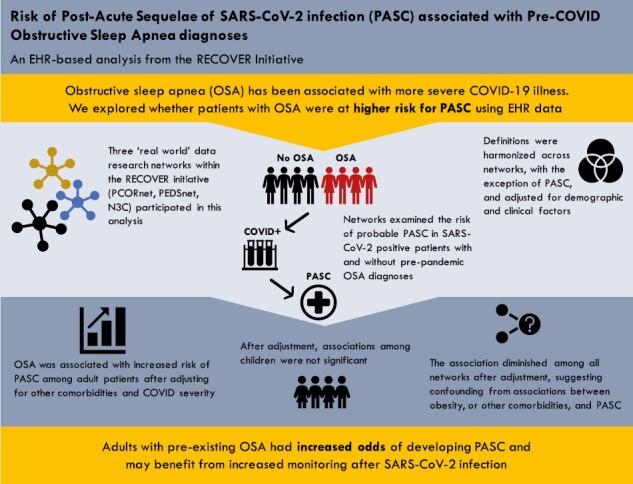
Abstract
Study Objectives
Obstructive sleep apnea (OSA) has been associated with more severe acute coronavirus disease-2019 (COVID-19) outcomes. We assessed OSA as a potential risk factor for Post-Acute Sequelae of SARS-CoV-2 (PASC).
Methods
We assessed the impact of preexisting OSA on the risk for probable PASC in adults and children using electronic health record data from multiple research networks. Three research networks within the REsearching COVID to Enhance Recovery initiative (PCORnet Adult, PCORnet Pediatric, and the National COVID Cohort Collaborative [N3C]) employed a harmonized analytic approach to examine the risk of probable PASC in COVID-19-positive patients with and without a diagnosis of OSA prior to pandemic onset. Unadjusted odds ratios (ORs) were calculated as well as ORs adjusted for age group, sex, race/ethnicity, hospitalization status, obesity, and preexisting comorbidities.
Results
Across networks, the unadjusted OR for probable PASC associated with a preexisting OSA diagnosis in adults and children ranged from 1.41 to 3.93. Adjusted analyses found an attenuated association that remained significant among adults only. Multiple sensitivity analyses with expanded inclusion criteria and covariates yielded results consistent with the primary analysis.
Conclusions
Adults with preexisting OSA were found to have significantly elevated odds of probable PASC. This finding was consistent across data sources, approaches for identifying COVID-19-positive patients, and definitions of PASC. Patients with OSA may be at elevated risk for PASC after SARS-CoV-2 infection and should be monitored for post-acute sequelae.
Keywords: Post-acute sequelae COVID-19, Long COVID, Chronic COVID-19 Syndrome, Late sequelae of COVID-19, Long haul COVID-19, Long-term COVID-19, Post COVID-19 syndrome, Post-acute COVID-19, Post-acute sequelae of SARS-CoV-2 infection, Sleep Apnea, Obstructive
Graphical Abstract
Statement of Significance.
Recent studies have identified a positive association between obstructive sleep apnea (OSA) and acute coronavirus disease-2019 (COVID-19) outcomes, but few studies have examined outcomes beyond acute illness. This is the first large-scale, multi-cohort collaboration to examine the role of preexisting OSA on risk for Post-Acute Sequelae of SARS-CoV-2 (often referred to as Long COVID). Notably, our analysis was conducted across multiple data sources, employed different approaches for identifying COVID-19-positive patients, and used multiple definitions of Long COVID. While we found consistent evidence of elevated risk for Long COVID among adults with preexisting OSA, additional research is needed to elucidate the role of severity, and treatment.
Introduction
Post-Acute Sequelae of SARS-CoV-2 infection (PASC) refers to ongoing, relapsing, or new symptoms, or other health effects occurring after the acute phase of SARS-CoV-2 infection (i.e. present four or more weeks after the acute infection). Recent studies estimate that between 7% and 54% of coronavirus disease-2019 (COVID-19) patients may develop PASC, and that risk may vary by sex, age, and certain preexisting conditions [1–3]. However, the impact of preexisting conditions on the risk of developing PASC is not well elucidated.
Obstructive sleep apnea (OSA) has been proposed as a potential risk factor for PASC, warranting further research [4]. OSA is characterized by repeated obstruction of airways during sleep, resulting in disrupted breathing, interrupted sleep, and blood oxygen desaturation. OSA is highly prevalent, affecting approximately twenty percent of adults in the United States, and is associated with a high risk of a number of comorbidities including obesity, hypertension, and diabetes [5].
