Abstract
A strain of Lactobacillus plantarum CQPC02 (LP-CQPC02) isolated from naturally fermented kimchi was utilized in this investigation. In order to construct an animal model of lupus nephritis, pristane was used. We then used a kit to identify markers in mouse blood and tissues and a quantitative polymerase chain reaction (qPCR) to measure the expression of genes associated to nuclear factor kappa-B (NF-κB) in mouse kidney tissue. According to the results of the experiments, oral administration of LP-CQPC02 LP-CQPC02 may lessen the lupus nephritis-related rise in urine protein as well as the cytokine levels that were rising in the serum and renal tissues, including IL-6, IL-12, tumor necrosis factor alpha, and interferon. Additionally, in mice with nephritis, LP-CQPC02 can lower serum creatinine (SCr), blood urea nitrogen (BUN), total cholesterol (TC), triglyceride (TG), and raise total protein (TP) and albumin (ALB) levels. In mice with nephritis, LP-CQPC02 can also reduce the positive rate of double-stranded deoxyribonucleic acid (dsDNA). Pathological sections were examined, and it was shown that LP-CQPC02 can lessen tissue damage such incomplete glomerular morphology and inflammatory infiltration brought on by nephritis. In the kidneys of mice with lupus nephritis, LP-CQPC02 can upregulate the expression of inhibitor of NF-κB (IκB-α), downregulate the expression of NF-κB, transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor (VEGF), intercellular cell adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1). Lactobacillus plantarum CQPC02 has been confirmed to have an intervention effect on nephritis in mice and has the potential as a probiotic.
Keywords: Lactobacillus plantarum, Lupus nephritis, NF-κB, dsDNA, Mice
Introduction
Sichuan kimchi is a traditional Chinese natural fermented vegetable. Its production dates back to about two thousand years. Its long history of production and consumption has made it a staple food in China. The area where kimchi is often eaten exceeds 20 million hectares [1]. Sichuan kimchi is rich in nutrients, vitamins, and mineral elements. In addition to being used as a side dish, it can also be used with other foods to make delicacies. The geographical environment of the Sichuan basin and the unique living habits of multi-ethnic mixed living gave rise to the natural fermentation process [2]. Studies have shown that the microorganisms found in naturally fermented kimchi exhibit a variety of biological activities including intestinal protection, weight loss, anti-inflammatory, and antioxidant effects that can also be used in the food industry [3–6]. These microorganisms are diverse and have value as a potential source of probiotic resources [7].
Systemic lupus erythematosus, a complicated immunological illness, is a kind of nephritis that includes lupus. Symptoms of glomerulonephritis will appear after the onset of lupus nephritis [8]. Lupus nephritis can cause immune decline, lymph node hyperplasia, glomerulonephritis, and often leads to renal failure, as the kidney tissue will experience glomerular sclerosis or diffuse hyperplasia, which will significantly impair the patient’s metabolism and detoxification ability. In severe cases, it can be life-threatening [9]. Most lupus nephritis patients show excessive activation of B and T cells after the onset of the disease. T cells differentiate into helper T cells, which can only play the role of an intermediate process in immunity, but the presence of probiotics can make it more important. Many T cells develop into regulatory T cells, which can resolve inflammation and directly interfere with nephritis [10]. Probiotics’ structural elements can also directly stimulate the immune system, which helps the body eliminate toxins and lessens inflammation [11]. Pristane was effectively employed to build a lupus nephritis model in experimental experiments, which was then used to investigate the impact of medications and wholesome diets on this disease. Pristane has the ability to boost inflammation and the immune system’s defenses. This increases the reactivity of B cells and the generation of a wide range of autoantibodies in animals, which leads to lupus nephritis [12, 13]. Our study team identified the lactic acid bacteria strain Lactobacillus plantarum CQPC02 from typical domestic handmade naturally fermented kimchi. Its survival rate in artificial gastric juice with pH 3.0 reached 91.88%, and in 0.3% artificial bile salt reached 18.02%. In vitro experiments have proved that Lactobacillus plantarum CQPC02 (LP-CQPC02) has good resistance and can be well colonized in the intestine to play its potential probiotic role [14]. Further experiments have also demonstrated that LP-CQPC02 can regulate intestinal health and thus play a role in the inhibition of constipation and weight loss [14, 15]. By assessing the levels of markers and inflammation-related cytokines in mice’s serum, the impact of LP-CQPC02 on lupus nephritis was verified. The effect of LP-CQPC02 was further elucidated by pathological examination and tissue mRNA expression detection.
Materials and methods
Microbiological strain
Lactobacillus plantarum CQPC02 was isolated and identified in common homemade naturally fermented Sichuan kimchi in Chongqing, China. The strain was preserved in the China General Microbiological Culture Collection Center (Beijing, China), with patent preservation number CGMCC No. 14491.
In vivo experiment
A controlled environment with a temperature of 20 ± 1°C and a humidity level of 30–40% was used to rear female C57BL/J6 mice. The test mice were given regular food and unlimited amounts of water. After the mice had become used to the diet for seven days, the experiment officially began. The normal group, the model group, the drug-positive (prednisone) control group, the LP-CQPC02 low-concentration treatment (LP-CQPC02-L) group, and the LP-CQPC02 high-concentration treatment (LP-CQPC02-H) group were formed from the fifty mice. Mice in the normal group were intraperitoneally injected with physiological saline solution on the first day after the commencement of the experiment, whereas mice in the other groups were intraperitoneally injected with 0.5 mL of pristane [16]. LP-CQPC02 was cultured after resuscitation, and then the cultured lactic acid bacteria were prepared into bacterial suspension with a concentration of 1.25×109 and 1.25×108 CFU/mL with normal saline for use. Prednisone 10 mg/kg solution was administered daily to the mice in the drug-positive control group, and 1.25×108 and 1.25×109 CFU/mL bacterial suspension were oral gavaged daily to the mice in the LP-CQPC02-L (108 CFU/kg) and LP-CQPC02-H (109 CFU/kg) groups. The mice were given prednisone and LP-CQPC02 for 12 weeks before being slaughtered through cervical dissection and their blood and internal organs taken.
