficacy.
Secondary outcomes
Within the first postoperative 48 h, the rate of NSAID rescue analgesia was higher in the VC-ESPB group (n = 16, 32.0%) than in the conventional group (n = 11, 21.6%); however, the difference was not statistically significant (32.0% vs. 21.6%, P = 0.24). This indicates that VC-ESPB could achieve potent opioid-free postoperative pain management with similar analgesic efficacy to that of the conventional opioid-based postoperative pain management; this was also reflected by the postoperative opioid (sufentanil) consumption (0 vs. 150.05 ± 22.62, P < 0.001, Table 4).
Table 4.
Secondary outcomes.
| Secondary outcomes | Study group |
P value | |
|---|---|---|---|
| VC-ESPB group |
Conventional group |
||
| (n = 50) | (n = 51) | ||
| Postoperative opioid consumption and rescue analgesia | |||
| Postoperative opioid consumption (sulfentaini, ug) | 0 (0) | 150.05 (22.62) | <0.001 |
| Rescue analgesic NSAIDs No. (%) | 17 (34.0) | 18 (35.3) | 0.89 |
| Within 48 h | 16 (32.0) | 11 (21.6) | 0.24 |
| After 48 h | 7 (14.0) | 15 (29.4) | 0.061 |
| Rescue analgesic Pethidine No. (%) | 1 (2.0) | 0 (0) | 1.00 |
| Postoperative anaesthesia events | |||
| Flush | 0 (0) | 3 (5.9) | 0.24 |
| Nausea | 2 (4.0) | 9 (17.6) | 0.028 |
| Vomiting | 1 (2.0) | 8 (15.7) | 0.031 |
| Dizziness | 4 (8.0) | 8 (15.7) | 0.22 |
| Bradycardia | 0 (0) | 0 (0) | 1.00 |
| Respiratory depression | 0 (0) | 0 (0) | 1.00 |
| Delirium | 0 (0) | 2 (3.9) | 0.50 |
| Spasticity | 0 (0) | 0 (0) | 1.00 |
| Postoperative recovery outcomes | |||
| PLOS | 8.16 (2.71) | 9.73 (3.22) | 0.008 |
| Time to resume | |||
| Off-bed | 2.36 (0.72) | 2.94 (0.73) | <0.001 |
| Bowel movement | 1.52 (0.65) | 1.86 (0.69) | 0.011 |
| Oral-intake | 2.02 (0.55) | 2.45 (0.64) | <0.001 |
| Postoperative complication | |||
| Bile leakage | 3 (6.0) | 2 (3.9) | 0.68 |
| Hemorrhage | 0 (0) | 1 (2.0) | 1.00 |
| Abscess | 4 (8.0) | 6 (11.8) | 0.74 |
| Ileus | 0 (0) | 2 (3.9) | 1.00 |
| Wound infection | 4 (8.0) | 6 (11.8) | 0.74 |
| Liver failure | 0 (0) | 0 (0) | 1.00 |
| Pneumonia | 8 (16.0) | 16 (31.4) | 0.070 |
| Pleural effusion | 4 (8.0) | 6 (11.8) | 0.76 |
| Arrhythmia | 0 (0) | 2 (3.9) | 1.00 |
| Renal insufficiency | 0 (0) | 1 (2.0) | 1.00 |
| Sepsis | 1 (2.0) | 2 (3.9) | 1.00 |
| Major complication | 4 (8.0) | 12 (23.5) | 0.033 |
| 30 d reoperation or readmission | 0 (0) | 1 (2.0) | 1.00 |
| 30 d death | 0 (0) | 0 (0) | 1.00 |
VC-ESPB, visualised continuous erector spinae plane block; PLOS, postoperative length of stay; NSAIDs, Non-steroidal anti-inflammatory drugs. Time to resume oral intake (the time to first semiliquid intake).
Notably, VAS-72 h (at rest: P = 0.006; at movement: P = 0.007), VAS-96 h (at rest: P = 0.004; at movement: P = 0.002), and the rate of NSAID rescue analgesia beyond 48 h (P = 0.061) were all significantly lower in the VC-ESPB group (n = 7, 14.0%) than that in the conventional group (n = 15, 29.4%), indicating a better analgesic aftereffect (P = 0.061, Table 3).
