Abstract
Objective
There is growing evidence for an association between anxiety and an increased risk of dementia, but it is not clear whether anxiety is a risk factor or a prodromic symptom. In this study, we investigated if clinically significant anxiety increases the risk of developing Alzheimer's disease (AD) up to 10 years later.
Methods
We used data from the longitudinal Zaragoza Dementia and Depression (ZARADEMP) Project. Excluding subjects with dementia at baseline left us with 3044 individuals aged >65 years. The Geriatric Mental State‐Automated Geriatric Examination for Computer Assisted Taxonomy (GMS‐AGECAT) package was used to identify cases and subcases of anxiety. AD was diagnosed by a panel of research psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM‐IV) criteria. Multivariate survival analysis with a competing risk regression model was performed.
Results
We observed a significant association between clinically significant anxiety at baseline and AD risk within a 10‐year follow‐up (SHR 2.82 [95% CI 1.21–6.58]), after controlling for confounders including depression. In contrast, isolated symptoms of anxiety were not significantly associated with an increased incidence of AD.
Conclusion
Our results support the hypothesis that clinically significant anxiety is an independent risk factor for AD and not just a prodromic symptom. Future studies should clarify if treating anxiety reduces the incidence of AD.
Keywords: Alzheimer's disease, competing risk, risk factor, ZARADEMP
1. INTRODUCTION
Dementia is currently one of the greatest sources of disability and dependence globally. It carries a stigma and social exclusion, places a significant burden on sufferers, caregivers and society, and entails significant economic costs (World Health Organization, 2015). The World Health Organisation (WHO) recognises dementia as a public health priority and, in the absence of current treatment, the implementation of preventive strategies targeting well‐identified risk factors is essential (2015).
Anxiety disorders are the most prevalent mental disorders and are associated with immense health care costs and a high burden of disease (Bandelow & Michaelis, 2015). Through several systematic reviews with meta‐analyses, we have shown an increased risk of all‐cause dementia (Santabárbara, Lipnicki, Olaya, Villagrasa, Bueno‐Notivol, et al., 2020), Alzheimer's disease (Santabárbara, Lipnicki, Bueno‐Notivol, et al., 2020), and vascular dementia (Santabárbara, Lipnicki, Olaya, Villagrasa, Gracia‐García, et al., 2020) in individuals with anxiety disorders. Depressive symptomatology and anxiety have also been linked to Parkinson's‐associated dementia (Riedel et al., 2010). Whether anxiety is a risk factor or a prodromal symptom of dementia remains unclear.
Symptoms or conditions present in the years approaching a dementia diagnosis might be risk factors but might also reflect common causes, preclinical disease effects, or prodromal changes (Singh‐Manoux et al., 2017). If associated with an increased risk of dementia when present closer to diagnosis, conditions like anxiety and depression are more likely to be prodromal and a consequence of emotional dysregulation within the construct of mild behavioural impairment that can arise prior to dementia (Ismail et al., 2016).
To date, few studies analyse the temporality of the relationship between anxiety and dementia. A recent systematic review (Gimson et al., 2018) reports on 4 articles that found a relationship between mid‐life anxiety and dementia diagnosed 10 years later. However, the included studies only investigated all‐cause dementia, or in one case dementia with Lewy bodies. Another review (Gulpers et al., 2016) found an increased risk of dementia associated with anxiety in later life and that was present closer to the time of diagnosis, suggesting that anxiety may be a prodromal symptom rather than a risk factor for dementia. Thus, whether anxiety is an independent risk factor for dementia or a prodromal symptom remains unknown, despite both hypotheses are not mutually excluding. Understanding the pathways through which individuals with anxiety develop progressive dementia is critical for the development of intervention strategies targeting dementia, and Alzheimer's disease (AD) in particular.
We have previously shown that clinically relevant anxiety increases the risk of AD during 4.5 years of follow‐up (Santabárbara, Villagrasa, et al., 2019). The current study aims to investigate if clinical anxiety is also a risk factor for AD after 10 years of follow‐up in the same cohort of community‐based individuals aged 65 years or older.
2. MATERIALS AND METHODS
This work follows STROBE (Von Elm et al., 2007) and SAMPL (Lang & Altman, 2013) guidelines for reporting observational studies in epidemiology and statistics, respectively.
2.1. Sample and procedure
We used data from the Zaragoza Dementia and Depression (ZARADEMP) Project, a longitudinal, population‐based study intended to document the incidence and risk factors of somatic and psychiatric diseases in adults aged ≥55 years. The Ethics Committee of the Institutional Review Board in our institutions (CEICA) approved the study according to Spanish Law, and principles of written informed consent, privacy, and confidentiality according to the Declaration of Helsinki were maintained throughout the Project.
The methods have been described in detail previously (Lobo et al., 2005, 2011). The representative sample was drawn from Spanish official census lists, was stratified with proportional allocation by age and sex and included institutionalised individuals. In the baseline, cross‐sectional study (starting in 1994), 4803 individuals were interviewed, the refusal rate being 20.5%. This paper reports results from the baseline study (Wave I) and three follow‐up waves (Waves II, III and IV; 2.5, 4.5 and 10 years later, respectively). A two‐phase, screening procedure was implemented in each of the waves. The first phase was carried out by well‐trained lay interviewers at the participant's home, or residence if they were institutionalised. In the second phase, a psychiatrist interviewed all subjects who were considered as a probable dementia cases or if information from the first phase was inconclusive. Validated Spanish versions of international instruments were used for the assessment in both phases and included the Mini‐Mental Status Examination (MMSE); Geriatric Mental State B (GMS‐B); Automated Geriatric Examination for Computer Assisted Taxonomy (AGECAT); History and Aetiology Schedule (HAS) (medical and psychiatric history data); Katz's Index for basic activities of daily living (bADL's) and the Lawton and Brody scale for instrumental activities (iADL's); and the European Studies of Dementia (EURODEM) Risk Factors Questionnaire (for medical conditions).