Recent studies have identified a positive association between OSA and acute COVID-19 outcomes [6–8]. In a 2022 meta-analysis, Hu et al. found that patients with OSA were at higher risk for fatal COVID-19 after adjusting for age and acute infection hospitalization status [7]. Similarly, Hariyanto and Kurniawan identified a significant association between OSA and severe COVID-19, intensive care unit admission, ventilation, and mortality in a meta-analysis of 21 studies [6]. Looking beyond acute outcomes, Labarca et al. identified significantly higher rates of abnormal pulmonary function and mental impairment among COVID-19 survivors with versus without preexisting comorbid OSA one year after COVID-19 infection [4], and multiple studies found increased sleep-related symptoms or disordered sleep in PASC patients [9, 10].
Prior studies have demonstrated that algorithms designed to identify patients with an OSA diagnosis from electronic health records (EHRs) have excellent validity. For example, in a multisite analysis of six institutions, Keenan et al reported a positive predictive value of 97.1 and negative predictive value of 95.5 using a diagnosis-based computable phenotype (CP) [11]. As part of the National Institutes of Health (NIH)-funded REsearching COVID to Enhance Recovery (RECOVER) Initiative, three “real-world data analysis” research teams collaborated to assess the impact of preexisting OSA on the risk for PASC in adults and children, through a harmonized analysis across all three RECOVER EHR research networks [12].
Methods
Participating institutions
RECOVER is a large NIH-funded initiative designed to investigate the long-term effects of COVID-19. RECOVER includes projects leveraging real-world data from EHRs to develop and validate algorithms to detect PASC for clinical and epidemiological characterization, risk factor prediction, and investigation of prevention and treatment opportunities. RECOVER’s EHR-based efforts include research teams from three participating research networks: the National COVID Cohort Collaborative (N3C); the National Patient-Centered Clinical Research Network (PCORnet) limited to adults aged 18 and older; and PCORnet’s pediatric population, anchored in PEDSnet, a pediatric learning health system within PCORnet. These networks are coordinated by a clinical science core (CSC) at NYU Langone Health. For this study, the RECOVER EHR networks and CSC collaborated to produce a harmonized analysis investigating the impact of preexisting OSA on the risk of probable PASC. N3C drew analyses from over 15 million patients across 77 data partners in the N3C Enclave [13]. Of the 77 participating sites, 13 were removed due to data quality issues. PCORnet selected from 11 million patients from 19 sites including institutions within the INSIGHT, ReachNet, OneFlorida, STAR, Greater Plain Collaborative, and PaTH Clinical Research Networks, and PEDSnet selected from 8.5 million patients across eight pediatric health systems.
Analysis Plan
For this study, in collaboration with the CSC, each network independently generated distinct CP definitions to identify probable PASC patients (Table 1) and applied those definitions in a harmonized analysis to examine the risk of probable PASC in patients with and without evidence of an OSA diagnosis prior to pandemic onset. CPs included a machine learning definition trained on patients who previously visited a Long COVID clinic [14], and rules-based definitions involving clinical diagnoses, labs, and medications.
Table 1.
PASC Computable Phenotype Definition Across Three EHR Networks
| Network | Approach | PASC computable phenotype |
|---|---|---|
| N3C | Machine-learning-based definition | Adult patients with all of the following within 100 to 190 days after the index date: • ≥18 years old • ≥2 days of data • ≥1 diagnosis and ≥1 medication in the pre-/post-index period Who are likely to have PASC based on a model trained to identify patients who have previously visited a Long COVID clinic |
| PCORnet | Rules-based definition | Adult patients with all of the following: • ≥1 diagnosis and ≥1 medication in the pre-/post-index period. • ≥1 incident diagnosis belonging to a list of 25 CCSR codes within 30 to 180 days after the index date (truncated at end of study period) with no prior diagnosis recorded before the index date. The 25 CCSR codes are established to have higher incidence rates in COVID positive patients, derived through prior literature and clinician review. |
| PEDSnet | Rules-based definition | Pediatric patients with any of the following within 28 to 179 days after the index date: • Clinician-assigned PASC or MIS-C diagnosis. • ≥2 occurrences of diagnosis codes for one of the following: ° Abnormal liver enzymes ° Myocarditis ° Anosmia ° Myositis ° Dysgeusia ° Pericarditis ° Chest pain ° Thrombophlebitis/thromboembolism ° COVID-19 |
Networks took different approaches to identify patients with probable PASC within the EHR. CCSR, Clinical Classifications Software Refined; MIS-C, Multisystem Inflammatory Syndrome in Children.
Patients were eligible for inclusion in this analysis if they had a documented COVID-19 infection within the study period (March 1, 2020–February 28, 2022), as determined by a positive SARS-CoV-2 polymerase chain reaction or antigen test. If there were multiple indicators of positivity for COVID-19 infection within the period, we considered the earliest instance to be the index event. We excluded patients with sparsely populated records, requiring all patients included to have two or more visits within the health system within the two years before their index event. As sufficient time is necessary to develop PASC after an acute infection, we also excluded patients if they died within 30 days of the index event. To clearly differentiate results for adults and children, N3C and PCORnet restricted their analyses to adults aged 21 and older, while PEDSnet was restricted to children under age 21 (Supplementary Figure 1).