Mouse urine protein test
After the experiment started, the mice were housed in metabolic cages every two weeks, their urine was collected during the first day, and a total protein (TP) kit (Coomassie brilliant blue method) was used to determine the amount of protein in the mouse urine within 24 h.
Mouse serum and tissue inflammatory cytokine determination
The top layer of serum was extracted after collecting mouse whole blood from the heart and centrifuged at 1500 rpm at 4°C for 10 min. Furthermore, 0.1 g of kidney tissue was weighed, 0.9 mL of saline was added, and the kidney tissue was homogenized at 4°C. The supernatant was collected after centrifugation (4000 rpm, 10 minutes). The levels of inflammatory cytokines IL-6, IL-12, tumor necrosis factor alpha (TNF-α), and interferon γ (IFN-γ) in serum and tissues were analyzed according to the manufacturer's instructions [17].
Determination of the levels of serum creatinine (SCr), blood urea nitrogen (BUN), total cholesterol (TC), triglyceride (TG), and albumin (ALB) in mouse serum
Whole blood was obtained from mice and centrifuged for 10 minutes at 1500 rpm at 4°C. The upper serum was collected, and the levels of SCr, BUN, TC, TG, and ALB in mouse serum were determined following the manufacturer's instructions [17].
Anti-double-stranded deoxyribonucleic acid (dsDNA) antibody assay
During the experiment, blood was obtained from the orbital socket every 2 weeks, and mouse serum was utilized to quantify anti-dsDNA antibody using a microtiter plate and an indirect immunofluorescence test [18].
Immunohistochemistry
Kidney tissues were preserved in 10% formalin after dissection. The tissue samples were dehydrated for 48 h before being embedded in paraffin, sectioned, and stained with hematoxylin-eosin (H&E). Using an optical microscope (BX43, Olympus, Tokyo, Japan), histopathological alterations were found [19].
Mouse tissue qPCR detection
The tissue combination was homogenized after 0.9 mL of normal saline was added to 0.1 g of mouse kidney tissue. 1.0 mL of RNAzol was used to extract RNA. The absorbance of the extracted RNA was measured at 260 and 280 nm to quantify RNA purity and concentration, and the concentration was adjusted to 1 μg/L. After reverse transcription to create cDNA, a reaction system solution of 1 L cDNA, 10 L SYBR Green PCR Master Mix, 7 μL sterile distilled water, and 1 μL upstream and downstream primer solutions is prepared. 95°C for 60 s; 95°C for 15 s for 40 cycles; 55°C for 30 s; 72°C for 35 s; 95°C for 30 s were the reaction conditions. The relative expression of the genes was determined using the 2−ΔΔCt technique, with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) serving as an internal reference (Table 1) [20].
Table 1.
Sequences of primers used in this experiment
| Gene Name | Forward sequence | Reverse sequence |
|---|---|---|
| TGF-β1 | 5′-GTCAACTGTGGAGCAACACG-3′ | 5′-TTCCGTCTCCTTGGTTCAGC-3′ |
| VEGF | 5′-AAGCTACTGCCGTCCGATT-3′ | 5′-GCTTCATCGTTACAGCAG-3′ |
| NF-κB | 5′-ATGGCAGACGATGATCCCTAC-3′ | 5′-CGGAATCGAAATCCCCTCTGTT-3′ |
| IκB-α | 5′-TGAAGGACGAGGAGTACGAGC-3′ | 5′-TGCAGGAACGAGTCTCCGT-3′ |
| ICAM-1 | 5′-TCCGCTACCATCACCGTGTAT-3′ | 5′-TAGCCAGCACCGTGAATGTG-3′ |
| VCAM-1 | 5′-TTGGGAGCCTCAACGGTACT-3′ | 5′-GCAATCGTTTTGTATTCAGGGGA-3′ |
| GAPDH | 5′-TGACCTCAACTACATGGTCTACA-3′ | 5′-CTTCCCATTCTCGGCCTTG-3′ |
TGF-β1 transforming growth factor beta 1; VEGF vascular endothelial growth factor; NF-κB nuclear factor kappa-B; IκB-α inhibitor of nuclear factor kappa-B alpha; ICAM-1 intercellular cell adhesion molecule-1; VCAM-1 vascular cell adhesion molecule-1; GAPDH glyceraldehyde-3-phosphate dehydrogenase
Statistical analysis
All indicators were tested three times in parallel, and the findings were reported as average value standard deviation. The index values for each group were then compared using one-way ANOVA. When P <0.05, significant differences were expected.
Results
Urine protein content of mouse
Over the course of the experiment, the quantity of protein in the urine of mice in the normal group did not vary considerably over time, whereas the amount of protein in the urine of mice in the other groups increased. The urine of mice induced by lupus nephritis was observed, and the foam in urine gradually increased, and the foam was characterized by small and dense. According to the clinical standard of human albuminuria, more than 1.35 g/L was considered as albuminuria in mice. Two weeks later, under the action of LP-CQPC02-L, LP-CQPC02-H, and prednisone, 7, 5, and 4 animals developed proteinuria, respectively, while all mice in the model group developed proteinuria (Table 2). The urine protein concentration of the model group mice (11.93±0.66 g/L) was greater after 12 weeks than that of the LP-CQPC02 and prednisone-treated animals, as well as the normal group mice (Table 3). The protein production in the urine of mice treated with high doses of LP-CQPC02 (LP-CQPC02-H, 7.01±0.49 g/L) and prednisone (6.64±0.91 g/L) was closest to that of normal mice (0.93±0.07 g/L), and the urine protein of LP-CQPC02-H animals was only slightly higher than that of prednisone mice.
Table 2.
