Abstract
Background
Human epidermal growth factor receptor 2 (HER2)-low expression in breast cancer has been recently identified as a new therapeutic target. However, it is unclear if HER2-low status has an independent impact on prognosis.
Materials and methods
A systematic literature research was carried out to identify studies comparing survival outcomes of patients affected by HER2-low versus HER2-zero breast cancer. Using random-effects models, pooled hazard ratios (HRs) and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for progression-free survival (PFS) and overall survival (OS) in the metastatic setting as well as disease-free survival (DFS), OS and pathological complete response (pCR) in the early setting. Subgroup analyses by hormone receptor (HoR) status were carried out. The study protocol is registered on PROSPERO (n.CRD42023390777).
Results
Among 1916 identified records, 42 studies including 1 797 175 patients were eligible. In the early setting, HER2-low status was associated with significant improved DFS (HR 0.86, 95% CI 0.79-0.92, P < 0.001) and OS (HR 0.90, 95% CI 0.85-0.95, P < 0.001) when compared to HER2-zero status. Improved OS was observed for both HoR-positive and HoR-negative HER2-low populations, while DFS improvement was observed only in the HoR-positive subgroup. HER2-low status was significantly associated with a lower rate of pCR as compared to HER2-zero status both in the overall population (OR 0.74, 95% CI 0.62-0.88, P = 0.001) and in the HoR-positive subgroup (OR 0.77, 95% CI 0.65-0.90, P = 0.001). In the metastatic setting, patients with HER2-low breast cancers showed better OS when compared with those with HER2-zero tumours in the overall population (HR 0.94, 95% CI 0.89-0.98, P = 0.008), regardless of HoR status. No significant PFS differences were found.
Conclusions
Compared with HER2-zero status, HER2-low status appears to be associated with a slightly increased OS both in the advanced and early settings, regardless of HoR expression. In the early setting, HER2-low tumours seem to be associated to lower pCR rates, especially if HoR-positive.
Key words: breast cancer, HER2-low, HER2-zero
Highlights
-
•
Patients with HER2-low tumours showed better OS than those with HER2-zero cancers in both settings.
-
•
No differences were found in terms of PFS between patients with HER2-low and HER2-zero tumours.
-
•
Patients with HER2-low tumours showed a lower rate of pCR compared to those with HER2-zero cancers.
Introduction
Breast cancer is one of the most common malignancies worldwide.1 It is traditionally classified into different subtypes, according to hormone receptor (HoR) expression and human epidermal growth factor receptor 2 (HER2) status: luminal-like (HoR-positive/HER2-negative), triple negative (HoR negative/HER2-negative) and HER2 positive (HoR-positive or negative), partially resembling the molecular luminal A, luminal B, HER2-enriched and basal-like subtypes.2,3 According to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines, HER2 positivity is defined by an immunohistochemical (IHC) score of 3+ or 2+ with in situ hybridization (ISH) amplification. An IHC score of 0, 1+ and 2+ without ISH amplification would define a tumour as HER2-negative.4
In recent years, a new concept has emerged in the breast cancer scenario: tumours characterized by an IHC score of 1+ and 2+ without ISH amplification are defined as HER2-low.5,6 These tumours, previously categorized as HER2-negative, have been recently identified as a therapeutic target for new HER2-targeting antibody-drug conjugates (ADCs), like trastuzumab deruxtecan (T-DXd). T-DXd was compared to a physician’s choice chemotherapy in HER2-low metastatic breast cancer patients treated with one or two previous lines of chemotherapy within the DESTINY-Breast04 phase III trial. The study showed notable improvements in progression-free survival (PFS) and overall survival (OS) with T-DXd in the overall population enrolled, as well as in the HoR-positive and triple-negative subcohorts, separately.7 Based on these results, T-DXd was recently approved by the Food and Drug Administration and European Medicines Agency for the treatment of patients with advanced HER2-low breast cancer, representing the first approved treatment indication in this subpopulation.8,9
Despite its therapeutic implications, it is unclear if HER2-low status has an independent impact on prognosis, both in the metastatic and early settings. Several studies have investigated the prognostic value of HER2-low status with conflicting results.10 In order to address this controversial topic, we conducted a systematic review and meta-analysis to assess the prognostic role of HER2-low status in breast cancer, both in early and advanced settings and according to HoR status.
Materials and Methods
We conducted a quantitative synthesis of data from studies evaluating the prognostic role of HER2-low status, in the early and advanced settings and according to HoR status.
Search strategy and study identification
We carried out a systematic literature research of PubMed and Cochrane databases with no language or date restriction up to 18 December 2022. We also retrieved abstracts from major international conferences of the past 2 years [American Society of Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO) and ESMO Breast, San Antonio Breast Cancer Symposium (SABCS)] in order to identify potentially eligible unpublished studies. The search strategy was carried out using the keywords ‘breast cancer’, ‘HER2-Low’, ‘ERBB2-low’, ‘human epidermal growth factor receptor 2 low’, ‘low level HER2’. The full search strategy used for each database is presented in the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2023.101592. The systematic literature research was carried out independently by two authors (CM and FJ) and any discrepancies were solved by discussion with a third author (EA). The present systematic review and meta-analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.11
This study is registered in the PROSPERO database (registration number CRD42023390777) and the protocol is available in the PROSPERO website.
Selection criteria and data extraction
To be included in the present meta-analysis, studies had to satisfy the following inclusion criteria: (i) studies including patients diagnosed with invasive breast cancer with any disease stage I-IV; (ii) studies reporting the prognosis of patients with HER2-low breast cancer in comparison to those with HER2-zero breast cancer. If more than one publication on the same dataset was available, data were extracted from the most updated record. Studies meeting one of the following criteria were excluded: (i) insufficient results on the association between HER2-low status and clinical outcomes; (ii) studies reporting on HER2-low status in patients not affected by breast cancer; (iii) studies published in languages other than English.
The following variables were extracted from the included studies, when available: author, year of publication, country, median follow-up, type of study, total number of patients, number of patients with HER2-low breast cancer, number of patients with HER2-zero breast cancer, number of patients with HER2-low/HoR-positive breast cancer, number of patients with HER2-zero/HoR-positive breast cancer, number of patients with HER2-low/HoR-negative breast cancer, number of patients with HER2-zero/HoR-negative breast cancer, type of comparison, disease-free survival (DFS), pathological complete response (pCR) and OS in the early setting for each patients’ subgroup, PFS and OS in the metastatic setting for each patients’ subgroup.
