Abstract
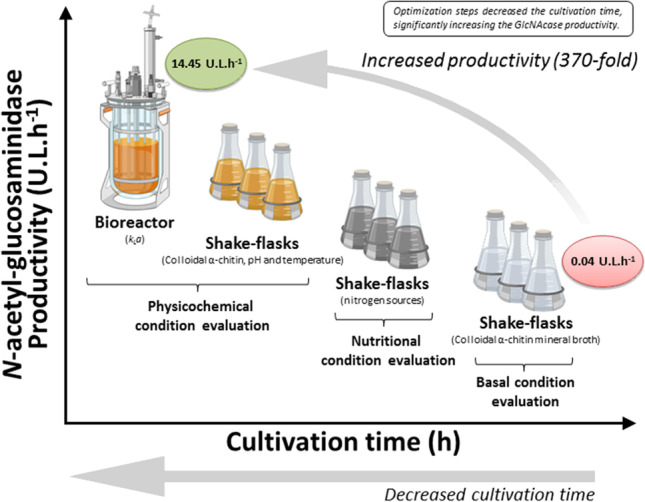
N-Acetyl-glucosaminidases (GlcNAcases) are exoenzymes found in a wide range of living organisms, which have gained great attention in the treatment of disorders related to diabetes, Alzheimer’s, Tay-Sachs’, and Sandhoff’s diseases; the control of phytopathogens; and the synthesis of bioactive GlcNAc-containing products. Aiming at future industrial applications, in this study, GlcNAcase production by marine Aeromonas caviae CHZ306 was enhanced first in shake flasks in terms of medium composition and then in bench-scale stirred-tank bioreactor in terms of physicochemical conditions. Stoichiometric balance between the bioavailability of carbon and nitrogen in the formulated culture medium, as well as the use of additional carbon and nitrogen sources, played a central role in improving the bioprocess, considerably increasing the enzyme productivity. The optimal cultivation medium was composed of colloidal α-chitin, corn steep liquor, peptone A, and mineral salts, in a 5.2 C:N ratio. Optimization of pH, temperature, colloidal α-chitin concentration, and kLa conditions further increased GlcNAcase productivity. Under optimized conditions in bioreactor (i.e., 34 °C, pH 8 and kLa 55.2 h−1), GlcNAcase activity achieved 173.4 U.L−1 after 12 h of cultivation, and productivity no less than 14.45 U.L−1.h−1 corresponding to a 370-fold enhancement compared to basal conditions.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s42770-023-01088-x.
Keywords: Aeromonas caviae, Bioproduction, Bioreactor, Chitinase, N-Acetyl-glucosaminidase
Introduction
N-Acetyl-glucosaminidases (GlcNAcases), also known as N-acetyl-hexosaminidases (EC 3.2.1.52), are exoenzymes belonging to the glycoside hydrolase group [1] and the family of chitinases. They not only catalyze the hydrolysis of N-acetyl-D-glucosamine (GlcNAc) and N-acetyl-D-galactosamine (GalNAc) from the non-reducing end of oligosaccharides, glycoproteins, glycolipids, and other glycoconjugates [1, 2], but also play a regulatory function in thousands of intracellular proteins, including those found in signal transduction, gene expression, cell cycle, and proteasomal degradation. The role of such enzymes in human physiology and diseases has been subject of extensive studies during the last decades. Their potential in the treatment of disorders related to type 2 diabetes, Alzheimer’s disease, different types of cancers [3], and Tay-Sachs’ [55] and Sandhoff’s [4] diseases, as well as neurodegenerative disorders caused by the accumulation of gangliosides especially in neurons [5], has recently gained a lot of attention among scientific and medical communities. Furthermore, different GlcNAcases isoforms have been reported as effective and highly specific tools for the enzymatic production of bioactive compounds like functional carbohydrates and glycomimetics for the food and pharmaceutical industries [6, 7].
Acknowledged as one of the most abundant organic compounds in nature and the main component of the exoskeleton from arthropods and fungal and algal cell walls, chitin, a polyglycan constituted by β (1 → 4)-2-acetamido-2-deoxy-D-glucose, is found in large amounts in seafood processing waste. Every year, 6 to 8 million tons of waste crab, shrimp, and lobster shells are produced globally [8]. Due to its very slow biodegradation, difficult disposal, and accumulation, such a chitin-based biowaste started to raise several concerns regarding its waste management and environmental impact. Aiming to increase competitiveness and expand industrial product family trees, while contributing to the implementation of carbon neutrality and circular economy, in recent decades, a significant number of new green and sustainable processes based on alternative chitin conversion pathways have been developed and proposed that explore a broad range of industrial potential applications [9, 10]. Among the numerous applications claimed for chitinous polymer derivatives, their conversion into value-added products for medical, pharmaceutical, biotechnological, and cosmetic use has aroused much industrial interest. Both chemical and enzymatic hydrolytic methods have been developed to exploit chitin derivatives. However, the acid hydrolytic methods have many industrial and environmental drawbacks, including acid wastes production, low yields, and high costs. On the other hand, given the enhanced functional properties and desirable biological activities of chitin byproducts, its enzymatic hydrolysis has been considered to offer many advantages [11].
Thanks to their role in chitin degradation in ocean ecosystems, marine chitinolytic bacteria are considered owners of great potential for producing chitinases [12]. Due to their ability to grow in wide ranges of temperature and pH, bacterial species belonging to the Aeromonas genus, which have a wide phenotypic diversity and are able to synthesize extracellular chitinases using different molecular inducers, are believed to have great biotechnological potential for large-scale GlcNAcases production. Standing as a great chitinase biosynthesis inducer, chitin, either in its α, β, or γ type and in the form of flakes, powder, colloidal matter, or oligomers, has been successfully used in a large number of studies [13]. When compared in terms of inductive efficacy, chitooligosaccharides, oligomers obtained from the hydrolysis of high molecular weight chitin molecules, have shown higher inductive profiles than those of chitin with higher crystalline polymorphic structures [14, 15]. However, colloidal α-chitin, a chemically pretreated chitin form with an enzymatically digestible structure obtained from relatively inexpensive raw materials such as seafood processing waste, is considered the most economically viable alternative [16–18].
This study reports the potential for GlcNAcase production by Aeromonas caviae CHZ306, a marine chitinolytic bacterium isolated from zooplankton samples from the coast of São Paulo state, Brazil, provides essential knowledge for the efficient production of GlcNAcases, and fills a gap in the current literature on the development of bioprocesses using chitinolytic bacteria. This enzyme had previously been successfully employed, together with other endochitinases and exochitinases, for the synergistic bioconversion of chitin into GlcNAc [19], an amino-monosaccharide used in the treatment of osteoarthritis and inflammatory bowel disease [16, 20, 21]. Considering the impacts of both growth conditions and culture medium composition on chitinase biosynthesis and activity [22, 23], the influence of different carbon and nitrogen sources, temperature, and pH was investigated either in shake flasks or bench-scale stirred-tank bioreactor, while the volumetric oxygen transfer coefficient (kLa) was optimized in the latter system aiming to maximize the GlcNAcase production yield.
Material and methods
Microorganism and pre-inoculum preparation
Aeromonas caviae CHZ306, a marine chitinolytic bacterium isolated from zooplankton samples from the coast of São Paulo state, Brazil, was used in the present study. The strain was initially grown on colloidal α-chitin agar plates at 28 °C for 96 h, according to the method described by Souza et. al. (2009) [18]. Five bacterial colonies were transferred to 250-mL Erlenmeyer flasks containing 100 mL of colloidal α-chitin broth and incubated at 28 °C for 24 h under orbital shaking (180 rpm). Cryotubes containing 1.0 mL of bacterial culture in 20% glycerol were preserved at − 80 °C and used as pre-inoculum.