The VC-ESPB group had significantly lower rates of postoperative nausea (n = 2, 4.0%, vs. n = 9, 17.6%, P = 0.028), vomiting (n = 1, 2.0% vs. n = 8, 15.7%, P = 0.031) and comparable rates of other postoperative anaesthesia events (Table 4). The VC-ESPB group had shorter hospital stay (8.16 ± 2.71 vs. 9.73 ± 3.22 d, P = 0.008), shorter time to off-bed (2.36 ± 0.72 vs. 2.94 ± 0.73 d, P < 0.001), earlier bowel movement (1.52 ± 0.65 vs. 1.86 ± 0.69 d, P = 0.011), earlier oral intake (2.02 ± 0.55 vs. 2.45 ± 0.64 d, P < 0.001), and lower incidence of major complications (n = 4, 8.0% vs. n = 6, 11.8%; P = 0.033). Moreover, VC-ESPB led to a significantly higher textbook outcome rate (n = 20, 40.0% vs. n = 8, 15.7%; P = 0.006, Supplementary Fig. S1A and B in Supplementary material), which was further confirmed by logistic regression analysis (OR [95CI]: 4.271 [1.587–11.499], P = 0.004, Supplementary Table S1 in Supplementary material). Furthermore, we compared the total hospitalisation expenses of the two group and found that the average expenses were slightly lower for the VC-ESPB group (5833.86 ± 490.86 vs. 6133.57 ± 697.51 USD; P = 0.015, Supplementary Table S2 in Supplementary material).
Discussion
Postoperative opioids increase the healthcare burden considering the additional expenses associated with various opioid-related adverse events and complications.4 Patients with HCC who need to undergo OMH often have cirrhosis, hypoproteinemia, decreased hepatic blood flow, reduced plasma protein binding capacity, and lower hepatic enzyme activity, all of which severely affect the clearance of opioids. In addition, unlike laparoscopic hepatectomy or donor liver resection, the limited residual liver volume after OMH also deceases the ability to metabolise opioids, which further precludes the use of opioids.6,9 An ideal opioid-sparing postoperative pain management approach is urgently needed for such patients.
To our knowledge, this is the first study to report that VC-ESPB is noninferior to conventional opioids in the postoperative pain management for OMH.9 This is the first indication that it is feasible to achieve the goal of opioid-free postoperative pain management. And the design of this study was rigorous and innovative.16,18,19,21,24,33, 34, 35 First, the VC-ESPB procedure takes take the long-lasting bilateral catheterisation with multiple long half-life ropivacaine intermittent injections mode, which greatly prolong the analgesia effect. Second, the visualised programme to determinate the drug diffusion by B-ultrasound and MRI further identified the successful blockade of VC-ESPB. Third, the intravenous opioid pump for postoperative pain management was designed to deliver only a basic dose and disallowed patient-controlled additional use, thus avoiding subjective bias and preventing opioid-overdose poisoning events. And We adapted a strict rescue analgesia strategy using flurbiprofen to indirectly determine the analgesic efficacy. Furthermore, considering that patients and investigators could distinguish the treatment allocation, which may cause subjective bias on outcomes. To minimize that, the primary outcome was recorded by a third staff, and secondary outcomes that need to be diagnosed by objective symptoms or objective examinations were used.
Herein, we showed that VC-ESPB has benefits for ERAS and improves textbook outcomes. Notably, opioid-sparing regimens are the cornerstone of ERAS, and the ERAS guidelines for patients undergoing hepatectomy recommend using a multimodal analgesia approach as a supplement for postoperative pain management, such as local anaesthetic wound infiltration.26,35 It is effective in suppressing incisional pain but often needs to be combined with patient-controlled opioid analgesia to manage visceral pain. Furthermore, the need and controversial position for its catheter placement makes it controversial as it carries the risk of compromising incision healing, particularly the longer incisions in OMH that often present with hypoproteinemia.36, 37, 38 We did not use it in this study, and the VC-ESPB could stay away from the incision. As VC-ESPB eliminates the need for patient-controlled opioid analgesia, it can reduce opioid use to a greater extent. As VC-ESPB is designed to block the sympathetic ganglia, thus more comprehensively covering both incisional and visceral pain.
