Abstract
Background
Trastuzumab deruxtecan (T-DXd) has been shown to benefit progression-free survival and overall survival in patients with metastatic breast cancer (mBC) after progression on ≥1 human epidermal growth factor receptor 2 (HER2)-targeted therapies. However, interstitial lung disease (ILD) and cardiotoxicity are the most significant toxicities associated with T-DXd. Therefore, we conducted a systematic review and meta-analysis to assess the incidence and severity of these toxicities in mBC patients treated with T-DXd.
Materials and methods
We searched PubMed, Cochrane, and Scopus databases, and conferences websites for randomized clinical trials and nonrandomized studies of intervention including HER2-low or HER2-positive mBC patients who received at least one dose of T-DXd. Statistical analysis was carried out using R software.
Results
We included 15 studies comprising 1970 patients with a mean follow-up of 13.3 months. Median age ranged from 53 to 59 years, 61.9% were non-Asian, and 67.4% had hormone receptor-positive mBC. In a pooled analysis, the incidence of ILD was 11.7% [222 patients; 95% confidence interval (CI) 9.1% to 15.0%]. Patients receiving T-DXd dose of 6.4 mg/kg developed a significantly higher rate of ILD (22.7%) compared to those receiving a dose of 5.4 mg/kg (9.3%) (P < 0.01). Most cases of ILD (80.2%; 174/217 patients) were mild (grade 1 or 2). Grade 3 or 4 ILD was reported in 29 patients (13.4%), and grade 5 in 14 patients (6.4%). The incidence of decreased left ventricular ejection fraction (LVEF) was 1.95% (95% CI 0.65% to 3.73%), and the QT interval (QTi) prolongation was 7.77% (95% CI 2.74% to 20.11%). Most patients were asymptomatic, but four had LV dysfunction and heart failure (0.26%).
Conclusions
In this meta-analysis of 1970 patients with mBC, treatment with T-DXd was associated with a 11.7% incidence of ILD, 7.7% incidence of prolonged QTi, and 1.9% incidence of reduced LVEF. Early detection and management of T-DXd-related toxicity by a multidisciplinary team may ultimately improve patient outcomes.
Key words: breast cancer, interstitial lung disease, cardiotoxicity, trastuzumab deruxtecan, antibody-drug conjugate
Highlights
-
•
The ILD incidence rate was 11.7% in mBC patients treated with T-DXd.
-
•
ILD grades 1-2, grades 3-5, and grade 5 had an incidence of 9.4%, 0.74%, and 0.09%, respectively.
-
•
The rate of ILD with a dose of 6.4 mg/kg was significantly higher compared with 5.4 mg/kg.
-
•
The incidence of prolonged QTi and decreased LVEF was 7.7% and 1.9%, respectively, and most patients were asymptomatic.
Introduction
Recently, a new antibody-drug conjugate directed at human epidermal growth factor receptor 2 (HER2), trastuzumab deruxtecan (T-DXd), has demonstrated an unprecedented benefit in progression-free survival (PFS) and overall survival (OS) in heavily pretreated patients with metastatic breast cancer (mBC).1,2 In the DESTINY-Breast03 study, which compared T-DXd versus trastuzumab emtansine (T-DM1) in patients with HER2-positive mBC previously treated with trastuzumab and a taxane, T-DXd reduced the risk of progression by 70% and the risk of death by 36%.3,4 Due to its bystander effect, T-DXd demonstrated significant clinical benefit in patients with low-HER2 expression, categorized as HER2-low, in luminal and triple-negative tumors.5 These findings establish T-DXd as the standard of care for second-line anti-HER2 treatment and beyond. Ongoing studies are evaluating the medication in various settings, including neoadjuvant (NCT04553770), adjuvant (NCT04622319), and first-line settings (NCT04784715), as well as in combination with other agents (NCT04539938).6
Interstitial lung disease (ILD) is the most significant toxicity related to T-DXd. It is a heterogeneous group of disorders of the lung parenchyma that present as inflammation and/or fibrosis, causing various respiratory symptoms or even asymptomatic conditions.7,8 However, ILD can be severe in some cases and progress to fibrosis, pulmonary hypertension, venous thromboembolism, congestive heart failure, respiratory failure, and death.7,8 The severe cases and deaths from ILD in the initial studies of T-DXd have raised concerns about the need to better understand and manage this adverse event.1,9,10 In addition, the variation in incidence rates between the recently published studies makes it challenging to identify the actual extent of the problem.
Cardiotoxicity is a well-known and frequently observed side-effect in patients with breast cancer receiving HER-2-targeted therapy, particularly trastuzumab.11,12 In the initial studies of trastuzumab and concomitant chemotherapy, ∼25% of patients experienced a decrease of >10% in their left ventricular ejection fraction (LVEF).13 Although these events were less frequent with sequential protocols and regular monitoring of cardiac function, the cardiotoxicity of T-DXd in mBC patients remains unclear.12 Therefore, we conducted this systematic review and meta-analysis to evaluate the incidence and severity of ILD and cardiotoxicity in mBC patients treated with T-DXd.
Materials and methods
This systematic review and meta-analysis followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement guidelines.14,15 We registered the study on PROSPERO under protocol number CRD42022370504.
Search strategy and data extraction
We comprehensively searched PubMed, Cochrane Central Register of Controlled Trials, and Scopus databases, and European Society for Medical Oncology (ESMO), ESMO Breast, American Society of Clinical Oncology (ASCO), and San Antonio Breast Cancer Symposium (SABCS) conference websites for studies published in English, with the following search terms: (‘breast cancer’) AND (‘trastuzumab deruxtecan’ OR ‘trastuzumab-deruxtecan’ OR ‘DS-8201a’). The searches were carried out from inception to 25 May 2023. The references of the included studies and relevant reviews, meta-analyses, and unpublished clinical trials were evaluated for any additional studies. Three authors (LRS, MMD, and MV) independently screened the search results and extracted the data according to prespecified search criteria and quality assessment. Disagreements were resolved by consensus among the other authors.