Study objectives
The primary objective of our meta-analysis was to assess the prognostic value of HER2-low status in breast cancer, both in the early and advanced settings. The primary objectives were to evaluate: (i) the association between HER2-low status and pCR rate, DFS and OS in the early setting; (ii) the association between HER2-low status and PFS and OS in the advanced setting.
Secondary objectives of our analysis were assessing (i) the association between HER2-low status and pCR rate, DFS and OS in the early setting according to the HoR status and (ii) the association between HER2-low status and PFS and OS in the metastatic setting, according to the HoR status.
Risk of bias assessment
The risk of bias (RoB) for each included study was evaluated by two investigators (CM and GNM). The RoB was assessed using the Quality in Prognosis Studies (QUIPS) tool,12 which includes six distinct domains regarding study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding, statistical analysis and reporting. Through this tool, each study was classified as having a low, moderate or high RoB.
Statistical analysis
We calculated the pooled hazard ratio (HR) comparing patients with HER2-low breast cancer and HER2-zero breast cancer for survival endpoints in the early setting (DFS and OS) and in the metastatic setting (PFS and OS), and the pooled odds ratio (OR) for the pCR endpoint. The random-effects model of DerSimonian and Laird was applied to compute the pooled estimates of HR and OR and their 95% confidence intervals (CIs). This model allowed us to estimate the amount of the variability between studies and accordingly provide suitable standard errors of pooled HR and pCR. We used the random-effects model even if the heterogeneity between studies was low since, when the studies included in a meta-analysis derived from the published literature, the assumption that they all share an identical true effect size and the differences are exclusively due to the sampling error, as required by the fixed-effects model, is too stringent. Nevertheless, when the heterogeneity is low, fixed- and random-effects models provide similar results.13 When available, HR based on multivariate analysis was used; if not available, we used HR based on univariate analysis. When the OR or HR estimates were not reported but the number of events for each group could be derived, ORs were computed as the odds of events between groups, whereas HRs were estimated using the method reported by Watkins and Bennett.14 Survival analyses were then repeated by excluding computed HRs and including only the studies reporting the HRs. The Higgins I2 index was computed to assess the degree of consistency of the results of the studies. Egger’s test was used to assess the likelihood of publication bias. To verify if some study strongly influenced the pooled estimates, sensitivity analyses were carried out, by excluding the studies one at a time and recalculating the pooled estimates. All statistical analyses and forest plot generations were carried out using STATA Software Version 13.1 (StataCorp LP, College Station, TX). Cohorts including merely HoR-positive tumours were only included in the HoR-positive subgroup analysis. Cohorts including exclusively HoR-negative tumours were only included in the HoR-negative subgroup analysis.
Results
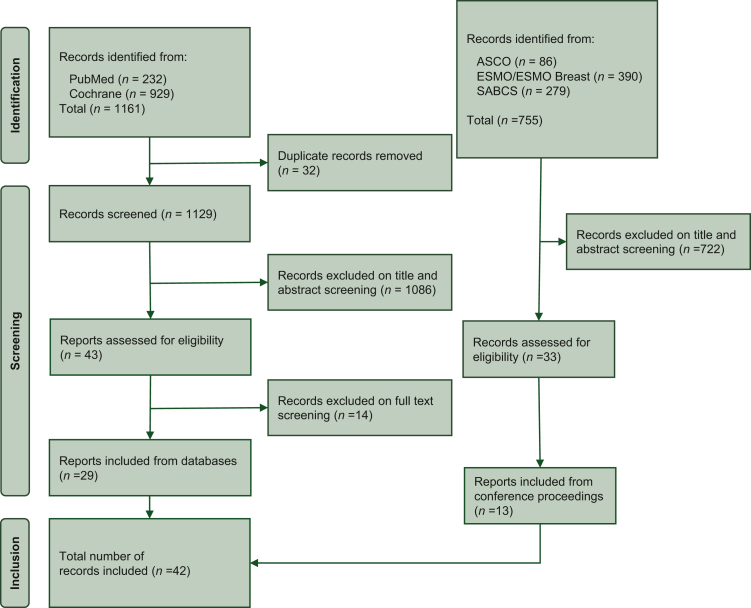
A total of 1916 records were identified from databases and conference proceedings by using the above-mentioned research criteria. After duplicate removal and exclusion of non-relevant records, 42 studies were included in the present meta-analysis (Figure 1). Among them, 12 studies included data from patients affected by metastatic breast cancer,15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 27 analysed data from patients with early breast cancer27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 and 3 studies analysed subjects in both settings.54, 55, 56 A total of 1 797 175 patients were eligible for this analysis, of whom 1 697 079 had early disease (1 118 389 HER2-low and 578 690 HER2-zero) and 100 096 had advanced disease (59 798 HER2-low and 40 298 HER2-zero).
Figure 1.
The PRISMA flow chart summarizing the process for the identification of eligible studies.
ASCO, American Society of Clinical Oncology; ESMO, European Society for Medical Oncology; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SABCS, San Antonio Breast Cancer Symposium.
Early setting
Pathological complete response
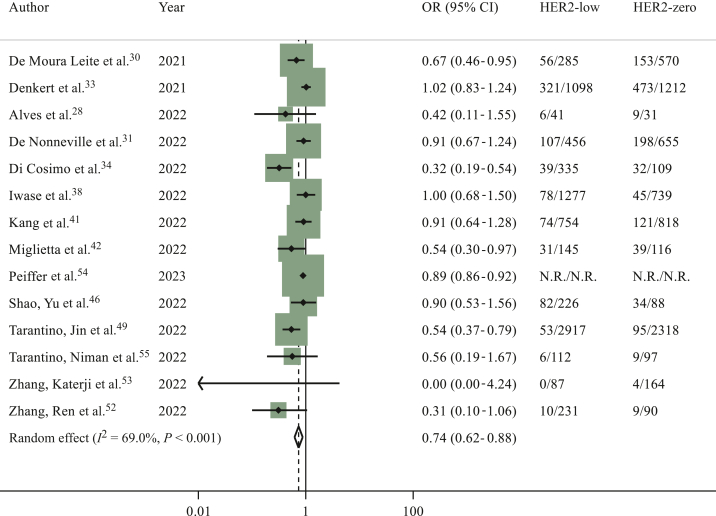
Considering the overall population, 14 studies including 114 754 patients28,30,31,33,34,38,41,42,46,49,52, 53, 54, 55 had available data regarding pCR. A total of 10 675 out of 68 059 (15.6%) patients with HER2-low breast cancer achieved pCR at surgery, compared to 10 593 out of 46 695 (22.6%) patients with HER2-zero breast cancer. A statistically significant difference in terms of pCR in favour of HER2-zero subgroup was found (OR 0.74, 95% CI 0.62-0.88, P = 0.001; I2 = 69%; P < 0.001) (Figure 2). The sensitivity analysis provided consistent results with similar OR estimates (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.101592). Egger’s test P value was 0.024, showing a potential publication bias.