Given challenges in leveraging polysomnography and symptom questionnaire data within multisite EHR-based analyses, we identified patients as having preexisting OSA if they had documentation of at least two records of any relevant International Statistical Classification of Diseases and Related Health Problems - Clinical Modification (ICD-10-CM) diagnostic codes (G47.30, G47.33, G47.39; ICD-9-CM: 327.20, 327.23, 327.29, 780.51, 780.53, and 780.57) or the equivalent Systematized Nomenclature of Medicine - Clinical Terms (SNOMED-CT) codes on separate days. This approach has been leveraged for prior analyses [11, 15] and demonstrated success over multiple sites. We sought evidence of OSA within two years prior to the study period (March 1, 2018– March 1, 2020), the duration of historical EHR data that was consistently available across all three cohorts.
Statistical analysis
We estimated unadjusted and adjusted odds ratios (ORs) using logistic regression models with 95% confidence intervals to evaluate the association between a preexisting diagnosis of OSA and probable PASC. We developed three adjusted models: (1) adjusting for age group (N3C and PCORnet: ([21–35, 36–45, 46–55, 56–65, and ≥65], PEDSnet [0–4, 5–9, 10–15, and 16–20]), sex, and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian, other, and unknown), (2) additionally adjusting for hospitalization status during the index SARS-CoV-2 infection as a proxy for infection severity, a risk factor for PASC [16], with documentation of hospitalization and COVID-19 or nonspecific active infection within one day prior to 16 days following the index event (not hospitalized, hospitalized, or hospitalized with evidence of Intensive Care Unit admission, ventilator use, or vasopressors), and (3) additionally adjusting for obesity (N3C and PCORnet: body mass index ≥30 or >1 diagnosis code, PEDSnet: body mass index percentile ≥ 95th using NHANES norms) and comorbidities (N3C and PCORnet: Charlson Comorbidity Index [CCI [17]], PEDSnet: Pediatric Medical Complexity Algorithm [PMCA [18]]) within two years prior to the index event. For this study, OSA codes were excluded from the PMCA.
As a sensitivity analysis, we then repeated our analyses using a wider set of eligibility criteria, identifying patients as COVID-19 positive based on lab results in addition to one inpatient or two outpatient instances of code U07.1 (ICD-10-CM). We also varied our initial models to investigate whether adjusting for (1) preexisting hypertension and diabetes instead of comorbidity score, (2) SARS-CoV-2 variant epoch of the index infection, or (3) pediatric asthma influenced the association, in addition to an interaction analysis by sex.
Results
We have summarized demographic characteristics of patients identified through positive lab tests from each network in Table 2. The N3C network comprised 1 783 940 COVID-19 patients. A total of 3.9% of N3C patients had preexisting OSA and 4.9% were identified as having probable PASC. The PCORnet network comprised 333 642 patients, of which 5.1% had preexisting OSA and 16.6% were identified with probable PASC. Finally, the PEDSnet network comprised 106 262 pediatric patients, of which 1.8% had preexisting OSA and 4.6% were identified as having probable PASC.
Table 2.
Patient Characteristics by EHR Network
| N3C | PCORnet | PEDSnet | |
|---|---|---|---|
| Total | 1 783 940 | 333 642 | 106 262 |
| Age | |||
| 0–4 | — | — | 37 852 (35.62%) |
| 5–9 | — | — | 23 937 (22.53%) |
| 10–15 | — | — | 28 374 (26.7%) |
| 16–20 | — | — | 16 099 (15.15%) |
| 21–35 | 493 596 (27.67%) | 86 503 (25.93%) | — |
| 36–45 | 328 361 (18.41%) | 57 766 (17.31%) | — |
| 46–55 | 327 465 (18.36%) | 56 181 (16.84%) | — |
| 56–65 | 310 112 (17.38%) | 58 837 (17.63%) | — |
| ≥66 | 324 406 (18.18%) | 74 355 (22.29%) | — |
| Sex | |||
| Male | 678 446 (38.03%) | 133 161 (39.91%) | 54 679 (51.46%) |
| Female | 1 104 905 (61.94%) | 200 393 (60.06%) | 51 577 (48.54%) |