Each group of mice’s urinary protein levels in the second week (g/L)
| Group | Normal | Model | LP-CQPC02-L | LP-CQPC02-H | Prednisone |
|---|---|---|---|---|---|
| Mice 1 | 0.88 | 4.34 | 1.38 | 2.98 | 1.11 |
| Mice 2 | 0.90 | 4.56 | 4.77 | 3.29 | 1.24 |
| Mice 3 | 0.94 | 4.98 | 5.23 | 1.25 | 1.05 |
| Mice 4 | 0.95 | 4.98 | 5.47 | 3.87 | 5.23 |
| Mice 5 | 0.87 | 4.67 | 1.36 | 1.3 | 4.89 |
| Mice 6 | 0.84 | 3.46 | 5.33 | 3.4 | 1.23 |
| Mice 7 | 0.87 | 4.94 | 1.39 | 3.04 | 4.83 |
| Mice 8 | 0.93 | 5.61 | 5.64 | 4.12 | 1.18 |
| Mice 9 | 0.86 | 5.2 | 5.72 | 3.12 | 5.12 |
| Mice 10 | 0.89 | 4.61 | 5.51 | 1.33 | 0.98 |
Table 3.
Each group of mice’s urinary protein levels throughout the experiment (g/L)
| Group | Week 2 | Week 4 | Week 6 | Week 8 | Week 10 | Week 12 |
|---|---|---|---|---|---|---|
| Normal | 0.89±0.04b | 0.88±0.05c | 0.88±0.07d | 0.92±0.04d | 0.92±0.07d | 0.93±0.07d |
| Model | 4.74±0.58a | 5.84±0.47a | 7.21±0.44a | 8.67±0.59a | 9.98±0.38a | 11.93±0.66a |
| LP-CQPC02-L | 4.16±1.98a | 5.25±0.46a | 5.89±0.55b | 7.22±0.69b | 7.68±0.94b | 9.24±0.92b |
| LP-CQPC02-H | 2.77±1.08ab | 3.76±0.43b | 4.73±0.47c | 5.31±0.60c | 5.79±0.45c | 7.01±0.49c |
| Prednisone | 2.69±2.01ab | 3.46±0.52b | 4.59±0.43c | 5.20±0.79c | 5.64±0.61c | 6.64±0.91c |
a–dThe same letters indicate no significant differences, whereas different letters show significant differences between the groups at the P<0.05 level. The following tables and figures are the same
IL-6, IL-12, TNF-α, and IFN-γ cytokine levels in mouse serum and kidney tissue
The levels of IL-6, IL-12, TNF-α, and IFN-γ cytokines in the model group's blood and kidney tissue were considerably greater than those in the other groups (P < 0.05; Tables 4 and 5). The levels of IL-6, IL-12, TNF-, and IFN- in lupus nephritis mice (model group) fell substantially (P < 0.05) following the action of LP-CQPC02 and prednisone, with the animals in the high concentration of LP-CQPC02 (LP-CQPC02-H) and prednisone dropping more. The levels of IL-6, IL-12, TNF-α, and IFN-γ in serum of LP-CQPCO2-H and prednisone groups were not significantly different (P > 0.05), but significantly lower than those of LP-CQPCO2-L group (P < 0.05). In the detection of renal cytokines, the levels of IL-12 and IFN-γ in the LP-CQPCO2-H group were significantly (P < 0.05) higher than those in the prednisone group, while the levels of IL-6 and TNF-α were not significantly (P > 0.05) different from those in the prednisone group. The levels of these cytokines in the LP-CQPCO2-H and prednisone groups were lower than those in the LP-CQPCO2-L group (P < 0.05).
Table 4.
Serum levels of IL-6, IL-12, TNF-α, and IFN-γ in mice with lupus nephritis
| Group | IL-6 (pg/mL) | IL-12 (ng/L) | TNF-α (ng/L) | IFN-γ (pg/mL) |
|---|---|---|---|---|
| Normal | 10.41±1.07d | 2.47±0.42d | 152.53±19.20d | 112.60±11.11d |
| Model | 93.19±4.37a | 14.69±0.95a | 795.51±41.13a | 645.21±38.40a |
| LP-CQPC02-L | 62.46±6.19b | 9.20±0.80b | 555.38±44.28b | 463.11±44.59b |
| LP-CQPC02-H | 31.17±4.60c | 5.12±0.75c | 368.90±40.12c | 322.81±50.23c |
| Prednisone | 27.52±8.33c | 5.05±1.24c | 361.57±93.02c | 300.82±84.76c |
a–dThe same letters indicate no significant differences, whereas different letters show significant differences between the groups at the P<0.05 level. The following tables and figures are the same. IL-6: interleukin 6; IL-12: interleukin 12; TNF-α: tumor necrosis factor alpha; IFN-γ: interferon gamma
Table 5.
Renal tissue levels of IL-6, IL-12, TNF-α, and IFN-γ in mice with lupus nephritis
| Group | IL-6 (pg/g) | IL-12 (pg/g) | TNF-α (pg/g) | TNF-α (pg/g) |
|---|---|---|---|---|
| Normal | 16.72±1.39d | 1.42±0.13e | 59.07±3.51d | 55.38±3.17e |
| Model | 83.58±5.66a | 26.88±3.07a | 223.31±15.04a | 238.14±10.98a |
| LP-CQPC02-L | 57.87±5.29b | 18.34±1.61b | 176.11±11.51b | 197.86±14.00b |
| LP-CQPC02-H | 42.34±6.44c | 8.06±0.63c | 95.63±5.68c | 129.43±7.78c |
| Prednisone | 32.14±7.08c | 5.88±0.68d | 89.36±11.15c | 105.75±7.86d |
a–dThe same letters indicate no significant differences, whereas different letters show significant differences between the groups at the P<0.05 level. The following tables and figures are the same. IL-6: interleukin 6; IL-12: interleukin 12; TNF-α: tumor necrosis factor alpha; IFN-γ: interferon gamma
Mouse serum SCr, BUN, TC, TG, TP, and ALB levels
The normal group's serum SCr, BUN, TC, and TG levels were considerably lower than those of the other groups, whereas the model group’s serum SCr, BUN, TC, and TG levels were the highest (P < 0.05, Table 6). Compared with the model group, LP-CQPC02 and prednisone treatment could reduce SCr, BUN, TC and TG levels in mice, but still higher than those in normal mice (P < 0.05). The levels of SCr, BUN, TC and TG in prednisone group were higher than those in LP-CQPC02-L and LP-CQPC02-H groups, but the above indexes in prednisone group were not significantly (P > 0.05) different from those in LP-CQPC02-H group, and were only significantly (P < 0.05) different from those in LP-CQPC02-L group. At the same time, serum TP and ALB levels revealed opposing patterns. Normal group had the highest TP and ALB levels (P < 0.05), LP-CQPC02-H and prednisone groups had significantly (P < 0.05) higher TP and ALB levels than LP-CQPC02-L group, and model group had the lowest TP and ALB levels (P < 0.05).