Since we were interested in “cognitively intact subjects”, individuals with dementia of any type were excluded from the follow‐up waves and were considered as censored cases. Moreover, subcases of dementia (subjects with any cognitive impairment) according to GMS‐AGECAT criteria and/or MMSE score under the standard cut‐off point (23/24) were also excluded at baseline (Wave I). For ease of comparison with the existing literature, we focused on participants aged 65 years and older.
2.2. Incident Alzheimer's disease assessment and diagnosis
Participants in the follow‐up waves (Waves II, III, and IV) were classified in phase I as ‘probable cases’ of dementia based on the GMS threshold ‘global’ score (1/2) and/or MMSE standard cut‐off points (23/24). Interviewers were blind to the results of the baseline interview. In phase II, all probable cases of dementia were reassessed in their place of residence by a research psychiatrist using the same methods, and a brief standardised neurological exam. Variables in the ZARADEMP interview were operationalised to conform to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) criteria, and used to diagnose cases of incident dementia and probable type of dementia (AD, vascular, other). The final DSM‐IV diagnosis was made by consensus, requiring agreement by at least three psychiatrists in a four‐member panel. Our previous studies have supported the validity of this diagnostic process (Lobo et al., 1995). Moreover, to document the accuracy of the panel, a proportion of cases were invited for a hospital diagnostic work‐up, which incorporated a neuropsychological diagnostic battery and neuroimaging. Agreement on the diagnosis of dementia and AD was reached in 95.8% and 86.7% of the cases respectively (Lobo et al., 2011).
For the purposes of this paper we focused on incident AD cases, diagnosed according to the National Institute of Neurological and Communicative Disorders and Stroke‐Alzheimer´s Disease and Related Disorders Association (NINCDS‐ADRDA) criteria. Cases of dementia other than AD were considered as censored cases.
2.3. Anxiety assessment and diagnosis
Diagnosis of anxiety was evaluated at baseline (“Wave I”) according to GMS‐AGECAT criteria. After symptom assessment, a computer program compared syndrome clusters (e.g., dementia, depression, anxiety) to reach a final diagnosis: Participants with AGECAT scores of 3, 4 and 5 on the 0–5 scale are considered to be likely “cases” of anxiety (clinically significant anxiety); those with scores of 1 or 2 are considered to be “subcases”, and those with a score of 0 are considered to be “non‐cases”. AGECAT caseness (score ≥3) has shown a good correlation with clinical diagnosis (Schaub et al., 2003) and implies “desirability of intervention” (Copeland et al., 2004). The “subcase” diagnostic category implies that symptoms are not severe enough to fulfil clinical diagnosis and/or require intervention.
2.4. Covariates
Potential confounding risk factors (covariates) were evaluated at baseline (Wave I), including socio‐demographic characteristics (age, sex, educational level and marital status), clinically significant depression, body mass index (BMI) and hearing loss, as previously reported (Lobo et al., 2005).
Educational status was categorised into three levels: “illiterate (unable to read and write, or with less than 2 years of formal education)”, “primary studies (complete or incomplete)” and “secondary education or above”. Marital status was defined as “single”, “married or living with their partner”, and “formerly married” (that includes “divorced or separated” or “widowed”). BMI was calculated as weight in kilograms divided by height in meters squared. A BMI greater than 30 kg/m2 was defined as “obesity”. Hearing loss was scored when the subject was almost or completely deaf. History of cardiovascular risk factors (angina, myocardial infarction or stroke) and diabetes was based on data from the EURODEM questionnaire (Launer et al., 1992). The diagnosis of clinically significant depression was based on the GMS‐AGECAT computer system (AGECAT score for depression ≥3) (Schaub et al., 2003).
2.5. Statistical analysis
Differences between baseline characteristics according to incident AD status were assessed using two‐tailed chi‐square tests on categorical data, and differences in variables with approximately normal distributions were assessed using two‐tailed t‐test.
The follow‐up period ended at the third follow‐up examination (Wave IV) for the cognitively intact individuals, at the date of invitation for refusals, at the date of moving away or death (based on data from the Civil Registry, ‘Padrón Municipal de Habitantes de Zaragoza’), or at the time of onset of dementia for cases, with onset estimated as the midpoint between diagnosis and the previous examination.
In the first step of the survival analysis, we built the cumulative incidence functions (CIF) for the anxiety disorder groups to estimate the probability of incident AD, taking into account the competing event (death) as time progressed, and with age as timescale with delayed entry (Thiébaut & Bénichou, 2004). Then, we used a competing risk regression (Pintilie, 2006) or sub‐distribution hazards regression to adjust estimates of incident AD risk taking into account the competing mortality risk (Austin et al., 2016) conveyed by the overall duration of follow‐up. All covariates were included in the multivariate model as they have all shown to be related to the risk of Alzheimer's disease (Kuo et al., 2020; Ralli et al., 2019; Sáiz‐Vázquez et al., 2021). The sub‐distribution hazard ratio (SHR) for assessing the risk of developing dementia for an individual factor was calculated using the cmprsk library in the R package (http://www.r‐project.org), which allows adjustment for socio‐demographic and clinical variables.
3. RESULTS
Figure 1 shows a flow diagram of the ZARADEMP Project. Of the 4803 subjects interviewed at baseline (Wave I), 1103 under the age of 65 and 746 subjects diagnosed with dementia were excluded from follow‐up, leaving a total of 3044 participants included in our initial sample. Between Wave I and Wave IV, 125 individuals (4.11%) were incident AD cases and 75 (2.46%) were incident cases of other dementias, 1382 (45.40%) died without developing AD and 776 (25.49%) were lost to follow‐up.
FIGURE 1.