Selection criteria
Type of studies
We searched for published manuscripts and unpublished studies that contained safety information on T-DXd therapy. Eligible studies included randomized controlled trials (RCTs) and non-RCTs in all phases of development, such as phase I, II, and III clinical trials. Given the still small number of published RCTs, we decided to include nonrandomized studies of interventions (NRSI) and real-world observational cohort studies. By including these studies, we aimed to build a robust body of evidence that would provide a more accurate estimate of the safety profile of T-DXd. We excluded reviews, case reports, case-control studies, and nonhuman studies.
Population of studies
We included patients with mBC who had received at least one dose of T-DXd, without any restrictions regarding age, histological type, or location of metastasis. Subjects with HER2-positive tumors [3+ on immunohistochemistry (IHC) analysis or positive in situ hybridization (ISH)] and HER2-low (1+ on IHC analysis or 2+ and negative results on ISH) were included. If a study had a subset of participants eligible for the meta-analysis, we included them only if the outcomes of interest for that subpopulation could be extracted. We excluded studies that focused on HER2-negative tumors or early breast cancer patients.
Intervention
The intervention of interest was the use of T-DXd at a dosage of 5.4 mg/kg or 6.4 mg/kg in the metastatic setting, regardless of the treatment line. We excluded studies investigating combination, neoadjuvant, and adjuvant therapies.
Outcomes and subanalyses
Outcomes of interest comprised ILD and cardiotoxicity, measured by decreased LVEF and the QT interval (QTi) changes. We included only studies that assessed at least one of the outcomes of interest. These adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE).16 We carried out a prespecified analysis of ILD according to CTCAE grade 1-2, grade 3-5, and grade 5 toxicity. The median time until the onset of lung disease and the median duration from the date of onset to the date of recovery were also collected.
Interstitial lung disease
ILD is a heterogeneous group of non-infectious lung diseases characterized by inflammation and fibrosis (scarring) of the lung interstitium, known as pneumonitis. The CTCAE classification categorizes ILD according to its severity and necessary intervention. Grades 1 and 2 indicate asymptomatic or mildly symptomatic patients who can be managed by observation or medical intervention. Grade 3 ILD indicates severe symptoms, while grade 4 represents life-threatening respiratory compromise requiring urgent intervention. Grade 5 refers to cases resulting in death. Patients with grades 3 and 4 of ILD typically require oxygen therapy and the administration of oral steroids or high doses of intravenous corticosteroids to reduce lung inflammation and control hypoxia.8,11,17
Cardiotoxicity
Assessment of cardiotoxicity was primarily based on the variation in LVEF during treatment. LVEF is the fraction of volume ejected during systole (stroke volume) relative to the volume of blood in the left ventricle at end-diastole (end-diastolic volume). It is the central measurement of left ventricular (LV) systolic function.18 The LVEF was categorized based on absolute numbers, the percentage of reduction, and the number of patients with reduced LVEF. We also recorded the incidence of prolonged QTi and heart failure associated with the decrease in LVEF.
Quality assessment
The methodological quality of each study was evaluated using the tool recommended by the Cochrane Collaboration Handbook.14 We assessed the risk of bias for NRSI using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool.19 For RCTs, we used the Cochrane Risk of Bias 2 Tool (RoB 2).20 Two independent reviewers (LRS and MMdD) carried out this assessment, and disagreements were resolved by consensus between the authors after reviewing the full article.
Statistical analysis
This was a single-arm proportion meta-analysis investigating the safety of T-DXd. Statistical analysis was carried out using R software and RStudio (version 2022.12.0+353; R Core Team, Viena, Austria). Random-effects modeling was used for analysis, assuming that the data come from varied populations with different distributions. DerSimonian and Laird’s random-effects model was employed to calculate the combined proportion of each AE and 95% confidence interval (CI). The results were presented as pooled analysis in forest plots.
We carried out prespecified subgroup analyses according to the dose given in the study (5.4 versus 6.4 mg/kg), the study type (phase III, II, and I clinical trial, and observational cohort studies), and the HER2 status (HER2-positive versus HER2-low). The comparison between different treatment groups was outside the scope of this study.
We used the Cochrane Q chi-square test and I2 statistic to examine heterogeneity across studies; P values <0.10 and I2 >30% were considered significant for heterogeneity. Leave-one-out sensitivity analysis was carried out by removing each study one at a time and recalculating the study results. Funnel plot analysis of point estimates based on study weights and a regression test for funnel plot asymmetry (Egger’s test) were used to explore publication bias.
