Abstract
Gekkotans are one of the major clades of squamate reptiles. As one of the earliest‐diverging lineages, they are crucial in studying deep‐level squamate phylogeny and evolution. Developmental studies can shed light on the origin of many important morphological characters, yet our knowledge of cranial development in gekkotans is very incomplete. Here, we describe the embryonic development of the skull in a parthenogenetic gekkonid, the mourning gecko (Lepidodactylus lugubris), studied using non‐acidic double staining and histological sectioning. Our analysis indicates that the pterygoid is the first ossifying bone in the skull, as in almost all other studied squamates, followed closely by the surangular and prearticular. The next to appear are the dentary, frontal, parietal and squamosal. The tooth‐bearing upper jaw bones, the premaxilla and maxilla, develop relatively late. In contrast to previous reports, the premaxilla starts ossifying from two distinct centres, reminiscent of the condition observed in diplodactylids and eublepharids. Only a single ossification centre of the postorbitofrontal is observed. Some of the endochondral bones of the braincase (prootic, opisthotic, supraoccipital) and the dermal parasphenoid are the last bones to appear. The skull roof is relatively poorly ossified near the time of hatching, with a large frontoparietal fontanelle still present. Many bones begin ossifying relatively later in L. lugubris than in the phyllodactylid Tarentola annularis, which suggests that its ossification sequence is heterochronic with respect to T. annularis.
Keywords: evo‐devo, homology, ossification, paedomorphosis, skeleton, squamates
We describe the embryonic development of the skull in a parthenogenetic gekkonid lizard, Lepidodactylus lugubris. The pterygoid is the first ossifying bone, followed closely by the surangular and prearticular. The tooth‐bearing upper jaw bones, the premaxilla and maxilla, develop relatively late. The premaxilla starts ossifying from two distinct centres, reminiscent of the condition present in diplodactylids and eublepharids.

1. INTRODUCTION
Encompassing more than 2000 currently accepted extant species (a number that continues to grow rapidly), the clade Gekkota is one of the major taxa of squamate reptiles (Uetz et al., 2022). This group contains morphologically very diverse lizards, from limbless pygopodids to ground‐dwelling taxa such as eublepharids to numerous arboreal, pad‐bearing lineages (e.g., Gamble et al., 2012). The phylogenetic position of this huge clade remains controversial. Most morphological phylogenetic analyses suggest that it is the sister group to all other squamates except the iguanians (e.g., Conrad, 2008; Estes et al., 1988; Gauthier et al., 2012), while molecular studies consistently place the gekkotans as sister to all other squamates (with the possible exception of the dibamids; e.g., Townsend et al., 2004; Vidal & Hedges, 2005; Wiens et al., 2010; Pyron et al., 2013; Reeder et al., 2015; Singhal et al., 2021). Some recent morphological studies (Simões et al., 2018; Tałanda et al., 2022)—based on the data matrix by Simões et al. (2018)—agree with molecular topologies, but the debate continues (Mongiardino Koch & Gauthier, 2018; Simões & Pyron, 2021; Skawiński & Borczyk, 2017).
In addition to their great taxonomic diversity and crucial phylogenetic position, the gekkotans show many peculiar anatomical characters. Except for the snakelike pygopodids, the gekkotan skull is relatively uniformly built; it shows large orbits, lacks the postorbital and upper temporal bars (and is therefore highly kinetic, showing a pronounced amphikinesis; e.g., Herrel et al., 2000), as well as the parietal foramen—features that are commonly attributed to paedomorphosis, that is, truncation of development (e.g., Evans, 2008). Besides these widely recognised paedomorphic traits, there are also examples of peramorphosis in the cranial skeleton in some gekkotans such as presence of sagittal crests, synostosis between parietals and novel circumorbital ossifications (e.g., Daza et al., 2015; Griffing et al., 2018). Despite the long history of studies on the gekkotan skull (see review in Evans, 2008), there are still many uncertainties about the identity and homology of some bones, especially those surrounding the orbit (e.g., Daza & Bauer, 2010). For example, the homology of the posterior dorsal circumorbital bone, usually called the postorbitofrontal (Daza & Bauer, 2010), is still debated (Daza & Bauer, 2010; Wise & Russell, 2010), as is the potential presence of the lacrimal and jugal (Daza & Bauer, 2010).
Developmental studies have the potential to shed additional light on the origins of gekkotan morphology, identity of contentious bones and the phylogenetic position of this important clade. However, most embryological studies concern the chondrocranial development (reviewed by Bellairs & Kamal, 1981), while detailed studies on the embryonic development of the bony skeleton are rare. For example, there is only one comprehensive description of the cranial ossification sequence in a gekkotan (the phyllodactylid Tarentola annularis; Khannoon & Evans, 2020), while most other studies are restricted to only a few selected stages or a certain skeletal region (e.g., El‐Toubi & Kamal, 1961; Wise & Russell, 2010). We attempt to fill part of this gap by describing the ossification sequence of the mourning gecko (Lepidodactylus lugubris), a species of parthenogenetic, all‐female gekkonid lizard and compare it to other squamates.
2. MATERIALS AND METHODS
2.1. Study species
The mourning gecko, Lepidodactylus lugubris (Duméril and Bibron, 1836), is a species of small gecko, native to Central Pacific islands but currently introduced to many tropical areas around the world (e.g., Karin et al., 2021). It belongs to the species‐rich genus Lepidodactylus (Uetz et al., 2022) which in turn belongs to the Gekkonidae and is closely related to Gekko, Luperosaurus and Pseudogekko (Gamble et al., 2012; Wood et al., 2020). It is a parthenogenetic, all‐female species, consisting of several clonal lineages, probably originating from a hybridogenetic event between L. moestus and L. pantai (Karin et al., 2021).
Morphological embryonic development of Lepidodactylus lugubris was described by Griffing et al. (2019), the development of its limb bones was studied by Rieppel (1994) and Griffing et al. (2022), and Kluge (1967) commented on ossification mode of the premaxilla.