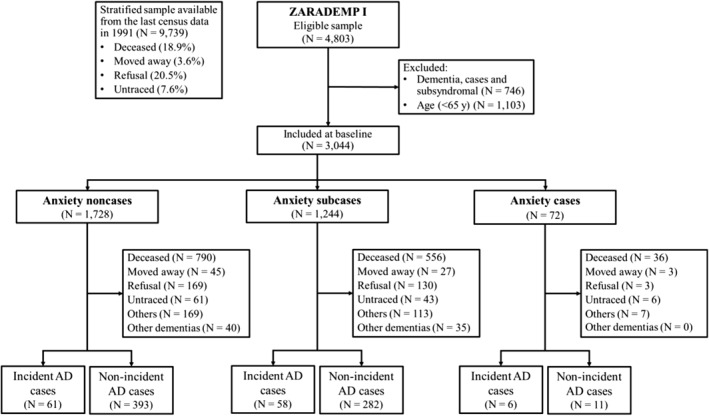
Zaragoza Dementia and Depression (ZARADEMP) study flow chart
The baseline sociodemographic characteristics of all participants by anxiety status, after excluding only those diagnosed with dementia, have been described in previous papers. In summary, participants who were considered cases and sub‐cases of anxiety were more likely to be female, have depression, report poor health status, and have disabilities in instrumental ADLs, compared to non‐cases.
Table 1 shows the baseline demographic characteristics according to incident AD status. The incident AD group was significantly older, more likely to be female and formerly married, compared to the non‐AD group.
TABLE 1.
Baseline characteristics according to incident Alzheimer's disease (AD) status
| No AD (N = 2919) | Incident AD (N = 125) | p‐Value | |
|---|---|---|---|
| Age (years) | 75.4 (7.7) | 81.2 (7.5) | <0.001 |
| Female sex | 1615 (55.3%) | 89 (71.2%) | 0.005 |
| Education | |||
| Illiterate | 240 (8.3%) | 26 (20.8%) | 0.774 |
| Primary school | 2189 (75.6%) | 86 (68.8%) | |
| High school or higher | 466 (16.1%) | 13 (10.4%) | |
| Marital status | |||
| Single | 286 (9.8%) | 5 (4.0%) | <0.001 |
| Married/in couple | 1670 (57.2%) | 45 (36.0%) | |
| Formerly married | 963 (32.9%) | 75 (60.0%) | |
| Depression | |||
| Noncase | 2571 (88.1%) | 109 (87.2%) | 0.767 |
| Case | 348 (11.9%) | 16 (12.8%) | |
| Anxiety | |||
| Noncase | 1667 (57.1%) | 61 (48.8%) | 0.058 |
| Subcase | 1186 (40.6%) | 58 (34.8%) | |
| Case | 66 (2.3%) | 6 (4.8%) | |
| BMI | 26.6 (6.6) | 25.9 (4.1) | 0.253 |
| Hearing loss | 22 (0.7%) | 2 (1.6%) | 0.595 |
Note: Bold entries mean the SHR is statistically significant. Data are given as mean (standard deviation) or number (%).
The median follow‐up period was 7.6 years (IQR: 2.9–11.3), over which time 125 participants developed AD. Table 2 shows the competing risk regression analysis of incident AD associated with anxiety status at baseline.
TABLE 2.
Anxiety status and risk of Alzheimer's disease (AD)
| Anxiety status at baseline | No. (%) of AD incident cases | Univariate model | Multivariate model | ||
|---|---|---|---|---|---|
| SHR (95% CI) a | p‐Value | SHR (95% CI) a | p‐Value | ||
| Non‐cases (n = 1728) | 61 (3.5) | 1 | ― | 1 | ― |
| Subcases (n = 1244) | 58 (4.7) | 1.23 (0.86–1.77) | 0.25 | 1.27 (0.88–1.86) | 0.2 |
| Cases (n = 72) | 6 (8.3) | 2.62 (1.14‐6.00) | 0.023 | 2.82 (1.21‐6.58) | 0.017 |
Note: Bold entries mean the SHR is statistically significant.
Abbreviations: AD, Alzheimer's disease; SHR, subdistribution hazard ratio.
Reported SHR of AD is related to non‐cases, CIs and p values related to SHR were from “normal approximation” of Wald χ 2 test with 1 df. Univariate Model include anxiety status and sex. Multivariate Model additionally included terms for educational level, marital status, depression, BMI and hearing loss.
When the competing risk of mortality is taken into account in the AD risk calculation, the crude comparison of the CIF by anxiety status showed that, at 10‐year follow‐up, compared to non‐anxiety cases, the probability of developing AD was higher in anxiety subcases and cases (Figure 2).
FIGURE 2.
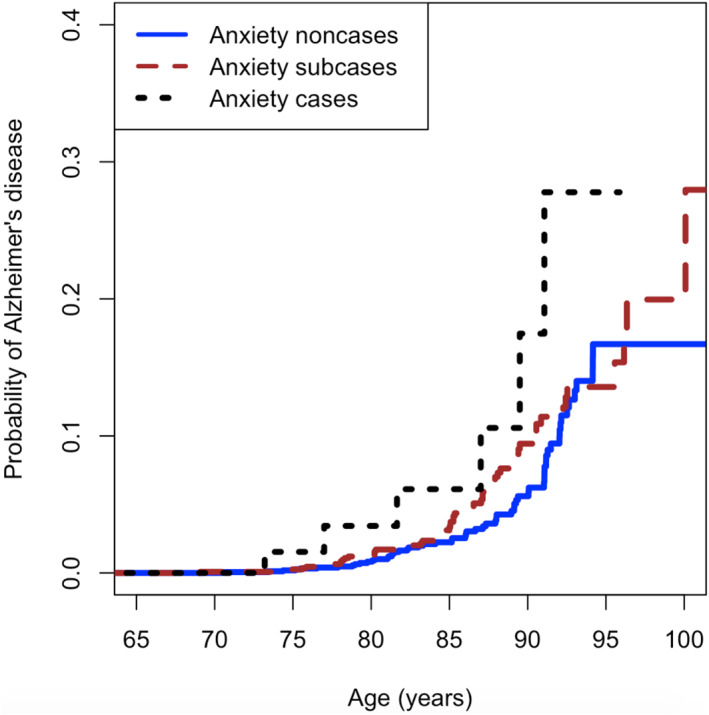
Probability of incident Alzheimer's disease (AD) by anxiety status at baseline in the study sample
The association shown in the univariate analysis of the risk of AD at 10 years of follow‐up (Wave IV) persisted in the multivariate analysis, which after the inclusion of all potential confounding factors showed a risk of AD 2.8 times higher in anxiety cases compared to non‐cases (p‐value = 0.017). There was no statistically significant difference between non‐cases and anxiety subcases in either analysis, univariate or multivariate.