Results
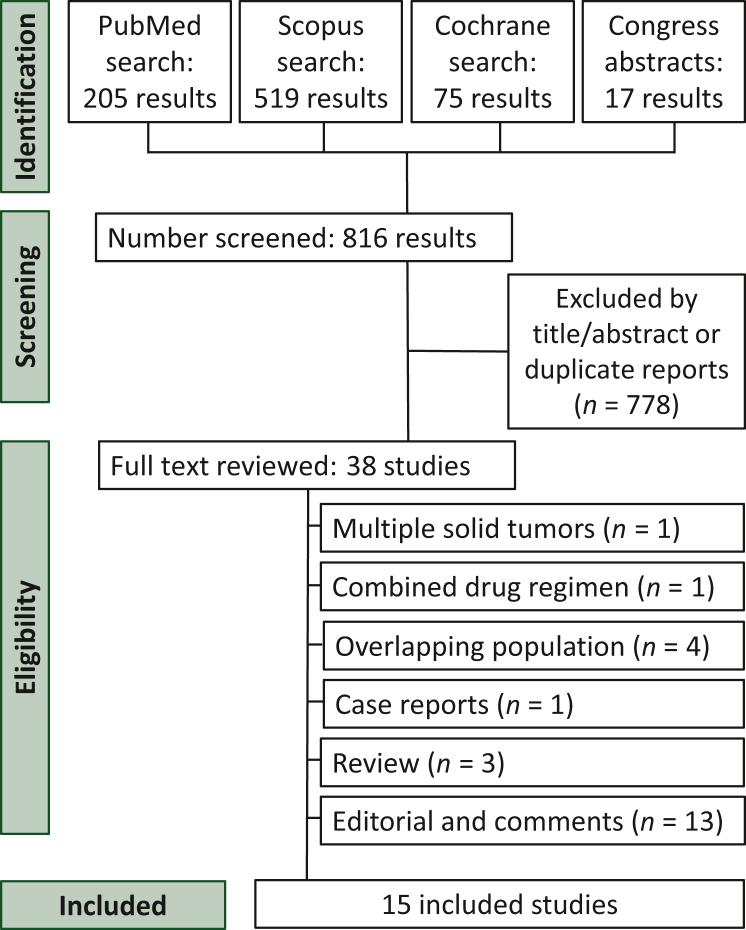
Our initial search found a total of 816 studies. After screening the titles and abstracts, we excluded 778 studies. Twenty-three studies were eliminated after reading the full text (Figure 1). Finally, this systematic review and meta-analysis included 15 studies involving 1970 patients with mBC. The characteristics of the included studies are described in Table 1.1, 10, 12, 2, 21, 22, 23, 24, 25, 26, 27, 28, 3, 4, 5,9,10,12,21, 22, 23, 24, 25, 26, 27, 28
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram of study screening and selection. The search strategy in PubMed, Scopus, and Cochrane databases, as well as conference websites, yielded 816 studies, of which 38 were fully reviewed based on the inclusion and exclusion criteria. A total of 15 studies were included in the meta-analysis.
Table 1.
Baseline characteristics of included studies
| Study | Design | n | Population | Intervention | Dose, mg/kg | Primary endpoint | Median follow-up, months (range) |
|---|---|---|---|---|---|---|---|
| DESTINY-01, 2019 | Non-RCT, phase II | 184 | HER2+, after T-DM1 | T-DXda | 5.4 | PFS | 11.1 (0.7-19.9) |
| DESTINY-02, 2022 | RCT, phase III | 608 | HER2+, after T-DM1 | T-DXd versus TPC | 5.4 | PFS | T-DXd: 21.5 (0.1-45.6); TPC: 18.6 (0-45.7) |
| DESTINY-03, 2022 | RCT, phase III | 524 | HER2+, after T&T | T-DXd versus T-DM1 | 5.4 | PFS | T-DXd: 28.4 (0-46.9); T-DM1: 26.5 (0-45.0) |
| DESTINY-04, 2022 | RCT, phase III | 713 | HER2-low, >1 line | T-DXd versus PCC | 5.4 | PFS | 18.4 (17.7-18.9) |
| DESTINY-07, 2022 | Non-RCT, phase Ib/II | 55 | HER2+, 1st line | T-DXd versus T-DXd + P | 5.4 | Safety and tolerability | 10.0 (NA) |
| TUXEDO-1, 2022 | Non-RCT, phase II | 15 | HER2+, with brain metastasis | T-DXda | 5.4 | ORR-IC | 12 (8-NA) |
| DEBBRAH, 2022 | Non-RCT, phase II | 21 | HER2+, with brain metastasis | T-DXdb | 5.4 | 16 weeks PFS, cohort 1/ORR-IC, cohorts 2 and 3 | 8.4 (1.4-12.6) |
| DAISY, 2021 | Non-RCT, phase II | 186 | HER2+, HER2-low and HER2−, >1 line | T-DXda | 5.4 | BOR | 10.1 (9.2-11.1) |
| Modi et al., 20209 | Non-RCT, phase I | 54 | HER2-low, >1 line | T-DXda | 5.4 (n = 21) 6.4 (n = 33) |
Safety and preliminary activity | NA |
| Shimomura et al., 202312 | Non-RCT, phase I | 51 | 92.2% HER2-low, >2 lines | T-DXda | 6.4 | QT/QTc interval and pharmacokinetics | NA |
| Tamura et al., 201910 | Non-RCT, phase I | 274 | HER2+, after T-DM1 | T-DXda | 5.4 (n = 49) 6.4 (n = 66) |
Safety and preliminary activity | 9.9 (6.9-14.3) |
| DE-REAL, 2023 | Retrospective cohort | 143 | HER2+ | T-DXda | 5.4 | PFS | 12 (1-31) |
| Nakajima et al., 202226 | Retrospective cohort | 22 | HER2+ | T-DXda | NA | PFS | 10.1 (8.4-12.0) |
| TREX-Old, 2023 | Retrospective cohort | 27 | HER2+ | T-DXda | 5.4 | PFS | 9.5 (1-29) |
| ROSET-BM, 2022 | Retrospective cohort | 104 | HER2+ | T-DXda | 5.4 | PFS | 11.2 |
BOR, best overall response; HER2, human epidermal growth factor receptor 2; n, number of patients; NA, not available; ORR-IC, intracranial objective response rate; P, pertuzumab; PCC, physician’s choice of chemotherapy; PFS, progression-free survival; RCT, randomized controlled trial; T-DXd, trastuzumab deruxtecan; T&T, trastuzumab and a taxane; TPC, treatment of physician’s choice.