Selection of the nitrogen sources for GlcNAcase production
The production of N-acetyl-glucosaminidase (GlcNAcase) by A. caviae CHZ306 was initially tested using twelve different inorganic and organic compounds as nitrogen sources (Table 1). Each culture medium was prepared with the following composition (g.L−1): specific nitrogen source, corresponding to 0.2 g of elemental nitrogen; KH2PO4, 0.2; K2HPO4, 1.6; MgSO4.7H2O, 0.2; NaCl, 0.1; FeSO4.7H2O, 0.01; CaCl2.2H2O, 0.02; and colloidal α-chitin, 10 (pH 7.0). For all tested culture media, the relative proportion of each compound was established based on its elemental composition and desired C:N ratio. The inoculum was prepared by addition of 1.0 mL of stock culture in 250-mL Erlenmeyer flasks containing 100 mL of colloidal α-chitin broth and incubation at 28 °C for 24 h under orbital shaking (180 rpm). Submerged cultures were carried out in triplicate adding 1.0 mL of inoculum (approx. 5.0 × 108 CFU L−1) in 250-mL Erlenmeyer flasks containing 100 mL of the different culture media and incubating at 28 °C for 96 h under orbital shaking (180 rpm). Aliquots of 500 μL were collected every 12 h to evaluate cell viability and quantify GlcNAcase activity.
Table 1.
Elemental composition of powdered nitrogen sources tested to select the best culture medium for GlcNAcase production by Aeromonas caviae CHZ306
| Culture medium | Nitrogen source | Main constituents | Carbon (%) | Nitrogen (%) | C:N ratioa |
|---|---|---|---|---|---|
| M1 | Ammonium sulfate | Nitrogen, hydrogen, sulfur, and hydrogen | 0 | 21 | 0.7 |
| M2 | Ammonium acetate | Carbon, hydrogen, nitrogen, and oxygen | 16 | 18 | 1.5 |
| M3 | Ammonium nitrate | Nitrogen, hydrogen, and oxygen | 0 | 35 | 0.7 |
| M4 | Urea | Carbon, hydrogen, nitrogen, and oxygen | 20 | 47 | 1.1 |
| M5 | Casamino acids | Amino acids | 33 | 11 | 3.5 |
| M6 | Meet extract | Proteins, amino acids, nucleotide fractions, organic acids, vitamins, and minerals | 42 | 13 | 3.7 |
| M7 | Yeast extract | Peptides, amino acids, carbohydrates, and vitamins | 39 | 11 | 3.9 |
| M8 | Bacto peptone | Peptone and amino acids | 43 | 15 | 3.3 |
| M9 | Peptone A | Peptone and amino acids | 42 | 13 | 3.6 |
| M10 | Peptone G | Peptone and amino acids | 44 | 16 | 3.2 |
| M11 | Corn steep liquor | Proteins, amino acids, carbohydrates, organic acids, vitamins, and minerals | 38 | 8 | 5.2 |
| M12 | Tryptone | Peptone and amino acids | 44 | 13 | 3.8 |
aC:N values represent the final carbon:nitrogen ratios in each culture medium, constituted by substrates and colloidal α-chitin. M1 refers to the conditions of the basal medium, used in our previous studies (results not shown)
Optimization of physicochemical conditions for GlcNAcase production in shake flasks
The influence of three independent variables, namely, pH, temperature, and colloidal α-chitin concentration, on GlcNAcase activity was investigated in shake flasks using three different factorial designs (Table 2), performed sequentially following the positive gradient of GlcNAcase productivity. To select the optimal initial pH of culture medium, two 23 full-factorial designs [54] with five replications at the central point were used. Moreover, a third 32 full-factorial design [24] with three replications at the central point was used to evaluate the influence of colloidal α-chitin concentration and temperature, at the optimal initial pH value previously established. Submerged cultures were carried out randomly by addition of 1.0 mL of inoculum suspension, prepared as previously described, in 250-mL Erlenmeyer flasks containing 100 mL of culture medium with the nitrogen sources selected in the Sect. 2.2 (peptone A and corn steep liquor), and subsequent incubation for 96 h under orbital shaking (180 rpm). Aliquots of 500 μL were collected every 12 h to evaluate cell viability and quantify GlcNAcase activity. Culture media were prepared with the following composition (g.L−1): peptone A, 0.54; corn steep liquor, 1.87; KH2PO4, 0.2; K2HPO4, 1.6; MgSO4.7H2O, 0.2; NaCl, 0.1; FeSO4.7H2O, 0.01; and CaCl2.2H2O, 0.02.
Table 2.
Experimental factorial designs used to evaluate the GlcNAcase production by Aeromonas caviae CHZ306 in shake flasks and stirred-tank bioreactor
| Laboratory-scale set-up | Design | Variables | Levels of variables a | ||
|---|---|---|---|---|---|
| Lower (− 1) | Center (0) | Higher (+ 1) | |||
| Shake flasks | 23-full factorial | Colloidal α-chitin (%) | 1 | 2 | 3 |
| pH | 6 | 7 | 8 | ||
| Temperature (°C) | 22 | 28 | 34 | ||
| 23-full factorial | Colloidal α-chitin (%) | 3 | 4 | 5 | |
| pH | 8 | 9 | 10 | ||
| Temperature (°C) | 34 | 38 | 42 | ||
| 32-full factorial | Colloidal α-chitin (%) | 5 | 6 | 7 | |
| Temperature (°C) | 32 | 34 | 36 | ||
| Stirred-tank bioreactor | 32-full factorial | Agitation (rpm) | 150 | 250 | 350 |
| Aeration (vvm) | 0.25 | 1.00 | 1.75 | ||
aLevels of variables do refer to actual values. Coded levels are written between brackets
Optimization of physicochemical conditions for GlcNAcase production in stirred-tank bioreactor
GlcNAcase production was then studied in bioreactor cultivation. In this step, the influence of volumetric oxygen transfer coefficient (kLa) on GlcNAcase production was evaluated combining different agitation speed and aeration rate conditions. For this purpose, a 32 full-factorial design [24] with three replications at the central point was used (Table 2), and all experiments were performed randomly. The inoculum was prepared by addition of 1.0 mL of stock culture in 500-mL Erlenmeyer flasks containing 200 mL of culture medium and incubation at 34 °C for 16 h under orbital shaking (180 rpm). The culture medium was prepared under optimal conditions, determined in shake flasks, with the following composition (g.L−1): peptone A, 0.54; corn-steep liquor, 1.87; KH2PO4, 0.2; K2HPO4, 1.6; MgSO4.7H2O, 0.2; NaCl, 0.1; FeSO4.7H2O, 0.01; CaCl2.2H2O, 0.02; and colloidal α-chitin, 50 (initial pH 8.0). Submerged cultures were carried out adding 200 mL of inoculum in a 3.0-L BioFlo 110 bioreactor (New Brunswick, Edison, NJ, USA) containing 1.8 L of culture medium. Sterilized antifoam Y-30 emulsion (0.002%, w/v) (Sigma-Aldrich, St. Louis, MO, USA) was initially added to avoid the formation of foam, which was continuously controlled during cultivation. During the experiments, temperature was automatically controlled at 34 °C. Dissolved oxygen concentration and pH were measured by electronic probes, and filtered air was continuously bubbled into the medium through a multi-point sparger. The medium pH was not controlled, and aliquots of 3.0 mL were collected over 36 h to evaluate cell viability and quantify GlcNAcase activity. All fermentation media used either in flasks or bioreactor were sterilized in autoclave at 121 °C for 20 min and equilibrated at 34 °C before inoculation.
Analytical methods
Viable cell count
Viable cell count was carried out according to the drop plate method [25]. After serial dilutions, 10 µL of cell suspension was inoculated (triplicate) on colloidal α-chitin agar plates [18] 19, at 34 °C for 96 h. Viable cell count was expressed as colony-forming units per liter (CFU.L−1).
GlcNAcase activity assay
GlcNAcase activity was quantified by the chitinase assay kit CS0980 (Sigma-Aldrich), according to the manufacturer’s instructions. Briefly, p-nitrophenyl-N-acetyl-β-D-glucosamine (pNP-GlcNAc) was used as a substrate, and one unit of GlcNAcase activity was defined as the amount of enzyme that released 1.0 µmol of p-nitrophenol per minute.
