Abstract
Background
Almost 100 novel cancer medicines have been approved in Europe over the last decade. Limited public health care resources in countries in Central and Eastern Europe (CEE) call for a prioritization of access to effective medicines. We investigated how both reimbursement status and waiting time to reimbursement correlate with the magnitude of clinical benefit provided by novel medicines in four selected countries (Czechia, Hungary, Poland, and Slovakia).
Materials and methods
A total of 124 indications of 51 cancer medicines with marketing authorization by the European Medicines Agency in 2011-2020 were included and followed up until 2022. Data on reimbursement status and waiting time to reimbursement (i.e. time from marketing authorization to national reimbursement approval) were collected for each country. Data were analyzed in relation to clinical benefit status (i.e. substantial versus nonsubstantial clinical benefit) of indications according to the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS).
Results
The degree of reimbursement differed between countries with 64% of indications with reimbursement in Czechia, 40% in Hungary, 51% in Poland, and 19% in Slovakia. In all countries, a significantly greater proportion of indications with a substantial clinical benefit was reimbursed (P < 0.05). The median waiting time to reimbursement ranged from 27 months in Poland to 37 months in Hungary. No significant differences in waiting time in relation to clinical benefit were observed in any country (P = 0.25-0.84).
Conclusions
Cancer medicines with a substantial clinical benefit are more likely to be reimbursed in all four CEE countries. Waiting times to reimbursement are equally long for medicines with or without a substantial clinical benefit, indicating a lack of prioritization of fast access to medicines delivering a substantial benefit. Incorporation of the ESMO-MCBS in reimbursement assessments and decisions could aid in better utilization of limited resources to deliver more effective cancer care.
Key words: cancer medicines, ESMO-MCBS, reimbursement decision, drug access, Central and Eastern Europe
Highlights
-
•
Limited public health care resources call for a prioritization of access to effective cancer medicines.
-
•
The degree of reimbursement of newer cancer medicines differs greatly between four countries in Central and Eastern Europe.
-
•
In all countries, a significantly greater proportion of indications with substantial ESMO-MCBS was reimbursed.
-
•
No significant differences in time to reimbursement from EMA approval in relation to ESMO-MCBS was observed in any country.
-
•
Incorporation of the ESMO-MCBS in reimbursement assessments and decisions could aid in better utilization of resources.
Introduction
The incidence of cancer is rising in Europe, mainly driven by population aging and unhealthy lifestyles.1 In 2020, an estimated 2.7 million new cancer cases were diagnosed and 1.3 million people died from cancer in the European Union (EU),2 making cancer the second leading cause of death.3 Despite improvements in survival rates of cancer in the past decades,4 the high number of deaths indicates a considerable unmet need for better treatment. Cancer medicines are an integral part of modern cancer care and patient access to effective medicines is crucial to achieve better health outcomes.5, 6, 7
The approval of novel cancer medicines by the European Medicines Agency (EMA) has accelerated in recent decades and reached almost 100 new medicines in the last decade.1 In addition to new medicines, new indications of previously approved medicines may become approved—in 2020, there were 17 such new indications on top of 10 new medicines.8 Granting access to all novel medicines/indications is increasingly challenging because cancer medicine expenditure has been rising considerably in Europe in the last decade.9 Furthermore, not all novel medicines/indications offer the same clinical benefit over the existing standard of care. The increasing number of medicines with varying clinical benefit challenges the financial sustainability of health care systems and calls for a prioritization of effective medicines, especially in countries with limited economic resources.10,11
Value frameworks, such as the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) launched in 2015, have been developed in response to the increasing number of cancer medicines.12 The ESMO-MCBS provides a scale of relative magnitude of clinical benefit that can be anticipated from a new treatment indication for solid tumors based on data derived from pivotal clinical trials or meta-analyses. The scale has been proposed by ESMO to be used as a tool for evaluating value in order to support the process of prioritization of access to cancer medicines by national health authorities when resources are constrained.12,13
Despite the central decision of marketing authorization (MA) of novel cancer medicines by the EMA in the EU,14 decisions regarding reimbursement approval are taken by public health care payers at the member state level. Therefore access to novel medicines through public health systems can differ considerably between European countries. Previous studies and reports have indicated worse access to novel cancer medicines in Central and Eastern Europe (CEE) compared with Northern and Western Europe, both in terms of availability and time from EMA MA to national reimbursement approval (NRA).1,15, 16, 17 There is, however, a lack of comparative studies evaluating access to cancer medicines in CEE at the level of indications rather than medicines as well as analyses with large samples and long follow-up time. Furthermore, multicountry studies examining the access situation in relation to the clinical benefit of medicines/indications in CEE are absent.
The aim of this study was to investigate how access to novel cancer medicines relates to clinical benefit in four selected countries in CEE (Czechia, Hungary, Poland, and Slovakia). For this purpose, we assessed reimbursement status and time to reimbursement (i.e. time from EMA MA to NRA) in relation to magnitude of clinical benefit (i.e. ESMO-MCBS) of novel cancer medicine indications in each country.
Materials and methods
Sample selection
This study covers four countries in CEE, namely, Czechia, Hungary, Poland, and Slovakia. They were selected based on closeness both from a political perspective (EU member states and working together in the Visegrád Group) and from an economic perspective [similar levels of economic wealth in terms of gross domestic product (GDP) per capita].