The analgesic efficacy of ESPB varies among existing researches because of the unclear mechanism of action in diverse surgical procedures. Kang et al. compared the either single or continuous ESPB with quadratus lumborum block, conventional method, and intrathecal morphine and did not find ESPB to be the superior alternative.18,20,39,40 Our VC-ESPB was different in design, and this study intended to analyse its noninferiority to conventional opioid-based postoperative pain management, with a focus on OMH but not laparoscopic hepatectomy. The procedures and administration in prone, multiple intermittent large administration volume, and visual drug infiltration assessment were also different. Regarding the mechanism, anatomical and cadaveric studies suggest that ESPB exerts a “paravertebral-like effect” caused by local anaesthetic infiltration into the paravertebral space through the space of erector spinae, thus resulting in an analgesic effect.41 However, previous studies did not verify this process. We herein used MRI to visually evaluate the infiltration of “paraspinal-like effect”, and our findings are in agreement with the findings of Schwartzmann et al. by coincidence.42, 43, 44 Our evidence has shown that successful blockade depends on the spread of the local anaesthetic through the space between the transverse costal ligament or through the transverse foramen to the nerve roots in the paravertebral space (Fig. 2A, 2B, 2G and 2H). This process is affected by differences in the anatomical structure around the transverse process, thus causing diffusion barriers and uncertain or inconsistent blockade.45, 46, 47, 48 We speculate that the local anaesthetic first diffuses longitudinally from the injection site and then to the paravertebral cavity after reaching the saturation level. This may also be affected by the local pression from injection volume as well as the concentration and gravity effect of the local anaesthetic, thus we preferred a multiple intermittent high-volume injection rather than a continuous pump.49,50 In addition, we administered the drug before anaesthesia induction, and the siphoning effect of chest negative pressure facilitated the infiltration of the drug into the paravertebral space.51, 52, 53 With these technical improvements, we obtained an improved diffusion rate of 92%.
Considering the differences in paravertebral anatomy and interstitial permeability, we defined any T5–T10 paravertebral space infiltration as successful drug diffusion. Unlike previous studies which used the lateral position, we kept the patient in prone position for 15 min (while factoring in the patient's tolerance to position) in an attempt to increase diffusion by gravity as much as possible. However, ropivacaine infiltration in some patients was not captured by MRI, which may be attributed to delayed infiltration (after 15 min) or other invisible blocking mechanisms, such as desensitisation of the sensory nerve fibres adjacent to the lymph nodes by local anaesthetics diffused through lymphatic reflux.43,54, 55, 56, 57, 58, 59 Even though drug diffusion was not captured in 8% of the cases, the results of regression analysis still showed that VC-ESPB did promote the textbook outcome, which is containing with an analgesic effect indicator. Therefore, we believe that as long as the longitudinal degree of intermuscular diffusion on MRI meets the innervation area of the incision, the block can still be considered effective after. Furthermore, we performed bilateral block to ensure effective analgesia for median incisions.
To our knowledge, this is the first study to report the superior aftereffects of VC-ESPB after 48 h reflecting by the VAS and a decreased need for rescue analgesia. We extended the observation time to 96 h postoperatively, which is longer than that reported in previous studies, and obtained such encouraging results. This may be attributed to the “tailing effect” of VC-ESPB and the long half-life of the local anaesthetic. Considering the duration of the whole intervention process in this study, we chose the time period from PACU to 48 h postoperatively and the AUC of the time–VAS curve as the primary outcomes rather than the VAS at certain time points; we believe this is a better approach as it is a more scientific and better reflection of the whole period of postoperative pain.60 The inclusion of more men than women is reasonable according to the incidence of HCC61; however, there was no difference between groups in terms of gender distribution. Thus, the differences in pain thresholds between men and women do not influence our results.
As the low-risk and the safety of ESPB described previously, no severe ESPB-related adverse events and no catheter dislocation events were observed in this study, which further upholds its safety.14 To avoid catheter malfunction, such as dislodgment, kinking, and clogging, we used local needle and thread fixation and added a large plaster with strong adhesion to fix catheters along the pipeline (Fig. 2D). In addition, the time of catheter removal was mostly after the patient’s first off-bed activity. However, a multi-centre cohort study with a large sample size is warranted to obtain an objective catheter drop rate.
