Abstract
Background
Polynucleotides (PN) are increasingly used for the treatment of facial erythema in the Republic of Korea. However, there are limited pre‐clinical and clinical data on the efficacy of polynucleotides for facial erythema. In this study, we investigated the current practice and perceived effectiveness of polynucleotide treatment for facial erythema among cosmetic physicians.
Methods
By conducting a survey among clinicians who use PN in clinical practice, we explored the current practices and assessed the perceived effectiveness of polynucleotides in treating facial erythema.
Results
A total of 557 physicians who used polynucleotides for facial erythema participated in the survey. Polynucleotides were used by 84.4%, 66.4%, and 47.4% of physicians for facial erythema caused by inflammatory facial dermatosis, repeated laser/microneedle radiofrequency, and steroid overuse, respectively. Among those users, 88.1%, 90%, and 83.7% respectively in those same categories answered that polynucleotides were “highly effective” or “effective.” Furthermore, they agreed that polynucleotides have the following properties: wound healing/regeneration (95.8%), protection of skin barrier (92.2%), hydration (90.5%), vascular stabilization (81.0%), and anti‐inflammation (79.5%).
Conclusion
Our findings showed that cosmetic physicians in the Republic of Korea have used PN as a part of combination treatment for facial erythema resulting from inflammatory facial dermatosis and repeated laser/ microneedle radiofrequency, rather than from steroid overuse. Also, most clinicians agreed that PN was effective for treatment of facial erythema. Given the lack of pre‐clinical and clinical trial evidence, the empirical responses of practicing physicians provide useful information to guide clinical practice and further research.
Keywords: effectiveness, erythema, polynucleotides, practice
1. INTRODUCTION
Facial erythema occurs when blood vessels in the skin dilate and blood flow increases. Although transient facial erythema is observed as a natural response to emotion, exercise, or heat exposure, chronic erythema is caused by inflammation or various medical conditions. 1 , 2 , 3 , 4
Skin conditions like rosacea, atopic dermatitis, acne, and seborrheic dermatitis can result in recurrent facial erythema. 1 Facial erythema is the most frequently occurring side effect of laser skin treatment. 2 Additionally, the repeated use of topical steroids on the face leads to skin thinning and results in the development of erythema. 3 Facial erythema persists for months and has a significant impact on the quality of life, self‐confidence, and self‐esteem of affected individuals. 5 This causes discomfort and is a source of concern for those affected, who are continually seeking out effective skincare products and treatments to improve the health and appearance of their skin.
Several treatment options, such as botulinum toxin, 6 injectable hyaluronic acid, 7 laser, 8 radiofrequency, 9 light‐emitting diodes (LED), 2 therapeutic ultrasound, 10 and plasma therapies, 11 are used for facial erythema. However, their efficacy is unclear due to limited pre‐clinical and clinical data. As these treatment options are not yet clearly established, there is a need for novel therapies or reliable aesthetic guidance.
Polynucleotides (PN) are extracted from the testes of Pacific salmon, rainbow trout fish, and sturgeons. 12 They undergo purification and are used in injection treatment products as natural DNA molecules. PN are composed of ≥ 13 covalently linked nucleotide monomers with a high molecular weight up to 800 kDa and have a viscoelastic texture. 13 Due to their ability to bind to the adenosine A2A receptor at the molecular level, PN exhibit anti‐inflammatory properties in in vitro models. 14 , 15 Various in vitro studies have demonstrated that PN show a trophic effect on human fibroblasts in primary culture and stimulate the secretion activity of collagen proteins and other proteins of the extracellular matrix (ECM). 16 PN have a hydrating effect by attracting moisture, restoring the ECM, and improving elasticity and tone. 17 Also, they enhance vascular endothelial growth factor (VEGF) within skin tissue, which strengthens microcirculation and promotes deoxyribonucleic acid (DNA) synthesis, enhancing remodeling of skin tissue. 18 As a result, they are expected to strengthen the skin barrier and have anti‐inflammatory effects, improving erythema.
With the proposed mechanism, PN are increasingly used for treatment of facial erythema in the Republic of Korea. However, there are limited pre‐clinical and clinical data on the efficacy of PN for facial erythema. Therefore, in this survey study, we investigated the current practice and perceived effectiveness of PN in treating facial erythema among cosmetic physicians.