| Unknown | 589 (0.03%) | 88 (0.03%) | 6 (0.01%) |
| Race/ethnicity | |||
| Non-Hispanic white | 1 215 790 (68.15%) | 167 431 (50.18%) | 45 953 (43.24%) |
| Non-Hispanic black | 229 908 (12.89%) | 60 424 (18.11%) | 23 404 (22.02%) |
| Hispanic | 189 593 (10.63%) | 45 544 (13.65%) | 17 684 (16.64%) |
| Non-Hispanic Asian | 28 251 (1.58%) | 10 881 (3.26%) | 3717 (3.5%) |
| Native Hawaiian or Other Pacific Islander | — | 533 (0.16%) | — |
| American Indian or Alaska Native | — | 834 (0.25%) | — |
| Other | 29 360 (1.65%) | 10 509 (3.15%) | 7998 (7.53%) |
| Missing/Unknown | 91 038 (5.10%) | 37 486 (11.24%) | 7506 (7.06%) |
| Hospitalization a | |||
| Hospitalized | 149 308 (8.37%) | 59,056 (17.70%) | 5009 (4.71%) |
| Hospitalized with ICU level care | 17 578 (0.99%) | 15,838 (4.75%) | 949 (0.89%) |
| Variant epoch of index infection | |||
| Alpha (Oct 1, 2020–Jun 30, 2021) | 687 524 (38.54%) | 119 952 (35.95%) | 6230 (5.86%) |
| Ancestral (Mar 1, 20/20–Sept 30, 2020) | 175 714 (9.85%) | 55 393 (16.60%) | 34 135 (32.12%) |
| Delta (Jul 1, 2021–Nov 30, 2021) | 346 083 (19.40%) | 48 562 (14.56%) | 21 661 (20.38%) |
| Omicron (Dec 1, 2021–Present) | 574 619 (32.21%) | 109 735 (32.89%) | 44 236 (41.63%) |
| PASC | |||
| Has probable PASC | 86 966 (4.87%) | 55 219 (16.55%) | 4902 (4.61%) |
| PMCA body system count b | |||
| 0 | — | — | 74 645 (70.25%) |
| 1 | — | — | 20 001 (18.82%) |
| 2 | — | — | 6187 (5.82%) |
| 3-4 | — | — | 3586 (3.37%) |
| 5-17 | — | — | 1843 (1.73%) |
| CCI scorec | — | ||
| Mean ± SD | 1.00 (1.88) | 0.52 (1.11) | — |
| Clinical conditions | |||
| Preexisting obstructive sleep apnea | 69 590 (3.90%) | 17 126 (5.13%) | 1860 (1.75%) |
| Obesityd | 458 025 (25.67%) | 102 963 (30.86%) | 16 583 (15.61%) |
| Essential hypertensione | 493 321 (27.65%) | 107 915 (32.34%) | 1292 (1.22%) |
| Diabetesf | 228 701 (12.82%) | 53 722 (16.10%) | 877 (0.83%) |
| Asthmag | — | — | 1134 (1.07%) |
Clinical and demographic characteristics of patients meeting the study inclusion criteria. CCI (Charlson Comorbidity Index).
aHospitalized with no indication of severe illness, or hospitalization with evidence of ventilation, admittance to the Intensive Care Unit, or vasopressors. Hospitalization must have taken place on the day prior through 16 days following the index event, with COVID-19 diagnosis, or other indication of active infection documented during the same timeframe.
bPediatric Medical Complexity Algorithm (PMCA) Body System Count was used as a measure of comorbidities in children.
cCCI Score was used as a measure of comorbidities in adults.
dMultiple diagnosis codes or a Body Mass Index ≥ 30 (adults), or Body Mass Index ≥ 95th percentile per Centers for Disease Control and Prevention guidelines (children), within two years prior to the index event.
eEncompasses essential hypertension in adults or children within two years prior to the index event.
fEncompasses Type 2 Diabetes in adults, and Type 1 or 2 Diabetes in children, within two years prior to the index event.
gFor pediatric patients aged ≥5 years at their most recent diagnosis or medication prescription, asthma within two years prior to the index event. Asthma status was determined using a computable phenotype described by Ashfar et al. [19] and modified for application to PEDSnet data. Patients were identified as having asthma if they met the following criteria: Either (1) asthma diagnoses on two separate dates, or (2) an asthma diagnosis code and asthma medication.
We estimated ORs for probable PASC by comparing patients with and without a preexisting OSA diagnosis (Table 3 and Supplementary Figure 2). The unadjusted odds of probable PASC were 1.41–3.93 times greater among adults with a preexisting OSA diagnosis than those without evidence of OSA (N3C, OR: 3.93, 95% CI: 3.84, 4.02; PCORnet, OR: 1.41, 95% CI: 1.36, 1.46). The association was attenuated yet still elevated after successively adjusting for demographic factors, hospitalization, obesity, and comorbidities.
Table 3.