Together with its low-risk and easy-to-perform make VC-ESPB a good choice for opioid-free postoperative pain management.14 However, it needs further real-world researches to clarify and uphold its application. In this study, we did not charge participants for VC-ESPB. However, the actual costs of VC-ESPB in clinical application will depend on the local medical fee standards and health insurance policy. However, even with the added fee of VC-ESPB, which is less than 200 USD according to the medical fee standards of our country, we think it remains financially favourable or comparable as it lowers other medical costs by facilitating shortened length of hospital stay and reduced complications.
To achieve opioid-free in postoperative pain management, VC-ESPB needs to be administered along with a rescue analgesic NSAID, as it was indicated that there was slightly more rescue analgesic NSAID use within the first 48 h in VC-ESPB group. Nevertheless, NSAIDS are more acceptable than opioids for patients underwent OMH. Thus, we claimed it can achieve the opioid-free postoperative pain management. The current study has several limitations. First, VC-ESPB is cumbersome to perform and requires good patient compliance; we will further simplify the procedures. Second, only patients with HCC undergoing OMH were enrolled in this study; whether the findings can be extrapolated to other abdominal surgeries remains to be evaluated. Finally, this was a single-centre RCT, and future studies involving multiple centres and larger sample sizes are required to validate the findings.
Taken together, our findings indicate noninferiority of VC-ESPB when compared with conventional opioid-based postoperative pain management for patients with HCC undergoing OMH, indicating that it is feasible to achieve completely opioid-free postoperative pain management. VC-ESPB is a safe and feasible approach with a better aftereffect, thus enhancing postoperative recovery and improving the textbook outcome of OMH.
Contributors
XC Z and DF W had full access to all data in the study and takes responsibility for the integrity of the data and accuracy of data analyses. XC Z and DF W have accessed and verified the underlying data. Concept and design: XC Z, DF W, S C, CY L, and YF T. Acquisition, analysis, and interpretation of data: All authors. Drafting of the manuscript: DF W and CY L. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: DF W and CY L. Administrative, technical, or material support: All authors. Study supervision: XC Z.
Data sharing statement
Data are available with publication from the corresponding author upon reasonable request.
Declaration of interests
All the authors declare that they have no competing interests.
Acknowledgements
This study was supported by the Joint Funds for the Innovation of Science and Technology, Fujian Province, China (Grant No. 2019Y9028, to XC Z); the Youth Scientific Research Project of Fujian Provincial Health Commission (Grant No. 2020QNA005, to DF W); and the Fujian Research and Training Grants for Young and Middle-aged Leaders in Healthcare [No.2021(60), to S C]. This work was sponsored by the Key Clinical Specialty Discipline Construction Program of Fujian, P.R.C.
We thank all the patients who participated in this study. We thank Dr. Chunjin Lin from Fujian Provincial Hospital for generating the VC-ESPB diagram (Fig. 2A and 2B). All the other diagrams were created with BioRender.com.
Footnotes
Translation: For the Chinese translation of the abstract see the Supplementary materials section.
Study protocol: see the Supplemental digital content section.
Supplementary tables and figures: see the Caption for supplementary material section.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102188.
Contributor Information
Shi Chen, Email: wawljwalj@163.com.
Xiaochun Zheng, Email: fjslyymzk@126.com.