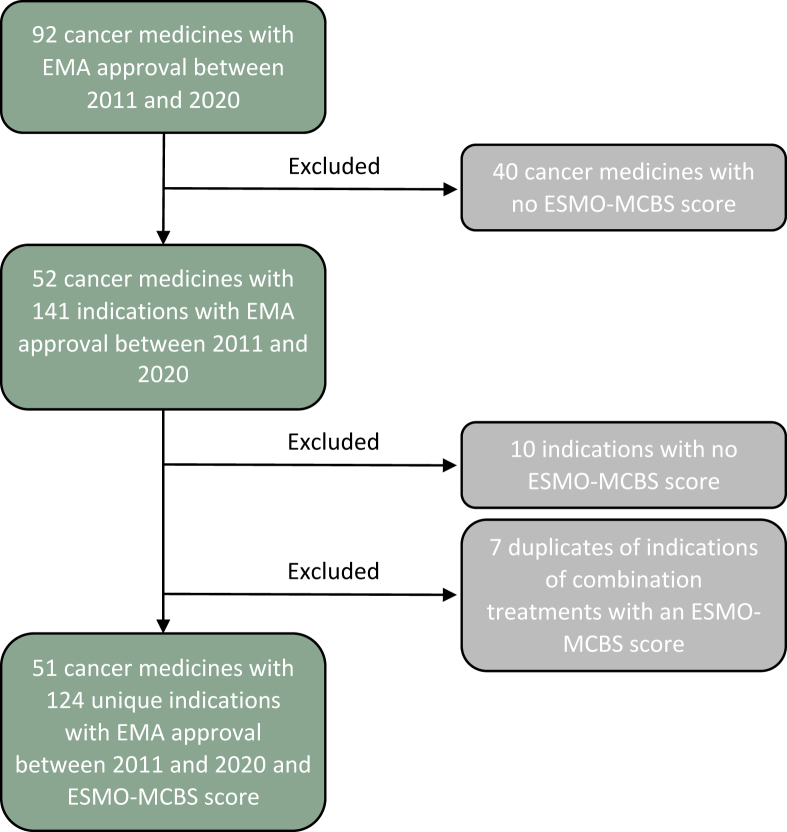
All 92 cancer medicines (new active substances) that received EMA MA between 1 January 2011 and 31 December 2020 were initially selected for the analysis. Medicines with missing ESMO-MCBS scores, such as all medicines exclusively used in hematologic cancers, were excluded. For the remaining medicines, all indications with EMA MA between 1 January 2011 and 31 December 2020 were selected. Indications with missing ESMO-MCBS scores were excluded. The analysis was conducted at the level of the indication rather than the medicine because EMA MA, national reimbursement decisions, and ESMO-MCBS scores are all specific for an indication. A flowchart of the selection of indications included in the analysis is presented in Figure 1.
Figure 1.
Flow diagram describing the selection of indications included in the study. Notes: See Table A.2 in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.101593 for the full list of included indications. EMA, European Medicines Agency; ESMO-MCBS, European Society for Medical Oncology Magnitude of Clinical Benefit Scale.
Data collection and definitions
Data on cancer medicines and indications approved by the EMA, including approval dates, were retrieved from EMA’s official website.18 Dates on NRA for each country were retrieved from official public databases.19, 20, 21, 22, 23, 24, 25, 26, 27, 28 Only reimbursement in the respective ‘national reimbursement list of medicinal products’ (issued in Czechia by the State Institute for Drug Control, in Hungary by the National Health Insurance Fund, and in Poland and Slovakia by the Ministry of Health) was considered. Reimbursement via so-called named-patient systems was not considered to ensure a common definition of standard reimbursement. These systems might be an important route for early patient access to new medicines before the inclusion in the national reimbursement list, yet decisions within these systems are often confidential. Data lock for reimbursement information was 1 July 2022.
In a subanalysis, a distinction was made between full and partial reimbursement in the NRA. Full reimbursement was defined as the wording of the approved EMA label of an indication being identical with the locally reimbursed indication text. The label of some EMA-approved indications contains a reference to ‘section 5.1’ in section 4.1 of the Summary of Product Characteristics. We followed the EMA regulatory procedural guidelines, in order to make a robust comparison between countries, which state ‘Information in section 5.1 should not constitute a new indication or a widening or restriction of an approved indication.’29 Therefore partial reimbursement was defined as any limiting provision (e.g. line of therapy, performance status, and treatment length) in the locally reimbursed indication text compared with the wording of the EMA label in section 4.1 of the Summary of Product Characteristics; see Table A.1 in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.101593 for an example. Some limitations could be minor and some major, but a quantification of the proportion of patients affected was not the subject of this study. Because of the complexity of assessing the extent of the limitations, only one common definition was used.
Waiting time to reimbursement for a specific indication was calculated as the time from EMA MA to NRA in each country. Thus only indications that had received positive (partial or full) reimbursement decisions were included.
The magnitude of clinical benefit was based on the ESMO-MCBS scoring system. Clinical benefit scores were retrieved from ESMO’s official website.30 The ESMO-MCBS score of the pivotal clinical trial and patient group underlying EMA MA was used for the indications included in the analysis. For indications approved in a noncurative treatment setting, the ESMO-MCBS score consists of a 5-point scale, where 5 represents the highest level of clinical benefit and 1 the lowest. For indications approved in a curative treatment setting, the ESMO-MCBS score is graded as A, B, or C, where A indicates the highest level of clinical benefit and C the lowest. Notably, ESMO categorizes scores 4 and 5 for noncurative treatments and scores A and B for curative treatments as indicating substantial improvement and a high level of proven clinical benefit.31 Following the ESMO-MCBS framework, indications with a score of 4, 5, A, or B were classified as having a ‘substantial clinical benefit’, whereas indications with scores 1-3 and C were referred to as having a ‘nonsubstantial clinical benefit’.
Statistical methods
Descriptive statistics were used to present results on reimbursement status and waiting time to reimbursement for each country. Reimbursement status was also analyzed descriptively in relation to reimbursement restrictions (i.e. partial or full reimbursement), type of medicine, cancer type, and treatment setting. The relationship between reimbursement status (i.e. proportion of indications with reimbursement versus no reimbursement) and clinical benefit status (i.e. nonsubstantial or substantial clinical benefit) was further analyzed statistically with a chi-square test for each country. The relationship between waiting time to reimbursement and clinical benefit status was analyzed statistically using a Mann–Whitney U test.