2. MATERIALS AND METHODS
2.1. Development of survey
We identified the current practice patterns of clinicians and their perceived effectiveness as the key content area relevant to understanding expert practice of PN treatments. To obtain opinions about current practice and perceived efficacy of PNs, we constructed items using reviews of current evidence. 14 , 19 , 20 , 21 Face‐to‐face consultation interviews were conducted with expert cosmetic clinicians, and discussions with other clinicians were performed to compose the survey draft.
We conducted a pilot study with a sample of seven cosmetic experts to test the survey draft and ensure its ease of use and external validity. Minor changes were made to the wording of the survey questions to increase clarity based on the feedback received from the pilot study. The pilot sample consisted of cosmetic clinicians who were not included in the main study.
The final survey consisted of structured questions regarding demographic information as well as level of agreement with various statements concerning current practice and perceived efficacy of PN.
The questions were designed to collect information from clinicians who use PN to treat erythema caused by various conditions, including different forms of inflammatory facial dermatosis, repeated laser or microneedle radiofrequency, and steroid overuse. Facial erythema can be associated with various inflammatory skin diseases and external stimuli that increase blood flow in the superficial capillaries and cause a change in the skin. The precise pathogenesis of erythema is unknown, but common etiology of facial erythema are inflammatory skin diseases (e.g., acne, rosacea, seborrheic dermatitis), post‐inflammatory and post‐procedural erythema. 22 Although patients with facial erythema might be mainly related to rosacea, the study tends to classify patients based on their causative etiology instead of diagnosis.
The first question asked if clinicians use PN for these contributing factors to erythema. If they answered “yes,” they were prompted to answer the second question on the number of cases treated with PN for this purpose. The answer options were presented in a multiple‐choice format, ranging from “fewer than 10 cases” to “more than 100 cases.” The third question asked the clinicians’ opinions on the effectiveness of PN for erythema. They chose from four options ranging from “highly effective” to “highly ineffective.”
We asked the respondent to indicate their level of agreement with the clinical efficacy of PN for purposes of 1) anti‐inflammation, 2) protection of skin barrier, 3) wound healing /regeneration, 4) vascular stabilization, and 5) hydration. For each question, there were four options to choose from 1) strongly agree, 2) agree, 3) disagree, and 4) strongly disagree. Responders were encouraged to select PN effects based on their experiential judgment when following up with patients after PN treatment.
Also, there was a set of questions that asked clinicians about their use of PN for treating erythema caused by various skin conditions such as inflammatory facial dermatosis and repeated laser/microneedle radiofrequency treatment. The question provided two options for use of PN: as a stand‐alone treatment or in combination with other treatments such as lesion targeting devices (laser, radiofrequency), regenerative devices (LED, therapeutic ultrasound, plasma), botulinum toxin, or hyaluronic acid.
We developed a structured single‐choice questionnaire about the procedure method of PN for treatment of facial erythema. The questionnaire covered treatment interval, frequency, dosage, injection device, injection site, and depth. Last, we asked clinicians to report the percentage of side effects they observed and allowed them to elaborate on these side effects if needed (Supplementary Material).
2.2. Data collection
This study was conducted at the 43rd Korean Association for Laser, Dermatology, and Trichology (KALDAT) held in Seoul, Republic of Korea, on April 23, 2023. The questionnaire was made available to clinicians attending the conference through a QR code, allowing them to complete the survey on their mobile device. Since the URL contained an encrypted, unique number for each individual, the survey participants could only respond to the survey once. All data were encrypted, and all personal identifying information was removed. All the participants were provided with a consent form for the use of personal information, an agreement to the use thereof, and consent to participate in the survey. The Institutional Review Board of Samsung Medical Center approved the protocol, study, and informed consent forms before enrollment (SMC‐2023‐02‐150‐001).
2.3. Statistical analysis
During analysis of the data from the survey, unequal selection probabilities and non‐response errors were corrected using complex sample design. Continuous data are presented as median (range) as appropriate. Categorical data are presented as a proportion.
Firstly, frequency analysis was conducted to understand the general characteristics of the study participants. Secondly, descriptive statistics and graphical representations were used to summarize data. All statistical analyses were performed using R ver. 4.3.0 software (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Baseline characteristics
A total of 557 physicians participated in the study. The participants in this study were predominately cosmetic physicians and male with one to five years of cosmetic procedure experience (see Figure 1).
FIGURE 1.