Appendix A. Supplementary data
References
- 1.Chen W., Zheng R., Baade P.D., et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Kasai M., Cipriani F., Gayet B., et al. Laparoscopic versus open major hepatectomy: a systematic review and meta-analysis of individual patient data. Surgery. 2018;163(5):985–995. doi: 10.1016/j.surg.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Hah J.M., Bateman B.T., Ratliff J., Curtin C., Sun E. Chronic opioid use after surgery: implications for perioperative management in the face of the opioid epidemic. Anesth Analg. 2017;125(5):1733–1740. doi: 10.1213/ANE.0000000000002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J.S., Vu J.V., Edelman A.L., et al. Health care spending and new persistent opioid use after surgery. Ann Surg. 2020;272(1):99–104. doi: 10.1097/SLA.0000000000003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kane-Gill S.L., Rubin E.C., Smithburger P.L., Buckley M.S., Dasta J.F. The cost of opioid-related adverse drug events. J Pain Palliat Care Pharmacother. 2014;28(3):282–293. doi: 10.3109/15360288.2014.938889. [DOI] [PubMed] [Google Scholar]
- 6.Tsukano Y., Sugita M., Hirata N., Yamamoto T. Future liver remnant volume is associated with postoperative fentanyl consumption following open donor hepatectomy: a retrospective multivariate analysis. J Anesth. 2022;36(6):731–739. doi: 10.1007/s00540-022-03110-2. [DOI] [PubMed] [Google Scholar]
- 7.Rudin A., Lundberg J.F., Hammarlund-Udenaes M., Flisberg P., Werner M.U. Morphine metabolism after major liver surgery. Anesth Analg. 2007;104(6):1409–1414. doi: 10.1213/01.ane.0000261847.26044.1d. table of contents. [DOI] [PubMed] [Google Scholar]
- 8.Moss C.R., Caldwell J.C., Afilaka B., et al. Hepatic resection is associated with reduced postoperative opioid requirement. J Anaesthesiol Clin Pharmacol. 2016;32(3):307–313. doi: 10.4103/0970-9185.188827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wrighton L.J., O'Bosky K.R., Namm J.P., Senthil M. Postoperative management after hepatic resection. J Gastrointest Oncol. 2012;3(1):41–47. doi: 10.3978/j.issn.2078-6891.2012.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudek P., Zawadka M., Andruszkiewicz P., Gelo R., Pugliese F., Bilotta F. Postoperative analgesia after open liver surgery: systematic review of clinical evidence. J Clin Med. 2021;10(16):3662. doi: 10.3390/jcm10163662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J., Pourrahmat M.M., Vasilyeva E., Kim P.T., Osborn J., Wiseman S.M. Efficacy and safety of patient-controlled analgesia compared with epidural analgesia after open hepatic resection: a systematic review and meta-analysis. Ann Surg. 2019;270(2):200–208. doi: 10.1097/SLA.0000000000003274. [DOI] [PubMed] [Google Scholar]
- 12.Jacquenod P., Wallon G., Gazon M., et al. Incidence and risk factors of coagulation profile derangement after liver surgery: implications for the use of epidural analgesia-A retrospective cohort study. Anesth Analg. 2018;126(4):1142–1147. doi: 10.1213/ANE.0000000000002457. [DOI] [PubMed] [Google Scholar]
- 13.Shontz R., Karuparthy V., Temple R., Brennan T.J. Prevalence and risk factors predisposing to coagulopathy in patients receiving epidural analgesia for hepatic surgery. Reg Anesth Pain Med. 2009;34(4):308–311. doi: 10.1097/AAP.0b013e3181ac7d00. [DOI] [PubMed] [Google Scholar]
- 14.White L.D., Riley B., Davis K., et al. Safety of continuous erector spinae catheters in chest trauma: a retrospective cohort study. Anesth Analg. 2021;133(5):1296–1302. doi: 10.1213/ANE.0000000000005730. [DOI] [PubMed] [Google Scholar]