Results
Sample characteristics
A total of 124 indications of 51 cancer medicines with EMA MA between 2011 and 2020 and with available ESMO-MCBS scores were included in the analysis; see Table A.2 in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.101593 for the full list of indications. Sample characteristics are presented in Table A.3 in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.101593. The majority of included indications were targeted therapies (60% of all indications), followed by immunotherapy (29%) and hormonal therapy (7%). The indications covered a variety of cancer types, including lung cancer (27% of all indications), breast cancer (15%), and gastrointestinal cancers (13%); 116 indications related to use in a noncurative treatment setting and the remaining 8 in a curative setting. Notably, 65 indications (52%) had a substantial clinical benefit according to the ESMO-MCBS, and 59 indications (48%) had a nonsubstantial clinical benefit.
Reimbursement status
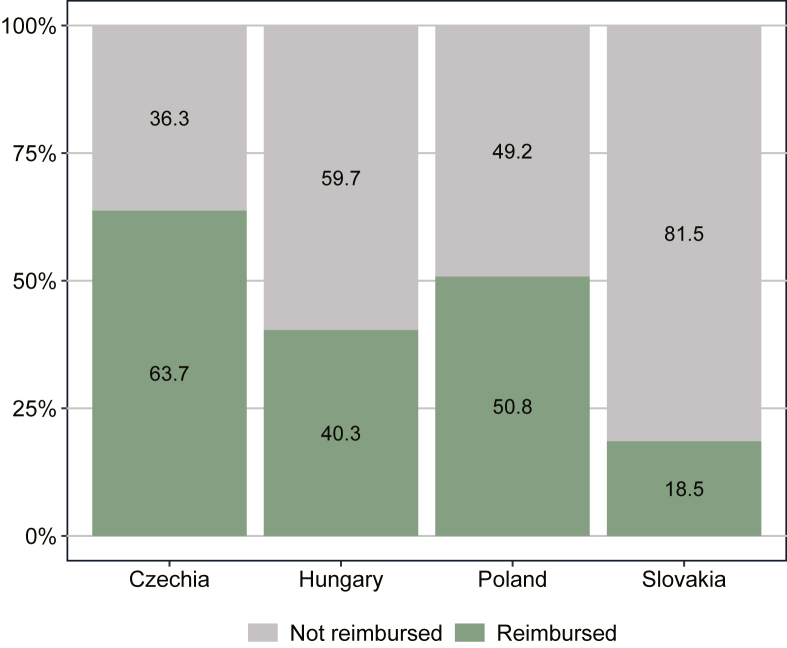
The proportion of indications by reimbursement status on 1 July 2022 in the four CEE countries is presented in Figure 2. There were considerable differences between countries. Czechia and Poland had the highest and second highest proportion of reimbursed indications with 64% and 51%, respectively. Hungary had 40% of all indications reimbursed. In Slovakia, only 19% of indications were reimbursed. Note that these numbers do not include reimbursement via named-patient systems in any of the countries.
Figure 2.
Reimbursement status of novel cancer medicine indications on 1 July 2022. Notes: The figure shows the proportion of indications with reimbursement (n = 124) on 1 July 2022 for indications that received EMA MA during the period 2011-2020. EMA, European Medicines Agency; MA, marketing authorization.
Far from all indications with reimbursement had full reimbursement (see Figure A.1 in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.101593). In Czechia and Poland, all indications were only partially reimbursed. In Slovakia, around three-quarters of all reimbursed indications were partially reimbursed and in Hungary around one-half of all reimbursed indications had partial reimbursement. The reimbursement status varied across and within countries depending on the type of medicine (see Figure A.2 in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.101593). In Czechia, Poland, and Slovakia, hormonal therapies had the largest proportion of reimbursed indications. Chemotherapies had the smallest proportion of reimbursed indications in Poland and Slovakia, but the largest in Hungary. Immunotherapies and targeted therapies had roughly similar proportions of reimbursed indications in all countries except for Slovakia, where only one indication of an immunotherapy was reimbursed. Furthermore, there were differences in reimbursement status depending on the cancer type (see Figure A.3 in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.101593). Skin cancer had the highest proportion of reimbursed indications in all countries followed by prostate cancer, except in Poland where lung cancer was in second place. Gastrointestinal cancers and urinary tract cancers had the lowest proportions of reimbursed indications. No major differences in the proportions of reimbursed indications were observed in the curative versus noncurative treatment setting (Figure A.4 in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.101593).
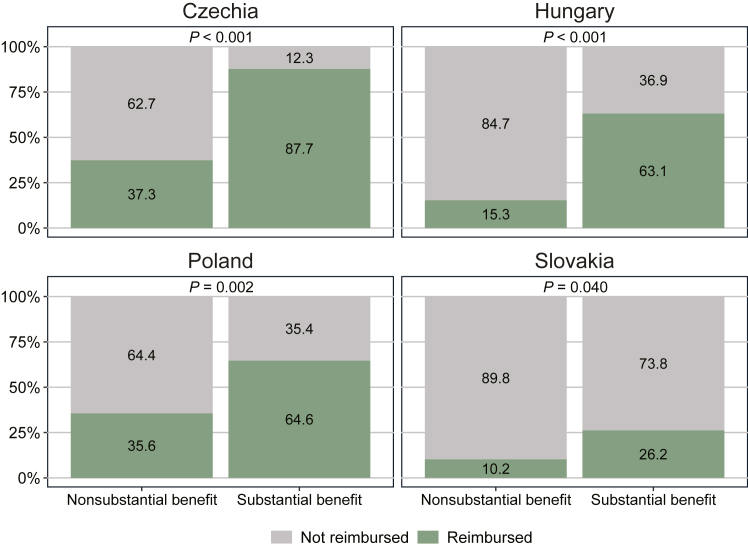
The reimbursement status of indications separated by clinical benefit status (i.e. non-substantial or substantial) is presented in Figure 3. In all four countries, the proportion of reimbursed indications was distinctly higher among indications with a substantial clinical benefit compared with those with a nonsubstantial clinical benefit. For instance, 88% of indications with a substantial clinical benefit had received reimbursement in Czechia compared with 37% with a nonsubstantial clinical benefit. A chi-square test confirmed these differences to be statistically significant (P < 0.05) in all countries.
Figure 3.