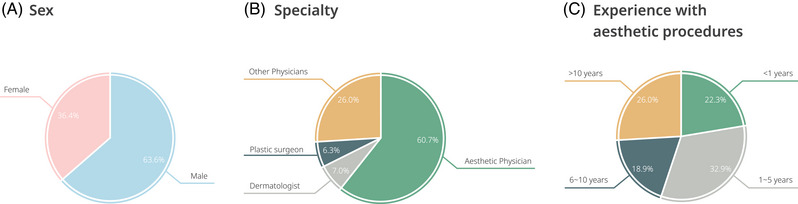
The characteristics of the 557 clinicians who participated in the survey.
3.2. Clinicians’ current practices and perceived effectiveness of PN in facial erythema caused by inflammatory facial dermatosis
Regarding the current practices utilized by clinicians, more than 80% of respondents used PN to treat facial erythema caused by inflammatory facial dermatosis (Figure 2). The majority of participants also agreed on the efficacy of PN, with 84.3% of clinicians reporting that PN were “effective” and 3.8% reporting that they were “highly effective” (based on the responses of 470 participants; see Figure 3A).
FIGURE 2.
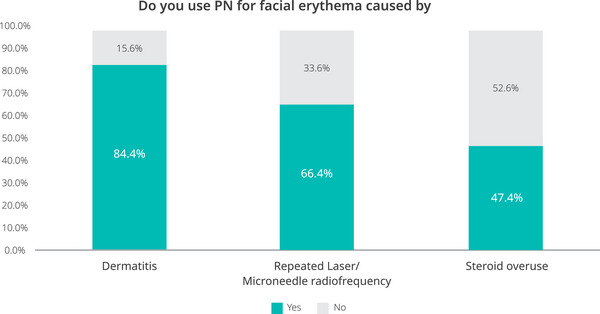
Clinician's use of PN for facial erythema caused by inflammatory facial dermatosis, repeated laser/microneedle radiofrequency, and steroid overuse.
FIGURE 3.
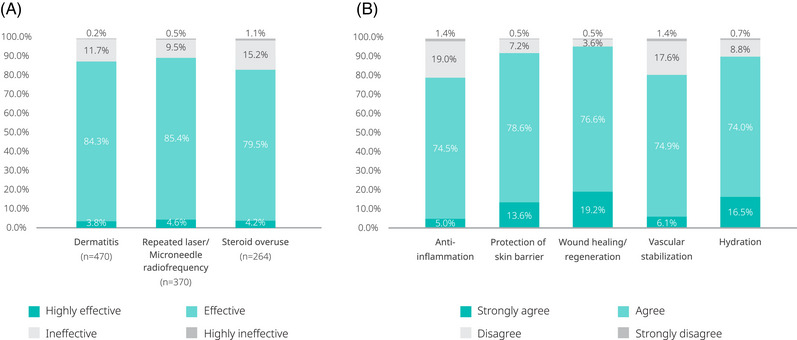
Clinician's perceived level of effectiveness of PN for treating facial erythema.
The majority of respondents used PN in combination with lesion targeting devices (ranging from 31.5% for atopic dermatitis to 48.3% for rosacea) or regenerative devices (ranging from 19.0% for acne to 28.9% for atopic dermatitis). A minority of respondents reported using PN as a stand‐alone treatment or second‐line treatment when other treatments had failed (Figure 4).
FIGURE 4.
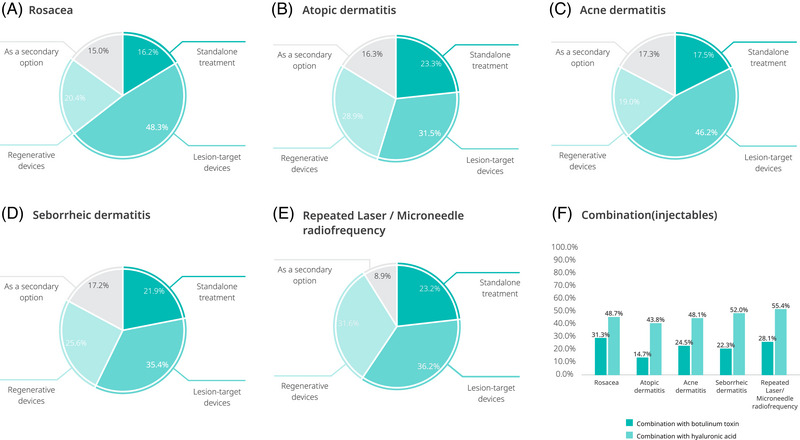
Clinicians’ PN procedure modalities for facial erythema caused by inflammatory facial dermatosis and repeated laser/microneedle radiofrequency.
