ABSTRACT
Background:
Hepatocellular adenomas (HCAs) are benign tumours that may be broken down into three different molecular pathogenic categories: catenin activator, hepatic cell nuclear agent 1 (HNF- 1) that has been inactivated, and Inflammatory hepatic adenomas are a genetic and pathological subtype of hepatic adenoma.
Methodology:
An analysis of 50 HCA cases was conducted to identify the magnetic resonance imaging characteristics that were specifically related to each HCA subtype IV. This method included 50 patients in total for the study, with 30 of them being new cases. Four cases involving medicine, pathology, surgery, and radiology were gathered and examined.
Results:
As per these analyses for inactivated HNF-1, the sure predictive esteem about homogeneous indicator spillage on the compound shift pictures could have been as high as 100%, negative predictive quality could have been as high as 94.7%, affectability could have been as high as 86.7%, and specificity could have been as high as 100%. Enhancement of the solid blood vessels to support the ongoing and future stages of the portal vein change. It took a certain predictive quality of 88.5%, a negative predictive worth of about 84%, an affectability of about 85.2%, and more specificity of about 87.5% to diagnose incendiary HCA from the predominant signs seen for T2W successions linked with late constant upgrades.
Conclusions:
Both HNF-1–mutated HCAs and incendiary HCAs need to be associated with specific magnetic resonance imaging phenotypes characterized independently as having diffused lipid repartition and sinusoidal expansion.
KEYWORDS: Hepatocellular adenomas, HNF-1, MRI
INTRODUCTION
Hepatocellular adenoma, also known as HCA, is an uncommon form of benign monoclonal liver tumour that primarily affects young women who are users of oral contraceptives. When it comes to biallelic-inactivating transmutations, the T cell factor 1 (TCF-1) gene that is responsible for inactivating hepatocyte atomic Figure 1 (HNF-1) needs an identifier of between 35% and 50% for HCAs.[1-4] In addition, HCAs that have HNF-1 mutations show an inhibition of gluconeogenesis, activation of glycolysis and the citrate cycle, and fatty liver synthesis, which leads to secondary estimated ratios of lipogenesis,[5] and Liver Fatty acid-binding protein (L-FABP), which stands for liver-type fatty acid–binding protein.[1] A -catenin transformation that activated the Wnt/-catenin pathway must also have been identified in more than 15% and up to 18% of HCA patients,[2-4,6] enacting changes about -catenin would likewise exist for 20% with 40% about hepatocellular carcinomas (HCCs), which means -catenin will be the practise every now and then actuated oncogene for HCC[7] (to review, see Laurent-Puig’s study) (for more information on this topics see \Laurent-Puig’s and Zucman-Rossi studies).[8]
Figure 1.
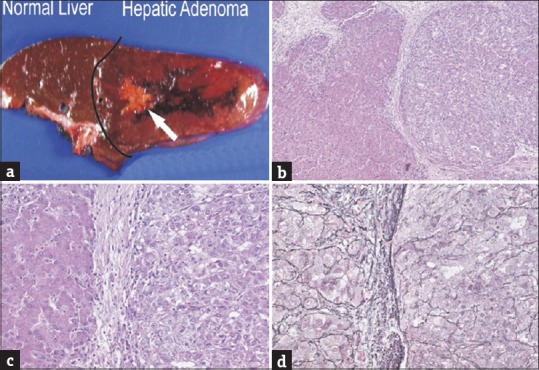
The hepatocellular carcinomas were recognized grossly in two cases. In this case, the carcinoma had a yellow stellate appearance as pointed out by the white arrow (panel a, tumor size is 2.5 cm); low power magnification of the interface between hepatic adenoma on the left and hepatocellular carcinoma on the right (panel b, original magnification × 20); the same field as panel b is shown at higher magnification (panel c, original magnification × 64); the same fields as panels b and c showing loss of reticulin in the carcinoma on the right of this image (panel d, original magnification × 64)
These tumours, which are occasionally related to obesity due to its association with poor diet and lack of exercise. In addition, the consumption of alcoholic beverages demonstrates a robust outflow of the acute phase of the incendiary response, with large amounts for both dispatchers running. Additionally, the protein for serum amyloid a (SAA) and C-reactive protein (CRP)[9] over you stop offering on that one over six instances of incendiary HCA also show the transformation of -catenin. There will be a significant emphasis placed on recording the extraordinarily variable presence of HCA for computed tomography (CT), magnetic resonance imaging (MRI), and contrast-enhanced ultrasonography filters. Which of the contrasting histological characteristics[10-15] are more likely to occur, starting with the most likely outcomes.