Reimbursement status of novel cancer medicine indications on 1 July 2022 by clinical benefit status. Notes: The figure shows proportions of indications with reimbursement on 1 July 2022 separated into indications with a nonsubstantial benefit (i.e. ESMO-MCBS scores 1-3 or C; n = 59) and with a substantial benefit (i.e. ESMO-MCBS scores 4, 5, A, or B; n = 65). A P value of <0.05 indicates a significant difference in reimbursement status between groups as determined by a chi-square test. ESMO-MCBS, European Society for Medical Oncology Magnitude of Clinical Benefit Scale.
Waiting time to reimbursement
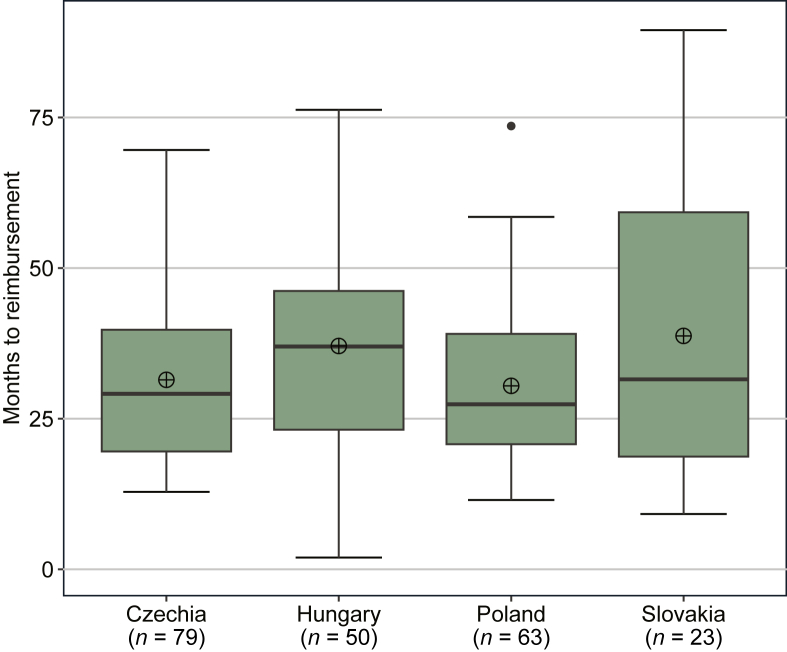
Waiting times to reimbursement (i.e. the time between EMA MA and NRA) for indications with reimbursement are shown in Figure 4. The median and mean waiting times were rather similar between countries; the median waiting time ranged from 27 months in Poland to 37 months in Hungary, whereas the mean waiting time ranged from 31 months in Poland to 39 months in Slovakia. However, there was a large span in waiting time to reimbursement between individual indications in all countries. The minimum/maximum waiting time was 13/70 months in Czechia, 2/76 months in Hungary, 12/74 months in Poland, and 9/90 months in Slovakia.
Figure 4.
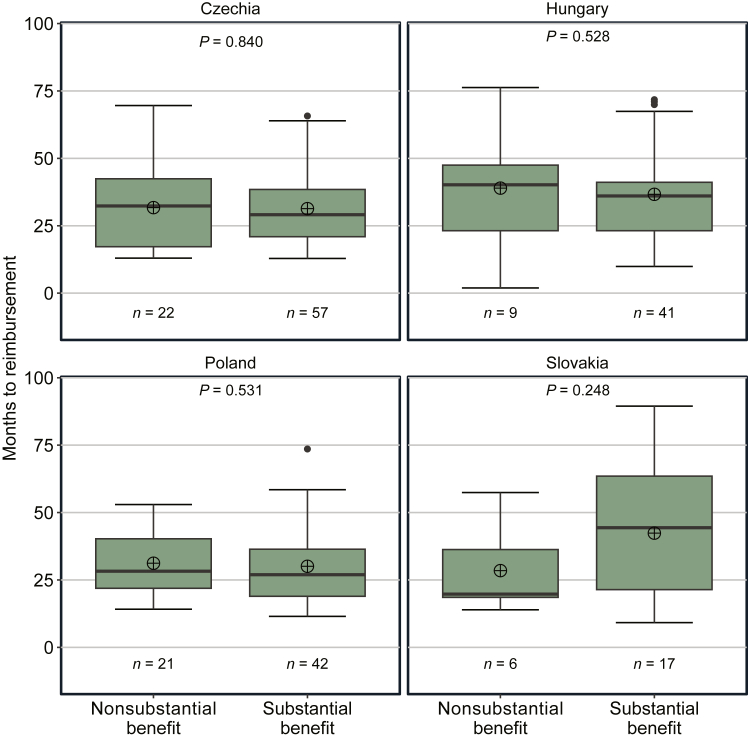
Waiting time to reimbursement for novel cancer medicine indications. Notes: The figure shows median (black line inside the green box), mean (circle with cross), interquartile range (IQR; green box), and minimum and maximum values based on the 1.5 IQR (whiskers) number of months from EMA MA to NRA for indications that received EMA MA during the period 2011-2020 and that received NRA until 1 July 2022. EMA, European Medicines Agency; MA, marketing authorization; NRA, national reimbursement approval.
Figure 5 presents the comparison of waiting times to reimbursement separated by clinical benefit status (i.e. nonsubstantial or substantial) for the four countries. Intracountry differences in median waiting times by clinical benefit status were small and slightly shorter for indications with a substantial clinical benefit in Czechia (32 months with a nonsubstantial clinical benefit versus 29 months with a substantial clinical benefit), Hungary (40 versus 36 months), and Poland (28 versus 27 months). By contrast, the median waiting time for indications with a substantial clinical benefit in Slovakia (44 months) was more than two times as long as for indications with a nonsubstantial clinical benefit (20 months). A Mann–Whitney U test showed that the observed differences in waiting times between indications with substantial and nonsubstantial clinical benefits were not statistically significant in any of the countries (P = 0.248-0.840). However, especially in Slovakia, the reliability of the statistical test is limited by the small number of indications with reimbursement.
Figure 5.