Figure 2.
Odds ratio (OR) for pathological complete response after neoadjuvant chemotherapy of HER2-low breast cancers versus HER2-zero breast cancers in the overall population (the size of the squares is proportional to the weight of each study).
CI, confidence interval; HER2, human epidermal growth factor receptor 2. Random effect: P = 0.001; Egger’s test: P = 0.024.
In the HoR-positive cohort, pCR data were reported by 13 studies.28,30,31,33,34,36,41,42,46,49,52,54,55 HER2-low status was significantly associated with a lower rate of pCR (OR 0.77, 95% CI 0.65-0.90, P = 0.001; I2 = 17.3%; P = 0.269) (Supplementary Figure S1, sensitivity analysis available in the Supplementary Material and Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.101592). In the HoR-negative cohort, pCR data were available for 15 studies.28,30,31,33, 34, 35,41,42,46,47,49,51,52,54,55 No statistically significant difference was found in pCR rates between patients with HER2-low and those with HER2-zero tumours (OR 0.95, 95% CI 0.81-1.11, P = 0.497; I2 = 42.5%; P = 0.042) (Supplementary Figure S2, sensitivity analysis available in the Supplementary Material and Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.101592). No significant publication bias was observed for pCR analyses both in HoR-positive and HoR-negative subanalyses (Egger’s test: P = 0.804 and P = 0.513, respectively).
Disease-free survival
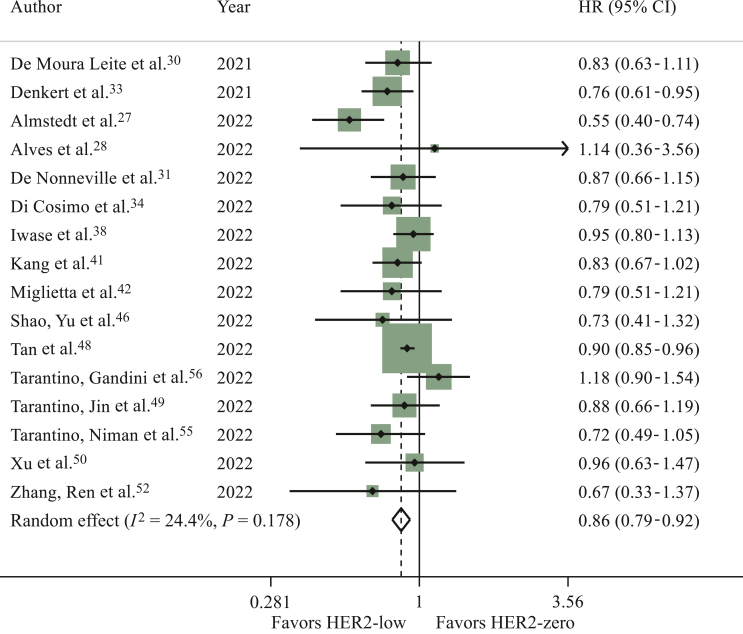
Sixteen studies reported DFS results in the overall population.27,28,30,31,33,34,38,41,42,46,48, 49, 50,52,55,56 HER2-low status was significantly associated with longer DFS as compared to HER2-zero status (HR 0.86, 95% CI 0.79-0.92, P < 0.001; I2 = 24.4%; P = 0.178) (Figure 3). Consistent results were reported in the sensitivity analysis (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2023.101592). Similar results were observed in the analysis where computed HRs were excluded (data not shown). No publication bias was detected (Egger’s test: P = 0.212).
Figure 3.
Hazard ratio for disease-free survival of HER2-low breast cancers versus HER2-zero breast cancers in the overall population (the size of the squares is proportional to the weight of each study).
CI, confidence interval; HER2, human epidermal growth factor receptor 2. Random effect: P < 0.001; Egger’s test: P = 0.212.
Among the 20 studies reporting DFS results in the HoR-positive cohort,27,29,30,32, 33, 34,36, 37, 38,41, 42, 43, 44, 45, 46,48,49,50,52,55 HER2-low status was significantly associated with longer DFS as compared to HER2-zero status (HR 0.86, 95% CI 0.80-0.93, P < 0.001; I2 = 17.8%; P = 0.232) (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2023.101592). Consistent results were reported in the sensitivity analysis (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2023.101592). No publication bias was found (Egger’s test: P = 0.357).
No statistically significant difference in terms of DFS was found between patients with HER2-low and those with HER2-zero tumours, analysing 17 studies reporting data from patients with HoR-negative disease (HR 0.90, 95% CI 0.78-1.04, P = 0.155; I2 = 35.6%; P = 0.073) (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2023.101592).27,30,33, 34, 35,37,39,41,42,44,46, 47, 48, 49, 50,52,55 Egger’s test P value was 0.928 showing no RoB. Sensitivity analysis showed a significant difference in favour of HER2-low tumours after the exclusion of the study by Di Cosimo et al.34 (HR 0.88, 95% CI 0.77-0.99, P = 0.038) (Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2023.101592).
Overall survival
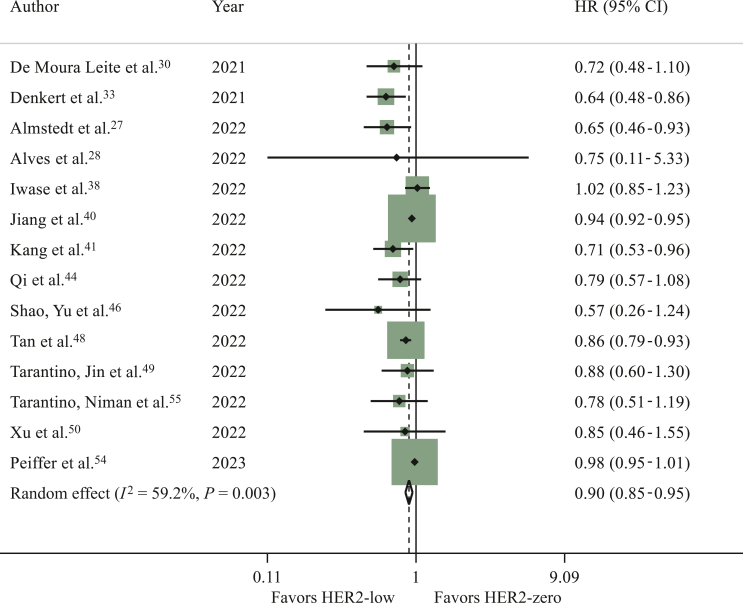
Fourteen studies reported OS data, comparing patients with HER2-low tumours and HER2-zero tumours.27,28,30,33,38,40,41,44,46,48, 49, 50,54,55 Patients with HER2-low tumours had significantly longer OS as compared to those with HER2-zero tumours (HR 0.90, 95% CI 0.85-0.95, P < 0.001; I2 = 59.2%; P = 0.003) (Figure 4; sensitivity analysis available in the Supplementary Material and Supplementary Table S7, available at https://doi.org/10.1016/j.esmoop.2023.101592). Similar results were observed in the analysis where computed HRs were excluded (data not shown). A potential publication bias was observed (Egger’s test: P = 0.031).