4. DISCUSSION
4.1. Main findings
Our findings from a sample of community‐dwelling adults over 65 years of age show that, compared to individuals without anxiety, those with clinically significant anxiety had a nearly threefold increased risk of developing AD within a 10‐year follow‐up, after adjusting for several covariates, including depression. In contrast, the presence of isolated anxiety symptoms was not significantly associated with an increased incidence of AD.
4.2. Comparison with previous studies
Our results for AD are in line with those reported for dementia more broadly after 10 years (Gimson et al., 2018), as well as with those reported in meta‐analyses (Gulpers et al., 2016; Kuring et al., 2020). One of these meta‐analyses (Gulpers et al., 2016) included six studies with a mean follow‐up of 4.3 years and reported a stronger association for smaller intervals between anxiety and dementia diagnosis, and for individuals older than 80 years, concluding that anxiety may represent a prodromal state of dementia. Although the effect size decreased with follow‐up time in the ZARADEMP Study, since we found that the risk of AD was multiplied by 4.5 (data not shown), 3.9 (Santabárbara, Villagrasa, et al., 2019), and 2.8 times among individuals with clinically significant anxiety over follow‐up periods of 2.5, 4.5 and 10 years, respectively; risk of developing AD remained increased for subjects with clinically significant anxiety even at the longest follow‐up and, in our study, it was greater than the reported by the meta‐analyses of Gulpers et al. (2016) and Kuring et al. (2020). We hypothesise that differences on effect size could be explained by methodological heterogeneity, specifically the fact that previous studies usually diagnose anxiety by symptomatic scales and they do not assess clinically significant anxiety. Moreover, we did not find in our sample a significantly increased risk of incident AD for anxiety symptoms (“subcases” of anxiety). The persistence of a significant association between clinically significant anxiety and AD after a long follow‐up period and the differences on AD risk in relation to anxiety severity, both support the hypothesis of anxiety being a risk factor for AD and not just a prodromal sign.
Distinguishing between risk and prodromal factors is a challenge in neurodegenerative diseases such as AD, where the prodromal phase may begin several years before cognitive impairment is manifest. Indeed, some research suggests that brain changes associated with AD may begin 20 years or more before the onset of dementia symptoms (Alzheimer's Association, 2017). It has also been reported that individuals who later develop AD exhibit particular trajectories of non‐cognitive symptoms, including anxiety, prior to diagnosis (Masters et al., 2015).
How anxiety may present as prodromal AD is still under investigation, but the most common symptoms may be worry, fear, tension, restlessness and fidgeting (Ismail et al., 2018) which are less likely to meet the criteria for clinically significant anxiety and more likely to be classified as isolated symptoms of anxiety.
4.3. Potential underlying biological mechanisms
There are many potential ways to explain the relationship of causality between anxiety and AD. Chronic anxiety disorders may generate atrophy and other changes that decrease brain reserve (Vance et al., 2010). Brain‐derived neurotrophic factor (BDNF) has been shown to be both reduced in AD (Teixeira et al., 2010) and protect against developing dementia (Weinstein et al., 2014). BDNF is also reduced in anxiety disorders (Domingos da Silveira da Luz et al., 2013). In addition, chronic anxiety may promote avoidance behaviours, with reduced exposure to mental and social stimuli resulting in decreased cognitive reserve (Stern, 2012).
Anxiety and stress elevate inflammatory cytokines (IL‐6 and TNF) that have negative effects on cognitive function (Ismail et al., 2018). Indeed, there is growing evidence for the role of CNS inflammation in the development of AD (Cuello, 2017), starting at very early stages even before Aβ deposits.
Elevated cortisol levels are also associated with anxiety disorders (Mathew et al., 2008) and generalised anxiety in late life (Gulpers et al., 2016; Mantella et al., 2008), and may contribute to pathological processes in AD including hippocampal atrophy, Aβ formation and tau accumulation (Gulpers et al., 2016; Ismail et al., 2018).
Other mechanisms potentially shared by anxiety and AD include serotonergic pathways implicated in depression and anxiety (Gallacher et al., 2009), and the apolipoprotein E4 allele commonly associated with AD risk may also be associated with elevated levels of anxiety (Raber, 2007). There are also links between amyloid B and anxiety. Higher amyloid load is associated with a greater increase in anxious and depressive symptoms in cognitively intact older people (Donovan et al., 2018), and higher levels of anxiety in Aβ‐positive individuals are associated with faster cognitive decline (Pietrzak et al., 2015).
Lastly, while some studies suggest that anxiolytics may reduce the risk of developing AD (Burke et al., 2017), others report that benzodiazepines, commonly prescribed for anxiety symptoms (Olfson et al., 2015), are linked to an increased risk of all‐cause dementia (Lucchetta et al., 2018) and AD (Ettcheto et al., 2020).
However, as Butters et al. (2008) suggested for depression, the concept of brain and cognitive reserve may integrate the multiple mechanisms connecting anxiety and AD. From this perspective, anxiety (or some drugs used for its treatment, such as benzodiazepines) would affect the threshold for manifesting clinical dementia.
4.4. Strengths and limitations
To our knowledge, this is the first study to assess the risk of AD for both clinically significant and subsyndromal anxiety over a period as long as 10 years. Rather than self‐rating scales, our study is advantaged by using an assessment method that identifies and distinguishes clinically significant anxiety from other mental disorders (Stewart, 2019). Furthermore, we controlled for clinically significant depression, which is consistently reported as a risk factor for AD (Gracia‐García et al., 2015; Ownby et al., 2006), and may inflate associations between anxiety and dementia if not accounted for (Burke et al., 2018). We also accounted for the competing risk of death, as not doing so may bias risk estimates, and is of vital importance when studying dementia risk (Licher et al., 2018).