Waiting time to reimbursement for novel cancer medicine indications by clinical benefit status. Notes: The figure shows median (black line inside the green box), mean (circle with cross), interquartile range (IQR; green box) and minimum and maximum values based on the 1.5 IQR (whiskers) number of months from EMA MA to NRA separated into indications with a nonsubstantial benefit (i.e. ESMO-MCBS scores 1-3 or C) and with a substantial benefit (i.e. ESMO-MCBS scores 4, 5, A, or B). A P value of <0.05 indicates a significant difference in waiting time to reimbursement between groups as determined by a Mann–Whitney U test. EMA, European Medicines Agency; ESMO-MCBS, European Society for Medical Oncology Magnitude of Clinical Benefit Scale; MA, marketing authorization; NRA, national reimbursement approval.
Discussion
This study found considerable country differences in the overall reimbursement level of indications of novel cancer medicines, ranging from 64% in Czechia down to 19% in Slovakia, despite all four countries sharing a fairly similar economic background in terms of GDP per capita at 69%-92% of the EU average in 2021.32 The fact that a considerable proportion is not getting reimbursed in these four countries has also been found in previous research by the Organisation for Economic Co-operation and Development (OECD) and the European Federation of Pharmaceutical Industries and Associations (EFPIA).16,17 Their research also indicated that the reimbursement level of novel cancer medicines in most Northern and Western European countries seems to be higher than in the four CEE countries in this study as well as other countries in the CEE region.16,17
Reasons for the low reimbursement levels of novel cancer medicines might be manyfold. First, an increasing number of novel cancer medicines is introduced every year. While the average annual number of new cancer medicines approved by the EMA was 4.4 in the period 2007-2011, it almost tripled to 11.0 medicines in the period 2017-2021.8 This rapid development holds the potential to improve patient outcomes, but it challenges health care systems to provide patients with all novel treatment options within constrained budgets. Second, the list prices of novel cancer medicines (i.e. annualized treatment costs per patient) have been increasing since 2000.33 Third, total expenditure on cancer medicines have been growing from €28 to €61 per capita (inflation-adjusted; based on list prices) between 2008 and 2018 in Europe.1 However, absolute spending on cancer medicines in the four CEE countries was below the European average at €15-40 per capita (based on list prices) in 2018.9 In addition, overall health care spending in relation to GDP is below the EU-27 average (10.9% in 2020) in all four CEE countries (Czechia 9.2%, Hungary 7.3%, Poland 6.5%, and Slovakia 7.2%).34 The limited resources in CEE imply a higher need to prioritize investment in novel cancer medicines with proven value.
The value of a novel cancer medicine/indication is determined by its clinical benefit compared with its costs.35 Within budget-constrained health care systems, considerations of value for money can guide reimbursement decisions by health care payers. Indeed, all four CEE countries in this study apply some form of health technology assessment (HTA) as part of their decision-making process for reimbursement.36 Cost-effectiveness (i.e. the ratio between incremental changes in costs and health over the current standard of care) and budget impact are main criteria for reimbursement in these countries.37 Varying cost-effectiveness thresholds, measured in costs per quality-adjusted life years (QALYs), are used. Czechia has applied a fixed amount of 1.2 million CZK per QALY as a cost-effectiveness threshold since 2013.38 Hungary used to apply a threshold of two to three times the GDP per capita in 2013-2017, three times the GDP per capita in 2017-2021, and multiple thresholds from 1.5 to 10 times the GDP per capita depending on the magnitude of health gain and the rarity of the disease since 2021.39, 40, 41 Poland applies a cost-effectiveness threshold of three times the GDP per capita.42 In Slovakia, the cost-effectiveness threshold was based on multiples of the average monthly salary until August 2022,43 and was lower than in the other countries.44 As of September 2022, the cost-effectiveness threshold in Slovakia has been raised and is now three to ten times the GDP per capita for orphan medicines and advanced therapy medicinal products, and two to three times the GDP per capita for other medicines. The level of threshold depends on the size of the incremental QALYs.45
The ESMO-MCBS was developed to support the process of HTA and to assist in rationalizing reimbursement decisions.12 Although the costs of medicines are not incorporated in the scale, the integration of the ESMO-MCBS in HTA could complement the reimbursement process and guide decisions toward medicines with considerable potential to significantly improve the health of patients with cancer. Conversely, the incorporation of the ESMO-MCBS could help to more clearly deprioritize medicines/indications with a low clinical benefit to save resources. Indeed, almost one-half (48%, 59 out of 124) of the indications included in the analysis had a nonsubstantial clinical benefit.
The results in our study showed a significantly larger proportion of reimbursement granted among indications with a substantial clinical benefit compared with those with a nonsubstantial benefit in all four CEE countries. This indicates that the degree of clinical benefit has had a distinct impact on reimbursement decisions in these countries and resembles findings from a study of reimbursement decisions in Israel in 2013-2015.46 Nonetheless, the four CEE countries reimbursed between 10% (Slovakia) and 37% (Czechia) of the 59 indications with a nonsubstantial clinical benefit.
The mean waiting times between EMA MA and NRA were ∼31-39 months in all four CEE countries. This is considerably longer compared with previous analysis in the EFPIA Patients W.A.I.T. Indicator, which indicated mean waiting times of ∼13-22 months in Czechia, Hungary, and Slovakia and 30 months in Poland.16 Reasons for these discrepancies between this study and the EFPIA report might be the more granular level of analysis on the basis of indications rather than medicines (and only their first indication with EMA MA) as well as the longer follow-up period of up to 11.5 years rather than up to 5 years. Previous analyses have also shown that mean waiting times are generally shorter in Northern and Western European countries compared with countries in CEE.16,47
Different reasons might contribute to long waiting times, such as delays in the reimbursement application of pharmaceutical companies after EMA MA, long HTA processes, and lengthy price negotiations.17,48 The ESMO-MCBS could support prioritizing assessment timelines of medicines/indications with a substantial clinical benefit, by accelerating the HTA process in view of anticipated EMA regulatory timelines. This process is similar to the use of horizon scanning systems that are commonly used in Western European countries to ensure health system readiness and prioritization of HTA reviews for selected medicines.49,50 Lastly, medicines/indications with a substantial clinical benefit could be attractive candidates for negotiating managed entry agreements to facilitate faster access.51
In our analysis, a substantial clinical benefit did not appear to speed up decision making, as no difference in the time from EMA MA to NRA was observed in relation to the clinical benefit status in any of the four CEE countries. The latter is in line with results from a study of reimbursement decisions in Slovenia in 2008-2018.51 Even outside of the EU, the time between MA and NRA was found to be independent of ESMO-MCBS scores in a sample of medicines evaluated in Canada in 2011-2018.52 Long waiting times until NRA of medicines with a substantial clinical benefit come with an opportunity cost.8 Continuing to treat patients with older and less effective medicines instead of novel medicines results in the loss of QALYs as well as productivity loss to the economy.53
Limitations
The inclusion of novel cancer medicines in the scope of this study relied on indications covered by the ESMO-MCBS. Only indications for solid tumors could be included as hemato-oncology indications are not covered by the ESMO-MCBS. A sizeable proportion of novel cancer medicines was therefore excluded from the analysis. Some indications for solid tumors lack an ESMO-MCBS score, especially for indications that garnered EMA approval prior to 2015. The results of this study are thus not necessarily representative of the overall access situation in oncology.