2.2. Sampling
Embryos were sourced from the non‐commercial breeding facility at the Faculty of Natural Sciences, University of Silesia in Katowice (most) and Zoo Wrocław. Details about the acquisition of the eggs were presented in a previous publication (Kaczmarek et al., 2021). The eggs were placed on a plastic Petri dish, cooled in the fridge (about 10 min), and then the Petri dish was put on ice while still in the fridge (8–10 min). The embryos were immediately isolated, sacrificed by decapitation, and then transferred to 4% paraformaldehyde (PFA) in phosphate‐buffered saline (PBS) for 4 h or to the Karnovsky fixative (a 1:1 mixture of 2.5% glutaraldehyde and 2% paraformaldehyde in phosphate buffer), both at 4°C. Then, the material was rinsed (in PBS or phosphate buffer) and transferred to 70% ethanol through an ethanol series of increasing concentration. The staging of the embryos followed the developmental table for another gekkonid, Paroedura picta (Noro et al., 2009), because a more detailed table made specifically for Lepidodactylus lugubris (Griffing et al., 2019) was not yet published. However, we attempted to align the stages between these two developmental tables, and in this article, both tables are used to indicate the developmental stage of the embryo (in the form of “Griffing et al. stage/Noro et al. stage”). More recently obtained material was staged according to both these tables.
The research was performed in accordance with Directive 2010/63/EU of the European Parliament and of the Council of September 22, 2010, on the protection of animals used for scientific purposes and the Act of January 15, 2015, on the protection of animals used for scientific or educational purposes (Journal of Laws 2015 item 266). In accordance with Directive 2010/63/EU (Journal of Laws 2015 item 266), the study does not require the permission of the Local Ethics Committee.
2.3. Preparation of specimens
Further preparation followed the protocol described by Dingerkus and Uhler (1977), with slight modifications. The embryos were stained with alcian blue for the presence of cartilage, digested in a sodium borate‐pancreatin solution and then stained with alizarin red S for the presence of calcifications. Double‐stained embryos were then transferred through a series of KOH‐glycerin solutions and ultimately stored in pure glycerin with the addition of thymol. Because the alcian blue is dissolved in an ethanol‐acetic acid solution, it may destroy tiny ossifications. Therefore, we stained some embryos either only with alizarin, with a very short period in alcian blue solution (1–2 h rather than 24 as in the original protocol; Dingerkus & Uhler, 1977), or using an acid‐free staining solution—also including alcian blue and alizarin—developed by Walker and Kimmel (2007) to minimise the risk of decalcification. At least one specimen per stage was stained using the latter method and these specimens constitute the main basis for the morphological descriptions. In total, we cleared and stained 54 embryos representing stages from 34/20 to 43/55–60 and several younger specimens that did not show any ossifications (Table 1).
TABLE 1.
Number of double‐stained embryos of Lepidodactylus lugubris.
| Stage | Number of studied embryos |
|---|---|
| 33/18 | 2 |
| 34/20–22 | 8 |
| 35/24 | 4 |
| 36/26 | 6 |
| 37–38/28–30 | 8 |
| 38–39/30 | 5 |
| 40/35 | 7 |
| 41/40–45 | 8 |
| 42/50 | 5 |
| 43/55–60 | 3 |
In addition, the histology of the embryos representing stages 33/18, 34/20–22, 36/26 and 37–38/28–30 was analysed under the light microscope. These embryos were fixed in Bouin's solution for 48 h, additionally decalcified in 10% formic acid for 2 weeks, rinsed in distilled water, dehydrated and embedded in paraffin. The transverse serial sections (7 μm thick) of the heads were prepared on a Leica RM2125RT rotary microtome and stained with Ehrlich's haematoxylin and eosin.
2.4. Imaging and identification
The whole‐mount specimens were observed under Zeiss Stemi SV 11 stereomicroscope while histological thin sections were analysed under Zeiss Axioskop 20. The photographs were taken with Zeiss AxioCam HRc mounted on either of these devices.
The identification and nomenclature of bones and anatomical structures follow Evans (2008) except the name for a posterior circumorbital ossification which is here called the ‘postorbitofrontal’, following Daza and Bauer (2010) (but see Wise & Russell, 2010 for a different interpretation).
3. RESULTS
3.1. Staining methods
The specimens stained using the Walker and Kimmel (2007) method generally showed more ossification than specimens of the same developmental stage stained using the Dingerkus and Uhler (1977) method. The cartilage was better stained in the specimens prepared using the latter method (Figure S1). Differences in bone staining were most apparent in earlier developmental stages (to the stage 40/35).
3.2. Developmental osteology
Stage 33/18. Calcified endolymph in dorsal endolymphatic sacs, present in some specimens, is the only element that stains with alizarin.
Stage 34/20–22 (Figures 1 and 2). At this stage, the facial prominences are fused and the upper lip is well‐formed. The mandible reaches the tip of the snout (for a detailed description of soft tissue development see Griffing et al., 2019; Kaczmarek et al., 2021). The pterygoid is the first ossification that appears in the skull. The posterior process (just posterior to the pupil in lateral view) is most intensely stained with alizarin which indicates that this part of the bone is most rich in calcium. Therefore, the ossification of the pterygoid probably starts there and proceeds anteriorly (Figure 1). Later in this stage, the surangular and prearticular ossify, as indicated by histological thin sections and some whole‐mount specimens. In histological sections, the ossification of the pterygoid is the most advanced which confirms that it is the first ossifying element. The surangular shows a trabecular structure, with a relatively large part of condensed (mesenchymal) cells, especially in the dorsal part (Figure 2). Meckel's cartilages remain unfused at the midline (Figure 1). The otoliths (calcifications in the inner ear) are present in some specimens.
FIGURE 1.

Lepidodactylus lugubris embryo at stage 34/20–22. (a) Ventral view, (b) right lateral view. en, calcified endolymph; Mc, Meckel's cartilage; pt, pterygoid. Scale bar = 2 mm.
FIGURE 2.

A histological transverse thin section of the head of a stage 34/20–22 Lepidodactylus lugubris embryo through a posterior eye region. Mc, Meckel's cartilage; pra, prearticular; pt, pterygoid; san, surangular. Scale bar = 100 μm.
Stage 35/24 (Figure 3). The ossification of the pterygoid advances. The bone is now S‐shaped (sigmoidal) in dorsal or ventral view, with the anterolateral process only beginning to ossify. A few further ossifications appear in the skull. The ossification of the parietal is restricted to the lateral margin which is faintly stained with alizarin. The lateral (orbital) margins of the frontal are also ossified. The ossification centre in the squamosal is barely noticeable. In the mandible, the dentary is ossified anteriorly, near the symphysis. The lateral surface of the surangular is somewhat porous (which is consistent with the trabecular structure observed in histological sections). The posterior end of the bone (just anterior to the future articular surface with the quadrate) is slightly upturned (Figure 3).