- 15.Pawa A., King C., Thang C., White L. Erector spinae plane block: the ultimate 'plan A' block? Br J Anaesth. 2023;130(5):497–502. doi: 10.1016/j.bja.2023.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Kot P., Rodriguez P., Granell M., et al. The erector spinae plane block: a narrative review. Korean J Anesthesiol. 2019;72(3):209–220. doi: 10.4097/kja.d.19.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang X., Wang J., Zhang J., Kang Y., Sandeep B., Yang J. Ultrasound-guided erector spinae plane block improves analgesia after laparoscopic hepatectomy: a randomised controlled trial. Br J Anaesth. 2022;129(3):445–453. doi: 10.1016/j.bja.2022.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Kim D., Kim J.M., Choi G.S., Heo G., Kim G.S., Jeong J.S. Ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic liver resection: a prospective, randomised controlled, patient and observer-blinded study. Eur J Anaesthesiol. 2021;38(Suppl 2):S106–S112. doi: 10.1097/EJA.0000000000001475. [DOI] [PubMed] [Google Scholar]
- 19.Fu J., Zhang G., Qiu Y. Erector spinae plane block for postoperative pain and recovery in hepatectomy: a randomized controlled trial. Medicine (Baltimore) 2020;99(41) doi: 10.1097/MD.0000000000022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang R., Chin K.J., Kim G.S., et al. Bilateral continuous erector spinae plane block using a programmed intermittent bolus regimen versus intrathecal morphine for postoperative analgesia in living donor laparoscopic hepatectomy: a randomized controlled trial. J Clin Anesth. 2021;75 doi: 10.1016/j.jclinane.2021.110479. [DOI] [PubMed] [Google Scholar]
- 21.Abu Elyazed M.M., Mostafa S.F., Abdelghany M.S., Eid G.M. Ultrasound-Guided erector spinae plane block in patients undergoing open epigastric hernia repair: a prospective randomized controlled study. Anesth Analg. 2019;129(1):235–240. doi: 10.1213/ANE.0000000000004071. [DOI] [PubMed] [Google Scholar]
- 22.Kang R., Chin K.J., Gwak M.S., et al. Bilateral single-injection erector spinae plane block versus intrathecal morphine for postoperative analgesia in living donor laparoscopic hepatectomy: a randomized non-inferiority trial. Reg Anesth Pain Med. 2019 doi: 10.1136/rapm-2019-100902. [DOI] [PubMed] [Google Scholar]
- 23.Forero M., Adhikary S.D., Lopez H., Tsui C., Chin K.J. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. 2016;41(5):621–627. doi: 10.1097/AAP.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 24.Aydin T., Balaban O., Acar A. Ultrasound guided continuous erector spinae plane block for pain management in pulmonary malignancy. J Clin Anesth. 2018;46:63–64. doi: 10.1016/j.jclinane.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Melloul E., Hubner M., Scott M., et al. Guidelines for perioperative care for liver surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg. 2016;40(10):2425–2440. doi: 10.1007/s00268-016-3700-1. [DOI] [PubMed] [Google Scholar]
- 26.Joliat G.R., Kobayashi K., Hasegawa K., et al. Guidelines for perioperative care for liver surgery: enhanced recovery after surgery (ERAS) society recommendations 2022. World J Surg. 2023;47(1):11–34. doi: 10.1007/s00268-022-06732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merath K., Chen Q., Bagante F., et al. Textbook outcomes among medicare patients undergoing hepatopancreatic surgery. Ann Surg. 2020;271(6):1116–1123. doi: 10.1097/SLA.0000000000003105. [DOI] [PubMed] [Google Scholar]
- 28.van Roessel S., Mackay T.M., van Dieren S., et al. Textbook outcome: nationwide analysis of a novel quality measure in pancreatic surgery. Ann Surg. 2020;271(1):155–162. doi: 10.1097/SLA.0000000000003451. [DOI] [PubMed] [Google Scholar]
- 29.Merath K., Chen Q., Bagante F., et al. A multi-institutional international analysis of textbook outcomes among patients undergoing curative-intent resection of intrahepatic cholangiocarcinoma. JAMA Surg. 2019;154(6) doi: 10.1001/jamasurg.2019.0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gorgec B., Benedetti Cacciaguerra A., Lanari J., et al. Assessment of textbook outcome in laparoscopic and open liver surgery. JAMA Surg. 2021;156(8) doi: 10.1001/jamasurg.2021.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn S. Understanding noninferiority trials. Korean J Pediatr. 2012;55(11):403–407. doi: 10.3345/kjp.2012.55.11.