Table 6.
Serum SCr, BUN, TC, TG, TP, and ALB levels in lupus nephritis-infected mice
| Group | SCr (μmol/L) | BUN (mmol/L) | TC (mmol/L) | TG (mmol/L) | TP (g/L) | ALB (g/L) |
|---|---|---|---|---|---|---|
| Normal | 52.82±4.63d | 1.26±0.32d | 2.00±0.27d | 1.10±0.23d | 72.02±5.36a | 46.58±3.62a |
| Model | 136.20±14.48a | 20.82±1.34a | 19.51±1.80a | 16.44±1.82a | 23.65±2.97d | 7.17±0.58d |
| LP-CQPC02-L | 109.66±13.29b | 12.95±1.67b | 13.28±1.11b | 11.35±1.18b | 42.37±3.61c | 11.63±1.49c |
| LP-CQPC02-H | 80.27±5.98c | 8.36±1.35c | 8.35±0.48c | 7.58±0.32c | 57.23±3.15b | 25.44±3.50b |
| Prednisone | 72.80±6.41c | 6.31±0.91c | 6.98±0.85c | 6.30±1.09c | 59.47±6.22b | 27.94±1.77b |
a–dThe same letters indicate no significant differences, whereas different letters show significant differences between the groups at the P<0.05 level. The following tables and figures are the same. SCr: serum creatinine; BUN: blood urea nitrogen; TC: total cholesterol; TG: triglyceride; TP: total protein; ALB: albumin
Positive rate of dsDNA in mouse
Autoantibody dsDNA was detected in mice at the second, fourth, sixth, seventh, tenth, and twelfth weeks following therapy. Beginning on the sixth weekend, all mice in the model group were positive, indicating that lupus nephritis had been successfully produced. Mice in the LP-CQPC02-L group tested positive on the eighth weekend, whereas mice in the LP-CQPC02-H and prednisone groups tested positive on the tenth weekend, indicating that LP-CQPC02 and prednisone reduced the rate at which mice developed lupus nephritis, and their effects were comparable (Table 7).
Table 7.
The proportion of lupus nephritis mice in each group that tested positive for double-stranded deoxyribonucleic acid (dsDNA)
| Group | Week 2 | Week 4 | Week 6 | Week 8 | Week 10 | Week 12 |
|---|---|---|---|---|---|---|
| Normal | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) |
| Model | 7/10 (70%) | 9/10 (90%) | 10/10 (100%) | 10/10 (100%) | 10/10 (100%) | 10/10 (100%) |
| LP-CQPC02-L | 5/10 (50%) | 7/10 (70%) | 8/10 (80%) | 10/10 (100%) | 10/10 (100%) | 10/10 (100%) |
| LP-CQPC02-H | 3/10 (30%) | 5/10 (50%) | 6/10 (60%) | 8/10 (80%) | 10/10 (100%) | 10/10 (100%) |
| Prednisone | 3/10 (30%) | 4/10 (40%) | 6/10 (60%) | 8/10 (80%) | 10/10 (100%) | 10/10 (100%) |
Histopathological observation of mouse kidney
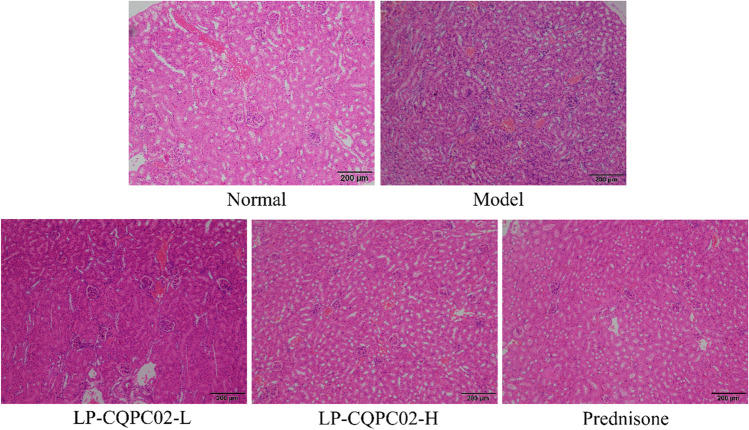
The renal tissue of the model mice revealed more severe damage. A high number of glomeruli were abnormal in form, some were ruptured, and there was significant inflammatory cell infiltration between the tissues (Fig. 1). Mice in the normal group had a complete glomerulus and cell structure. Both LP-CQPC02 and prednisone have been shown to diminish renal tissue lesions produced by lupus nephritis, as well as kidney damage. Simultaneously, high-concentration LP-CQPC02 (LP-CQPC02-H) and prednisone have a greater impact, and both can enhance kidney tissue shape to resemble that of the normal group.
Fig. 1.