This study also comes with several limitations. Firstly, by defining anxiety based on symptomatic severity we did not differentiate between types of anxiety disorders. Different anxiety disorders exhibit different brain alterations (Ferrari et al., 2008), and their associated risk of AD may also differ. However, this syndromic approach could be more interesting for research than analysis by diagnostic categories, based on consensus rather than on evidence and validated scientific bases (National Institute of Mental Health, n.d). Secondly, by assessing anxiety only at baseline, we do not know whether it was brief and episodic or chronic (Ismail et al., 2018). Nor did we assess how the risk of AD was associated with persistent or recurrent anxiety throughout follow‐up. Thirdly, while evidence suggests an increased risk of AD associated with benzodiazepines and Z‐drugs (Ettcheto et al., 2020), we did not control for their use. We also note that a relatively small number of anxiety cases developed AD and that a hospital diagnosis was not made in all cases classified as dementia. Otherwise, in the current study we calculated AD risk on the basis of cumulative incidence of AD at a 10‐year follow‐up, which means that AD became manifest at any time within the overall follow‐up. Lastly, a specific diagnosis of Alzheimer's dementia can be difficult in both epidemiological and clinical contexts. However, we previously reported in a study based on the same sample and similar methodology, also incorporating a competing risk of death and with a shorter follow‐up time, that anxiety increases the risk for global dementia (i.e., dementia of any cause) (Santabárbara, Lopez‐Anton, et al., 2019). These results (SHR: 2.74; 95% CI: 1.18–6.35) are very similar to those found in this study for Alzheimer's Dementia.
4.5. Clinical, public health and future research implications
Our results support anxiety being a risk factor for AD rather than just a prodromal symptom and thus suggest the potential to reduce or delay the incidence of AD by addressing anxiety. This could be done clinically with preventive and therapeutic strategies (either pharmacological or psychotherapeutic), and at a population level by detecting at‐risk subpopulations and implementing targeted prevention programmes. However, it remains to be elucidated whether reducing anxiety does translate into a lower risk of AD. There are currently no studies that specifically address the risk of dementia or AD for anxiety regarding treatment status and response to treatment. In any case, our results highlight the need of following individuals presenting clinically significant anxiety in old age for future decline. Future research should also aim to create a better understanding of the mechanisms underlying the association between anxiety and AD, and from which efforts to develop preventive or therapeutic interventions would benefit.
5. CONCLUSIONS
Our study has found that clinically significant anxiety is associated with an increased risk of AD, even at a 10‐year follow‐up period and when controlling for confounding factors. This supports the hypothesis that anxiety is a risk factor for AD, but does not exclude anxiety also being a prodrome when present closer to a diagnosis of AD. Future research should aim to determine the mechanisms underpinning this association, as well as the extent to which the prevention and treatment of anxiety disorders would benefit public health by reducing the incidence and burden of AD.
AUTHOR CONTRIBUTIONS
Conceptualisation: Patricia Gracia‐García, Juan Bueno‐Notivol, Antonio Lobo and Javier Santabárbara; methodology: Patricia Gracia‐García, Juan Bueno‐Notivol, Darren M. Lipnicki and Javier Santabárbara; software, Javier Santabárbara; validation: Darren M. Lipnicki and Javier Santabárbara; formal analysis, Javier Santabárbara; investigation: Patricia Gracia‐García, Juan Bueno‐Notivol, Concepción de la Cámara, Antonio Lobo and Javier Santabárbara; resources: Antonio Lobo; data curation: Patricia Gracia‐García, Juan Bueno‐Notivol, Concepción de la Cámara, Javier Santabárbara; writing—original draft preparation: Patricia Gracia‐García and Juan Bueno‐Notivol; writing—review and editing: Patricia Gracia‐García, Juan Bueno‐Notivol, Darren M. Lipnicki and Javier Santabárbara; visualisation: Patricia Gracia‐García, Juan Bueno‐Notivol, Darren M. Lipnicki, Concepción de la Cámara and Javier Santabárbara; supervision: Patricia Gracia‐García, Darren M. Lipnicki, Antonio Lobo and Javier Santabárbara; project administration: Antonio Lobo; funding acquisition: Concepción de la Cámara and Antonio Lobo. All authors have read and agreed to the published version of the manuscript.
CONFLICTS OF INTEREST
We declare that P. Gracia‐García has received financial support to attend scientific meetings from Servier, Pfizer, Lundbeck, Nutrición Médica, Esteve and Angelini. C. de la Cámara has received financial support to attend scientific meetings from Janssen‐Cilag, Almirall, Eli Lilly, Lundbeck, Rovi, Esteve, Novartis and Astrazeneca. None of these activities are related to the current project. For the remaining authors, no conflicts of interest were declared. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
ETHICS STATEMENT
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the CEIC Aragón (protocol code CP16/2012, September 19, 2012).
INFORMED CONSENT STATEMENT
Informed consent was obtained from all subjects involved in the study.
ACKNOWLEDGEMENTS
The authors acknowledge the contribution of the lay interviewers, senior medical students, and members of the ZARADEMP Workgroup who participated in the study. Supported by Grants from the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness, Madrid, Spain (grants 94/1562, 97/1321E, 98/0103, 01/0255, 03/0815, 06/0617, 12/02254, 16/00896, PI/19/01874, G03/128) and from the Fondo Europeo de Desarrollo Regional (FEDER) of the European Union “Una manera de hacer Europa” (Project number PI16/00896) and Gobierno de Aragón (grant B15_17R).