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quartagno M., Carpenter J.R., Walker A.S., Clements M., Parmar M.K. The DURATIONS randomised trial design: estimation targets, analysis methods and operating characteristics. Clin Trials. 2020;17(6):644–653. doi: 10.1177/1740774520944377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar K., Kirksey M.A., Duong S., Wu C.L. A review of opioid-sparing modalities in perioperative pain management: methods to decrease opioid use postoperatively. Anesth Analg. 2017;125(5):1749–1760. doi: 10.1213/ANE.0000000000002497. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J., He Y., Wang S., et al. The erector spinae plane block causes only cutaneous sensory loss on ipsilateral posterior thorax: a prospective observational volunteer study. BMC Anesthesiol. 2020;20(1):88. doi: 10.1186/s12871-020-01002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altiparmak B., Korkmaz Toker M., Uysal A.I., Kuscu Y., Gumus Demirbilek S. Ultrasound-guided erector spinae plane block versus oblique subcostal transversus abdominis plane block for postoperative analgesia of adult patients undergoing laparoscopic cholecystectomy: randomized, controlled trial. J Clin Anesth. 2019;57:31–36. doi: 10.1016/j.jclinane.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Ozturk E., Yilmazlar A., Coskun F., Isik O., Yilmazlar T. The beneficial effects of preperitoneal catheter analgesia following colon and rectal resections: a prospective, randomized, double-blind, placebo-controlled study. Tech Coloproctol. 2011;15(3):331–336. doi: 10.1007/s10151-011-0720-6. [DOI] [PubMed] [Google Scholar]
- 37.Gross M.E., Nelson E.T., Mone M.C., et al. A comparison of postoperative outcomes utilizing a continuous preperitoneal infusion versus epidural for midline laparotomy. Am J Surg. 2011;202(6):765–769. doi: 10.1016/j.amjsurg.2011.05.016. discussion 70. [DOI] [PubMed] [Google Scholar]
- 38.Wu C.L., Partin A.W., Rowlingson A.J., Kalish M.A., Walsh P.C., Fleisher L.A. Efficacy of continuous local anesthetic infusion for postoperative pain after radical retropubic prostatectomy. Urology. 2005;66(2):366–370. doi: 10.1016/j.urology.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 39.Lee S., Kang R.A., Kim G.S., et al. Comparison of postoperative analgesic effects of posterior quadratus lumborum block and intrathecal morphine in laparoscopic donor hepatectomy: a prospective randomized non-inferiority clinical trial. Reg Anesth Pain Med. 2022;47(9):527–533. doi: 10.1136/rapm-2022-103577. [DOI] [PubMed] [Google Scholar]
- 40.Kang R., Lee S., Kim G.S., et al. Comparison of analgesic efficacy of erector spinae plane block and posterior quadratus lumborum block in laparoscopic liver resection: a randomized controlled trial. J Pain Res. 2021;14:3791–3800. doi: 10.2147/JPR.S343366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sivakumar R.K., Karmakar M.K. Variable anterior spread of local anesthetic after erector spinae plane block (ESPB): time to turn the spotlight on the 'retro-SCTL space'. Reg Anesth Pain Med. 2023;48(9):483–484. doi: 10.1136/rapm-2023-104362. [DOI] [PubMed] [Google Scholar]
- 42.Schwartzmann A., Peng P., Maciel M.A., Alcarraz P., Gonzalez X., Forero M. A magnetic resonance imaging study of local anesthetic spread in patients receiving an erector spinae plane block. Can J Anaesth. 2020;67(8):942–948. doi: 10.1007/s12630-020-01613-8. [DOI] [PubMed] [Google Scholar]
- 43.Schwartzmann A., Peng P., Maciel M.A., Forero M. Mechanism of the erector spinae plane block: insights from a magnetic resonance imaging study. Can J Anaesth. 2018;65(10):1165–1166. doi: 10.1007/s12630-018-1187-y. [DOI] [PubMed] [Google Scholar]
- 44.Schwartzmann A., Peng P., Antunez Maciel M., Forero M. Bilateral erector spinae plane block (ESPB) epidural spread. Reg Anesth Pain Med. 2019;44(1):131. doi: 10.1136/rapm-2018-000030. [DOI] [PubMed] [Google Scholar]