Approximately half of the clinicians used PN in combination with hyaluronic acid to treat facial erythema. The combination of PN with botulinum toxin was used by 15–31% of clinicians (Figure 4).
3.3. Clinicians’ current practices and perceived effectiveness of PN in facial erythema caused by repeated laser/ microneedle radiofrequency
Approximately two‐thirds (66.4%) of the clinician respondents used PN to treat facial erythema caused by repeated laser or microneedle radiofrequency (Figure 2). Most participants agreed on effectiveness, with 85.4% of clinicians reporting that PN were “effective,” and 4.6% reporting that they were “highly effective” (Figure 3A).
Most physicians reported PN use in combination with lesion targeting devices (36.2%) or regenerative devices (31.6%). A minority of respondents used PN as a stand‐alone (23.2%) or second‐line therapy (8.9%) (Figure 4). More than half (55.4%) of the clinicians used PN in combination with hyaluronic acid, and 28.1% used PN in combination with botulinum toxin to treat facial erythema caused by repeated laser or microneedle radiofrequency treatments.
3.4. Clinicians’ current practices and perceived effectiveness of PN in facial erythema caused by steroid overuse
In the survey, 47.4% of clinician respondents had used PN to treat facial erythema caused by steroid overuse (Figure 2). Most of them reported that PN were effective (79.5%) or highly effective (4.2%) (Figure 3A).
3.5. Clinicians’ perceived clinical effectiveness of PN in facial erythema
Regarding the perceived clinical effectiveness of PN in facial erythema, most respondents “strongly agreed” or “agreed” with PN effects on wound healing and regeneration (19.2% and 76.6%, respectively), protection of skin barrier (13.6% and 78.6%), hydration (16.5% and 74.0%), vascular stabilization (6.1% and 74.9%), and anti‐inflammation (5.0% and 74.5%) (Figure 3B).
3.6. Clinicians’ current practice of PN in facial erythema
The most common clinical practice approach taken to enhance the improvement of erythema involved injecting 2 cc of PN every three to four weeks, for a total of three to four sessions. They typically used needle injections in the intradermal layer of the skin, applying it evenly over the entire face but focusing on the area of erythema (Figure 5).
FIGURE 5.
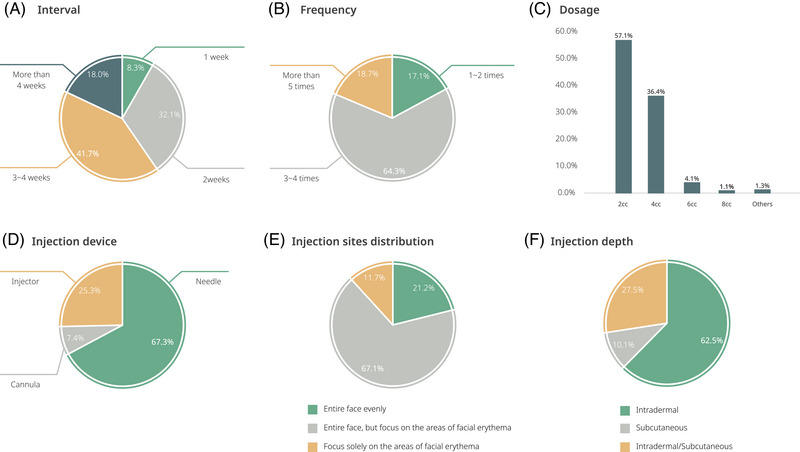
Clinicians’ PN procedure methods in treatments for facial erythema.
3.7. Clinicians’ experience of side effects
In this study, 96.6% of the respondents said they had rarely experienced adverse reactions, and most of the adverse reactions were mild and already described in the package insert. The most frequent adverse events were linked to local injection reactions.
4. DISCUSSION
To the best of our knowledge, this study is the first to investigate the current patterns of clinicians and their perceived effectiveness of PN use for facial erythema. Given a lack of pre‐clinical and clinical trial evidence for PN use in treatment of facial erythema, a survey among physicians who used PN in clinical practice would provide useful information to guide clinical practice and further research.
We observed that most cosmetic physicians use PN to treat facial erythema caused by inflammatory facial dermatosis, repeated laser treatments, or steroid overuse. Notably, the majority of physicians who use PN for erythema responded that it is “highly effective” and “effective” (approximately 90%). In addition, approximately 80% of physicians agreed on the following effects of PN in the following decreasing order: wound healing and regeneration, protection of skin barrier, hydration, vascular stabilization, and anti‐inflammation.