Single-arm study.
Multicohort study.
Most of the studies included patients with a median age of 53-59 years, non-Asian origin (61.9%), and hormone receptor-positive tumors (67.4%). The proportion of HER2-low patients was 27.8%. The mean follow-up time across the studies was 13.3 months, ranging from 8.4 to 28.4 months. Table 2 details the baseline characteristics of patients who received T-DXd.
Table 2.
Baseline characteristics of patients treated with T-DXd
| Study | n | Period of enrollment | Age, years, median (range) | Asian, n (%) | HR+, n (%) | Metastasis | ECOG 0, n (%) |
|---|---|---|---|---|---|---|---|
| DESTINY-01, 2019 | 184 | 2017-2018 | 55.0 (28.0-96.0) | 63 (34.2) | 97 (52.7) | Visceral 91.8%/CNS 13.0% | 102 (55.4) |
| DESTINY-02, 2022 | 406 | 2018-2020a | 54.2 (22.4-88.5) | 112 (27.6) | 238 (58.6) | Visceral 77.8%/CNS 18.2% | 228 (56.2) |
| DESTINY-03, 2022 | 261 | 2018-2020 | 54.3 (27.9-83.1) | 152 (58.2) | 131 (50.2) | Visceral 70.4%/CNS 16.4% | 154 (59.0) |
| DESTINY-04, 2022 | 374 | 2019b-2021 | 57.5 (31.5-80.2) | 151 (40.5) | 333 (89.3) | Liver 71.3%/lung 32.2%/CNS 6.4% | 200 (53.6) |
| DESTINY-07, 2022 | 23 | 2020c-2023d | NA | NA | NA | NA | NA |
| TUXEDO-1, 2022 | 15 | 2020-2021 | 69 (30-76) | 0 (0) | 12 (80) | Visceral 80.0%/CNS 100.0% | 9 (60.0) |
| DEBBRAH, 2022 | 21 | 2020-2021 | 53.0 (36.0-77.0) | 0 (0) | 16 (76.2) | Non-CNS 76.2%/CNS 100% | 15 (71.4) |
| DAISY, 2021 | 179 | 2019-2021 | 55 (24-82) | 0 (0) | NA | NA | NA |
| Modi et al., 20209 | 54 | 2016-2018 | 56.6 (33-75) | 27 (50.0) | 47 (87.0) | Bone 63%/visceral 100%/CNS 9.3% | 36 (66.7) |
| Shimomura et al., 202312 | 51 | 2018e | 56 (31-79) | 51 (100) | 43 (84.3) | NA | 31 (60.8) |
| Tamura et al., 201910 | 115 | 2015-2018 | 55.0 (47.0-66.0) | 62 (54.0) | 81 (70) | NA | 72 (63) |
| DE-REAL, 2023 | 143 | 2020-2023 | 66 (33-84) | 0 (0) | 108 (75) | NA | NA |
| Nakajima et al., 202226 | 22 | 2020-2021 | 59.5 (42-78) | 22 (100) | 15 (68.2) | Bone 68.2%/CNS 40.9% | 6 (27.3) |
| TREX-Old, 2023 | 27 | 2021-2023 | 74 (70-81) | 0 (0) | NA | Visceral 70.0% | 6 (22) |
| ROSET-BM, 2022 | 104 | 2015-2021 | NAf | 104 (100) | 59 (56.7) | Visceral 76.0%/CNS 100% | 27 (26.0) |
CNS, central nervous system; ECOG, Eastern Cooperative Oncology Group; HR+, hormone receptor positive; NA, not available; T-DXd, trastuzumab deruxtecan.
The recruitment was completed on 7 January 2021 (https://clinicaltrials.gov/ct2/show/NCT03523585).
The first patient was enrolled on 27 December 2018.
The first patient was enrolled on 28 December 2020.
The recruitment is ongoing.
The first patient was enrolled on 26 December 2017.
Twenty-nine patients (27.9%) with age ≥65 years.
ILD
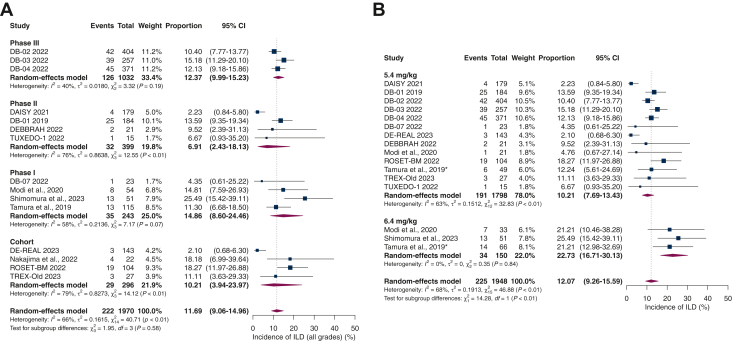
Of the 1970 patients included in the safety analysis of the studies, 222 cases of ILD were recorded. A pooled analysis revealed an overall ILD incidence rate of 11.7% (95% CI 9.06% to 14.96; I2 66%). When stratified by study type, the estimated incidence varied between subgroups. We found an ILD rate of 12.4% among the phase III trials (95% CI 9.99% to 15.23; I2 40%), 6.91% in the phase II trials (95% CI 2.43% to 18.13%; I2 76%), 14.86% in the phase I trials (95% CI 8.60% to 24.46%; I2 58%), and 10.21% in the observational cohort study group (95% CI 3.94% to 23.97%; I2 79%), as depicted in Figure 2A.
Figure 2.