FIGURE 3.
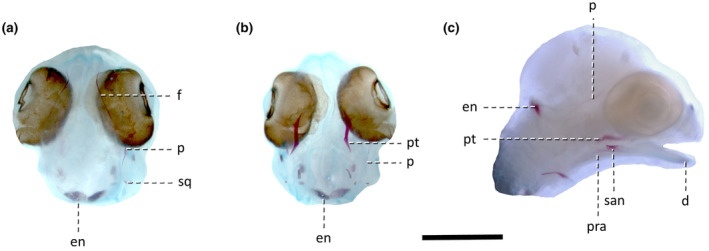
Lepidodactylus lugubris embryos at stage 35/24. (a) Dorsal view, (b) ventral view, (c) right lateral view. Abbreviations: d, dentary; en, calcified endolymph; f, frontal; p, parietal; pra, prearticular; pt, pterygoid; san, surangular; sq, squamosal. Scale bar = 2 mm.
Stage 36/26 (Figure 4). Meckel's cartilages are now fused at the midline. The dentaries start to ossify along them, with the strongest ossification in the most anterior (symphyseal) part. More posteriorly, the ossification proceeds along the dorsal and ventral margins of Meckel's cartilage. These two ‘branches’ of the dentary meet again in the most posterior part of the bone. The ossifications of the nasal (weakly stained) and prefrontal (well stained) appear. The dorsal process of the prefrontal elongates and its posterior end reaches approximately the anterior end of the pupil. At the level of the mid‐eye and anterior to that, the ossifications of the frontals start to expand towards the midline. The squamosal is now much larger and more visible (Figure 4). The egg teeth first appear during this stage, although they are poorly visible in whole‐mount specimens (detailed description of the development of the egg teeth was presented by Hermyt et al., 2020). Later during this stage, the maxilla and vomer start ossifying. They are very difficult to observe in double‐stained specimens but are better distinguishable in histological sections.
FIGURE 4.

Lepidodactylus lugubris embryo at stage 36/26. (a) Dorsal view, (b) ventral view, (c) right lateral view. d, dentary; en, calcified endolymph; f, frontal; n, nasal; p, parietal; pra, prearticular; prf, prefrontal; pt, pterygoid; san, surangular; sq, squamosal. Scale bar = 2 mm.
Stage 37–38/28–30 (Figures 5 and 6). Many bones ossify during this stage. The palatine, septomaxilla and premaxilla appear (the septomaxilla starts ossifying slightly before the premaxilla). The maxilla first ossifies along the ventral (alveolar) margin but later in this stage expands dorsally (Figure 5). The premaxilla starts ossifying from two different centres, each bearing a single egg tooth (Figure 6). The nasal and anterior part of the frontal expand their ossification towards the midline (though they do not contact), so the anterior skull roof is much better ossified than in the previous stage. The postorbitofrontal is present as a single, approximately triangular ossification, with a slightly longer anterior (frontal) process. Later during this stage, both the dorsal (parietal) and especially the frontal processes elongate, so the bone becomes more V‐shaped. The jugal appears as a minor ossification below the orbit, just posterior to the posterior end of the maxilla. The middle part of the epipterygoid shaft and the quadrate also ossify. The exoccipitals develop as the first ossifications in the braincase. In the mandible, the coronoid appears but is only loosely connected to other mandibular bones. The splenial also appears. The surangular consolidates and is much less porous than in the previous stage. The first teeth in the maxilla and dentary stain with alizarin. In the hyoid apparatus, the first ceratobranchials are the only ossifications (Figure 5).
FIGURE 5.

Lepidodactylus lugubris embryo at stage 37–38/28–30. (a) Dorsal view, (b) ventral view, (c) right lateral view. cb, first ceratobranchial; co, coronoid; d, dentary; en, calcified endolymph; eo, exoccipital; ept, epipterygoid; f, frontal; j, jugal; mx, maxilla; n, nasal; p, parietal; pa, palatine; pmx, premaxilla; pof, postorbitofrontal; pra, prearticular; prf, prefrontal; pt, pterygoid; q, quadrate; san, surangular; smx, septomaxilla; sp, splenial; sq, squamosal; v, vomer. Scale bar = 2 mm.
FIGURE 6.

Development of the premaxilla in Lepidodactylus lugubris. (a) Close‐up of two premaxillary ossification centres in a stage 37–38/28–30 embryo, each bearing a single egg tooth. Ventral view. Scale bar = 0.5 mm. (b) A histological transverse thin section taken at the egg tooth level. Scale bar = 50 μm. et, egg tooth; pmx, premaxilla.
Stage 38–39/30. There is no noticeable advance in ossification over the preceding stage.
Early stage 40/35 (Figure 7). The ectopterygoid is visible in whole‐mount specimens. It is present as a narrow splint of bone located anterior to the anterolateral process of the pterygoid. The basisphenoid ossifies from three centres, one in the crista sellaris and two located in polar cartilages (future basipterygoid processes). The basioccipital appears as an approximately V‐shaped bone with its ossification most advanced in its posteriormost part (at the midline). Both anterior processes are more poorly stained. The basicranial fenestra between the basioccipital and basisphenoid is very wide (Figure 7).
FIGURE 7.

Lepidodactylus lugubris embryo at stage 40/35. (a) Dorsal view, (b) ventral view, (c) left lateral view (mirrored). art, articular; bo, basioccipital; bs, basisphenoid; co, coronoid; d, dentary; en, calcified endolymph; eo, exoccipital; ept, epipterygoid; f, frontal; mx, maxilla; n, nasal; p, parietal; pmx, premaxilla; pof, postorbitofrontal; prf, prefrontal; pro, prootic; pt, pterygoid; q, quadrate; san, surangular; sq, squamosal. Scale bar = 2 mm.