The ESMO-MCBS enables a distinction between indications with substantial and nonsubstantial clinical benefits. Although the ESMO-MCBS is a robust tool, it has some limitations. There might be unmet clinical needs or situations where it is difficult to prove substantial clinical benefit (e.g. in the treatment of testicular cancer or sarcomas). The ESMO-MCBS scores might also change over time when new clinical evidence emerges for an indication or when the entire method of the scoring system is updated.
A caveat of this study is that the results on reimbursement status and time to reimbursement only represent the national perspective of access to medicines, which may differ from access for patients. Even though a medicine was or has been granted reimbursement, it does not necessarily translate into immediate access for all clinically eligible patients across a country. There are many potential barriers following the reimbursement decision that may limit utilization and adoption of novel medicines and hence patient access. One barrier highlighted in this study is the restriction of reimbursement to a particular patient population differing in size compared with that outlined by EMA MA (called ‘partial reimbursement’). Such restrictions could be requirements on performance status of patients, use in a particular line of therapy, or treatment length. All reimbursed indications in Czechia and Poland exhibited some form of restrictions, although the nature of a restriction can vary across indications and countries and therefore have different implications for the size of the patient population affected (see Table A.1 in the Supplementary Appendix, available at https://doi.org/10.1016/j.esmoop.2023.101593 for an example). Other barriers described in previous research that limit patient access to novel reimbursed medicines are suboptimal care pathways, lack of multidisciplinary assessment, lack of reimbursement of companion diagnostics of an already reimbursed medicine, limited annual public budgets for medicines and hospital budgets, slow change of clinical routines, and use of outdated clinical guidelines.54,55
Another caveat is that access to novel cancer medicines through named-patient systems was not considered in the analysis. This might make the access situation look different than it actually is. Through such schemes, the treating physician needs to file an application for every patient to the public insurance body or the health authorities to get approval for the use of a particular medicine that is not on the standard reimbursement list. This bureaucratic hurdle might restrict patient access to varying degrees in different countries. In Slovakia, medicines and indications not included in the reimbursement list (and even some incorporated into the reimbursement list) must be approved by health insurance companies (HICs) on request of the treating oncologist for each patient. Approval from HICs is not granted for the whole treatment period, but repeated requests have to be sent to HICs, usually after 3-6 months of therapy. This system puts an administrative load on treating oncologists and is sometimes a barrier to uninterrupted treatment. Because of confidentiality clauses in named-patient systems, it is not clear which medicines/indications are covered by these systems and at what time point they were included. Therefore it was not possible to determine whether this separate route to patient access has had a greater focus on indications with a substantial clinical benefit.
Conclusion
This study found noticeable differences in access to novel cancer medicines between four countries in CEE despite a fairly similar economic background. The proportion of reimbursed indications with EMA MA between 2011 and 2020 ranged from 19% in Slovakia to 64% in Czechia. The median time from EMA MA to NRA took 2-3 years in all countries. Indications with a substantial clinical benefit were more likely to be reimbursed than those with a nonsubstantial clinical benefit in all countries. Waiting times to reimbursement were equally long for indications with or without a substantial clinical benefit, indicating a lack of prioritization of fast access to treatment options delivering a substantial benefit. The incorporation of the ESMO-MCBS in reimbursement assessments and decisions could be considered to improve access to indications with a substantial clinical benefit and to aid better utilization of limited resources to deliver effective cancer care.
Acknowledgements
The authors acknowledge Maria Reckova, National Oncology Institute, Slovakia, and POKO Poprad, Slovakia, for her valuable comments and Barbora Husakova, MSD, s.r.o., Slovakia, for her technical help and valuable comments, Andrea Manzano, IHE, Sweden, for her technical help, and Karin Wahlberg, IHE, Sweden for her writing assistance.
Funding
This work was supported by MSD International Business GmbH (no grant number).
Disclosure
TH and AG are employees at IHE, an independent research organization receiving funding from both public and private sector organizations; and report institutional fees from MSD to IHE for the preparation of this manuscript. PS is an employee of MSD, Slovakia, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stocks and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. TD is a founder of Value Outcomes, an independent research organization receiving funding from both public and private sector organizations; and reports institutional fees from MSD to Value Outcomes for providing the Czech data for the analysis. PR has received honoraria for lectures and advisory boards from BMS, MSD, Novartis, Pierre Fabre, Merck, Sanofi, Astra Zeneca, and Philogen outside the scope of this study. CB is a former employee of MSD (UK) Limited, London, UK at the time the work was done and may own stock/holds stock options in Merck & Co., Inc., Rahway, NJ, USA. EK is an employee of MSD, Greece, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stocks and/or stock options in Merck & Co., Inc., Rahway, NJ, USA.