Association of preexisting OSA and PASC by EHR Network
| Prior OSA | With PASC | Without PASC | Unadjusted OR (95% CI) |
Model 1a OR (95% CI) | Model 2b OR (95% CI) | Model 3c OR (95% CI) |
|---|---|---|---|---|---|---|
| Yes | N3C: 10 756 (15.5%) PCORnet: 3681 (21.5%) PEDSnet: 247 (13.3%) |
N3C: 58 834 (84.5%) PCORnet: 13 445 (78.5%) PEDSnet: 1613 (86.7%) |
N3C: 3.93 (3.84, 4.02) PCORnet: 1.41 (1.36, 1.46) PEDSnet: 3.28 (2.85, 3.76) |
3.15 (3.08, 3.22) 1.22 (1.18, 1.27) 3.26 (2.83, 3.73) |
2.92 (2.85, 2.99) 1.17 (1.13, 1.22) 2.24 (1.92, 2.61) |
1.75 (1.71, 1.80) 1.12 (1.08, 1.16) 1.05 (0.89, 1.24) |
| No | N3C: 76 210 (4.4%) PCORnet: 51 538 (16.3%) PEDSnet: 4655 (4.5%) |
N3C: 1 638 140 (95.6%) PCORnet: 264 978 (83.7%) PEDSnet: 99 747 (95.5%) |
Odds ratios and 95% CI for association of preexisting OSA and probable PASC. CI, Confidence Interval.
aAdjusted for age group, sex, and race/ethnicity.
bAdjusted for age group, sex, race/ethnicity, and hospitalization status.
cAdjusted for age group, sex, race/ethnicity, hospitalization status, obesity, and comorbidities.
Results followed a similar pattern in children (PEDSnet unadjusted OR: 3.28, 95% CI: 2.85, 3.76). This association was attenuated yet still elevated after adjusting for demographic factors alone and together with hospitalization. After additionally adjusting for obesity and comorbidities, no significant difference in the odds of probable PASC was found when comparing children with and without OSA (PEDSnet: 1.05, 95% CI: 0.89, 1.24).
In a subsequent sensitivity analysis, we reexamined the association between preexisting OSA and probable PASC development among patients with either a positive lab test or a diagnosis indicating COVID-19, to assess the impact of the inclusion criteria on the study outcome (Supplementary Table 1). Through this expanded inclusion criteria, an additional 2999 PEDSnet patients, 78 103 PCORnet patients, and 185 928 N3C patients were included in the analysis. The resulting ORs from the three sites generated similar results to those of the primary analysis: the odds of developing probable PASC were higher among patients with preexisting OSA compared to those without preexisting OSA after adjusting for sex, race/ethnicity, age group, and hospitalization at the time of the index infection.
Further sensitivity analyses, additionally adjusting for pediatric asthma or variant epoch of the index infection, did not meaningfully change the results (Supplementary Table 2). When adjusting for hypertension and diabetes instead of comorbidity score, the association changed minimally for adults though was no longer significant in PCORnet (1.03, 95% CI: 1.00, 1.07 vs. 0.91, 95% CI: 0.88, 0.95). Within the pediatric population, this modification strengthened the association (PEDSnet: 1.07, 95% CI: 0.92, 1.25 vs. 1.95 vs. 95% CI: 1.67, 2.27).
Finally, interaction analysis results were not significant in PEDSnet or PCORnet. In N3C, results indicated that evidence of OSA in men increases odds of PASC by 59%, compared with an 89% increase in women (p < .001).
Discussion
In this harmonized analysis, we found that preexisting OSA was associated with increased risk of PASC-like conditions among adult patients in the RECOVER EHR research networks, despite different approaches for identification of probable PASC patients. The positive associations between preexisting OSA and probable PASC among adults were attenuated but remained significant after adjusting for other comorbidities. In contrast, associations among children were no longer significant after comorbidity adjustment. Sensitivity analyses modifying covariates did not meaningfully alter the results for adults. However, sensitivity analyses for children demonstrated the susceptibility of the association to the potential confounding influence of various comorbidities, with different effect estimates when adjusting for asthma, PMCA, or hypertension and diabetes.
Indeed, we observed that the strength of the association diminished among all three networks after adjusting for obesity and comorbidities, which suggests that the observed risk may in part be due to confounding from underlying associations between obesity, or other comorbidities, and PASC. Obesity is a particularly well-known risk factor for both OSA and severity of acute COVID infections, and was most prevalent in PCORnet, suggesting that obesity may account for some of the apparent difference in OSA-associated risk for PASC outcomes between networks [20, 21].
A notable strength of our study is the consistency of positive associations between OSA and PASC risk among adults across multiple data sources, approaches for identifying COVID-19-positive patients, and definitions of probable PASC. Because identification of PASC within patient populations has proved challenging due to heterogeneity of symptoms and lack of an accepted case definition, this study included a range of PASC definitions that were either more sensitive or more specific to examine the association [22].