Portions of a mouse’s kidney stained in hematoxylin-eosin (H&E) and showing lupus nephritis. Magnification 40×, Olympus BX43 microscope
mRNA expression in mouse kidney tissue
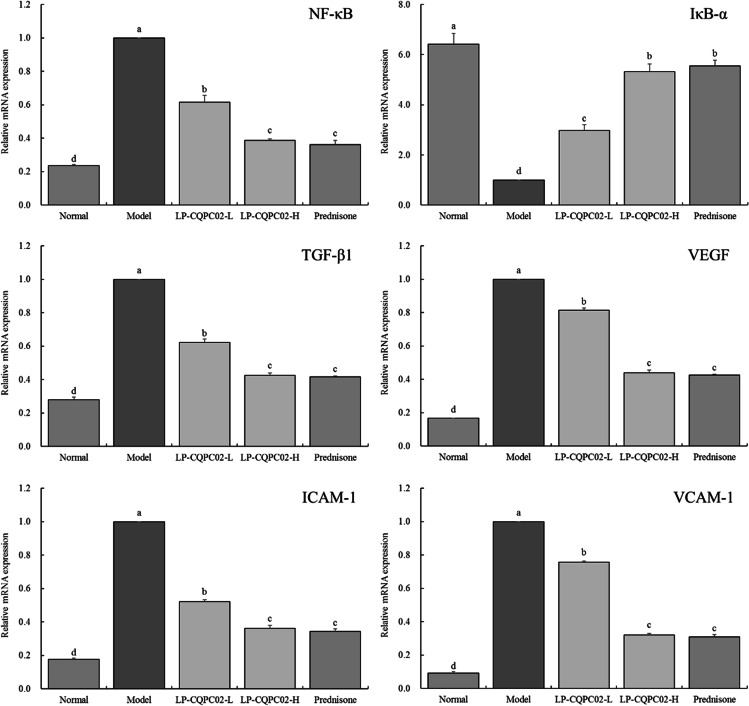
The mRNA expression intensity of NF-κB, TGF-β1, VEGF, ICAM-1 and VCAM-1 was the weakest in normal group, while the expression of IκB-α was the strongest (P < 0.05). The expression of IκB-α was the weakest in the model group, while the expression of NF-κB, TGF-β1, VEGF, ICAM-1 and VCAM-1 was the strongest (P < 0.05). Compared with the model group, LP-CQPC02 (LP-CQPC02-L AND LP-CQPC02-H) and prednisone significantly (P < 0.05) up-regulated the expression of IκB-α and down-regulated the expression of NF-κB, TGF-β1, VEGF, ICAM-1 and VCAM-1 in the kidney tissue of the model group. The ability of LP-CQPC02-H and prednisone to regulate the above expression was stronger than that of LP-CQPC02-L (P < 0.05). LP-CQPC02-H and prednisone could make the mRNA expression of NF-κB, TGF-β1, VEGF, ICAM-1, VCAM-1 weaker than that of LP-CQPC02-L and make the expression of IκB-α stronger than that of LP-CQPC02-L (P < 0.05) (Fig. 2).
Fig. 2.
mRNA expression in a mouse model of lupus nephritis’ kidney. a–d The same letters indicate no significant differences, whereas different letters show significant differences between the groups at the P<0.05 level. The following tables and figures are the same. TGF-β1: transforming growth factor beta 1; VEGF: vascular endothelial growth factor; NF-κB: nuclear factor kappa-B; IκB-α: inhibitor of nuclear factor kappa-B alpha; ICAM-1: intercellular cell adhesion molecule-1; VCAM-1: vascular cell adhesion molecule-1
Discussion
Pristane is a medium-length alkyl chain that acts by a similar mechanism to an adjuvant, causing inflammation and enhancing the immune response. After intraperitoneal injection of pristane, pristane oil drops were engulfed by mononuclear phagocytes, and T and B lymphocytes proliferated and aggregated to form granuloma. T cells are highly activated and B cells are highly reactive, producing a variety of autoantibodies. It has also been suggested that pristane can induce cell apoptosis through mitochondrial damage pathway, and the nuclear antigen produced initiates the autoimmune response. At the same time, pristane induced the typical symptoms of lupus nephritis in mice, so pristane was used as the inducer to induce lupus nephritis in mice to establish the animal model [12]. Therefore, pristane was also used in this study to induce lupus nephritis for follow-up experiments.
Urinary protein is significant in the onset and progression of renal disease. One of the most common signs of complicated comprehensive nephropathy is excessive proteinuria. Proteinuria is the most common clinical symptom of lupus nephritis [21]. The lupus nephritis mice used in this study also had proteinuria, and both LP-CQPC02 and prednisone may lower the quantity of protein in the urine while simultaneously helping to relieve lupus nephritis. The impact of LP-CQPC02 increased with increasing concentration.
The byproducts of protein metabolism are the nitrogen-containing chemical molecules serum creatinine and urea. These tiny molecules are filtered from the glomerulus when kidney function is normal. The glomerulus’s ability to filter becomes less effective when the kidney is injured. As a result, an indicator for the clinical diagnosis of renal impairment may be established using the rise in blood creatinine and urea levels [22].
Hyperlipidemia is brought on by high triglyceride and cholesterol levels. The features of hyperkalemia will coexist when renal disease deteriorates to a certain degree. As a result, triglycerides and cholesterol can be used as markers for kidney damage and functioning [23]. Due to chronic proteinuria, patients with nephrotic syndrome have significantly lower serum TP [24]. The most prevalent protein in serum is albumin. Albumin is frequently utilized to treat critical illnesses, including as kidney disease-related edema and the loss of TP and albumin content in cases of renal insufficiency. Normal renal function can be maintained in part by maintaining albumin and TP serum content [24]. Prednisone and LP-CQPC02 both showed in this trial their capacity to prevent lupus nephritis from increasing blood creatinine, urea, cholesterol, and triglycerides while decreasing albumin and TP levels. Prednisone and LP-CQPC02 may control certain renal disease-related signs, which help to safeguard the kidneys.