Determination of the initial volumetric oxygen transfer coefficient
The initial volumetric oxygen transfer coefficient (kLa) was determined by the dynamic gassing-out (physical) method [26]. Pure nitrogen was bubbled into the non-inoculated medium to remove dissolved oxygen. Agitation speed and airflow conditions were set under the same conditions as those of fermentation experiments. The dissolved oxygen concentration was measured every 5 s throughout the reaeration process employing a sterilizable galvanic electrode connected to a Teflon-silicone-Teflon membrane. The equipment was previously calibrated at atmospheric pressure. The dissolved oxygen mass balance in the liquid phase can be written as:
| 1 |
where CL is the dissolved oxygen concentration, C* is the oxygen concentration at saturation in the liquid, OTR is the oxygen transfer rate, and rO2 = OUR is the oxygen uptake rate. When OUR = 0, the oxygen mass balance in the liquid phase, in the absence of cells, can be simplified to:
| 2 |
The initial kLa value was then obtained from the slope of the straight line describing the oxygen mass variation versus time according to the integrated equation:
| 3 |
Statistical analysis
The Statistica software 7.0 (Statsoft, Tulsa, OK, USA) was used for experimental designs, data regression, and graphical analysis of the shake flask optimization experiments. The maximum likelihood estimates of the bioreactor model coefficients, as well as their standard errors, t-statistic values, and significance scores, were calculated using the system fit package in R [27]. The statistical significance of the regression coefficients was determined by the Fischer’s test for analysis of variance (ANOVA) at a significance level (P) ≤ 0.05, and the extent of variance explained by each model was given by the determination coefficient (R2). To minimize the error of ANOVA, tests corresponding to the central point of the shake flask optimization experiments were repeated five times. To perform the statistical analysis, the actual values of each independent variable (Xi) were coded according to the equation:
| 4 |
where xi are the coded values, Xo the actual value at the central point, and ΔXi the step change value.To identify the best conditions for GlcNAcase production in the shake flask experiments, the following quadratic model was used:
| 5 |
where ŷi are the predicted values for each response, while bo, bi, bii, and bij are the intercept, linear, quadratic, and interaction coefficients, respectively. The Pareto analysis was performed to estimate the effects of the independent variables and their interactions on GlcNAcase production at a confidence level of 95%.
Results
Optimization of culture medium for GlcNAcase production
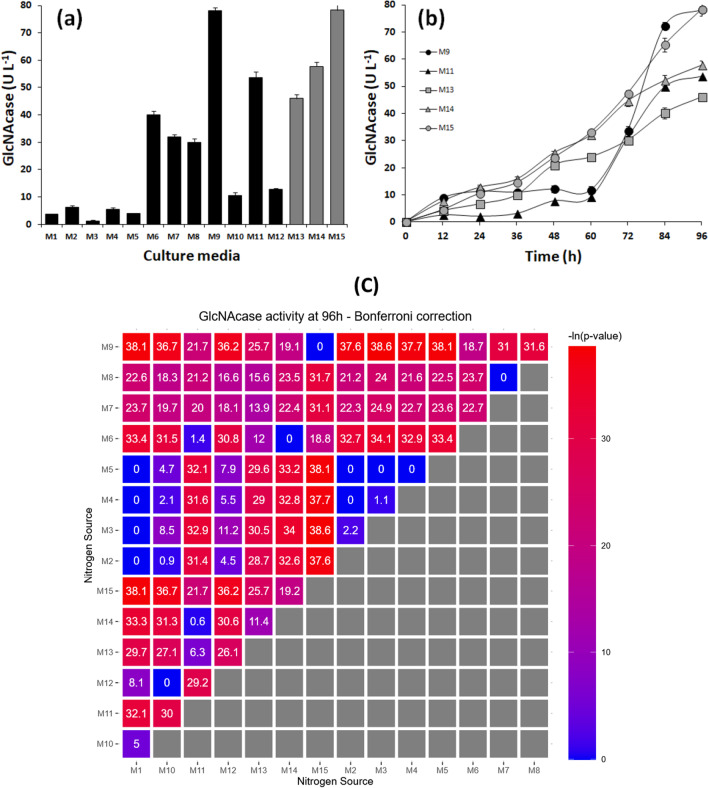
Culture medium for GlcNAcase production by A. caviae CHZ306 was initially tested using twelve different compounds as nitrogen sources, previously analyzed in elemental composition for carbon (C) and nitrogen (N). Indeed, nitrogen supplementation was based on inorganic and organic compounds widely applied in microbial bioprocesses, i.e., M1 (ammonium sulfate); M2 (ammonium acetate); M3 (ammonium nitrate); M4 (urea); M5 (casamino acids); M6 (meat extract); M7 (yeast extract); M8 (bacto peptone); M9 (peptone A); M10 (peptone G); M11 (corn steep liquor); and M12 (tryptone). Based on the obtained results (Fig. 1a), all the culture media proved capable of providing suitable conditions for A. caviae CHZ306 growth (107 to 1010 CFU.L−1) and GlcNAcase production, since the maximum enzyme activity was reached only after 96 h of incubation. Among the different tested nitrogen sources, the medium containing peptone A (M9) and corn steep liquor (M11) in 4.4 and 5.2 C:N ratios, respectively, showed the highest GlcNAcase activities (78 ± 1.0 and 54 ± 2.1 U.L−1, respectively).
Fig. 1.
GlcNAcase production by Aeromonas caviae CHZ306 cultivated in the chitin-containing medium under different nitrogen supplementations (viz. M1–M12). a GlcNAcase activity at 12 h A. caviae CHZ306 cultivation. b Time-course profiles of GlcNAcase activity during A. caviae CHZ306 cultivation in the medium M9, M11, M13, M14, and M15. M1 (ammonium sulfate); M2 (ammonium acetate); M3 (ammonium nitrate); M4 (urea); M5 (casamino acids); M6 (meat extract); M7 (yeast extract); M8 (bacto peptone); M9 (peptone A); M10 (peptone G); M11 (corn steep liquor); M12 (tryptone); M13 (1:1 (w/w) corn steep liquor:peptone A); M14 (1:2 (w/w) corn steep liquor:peptone A); M15 (2:1 (w/w) corn steep liquor:peptone A). Corn steep liquor and peptone A combinations in different proportions (M13, M14, and M15) are depicted by gray curves and bars. c Bonferroni pairwise comparison correction of the GlcNAcase activities at 96 h obtained with different nitrogen sources. Each square represents the negative natural log of the p value of the t-test pairwise comparison between the GlcNAcase activity obtained using each nitrogen source. Since -ln(0.05) = 2.996, any value on this matrix above 3 will cross the statistical significance threshold
In order to detect possible synergistic effects arising from the combination of these two nitrogen sources, additional tests were carried out using them in different proportions, namely, 1:1 (M13), 1:2 (M14), and 2:1 (M15) (w/v) in 4.2, 3.9, and 4.4 C:N ratios, respectively. Compared to individual use of peptone A (M9) and corn steep liquor (M11) (Fig. 1a), none of these combinations allowed improving GlcNAcase activity after 96 h of incubation. However, in all tested proportions, it was observed that the interaction of these two nitrogen sources promoted a significant improvement in the kinetics of GlcNAcase production after 36 h of cultivation (Fig. 1b). In the individual use of peptone A (M9) and corn steep liquor (M11), the production kinetics was only observed after 60 h of cultivation. Particularly, after 60 h of cultivation, GlcNAcase activity using M13, M14, and M15 was 24 ± 0.9, 32 ± 0.4, and 33 ± 0.9 U.L−1, respectively, which represents approximately 2.4- to 3.3-fold activity increases compared to M9 and M11. Compared to the GlcNAcase activity achieved after 96 h in the cultivation performed on the basal medium (M1) (3.7 U.L−1) (Table 3), the M15 medium allowed a 21-fold increase (78 U.L−1), a 4.4 C:N ratio, and exhibited the best production profile among all tested formulations (Fig. 1b).
Table 3.