Late stage 40/35 (see Figure 8). Ossification of the snout further progresses. The nasals are well‐developed but do not yet contact each other at the midline. The midline ossification of the frontal extends posteriorly approximately to the level of the middle of the eye. The ossification of the postorbitofrontal progresses, especially its anterior process, which elongates and reaches approximately the posterior third of the eye. The prootic and opisthotic ossify; the alar process of the former remains cartilaginous. Neither of these two bones contact each other nor the exoccipitals. The supraoccipital appears as a relatively thin and anteroposteriorly short bone. The basioccipital is an approximately V‐shaped bone in ventral view, with a small anterior midline projection. The lateral parts of the basisphenoid expand posteriorly, creating the posterolateral processes. The basicranial fenestra is large and resembles a rectangle with rounded corners. A very short anterior median process (parasphenoid rostrum) starts to ossify from the sphenoid. The articular ossifies and begins to fuse with the prearticular but this incipient compound structure (also called the articular) is not yet firmly attached to the other mandibular bones.
FIGURE 8.

Lepidodactylus lugubris embryo at stage 41/40–45. (a) Dorsal view, (b) ventral view, (c) right lateral view. art, articular; bo, basioccipital; bs, basisphenoid; co, coronoid; d, dentary; en, calcified endolymph; ept, epipterygoid; f, frontal; mx, maxilla; n, nasal; ot, otooccipital; p, parietal; pmx, premaxilla; pof, postorbitofrontal; prf, prefrontal; pro, prootic; pt, pterygoid; san, surangular; so, supraoccipital; sq, squamosal. Scale bar = 2 mm.
Stage 41/40–45 (Figure 8). There is little progress over the preceding stage. The lateral wall of the snout, composed of the maxilla and prefrontal, reaches an almost complete state of ossification. The dorsal process of the prefrontal almost reaches the middle of the eye. The lateral ridges of the supraoccipital start to develop. The exoccipitals and opisthotics fuse into otooccipitals (Figure 8).
Stage 42/50 (Figure 9). The skull looks very similar to the previous stage. In dorsal view, the nasals begin to tightly contact each other at the midline along almost their entire length (except the most posterior end where there is a very small gap between them). The ossification of the prootic further progresses and now almost the entire bone is well ossified, except for the tip of the alar process. The lateral ridges of the supraoccipital are more pronounced than in the previous stage. The basisphenoid and basioccipital also further develop. The expanded ends of the basipterygoid processes begin to ossify. The anterolateral processes of the basioccipital get more robust and elongate so that the gaps between the lateral parts of the basisphenoid and basioccipital are much narrower than in the previous stage (Figure 9).
FIGURE 9.

Lepidodactylus lugubris embryo at stage 42/50. (a) Dorsal view, (b) ventral view, (c) right lateral view. art, articular; bo, basioccipital; bs, basisphenoid; co, coronoid; d, dentary; ept, epipterygoid; f, frontal; mx, maxilla; n, nasal; ot, otooccipital; p, parietal; pmx, premaxilla; pof, postorbitofrontal; prf, prefrontal; pro, prootic; pt, pterygoid; san, surangular; so, supraoccipital; sq, squamosal. Scale bar = 2 mm.
Stage 43/55–60 (Figure 10). The ossification further progresses and the skull consolidates. The nasals remain unfused at the midline. The frontals start to contact each other at the level of the middle and anterior part of the eye. The midline ossification of the frontal extends approximately to the middle of the eye. The posterior margin of the parietal starts to ossify; initially, from an additional ossification centre at the midline, from which the ossification spreads laterally, so that the parietals first fuse at their posterior margin. The ossification of the basisphenoid and basioccipital significantly advances; the basicranial fenestra is now restricted to a gap between them, a small posterior notch in the basisphenoid, and a longer anterior notch in the basioccipital. In near‐hatching specimens, the tips of the basipterygoid processes still are not ossified and not in contact with the pterygoids (Figure 10).
FIGURE 10.

Lepidodactylus lugubris embryo at stage 43/55–60. (a) A younger specimen in dorsal view. An older specimen in (b) dorsal view, (c) ventral view, (d) left lateral view (mirrored). art, articular; bo, basioccipital; bs, basisphenoid; d, dentary; mx, maxilla; n, nasal; ot, otooccipital; p, parietal; pmx, premaxilla; pof, postorbitofrontal; prf, prefrontal; pro, prootic; pt, pterygoid; q, quadrate; san, surangular; so, supraoccipital; sq, squamosal. Scale bar = 2 mm.
4. DISCUSSION
4.1. Comparative ossification sequence
The ossification sequences are often considered conserved among squamates (e.g., Khannoon & Evans, 2020) and reptiles (e.g., Chapelle et al., 2020) or even tetrapods (Schoch, 2006) in general. Recently, Chapelle et al. (2020) stated that there is a “deep‐time conservation of cranial ossification sequences in saurians”. More specifically, based on a comparison of the embryonic skull development in iguanian Pogona vitticeps, tortoise Centrochelys sulcata, crocodile Crocodylus niloticus and chicken Gallus gallus, they concluded that “the bones of the snout are the first to ossify, including the premaxilla, maxilla and dentary. They are followed by the remaining bones of the mandible (…) and most of the lateral bones of the face (…) and the palatal bones” (Chapelle et al., 2020: p. 5). The sequence observed in Lepidodactylus lugubris is not consistent with this pattern. Of the jaw bones, only the dentary ossifies early, while the ossification of the premaxilla and maxilla is delayed. The first bone to appear is the pterygoid, one of the bones of the palate. The very early ossification of the pterygoid (usually as the first or one of a few first bones) is an almost universal character in studied squamates, present in iguanian (e.g., Ollonen et al., 2018), gekkotan (Rieppel, 1994 and this work), scincoid (e.g., Hugi et al., 2012), lacertiform (e.g., Hernández‐Jaimes et al., 2012; Roscito & Rodrigues, 2012), and anguimorph (e.g., Good, 1995; Werneburg et al., 2015) lizards, as well as in many snakes (e.g., Buchtová et al., 2007; Polachowski & Werneburg, 2013) (see Table S1 for a full list). Because of this, the pterygoid was reconstructed to be ancestrally the first ossifying bone in squamates (Werneburg et al., 2015; Werneburg & Sánchez‐Villagra, 2015), a conclusion supported by our data. Very early appearance of the surangular and prearticular is also known in other squamates, for example, in Pogona vitticeps (Ollonen et al., 2018), Liopholis whitii, Lerista bougainvillii, Hemiergis peronii, Saiphos equalis (Hugi et al., 2012), Calyptommatus sinebrachiatus and Nothobachia ablephara (Roscito & Rodrigues, 2012).