Polydeoxyribonucleotide (PDRN), derived from salmon germ cells, is a mixture of deoxyribonucleotides. It exhibits anti‐inflammatory properties by binding to adenosine A2A receptors, as demonstrated in various in vitro clinical models. 23 PDRN is known to accelerate the repair of damaged DNA caused by ultraviolet B (UVB) or reactive oxygen species (ROS) through the de novo salvage pathway. 24 To enhance their effect, PN are developed through controlled depolymerization, resulting in higher molecular weight and a viscoelastic texture. The pharmacological mechanism of action of PN is not fully understood. However, due to the structural similarity between PN and PDRN, similar effects have been suggested. Nevertheless, further experiments or independent research comparing the effects of PN to PDRN are necessary.
The precise pathogenesis of erythema in inflammatory facial dermatosis is unknown, but one proposed etiology is that repeated episodes of microcapillary dilation result in loss of vascular tone and subsequent permanent microcapillary dilatation. 6 This may lead to vascular instability and the release of inflammatory cytokines and reactive oxygen species (ROS). 25 , 26 Our findings demonstrated that most clinicians perceived PN use as effective in vascular stabilization and anti‐inflammation.
This is supported by a previous in vitro study by Bitto et al., which suggested that PDRN had the potential to reduce the level of proinflammatory cytokine TNF‐α, as determined by western blotting. 27 Also, a randomized controlled trial from a single center in the Republic of Korea reported that the vascularity on post‐operative scars decreased in the PN administration group compared to the control. 21 Considering the biological functions of PN, research on this therapeutic agent may open up promising methods for vascular stabilization and anti‐inflammation treatment. This is particularly relevant for inflammatory facial dermatosis.
Patients often experience dryness after laser exposure, 28 which can lead to an increase in skin sensitivity, as erythema is the most common side effect of fractional laser treatment. 2 Also, the side effects of microneedle radiofrequency may occur due to destruction of the skin barrier. 29 In this study, we observed that our respondents used PN to treat facial erythema caused by repeated laser and microneedle radiofrequency procedures.
Previous studies have indicated that 10 to 30 water molecules per phosphate are involved in the interaction with DNA. 30 The hydrating properties of nucleic acids suggest that PN can be beneficial for individuals with dry skin that has weakened scaffolding. Also, PN serve as both barrier organelles and structural scaffolds that support the proper accumulation of ECM during the process of wound healing. 21 An in vivo study from Mi Yu et al. demonstrated that intraperitoneal injection of PDRN had effects on laser‐induced skin erythema, increased epithelial confluence, and decreased crusting in a rat model. 31 In agreement with these findings, PN are beneficial therapeutic agents for dryness and skin damage caused by repeated laser and microneedle radiofrequency treatments.
Prolonged use of steroids can lead to complications such as skin atrophy and telangiectasia. Recent studies showed the impact of glucocorticoids on connective tissue, revealing that skin atrophy is primarily caused by a reduction in collagen synthesis and subsequent altering of the skin's collagen framework. 32 , 33 The treatment of facial erythema resulting from steroid overuse is extremely challenging, and there is no standard of care. However, in our research, approximately half of the physicians utilized PN for managing skin erythema caused by steroid use.
Previous studies have reported that PDRN increase collagen I expression 34 through the activation of membrane‐bound adenosine receptors and to stimulate cell growth and angiogenesis. 35 PN possess the capacity to stimulate the secretion of collagen proteins and other proteins in the extracellular matrix. 36
Lesion target devices and regenerative devices, including lasers, radiofrequency, LED, therapeutic ultrasound, plasma, are widely used for facial erythema. 37 More than half of physicians reported PN use in combination with devices to treat facial erythema in this study. Previous studies confirmed the synergistic effect of PN when used in conjunction with devices. Kim et al. demonstrated that the combination of PN, non‐ablative fractional erbium glass laser, and ablative fractional CO2 laser therapy significantly softened skin contraction and improved skin mobility. 21 Also, previous studies observed that PN, coupled with laser, improved pigmentation, vascularity, scar size, and the erythema index. 21 , 38 The potential applicability of PN as an adjuvant therapeutic agent for treating facial erythema was demonstrated in this study.