(A) Overall incidence of interstitial lung disease (ILD) (all grades) and ILD stratified by study type. (B) Subgroup analysis according to administered dosing of trastuzumab deruxtecan (T-DXd) revealed a significantly higher rate of ILD for 6.4 mg/kg compared to 5.4 mg/kg (22.7% versus 10.2%; P < 0.01). CI, confidence interval.
Then, we conducted a subgroup analysis aiming to investigate the rates of ILD according to the administered doses of T-DXd (Figure 2B). Patients who received T-DXd at a dose of 6.4 mg/kg developed a significantly higher ILD rate of 22.7% compared to 10.2% among those with a T-DXd dose of 5.4 mg/kg (P < 0.01, test for subgroup analysis differences).
Out of the cases of ILD, 80.2% (174/217 patients) were categorized as grade 1 or 2 and treated with supportive medical care, glucocorticoids, and/or discontinuation of T-DXd. Additionally, 13.4% (29) of cases were classified as grade 3 or 4, and 6.4% (14) were classified as grade 5. The incidence rates of ILD grades 1-2, 3-5, and 5 were 9.44%, 0.74%, and 0.09%, respectively (Supplementary Figure S1A-C, available at https://doi.org/10.1016/j.esmoop.2023.101613). Most studies confirmed the possible cases of ILD through an independent external analysis and established clear criteria for diagnosis and patient management (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101613).
According to a pooled analysis of five studies,1,2,4,5,10 the estimated median time for the onset of lung disease was 209.5 days (95% CI 129-251.5 days). The median duration from onset to recovery was 34 days, ranging from 3 to 179 days, which was reported only by the DB-01 study.1
Cardiotoxicity
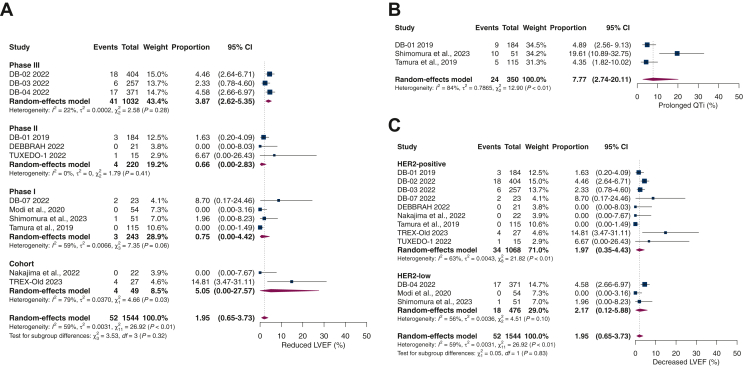
The overall incidence of reduction in LVEF was 1.95% (95% CI 0.65% to 3.73%; I2 59%). Among the patients in the phase III RCTs (67% of the population), the rate of decreased LVEF was 3.9%, which was significantly higher compared to those in phase II (1.9%) and phase I trials (0.1%). The observational cohort studies group represented only 3% of the population and had the highest incidence of reduced LVEF (5.0%). We found a significant difference in this subgroup analysis by study type (P = 0.02), which may be attributed to some specific population features or selection bias in the nonrandomized small-sized studies (Figure 3A).
Figure 3.
(A) Overall incidence of reduced left ventricular ejection fraction (LVEF) and subgroups by study type. (B) Incidence of prolonged QT interval (QTi). (C) Reduction in LVEF in human epidermal growth factor receptor 2 (HER2)-positive versus HER2-low metastatic breast cancer patients (P = 0.83). CI, confidence interval.
The incidence of prolonged QTi was 7.77% (95% CI 2.74% to 20.11%; I2 84%; Figure 3B). Three studies reported a total of 24 cases out of 350 patients. Among these cases, 22 were classified as grades 1 and 2, and 2 as grade 3. Most patients with cardiac toxicity were asymptomatic, but four had LV dysfunction and heart failure (0.26%). Two patients experienced cardiac failure in the DB-02 study and another two in the DB-04 study; half of these cases were grade 3.2,5 Additional information regarding cardiotoxicity can be found in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.101613.
Subgroup analysis of reduced LVEF comparing HER2-positive with HER2-low patients revealed no significant differences. Among 1068 HER2-positive patients, 34 developed cardiac toxicity, yielding an incidence rate of 1.97% (95% CI 0.35% to 4.43%; I2 63%). In the HER2-low group, 18 of 476 patients had a decrease in LVEF, with an incidence rate of 2.17 (95% CI 0.12-5.88; I2 56%), as demonstrated in Figure 3C.
Quality assessment
Most published studies were categorized as having a low or moderate risk of bias (Supplementary Tables S3 and S4, available at https://doi.org/10.1016/j.esmoop.2023.101613). Two RCTs were considered at low risk of bias (DB-03 and DB-04). The risk of bias for nonrandomized studies was considered moderate for all included studies. Regarding the unpublished studies, including abstracts and conference presentations, the risk of bias was considered high for retrospective studies, likely due to missing or incomplete information at the time of assessment (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2023.101613). DB-02 had some concerns due to incomplete information about the randomization process.2
Considering the high heterogeneity in our results, we carried out a sensitivity analysis according to published and unpublished studies (Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2023.101613). We also conducted leave-one-out sensitivity tests by systematically removing each study from the pooled estimates of our primary outcomes. It did not substantially alter the results for ILD or cardiotoxicity analyses (Supplementary Figure S3A and B, available at https://doi.org/10.1016/j.esmoop.2023.101613).