Supplementary data
References
- 1.Hofmarcher T., Brådvik G., Svedman C., Lindgren P., Jönsson B., Wilking N. IHE; Lund, Sweden: 2019. Comparator Report on Cancer in Europe 2019 – Disease Burden, Costs and Access to Medicines. IHE Report 2019:7. 7. [Google Scholar]
- 2.Dyba T., Randi G., Bray F., et al. The European cancer burden in 2020: incidence and mortality estimates for 40 countries and 25 major cancers. Eur J Cancer. 2021;157:308–347. doi: 10.1016/j.ejca.2021.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eurostat. Causes of death statistics. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Causes_of_death_statistics Available at.
- 4.Allemani C., Matsuda T., Di Carlo V., et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofmarcher T., Jönsson B., Wilking N. IHE; Lund, Sweden: 2014. Access to High-Quality Oncology Care Across Europe. IHE Report 2014:2. 2. [Google Scholar]
- 6.Lichtenberg F. Has medical innovation reduced cancer mortality? CESifo Econ Stud. 2014;60(1):135–177. [Google Scholar]
- 7.Lichtenberg F. The impact of new drug launches on life-years lost in 2015 from 19 types of cancer in 36 countries. J Demogr Econ. 2018;84:309–354. [Google Scholar]
- 8.Hofmarcher T., Ericsson O., Lindgren P. IHE; Lund, Sweden: 2022. Cancer in Ireland – Disease Burden, Costs and Access to Medicines. IHE Report 2022:4. 4. [Google Scholar]
- 9.Hofmarcher T., Lindgren P., Wilking N., Jonsson B. The cost of cancer in Europe 2018. Eur J Cancer. 2020;129:41–49. doi: 10.1016/j.ejca.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Godman B., Hill A., Simoens S., et al. Potential approaches for the pricing of cancer medicines across Europe to enhance the sustainability of healthcare systems and the implications. Expert Rev Pharmacoecon Outcomes Res. 2021;21(4):527–540. doi: 10.1080/14737167.2021.1884546. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . World Health Organization; Geneva, Switzerland: 2018. Technical Report: Pricing of Cancer Medicines and Its Impacts: A Comprehensive Technical Report for the World Health Assembly Resolution 70.12: Operative Paragraph 2.9 on Pricing Approaches and Their Impacts on Availability and Affordability of Medicines for the Prevention and Treatment of Cancer. [Google Scholar]
- 12.Cherny N.I., Sullivan R., Dafni U., et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS) Ann Oncol. 2015;26(8):1547–1573. doi: 10.1093/annonc/mdv249. [DOI] [PubMed] [Google Scholar]
- 13.Cherny N.I., Dafni U., Bogaerts J., et al. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol. 2017;28(10):2340–2366. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency Authorisation of medicines. https://www.ema.europa.eu/en/about-us/what-we-do/authorisation-medicines Available at.
- 15.Cufer T., Ciuleanu T.E., Berzinec P., et al. Access to novel drugs for non-small cell lung cancer in central and southeastern Europe: a central European cooperative oncology group analysis. Oncologist. 2020;25(3):e598–e601. doi: 10.1634/theoncologist.2019-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton M., Scott K., Troein P. EFPIA Patients W.A.I.T. Indicator 2021 Survey. IQVIA; Durham, NC: 2022. [Google Scholar]
- 17.OECD . OECD Publishing; Paris, France: 2020. Addressing Challenges in Access to Oncology Medicines. [Google Scholar]
- 18.European Medicines Agency Union Register of medicinal products for human use. https://ec.europa.eu/health/documents/community-register/html/reg_hum_act.htm?sort=a Available at.
- 19.Ministry of Health of the Slovak Republic List of reimbursed drugs (from 2016 onwards) https://www.health.gov.sk/?zoznam-kategorizovanych-liekov Available at.
- 20.Ministry of Health of the Slovak Republic Reimbursement and officially determined prices (from 2011 until 2015) https://www.health.gov.sk/?kategorizecia-a-uuc Available at.
- 21.State Institute for Drug Control List of medicines and food for special medical purposes covered by health insurance. https://www.sukl.cz/sukl/seznam-leciv-a-pzlu-hrazenych-ze-zdrav-pojisteni Available at.
- 22.Ministry of Health of Poland Announcements of the Minister of Health - list of reimbursed drugs. https://www.gov.pl/web/zdrowie/obwieszczenia-ministra-zdrowia-lista-lekow-refundowanych Available at.
- 23.The National Health Insurance Fund of Hungary. Archive - Public Medicines Register. https://neak.gov.hu/felso_menu/szakmai_oldalak/gyogyszer_segedeszkoz_gyogyfurdo_tamogatas/egeszsegugyi_vallalkozasoknak/pupha/PUPHAarch Available at.
- 24.National Legislation Database 7/2014. (I. 29.) EMMI decree on the amendment of certain pharmaceutical-related and other regulations related to the support of civil organizations. https://njt.hu/jogszabaly/2014-7-20-5H Available at.
- 25.National Legislation Database. 32/2016. (X. 27.) EMMI decree on the amendment of certain ministerial decrees on health insurance. https://njt.hu/jogszabaly/2016-32-20-5H Available at.
- 26.National Legislation Database. 35/2018. (X. 12.) EMMI decree on the amendment of ministerial decrees on the social insurance support of certain medicines. https://njt.hu/jogszabaly/2018-35-20-5H Available at.
- 27.National Legislation Database 36/2019. (XII. 30.) EMMI decree on the amendment of the ministerial decrees on the social security support of certain medicines. https://njt.hu/jogszabaly/2019-36-20-5H Available at.
- 28.National Legislation Database 55/2021. (XII. 22.) EMMI decree on the amendment of the ministerial decrees on the social security support of certain medicines. https://njt.hu/jogszabaly/2021-55-20-5H Available at.
- 29.European Medicines Agency Question and answer on the information contained within section 5.1 of the SPC on pharmacodynamic properties for pharmaceutical products. 2017. https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/question-answer-information-contained-within-section-51-summary-product-characteristics_en.pdf Available at.
- 30.European Society for Medical Oncology ESMO-MCBS scorecards. https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-scorecards Available at.
- 31.European Society for Medical Oncology About the ESMO-MCBS. https://www.esmo.org/guidelines/esmo-mcbs/about-the-esmo-mcbs Available at.