Approximately 15–30% of clinicians reported using PN in combination with botulinum toxin (BoNT) to treat facial erythema. BoNT has been reported as a treatment leading to a significant decrease in erythema. 39 However, there have been limited clinical studies investigating the effectiveness on this combination therapy. Despite the lack of sufficient evidence, clinicians utilize this combination therapy in a manner that is clinically relevant. Further randomized control trials (RCTs) and observational clinical studies are required to confirm our findings.
In this study, half of respondents used a combination therapy with hyaluronic acid instead of using PN alone for the treatment of erythema. A recent clinical study from two centers in Italy reported that a combination of polynucleotide and hyaluronic acid is able to speed the healing rate of wounds. This combination therapy led to a decrease in inflammation of perilesional areas and promoted wound contraction and epithelialization. 40 Our results indicated that the combination of PN and hyaluronic acid may be a treatment option for facial erythema.
Despite the strengths and clinical implications of our study, there are some limitations. First, the participants were exclusively from the Republic of Korea, which restricts the generalizability of our findings to other countries. Second, we were unable to survey non‐responders and compared their responses with those of the participants who did respond. This missing data could potentially introduce bias and affect the overall conclusions of the study.
However, this is the first study to explore the perspectives of aesthetic clinicians regarding PN in Korea. Further studies, such as pre‐clinical or clinical trials, should confirm the mechanism of PN on facial erythema.
5. CONCLUSION
Republic of Korea cosmetic physicians have used PN as a combination treatment for facial erythema resulting from inflammatory facial dermatosis, repeated laser/microneedle radiofrequency, and steroid overuse. Also, most of the clinicians agreed that PN had an effect on facial erythema. This study also provides a reliable guideline outlining the most effective way to utilize PN for facial erythema arising from different causes. This finding contributes to the evidence of the beneficial effect of off‐label treatment of erythema using PN in the Republic of Korea. Our results indicated that monotherapy or combined administration of PN demonstrated promising efficacy as a treatment option for facial erythema, according to responders in a large scaly survey.
CONFLICT OF INTEREST STATEMENT
Michael J. Kim, Hyun Jun Park, Gong Chan Rah, Hosung Choi, Sang‐Tae Anh, Gun Hyon Ji, Min Seong Kim, Geebum Kim, and Seung Min Oh are on the advisory board of PharmaResearch Co. Ltd. (Republic of Korea).
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was supported by PharmaResearch Co. Ltd. (grant number S‐2023‐0690‐0000‐01).
Lee D, Kim MJ, Park HJ, et al. Current practices and perceived effectiveness of polynucleotides for treatment of facial erythema by cosmetic physicians. Skin Res Technol. 2023;29:e13466. 10.1111/srt.13466
Dagyeong Lee, Michael J. Kim, Dong Wook Shin and Seung Min Oh contributed equally.
Contributor Information
Dong Wook Shin, Email: dwshin.md@gmail.com.
Seung Min Oh, Email: smkosomi@naver.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon reasonable request from the corresponding author.
REFERENCES
- 1. Dessinioti C, Antoniou C. The “red face”: Not always rosacea. Clin Dermatol. 2017;35(2):201‐206. [DOI] [PubMed] [Google Scholar]
- 2. Alster TS, Wanitphakdeedecha R. Improvement of postfractional laser erythema with light‐emitting diode photomodulation. Dermatol Surg. 2009;35(5):813‐815. [DOI] [PubMed] [Google Scholar]
- 3. Leyden JJ, Thew M, Kligman AM. Steroid Rosacea. Arch Dermatol. 1974;110(4):619‐622. 10.1001/archderm.1974.01630100075019 [DOI] [PubMed] [Google Scholar]
- 4. Maloney BP, Millman B, Monheit G, McCOLLOUGH EG. The etiology of prolonged erythema after chemical peel. Dermatol Surg. 1998;24(3):337‐341. [DOI] [PubMed] [Google Scholar]