Comparison of optimal conditions at each step of GlcNAcase production by Aeromonas caviae CHZ306
| Variables | Evaluation steps | |||
|---|---|---|---|---|
| Basal conditionsa | Nutritional conditions | Physicochemical conditions | ||
| Cultivation system | Shake flasks | Shake flasks | Shake flasks | Stirred-tank bioreactor |
| Primary carbon source | Colloidal α-chitin | Colloidal α-chitin | Colloidal α-chitin | Colloidal α-chitin |
| Secondary carbon source | - | Corn steep liquor, peptone A | Corn steep liquor, peptone A | Corn steep liquor, peptone A |
| Nitrogen source | (NH4)2SO4 | Corn steep liquor, peptone A | Corn steep liquor, peptone A | Corn steep liquor, peptone A |
| C:N ratio | 0.7 | 4.4 | 5.2 | 5.2 |
| Colloidal α-chitin (%) | 1.0 | 1.0 | 5.0 | 5.0 |
| pH | 7.0 | 7.0 | 8.0 | 8.0 |
| Temperature (°C) | 28 | 28 | 34 | 34 |
| Agitation (rpm) | 180 | 180 | 180 | 250 |
| Aeration (vvm) | - | - | - | 1.75 |
| kLa (h−1) | - | - | - | 55.2 |
| Time of max. activity (h) | 96 | 96 | 36 | 12 |
| GlcNAcase activity (U.L−1) | 3.7 | 78.3 | 192.5 | 173.4 |
| GlcNAcase productivity (U.L−1.h−1) | 0.04 | 0.82 | 5.35 | 14.45 |
aBasal conditions refer to those used in our previous studies (data not shown)
The statistical significance of these comparisons was assessed using the Bonferroni correction for multiple comparisons. Figure 1c shows the Bonferroni matrix, where each square shows the negative natural log of the p value of each pairwise t-test comparison (calculated using pairwise t-test (p.adjust.method = “bonferroni”) function in R and plotted using the package ggplot2). The p value was plotted in the logarithmic scale to facilitate the interpretation of the results. Since -ln (0.05) = 2.996, any value on this matrix above 3 will cross the statistical significance threshold. As shown in Fig. 1c, the GlcNAcase activities at 96 h that resulted from M9 and M15 are statistically significantly larger (based on pairwise comparisons) than those obtained using all remaining nitrogen sources. The only exception was the difference between the GlcNAcase activity obtained at 96 h using M9 and M15 (the two highest ones), whose difference was not statistically significant based on a t-test pairwise comparison.
Optimization of culture conditions for GlcNAcase production in shake flasks
Culture conditions for GlcNAcase production by A. caviae CHZ306 were investigated in shake flasks in terms of colloidal α-chitin concentration, pH, and temperature using three factorial experimental designs. Within the condition ranges investigated in the first experimental design, A. caviae CHZ306 growth was not significantly influenced by any of the variables tested, reaching viable cell counts around 109 to 1010 CFU.L−1 (results not shown). Despite the absence of any significant effect upon microbial growth, all the independent variables as well as their interactions exerted significant positive effects (Fig. S1 of the Supplementary Material) on A. caviae CHZ306 GlcNAcase activity over cultivation time (Fig. 2). As shown in Table S1 of the Supplementary Material, where the run numbering and conditions are provided in detail, GlcNAcase activity was in fact progressively increased with the increase in colloidal α-chitin concentration, pH, and temperature. In the run 8, carried out at the highest levels of the three selected variables, not only was an almost 40-fold increase in GlcNAcase activity (141.1 U.L−1) observed, but the production time (48 h) was also even halved (Fig. 2a) compared to basal conditions (Table 3), thus leading to a 75-fold enzyme productivity raise (2.94 U.L−1.h−1).
Fig. 2.
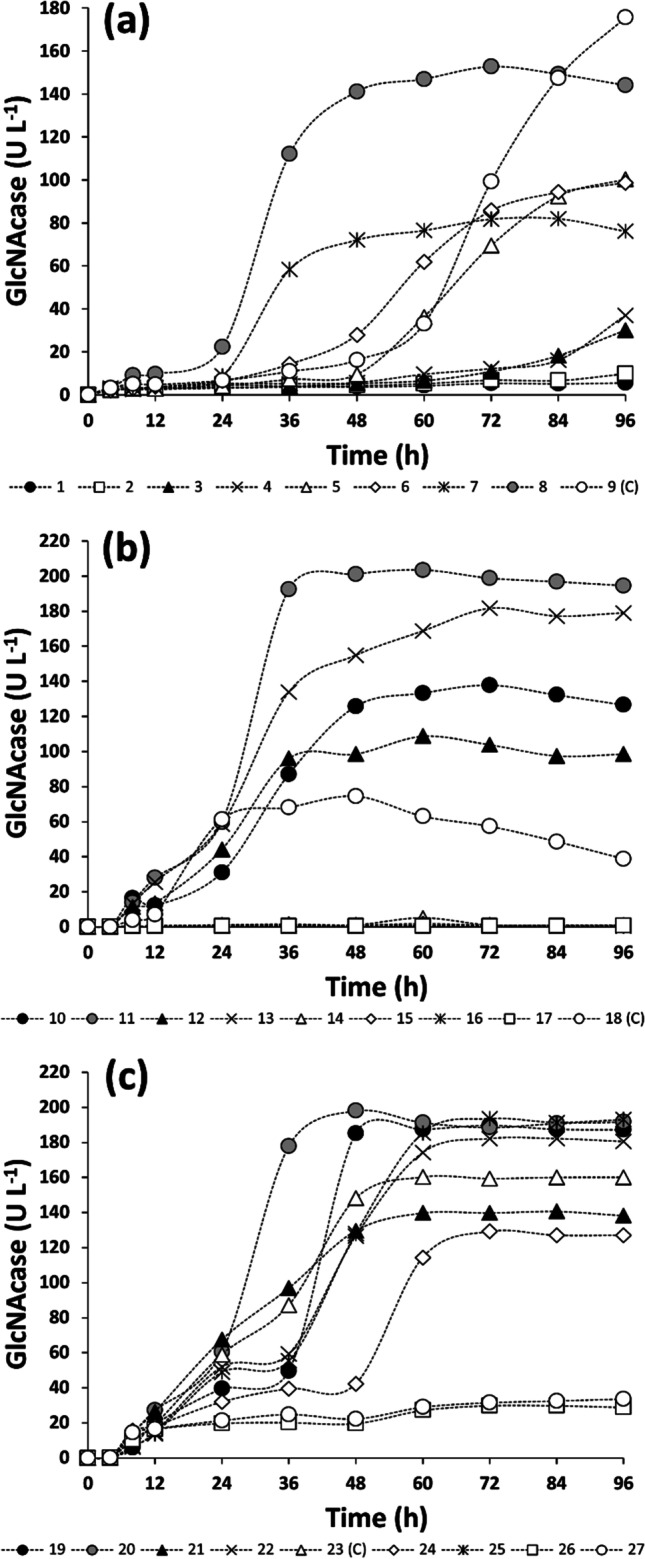
Influence of colloidal α-chitin concentration, pH, and temperature on GlcNAcase production by A. caviae CHZ306 in shake flasks. GlcNAcase production profiles from 1st, 2nd, and 3rd experimental designs are represented in panels a, b, and c, respectively. GlcNAcase productions marked with gray filled circles do refer to the best conditions of each experimental design. Legend numbers are the same as those of runs performed according to the experimental designs (Table S1)
To reach even higher GlcNAcase production levels, higher ranges of temperature (34–42 °C), colloidal α-chitin concentrations (3–5%), and pH values (8–10) were tested according to a second 23-experimental design. Whereas colloidal α-chitin concentration continued to exert a statistically significant positive effect (Fig. S1), further increases in temperature and pH were detrimental for enzyme production. As a consequence, the run 11 carried out at colloidal α-chitin concentration of 5%, pH 8.0, and 34 °C ensured the highest GlcNAcase production (192.5 U.L−1) after only 36 h (Table S1 and Fig. 2c), corresponding to a 137-fold enzyme productivity raise (5.35 U.L−1.h−1) compared to the initial experimental conditions (Table 3).
Based on these results, the initial pH was fixed at this optimum value (8.0) in a third 32-experimental design where temperature and colloidal α-chitin concentrations were varied within lower (32–36 °C) and higher (5–7%) ranges, respectively. GlcNAcase production was affected by increasing colloidal α-chitin concentration (6–7%), and despite the positive effect of temperature on GlcNAcase production highlighted by the Pareto chart (Fig. S1), the run 20, which was a replication of the run 11 of the previous design (α-chitin concentration of 5%, pH 8.0, and 34 °C), provided the best results (Table S1); therefore, these conditions were finally selected as the best ones for GlcNAcase production in shake flasks. Under these conditions, the optimum concentration of colloidal α-chitin determinated ensured a C:N ratio in the culture medium (5.2) above the minimum threshold estimated earlier (4.4).
Optimization of culture conditions for GlcNAcase production in stirred-tank bioreactor
The influence of the volumetric oxygen transfer coefficient (kLa) on GlcNAcase production by A. caviae CHZ306 was investigated in stirred-tank bioreactor using a 32 full-factorial design, by combining different agitation speeds (rpm) and aeration rates (vvm). Figure 3 shows the profiles of GlcNAcase production, cell growth, and dissolved oxygen level (dO2) versus time.