As a result, we conducted research on 50 different HCA cases to identify MRI characteristics that were particularly connected to the various HCA subtypes IV.
MATERIALS AND METHODS
One hundred and twenty four people who were affected by HCA underwent surgery at our foundation or one of our subsidiary clinics. Prior to this, 48 cases were identified, all of which featured liver MRI characteristics. In addition, material pertaining to suitable obsessiveness was easily accessible. The remaining 76 cases were not included in this analysis because either there were insufficient MRI data (n = 70) or the nodules were either completely hemorrhagic or necrotic (n = 6). Both of these reasons led to the exclusion of these cases. They are accompanied in turn by 20 HCA cases which did not undergo resection (additional previous late cases); liver biopsies which were required were performed, and fitting MRI information was accessible for both from claiming these instances. In total, there were 50 patients involved in the study using this method; 30 of them claimed to have previously served as a news person. We gathered and analyzed information pertaining to medical, pathological, surgical, and radiological procedures.
Histopathological Examination: To prepare the tissue examples, which included 48 careful examples and two biopsy examples, the following steps were taken: Masson’s trichrome, hematoxylin and eosin, reticulin and Perl’s stain, and CK 7 and immune staining for path/atomic neurotic characterization; these are the same markers as L-FABP, -catenin, glutamine synthetase (GS), SAA, and CRP, which have already been covered. When placing our order for the knobs, we made use of the accompanying staining information.
The evaluations were carried out using a closed magnet framework with a strength of 1.5 T. At the Hospital of Bordeaux University (Sonata, Siemens Medical System, Erlangen, Germany; Achieva, Philips Medical Systems, Best, The Netherlands), preactive MRI evaluations were performed on 32 patients using committed stomach-staged cluster curls for signal gathering.
Image Assessment: All of the MRI information surveys were carried out by two stomach radiologists (H. L. and H. T.) who were unaware of the obsessive outcomes and order, and they came to a consensus on what should be done with the data. We did this in a number of different sores, but the largest resected HCA was the one we broke down.
In the statistical analysis, the Student’s t-test is used to determine whether or not there was a difference in the diverse subtype’s average size of HCAs that was statistically significant.
RESULTS
There were only two males among the total of 50 samples. The average age was 38.5 years with a standard deviation of 7.7 years; 20 of the patients had a knob, 22 patients had between two and 10 knobs, and eight patients had more than 10 knobs [Table 1]. The largest knob had an average size of 64.7 mm, plus or minus 27 millimetres. There was not a significant difference in the size of the largest knob between HNF-1–deactivated (67 mm 32) and fiery (57 mm 21) subtypes (P =0.23) or between HCAs with no markers (63 mm 32) and HNF-1-deactivated or provocative HCAs (P =0.63 and. 73, respectively). A total of 10 HCAs, four of which were incendiary, two of which were HNF-1–inactivated, and four of which lacked any markers, were found to contain both hemorrhagic and necrotic components. The HCA had an average size of 75 mm 36 mm when there was rot or subacute drain present. There was not a significant difference in mean size between HCAs that were necrotic and hemorrhagic and HCAs that were not necrotic and hemorrhagic (P =.15). In 10 of the liver samples, we discovered steatosis in the solid portions of the organ (eight with incendiary HCAs; two with HCAs with no markers). In the parenchyma of liver contiguous HNF-1–deactivated HCAs, steatosis had never been observed; however, eight of the 26 people who were affected by the condition had fiery HCAs (P =.014).
Table 1.