IL-12, a marker of an autoimmune reaction, rises during the early stages of lupus nephritis. The presence of many autoantibodies is one of the hallmarks of lupus nephritis. The direct cellular generation of autoantibodies can be encouraged by IL-12. The condition is made worse by the increased mass synthesis of this autoantibody caused by the rise in IL-12 levels [25]. IFN-γ engages in the whole immune-inflammatory process of nephritis as an inflammatory mediator. IFN-γ levels have clinically considerably raised in glomerulonephritis patients [26]. Nephritis changes the way that inflammation-related cytokines behave, and it also greatly increases the amount of these cytokines in the blood, including IL-6, IL-12, TNF-α, and IFN-γ [27]. In this study, inflammatory cytokines IL-6, IL-12, TNF-α, and IFN-γ levels considerably rose in mice with lupus nephritis, and LP-CQPC02 and prednisone were able to effectively suppress this shift.
In order to produce autoreactive antibodies in lupus nephritis, immunoglobulin G must undergo mutation (IgG). Kidney damage brought on by the significant accumulation of IgG, anti-dsDNA antibodies, and immune complexes from plasma in the glomerulus. Additionally, it has been shown that systemic lupus nephritis is almost exclusively associated with large amounts of dsDNA antibodies, and these antibodies have been shown to be specific for this condition [28]. Both prednisone and LP-CQPC02 can reduce the appearance of dsDNA antibody, protect kidney tissue and alleviate nephritis.
The NF-κB signal transduction pathway is involved in a variety of pathological processes, so it has become a potential target for the intervention of drugs or biologically active substances [29]. NF-κB is an important transcription factor that activates a variety of immune and inflammation-related genes, including TNF, IL-1, IL-2, IL-6, IL-8, IL-10, and adhesion molecules (ICAM-1, VCAM-1) [30]. NF-κB is involved in a variety of physiological and pathological processes and the pathogenesis of kidney disease. In nephritis, the body's inflammatory response is too strong, leading to damage to the kidney's own tissues. During treatment, the activity of NF-κB should be inhibited to reduce inflammatory damage [31]. The activity of NF-κB is closely related to IκB-α. When the expression of IκB-α is weakened, NF-κB is dissociated from IκB-α, and harms cells and tissues; when the expression of IκB-α increases, the dissociated NF-κB will quickly bind to the newly synthesized IκB, which can reduce the activity of NF-κB and protect the body [32]. The activation of NF-κB can cause inflammatory growth factors including TGF-β1, and TGF-β1 can act as a regulatory product to activate NF-κB through a positive feedback pathway [33]. The kidney fibrosis is associated with TGF-β1, the most significant pro-fibrotic factor in the body. TGF-β1 is frequently expressed improperly in renal disorders. In the kidney, podocytes and glomerular mesangial cells release TGF-β1 (GMCs). While GMCs express immunoglobulins (IgA) that trigger TGF-β1 production, podocytes secrete TGF-β1 as a result of prior interactions with endothelial cells [34]. Glomerular endothelial cells are stimulated by VEGF to release TGF-β1 when kidney injury has occurred. After the start of lupus nephritis, abnormal expression of TGF-β1 and VEGF is a typical symptom. As inflammatory cytokines have an inhibitory impact, regulating these two expressions can therefore effectively regulate lupus nephritis and is effective for IL-6, IL-12, TNF-α, and IFN-γ [35].
NF-κB migrates into the nucleus and binds its target sequence, regulating the transcriptional activity of related genes, such as ICAM-1, VCAM-1, TNF-α, and IL-6. The enhancers and promoters of the genes of these factors contain binding sites for NF-κB [30]. Two adhesion molecules from the immunoglobulin superfamily are ICAM-1 and VCAM-1. ICAM-1 and VCAM-1 bind to receptors on the surface of leukocytes to facilitate the adherence of leukocytes to endothelial cells, encouraging following leukocytes to engage in trans-endothelial cell transfer. The accumulation of white blood cells blocks the capillaries, and the activated white blood cells can release a large amount of toxic substances to damage neurons and glial cells, thereby aggravating tissue damage and exacerbating nephritis [36]. In addition, white blood cells also release some inflammatory mediators and cytokines, aggravate the inflammatory response, and attract more white blood cells into the tissues, forming a vicious circle [37].
Conclusion
LP-CQPC02 is a newly discovered lactic acid bacteria isolated and identified from natural pickles in Sichuan, China, [15] and confirmed its ability to treat lupus nephritis in an animal model of the condition. The outcomes of the investigation demonstrated that LP-CQPC02 can reduce lupus nephritis-related tissue and serum inflammatory lesions in mice. Particularly, LP-CQPC02 can control TGF-1 expression, which is a defining feature of lupus nephritis. There are certain negative effects associated with the medications used to treat lupus nephritis in clinical settings today. Prednisone was utilized as a drug positive control in this study because it has less negative effects than other medications. The effect of LP-CQPC02 could be close to that of prednisone, and make lupus nephritis patients healthier. Lactobacillus plantarum CQPC02 has been shown to interfere with nephritis in mice and has the potential as a probiotic, but this needs to be further studied through human trials.
Author contribution
Investigation, methodology, writing-original draft: YW; methodology, writing-original draft: XY; Formal analysis, methodology, writing-review, and editing: YW; Conceptualization, supervision, writing-review, and editing: XCZ; Formal analysis: XZ; writing-original draft: Xin Ma. All authors read and approved the final manuscript.
Funding information
This study was supported by the Science and Technology Research Program of Chongqing Municipal Education Commission (Grant No. KJZD-K202001601).
Data Availability
Not applicable.
Declarations
Ethical statement
This study was approved by the Ethics Committee of Chongqing Medical University, (202111012B, Chongqing, China), and it was in accordance with the national standard of the People’s Republic of China (GB/T 35892–2018) laboratory animal guidelines for ethical review of animal welfare.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xi Yao, Email: 156543868@qq.com.
Yan Wen, Email: wenyanya@163.com.