Our analysis had several limitations. First, COVID diagnoses may not be well documented within the EHR, especially given limited availability of testing during early months of the pandemic, lower testing accuracy among women and people under 40 [23], and, more recently, increasingly widespread use of at-home testing.
Second, our analysis did not adjust for COVID vaccination status, as few EHR data networks adequately capture this, and the networks in this collaboration are still establishing data linkages to statewide vaccination registries to improve COVID vaccination data quality. Race and ethnicity data also varied in completeness across networks, which is consistent with prior studies [24, 25]. After initial exploratory analysis, we chose not to pursue whether active treatment of OSA altered findings, due to concerns that continuous positive airway pressure (CPAP) machine orders are not being routinely transmitted from participating sites to EHR networks and would be more reliably explored using claims data. Finally, our CP could only identify patients with diagnosed OSA, and OSA severity could not be ascertained due to the lack of polysomnography and sleep-specific symptom data. This means that results may not be generalizable across the spectrum of OSA severity or phenotype.
We also noted that the prevalence of OSA in our analyses was lower than in some published studies [15], though consistent with estimated prevalence of OSA in EHR-based data warehouses and claims databases [26]. This discrepancy may be due to the limited (two year) lookback period that was used for this analysis. It is possible that individuals diagnosed with sleep apnea prior to a SARS-CoV-2 infection are more likely to seek care from a Long COVID clinic or be diagnosed with PASC, due to increased encounters with the healthcare system. Nevertheless, OSA is often underdiagnosed in clinical practice and therefore might be present in patients not detected by our computable phenotyping strategy.
PASC is not one cohesive condition, but rather multiple constellations of symptoms or sub-phenotypes [27]. This analysis did not determine what symptoms were most prevalent in patients with OSA who develop probable PASC, but future research could examine the association of OSA and other preexisting conditions with specific PASC variations and the trajectory of impacted patients. The RECOVER Initiative also includes the recruitment and longitudinal follow-up of large cohorts of adult and pediatric participants with and without history of SARS-CoV-2 infection (>10 000 participants in each cohort) [28]. In these cohorts, patient history of OSA prior to SARS-CoV-2 infection and the development of probable PASC will be assessed directly without relying solely on EHR diagnoses. Nonetheless, these findings suggest that patients with OSA may be at elevated risk for PASC after SARS-CoV-2 and should be monitored for post-acute infection sequelae.
Supplementary Material
Supplementary material is available at SLEEP online.
Acknowledgments
We would like to thank the National Community Engagement Group (NCEG), all patient, caregiver and community Representatives, and all the participants enrolled in the RECOVER Initiative.
Contributor Information
Hannah L Mandel, Department of Population Health, New York University Grossman School of Medicine, New York, NY, USA.
Gunnar Colleen, Department of Population Health, New York University Grossman School of Medicine, New York, NY, USA.
Sajjad Abedian, Information Technologies and Services Department, Weill Cornell Medicine, New York, NY, USA.
Nariman Ammar, Department of Pediatrics, University of Tennessee Health Science Center College of Medicine Memphis, Memphis, TN, USA.
L Charles Bailey, Applied Clinical Research Center, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Tellen D Bennett, Department of Pediatrics, Children’s Hospital Colorado, Aurora, CO, USA.
M Daniel Brannock, Center for Data Science and AI, RTI International, Durham, NC, USA.
Shari B Brosnahan, Division of Pulmonary, Department of Medicine, Critical Care and Sleep Medicine, NYU Langone Health, New York, NY, USA¸.
Yu Chen, Department of Population Health, New York University Grossman School of Medicine, New York, NY, USA.
Christopher G Chute, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, MD, USA.
Jasmin Divers, Department of Foundations of Medicine, New York University Long Island School of Medicine, Mineola, NY, USA.
Michael D Evans, Clinical and Translational Science Institute, University of Minnesota, Minneapolis, MN, USA.
Melissa Haendel, Biomedical Informatics, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Margaret A Hall, Department of Biomedical Informatics, Stony Brook University, Stony Brook, NY, USA.
Kathryn Hirabayashi, Applied Clinical Research Center, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Mady Hornig, Department of Epidemiology, Columbia University Mailman School of Public Health, New York, NY, USA.
Stuart D Katz, Leon H. Charney Division of Cardiology, Department of Medicine, NYU Langone Health, New York, NY, USA.
Ana C Krieger, Departments of Medicine, Neurology, and Genetic Medicine, Weill Cornell Medical College, New York, NY, USA.
Johanna Loomba, Integrated Translational Health Research Institute, University of Virginia, Charlottesville, VA, USA.
Vitaly Lorman, Applied Clinical Research Center, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Diego R Mazzotti, Division of Pulmonary Critical Care and Sleep Medicine, Department of Internal Medicine, University of Kansas Medical Center, Kansas City, KS, USA.