- 5. Amiri R, Khalili M, Mohammadi S, Iranmanesh B, Aflatoonian M. Treatment protocols and efficacy of light and laser treatments in post‐acne erythema. J Cosmet Dermatol. 2022;21(2):648‐656. [DOI] [PubMed] [Google Scholar]
- 6. Hanna E, Xing L, Taylor JH, Bertucci V. Role of botulinum toxin A in improving facial erythema and skin quality. Arch Dermatol Res. 2021:1‐10. [DOI] [PubMed] [Google Scholar]
- 7. Judodihardjo H, Dykes P. Objective and subjective measurements of cutaneous inflammation after a novel hyaluronic acid injection. Dermatol Surg. 2008;34:S110‐S114. [DOI] [PubMed] [Google Scholar]
- 8. Al‐Niaimi F, Glagoleva E, Araviiskaia E. Pulsed dye laser followed by intradermal botulinum toxin type‐A in the treatment of rosacea‐associated erythema and flushing. Dermatol Ther. 2020;33(6):e13976. [DOI] [PubMed] [Google Scholar]
- 9. Min S, Park SY, Yoon JY, Kwon HH, Suh DH. Fractional microneedling radiofrequency treatment for acne‐related post‐inflammatory erythema. Acta Derm Venereol. 2016;96(1):87‐91. [DOI] [PubMed] [Google Scholar]
- 10. Kim YJ, Moon IJ, Lee HW, et al. The efficacy and safety of dual‐frequency ultrasound for improving skin hydration and erythema in patients with rosacea and acne. J Clin Med. 2021;10(4):834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. JI NA, JW CHOI, HR CHOI, et al. Rapid healing and reduced erythema after ablative fractional carbon dioxide laser resurfacing combined with the application of autologous platelet‐rich plasma. Dermatol Surg. 2011;37(4):463‐468. [DOI] [PubMed] [Google Scholar]
- 12. Kim T‐H, Heo S‐Y, Oh G‐W, Heo S‐J, Jung W‐K. Applications of marine organism‐derived polydeoxyribonucleotide: its potential in biomedical engineering. Mar Drugs. 2021;19(6):296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee YJ, Kim HT, Lee YJ, et al. Comparison of the effects of polynucleotide and hyaluronic acid fillers on periocular rejuvenation: a randomized, double‐blind, split‐face trial. J Dermatol Treat. 2022;33(1):254‐260. [DOI] [PubMed] [Google Scholar]
- 14. Bitto A, Oteri G, Pisano M, et al. Adenosine receptor stimulation by polynucleotides (PDRN) reduces inflammation in experimental periodontitis. J Clin Periodontol. 2013;40(1):26‐32. [DOI] [PubMed] [Google Scholar]
- 15. Altavilla D, Bitto A, Polito F, et al. Polydeoxyribonucleotide (PDRN): a safe approach to induce therapeutic angiogenesis in peripheral artery occlusive disease and in diabetic foot ulcers. Cardiovasc Hematol Agents Med Chem (Formerly Current Medicinal Chemistry‐Cardiovascular & Hematological Agents). 2009;7(4):313‐321. [DOI] [PubMed] [Google Scholar]
- 16. Sini P, Denti A, Cattarini G, Daglio M, Tira M, Balduini C. Effect of polydeoxyribonucleotides on human fibroblasts in primary culture. Cell Biochem Funct. 1999;17(2):107‐114. [DOI] [PubMed] [Google Scholar]
- 17. Palmieri IP, Raichi M. Biorevitalization of postmenopausal labia majora, the polynucleotide/hyaluronic acid option. Obstet Gynecol Rep. 2019;3:1‐5. [Google Scholar]
- 18. Saraiva SM, Castro‐López V, Pañeda C, Alonso MJ. Synthetic nanocarriers for the delivery of polynucleotides to the eye. Eur J Pharm Sci. 2017;103:5‐18. [DOI] [PubMed] [Google Scholar]
- 19. Rathbone MP, Middlemiss PJ, Gysbers JW, DeForge S, Costello P, Del Maestro RF. Purine nucleosides and nucleotides stimulate proliferation of a wide range of cell types. In Vitro Cell Dev Biol. 1992:529‐536. [DOI] [PubMed] [Google Scholar]
- 20. Jin H, Seo J, Eun SY, et al. P2Y2R activation by nucleotides promotes skin wound‐healing process. Exp Dermatol. 2014;23(7):480‐485. [DOI] [PubMed] [Google Scholar]
- 21. Kim JH, Jeong JJ, Lee YI, et al. Preventive effect of polynucleotide on post‐thyroidectomy scars: a randomized, double‐blinded, controlled trial. Lasers Surg Med. 2018;50(7):755‐762. [DOI] [PubMed] [Google Scholar]
- 22. Ohanenye C, Taliaferro S, Callender VD. Diagnosing Disorders of Facial Erythema. Dermatol Clin. 2023;41(3):377‐392. [DOI] [PubMed] [Google Scholar]