References
- 1.Gan Y, Tong J, Zhou X, Long X, Pan Y, Liu W, Zhao X. Hepatoprotective effect of Lactobacillus plantarum HFY09 on ethanol-induced liver injury in mice. Front Nutr. 2021;8:684588. doi: 10.3389/fnut.2021.684588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C, Fan Y, Li S, Zhou X, Park KY, Zhao X. Antioxidant effect of soymilk fermented by Lactobacillus plantarum HFY01 on D-galactose-induced premature aging mouse model. Front Nutr. 2021;8:667643. doi: 10.3389/fnut.2021.667643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Chen L, Zhang L, Chen Q, Tan F, Zhao X. Effect of Lactobacillus fermentum HFY03 on the antifatigue and antioxidation ability of running exhausted mice. Oxid Med Cell Longe. 2021;2021:8013681. doi: 10.1155/2021/8013681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan Q, Hu J, Zhang Y, Wan Y, Zhang C, Liu X, Long X, Tan F, Zhao X. Inhibitory effect of Lactococcus lactis subsp. lactis HFY14 on diphenoxylate-induced constipation in mice by regulating the VIP-cAMP-PKA-AQP3 signaling pathway. Drug Des Devel Ther. 2021;15:1971–1980. doi: 10.2147/DDDT.S309675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Liu H, Yang J, Mu J, Wang R, Zhao X. Effect of soybean milk fermented with Lactobacillus plantarum HFY01 isolated from yak yogurt on weight. RSC Adv. 2020;10:34276–34289. doi: 10.1039/D0RA06977A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li F, Lu D, Zhong Q, Tan F, Li W, Liao W, Zhao X. Lactobacillus fermentum HFY06 reduced CCl4-induced hepatic damage in Kunming mice. RSC Adv. 2020;10:1–9. doi: 10.1039/C9RA08789C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi R, Tan F, Liao W, Wang Q, Mu J, Zhou X, Yang Z, Zhao X. Isolation and identification of Lactobacillus plantarum HFY05 from natural fermented yak yogurt and its effect on alcoholic liver injury in mice. Microorganisms. 2019;7:530. doi: 10.3390/microorganisms7110530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Y, Zeng Y, Xue W, Chen Y, Li Q, Bian Z, Tang L, Tang T, Chen C, Gao X, Guo W. Anti−IL-12/23 p40 antibody attenuates chronic graft-versus-host disease with lupus nephritis via inhibiting Tfh cell in mice. Biomed Pharmacother. 2020;129:110396. doi: 10.1016/j.biopha.2020.110396. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Xiao B, Zhong M, Li Q, Chen J, Huang J, Rao H. LncRNA NEAT1 accelerates renal mesangial cell injury via modulating the miR-146b/TRAF6/NF-κB axis in lupus nephritis. Cell Tissue Res. 2020;382:627–638. doi: 10.1007/s00441-020-03248-z. [DOI] [PubMed] [Google Scholar]
- 10.Sfikakis PP, Boletis JN, Lionaki S, Vigklis V, Fragiadaki KG, Iniotaki A, Moutsopoulos HM. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52:501–513. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 11.Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr Pharm Design. 2009;15:1428–1518. doi: 10.2174/138161209788168155. [DOI] [PubMed] [Google Scholar]
- 12.Bonomini F, Dos Santos M, Veronese FV, Rezzani R. NLRP3 inflammasome modulation by melatonin supplementation in chronic pristane-induced lupus nephritis. Int J Mol Sci. 2014;20:3466. doi: 10.3390/ijms20143466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu D, Senouthai S, Wang J, You Y. Vasoactive intestinal peptide ameliorates renal injury in a pristane-induced lupus mouse model by modulating Th17/Treg balance. BMC Nephrol. 2019;20:350. doi: 10.1186/s12882-019-1548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao X, Zhang J, Yi S, Li X, Guo Z, Zhou X, Mu J, Yi R. Lactobacillus plantarum CQPC02 prevents obesity in mice through the PPAR-α signaling pathway. Biomolecules. 2019;9:407. doi: 10.3390/biom9090407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B, Yang X, Guo L, Zhang J, Zhou X, Yi R, Zhao X. Inhibitory effect of Lactobacillus plantarum CQPC02 isolated from Chinese Sichuan pickles (Paocai) on constipation in mice. J Food Quality. 2019;2019:9781914. doi: 10.1155/2019/9781914. [DOI] [Google Scholar]
- 16.Shen H, Zhu YQ, Kong Y, Wang J, Zhu H, Yu G, Cai L, Zhu Y, Wang Z, Qiu Y. Immune intervention effect of human-mouse chimeric antibody B7-1 against murine lupus nephritis model. Chinese J Immunol. 2015;2015:1200–1205. doi: 10.3969/j.issn.1000-484X.2015.09.011. [DOI] [Google Scholar]
- 17.Wan R, Zeng X, Liu B, Yi R, Zhou X, Mu J, Zhao X. Prophylactic effect of Lactobacillus plantarum KSFY06 on HCl/ethanol-induced gastric injury in mice. Food Funct. 2020;11:2679. doi: 10.1039/C9FO02474C. [DOI] [PubMed] [Google Scholar]
- 18.Zheng ZW, Chen C, Wang HM, Ding GH. Correlation of Serumβ2-microglobulin level with disease activity of systemic lupus erythematosus and degree of lupus nephritis. Chinese Gene Pract. 2019;22:2058–2063. doi: 10.12114/j.issn.1007-9572.2018.00.310. [DOI] [Google Scholar]
- 19.Wang R, Sun F, Ren C, Zhai L, Xiong R, Yang Y, Yang W, Yi R, Li C, Zhao X. Hunan Insect tea polyphenols provide protection against gastric injury induced by HCl/ethanol through an antioxidant mechanism in mice. Food Funct. 2021;12:747–760. doi: 10.1039/D0FO02677H. [DOI] [PubMed] [Google Scholar]