The parietal is often described as a late‐ossifying bone in sauropsids (Chapelle et al., 2020; Schoch, 2006). In Lepidodactylus lugubris, interestingly, it is one of the first bones to appear (approximately simultaneously with the dentary). However, such condition is not exceptional among squamates, as the relatively early appearance of this bone (before the premaxilla and/or no later than the maxilla) was described, for example, in Pogona vitticeps (Ollonen et al., 2018), Lerista bougainvillii, Saiphos equalis (Hugi et al., 2012), Calyptommatus sinebrachiatus, Nothobachia ablephara (Roscito & Rodrigues, 2012), Elgaria coerulea (Good, 1995) and Anguis fragilis (Skawiński et al., 2021). As in other squamates (Werneburg et al., 2015), some of the endochondral bones of the neurocranium (prootic, opisthotic and supraoccipital) and the dermal parasphenoid are the last bones to appear during embryonic development in L. lugubris.
Lepidodactylus lugubris is similar to another gekkonid, Gehyra oceanica, in a very early appearance of the pterygoid (Rieppel, 1994). Unfortunately, it is difficult to compare the ossification sequence of L. lugubris to that of Tarentola annularis, the only other gekkotan described from a comparable sample size (Khannoon & Evans, 2020), because the latter was studied using somewhat different methods (most notably, a long fixation in PFA and staining that used acidic conditions which might have affected the detectability of small ossifications). The authors described only three ossification events during which the individual bones appeared during ontogeny (Khannoon & Evans, 2020), in contrast to at least nine such events present in L. lugubris (Table 2). Khannoon and Evans (2020) are likely correct in stating that this is a result of insufficient sampling from the early period of skeletogenesis (in this case, between 15 and 23 days after oviposition). The first bones begin to ossify approximately at the same time (stage 34) in L. lugubris and T. annularis. However, it is important to note that ‘homologisation’ (that is, a direct alignment) of developmental stages in different species is almost impossible due to the widespread occurrence of heterochronic events (Alberch, 1985; Andrews et al., 2013; Skawiński & Borczyk, 2017). Most skull bones were already ossified to some extent in stage 34 in T. annularis as indicated by histological thin‐sectioning. According to the authors, this lack of delay in ossification does not support paedomorphosis in the gekkotan skull (Khannoon & Evans, 2020). In contrast to T. annularis, most skull bones in L. lugubris begin their ossification noticeably later. In our opinion, it is preliminary to say whether this does or does not suggest an important role of paedomorphosis in gekkotans. In addition, many of the currently available descriptions of cranial development in squamates used staining in acidic conditions which probably affects interpretations about the timing of ossification.
TABLE 2.
Summary of the stage and rank (i.e., order) in which cranial elements begin to ossify (or calcify) during embryonic development in Lepidodactylus lugubris.
| Bone | Stage | Rank |
|---|---|---|
| Nasal (d) | 36/26 | 5 |
| Frontal (d) | 35/24 | 4 |
| Parietal (d) | 35/24 | 4 |
| Premaxilla (d) | 37–38/28 | 7 |
| Maxilla (d) | 36/26 | 6 |
| Prefrontal (d) | 36/26 | 5 |
| Postorbitofrontal (d) | 37–38/28 | 7 |
| Jugal (d) | 37–38/28 | 7 |
| Squamosal (d) | 35/24 | 4 |
| Epipterygoid (e) | 37–38/28 | 7 |
| Quadrate (e) | 37–38/28 | 7 |
| Vomer (d) | 36/26 | 6 |
| Palatine (d) | 37–38/28 | 7 |
| Pterygoid (d) | 34/20–22 | 1 |
| Ectopterygoid (d) | 40/35 | 8 |
| Septomaxilla (d) | 37–38/28 | 7 |
| Basisphenoid (e) | 40/35 | 8 |
| Basioccipital (e) | 40/35 | 8 |
| Parasphenoid (d) | 40/35 | 9 |
| Opisthotic (e) | 40/35 | 9 |
| Prootic (e) | 40/35 | 9 |
| Exoccipital (e) | 37–38/28 | 7 |
| Supraoccipital (e) | 40/35 | 9 |
| Dentary (d) | 35/24 | 3 |
| Surangular (d) | 34/20–22 | 2 |
| Coronoid (d) | 37–38/28 | 7 |
| Splenial (d) | 37–38/28 | 7 |
| Articular (e) | 40/35 | 8 |
| Prearticular (d) | 34/20–22 | 2 |
| First ceratobranchial (e) | 37–38/28 | 7 |
| Other elements | ||
| Calcified endolymph | 33/18 | ‐ |
| Otoliths | 34/20–22 | ‐ |
Abbreviations: d, dermal bone; e, endochondral bone.
4.2. Bone development
According to current knowledge, the premaxilla starts ossifying from a single centre in phyllodactylids, sphaerodactylids and gekkonids (El‐Toubi & Kamal, 1961; Khannoon & Evans, 2020; Kluge, 1967), in contrast to diplodactylids and eublepharids, in which it is a paired bone (Kluge, 1967). Such mode of development was described for gekkonids by Kluge (1967) who illustrated it based on Lepidodactylus lugubris. However, our data show that the premaxilla starts ossifying from two separate centres, as indicated by both alizarin‐stained whole‐mount specimens (Figure 6a) and histological thin sections (Figure 6b, see also Figure 5a in Hermyt et al., 2020). What is the reason for these differences? Firstly, the two premaxillary ossification centres quickly fuse to form a single bone so it is difficult to capture this transient moment during sampling. In addition, at the beginning, both ossification centres are relatively small. Therefore, it seems possible that previous authors—who used staining methods employing acidic conditions—could not observe these two centres because they were dissolved in the acid. During later stages, when the bones attained larger size and could withstand the effect of acid, they were already fused. In turn, histological studies usually use a smaller sample size than those using staining, so this brief stage in which both premaxillary centres are present has a much greater chance of being missed. In light of the presented data, it seems most likely that the presence of separate premaxillary ossification centres is the ancestral state for gekkotans as it is present in pygopodoid diplodactylids, and gekkonoid eublepharids (Kluge, 1967) and gekkonids (this work). If this scenario is correct, the presence of a single ossification is either a derived state of sphaerodactylids (Kluge, 1967) and phyllodactylids (Khannoon & Evans, 2020) or a sampling or methodological artefact. This needs to be tested using a densely‐sampled series of embryos which have not been subjected to the acidic conditions.