Fig. 3.
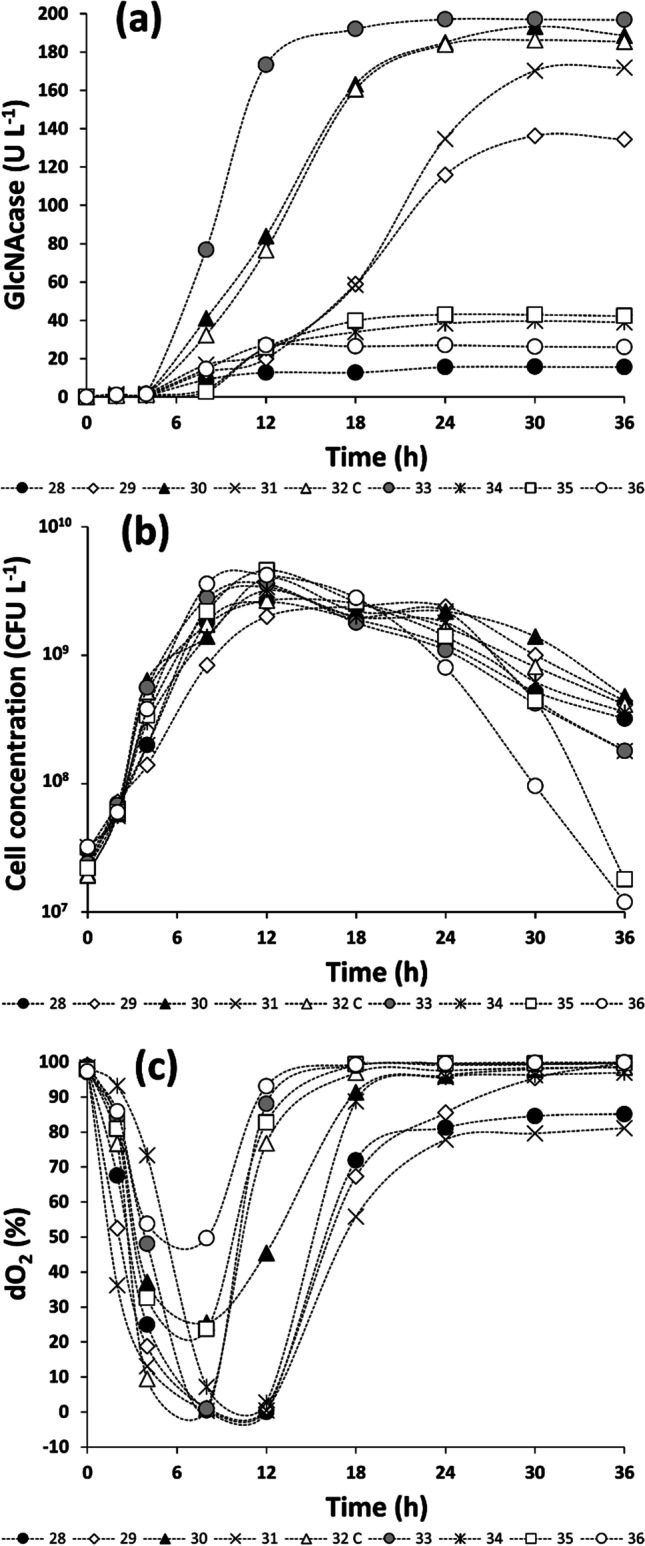
Influence of the volumetric oxygen transfer coefficient (kLa) on GlcNAcase production by A. caviae CHZ306 in stirred-tank bioreactor. Profiles of GlcNAcase production, cell growth and dissolved oxygen (expressed as a percentage of the saturation level) are illustrated in panels a, b, and c, respectively. Profiles marked with gray filled circles do refer to the run 33 that ensured the highest GlcNAcase production
GlcNAcase production, which varied from 0.53 to 197.10 U.L−1 depending on the operational conditions (Table S2 of the Supplementary Material), was the highest after 12 h of cultivation in runs 30, 32, and 33 carried out at kLa in the range of 35–55 h−1 (Fig. 3a), while cell growth (107 to 109 CFU.L−1) was poorly affected either by low, medium, or high kLa conditions (Fig. 3b). A qualitatively similar dO2 profile was observed in all the runs (Fig. 3c), describing an initial decrease in the first 6–12 h of cultivation, corresponding to both the exponential growth phase and the highest GlcNAcase productivities, a subsequent progressive increase, and an almost constant value at the end of cultivation.
Either lower or higher kLa values than the above optimum range negatively affected GlcNAcase production over time. For instance, in the run 28, kLa conditions (8.28 h−1) were able to support cell growth, but not trigger GlcNAcase biosynthesis. On the other hand, runs 35 and 36, carried out at the highest kLa values (70.20 and 96.12 h−1), resulted in poor GlcNAcase production.
On the other hand, the run 33 carried out at 55.20 h−1 kLa resulted in the highest GlcNAcase production (173.38 U.L−1) after only 12 h (Fig. 3a), corresponding to a 370-fold productivity increase (14.4 U.L−1.h−1) compared to the basal conditions (Table 3).
To better visualize the effects of kLa and cultivation time on GlcNAcase productivity in bioreactor (Table S3 of the Supplementary Material), the results obtained were used to construct a response surface model. Due to the large number of kLa levels tested and time points measured, several different options of polynomial functions were evaluated in their capacity to model the productivity values. Although several polynomial functions of orders 3 and 4 fitted the data with an R2 around 0.8, following the principle of parsimony, the model below was chosen, which allowed to obtain a good fit with the smallest number of free parameters:
where x1 is the kLa [h−1], x2 is the cultivation time [h], and y is the productivity [U.L−1.h−1].
This model was able to fit reasonably well the complex geometry of data observed with an R2 of 0.77, while models of higher order tended to overestimate the productivity values at the peaks and underestimate them at the throughs. Table S4 shows the maximum likelihood estimation values of the model coefficients, as well as their standard errors, estimated t-statistic values, and significance scores calculated using the Systemfit package in R [27]. Although the highest experimental productivity was reached after 12 h at a kLa of 55.20 h−1, the optimal productivity predicted by the response surface model is expected to occur after 16.6 h at a kLa of 36.4 h−1.
Discussion
GlcNAcases are exoenzymes with biotechnological potential belonging to the chitinase family and commonly secreted at low concentrations in bacterial culture media. Its biosynthesis in bacteria is influenced by inducers (e.g., chitin and its hydrolysis products), the composition of the culture medium (e.g., carbon and nitrogen sources), and various other physical factors (e.g., temperature, pH, speed agitation, and aeration). However, some of these factors, as well as products of chitin hydrolysis itself, can also repress chitinase biosynthesis [13, 28, 29]. Thus, the choice of the type of inducer, the composition of the culture medium, and the type of culture are determining characteristics in the production of these enzymes.
In the present study, GlcNAcase production by A. caviae CHZ306 was explored and discussed based on the most relevant aspects involved in the lab-scale upstream bioprocess. Colloidal α-chitin was used as a low-cost carbon source and inducer of GlcNAcase synthesis, and the most suitable nitrogen source for enzyme production was evaluated using different culture media in shake flasks. The M15 medium allowed a 21-fold increase on GlcNAcase production (78 U.L−1) compared to the M1 basal medium (4.0 U.L−1) and exhibited the best production profile among all tested formulations (Fig. 1b). Such an increase could probably be due to the higher bioavailability of substrates on M15 medium, such as proteins, peptone, amino acids, carbohydrates, organic acids, vitamins, and minerals, from peptone A and corn-steep liquor. Several studies addressing the improvement of chitinase production using different nitrogen sources (Table 4) unexpectedly demonstrated that some additional carbon sources are more readily assimilable than colloidal α-chitin, thus representing an important contribution for chitinase production. For instance, yeast extract significantly improved chitinase production by Streptomyces griseorubens C9 on colloidal α-chitin [30]. A mixture of colloidal chitin, ammonium sulfate, and yeast extract maximized chitinase production by Bacillus licheniformis AT6 [31]. The chitinase production by Bacillus amyloliquefaciens Z7 was enhanced using chitin, starch, and yeast extract [32], and a mixture of swollen chitin, sucrose, yeast extract, and ammonium sulfate was used to optimize the chitinase production by Escherichia fergusonii [33].