Figure 4.
Hazard ratio (HR) for overall survival of HER2-low breast cancers versus HER2-zero breast cancers in the overall population in the early setting (the size of the squares is proportional to the weight of each study).
CI, confidence interval; HER2, human epidermal growth factor receptor 2. Random effect: P < 0.001; Egger’s test: P = 0.031.
Data about OS in the HoR-positive population were reported in 15 studies.27,30,32,37,38,40,41,43,44,46,48, 49, 50,54,55 HER2-low tumours were associated with better OS than HER2-zero tumours (HR 0.94, 95% CI 0.90-0.98, P = 0.003; I2 = 47.4%; P = 0.021) (Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2023.101592). Consistent results were reported in the sensitivity analysis (Supplementary Table S8, available at https://doi.org/10.1016/j.esmoop.2023.101592). Egger’s test P value was <0.001 showing risk of publication bias. OS data in patients with HoR-negative disease were available in 16 studies.27,30,33,35,37,39, 40, 41,44,46, 47, 48, 49, 50,54,55 Again, a significant difference in OS was found between the two groups, in favour of HER2-low tumours (HR 0.88, 95% CI 0.82-0.95, P = 0.001; I2 = 36.5%; P = 0.072; Egger’s test: P = 0.378) (Supplementary Figure S6, available at https://doi.org/10.1016/j.esmoop.2023.101592). Sensitivity analysis showed the same results after excluding each study one by one (Supplementary Table S9, available at https://doi.org/10.1016/j.esmoop.2023.101592).
Metastatic setting
Progression-free survival
Three studies reported data regarding PFS in the overall population.18,19,56 No significant difference was found in terms of PFS in the first line between HER2-low and HER2-zero tumours (HR 0.99, 95% CI 0.96-1.03, P = 0.710; I2 = 0.0%; P = 0.541. Egger’s test: P = 0.300) (Supplementary Figure S7, sensitivity analysis available in the Supplementary Material and Supplementary Table S10, available at https://doi.org/10.1016/j.esmoop.2023.101592). Five studies reported PFS data for the HoR-positive cohort.15,16,18,19,26 Consistent with the results obtained for the overall population, there was no significant difference in terms of PFS in the HoR-positive cohort (HR 1.13, 95% CI 0.94-1.35, P = 0.192; I2 = 70.8%; P = 0.008; Egger’s test: P = 0.259) (Supplementary Figure S8, available at https://doi.org/10.1016/j.esmoop.2023.101592). Sensitivity analysis demonstrated similar results (Supplementary Table S11, available at https://doi.org/10.1016/j.esmoop.2023.101592). PFS data in the HoR-negative cohort were available in two studies18,19 and the difference between HER2-low and HER2-zero status was not significant (HR 0.92, 95% CI 0.84-1.02, P = 0.103; Egger’s test: not computable, sensitivity analysis not carried out) (Supplementary Figure S9, available at https://doi.org/10.1016/j.esmoop.2023.101592).
Overall survival
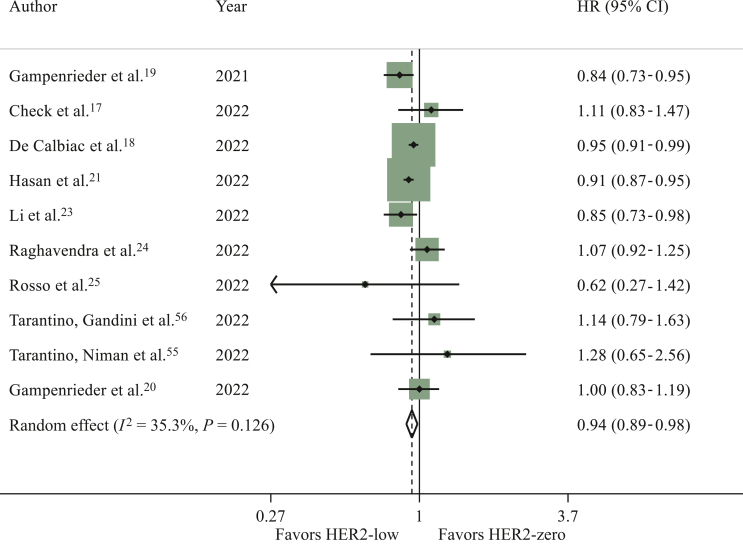
OS data for the overall population were reported in 10 studies.17, 18, 19, 20, 21,23, 24, 25,55,56 A significant difference in terms of OS in favour of patients with HER2-low breast cancer was found in the overall population (HR 0.94, 95% CI 0.89-0.98, P = 0.008; I2 = 35.3%; P = 0.126; Egger’s test: P = 0.540) (Figure 5; sensitivity analysis available in the Supplementary Material and Supplementary Table S12, available at https://doi.org/10.1016/j.esmoop.2023.101592).
Figure 5.
Hazard ratio (HR) for overall survival of HER2-low breast cancers versus HER2-zero breast cancers in the overall population in the metastatic setting (the size of the squares is proportional to the weight of each study).
CI, confidence interval; HER2, human epidermal growth factor receptor 2. Random effect: P = 0.008; Egger’s test: P = 0.540.