The postorbitofrontal ossified from a single centre, as in Tarentola annularis (Khannoon & Evans, 2020). The same condition was also described in a distantly related alopoglossid, Alopoglossus bicolor, which has a single postorbitofrontal, even though its close relatives retain a separate postfrontal and postorbital (Hernández‐Jaimes et al., 2012). Wise and Russell (2010) described a different pattern in Eublepharis macularius, in which the postfrontal fuses with the frontal and the separate ossification in the adult skull is the postorbital. We did not observe such a pattern in Lepidodactylus lugubris. There are several potential explanations for these differences; for example, there might be a genuine variation within gekkotans, the small postfrontal ossification might be lost in T. annularis (Khannoon & Evans, 2020) and L. lugubris or its absence in our sample might be a result of insufficient sampling. Further studies could shed more light on this problem.
The frontoparietal fontanelle is of variable size around the time of hatching in gekkotans. In most cases, it is wide, with the parietals restricted to narrow lateral bars. Such condition was described in a late embryo of Ptyodactylus hasselquistii (El‐Toubi & Kamal, 1961), Tarentola annularis (Khannoon & Evans, 2020), perinate of Gonatodes albogularis (Maisano, 2001) and hatchling of Aristelliger praesignis (Griffing et al., 2018). However, in some other gekkotans—Bunopus tuberculatus, Hemidactylus homoeolepis (Mohammed, 1989) and Cyrtodactylus pubisculus (Rieppel, 1992)—the fontanelle is almost closed. For example, in the last species, even though the ossification of the skull roof is relatively advanced, the parietals are still not fused at the midline (Rieppel, 1992). Lepidodactylus lugubris is closer to the less‐ossified extreme in this regard. However, the posterior margin of the parietals is almost completely ossified so that the posterior skull roof is at more advanced developmental stage than in the oldest described embryos of T. annularis (Khannoon & Evans, 2020).
AUTHOR CONTRIBUTIONS
TS designed the project and performed double staining of the embryos, PK collected and staged the embryos and prepared most of the histological thin sections. TS and BB analysed the double‐stained embryos and prepared figures. TS wrote the manuscript with input from BB and PK. All of the authors reviewed and accepted the draft.
CONFLICT OF INTEREST STATEMENT
Authors have no conflicts of interests to declare.
Supporting information
Figure S1.
Table S1.
ACKNOWLEDGMENTS
We thank Ewa Serwa for help with histological staining of some thin sections. Marek Pastuszek and Magda Fabiszewska‐Jerzmańska (ZOO Wrocław) generously gave us several embryos. We are grateful to two anonymous reviewers for their helpful comments. This work was supported by two grants for young scientists awarded to TS by the University of Wrocław (grant numbers: 0420/2316/17, 0420/2566/18).
Skawiński, T. , Kaczmarek, P. & Borczyk, B. (2023) Embryonic development of the skull in a parthenogenetic lizard, the mourning gecko (Lepidodactylus lugubris). Journal of Anatomy, 243, 618–629. Available from: 10.1111/joa.13871
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Alberch, P. (1985) Problems with the interpretation of developmental sequences. Systematic Zoology, 34, 46–58. Available from: 10.2307/2413344 [DOI] [Google Scholar]
- Andrews, R.M. , Brandley, M.C. & Greene, V.W. (2013) Developmental sequences of squamate reptiles are taxon specific. Evolution & Development, 15, 326–343. Available from: 10.1111/ede.12042 [DOI] [PubMed] [Google Scholar]
- Bellairs, A.d.'A. & Kamal, A.M. (1981) The chondrocranium and the development of the skull in recent reptiles. In: Gans, C. & Parsons, T.S. (Eds.) Biology of the Reptilia. Volume 11, Morphology F. London, New York, Toronto, Sydney, San Francisco: Academic Press, pp. 1–263. [Google Scholar]
- Buchtová, M. , Boughner, J.C. , Fu, K. , Diewert, V.M. & Richman, J.M. (2007) Embryonic development of Python sebae—II: craniofacial microscopic anatomy, cell proliferation and apoptosis. Zoology, 110, 231–251. Available from: 10.1016/j.zool.2007.01.006 [DOI] [PubMed] [Google Scholar]
- Chapelle, K.E. , Fernandez, V. & Choiniere, J.N. (2020) Conserved in‐ovo cranial ossification sequences of extant saurians allow estimation of embryonic dinosaur developmental stages. Scientific Reports, 10, 4224. Available from: 10.1038/s41598-020-60292-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, J.L. (2008) Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bulletin of the American Museum of Natural History, 310, 1–182. Available from: 10.1206/310.1 [DOI] [Google Scholar]
- Daza, J.D. & Bauer, A.M. (2010) The circumorbital bones of the Gekkota (Reptilia: Squamata). Anatomical Record, 293, 402–413. Available from: 10.1002/ar.21039 [DOI] [PubMed] [Google Scholar]
- Daza, J.D. , Mapps, A.A. , Lewis, P.J. , Thies, M.L. & Bauer, A.M. (2015) Peramorphic traits in the tockay gecko skull. Journal of Morphology, 276, 915–928. Available from: 10.1002/jmor.20389 [DOI] [PubMed] [Google Scholar]
- Dingerkus, G. & Uhler, L.D. (1977) Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technology, 52, 229–232. Available from: 10.3109/10520297709116780 [DOI] [PubMed] [Google Scholar]
- El‐Toubi, M.R. & Kamal, A.M. (1961) The development of the skull of Ptyodactylus hasselquistii. III. The osteocranium of a late embryo. Journal of Morphology, 108, 193–201. Available from: 10.1002/jmor.1051080205 [DOI] [PubMed] [Google Scholar]