Table 4.
Comparative summary of conditions for chitinase production by chitinolytic bacteria
| Specie | Carbon source | Nitrogen source | Optimum condition | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Main | Additional | Main | Additional | pH | Temp. (°C) | Chitin (%) | ||
| A. caviae CHZ306 | Colloidal chitin | Corn steep liquor and peptone A | Corn steep liquor | Peptone A | 6.0 | 34 | 5.0 | This study |
| A. hydrophila HS4 | Colloidal chitin | Starch | Malt extract | - | 8.0 | 37 | 0.3 | [39] |
| A. punctata HS6 | Colloidal chitin | Starch | Yeast extract | - | 7.0 | 37 | 0.3 | [39] |
| Aeromonas sp. JK1 | Colloidal chitin | Triton X-100 | Ammonium sulfate | - | 8.0 | 30 | 0.75 | [40] |
| Aeromonas sp. ZD_05 | Colloidal chitin | - | Peptone | - | 7,0 | 30 | 1.0 | [41] |
| B. amyloliquefaciens Z7 | Chitin | Starch | Yeast extract | - | 6.5 | 37 | 0.88 | [32] |
| B. laterosporous MML2270 | Colloidal chitin | - | Yeast extract | - | 8.0 | 35 | 0.3 | [44] |
| B. licheniformis AT6 | Colloidal chitin | - | Ammonium sulfate | Yeast extract | 7.0 | 35 | 0.5 | [31] |
| B. licheniformis NM120–17 | Colloidal chitin | Lactose | Casein | - | 8.0 | 30 | 1.5 | [45] |
| B. thuringiensis NM101–19 | Colloidal chitin | Galactose | Casein | - | 7.0 | 30 | 1.5 | [45] |
| B. thuringiensis R 176 | Ball-milled chitin | Rice Straw | Ammonium sulfate | Rice Straw | 7.0 | 37 | 0.5 | [51] |
| E. fergusonii | Swollen chitin | Sucrose | Ammonium sulfate | Yeast extract | 7.0 | 30 | 1.0 | [33] |
| S. marcescens ATCC27117 | Colloidal chitin | - | Yeast extract | Tryptone | 7.5 | 30 | 1,5 | [52] |
| S. marcescens KY | Colloidal chitin | - | Yeast extract | Tryptone | 6.0–7.0 | 30 | 1.5 | [52] |
| S. griseorubens C9 | Colloidal chitin | - | Yeast extract | - | 5.0–9.0 | 40 | 2.0 | [30] |
| S. viridificans | Colloidal chitin | Arabinose | Ammonium sulfate | Yeast extract | 6.0 | 30 | 1.5 | [53] |
Another factor related to the increased production of chitinases could be related to the different C:N ratios between M1 (0.7) and M15 (4.4). Even though several studies have shown that the C:N ratio plays a central role in microbial growth, respiration and biosynthesis [34–38], only few attempts were made to improve chitinase production taking into account the stoichiometric balance between the bioavailability of carbon and nitrogen present in the culture medium and their consumption. However, knowing the elemental cell composition, it is possible to estimate the concentrations of nutrients that will make up the culture medium. Considering that bacterial cells are composed of approximately 50% carbon and 12% nitrogen, under aerobiosis about 50–60% of carbon and nitrogen contained in the culture medium is incorporated into cell biomass, while under anaerobiosis only 10–20%. Based on such considerations, it would be appropriate to prepare a culture medium with a C:N ratio of approximately 4.2, in which 2.3 would be required only for bacterial morphological plasticity under aerobiosis and 0.6 under anaerobiosis. In the present study, as the M1, M2, M3, and M4 culture media had C:N ratios (< 1.5) significantly lower than the nutritional requirements of a bacterial cell (Table 1), they led to the lowest GlcNAcase productions (Fig. 1a). Contrarily, the M15 culture medium, which had a C:N ratio (4.4) higher than the above threshold value, was able to meet A. caviae CHZ306 nutritional requirements, greatly improving its performance in terms of GlcNAcase production.
Studies have also shown the important effects of pH, temperature, and colloidal α-chitin concentrations on chitinase biosynthesis by different bacterial species (Table 4). The production of chitinases by A. hydrophila HS4 [39], A. punctata HS6 [39], Aeromonas. sp. JK1 [40], and Aeromonas sp. ZD_05 [41], for example, was evaluated under different conditions of pH (4–12), temperature (18–55 °C), and colloidal α-chitin concentrations (0.1–3.0%) and showed optimal production conditions of chitinase in ranges of 7.0–8.0, pH; 30–37 °C, temperature; and 0.3–1.0%, chitin concentration. These conditions were further investigated in this study, in which the A. caviae CHZ306 growth was not significantly influenced by any of the variables tested. These results are qualitatively in line with those reported in the literature for Aeromonas genus growth in wide ranges of temperature (22–37 °C) and pH (4.5–9.0) [42, 43]. As expected, the optimum concentration of colloidal α-chitin determined ensured a C:N ratio in the culture medium above the minimum threshold estimated in the first steps of the study (Table 3). Moreover, as in some previous reports [39, 40, 44, 45], the alkaline pH sustained GlcNAcase production, while temperatures either higher or lower than the optimal one led, as for any enzyme system, to a significant activity decrease. An increase in colloidal α-chitin concentration up to 5% improved GlcNAcase production; however, it appeared to inhibit enzyme synthesis beyond this maximum threshold and over time, probably due to the high concentration of GlcNAc, i.e., the product of GlcNAcase-catalyzed reaction. According to some studies [28, 29], GlcNAc appears to directly or indirectly induce or repress a variety of cellular processes, including chitin utilization, energy metabolism, biofilm formation, and pathogenicity. For instance, in marine-derived Vibrio parahaemolyticus, GlcNAc induced and repressed no less than 81 and 55 genes, respectively [29]; in addition, chitinase secretion seems to be unaffected by low GlcNAc levels, but strongly inhibited by high GlcNAc levels [46].
An extensive literature on the operational design of bioprocesses is nowadays available, showing that a scale-up in bioreactors is highly dependent on the ability to transfer O2 from the gas phase to the liquid phase [47]. Therefore, GlcNAcase production was finally tested in stirred-tank bioreactor, known for providing high performance in bioprocesses, where the influence of the volumetric oxygen transfer coefficient (kLa) was investigated, by combining different agitation speeds (rpm) and aeration rates (vvm).
Under the best experimental conditions, the balance between agitation speed (250 rpm) and aeration rate (1.75 vvm) expressed in kLa (55.20 h−1) was essential to increase productivity, reducing the production time of GlcNAcase to 12 h. Either lower or higher kLa values than the above optimum range negatively affected GlcNAcase production over time. Lower kLa conditions were able to support cell growth, but not trigger GlcNAcase biosynthesis. Under these conditions, the low agitation speeds and aeration rates, together with the insolubility of colloidal α-chitin, caused cell adhesion to the bioreactor wall. On the other hand, the highest kLa conditions resulted in poor GlcNAcase production, probably due to mechanical stress to cells driven by excess agitation intensity [48] and/or inhibition of chitinase biosynthesis by excess oxygen [49]. Similar results were reported by [50], who evaluated the influence of agitation speed (100, 200, and 300 rpm) and aeration rate (1, 2, and 3 vvm) on the production of Paenibacillus sp. CHE-N1 chitinase.
According to the data analysis, as mentioned previously, the highest experimental productivity was reached after 12 h at a kLa of 55.20 h−1, and the optimal productivity is expected to occur after 16.6 h at a kLa of 36.4 h−1. However, given that the response surface model underestimates the productivity at short fermentation times and struggles to fit the abrupt reduction of productivity observed increasing kLa from 55.20 to 70.20 h−1, this reduction was probably caused by mechanical cell damage and excess oxygen. Taking into consideration both experimental data and mathematical modeling, it is likely that the actual optimal operating conditions are at slightly higher kLa and slightly lower times than those predicted by the response surface model, although not too far from them.