MRI characteristics of HCA Subclass
| Group 1: L-FABP (n=15) | Group 2: SAA + (n=27) | Group 3: β-Catenin + (n=2) | Group 4: Unclassified (n=7) | |
|---|---|---|---|---|
| Diameter (mm) | 57+21 | 67±30 | 101±11 | 63±2 |
| Necrotic/hemorrhagic component | 12 (2) | 25 (4) | 0 | 71 (5) |
| Liver steatosis | 0 | 31 (8) | 0 | 29 (2) |
| T1W signals | ||||
| Hypo/Iso | 87 (13) | 74 (20) | 100 (2) | 43 (3) |
| Dropouts of signals | 93 (14) | 11 (3) | 0 | 14 (1) |
| Hyper | 13 (2) | 26 (7) | 0 | 57 (4) |
| OP T1W | ||||
| Homogeneous repartition of dropouts of signal | 87 (13) | 0 | 0 | 0 |
| T2W signals | ||||
| Homogenous | 87 (13) | 44 (12) | 0 | 0 |
| Hyper | 53 (0) | 15 (4) | 0 | 43 (0) |
| Hypo/Iso | 47 (7) | 0 | 100 (2) | 57 (4) |
| High signals peripheral rim | 0 | 81 (22) | 0 | 0 |
| Noticeably hyper intense | 0 | 85 (23) | Focal 100 (2) | 0 |
Each and every one of the knobs from a similar patient that were examined using immunohistochemistry belonged to the same subtype. This was the case in each and every instance of different knobs being arranged in the HNF-1–transformed and provocative gatherings. However, the severity of SAA and CRP overproductions varied between knobs obtained from different affected individuals who had varying degrees of incendiary HCA injuries. In a patient who had multiple incendiary HCA, some of the knobs also tested positive for -catenin (using immune histochemistry and atomic science), whereas other knobs did not. In light of the fact that these two cancers had a comparative immune histochemical profile and unique MRI highlights, we analyzed the images of two resected HCAs from a total of 26 samples. We looked into one HCA for each individual patient across the broad spectrum of possible scenarios. HCA knobs were found to be associated with three different types of liver tumours in 10 patients: common Focal nodular hyperplasia (FNH) (found in four patients), hemangioma (found in five patients), and hepatobiliary cystadenoma (one patient). Within the scope of this affiliation is the performance of immunohistochemical staining of the FNH. There was no increased production of either SAA or CRP in any of the FNHs that exhibited typical immune staining for L-FABP, similar to that which was observed in the surrounding nontumoral liver.
To begin, a signal failure was found on the substance shift arrangement by surveying greasy hepatocytes in 14 of the 15 HNF-1–deactivated HCAs, but in only three provocative HCAs with zero -catenin-activated HCAs or uncategorized HCAs (P =0.001). The diffused and homogeneous sign was present in certain HNF-1–inactivated HCAs (13 of 14 cases), but it was heterogeneous and central in the three positive incendiary HCAs (P =0.0001), which is a statistically significant difference. There was not a single instance of consistent sign failure in any of the unclassified or -catenin-activated HCAs. The affectability appeared to be 86.7%, and the explicitness was 100%. The positive MRI highlight prescient worth (homogeneous sign failure of the growth on synthetic shift pictures) for the determination of HNF-1–deactivated HCA in the 51 HCAs populace reaches 100%, the negative prescient worth was 94.7%, and the explicitness was 100%.
Second, in contrast to incendiary HCAs (see note below), the majority of HNF-1–inactivated HCAs (13 of 15 wounds) showed only a moderate improvement in the blood vessels, and none of the wounds showed consistent improvement during the delayed stage. There were no other MRI highlights overall that were connected with HNF-1 that was inactivated [Table 1].
X-ray contains the potentially inflammatory HCA. Essentially, fiery HCA was connected to the accompanying MRI highlights.
All incendiary HCAs (27 broken down cases) had a signal of hyperintense on T2W pictures, but only eight HNF-1–deactivated HCAs did (P =0.001 for both groups). The sign was particularly hyper intense in 23 of 27 fiery HCAs, however in none of the HNF-1α–inactivated HCAs (P <.001). An objective component with a more grounded signal in the external portion of the injury was found in 22 fiery HCAs, but it was not found in any of the HCAs whose HNF-1 had been inactivated. It was found that 25 of 27 fiery HCAs had improved blood vessel function, while only two HNF-1–deactivated HCAs did (P =0.001 for both comparisons). Continuous improvement during the deferred stage was observed in 24 of the 25 HCAs that yielded positive incendiary results. A relationship of uniquely hyperintense signals on T2W pictures and constant improvement on deferred stage T1W pictures in 23 of 27 fiery HCAs, but only in three HNF-1–deactivated HCAs and three uncategorized HCAs (P =.0001) was discovered. In this population of 51 HCAs, the positive predictive value of dual MRI highlights for the conclusion of provocative telangiectatic HCA reached 88.5%, the negative predictive value was 84%, the affectability was 85.2%, and the particularity was 87.5%. Additional MRI highlights that were not observed to be fundamentally connected with incendiary HCA are presented in Table 1.
DISCUSSION
As per this study, an HNF-1–inactivated HCA can be positively identified on MRI if there is observed homogeneous fat dissemination, particularly on compound shift arrangements. Exhaustive investigations into a large number of HCA have revealed stamped steatosis to be a component that is common to almost all instances of HNF-1–inactivated HCA.[3,4] This steatosis is brought on by the beginning of the lipogenic process.[5] The combination of especially hyperintense signals on T2W pictures and persistent improvement in the delayed stage were observed as sensitive (85.2% of radiological findings of fiery HCA in the population of 50 HCAs) and explicit (87.5% of radiological findings of fiery HCA, respectively). An objective-like component was seen for the vast majority of these wounds on the T2W images, with an exceptionally concentrated energy signal framing an edge in the external portion of the wound. The edges and the telangiectatic region appeared to have a reasonable relationship with one another.