- Estes, R. , de Queiroz, K. & Gauthier, J. (1988) Phylogenetic relationships within Squamata. In: Estes, R. & Pregill, G.K. (Eds.) Phylogenetic relationships of the lizard families: essays commemorating Charles L. Camp. Stanford: Stanford University Press, pp. 119–281. [Google Scholar]
- Evans, S.E. (2008) The skull of lizards and tuatara. In: Gans, C. , Gaunt, A.S. & Adler, K. (Eds.) Biology of the Reptilia. Volume 20, Morphology H. Ithaca: Society for the Study of Amphibians and Reptiles, pp. 1–347. [Google Scholar]
- Gamble, T. , Greenbaum, E. , Jackman, T.R. , Russell, A.P. & Bauer, A.M. (2012) Repeated origin and loss of adhesive toepads in geckos. PLoS One, 7, e39429. Available from: 10.1371/journal.pone.0039429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier, J.A. , Kearney, M. , Maisano, J.A. , Rieppel, O. & Behlke, A.D.B. (2012) Assembling the squamate tree of life: perspectives from the phenotype and the fossil record. Bulletin of the Peabody Museum of Natural History, 53, 3–308. Available from: 10.3374/014.053.0101 [DOI] [Google Scholar]
- Good, D.A. (1995) Cranial ossification in the northern alligator lizard, Elgaria coerulea (Squamata, Anguidae). Amphibia‐Reptilia, 16, 157–166. Available from: 10.1163/156853895X00334 [DOI] [Google Scholar]
- Griffing, A.H. , Daza, J.D. , Deboer, J.C. & Bauer, A.M. (2018) Developmental osteology of the parafrontal bones of the Sphaerodactylidae. Anatomical Record, 301, 581–606. Available from: 10.1002/ar.23749 [DOI] [PubMed] [Google Scholar]
- Griffing, A.H. , Gamble, T. , Bauer, A.M. & Russell, A.P. (2022) Ontogeny of the paraphalanges and derived phalanges of Hemidactylus turcicus (Squamata: Gekkonidae). Journal of Anatomy, 241, 1039–1053. Available from: 10.1111/joa.13735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffing, A.H. , Sanger, T.J. , Daza, J.D. , Nielsen, S.V. , Pinto, B.J. , Stanley, E.L. et al. (2019) Embryonic development of a parthenogenetic vertebrate, the mourning gecko (Lepidodactylus lugubris). Developmental Dynamics, 248, 1070–1090. Available from: 10.1002/dvdy.72 [DOI] [PubMed] [Google Scholar]
- Hermyt, M. , Metscher, B. & Rupik, W. (2020) Do all geckos hatch in the same way? Histological and 3D studies of egg tooth morphogenesis in the geckos Eublepharis macularius Blyth 1854 and Lepidodactylus lugubris Duméril & Bibron 1836. Journal of Morphology, 281, 1313–1327. Available from: 10.1002/jmor.21249 [DOI] [PubMed] [Google Scholar]
- Hernández‐Jaimes, C. , Jerez, A. & Ramírez‐Pinilla, M.P. (2012) Embryonic development of the skull of the Andean lizard Ptychoglossus bicolor (Squamata, Gymnophthalmidae). Journal of Anatomy, 221, 285–302. Available from: 10.1111/j.1469-7580.2012.01549.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrel, A. , Aerts, P. & De Vree, F. (2000) Cranial kinesis in geckoes: functional implications. Journal of Experimental Biology, 203, 1415–1423. Available from: 10.1242/jeb.203.9.1415 [DOI] [PubMed] [Google Scholar]
- Hugi, J. , Hutchinson, M.N. , Koyabu, D. & Sánchez‐Villagra, M.R. (2012) Heterochronic shifts in the ossification sequences of surface‐ and subsurface‐dwelling skinks are correlated with the degree of limb reduction. Zoology, 115, 188–198. Available from: 10.1016/j.zool.2011.10.003 [DOI] [PubMed] [Google Scholar]
- Kaczmarek, P. , Metscher, B. & Rupik, W. (2021) Embryology of the naso‐palatal complex in Gekkota based on detailed 3D analysis in Lepidodactylus lugubris and Eublepharis macularius . Journal of Anatomy, 238, 249–287. Available from: 10.1111/joa.13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin, B.R. , Oliver, P.M. , Stubbs, A.L. , Arifin, U. , Iskandar, D.T. , Arida, E. et al. (2021) Who's your daddy? On the identity and distribution of the paternal hybrid ancestor of the parthenogenetic gecko Lepidodactylus lugubris (Reptilia: Squamata: Gekkonidae). Zootaxa, 4999, 87–100. Available from: 10.11646/zootaxa.4999.1.6 [DOI] [PubMed] [Google Scholar]
- Khannoon, E.R. & Evans, S.E. (2020) Embryonic skull development in the gecko, Tarentola annularis (Squamata: Gekkota: Phyllodactylidae). Journal of Anatomy, 237, 504–519. Available from: 10.1111/joa.13213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge, A.G. (1967) Higher taxonomic categories of gekkonid lizards and their evolution. Bulletin of the American Museum of Natural History, 135, 1–60. [Google Scholar]
- Maisano, J.A. (2001) A survey of state of ossification in neonatal squamates. Herpetological Monographs, 15, 135–157. Available from: 10.2307/1467041 [DOI] [Google Scholar]
- Mohammed, M.B.H. (1989) Some observations on the cartilaginous structures of the head skeleton in some geckos (Reptilia: Gekkonidae). Zoologischer Anzeiger, 222, 3–11. [Google Scholar]
- Mongiardino Koch, N. & Gauthier, J.A. (2018) Noise and biases in genomic data may underlie radically different hypotheses for the position of Iguania within Squamata. PLoS One, 13, e0202729. Available from: 10.1371/journal.pone.0202729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noro, M. , Uejima, A. , Abe, G. , Manabe, M. & Tamura, K. (2009) Normal developmental stages of the Madagascar ground gecko Paroedura pictus with special reference to limb morphogenesis. Developmental Dynamics, 238, 100–109. Available from: 10.1002/dvdy.21828 [DOI] [PubMed] [Google Scholar]