The visual inspection of the funnel plot with all studies (ILD analysis) indicated a likely asymmetry toward the left side, and the regression (Egger’s) test was statistically significant (P = 0.036; Supplementary Figure S4A, available at https://doi.org/10.1016/j.esmoop.2023.101613). We hypothesized that this significant asymmetry might be related to (i) the different doses of T-DXd among studies; for example, in Shimomura et al. 2023,12 patients were treated with a high dose of 6.5 mg/kg, which yielded a higher rate of ILD compared with other studies; (ii) T-DXd is a relatively new medication; hence after finding the ideal dose and learning about its side-effects, clinicians are becoming more familiar with identifying the important toxicities early and dealing with dose reductions and AEs. Consequently, the ILD rate may decrease in future studies and contribute to a shift toward the left side of the funnel plot. Additionally, this could represent a publication bias due to studies not yet published.
The funnel plot of the reduced LVEF analysis showed a predominantly symmetrical distribution of studies with similar weights around the central pooled estimate. The regression test for funnel plot asymmetry (Egger’s test) did not reveal a statistically significant result (P = 0.464), indicating no evidence suggestive of significant publication bias (Supplementary Figure S4B, available at https://doi.org/10.1016/j.esmoop.2023.101613).
Discussion
Our systematic review and meta-analysis comprehensively analyzed the safety of T-DXd therapy in mBC patients. We included data from 15 studies with 1970 patients and conducted a focused assessment of two significant AEs associated with T-DXd: ILD and decreased LVEF. Our main findings were: (i) the overall incidence of ILD was 11.7%, which was significantly higher in patients receiving T-DXd at a dose of 6.4 mg/kg (22.7%) compared to 5.4 mg/kg (10.2%). Moreover, the majority (80%) of ILD cases were mild, with ∼20% being serious AEs. The incidence rates of grades 1 and 2 ILD were 9.4%, and for grades 3 and 4, 0.74%, with a mortality rate of 0.09%. (ii) We observed that 1.95% of patients treated with T-DXd experienced a reduction in LVEF, while 7.7% had a prolonged QTi, with most cases being mild and asymptomatic. HER2-positive and HER2-low patients had a similar rate of reduced LVEF. LV dysfunction and heart failure occurred in 0.26% of patients.
ILD, also known as pneumonitis, is a serious side-effect that can occur in patients receiving T-DXd, and it has been associated with a higher risk of death than other adverse effects.1,29 Lung opacity, typically presenting as consolidations in the subpleural areas, is the most common chest imaging finding in T-DXd-induced ILD. Even if asymptomatic, these findings in patients undergoing T-DXd treatment should raise suspicion of drug-induced ILD.30
In the phase II DESTINY-Breast01 study,1 the ILD rate was 13.6%, similar to the phase I studies.9,10 Four deaths (2.1%) ILD-related were initially reported as respiratory failure (two cases), lymphangitis (one case), and pneumonitis (one case), highlighting the difficulty of etiological diagnosis and the need for standardization of radiological findings.
In the DESTINY-Breast03 trial, which compared T-DXd with T-DM1 in the second line or later, a 15.2% rate of ILD was observed.4 However, unlike other trials, DB-03 had no grade 4 or 5 ILD/pneumonitis events, which suggests that the growing experience of investigators has reduced the incidence of serious AEs over time.
Similar to our results, the phase I study by Tamura et al. showed that the proportion of ILD was lower for those who received 5.4 mg/kg dose than for those who received 6.4 mg/kg dose, favoring the choice of the lowest dose (5.4 mg/kg) in clinical practice.10 In a previous meta-analysis including individual data from nine studies using T-DXd to treat patients with breast cancer and other sites, several factors were potentially related to ILD, such as age younger than 65 years, recruitment in Japan, a dose of >6.4 mg/kg, and the presence of pulmonary comorbidities.31
Several guidelines have been published in recent years to provide clinical guidance for the early diagnosis, monitoring, and management of T-DXd-related pneumonitis for clinicians and clinical research.7,32, 33, 34 These guidelines emphasize the importance of closely monitoring signs and symptoms during the administration of T-DXd and provide orientations for the use of corticosteroid therapy to reduce the risk of fatal outcomes.7,32, 33, 34 Although most cases of ILD were mild, 14 patients (0.09%) died from T-DXd-related pneumonitis in this meta-analysis.
As reported in five studies, most ILD episodes occur within the first 12 months of exposure. After 12 and 18 months, the risk of late-onset disease decreases to 7.0% and 1.4%, respectively.31 However, DB-03 demonstrated a rising incidence of ILD with increasing follow-up time, from 10.5% at 16.2 months3 to 15.2% at 28.4 months.4 Although ILD is more common in the first 12 months of exposure, these data highlight the importance of constant surveillance, monitoring for late toxicities, and updating long-term safety data.31
Even though the pathogenic mechanisms are not yet fully understood, there are accepted theories about T-DXd’s immunogenic effect or direct toxic action on endothelial and epithelial cells, causing an acute inflammatory reaction and lung injury.7,8 Animal models have identified T-DXd in alveolar macrophages rather than lung epithelial cells, suggesting a target-independent effect of T-DXd.7,35 In the future, a better understanding of pathogenic factors, new technologies for early diagnosis, and advances in targeted treatment may reduce ILD rates in T-DXd patients.
Our pooled analysis revealed a low incidence of cardiotoxicity with T-DXd, with an incidence rate of 1.95% of LVEF reduction (mostly asymptomatic cases). However, it is essential to carry out a comprehensive assessment of cardiovascular risk factors before treatment and monitor cardiac function periodically during treatment and as needed based on new signs and symptoms.11 In asymptomatic patients with LVEF between 40% and 50%, treatment with T-DXd requires rigorous cardiac monitoring and interruption of medication in case of an LVEF reduction >10%.36
Another cardiac outcome assessed was QTi prolongation, which can occur with various anticancer medications. Although it was reported in only three studies, its combined incidence was low, in agreement with a pharmacokinetic study using T-DXd at a dose of 6.4 mg/kg.12 Additionally, the predominance of mild and asymptomatic cases reduces the clinical significance of these instances, given that they typically do not necessitate specific treatment or the discontinuation of T-DXd.12 Furthermore, the low occurrence of heart failure supports the cardiological safety profile of the new anti-HER2 therapies.