Nine studies reported OS data in the HoR-positive cohort.16,18,19,21, 22, 23,26,54,55 As in the overall population, HER2-low status appeared to be associated with better OS when compared to HER2-zero status (HR 0.92, 95% CI 0.87-0.98, P = 0.013; I2 = 71.3%, P < 0.001) (Supplementary Figure S10, sensitivity analysis available in the Supplementary Material and Supplementary Table S13, available at https://doi.org/10.1016/j.esmoop.2023.101592). Data about OS in HoR-negative patients were available in six studies.18, 19, 20,23,54,55 Again, patients affected by HER2-low tumours showed longer OS when compared to patients with HER2-zero tumours (HR 0.91, 95% CI 0.87-0.95, P < 0.001; I2 = 0.0%, P = 0.981) (Supplementary Figure S11, sensitivity analysis available in the Supplementary Material and Supplementary Table S14, available at https://doi.org/10.1016/j.esmoop.2023.101592). No significant publication bias was observed in both HoR-positive and HoR-negative subanalyses (Egger’s test: P = 0.259 and P = 0.746, respectively).
Risk of bias and publication bias
Eleven studies included were considered to have an overall high RoB,17,20,21,28,32,36,38,40,42,45,50 while 19 studies were classified as having a moderate RoB15,16,19,22, 23, 24,29, 30, 31,41,43,44,46,47,52, 53, 54, 55, 56 and 12 studies were considered to have a low RoB.18,25, 26, 27,33, 34, 35,37,39,48,49,51 A detailed RoB assessment57 for each study is reported in the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2023.101592.
Discussion
In the past 2 years, HER2-low status has been identified as a new therapeutic target after the impressive results obtained by T-DXd in the phase III DESTINY-Breast04 trial.7 These data prompted a relevant debate to define whether HER2-low breast cancer could be considered as a new clinico-pathological entity or not.10 This meta-analysis aimed to clarify the prognostic role of HER2-low status. Overall, we included 42 studies with a total of 1 797 175 patients. We observed that HER2-low status appeared to be associated with improved OS regardless of HoR status, both in the advanced and early settings. Moreover, HER2-low status appeared to be associated with a lower rate of pCR as compared to HER2-zero status, in the overall population and HoR-positive subset, but not in triple-negative cases.
In the early setting, HER2-low status was associated with longer DFS in the overall population and in patients with HoR-positive disease, while no significant difference was found in the HoR-negative cohort. Among patients with advanced breast cancer, despite the improvement demonstrated in OS, no significant difference was detected in terms of PFS, regardless of HoR status.
An explanation for the slightly better prognosis observed in patients with HER2-low tumours might reside in HER2-low tumour biology, apparently strictly associated to HoR status. A lower prevalence of prognostically unfavoured non-luminal tumours in HoR-positive/HER2-low versus HoR-positive/HER2-zero and a direct correlation between HER2-low prevalence and HoR levels have been observed, while no molecular differences have been found in triple-negative HER2-zero versus HER2-low tumours.6,49,58 A higher prevalence of basal-like tumours in HER2-zero versus HER2-low breast cancer, driven by the higher prevalence of triple-negative disease in this former IHC category, was also reported.6,58 Hence, the more favourable prognosis of HER2-low disease might have been influenced by these underlying biological features. At the same time, the relative difference in survival between HER2-low and HER2-zero breast cancer patients is very limited and the statistical significance could be due to the high number of patients included in the analysis and heterogeneity of treatments administered. For these reasons, the better outcomes of HER2-low subgroup may probably translate into limited clinical differences.
We also evaluated the association between HER2 status and pCR. HER2-low status appeared to be associated with a lower rate of pCR as compared to HER2-zero status, regardless of HoR status. A substantial heterogeneity was detected in the pCR evaluation among the overall population, while it appeared to be low in the HoR-positive cohort analysis. The results detected in the HoR-positive population are consistent with the data published by Schettini et al.6 According to their prediction analysis of microarray 50 (PAM50) analysis, only 28.7% of HER2-zero tumours were classified as luminal A. The rate of luminal A subtypes increases when analysing HER2-low IHC 1+ cancers (49%) and HER2-low IHC 2+/ISH not amplified tumours (54.2%).6 Agostinetto et al. analysed 789 samples with available PAM50 data: among luminal A tumours, the great majority were represented by HER2-low/HoR-positive cancers (54.4%), while 33.7% were HER2-zero/HoR-positive cancers.58 These data could justify our findings, considering that luminal A breast cancer is characterized by a lower response to chemotherapy and better prognosis than the other subtypes.59,60 Considering that the HoR-positive tumours represent the majority of HER2-low breast cancer (from 64% to 93% according to literature),10 the overall population results could be mostly driven by the HoR-positive cohort. Consistently, in the HoR-negative subgroup analysis, no difference in terms of pCR was detected between HER2-low and HER2-zero breast cancer. According to the studies carried out by Schettini et al. and Agostinetto et al., the majority of triple-negative breast cancers were basal-like through PAM50 analysis, with no significant differences based on HER2 status.6,58 Coherently, within the basal-like subtype, the rates of HER2-low and HER2-zero tumours were quite similar (41.7% and 40.3%, respectively).58 Considering that triple-negative and basal-like breast cancers seem to have a good response to chemotherapy, it is not surprising that no difference was observed in pCR, irrespective of HER2 status.
Our data are overall consistent with those published by Denkert et al., who showed that patients with HER2-zero tumours not reaching pCR were those at worst prognosis.33
As regards the PFS results in the metastatic setting, no differences were found between HER2-low and HER2-zero tumours, in the overall population and regardless of HoR status. In three15,16,26 out of five studies included in the HoR-positive cohort analysis, the whole cohort was treated in the first line with cyclin-dependent kinase (CDK) 4/6 inhibitors and endocrine therapy. In the study by Gampenrieder et al., 42.7% of patients were treated with this regimen;19 only 63 out of 15 054 patients received first-line CDK 4/6 inhibitors in the study conducted by de Calbiac et al.18 These results are particularly interesting since researchers are actively looking for validated biomarkers to predict the response to CDK 4/6 inhibitors. In the overall population and HoR-negative cohort, data regarding first-line treatments were scarce. Considering the triple-negative subgroup, different regimens could be used as first-line treatment, thus preventing us from drawing solid conclusions.
Our meta-analysis has some limitations that should be considered. Firstly, our study is not an individual patient-level data meta-analysis, though it has been shown that individual-level and trial-level pooled analysis results do not diverge significantly, especially for survival data.61, 62, 63 Secondly, almost every study included in our meta-analysis was a retrospective analysis; only one study was prospective45 and data of two papers were derived from prospective/retrospective registries.19,26 Most of the data are derived from national registries, including cancers diagnosed through different decades. A central review of the tumour samples specifically for the considered analysis has been carried out only in two studies.23,39 Before the discovery of HER2-low status as a therapeutic target, the pathologists were unaware that the distinction of HER2-zero and HER2-low status could guide patient’s treatment, so that the historical scores could not be accurate enough to be fully trustable. Moreover, the staining technique and the interpretation (observer-dependent) have been slightly modified over time4 and significant discordance among pathologists in the evaluation of HER2 status at immunohistochemistry has been demonstrated, especially for HER2 1+ and 2+ categories.6,64
Furthermore, in the DAISY phase II study, a subgroup of HER2-zero breast tumours partially responded to T-DXd, with a median PFS of 4.2 months.65 These results strongly suggest that better ways of assessing which patients might benefit from T-DXd are urgently needed.