These three cancers displayed specific MRI characteristics of provoking HCA, including extremely hyperintense signals on T2W images and a persistent progression in the delayed stage.[14,15]
CONCLUSIONS
This study shows MRI information and genotype/aggregate grouping are decently connected for the two significant subtypes. Now, clinical and biochemical information might vary between HNF-1α–changed HCA and fiery HCA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rebouissou S, Bioulac-Sage P, Zucman-Rossi J. Molecular pathogenesis of focal nodular hyperplasia and hepatocellular adenoma. J Hepatol. 2008;48:163–70. doi: 10.1016/j.jhep.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Bluteau O, Jeannot E, Bioulac-Sage P, Marqués JM, Blanc JF, Bui H, et al. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet. 2002;32:312–5. doi: 10.1038/ng1001. [DOI] [PubMed] [Google Scholar]
- 3.Marquardt JU, Thorgeirsson SS. Next-generation genomic profiling of hepatocellular adenomas:A new era of individualized patient care. Cancer Cell. 2014;25:409–11. doi: 10.1016/j.ccr.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, et al. Genotype-phenotype correlation in hepatocellular adenoma:new classification and relationship with HCC. Hepatology. 2006;43:515–24. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 5.Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa Cunha A, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–8. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 6.Rebouissou S, Imbeaud S, Balabaud C, Boulanger V, Bertrand-Michel J, Tercé F, et al. HNF1alpha inactivation promotes lipogenesis in human hepatocellular adenoma independently of SREBP-1 and carbohydrate-response element-binding protein (ChREBP) activation. J Biol Chem. 2007;282:14437–46. doi: 10.1074/jbc.M610725200. [DOI] [PubMed] [Google Scholar]
- 7.Chen YW, Jeng YM, Yeh SH, Chen PJ. P53 gene and Wnt signaling in benign neoplasms:Beta-catenin mutations in hepatic adenoma but not in focal nodular hyperplasia. Hepatology. 2002;36:927–35. doi: 10.1053/jhep.2002.36126. [DOI] [PubMed] [Google Scholar]
- 8.Damalas A, Ben-Ze'ev A, Simcha I, Shtutman M, Leal JF, Zhurinsky J, et al. Excess beta-catenin promotes accumulation of transcriptionally active p53. EMBO J. 1999;18:3054–63. doi: 10.1093/emboj/18.11.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niu ZS, Niu XJ, Wang WH. Genetic alterations in hepatocellular carcinoma:An update. World J Gastroenterol. 2016;22:9069–95. doi: 10.3748/wjg.v22.i41.9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bioulac-Sage P, Laumonier H, Laurent C, Zucman-Rossi J, Balabaud C. Hepatocellular adenoma:What is new in 2008. Hepatol Int. 2008;2:316–21. doi: 10.1007/s12072-008-9075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van der Borght S, Libbrecht L, Katoonizadeh A, Aerts R, Nevens F, Verslype C, Roskams TA. Nuclear beta-catenin staining and absence of steatosis are indicators of hepatocellular adenomas with an increased risk of malignancy. Histopathology. 2007;51:855–6. doi: 10.1111/j.1365-2559.2007.02862.x. [DOI] [PubMed] [Google Scholar]
- 12.Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, et al. Imaging for the diagnosis of hepatocellular carcinoma:A systematic review and meta-analysis. Hepatology. 2018;67:401–21. doi: 10.1002/hep.29487. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad NA, Kochman ML, Lewis JD, Ginsberg GG. Can EUS alone differentiate between malignant and benign cystic lesions of the pancreas? Am J Gastroenterol. 2001;96:3295–300. doi: 10.1111/j.1572-0241.2001.05328.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang N-W, Shen S, Qiu L, Wang F, Liu S. Triphasic dynamic computed tomography of hepatica adenoma and analysis of pathologic characteristics. J Med Imaging Health Inform. 2017;7:1277–80. [Google Scholar]
- 15.Laumonier H, Bioulac-Sage P, Laurent C, Zucman-Rossi J, Balabaud C, Trillaud H. Hepatocellular adenomas:Magnetic resonance imaging features as a function of molecular pathological classification. Hepatology. 2008;48:808–18. doi: 10.1002/hep.22417. [DOI] [PubMed] [Google Scholar]