Gracia‐García, P. , Bueno‐Notivol, J. , Lipnicki, D. M. , de la Cámara, C. , Lobo, A. , & Santabárbara, J. (2023). Clinically significant anxiety as a risk factor for Alzheimer's disease: Results from a 10‐year follow‐up community study. International Journal of Methods in Psychiatric Research, 32(3), e1934. 10.1002/mpr.1934
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Alzheimer’s Association . (2017). 2017 Alzheimer’s disease facts and figures. Alzheimer's and Dementia, 13(4), 325–373. 10.1016/j.jalz.2017.02.001 [DOI] [Google Scholar]
- Austin, P. C. , Lee, D. S. , D’Agostino, R. B. , & Fine, J. P. (2016). Developing points‐based risk‐scoring systems in the presence of competing risks. Statistics in Medicine, 35(22), 4056–4072. 10.1002/sim.6994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow, B. , & Michaelis, S. (2015). Epidemiology of anxiety disorders in the 21st century. Dialogues in Clinical Neuroscience, 17(3), 327–335. 10.31887/DCNS.2015.17.3/bbandelow [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, S. L. , Cadet, T. , Alcide, A. , O’Driscoll, J. , & Maramaldi, P. (2018). Psychosocial risk factors and Alzheimer’s disease: The associative effect of depression, sleep disturbance, and anxiety. Aging & Mental Health, 22(12), 1577–1584. 10.1080/13607863.2017.1387760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, S. L. , O’Driscoll, J. , Alcide, A. , & Li, T. (2017). Moderating risk of Alzheimer’s disease through the use of anxiolytic agents. International Journal of Geriatric Psychiatry, 32(12), 1312–1321. 10.1002/gps.4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters, M. A. , Young, J. B. , Lopez, O. , Aizenstein, H. J. , Mulsant, B. H. , Reynolds, C. F., III , DeKosky, S. T. , & Becker, J. T. (2008). Pathways linking late‐life depression to persistent cognitive impairment and dementia. Dialogues in Clinical Neuroscience, 10(3), 345–357. 10.31887/DCNS.2008.10.3/mabutters [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland, J. R. M. , Beekman, A. T. F. , Braam, A. W. , Dewey, M. E. , Delespaul, P. , Fuhrer, R. , Hooijer, C. , Lawlor, B. A. , Kivela, S.‐L. , Lobo, A. , Magnusson, H. , Mann, A. H. , Meller, I. , Prince, M. J. , Reischies, F. , Roelands, M. , Skoog, I. , Turrina, C. , DeVries, M. W. , & Wilson, K. C. M. (2004). Depression among older people in Europe: The EURODEP studies. World Psychiatry, 3(1), 45–49. [PMC free article] [PubMed] [Google Scholar]
- Cuello, A. C. (2017). Early and late CNS inflammation in Alzheimer’s disease: Two extremes of a continuum? Trends in Pharmacological Sciences, 38(11), 956–966. 10.1016/j.tips.2017.07.005 [DOI] [PubMed] [Google Scholar]
- Domingos da Silveira da Luz, A. C. , Pereira Dias, G. , do Nascimento Bevilaqua, M. C. , Cocks, G. , Gardino, P. F. , Thuret, S. , & Nardi, A. E. (2013). Translational findings on brain‐derived neurotrophic factor and anxiety: Contributions from basic research to clinical practice. Neuropsychobiology, 68(3), 129–138. 10.1159/000353269 [DOI] [PubMed] [Google Scholar]
- Donovan, N. J. , Locascio, J. J. , Marshall, G. A. , Gatchel, J. , Hanseeuw, B. J. , Rentz, D. M. , Johnson, K. A. , & Sperling, R. A. (2018). Longitudinal association of amyloid beta and anxious‐depressive symptoms in cognitively normal older adults. American Journal of Psychiatry, 175(6), 530–537. 10.1176/appi.ajp.2017.17040442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettcheto, M. , Olloquequi, J. , Sánchez‐López, E. , Busquets, O. , Cano, A. , Manzine, P. R. , Beas‐Zarate, C. , Castro‐Torres, R. D. , García, M. L. , Bulló, M. , Auladell, C. , Folch, J. , & Camins, A. (2020). Benzodiazepines and related drugs as a risk factor in Alzheimer’s disease dementia. Frontiers in Aging Neuroscience, 11. 10.3389/fnagi.2019.00344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari, M. C. F. , Busatto, G. F. , McGuire, P. K. , & Crippa, J. A. S. (2008). Structural magnetic resonance imaging in anxiety disorders: An update of research findings. Revista Brasileira de Psiquiatria, 30(3), 251–264. 10.1590/S1516-44462008000300013 [DOI] [PubMed] [Google Scholar]
- Gallacher, J. , Bayer, A. , Fish, M. , Pickering, J. , Pedro, S. , Dunstan, F. , Ebrahim, S. , & Ben‐Shlomo, Y. (2009). Does anxiety affect risk of dementia? Findings from the Caerphilly Prospective Study. Psychosomatic Medicine, 71(6), 659–666. 10.1097/PSY.0b013e3181a6177c [DOI] [PubMed] [Google Scholar]
- Gimson, A. , Schlosser, M. , Huntley, J. D. , & Marchant, N. L. (2018). Support for midlife anxiety diagnosis as an independent risk factor for dementia: A systematic review. BMJ Open, 8(4), e019399. 10.1136/bmjopen-2017-019399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia‐García, P. , De‐la‐Cámara, C. , Santabárbara, J. , Lopez‐Anton, R. , Quintanilla, M. A. , Ventura, T. , Marcos, G. , Campayo, A. , Saz, P. , Lyketsos, C. , & Lobo, A. (2015). Depression and incident Alzheimer disease: The impact of disease severity. The American Journal of Geriatric Psychiatry, 23(2), 119–129. 10.1016/j.jagp.2013.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulpers, B. , Ramakers, I. , Hamel, R. , Köhler, S. , Oude Voshaar, R. , & Verhey, F. (2016). Anxiety as a predictor for cognitive decline and dementia: A systematic review and meta‐analysis. The American Journal of Geriatric Psychiatry, 24(10), 823–842. 10.1016/j.jagp.2016.05.015 [DOI] [PubMed] [Google Scholar]