- 23. Colangelo MT, Galli C, Guizzardi S. The effects of polydeoxyribonucleotide on wound healing and tissue regeneration: a systematic review of the literature. Regener Med. 2020;15(6):1801‐1821. [DOI] [PubMed] [Google Scholar]
- 24. Belletti S, Uggeri J, Gatti R, Govoni P, Guizzardi S. Polydeoxyribonucleotide promotes cyclobutane pyrimidine dimer repair in UVB‐exposed dermal fibroblasts. Photodermatol Photoimmunol Photomed. 2007;23(6):242‐249. [DOI] [PubMed] [Google Scholar]
- 25. Müllegger RR, McHugh G, Ruthazer R, Binder B, Kerl H, Steere AC. Differential expression of cytokine mRNA in skin specimens from patients with erythema migrans or acrodermatitis chronica atrophicans. J Invest Dermatol. 2000;115(6):1115‐1123. [DOI] [PubMed] [Google Scholar]
- 26. Kim HM, Byun K‐A, Oh S, et al. A mixture of topical forms of polydeoxyribonucleotide, vitamin C, and niacinamide attenuated skin pigmentation and increased skin elasticity by modulating nuclear factor erythroid 2‐like 2. Molecules. 2022;27(4):1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bitto A, Galeano M, Squadrito F, et al. Polydeoxyribonucleotide improves angiogenesis and wound healing in experimental thermal injury. Crit Care Med. 2008;36(5):1594‐1602. [DOI] [PubMed] [Google Scholar]
- 28. Freedman BM. Topical polyphenolic antioxidants reduce the adverse effects of intense pulsed light therapy. J Cosmet Laser Ther. 2009;11(3):142‐145. [DOI] [PubMed] [Google Scholar]
- 29. Wang B, Deng Y‐x, Li P‐y, et al. Efficacy and safety of non‐insulated fractional microneedle radiofrequency for treating difficult‐to‐treat rosacea: a 48‐week, prospective, observational study. Arch Dermatol Res. 2022;314(7):643‐650. [DOI] [PubMed] [Google Scholar]
- 30. Chalikian TV, Völker J. Nucleic Acids: Hydration. Wiley Encyclopedia of Chemical Biology. 2007:1‐8. [Google Scholar]
- 31. Yu M, Lee JY. Polydeoxyribonucleotide improves wound healing of fractional laser resurfacing in rat model. J Cosmet Laser Ther. 2017;19(1):43‐48. [DOI] [PubMed] [Google Scholar]
- 32. OIKARINEN A, AUTIO P. New aspects of the mechanism of corticosteroid–induced dermal atrophy. Clin Exp Dermatol. 1991;16(6):416‐419. [DOI] [PubMed] [Google Scholar]
- 33. Kim MJ, Park HJ, Oh SM, Yi KH. Polynucleotide injection treatment for iatrogenic fat atrophy in two patients: potential for safe volumization in aesthetic medicine. Skin Res Technol. 2023;29(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gennero L, De Siena R, Denysenko T, et al. A novel composition for in vitro and in vivo regeneration of skin and connective tissues. Cell Biochem Funct. 2011;29(4):311‐333. [DOI] [PubMed] [Google Scholar]
- 35. Perez‐Aso M, Mediero A, Cronstein BN. Adenosine A2A receptor (A 2A R) is a fine‐tune regulator of the collagen1: collagen3 balance. Purinergic Signal. 2013;9:573‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim JH, Kwon T‐R, Lee SE, et al. Comparative evaluation of the effectiveness of novel hyaluronic acid‐polynucleotide complex dermal filler. Sci Rep. 2020;10(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Loyal J, Carr E, Almukhtar R, Goldman MP. Updates and best practices in the management of facial erythema. Clin Cosmet Invest Dermatol. 2021:601‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yogya Y, Wanitphakdeedecha R, Wongdama S, Nanchaipruek Y, Yan C, Rakchart S. Efficacy and safety of using noninsulated microneedle radiofrequency alone versus in combination with polynucleotides for treatment of periorbital wrinkles. Dermatol Ther. 2022;12(5):1133‐1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim YS, Hong ES, Kim HS. Botulinum toxin in the field of dermatology: novel indications. Toxins. 2017;9(12):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. De Caridi G, Massara M, Acri I, et al. Trophic effects of polynucleotides and hyaluronic acid in the healing of venous ulcers of the lower limbs: a clinical study. Int Wound J. 2016;13(5):754‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the corresponding author.