Another issue we had to consider was the heterogeneity between studies, which was high on four occasions when the pooled estimate was statistically significant (Figures 2 and 4, Supplementary Figures S5 and S10, available at https://doi.org/10.1016/j.esmoop.2023.101592). However, only one study result conflicted with the pooled estimate, and such a merely quantitative heterogeneity did not affect the direction of the pooled estimate. In another case (Supplementary Figure S10, available at https://doi.org/10.1016/j.esmoop.2023.101592), three out of nine studies diverged from the pooled estimate. Yet, they were the least powerful studies and only one reported a statistically significant result, thus not affecting the reliability of the pooled estimate.
As regards the metastatic setting, in some studies the HER2 status was assessed on the primary tumour sample,23, 24, 25 in others on the sample of the biopsy carried out on the metastatic site if available and on the primary tumour block if the metastatic tissue was not available.16,18,19,22,26,55 This could be impactful considering the potential significant discordance in terms of HER2 status between primary and metastatic disease, with 44% of breast cancers changing HER2 status from HER2-zero to HER2-low and 22% vice versa.56
By contrast, the strength of our meta-analysis is the number of patients included, amounting to 1 797 175 subjects. To the best of our knowledge, our study is the largest and most up-to-date meta-analysis assessing the prognostic value of HER2-low status as compared with HER2-zero, both in the early and metastatic settings. Furthermore, we provided a comprehensive analysis of the impact of HER2-low status on different clinical outcomes, in both the advanced and early settings. Finally, we found a specific prognostic implication in terms of OS which is consistent across both settings and all subgroups.
In conclusion, HER2-low breast cancer cannot be considered a new biologic entity and its differential prognostic features in reference to HER2-zero disease are limited and likely driven by HoR status and its underlying biology. Nevertheless, its role as a therapeutic target for novel anti-HER2 ADCs is unquestionable, though probably related only to the presence of some levels of HER2 in the tumour cell membrane. In any case, further investigations are needed to establish the possibility of de-escalating treatment in HER2-low breast cancer due to a potential slightly better prognosis over HER2-zero tumours. Ensuring the proper identification of patients with HER2-low disease has become essential to not deny patients a highly effective treatment with novel targeted agents. To achieve this goal, education and training of pathologists is an urgent need, because they should dismiss the traditional binary distinction of HER2-positive and HER2-negative disease, and accurately and reproducibly report HER2 status according to the scores of the current ASCO/CAP recommendations.4
Acknowledgements
FS is supported by a Rio Hortega contract from the Instituto de Salud Carlos III.
Funding
None.
Disclosure
CM reports support to attend medical conferences from Gilead and honoraria from Novartis and Lilly (all outside the submitted work). EA reports consultancy fees/honoraria from Eli Lilly, Sandoz, AstraZeneca, research grant to her institution from Gilead and support for attending medical conferences from Novartis, Roche, Eli Lilly, Genetic, Istituto Gentili, Daiichi Sankyo, AstraZeneca. GNM reports support to attend medical conferences from Roche and Bayer (all outside the submitted work). AP reports advisory and consulting fees from Roche, Pfizer, Novartis, Amgen, BMS, Puma, Oncolytics Biotech, MSD, Guardant Health, Peptomyc and Lilly, lecture fees from Roche, Pfizer, Novartis, Amgen, BMS, Nanostring Technologies and Daiichi Sankyo, institutional financial interests from Boehringer, Novartis, Roche, Nanostring, Sysmex Europe GmbH, Medica Scientia inno. Re-search, SL, Celgene, Astellas and Pfizer; and shares ownership and a leadership role in Reveal Genomics, SL. EB reports funding to her institution from Gilead Science. FS declares personal fees for educational activities from Novartis and Gilead and travel expenses from Gilead, Novartis and Daiichy Sankyo. GV received honoraria for advisory boards and consulting fees from Roche, AstraZeneca, Daiichi Sankyo, MSD Oncology and Pfizer. LDM reports institutional research grant from Eli Lilly, Novartis, Roche, Daiichi Sankyo and Seagen, consulting fees from Eli Lilly; honoraria from Roche, Novartis, Pfizer, Eli Lilly, AstraZeneca, Merck Sharp and Dohme, Seagen, Gilead, Pierre Fabre, Eisa, Exact Sciences and Ipsen and support for attending meetings from Roche, Pfizer and Eisai; and fees for participation on a data safety monitoring board or advisory board from Novartis, Roche, Eli Lilly, Pfizer, Daiichi-Sankyo, Exact Sciences, Gilead, Pierre Fabre, Eisai, AstraZeneca and Agendia. ML played an advisory role for Roche, Lilly, Novartis, Astrazeneca, Pfizer, Seagen, Gilead, MSD and Exact Sciences and received speaker honoraria from Roche, Daiichi Sankyo, Lilly, Novartis, Pfizer, Sandoz, Libbs and Takeda and travel grants from Gilead outside the submitted work. EDA received honoraria and/or participated to advisory board from Roche/GNE, Novartis, Seattle Genetics, Zodiac, Libbs and Pierre Fabre; received travel grants from Roche/GNE and GSK/Novartis; and received research grant to his institution from Roche/GNE, AstraZeneca, GSK/Novartis and Servier. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Harbeck N., Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 3.Schettini F., Brasó-Maristany F., Kuderer N.M., Prat A. A perspective on the development and lack of interchangeability of the breast cancer intrinsic subtypes. NPJ Breast Cancer. 2022;8(1):85. doi: 10.1038/s41523-022-00451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolff A.C., Hammond M.E.H., Allison K.H., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36(20):2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 5.Tarantino P., Hamilton E., Tolaney S.M., et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38(17):1951–1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 6.Schettini F., Chic N., Brasó-Maristany F., et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(1):1. doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modi S., Jacot W., Yamashita T., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387(1):9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration FDA Approves First Targeted Therapy for HER2-Low Breast Cancer. 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-her2-low-breast-cancer Available at.