- Ismail, Z. , Gatchel, J. , Bateman, D. R. , Barcelos‐Ferreira, R. , Cantillon, M. , Jaeger, J. , Donovan, N. J. , & Mortby, M. E. (2018). Affective and emotional dysregulation as pre‐dementia risk markers: Exploring the mild behavioral impairment symptoms of depression, anxiety, irritability, and euphoria. International Psychogeriatrics, 30(2), 185–196. 10.1017/S1041610217001880 [DOI] [PubMed] [Google Scholar]
- Ismail, Z. , Smith, E. E. , Geda, Y. , Sultzer, D. , Brodaty, H. , Smith, G. , Agüera‐Ortiz, L. , Sweet, R. , Miller, D. , & Lyketsos, C. G. (2016). Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimer's and Dementia, 12(2), 195–202. 10.1016/j.jalz.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, C.‐Y. , Stachiv, I. , & Nikolai, T. (2020). Association of late life depression, (non‐) modifiable risk and protective factors with dementia and Alzheimer’s disease: Literature review on current evidences, preventive interventions and possible future trends in prevention and treatment of dementia. International Journal of Environmental Research and Public Health, 17(20), 7475. 10.3390/ijerph17207475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuring, J. K. , Mathias, J. L. , & Ward, L. (2020). Risk of dementia in persons who have previously experienced clinically‐significant depression, anxiety, or PTSD: A systematic review and meta‐analysis. Journal of Affective Disorders, 274, 247–261. 10.1016/j.jad.2020.05.020 [DOI] [PubMed] [Google Scholar]
- Lang, T. A. , & Altman, D. G. (2013). Basic statistical reporting for articles published in clinical medical journals: The SAMPL guidelines. In Smart P., Maisonneuve H., & Polderman A. (Eds.), Science editors’ handbook. European Association of Science. [Google Scholar]
- Launer, L. J. , Brayne, C. , Dartigues, J. F. , & Hofman, A. (1992). European studies on the incidence of dementing diseases, a report of the EURODEM research group. Neuroepidemiology, 11(Suppl), 1–22. 10.1159/issn.0251-5350 [DOI] [Google Scholar]
- Licher, S. , Yilmaz, P. , Leening, M. J. G. , Wolters, F. J. , Vernooij, M. W. , Stephan, B. C. M. , Ikram, M. K. , & Ikram, M. A. (2018). External validation of four dementia prediction models for use in the general community‐dwelling population: A comparative analysis from the Rotterdam study. European Journal of Epidemiology, 33(7), 645–655. 10.1007/s10654-018-0403-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo, A. , Lopez‐Anton, R. , Santabárbara, J. , De‐la‐Cámara, C. , Ventura, T. , Quintanilla, M. A. , Roy, J. F. , Campayo, A. J. , Lobo, E. , Palomo, T. , Rodriguez‐Jimenez, R. , Saz, P. , & Marcos, G. (2011). Incidence and lifetime risk of dementia and Alzheimer’s disease in a Southern European population. Acta Psychiatrica Scandinavica, 124(5), 372–383. 10.1111/j.1600-0447.2011.01754.x [DOI] [PubMed] [Google Scholar]
- Lobo, A. , Saz, P. , Marcos, G. , Día, J. L. , & de la Cámara, C. (1995). The prevalence of dementia and depression in the elderly community in a Southern European population. Archives of General Psychiatry, 52(6), 497. 10.1001/archpsyc.1995.03950180083011 [DOI] [PubMed] [Google Scholar]
- Lobo, A. , Saz, P. , Marcos, G. , Día, J.‐L. , De‐la‐Cámara, C. , Ventura, T. , Montañés, J. A. , Lobo‐Escolar, A. , & Aznar, S. (2005). The ZARADEMP project on the incidence, prevalence and risk factors of dementia (and depression) in the elderly community: II. Methods and first results. The European Journal of Psychiatry, 19(1), 40–54. 10.4321/S0213-61632005000100004 [DOI] [Google Scholar]
- Lucchetta, R. C. , da Mata, B. P. M. , & Mastroianni, P. D. C. (2018). Association between development of dementia and use of benzodiazepines: A systematic review and meta‐analysis. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 38(10), 1010–1020. 10.1002/phar.2170 [DOI] [PubMed] [Google Scholar]
- Mantella, R. C. , Butters, M. A. , Amico, J. A. , Mazumdar, S. , Rollman, B. L. , Begley, A. E. , Reynolds, C. F. , & Lenze, E. J. (2008). Salivary cortisol is associated with diagnosis and severity of late‐life generalized anxiety disorder. Psychoneuroendocrinology, 33(6), 773–781. 10.1016/j.psyneuen.2008.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters, M. C. , Morris, J. C. , & Roe, C. M. (2015). “Noncognitive” symptoms of early Alzheimer disease: A longitudinal analysis. Neurology, 84(6), 617–622. 10.1212/WNL.0000000000001238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew, S. J. , Price, R. B. , & Charney, D. S. (2008). Recent advances in the neurobiology of anxiety disorders: Implications for novel therapeutics. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 148C(2), 89–98. 10.1002/ajmg.c.30172 [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health . (n.d.). About RDoC. Retrieved January 18, 2022, from https://www.nimh.nih.gov/research/research‐funded‐by‐nimh/rdoc/about‐rdoc
- Olfson, M. , King, M. , & Schoenbaum, M. (2015). Benzodiazepine use in the United States. JAMA Psychiatry, 72(2), 136. 10.1001/jamapsychiatry.2014.1763 [DOI] [PubMed] [Google Scholar]
- Ownby, R. L. , Crocco, E. , Acevedo, A. , John, V. , & Loewenstein, D. (2006). Depression and risk for Alzheimer disease: Systematic review, meta‐analysis, and metaregression analysis. Archives of General Psychiatry, 63(5), 530. 10.1001/archpsyc.63.5.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak, R. H. , Lim, Y. Y. , Neumeister, A. , Ames, D. , Ellis, K. A. , Harrington, K. , Lautenschlager, N. T. , Restrepo, C. , Martins, R. N. , Masters, C. L. , Villemagne, V. L. , Rowe, C. C. , & Maruff, P. (2015). Amyloid‐β, anxiety, and cognitive decline in preclinical Alzheimer disease. JAMA Psychiatry, 72(3), 284. 10.1001/jamapsychiatry.2014.2476 [DOI] [PubMed] [Google Scholar]