Julie McMurry, Biomedical Informatics, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Richard A Moffitt, Department of Biomedical Informatics, Stony Brook University, Stony Brook, NY, USA.
Nathan M Pajor, Division of Pulmonary Medicine Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH, USA.
Emily Pfaff, Department of Medicine, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, USA.
Jeff Radwell, Department of Population Health, New York University Grossman School of Medicine, New York, NY, USA.
Hanieh Razzaghi, Applied Clinical Research Center, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA.
Susan Redline, Division of Sleep and Circadian Disorders, Department of Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Elle Seibert, Rose International, Los Angeles, CA, USA.
Suchetha Sharma, Integrated Translational Health Research Institute, University of Virginia, Charlottesville, VA, USA.
Tanayott Thaweethai, Biostatistics Center, Massachusetts General Hospital, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA.
Mark G Weiner, Department of Medicine, Weill Cornell Medical College, New York, NY, USA.
Yun Jae Yoo, Department of Biomedical Informatics, Stony Brook University, Stony Brook, NY, USA.
Andrea Zhou, Integrated Translational Health Research Institute, University of Virginia, Charlottesville, VA, USA.
Lorna E Thorpe, Department of Population Health, New York University Grossman School of Medicine, New York, NY, USA.
Funding
This study is part of the NIH Researching COVID to Enhance Recovery (RECOVER) Initiative, which seeks to understand, treat, and prevent the post-acute sequelae of SARS-CoV-2 infection (PASC). For more information on RECOVER, visit https://recovercovid.org/. This research was funded by the National Institutes of Health (NIH) Agreement OTA OT2HL161847 as part of the Researching COVID to Enhance Recovery (RECOVER) research program. This research was supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences.
Author Contribution
Authorship has been determined according to ICMJE recommendations. The content is solely the responsibility of the authors and does not necessarily represent the official views of the RECOVER Program, the NIH, or other funders.
Disclosure Statement
Financial disclosure: SBB receives funding from the Stony Wold-Hubert Fund and NYU distributed Doris Duke Fund to Retain Clinician Scientist. Nonfinancial disclosure: none.
Data Availability
The N3C data transfer to NCATS has been performed under a Johns Hopkins University reliance protocol (IRB00249128) or individual site agreements with the NIH. The N3C Data Enclave is managed under the authority of the NIH; more information can be found at ncats.nih.gov/n3c/resources. All data is available in the N3C Data Enclave to those with an approved protocol and data use request from an institutional review board. Data access is governed under the authority of the National Institutes of Health; more information on accessing the data can be found at https://covid.cd2h.org/for-researchers. Please send all other data access requests to the corresponding author, who will direct them accordingly.
References
- 1. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B.. Global prevalence of post COVID-19 condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:jiac136. doi: 10.1093/infdis/jiac136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4(10):e2128568e2128568. doi: 10.1001/jamanetworkopen.2021.28568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie Y, Bowe B, Al-Aly Z.. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12(1):6571. doi: 10.1038/s41467-021-26513-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Labarca G, Henríquez-Beltrán M, Lamperti L, et al. Impact of obstructive sleep apnea (OSA) in COVID-19 survivors, symptoms changes between 4-months and 1 year after the COVID-19 infection. Front Med (Lausanne). 2022;9:884218. doi: 10.3389/fmed.2022.884218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Franklin KA, Lindberg E.. Obstructive sleep apnea is a common disorder in the population—a review on the epidemiology of sleep apnea. J Thorac Dis. 2015;7(8):1311–1322. doi: 10.3978/j.issn.2072-1439.2015.06.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hariyanto TI, Kurniawan A.. Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Sleep Med. 2021;82:47–53. doi: 10.1016/j.sleep.2021.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu M, Han X, Ren J, Wang Y, Yang H.. Significant association of obstructive sleep apnoea with increased risk for fatal COVID-19: a quantitative meta-analysis based on adjusted effect estimates. Sleep Med Rev. 2022;63:101624. doi: 10.1016/j.smrv.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pena Orbea C, Wang L, Shah V, et al. Association of sleep-related hypoxia with risk of COVID-19 hospitalizations and mortality in a large integrated health system. JAMA Netw Open. 2021;4(11):e2134241e2134241. doi: 10.1001/jamanetworkopen.2021.34241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deer RR, Rock MA, Vasilevsky N, et al. Characterizing long COVID: deep phenotype of a complex condition. EBioMedicine. 2021;74:103722. doi: 10.1016/j.ebiom.2021.103722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ashkenazi-Hoffnung L, Shmueli E, Ehrlich S, et al. Long COVID in children: observations from a designated pediatric clinic. Pediatr Infect Dis J. 2021;40(12):e509–e511. doi: 10.1097/INF.0000000000003285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keenan BT, Kirchner HL, Veatch OJ, et al. Multisite validation of a simple electronic health record algorithm for identifying diagnosed obstructive sleep apnea. J Clin Sleep Med. 2020;16(2):175–183. doi: 10.5664/jcsm.8160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. RECOVER: Researching COVID to Enhance Recovery. RECOVER: Researching COVID to Enhance Recovery. https://recovercovid.org. Accessed April 21, 2022.