- 9.European Medicines Agency Assessment report. https://www.ema.europa.eu/en/medicines/human/EPAR/enhertu Available at.
- 10.Molinelli C., Jacobs F., Marchiò C., et al. HER2-low breast cancer: where are we? Breast Care. 2022;17(6):533–545. doi: 10.1159/000527391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayden J.A., van der Windt D.A., Cartwright J.L., Côté P., Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 13.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Method. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 14.Watkins C., Bennett I. A simple method for combining binomial counts or proportions with hazard ratios for evidence synthesis of time-to-event data. Res Synth Methods. 2018;9(3):352–360. doi: 10.1002/jrsm.1301. [DOI] [PubMed] [Google Scholar]
- 15.Bao K.K.H., Sutanto L., Tse S.S.W., Man Cheung K., Chan J.C.H. The association of ERBB2-low expression with the efficacy of cyclin-dependent kinase 4/6 inhibitor in hormone receptor-positive, ERBB2-negative metastatic breast cancer. JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.33132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlino F., Diana A., Ventriglia A., et al. HER2-low status does not affect survival outcomes of patients with metastatic breast cancer (MBC) undergoing first-line treatment with endocrine therapy plus palbociclib: results of a multicenter, retrospective cohort study. Cancers (Basel) 2022;14(20):4981. doi: 10.3390/cancers14204981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Check D.K., Jackson B.E., Spees L., et al. Treatment patterns and health care resource use of patients with metastatic breast cancer with HER2-low expression: a cancer registry-linked insurance claims study. J Clin Oncol. 2022;40(suppl 28):399. [Google Scholar]
- 18.de Calbiac O., Lusque A., Mailliez A., et al. Comparison of management and outcomes in ERBB2-low vs ERBB2-zero metastatic breast cancer in France. JAMA Netw Open. 2022;5(9) doi: 10.1001/jamanetworkopen.2022.31170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gampenrieder S.P., Rinnerthaler G., Tinchon C., et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 2021;23(1):112. doi: 10.1186/s13058-021-01492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gampenrieder S.P., Dezentjé V., Lambertini M., et al. 177P Low HER2 expression does not influence prognosis in metastatic triple-negative breast cancer: results from an international, multicenter analysis coordinated by the Austrian Group Medical Tumor Therapy (AGMT) Ann Oncol. 2022;33:S208. [Google Scholar]
- 21.Hasan S., Neubauer Z., Press R.H., et al. Prognostic implications of HER2Neu-low in metastatic breast cancer. J Clin Oncol. 2022;40(suppl 16):1044. doi: 10.1002/cam4.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holthuis E.I., Vondeling G.T., Kuiper J.G., et al. Real-world data of HER2-low metastatic breast cancer: a population based cohort study. Breast. 2022;66:278–284. doi: 10.1016/j.breast.2022.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Abudureheiyimu N., Mo H., et al. In real life, low-level HER2 expression may be associated with better outcome in HER2-negative breast cancer: a study of the National Cancer Center, China. Front Oncol. 2022;11 doi: 10.3389/fonc.2021.774577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raghavendra AS, Liu DD, Mouabbi JA, Tripathy D. Prevalence of HER2-low among metastatic breast cancer patients and their outcomes compared to HER2 IHC 0. Paper presented at the San Antonio Breast Cancer Symposium. December 6-12, 2022; Texas, USA.
- 25.Rosso C., Voutsadakis I.A. Characteristics, clinical differences and outcomes of breast cancer patients with negative or low HER2 expression. Clin Breast Cancer. 2022;22(4):391–397. doi: 10.1016/j.clbc.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Zattarin E. HER2-low status is associated with worse clinical outcomes in hormone receptor-positive, HER2-negative advanced breast cancer patients treated with first-line cyclin-dependent kinase 4/6 inhibitors plus endocrine therapy. Paper presented at the San Antonio Breast Cancer Symposium. December 6-12, 2022; Texas, USA.
- 27.Almstedt K., Heimes A.S., Kappenberg F., et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur J Cancer. 2022;173:10–19. doi: 10.1016/j.ejca.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Alves F.R., Gil L., Vasconcelos de Matos L., et al. Impact of human epidermal growth factor receptor 2 (HER2) low status in response to neoadjuvant chemotherapy in early breast cancer. Cureus. 2022;14(2) doi: 10.7759/cureus.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen M., Chen W., Liu D., et al. Prognostic values of clinical and molecular features in HER2 low-breast cancer with hormonal receptor overexpression: features of HER2-low breast cancer. Breast Cancer. 2022;29(5):844–853. doi: 10.1007/s12282-022-01364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Moura Leite L., Cesca M.G., Tavares M.C., et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res Treat. 2021;190(1):155–163. doi: 10.1007/s10549-021-06365-7. [DOI] [PubMed] [Google Scholar]
- 31.de Nonneville A., Houvenaeghel G., Cohen M., et al. Pathological complete response rate and disease-free survival after neoadjuvant chemotherapy in patients with HER2-low and HER2-0 breast cancers. Eur J Cancer. 2022;176:181–188. doi: 10.1016/j.ejca.2022.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Denkert. Outcome analysis of HER2-zero or HER2-low hormone receptor-positive (HR+) breast cancer patients - characterization of the molecular phenotype in combination with molecular subtyping. Paper presented at the San Antonio Breast Cancer Symposium. December 6-12, 2022; Texas, USA.
- 33.Denkert C., Seither F., Schneeweiss A., et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–1161. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
- 34.Di Cosimo S., La Rocca E., Ljevar S., et al. Moving HER2-low breast cancer predictive and prognostic data from clinical trials into the real world. Front Mol Biosci. 2022;9 doi: 10.3389/fmolb.2022.996434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domergue C., Martin E., Lemarié C., et al. Impact of HER2 status on pathological response after neoadjuvant chemotherapy in early triple-negative breast cancer. Cancers. 2022;14(10):2509. doi: 10.3390/cancers14102509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douganiotis G., Kontovinis L., Markopoulou E., et al. Prognostic significance of low HER2 expression in patients with early hormone receptor positive breast cancer. Cancer Diagn Progn. 2022;2(3):316–323. doi: 10.21873/cdp.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horisawa N., Adachi Y., Takatsuka D., et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer. 2022;29(2):234–241. doi: 10.1007/s12282-021-01303-3. [DOI] [PubMed] [Google Scholar]
- 38.Iwase T, Fujii T, Yam C, et al. Quantitative estrogen receptor expression affects pathologic complete response to neoadjuvant chemotherapy in patients with early-stage breast cancer with low expression of HER2. Paper presented at the San Antonio Breast Cancer Symposium. December 6-12, 2022; Texas, USA.