Conclusion
Given the functional properties, features, and recognized high technological and economic potential of GlcNAcase, the development of a bioprocess aimed at its large-scale production is of great importance for the consolidation of its current and emerging biotechnological applications. The enhanced production of GlcNAcase by marine Aeromonas caviae CHZ306 in bioreactor was successfully attained in the present work. A stoichiometric balance between the bioavailability of carbon and nitrogen in the formulated culture medium with colloidal α-chitin as well as the use of additional carbon and nitrogen sources played a central role in improving the bioprocess, considerably increasing the enzyme productivity. Optimization of physicochemical conditions and kLa values of the bioprocess further increased GlcNAcase productivity. Intermediate cultivation conditions acting synergistically, without exposing the cells to extreme temperature, cell damage, and excess oxygen, were fundamental to achieve such performance. Future efforts should be directed towards controlling the concentration of colloidal α-chitin, because high amounts of its hydrolysis products, such as GlcNAc, can lead to metabolic repression of the synthesis of this enzyme.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
This work was supported by the Sao Paulo Research Foundation (FAPESP), Brazil, [grant numbers 2012/16824–0 and 2013/18773–6].
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Flávio Augusto Cardozo and Valker Araujo Feitosa shared first co-author.
Responsible Editor: Solange I. Mussatto
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Slámová K, Bojarová P, Petrásková L, Křen V. β-N-Acetylhexosaminidase: what’s in a name…? Biotechnol Adv. 2010;28(6):682–693. doi: 10.1016/J.BIOTECHADV.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Hyskova V, Ryšlavá H. Different roles of β-N-Acetylhexosaminidase in metabolism. Biochem Anal Biochem. 2015;04(04):1–2. doi: 10.4172/2161-1009.1000215. [DOI] [Google Scholar]
- 3.Li B, Li H, Lu L, Jiang J. Structures of human O-GlcNAcase and its complexes reveal a new substrate recognition mode. Nat Struct Mol Biol. 2017;24(4):362–369. doi: 10.1038/nsmb.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson MC (2014) Hexosaminidase deficiency. In: Encyclopedia of the Neurological Sciences. Elsevier Inc. 564–565. 10.1016/B978-0-12-385157-4.00098-1
- 5.Kim C, Nam DW, Park SY, et al. O-linked β-N-acetylglucosaminidase inhibitor attenuates β-amyloid plaque and rescues memory impairment. Neurobiol Aging. 2013;34(1):275–285. doi: 10.1016/J.NEUROBIOLAGING.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Hušáková L, Riva S, Casali M, et al. Enzymatic glycosylation using 6-O-acylated sugar donors and acceptors: β-N-acetylhexosaminidase-catalysed synthesis of 6-O, N, N′-triacetylchitobiose and 6′-O, N, N′-triacetylchitobiose. Carbohyd Res. 2001;331(2):143–148. doi: 10.1016/S0008-6215(01)00027-1. [DOI] [PubMed] [Google Scholar]
- 7.Slámová K, Bojarová P (2017) Engineered N-acetylhexosamine-active enzymes in glycoscience. Biochim et Biophysica Acta (BBA) - General Subject 8:2070–2087. 10.1016/j.bbagen.2017.03.019 [DOI] [PubMed]
- 8.Yan N, Chen X. Sustainability: don’t waste seafood waste. Nature. 2015;524(7564):155–157. doi: 10.1038/524155a. [DOI] [PubMed] [Google Scholar]
- 9.Riehle F, Hoenders D, Guo J, Eckert A, Ifuku S, Walther A. Sustainable chitin nanofibrils provide outstanding flame-retardant nanopapers. Biomacromol. 2019;20(2):1098–1108. doi: 10.1021/acs.biomac.8b01766. [DOI] [PubMed] [Google Scholar]
- 10.Namboodiri MMT, Pakshirajan K (2022) Valorization of waste biomass for chitin and chitosan production. In: Waste Biorefinery. Elsevier 241–266. 10.1016/b978-0-12-818228-4.00010-1
- 11.Kaczmarek MB, Struszczyk-Swita K, Li X, Szczęsna-Antczak M, Daroch M. Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front Bioeng Biotechnol. 2019;7(SEP):243. doi: 10.3389/fbioe.2019.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Souza CP, Almeida BC, Colwell RR, Rivera ING. The importance of chitin in the marine environment. Mar Biotechnol. 2011;13(5):823–830. doi: 10.1007/s10126-011-9388-1. [DOI] [PubMed] [Google Scholar]
- 13.Stoykov YM, Pavlov AI, Krastanov AI. Chitinase biotechnology: production, purification, and application. Eng Life Sci. 2015;15(1):30–38. doi: 10.1002/elsc.201400173. [DOI] [Google Scholar]
- 14.Sato Y, Araki Y (2008) Identification of inducers for chitinase B (ChiB) production in Bacillus cereus CH and estimation of its induction mechanism. J Environ Biotechnol 8(2):119–121. Accessed June 16, 2019. https://www.jseb.jp/wordpress/wp-content/uploads/08-02-119.pdf
- 15.Sato Y, Araki Y (2007) Analysis of ChiA and ChiB production by bacillus cereus CH: induction, gene expression, and localization of two chitinases. J Environ Biotechnol 7(1):27–32. https://www.jseb.jp/wordpress/wp-content/uploads/07-01-27.pdf. Accessed 16 Jun 2019
- 16.Chen JK, Shen CR, Liu CL. N-Acetylglucosamine: production and applications. Mar Drugs. 2010;8(9):2493–2516. doi: 10.3390/md8092493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirano S, Nagao N. An improved method for the preparation of colloidal chitin by using methanesulfonic acid. Agric Biol Chem. 1988;52(8):2111–2112. doi: 10.1080/00021369.1988.10868977. [DOI] [Google Scholar]
- 18.Souza CP, Burbano-Rosero EM, Almeida BC, Martins GG, Albertini LS, Rivera ING. Culture medium for isolating chitinolytic bacteria from seawater and plankton. World Journal of Microbiology and Biotechnology. 2009;25(11):2079–2082. doi: 10.1007/s11274-009-0098-z. [DOI] [Google Scholar]
- 19.Cardozo FA, Facchinatto WM, Colnago LA, Campana-Filho SP, Pessoa A. Bioproduction of N-acetyl-glucosamine from colloidal α-chitin using an enzyme cocktail produced by Aeromonas caviae CHZ306. World J Microbiol Biotechnol. 2019;35(8):114. doi: 10.1007/s11274-019-2694-x. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Liu Y, Shin H, dong, , et al. Microbial production of glucosamine and N-acetylglucosamine: advances and perspectives. Appl Microbiol Biotechnol. 2013;97(14):6149–6158. doi: 10.1007/s00253-013-4995-6. [DOI] [PubMed] [Google Scholar]
- 21.Jung WJ, Park RD. Bioproduction of chitooligosaccharides: present and perspectives. Mar Drugs. 2014;12(11):5328–5356. doi: 10.3390/md12115328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahiya N, Tewari R, Hoondal GS. Biotechnological aspects of chitinolytic enzymes: a review. Appl Microbiol Biotechnol. 2006;71(6):773–782. doi: 10.1007/s00253-005-0183-7. [DOI] [PubMed] [Google Scholar]
- 23.Das S, Roy D, Sen R. Utilization of chitinaceous wastes for the production of chitinase. Adv Food Nutr Res. 2016;78:27–46. doi: 10.1016/bs.afnr.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Box GEP, Behnken DW. Simplex-sum designs: a class of second order rotatable designs derivable from those of first order. Ann Math Stat. 1960;31(4):838–864. doi: 10.1214/aoms/1177705661. [DOI] [Google Scholar]
- 25.Barbosa HR, Rodrigues MFA, Campos CC, et al. Counting of viable cluster-forming and non cluster-forming bacteria: a comparison between the drop and the spread methods. J Microbiol Methods. 1995;22(1):39–50. doi: 10.1016/0167-7012(94)00062-C. [DOI] [Google Scholar]
- 26.Pirt SJ (1976) Principles of microbe and cell cultivation. Halsted Pres, Division of John Wiley and Son. https://openlibrary.org/books/OL5063023M/Principles_of_microbe_and_cell_cultivation. Accessed 23 Sept 2019