- 13. Haendel MA, Chute CG, Bennett TD, et al.; N3C Consortium. The National COVID Cohort Collaborative (N3C): rationale, design, infrastructure, and deployment. J Am Med Inform Assoc. 2021;28(3):427–443. doi: 10.1093/jamia/ocaa196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pfaff ER, Girvin AT, Bennett TD, et al.; N3C Consortium. Identifying who has long COVID in the USA: a machine learning approach using N3C data. Lancet Digit Health. 2022;4(7):e532–e541. doi: 10.1016/S2589-7500(22)00048-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cade BE, Dashti HS, Hassan SM, Redline S, Karlson EW.. Sleep apnea and COVID-19 mortality and hospitalization. Am J Respir Crit Care Med. 2020;202(10):1462–1464. doi: 10.1164/rccm.202006-2252le [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ.. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9):e1003773e1003773. doi: 10.1371/journal.pmed.1003773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charlson M, Wells MT, Ullman R, King F, Shmukler C.. The Charlson comorbidity index can be used prospectively to identify patients who will incur high future costs. PLoS One. 2014;9(12):e112479. doi: 10.1371/journal.pone.0112479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simon TD, Cawthon ML, Stanford S, et al.; Center of Excellence on Quality of Care Measures for Children with Complex Needs (COE4CCN) Medical Complexity Working Group. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647–e1654. doi: 10.1542/peds.2013-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Afshar M, Press VG, Robison RG, et al. A computable phenotype for asthma case identification in adult and pediatric patients: external validation in the Chicago Area Patient-Outcomes Research Network (CAPriCORN). J Asthma. 2018;55(9):1035–1042. doi: 10.1080/02770903.2017.1389952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sattar N, Valabhji J.. Obesity as a risk factor for severe COVID-19: summary of the best evidence and implications for health care. Curr Obes Rep. 2021;10(3):282–289. doi: 10.1007/s13679-021-00448-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 22. Rando HM, Bennett TD, Byrd JB, et al. Challenges in defining Long COVID: striking differences across literature, Electronic Health R ecords, and patient-reported information. MedRxiv Prepr Serv Health Sci. 2021;. doi: 10.1101/2021.03.20.21253896 [DOI] [Google Scholar]
- 23. Levine-Tiefenbrun M, Yelin I, Uriel H, et al. SARS-CoV-2 RT-qPCR test detection rates are associated with patient age, sex, and time since diagnosis. J Mol Diagn. 2022;24(2):112–119. doi: 10.1016/j.jmoldx.2021.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cook LA, Sachs J, Weiskopf NG.. The quality of social determinants data in the electronic health record: a systematic review. J Am Med Inform Assoc. 2022;29(1):187–196. doi: 10.1093/jamia/ocab199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Polubriaginof FCG, Ryan P, Salmasian H, et al. Challenges with quality of race and ethnicity data in observational databases. J Am Med Inform Assoc JAMIA. 2019;26(8–9):730–736. doi: 10.1093/jamia/ocz113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazzotti D, Waitman LR, Gozal D, Song X.. 0774 Positive airway pressure utilization, major adverse cardiovascular events incidence risk and mortality in medicare beneficiaries with obstructive sleep apnea. Sleep. 2022;45(suppl_1) :A336–A337. doi: 10.1093/sleep/zsac079.770 [DOI] [Google Scholar]
- 27. Peluso MJ, Kelly JD, Lu S, et al. Persistence, magnitude, and patterns of postacute symptoms and quality of life following onset of sars-cov-2 infection: cohort description and approaches for measurement. Open Forum Infect Dis. 2021;9(2):ofab640. doi: 10.1093/ofid/ofab640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. RECOVER Research Protocols. RECOVER Research Protocols.https://recovercovid.org/protocols. Accessed November 27, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The N3C data transfer to NCATS has been performed under a Johns Hopkins University reliance protocol (IRB00249128) or individual site agreements with the NIH. The N3C Data Enclave is managed under the authority of the NIH; more information can be found at ncats.nih.gov/n3c/resources. All data is available in the N3C Data Enclave to those with an approved protocol and data use request from an institutional review board. Data access is governed under the authority of the National Institutes of Health; more information on accessing the data can be found at https://covid.cd2h.org/for-researchers. Please send all other data access requests to the corresponding author, who will direct them accordingly.