- 32.Eurostat GDP per capita, consumption per capita and price level indices. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=GDP_per_capita,_consumption_per_capita_and_price_level_indices Available at.
- 33.IQVIA Institute for Human Data Science . IQVIA; Parsippany, NJ: 2019. Global Oncology Trends 2019 - Therapeutics, Clinical Development and Health System Implications. [Google Scholar]
- 34.OECD/European Union . OECD Publishing; Paris, France: 2022. Health at a Glance: Europe 2022: State of Health in the EU Cycle. [Google Scholar]
- 35.Porter M.E. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 36.European Commission . Publications Office of the European Union; Luxembourg City, Luxembourg: 2017. Mapping of HTA National Organisations, Programmes and Processes in EU and Norway. [Google Scholar]
- 37.World Health Organization . WHO Regional Office for Europe; Copenhagen, Denmark: 2018. Medicines Reimbursement Policies in Europe. [Google Scholar]
- 38.2022. State Institute for Drug Control. SP-CAU-028 Assessment procedure for cost-effectiveness analysis [SP-CAU-028 Postup pro posuzování analýzy nákladové efektivity]https://www.sukl.cz/leciva/sp-cau-028 Available at. [Google Scholar]
- 39.Ministry of Human Resources Guidelines on conducting health economic analyses [Az Emberi Erőforrások Minisztériuma szakmai irányelve az egészség-gazdaságtani elemzések készítéséhez] Egészségügyi Közlöny. 2013;62(3):579–600. [Google Scholar]
- 40.Ministry of Human Resources Guidelines on the methodology of health technology assessment and economic evaluation [Az Emberi Erőforrások Minisztériuma szakmai irányelve az egészségügyi technológia értékelés módszertanáról és ennek keretében költséghatékonysági elemzések készítéséről] Egészségügyi Közlöny. 2017;66(3):821–842. [Google Scholar]
- 41.Ministry of Human Resources Health care guideline – on conducting and evaluating health economic analyses [Egészségügyi szakmai irányelv – Az egészség-gazdaságtani elemzések készítéséhez és értékeléséhez] Egészségügyi Közlöny. 2021;71(21):2178–2200. [Google Scholar]
- 42.Cameron D., Ubels J., Norstrom F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action. 2018;11(1) doi: 10.1080/16549716.2018.1447828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skoupa J., Annemans L., Hajek P. Health economic data requirements and availability in the European Union: results of a survey among 10 European countries. Value Health Reg Issues. 2014;4:53–57. doi: 10.1016/j.vhri.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Pažitný, Kandilaki . Pažitný & Kandilaki; Bratislava, Slovakia: 2022. Drug Policy in Broader Budgetary Context (Slovakia) [Google Scholar]
- 45.Ministry of Health of the Slovak Republic. Decree of the Ministry of Health of the Slovak Republic No. 298/2022 Coll. stipulating the details on calculation of the relevant multiple of gross domestic product for determining the value threshold for the assessed medicinal product [Vyhláška Ministerstva zdravotníctva Slovenskej republiky 298/2022 Z. z. ktorou sa ustanovujú podrobnosti výpočtu príslušného násobku hrubého domáceho produktu pre stanovenie prahovej hodnoty posudzovaného lieku] https://www.slov-lex.sk/static/SK/ZZ/2022/298/vyhlasene_znenie.html Available at.
- 46.Hammerman A., Greenberg-Dotan S., Feldhamer I., Birnbaum Y., Cherny N.I. The ESMO-Magnitude of Clinical Benefit Scale for novel oncology drugs: correspondence with three years of reimbursement decisions in Israel. Expert Rev Pharmacoecon Outcomes Res. 2018;18(1):119–122. doi: 10.1080/14737167.2017.1343146. [DOI] [PubMed] [Google Scholar]
- 47.Uyl-de Groot C.A., Heine R., Krol M., Verweij J. Unequal access to newly registered cancer drugs leads to potential loss of life-years in Europe. Cancers (Basel) 2020;12(8):2313. doi: 10.3390/cancers12082313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.European Federation of Pharmaceutical Industries and Associations . The Root Cause of Unavailability and Delay to Innovative Medicines: Reducing the Time Before Patients Have Access to Innovative Medicines. EFPIA; Brussels, Belgium: 2022. [Google Scholar]
- 49.Vogler S. “Ready for the future?” - Status of national and cross-country horizon scanning systems for medicines in European countries. Ger Med Sci. 2022;20:Doc05. doi: 10.3205/000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wild C., Langer T. Emerging health technologies: informing and supporting health policy early. Health Policy. 2008;87(2):160–171. doi: 10.1016/j.healthpol.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Janzic U., Knez L., Janzic A., Cufer T. Time to access to novel anticancer drugs and the correlation with ESMO-Magnitude of Clinical Benefit Scale in Slovenia. Expert Rev Pharmacoecon Outcomes Res. 2019;19(6):717–723. doi: 10.1080/14737167.2019.1702879. [DOI] [PubMed] [Google Scholar]
- 52.Thomson S., Everest L., Witzke N., et al. Examining the association between oncology drug clinical benefit and the time to public reimbursement. Cancer Med. 2022;11(2):380–391. doi: 10.1002/cam4.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rachev B., Wilking N., Kobelt G., et al. Budget projections and clinical impact of an immuno-oncology class of treatments: experience in four EU markets. J Cancer Policy. 2021;28 doi: 10.1016/j.jcpo.2021.100279. [DOI] [PubMed] [Google Scholar]
- 54.Hofmarcher T., Lindgren P., Wilking N. IHE; Lund, Sweden: 2022. Diagnosed But Not Treated: How to Improve Patient Access to Advanced NSCLC Treatment in Europe. IHE Report 2022:2. 2. [Google Scholar]
- 55.Hofmarcher T., Lindgren P., Wilking N. Systemic anti-cancer therapy patterns in advanced non-small cell lung cancer in Europe. J Cancer Policy. 2022;34 doi: 10.1016/j.jcpo.2022.100362. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.