- 39.Jacot W., Maran-Gonzalez A., Massol O., et al. Prognostic value of HER2-low expression in non-metastatic triple-negative breast cancer and correlation with other biomarkers. Cancers. 2021;13(23):6059. doi: 10.3390/cancers13236059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang C., Perimbeti S., Deng L., Shapiro C.L., Gandhi S. Abstract 4124: Clinical outcomes in women with resectable HER2-low breast cancer in the real world. Cancer Res. 2022;82(suppl 12):4124. [Google Scholar]
- 41.Kang S., Lee S.H., Lee H.J., et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur J Cancer. 2022;176:30–40. doi: 10.1016/j.ejca.2022.08.031. [DOI] [PubMed] [Google Scholar]
- 42.Miglietta F., Griguolo G., Bottosso M., et al. HER2-low-positive breast cancer: evolution from primary tumor to residual disease after neoadjuvant treatment. NPJ Breast Cancer. 2022;8(1):66. doi: 10.1038/s41523-022-00434-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mutai R., Barkan T., Moore A., et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast. 2021;60:62–69. doi: 10.1016/j.breast.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi W.X., Chen L., Cao L., Xu C., Cai G., Chen J. Ki-67 index provides long-term survival information for early-stage HER2-low-positive breast cancer: a single-institute retrospective analysis. J Oncol. 2022;2022:1–9. doi: 10.1155/2022/4364151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothschild HT. HER-2 low status in early stage invasive lobular carcinoma of the breast: associated factors and outcomes in an institutional series. Paper presented at the San Antonio Breast Cancer Symposium. December 6-12, 2022; Texas, USA. [DOI] [PMC free article] [PubMed]
- 46.Shao Y., Yu Y., Luo Z., et al. Clinical, pathological complete response, and prognosis characteristics of HER2-low breast cancer in the neoadjuvant chemotherapy setting: a retrospective analysis. Ann Surg Oncol. 2022;29(13):8026–8034. doi: 10.1245/s10434-022-12369-4. [DOI] [PubMed] [Google Scholar]
- 47.Sierra M. Magnetic resonance imaging (MRI) and clinicopathological analysis of triple-negative breast cancer (TNBC) patients (pts) treated with primary anthracyclines (A)/taxanes (TX)-based chemotherapy. Paper presented at the San Antonio Breast Cancer Symposium. December 6-12, 2022; Texas, USA.
- 48.Tan R.S.Y.C., Ong W.S., Lee K.H., et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis. BMC Med. 2022;20(1):105. doi: 10.1186/s12916-022-02284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tarantino P., Jin Q., Tayob N., et al. Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol. 2022;8(8):1177–1183. doi: 10.1001/jamaoncol.2022.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu H., Han Y., Wu Y., et al. Clinicopathological characteristics and prognosis of HER2-low early-stage breast cancer: a single-institution experience. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.906011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yam C, Li Z, Korkut A, et al. Clinical and molecular characteristics of HER2-low/zero early stage triple-negative breast cancer. Paper presented at the San Antonio Breast Cancer Symposium. December 6-12, 2022; Texas, USA.
- 52.Zhang G., Ren C., Li C., et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. 2022;20(1):142. doi: 10.1186/s12916-022-02346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H., Katerji H., Turner B.M., Audeh W., Hicks D.G. HER2-low breast cancers: incidence, HER2 staining patterns, clinicopathologic features, MammaPrint and BluePrint genomic profiles. Mod Pathol. 2022;35(8):1075–1082. doi: 10.1038/s41379-022-01019-5. [DOI] [PubMed] [Google Scholar]
- 54.Peiffer D.S., Zhao F., Chen N., et al. Clinicopathologic characteristics and prognosis of ERBB2-low breast cancer among patients in the National Cancer Database. JAMA Oncol. 2023;9(4):500–510. doi: 10.1001/jamaoncol.2022.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarantino P., Niman S.M., Erick T.K., et al. HER2-low inflammatory breast cancer: clinicopathologic features and prognostic implications. Eur J Cancer. 2022;174:277–286. doi: 10.1016/j.ejca.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Tarantino P., Gandini S., Nicolò E., et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer. 2022;163:35–43. doi: 10.1016/j.ejca.2021.12.022. [DOI] [PubMed] [Google Scholar]
- 57.McGuinness L.A., Higgins J.P.T. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Syn Meth. 2021;12(1):55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 58.Agostinetto E., Rediti M., Fimereli D., et al. HER2-low breast cancer: molecular characteristics and prognosis. Cancers (Basel) 2021;13(11):2824. doi: 10.3390/cancers13112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prat A., Pineda E., Adamo B., et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24:S26–S35. doi: 10.1016/j.breast.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Jensen M.B., Lænkholm A.V., Nielsen T.O., et al. The Prosigna gene expression assay and responsiveness to adjuvant cyclophosphamide-based chemotherapy in premenopausal high-risk patients with breast cancer. Breast Cancer Res. 2018;20(1):79. doi: 10.1186/s13058-018-1012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gulia S., Kannan S., Ghosh J., Rath S., Maheshwari A., Gupta S. Maintenance therapy with a poly(ADP-ribose) polymerase inhibitor in patients with newly diagnosed advanced epithelial ovarian cancer: individual patient data and trial-level meta-analysis. ESMO Open. 2022;7(5) doi: 10.1016/j.esmoop.2022.100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tierney J.F., Fisher D.J., Burdett S., Stewart L.A., Parmar M.K.B. Comparison of aggregate and individual participant data approaches to meta-analysis of randomised trials: an observational study. PLoS Med. 2020;17(1) doi: 10.1371/journal.pmed.1003019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tudur Smith C., Clarke M., Marson T., et al. A framework for deciding if individual participant data are likely to be worthwhile. Abstracts of the 23rd Cochrane Colloquium, Vienna, Austria. Cochrane Database Syst Rev. 2015;10(suppl) RO 6.1. [Google Scholar]
- 64.Robbins C.J., Fernandez A.I., Han G., et al. Multi-institutional assessment of pathologist scoring HER2 immunohistochemistry. Mod Pathol. 2023;36(1) doi: 10.1016/j.modpat.2022.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosele M.F., Lusque A., Dieras V., et al. LBA1 Unraveling the mechanism of action and resistance to trastuzumab deruxtecan (T-DXd): biomarker analyses from patients from DAISY trial. Ann Oncol. 2022;33:S123. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.