The DESTINY-Breast02 trial evaluated mBC patients who had progressed while on or after T-DM1. The safety profile was consistent with the previous phase III trials. LVEF dysfunction was observed in 4.5% of patients, with only two (0.5%) patients experiencing grade ≥3 events.2 Phase III RCTs demonstrated significant benefits of T-DXd, including prolonged OS, for patients with mBC. Following the robust evidence from these large trials, T-DXd has been approved in the United States, Europe, and several other countries worldwide.17,37
One observational retrospective cohort study in our meta-analysis evaluated patients who received T-DXd after disease progression on trastuzumab, pertuzumab, and T-DM1. This study reported a high rate of ILD of 18.2%.26 However, all patients recovered from drug-related ILD, with no treatment-related deaths. Cardiac toxicity was also assessed, and none of the patients had a treatment-related decrease in LVEF. Interestingly, one patient with an LVEF of 41% received T-DXd with close monitoring and remained on treatment without cardiotoxicity.26 The real-world studies in our meta-analysis support the safety profile of T-DXd found in clinical trials.
Our study has limitations. Firstly, including unpublished, observational cohorts, small-sized, and early-phase studies could introduce selection bias into our meta-analysis. However, considering the methodological quality of most studies and the relatively small number of RCTs published so far, these smaller studies have contributed greatly to building robust and consistent evidence about the safety of T-DXd. Nonetheless, further off-trial data, such as real-world cohort studies, are necessary to provide additional evidence about T-DXd safety in unselected patients.
Additionally, while all published studies reported ILD according to the CTCAE classification, none presented data on toxicities by subgroups of interest, such as age, ethnicity, or previous cardiac or pulmonary comorbidities. Because we did not have individualized patient data, we could not carry out subgroup analysis to assess risk factors for ILD and cardiotoxicity.
Conclusion
Our comprehensive systematic review and meta-analysis support the safety of T-DXd as a treatment option for mBC patients. The overall incidence of cardiac toxicity was low, and most cases were asymptomatic. Although ILD occurred in 11.7% of patients, most of the cases were mild and effectively managed. Notably, the 5.4 mg/kg dosing of T-DXd was significantly safer than the 6.4 mg/kg dosing. However, our analysis revealed that severe ILD-related toxicity, including grade 5, can occur.
These findings highlight the importance of closely monitoring patients receiving T-DXd therapy for early diagnosis, prompt management, and potential treatment interruption if necessary. We recommend that a multidisciplinary team manages patients according to the most recent guidelines to improve outcomes and prevent ILD-related deaths. Further research, including real-world cohort studies, will help to enhance our understanding of T-DXd safety in diverse patient populations.
Acknowledgements
The authors would like to thank Rhanderson Cardoso, MD, for his support in conducting this research. Medical writing and editorial support were provided by Maio Consultoria e Assessoria Medica e Cientifica Ltd. and funding for publication was provided by Daiichi Sankyo Co., Ltd. We are grateful to Daiichi Sankyo Co., Ltd for academic support. All authors take responsibility for the reliability, freedom from bias, and interpretation of the data presented.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors. However, after completion of the research, Daiichi Sankyo Co. Ltd. supported the editorial process without any participation or interference in the content of the paper.
Disclosure
The authors have declared no conflicts of interest.
Data Sharing
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Supplementary data
References
- 1.Modi S., Saura C., Yamashita T., et al. DESTINY-Breast01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krop I, Park YH, Kim SB, et al. GS2-01 Trastuzumab deruxtecan vs physician’s choice in patients with HER2+ unresectable and/or metastatic breast cancer previously treated with trastuzumab emtansine: primary results of the randomized, phase 3 study DESTINY-Breast02 [abstract]. In: Proceedings of the 2022 San Antonio Breast Cancer Symposium. December 6-10, 2022; San Antonio, TX. Philadelphia, PA: AACR; Cancer Res. 2023;83(suppl 5):Abstract nr GS2-01.
- 3.Cortés J., Kim S.B., Chung W.P., et al. DESTINY-Breast03 Trial Investigators. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 4.Hurvitz S.A., Hegg R., Chung W.P., et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105–117. doi: 10.1016/S0140-6736(22)02420-5. [DOI] [PubMed] [Google Scholar]
- 5.Modi S., Jacot W., Yamashita T., et al. DESTINY-Breast04 Trial Investigators. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trastuzumab deruxtecan clinical trials. ClinicalTrials.gov [cited 2023 Feb 22]. Available at https://clinicaltrials.gov/search?term=trastuzumab%20deruxtecan&aggFilters=status. Accessed July 15, 2023.
- 7.Swain S.M., Nishino M., Lancaster L.H., et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)-related interstitial lung disease/pneumonitis-focus on proactive monitoring, diagnosis, and management. Cancer Treat Rev. 2022;106 doi: 10.1016/j.ctrv.2022.102378. [DOI] [PubMed] [Google Scholar]
- 8.Conte P., Ascierto P.A., Patelli G., et al. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. ESMO Open. 2022;7(2) doi: 10.1016/j.esmoop.2022.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modi S., Park H., Murthy R.K., et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38(17):1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura K., Tsurutani J., Takahashi S., et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):816–826. doi: 10.1016/S1470-2045(19)30097-X. [DOI] [PubMed] [Google Scholar]
- 11.Hackshaw M.D., Danysh H.E., Singh J., et al. Incidence of pneumonitis/interstitial lung disease induced by HER2-targeting therapy for HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2020;183(1):23–39. doi: 10.1007/s10549-020-05754-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimomura A., Takano T., Takahashi S., et al. Effect of trastuzumab deruxtecan on QT/QTc interval and pharmacokinetics in HER2-positive or HER2-low metastatic/unresectable breast cancer. Clin Pharmacol Ther. 2023;113(1):160–169. doi: 10.1002/cpt.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buzdar A.U., Suman V.J., Meric-Bernstam F., et al. American College of Surgeons Oncology Group investigators. Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14(13):1317–1325. doi: 10.1016/S1470-2045(13)70502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J., Thomas J., Chandler J., et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; Chichester (UK): 2019. [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G., Prisma Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version. 5.0.p.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf Available at.