- Pintilie, M. (2006). Competing risks: A practical perspective. Wiley. [Google Scholar]
- Raber, J. (2007). Role of apolipoprotein E in anxiety. Neural Plasticity, 2007, 1–7. 10.1155/2007/91236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralli, M. , Gilardi, A. , Di Stadio, A. , Severini, C. , Salzano, F. A. , Greco, A. , & de Vincentiis, M. (2019). Hearing loss and Alzheimer’s disease: A review. The International Tinnitus Journal, 23(2), 79–85. 10.5935/0946-5448.20190014 [DOI] [PubMed] [Google Scholar]
- Riedel, O. , Heuser, I. , Klotsche, J. , Dodel, R. , & Wittchen, H.‐U. (2010). Occurrence risk and structure of depression in Parkinson disease with and without dementia: Results from the GEPAD study. Journal of Geriatric Psychiatry and Neurology, 23(1), 27–34. 10.1177/0891988709351833 [DOI] [PubMed] [Google Scholar]
- Sáiz‐Vázquez, O. , Gracia‐García, P. , Ubillos‐Landa, S. , Puente‐Martínez, A. , Casado‐Yusta, S. , Olaya, B. , & Santabárbara, J. (2021). Depression as a risk factor for Alzheimer’s disease: A systematic review of longitudinal meta‐analyses. Journal of Clinical Medicine, 10(9), 1809. 10.3390/jcm10091809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santabárbara, J. , Lipnicki, D. M. , Bueno‐Notivol, J. , Olaya‐Guzmán, B. , Villagrasa, B. , & López‐Antón, R. (2020). Updating the evidence for an association between anxiety and risk of Alzheimer’s disease: A meta‐analysis of prospective cohort studies. Journal of Affective Disorders, 262(September), 397–404. 10.1016/j.jad.2019.11.065 [DOI] [PubMed] [Google Scholar]
- Santabárbara, J. , Lipnicki, D. M. , Olaya, B. , Villagrasa, B. , Bueno‐Notivol, J. , Nuez, L. , López‐Antón, R. , & Gracia‐García, P. (2020). Does anxiety increase the risk of all‐cause dementia? An updated meta‐analysis of prospective cohort studies. Journal of Clinical Medicine, 9(6), 1791. 10.3390/jcm9061791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santabárbara, J. , Lipnicki, D. M. , Olaya, B. , Villagrasa, B. , Gracia‐García, P. , Bueno‐Notivol, J. , Lobo, A. , & López‐Antón, R. (2020). Association between anxiety and vascular dementia risk: New evidence and an updated meta‐analysis. Journal of Clinical Medicine, 9(5), 1368. 10.3390/jcm9051368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santabárbara, J. , Lopez‐Anton, R. , de la Cámara, C. , Lobo, E. , Gracia‐García, P. , Villagrasa, B. , Bueno‐Notivol, J. , Marcos, G. , & Lobo, A. (2019). Clinically significant anxiety as a risk factor for dementia in the elderly community. Acta Psychiatrica Scandinavica, 139(1), 6–14. 10.1111/acps.12966 [DOI] [PubMed] [Google Scholar]
- Santabárbara, J. , Villagrasa, B. , López‐Antón, R. , Olaya, B. , Bueno‐Notivol, J. , de la Cámara, C. , Gracia‐García, P. , Lobo, E. , & Lobo, A. (2019). Clinically relevant anxiety and risk of Alzheimer’s disease in an elderly community sample: 4.5 years of follow‐up. Journal of Affective Disorders, 250(February), 16–20. 10.1016/j.jad.2019.02.050 [DOI] [PubMed] [Google Scholar]
- Schaub, R. T. , Linden, M. , & Copeland, J. R. M. (2003). A comparison of GMS‐A/AGECAT, DSM‐III‐R for dementia and depression, including subthreshold depression (SD) ‐ Results from the Berlin Aging Study (BASE). International Journal of Geriatric Psychiatry, 18(2), 109–117. 10.1002/gps.799 [DOI] [PubMed] [Google Scholar]
- Singh‐Manoux, A. , Dugravot, A. , Fournier, A. , Abell, J. , Ebmeier, K. , Kivimäki, M. , & Sabia, S. (2017). Trajectories of depressive symptoms before diagnosis of dementia. JAMA Psychiatry, 74(7), 712. 10.1001/jamapsychiatry.2017.0660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer’s disease. The Lancet Neurology, 11(11), 1006–1012. 10.1016/S1474-4422(12)70191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, R. (2019). Anxiety and dementia: Cause or effect? Acta Psychiatrica Scandinavica, 139(1), 3–5. 10.1111/acps.12992 [DOI] [PubMed] [Google Scholar]
- Teixeira, A. L. , Barbosa, I. G. , Diniz, B. S. , & Kummer, A. (2010). Circulating levels of brain‐derived neurotrophic factor: Correlation with mood, cognition and motor function. Biomarkers in Medicine, 4(6), 871–887. 10.2217/bmm.10.111 [DOI] [PubMed] [Google Scholar]
- Thiébaut, A. C. M. , & Bénichou, J. (2004). Choice of time‐scale in Cox’s model analysis of epidemiologic cohort data: A simulation study. Statistics in Medicine, 23(24), 3803–3820. 10.1002/sim.2098 [DOI] [PubMed] [Google Scholar]
- Vance, D. E. , Roberson, A. J. , McGuinness, T. M. , & Fazeli, P. L. (2010). How neuroplasticity and cognitive reserve protect cognitive functioning. Journal of Psychosocial Nursing and Mental Health Services, 48(4), 23–30. 10.3928/02793695-20100302-01 [DOI] [PubMed] [Google Scholar]
- Von Elm, E. , Altman, D. G. , Egger, M. , Pocock, S. J. , Gøtzsche, P. C. , & Vandenbroucke, J. P. (2007). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Medicine, 4(10), 1623–1627. 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein, G. , Beiser, A. S. , Choi, S. H. , Preis, S. R. , Chen, T. C. , Vorgas, D. , Au, R. , Pikula, A. , Wolf, P. A. , DeStefano, A. L. , Vasan, R. S. , & Seshadri, S. (2014). Serum brain‐derived neurotrophic factor and the risk for dementia. JAMA Neurology, 71(1), 55. 10.1001/jamaneurol.2013.4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2015). First WHO Ministerial conference on global action against dementia. Meeting Report. WHO Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