- 27.Henningsen A, Hamann JD. Systemfit: a package for estimating systems of simultaneous equations in R. J Stat Software. 2007;23(4):1–40. doi: 10.18637/jss.v023.i04. [DOI] [Google Scholar]
- 28.Keyhani NO, Roseman S. Physiological aspects of chitin catabolism in marine bacteria. Biochimica et Biophysica Acta (BBA) General Subjects. 1999;1473(1):108–122. doi: 10.1016/S0304-4165(99)00172-5. [DOI] [PubMed] [Google Scholar]
- 29.Thompson FL, Neto AA, de Santos EO, Izutsu K, Iida T. Effect of N-acetyl-D-glucosamine on gene expression in Vibrio parahaemolyticus. Microbes Environ. 2011;26(1):61–66. doi: 10.1264/jsme2.ME10152. [DOI] [PubMed] [Google Scholar]
- 30.Meriem G, Mahmoud K. Optimization of chitinase production by a new Streptomyces griseorubens C9 isolate using response surface methodology. Annal Microbiol. 2017;67(2):175–183. doi: 10.1007/s13213-016-1249-8. [DOI] [Google Scholar]
- 31.Aounallah MA, Slimene-Debez I, ben, Djebali K, , et al. Enhancement of exochitinase production by Bacillus licheniformis AT6 strain and improvement of N-acetylglucosamine production. Appl Biochem Biotechnol. 2017;181(2):650–666. doi: 10.1007/s12010-016-2239-9. [DOI] [PubMed] [Google Scholar]
- 32.He Y, Xu J, Wang S, Zhou G, Liu J. Optimization of medium components for production of chitin deacetylase by Bacillus amyloliquefaciens Z7, using response surface methodology. Biotechnol Biotechnol Equip. 2014;28(2):242–247. doi: 10.1080/13102818.2014.907659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim TI, Lim DH, Baek KS, Jang SS, Park BY, Mayakrishnan V. Production of chitinase from Escherichia fergusonii, chitosanase from Chryseobacterium indologenes, Comamonas koreensis and its application in N-acetylglucosamine production. Int J Biol Macromolecules. 2018;112:1115–1121. doi: 10.1016/J.IJBIOMAC.2018.02.056. [DOI] [PubMed] [Google Scholar]
- 34.Egli Th, Quayle JR. Influence of the carbon: nitrogen ratio of the growth medium on the cellular composition and the ability of the methylotrophic yeast Hansenula polymorpha to utilize mixed carbon sources. Microbiology. 1986;132(7):1779–1788. doi: 10.1099/00221287-132-7-1779. [DOI] [Google Scholar]
- 35.Vrede T. Elemental composition (C:N:P) and growth rates of bacteria and Rhodomonas grazed by Daphnia. J Plankton Res. 1998;20(3):455–470. doi: 10.1093/plankt/20.3.455. [DOI] [Google Scholar]
- 36.Touratierl F, Egendre’ L, Vezina A (1999) Model of bacterial growth influenced by substrate C:N ratio and concentration. Aquat Microb Ecol 19:105–118. https://pdfs.semanticscholar.org/7aed/6e0956822101a43a4999d1703c2ab667472d.pdf. Accessed 17 Jun 2019
- 37.Vrede K, Heldal M, Norland S, Bratbak G. Elemental composition (C, N, P) and cell volume of exponentially growing and nutrient-limited bacterioplankton. Appl Environ Microbiol. 2002;68(6):2965–2971. doi: 10.1128/aem.68.6.2965-2971.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mwangi ESK, Gatebe EG, Ndung’u MW (2012) Impact of nutritional (C: N ratio and source) on growth, oxalate accumulation, and culture pH by Sclerotinia Sclerotiorum. J Bio Agri Healthcare 2(10):136–146. https://www.iiste.org/Journals/index.php/JBAH/article/view/3284/3330. Accessed 17 Jun 2019
- 39.Saima KM, Roohi AI. Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J Gen Eng Biotechnol. 2013;11(1):39–46. doi: 10.1016/J.JGEB.2013.03.001. [DOI] [Google Scholar]
- 40.Jami Al Ahmadi K, Tabatabaei Yazdi M, Fathi Najafi M, et al. Optimization of medium and cultivation conditions for chitinase production by the newly isolated: Aeromonas sp. Biotechnology. 2008;7(2):266–272. doi: 10.3923/biotech.2008.266.272. [DOI] [Google Scholar]
- 41.Ghasemi Y, Dehdari Z, Mohkam M, Kargar M (2013) Isolation and optimization of cultivation conditions for production of chitinase by Aeromonas sp. ZD_05 from the Persian Gulf. J Pure App Microbiol 7:913–918. https://microbiologyjournal.org/archive_mg/jmabsread.php?snoid=1226&month=&year=. Accessed 17 Jun 2019
- 42.Palumbro SA, Morgan DR, Buchanan RL. Influence of temperature, NaCI, and pH on the growth of Aeromonas Hydrophila. J Food Sci. 1985;50(5):1417–1421. doi: 10.1111/j.1365-2621.1985.tb10490.x. [DOI] [Google Scholar]
- 43.Häunninen ML. Phenotypic characteristics of the three hybridization groups of Aeromonas hydrophila complex isolated from different sources. J Appl Bacteriol. 1994;76(5):455–462. doi: 10.1111/j.1365-2672.1994.tb01102.x. [DOI] [Google Scholar]
- 44.Shanmugaiah V, Mathivanan N, Balasubramanian N, Manoharan PT (2008) Optimization of cultural conditions for production of chitinase by Bacillus laterosporous MML2270 isolated from rice rhizosphere soil. African J Biotechnol 7(15):2562–2568. http://www.academicjournals.org/AJB. Accessed 17 Jun 2019
- 45.Gomaa EZ. Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: their potential in antifungal biocontrol. J Microbiol. 2012;50(1):103–111. doi: 10.1007/s12275-012-1343-y. [DOI] [PubMed] [Google Scholar]
- 46.Itoh T, Hibi T, Fujii Y, et al. Cooperative degradation of chitin by extracellular and cell surface-expressed chitinases from Paenibacillus sp. Strain FPU-7. Appl Environ Microbiol. 2013;79(23):7482–7490. doi: 10.1128/AEM.02483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Ochoa F, Gomez E. Bioreactor scale-up and oxygen transfer rate in microbial processes: an overview. Biotechnol Adv. 2009;27(2):153–176. doi: 10.1016/J.BIOTECHADV.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Rosa JC, Neto AB, Hokka CO, Badino AC. Influence of dissolved oxygen and shear conditions on clavulanic acid production by Streptomyces clavuligerus. Bioprocess Biosyst Eng. 2005;27(2):99–104. doi: 10.1007/s00449-004-0386-9. [DOI] [PubMed] [Google Scholar]
- 49.Baez A, Shiloach J. Effect of elevated oxygen concentration on bacteria, yeasts, and cells propagated for production of biological compounds. Microb Cell Fact. 2014;13:181. doi: 10.1186/S12934-014-0181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kao PM, Chen CI, Huang SC, Chang YC, Tsai PJ, Liu YC. Effects of shear stress and mass transfer on chitinase production by Paenibacillus sp CHE-N1. Bioche Eng J. 2007;34(2):172–178. doi: 10.1016/J.BEJ.2006.11.028. [DOI] [Google Scholar]
- 51.Chaiharn M, Lumyong S, Hasan N, Plikomol A. Solid-state cultivation of Bacillus thuringiensis R 176 with shrimp shells and rice straw as a substrate for chitinase production. Annal Microbiol. 2013;63(2):443–450. doi: 10.1007/s13213-012-0488-6. [DOI] [Google Scholar]
- 52.Cha JM, Cheong KH, Cha WS, Choi D, Roh SH, Kim S, ll, (2004) Optimal conditions for chitinase production by Serratia marcescens. Biotechnol Bioproc Eng 9(4):297–302. 10.1007/BF02942347
- 53.Gupta R, Saxena RK, Chaturvedi P, Virdi JS (1995) Chitinase production by Streptomyces viridificans: its potential in fungal cell wall lysis. J Appl Bacteriol 78(4):378–383. http://www.ncbi.nlm.nih.gov/pubmed/7744723. Accessed 17 Jun 2019 [DOI] [PubMed]
- 54.Box GEP, Hunter WG, Hunter JS. Statistics for Experimenters: An Introduction to Design, Data Analysis, and Model Building. New Jersey: John Wiley & Sons; 1978. [Google Scholar]
- 55.Tews I, Perrakis A, Oppenheim A, Dauter Z, Wilson KS, Vorgias CE. Bacterial chitobiase structure provides insight into catalytic mechanism and the basis of Tay-Sachs disease. Nat Struct Biol. 1996;3(7):638–648. doi: 10.1038/nsb0796-638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.