- 17.Perez J., Garrigós L., Gion M., et al. Trastuzumab deruxtecan in HER2-positive metastatic breast cancer and beyond. Expert Opin Biol Ther. 2021;21(7):811–824. doi: 10.1080/14712598.2021.1890710. [DOI] [PubMed] [Google Scholar]
- 18.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Sterne J.A., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 21.Diéras V, Deluche E, Lusque A, et al. Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: a phase II study with biomarkers analysis (DAISY) [abstract]. In: Proceedings of the 2021 San Antonio Breast Cancer Symposium. December 7-10, 2021; San Antonio, TX. Philadelphia, PA: AACR; Cancer Res. 2022;82(suppl 4):Abstract nr PD8-02.
- 22.Bartsch R., Berghoff A.S., Furtner J., et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022;28(9):1840–1847. doi: 10.1038/s41591-022-01935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez-García J.M., Batista M.V., Cortez P., et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro Oncol. 2023;25(1):157–166. doi: 10.1093/neuonc/noac144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton E, Jhaveri K, Loi S, et al. Dose-expansion study of trastuzumab deruxtecan as monotherapy or combined with pertuzumab in patients with metastatic human epidermal growth factor receptor 2-positive (HER2+) breast cancer in DESTINY-Breast07 (DB-07) [abstract]. In: Proceedings of the 2022 San Antonio Breast Cancer Symposium. December 6-10, 2022; San Antonio, TX. Philadelphia, PA: AACR; Cancer Res. 2023;83(suppl 5):Abstract nr PD18-11.
- 25.Botticelli A., Pisegna S., Scagnoli S., et al. 236P Trastuzumab deruxtecan in Italian real-world experience: updated analysis from DE-REAL study. ESMO Open. 2023;8(suppl 4) [Google Scholar]
- 26.Nakajima H., Harano K., Nakai T., et al. Impacts of clinicopathological factors on efficacy of trastuzumab deruxtecan in patients with HER2-positive metastatic breast cancer. Breast. 2022;61:136–144. doi: 10.1016/j.breast.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buono G., Deleuze A., Klocker E.V., et al. 237P Real-world safety and efficacy of trastuzumab-deruxtecan (T-DXd) in HER2-positive advanced breast cancer (ABC) elderly patients (pts): the TREX-Old retrospective registry. ESMO Open. 2023;8(suppl 4) [Google Scholar]
- 28.Yamanaka T, Niikura N, Nomura H, et al. Trastuzumab deruxtecan for the treatment of patients with HER2-positive breast cancer with brain and/or leptomeningeal metastases: a multicenter retrospective study (ROSET-BM study) [abstract]. In: Proceedings of the 2022 San Antonio Breast Cancer Symposium. December 6-10, 2022; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res. 2023;83(suppl 5):Abstract nr PD7-01.
- 29.Tsurutani J., Iwata H., Krop I., et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 2020;10(5):688–701. doi: 10.1158/2159-8290.CD-19-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang Y.J., Jeong J.H., Kim J.E., Ahn J.H., Jung K.H., Kim S.B. CT findings from interstitial lung diseases in patients with metastatic breast cancer treated with fam-trastuzumab deruxtecan: a single institutional experience. J Breast Cancer. 2022;25(1):49–56. doi: 10.4048/jbc.2022.25.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powell C.A., Modi S., Iwata H., et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open. 2022;7(4) doi: 10.1016/j.esmoop.2022.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kubo K., Azuma A., Kanazawa M., et al. Consensus statement for the diagnosis and treatment of drug-induced lung injuries. Respir Investig. 2013;51:260–277. doi: 10.1016/j.resinv.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Tarantino P., Modi S., Tolaney S.M., et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: a review. JAMA Oncol. 2021;7(12):1873–1881. doi: 10.1001/jamaoncol.2021.3595. [DOI] [PubMed] [Google Scholar]
- 34.Rugo H.S., Bianchini G., Cortes J., Henning J.W., Untch M. Optimizing treatment management of trastuzumab deruxtecan in clinical practice of breast cancer. ESMO Open. 2022;7(4) doi: 10.1016/j.esmoop.2022.100553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumagai K., Aida T., Tsuchiya Y., Kishino Y., Kai K., Mori K. Interstitial pneumonitis related to trastuzumab deruxtecan, a human epidermal growth factor receptor 2-targeting Ab-drug conjugate, in monkeys. Cancer Sci. 2020;111(12):4636–4645. doi: 10.1111/cas.14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ENHERTU® (fam-trastuzumab deruxtecan-nxki) for Injection, for Intravenous Use: Highlights of Prescribing Information [package insert]. Wilmington, DE: AstraZeneca; 2019. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761139s000lbl.pdf Available at.
- 37.Narayan P., Osgood C.L., Singh H., et al. FDA approval summary: fam-trastuzumab deruxtecan-nxki for the treatment of unresectable or metastatic HER2-positive breast cancer. Clin Cancer Res. 2021;27(16):4478–4485. doi: 10.1158/1078-0432.CCR-20-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.