- 45.Aponte A., Sala-Blanch X., Prats-Galino A., Masdeu J., Moreno L.A., Sermeus L.A. Anatomical evaluation of the extent of spread in the erector spinae plane block: a cadaveric study. Can J Anaesth. 2019;66(8):886–893. doi: 10.1007/s12630-019-01399-4. [DOI] [PubMed] [Google Scholar]
- 46.Harbell M.W., Seamans D.P., Koyyalamudi V., Kraus M.B., Craner R.C., Langley N.R. Evaluating the extent of lumbar erector spinae plane block: an anatomical study. Reg Anesth Pain Med. 2020;45(8):640–644. doi: 10.1136/rapm-2020-101523. [DOI] [PubMed] [Google Scholar]
- 47.Willard F.H., Vleeming A., Schuenke M.D., Danneels L., Schleip R. The thoracolumbar fascia: anatomy, function and clinical considerations. J Anat. 2012;221(6):507–536. doi: 10.1111/j.1469-7580.2012.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivanusic J., Konishi Y., Barrington M.J. A cadaveric study investigating the mechanism of action of erector spinae blockade. Reg Anesth Pain Med. 2018;43(6):567–571. doi: 10.1097/AAP.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 49.Farag E., Choi Y.-J., Kwon H.-J., et al. Influence of injectate volume on paravertebral spread in erector spinae plane block: an endoscopic and anatomical evaluation. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0224487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwenk E.S., Lam E., Abulfathi A.A., et al. Population pharmacokinetic and safety analysis of ropivacaine used for erector spinae plane blocks. Reg Anesth Pain Med. 2023;48(9):454–461. doi: 10.1136/rapm-2022-104252. [DOI] [PubMed] [Google Scholar]
- 51.Chin K.J., El-Boghdadly K. Mechanisms of action of the erector spinae plane (ESP) block: a narrative review. Can J Anaesth. 2021;68(3):387–408. doi: 10.1007/s12630-020-01875-2. [DOI] [PubMed] [Google Scholar]
- 52.Gadsden J. The erector spinae plane block: the case of the elusive mechanism of action. Can J Anaesth. 2021;68(3):288–292. doi: 10.1007/s12630-020-01876-1. [DOI] [PubMed] [Google Scholar]
- 53.Elsharkawy H., Pawa A., Mariano E.R. Interfascial plane blocks: back to basics. Reg Anesth Pain Med. 2018;43(4):341–346. doi: 10.1097/AAP.0000000000000750. [DOI] [PubMed] [Google Scholar]
- 54.Zhu Y., Wang S., Wu H., Wu Y. Effect of perioperative parecoxib on postoperative pain and local inflammation factors PGE2 and IL-6 for total knee arthroplasty: a randomized, double-blind, placebo-controlled study. Eur J Orthop Surg Traumatol. 2014;24(3):395–401. doi: 10.1007/s00590-013-1203-4. [DOI] [PubMed] [Google Scholar]
- 55.Chin K.J., Malhas L., Perlas A. The erector spinae plane block provides visceral abdominal analgesia in bariatric surgery: a report of 3 cases. Reg Anesth Pain Med. 2017;42(3):372–376. doi: 10.1097/AAP.0000000000000581. [DOI] [PubMed] [Google Scholar]
- 56.Sullivan T.R., Kanda P., Gagne S., Costache I. Harlequin syndrome associated with erector spinae plane block. Anesthesiology. 2019;131(3):665. doi: 10.1097/ALN.0000000000002733. [DOI] [PubMed] [Google Scholar]
- 57.Elkoundi A., Eloukkal Z., Bensghir M., Belyamani L., Lalaoui S.J. Erector spinae plane block for hyperalgesic acute pancreatitis. Pain Med. 2019;20(5):1055–1056. doi: 10.1093/pm/pny232. [DOI] [PubMed] [Google Scholar]
- 58.Celik M., Tulgar S., Ahiskalioglu A., Alper F. Is high volume lumbar erector spinae plane block an alternative to transforaminal epidural injection? Evaluation with MRI. Reg Anesth Pain Med. 2019 doi: 10.1136/rapm-2019-100514. [DOI] [PubMed] [Google Scholar]
- 59.Otero P.E., Fuensalida S.E., Russo P.C., Verdier N., Blanco C., Portela D.A. Mechanism of action of the erector spinae plane block: distribution of dye in a porcine model. Reg Anesth Pain Med. 2020;45(3):198–203. doi: 10.1136/rapm-2019-100964. [DOI] [PubMed] [Google Scholar]
- 60.Chang T.K., Huang C.W., Su W.C., et al. Extended-release dinalbuphine sebacate versus intravenous patient-controlled analgesia with fentanyl for postoperative moderate-to-severe pain: a randomized controlled trial. Pain Ther. 2020;9(2):671–681. doi: 10.1007/s40122-020-00197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.