- 20.Long X, Sun F, Wang Z, Liu T, Gong J, Kan X, Zou Y, Zhao X. Lactobacillus fermentum CQPC08 protects rats from lead-induced oxidative damage by regulating the Keap1/Nrf2/ARE pathway. Food Funct. 2021;12:6029. doi: 10.1039/D1FO00589H. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton P, Myers J, Gillham J, Ayers G, Brown N, Venning M. Urinary protein selectivity in nephrotic syndrome and pregnancy: resurrection of a biomarker when renal biopsy is contraindicated. Clin Kidney J. 2014;7:595–598. doi: 10.1093/ckj/sfu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li XY, Kong FY, Zhang HQ, Tang RX, Zheng KY. The duplication and identification of anti-glomerular basement membrane (GBM ) nephritis model in mice. Acta Academiae Med Xuzhou. 2011;31:527–530. doi: 10.3969/j.issn.1000-2065.2011.08.007. [DOI] [Google Scholar]
- 23.Chou A, Zhou JY, Zhou Y, Hua J, Liu JB. Therapeutic effect of Zhenwu decoction on chronic glomerulonephritis rat model induced by cationization bovine serum albumin osmotic pump. Tradit Chinese Drug Res Clin Pharmacol. 2012;23:626–630. doi: 10.3969/j.issn.1003-9783.2012.06.009. [DOI] [Google Scholar]
- 24.Zhou T, Lin S, Yang S, Lin W. Efficacy and safety of tacrolimus in induction therapy of patients with lupus nephritis. Drug Des Devel Ther. 2019;13:857–869. doi: 10.2147/DDDT.S189156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li ZJ, Li YJ, Yang QQ, Yang X, Xu YW, Yu XQ. Significance of levels of IL 12 and IgG in patients with lupus nephritis. Li New Chinese Med. 2002;33:19–20. doi: 10.3969/j.issn.0253-9802.2002.01.011. [DOI] [Google Scholar]
- 26.Xiang L, Gao XX, Pan JR. Serum levels of interferon-gamma and interleukin-10 in patients with chronic glomerulonephritis and their clinical significance. Chinese J Clin Med. 2006;13(269-270):272. doi: 10.3969/j.issn.1008-6358.2006.02.057. [DOI] [Google Scholar]
- 27.Córdova C, JrF L-E-S, Pires AS, Souza VC, Brito CJ, Moraes CF, Sposito AC, Nóbrega OT. Long-term resistance training is associated with reduced circulating levels of IL-6, IFN-γ and TNF-α in elderly women. Neuroimmunomodulation. 2011;18:165–170. doi: 10.1159/000323396. [DOI] [PubMed] [Google Scholar]
- 28.Du J, Wang QS, Jia RH. Glomerular cell proliferation and apoptosis in experimental glomerulosclerosis. J Pract Med. 2005;21:1623–1625. doi: 10.3969/j.issn.1006-5725.2005.15.006. [DOI] [Google Scholar]
- 29.Edelbauer M, Kshirsagar S, Riedl M, Billing H, Tönshoff B, Haffner D, Dötsch J, Wechselberger G, Weber LT, Steichen-Gersdorf E. Soluble VEGF receptor 1 promotes endothelial injury in children and adolescents with lupus nephritis. Pediatr Nephrol. 2012;27:793–800. doi: 10.1007/s00467-011-2062-z. [DOI] [PubMed] [Google Scholar]
- 30.Zhou XJ, Zhao XH, Zhang AR. NF-κB signaling pathways in spinal cord injury inflammation. Chinese. J Biochem Mol Biol. 2020;36:1159–1164. doi: 10.13865/j.cnki.cjbmb.2020.06.1171. [DOI] [Google Scholar]
- 31.Lee EK, Kim JM, Choi J, Jung KJ, Kim DH, Chung SW, Ha YM, Yu BP, Chung HY. Modulation of NF-κB and FOXOs by baicalein attenuates the radiation-induced inflammatory process in mouse kidney. Free Radic Res. 2011;45:507–517. doi: 10.3109/10715762.2011.555479. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q, Sun P, Wang R, Zhao X. Therapeutic effect of Dendrobium candidum on lupus nephritis in mice. Pharmacogn Mag. 2017;13:129–135. doi: 10.4103/0973-1296.197653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pu Y, Zhao H, Wu X, Mei M, Shen B. The long noncoding RNA Ptprd-IR is a novel molecular target for TGF-β1-mediated nephritis. Int J Biochem Cell Biol. 2020;122:105742. doi: 10.1016/j.biocel.2020.105742. [DOI] [PubMed] [Google Scholar]
- 34.Ravinal RC, Costa RS, Coimbra TM, Dantas M, dos Reis MA. Mast cells, TGF-beta1 and myofibroblasts expression in lupus nephritis outcome. Lupus. 2005;14:814–821. doi: 10.1191/0961203305lu2188oa. [DOI] [PubMed] [Google Scholar]
- 35.Fujino A, Moriya Y, Morikawa Y, Hoshino K, Watanabe T, Shimojima N, Kitajima M. A role of cytokines in OK-432 injection therapy for cystic lymphangioma: an approach to the mechanism. J Pediatr Surg. 2003;38:1806–1809. doi: 10.1016/j.jpedsurg.2003.08.041. [DOI] [PubMed] [Google Scholar]
- 36.Deng WJ, Xu MW, Meng QY, Li Z, Qiu XN, Yin SL, Sun D, Dai C, Liu Y. CD8+CD103+ iTregs inhibit the progression of lupus nephritis by attenuating glomerular endothelial cell injury. Rheumatology. 2019;58:2039–2050. doi: 10.1093/rheumatology/kez112. [DOI] [PubMed] [Google Scholar]
- 37.Fang X, Dorcely B, Ding XP, Yin S, Son NH, Hu SL, Goldberg IJ. Glycemic reduction alters white blood cell counts and inflammatory gene expression in diabetes. J Diabetes Complications. 2018;32:1027–1034. doi: 10.1016/j.jdiacomp.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.