- Ollonen, J. , Da Silva, F.O. , Mahlow, K. & Di‐Poï, N. (2018) Skull development, ossification pattern, and adult shape in the emerging lizard model organism Pogona vitticeps: a comparative analysis with other squamates. Frontiers in Physiology, 9, 278. Available from: 10.3389/fphys.2018.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polachowski, K.M. & Werneburg, I. (2013) Late embryos and bony skull development in Bothropoides jararaca (Serpentes, Viperidae). Zoology, 116, 36–63. Available from: 10.1016/j.zool.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Pyron, R.A. , Burbrink, F.T. & Wiens, J.J. (2013) A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology, 13, 93. Available from: 10.1186/1471-2148-13-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder, T.W. , Townsend, T.M. , Mulcahy, D.G. , Noonan, B.P. , Wood, P.L. , Sites, J.W. et al. (2015) Integrated analyses resolve conflicts over squamate reptile phylogeny and reveal unexpected placements for fossil taxa. PLoS One, 10, e0118199. Available from: 10.1371/journal.pone.0118199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieppel, O. (1992) Studies on skeleton formation in reptiles. I. The postembryonic development of the skeleton in Cyrtodactylus pubisculus (Reptilia: Gekkonidae). Journal of Zoology, 227, 87–100. Available from: 10.1111/j.1469-7998.1992.tb04346.x [DOI] [Google Scholar]
- Rieppel, O. (1994) Studies on skeleton formation in reptiles. Patterns of ossification in the limb skeleton of Gehyra oceanica (Lesson) and Lepidodactylus lugubris (Duméril & Bibron). Annales Des Sciences Naturelles, Zoologie, 15, 83–91. [Google Scholar]
- Roscito, J.G. & Rodrigues, M.T. (2012) Skeletal development in the fossorial gymnophthalmids Calyptommatus sinebrachiatus and Nothobachia ablephara . Zoology, 115, 289–301. Available from: 10.1016/j.zool.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Schoch, R.R. (2006) Skull ontogeny: developmental patterns of fishes conserved across major tetrapod clades. Evolution & Development, 8, 524–536. Available from: 10.1111/j.1525-142X.2006.00125.x [DOI] [PubMed] [Google Scholar]
- Simões, T. , Caldwell, M.W. , Tałanda, M. , Bernardi, M. , Palci, A. , Vernygora, O. et al. (2018) The origin of squamates revealed by a Middle Triassic lizard from the Italian Alps. Nature, 557, 706–709. Available from: 10.1038/s41586-018-0093-3 [DOI] [PubMed] [Google Scholar]
- Simões, T.R. & Pyron, R.A. (2021) The squamate tree of life. Bulletin of the Museum of Comparative Zoology, 163, 47–95. Available from: 10.3099/0027-4100-163.2.47 [DOI] [Google Scholar]
- Singhal, S. , Colston, T.J. , Grundler, M.L. , Smith, S.A. , Costa, G.C. , Colli, G.R. et al. (2021) Congruence and conflict in higher‐level phylogenetics of squamate reptiles: an expanded phylogenomic perspective. Systematic Biology, 70, 542–557. Available from: 10.1093/sysbio/syaa054 [DOI] [PubMed] [Google Scholar]
- Skawiński, T. & Borczyk, B. (2017) Evolution of developmental sequences in lepidosaurs. PeerJ, 5, e3262. Available from: 10.7717/peerj.3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skawiński, T. , Skórzewski, G. & Borczyk, B. (2021) Embryonic development and perinatal skeleton in a limbless, viviparous lizard, Anguis fragilis (Squamata: Anguimorpha). PeerJ, 9, e11621. Available from: 10.7717/peerj.11621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tałanda, M. , Fernandez, V. , Panciroli, E. , Evans, S.E. & Benson, R.B.J. (2022) Synchrotron tomography of a stem lizard elucidates early squamate anatomy. Nature, 611, 99–104. Available from: 10.1038/s41586-022-05332-6 [DOI] [PubMed] [Google Scholar]
- Townsend, T.M. , Larson, A. , Louis, E. & Macey, J.R. (2004) Molecular phylogenetics of Squamata: the position of snakes, amphisbaenians, and dibamids, and the root of the squamate tree. Systematic Biology, 53, 735–757. Available from: 10.1080/10635150490522340 [DOI] [PubMed] [Google Scholar]
- Uetz, P. , Freed, P. & Hošek, J. (2022) The reptile database. Available at: http://www.reptile‐database.org
- Vidal, N. & Hedges, S.B. (2005) The phylogeny of squamate reptiles (lizards, snakes, and amphisbaenians) inferred from nine nuclear protein‐coding genes. Comptes Rendus Biologies, 328, 1000–1008. Available from: 10.1016/j.crvi.2005.10.001 [DOI] [PubMed] [Google Scholar]
- Walker, M.B. & Kimmel, C.B. (2007) A two‐color acid‐free cartilage and bone stain for zebrafish larvae. Biotechnic & Histochemistry, 82, 23–28. Available from: 10.1080/10520290701333558 [DOI] [PubMed] [Google Scholar]
- Werneburg, I. , Polachowski, K.M. & Hutchinson, M.N. (2015) Bony skull development in the Argus monitor (Squamata, Varanidae, Varanus panoptes) with comments on developmental timing and adult anatomy. Zoology, 118, 255–280. Available from: 10.1016/j.zool.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Werneburg, I. & Sánchez‐Villagra, M.R. (2015) Skeletal heterochrony is associated with the anatomical specializations of snakes among squamate reptiles. Evolution, 69, 254–263. Available from: 10.1111/evo.12559 [DOI] [PubMed] [Google Scholar]
- Wiens, J.J. , Kuczynski, C.A. , Townsend, T. , Reeder, T.W. , Mulcahy, D.G. & Sites, J.W., Jr. (2010) Combining phylogenomics and fossils in higher‐level squamate reptile phylogeny: molecular data change the placement of fossil taxa. Systematic Biology, 59, 674–688. Available from: 10.1093/sysbio/syq048 [DOI] [PubMed] [Google Scholar]
- Wise, P.A.D. & Russell, A.P. (2010) Development of the dorsal circumorbital bones in the leopard gecko (Eublepharis macularius) and its bearing on the homology of these elements in the Gekkota. Anatomical Record, 293, 2001–2006. Available from: 10.1002/ar.21277 [DOI] [PubMed] [Google Scholar]
- Wood, P.L. , Guo, X. , Travers, S.L. , Su, Y.‐C. , Olson, K.V. , Bauer, A.M. et al. (2020) Parachute geckos free fall into synonymy: Gekko phylogeny, and a new subgeneric classification, inferred from thousands of ultraconserved elements. Molecular Phylogenetics and Evolution, 146, 106731. Available from: 10.1016/j.ympev.2020.106731 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Table S1.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


