Summary
Background
Patients with inflammatory bowel disease (IBD) and healthy controls received primary SARS-CoV-2-mRNA vaccination and a booster after six months. Anti-TNF-α-treated patients showed significantly lower antibody (Ab) levels and faster waning than α4β7-integrin-antagonist recipients and controls. This prospective cohort study aimed to elucidate the underlying mechanisms on the basis of circulating T-follicular helper cells (cTfh) and B memory cells.
Methods
We measured SARS-CoV-2- Wuhan and Omicron specific Abs, B- and T-cell subsets at baseline and kinetics of Spike (S)-specific B memory cells along with distributions of activated cTfh subsets before and after primary and booster vaccination.
Findings
Lower and faster waning of Ab levels in anti-TNF-α treated IBD patients was associated with low numbers of total and naïve B cells vs. expanded plasmablasts prior to vaccination. Along with their low Ab levels against Wuhan and Omicron VOCs, reduced S-specific B memory cells were identified after the 2nd dose which declined to non-detectable after 6 months. In contrast, IBD patients with α4β7-integrin-antagonists and controls mounted and retained high Ab levels after the 2nd dose, which was associated with a pronounced increase in S-specific B memory cells that were maintained or expanded up to 6 months. Booster vaccination led to a strong increase of Abs with neutralizing capacity and S-specific B memory cells in these groups, which was not the case in anti-TNF-α treated IBD patients. Of note, Ab levels and S-specific B memory cells in particular post-booster correlated with the activation of cTfh1 cells after primary vaccination.
Interpretations
The reduced magnitude, persistence and neutralization capacity of SARS-CoV-2 specific Abs after vaccination in anti-TNF-α-treated IBD patients were associated with impaired formation and maintenance of S-specific B memory cells, likely due to absent cTfh1 activation leading to extra-follicular immune responses and diminished B memory cell diversification. These observations have implications for patient-tailored vaccination schedules/vaccines in anti-TNF-α-treated patients, irrespective of their underlying disease.
Funding
The study was funded by third party funding of the Institute of Specific Prophylaxis and Tropical Medicine at the Medical University Vienna. The funders had no role in study design, data collection, data analyses, interpretation, or writing of report.
Keywords: SARS-CoV-2 vaccination, Inflammatory bowel disease, Immune response, Immunosuppression, T follicular helper cells, B memory cells
Research in context.
Evidence before this study
We searched PubMed and Embase, without language restrictions, for studies published between January 1, 2000, and October 20, 2022, investigating humoral or B memory or Tfh responses to SARS-CoV-2 vaccination in immunosuppressed IBD patients. Search terms (“COVID-19” OR “SARS-CoV-2”) AND (“vaccine” OR “vaccination”) AND (“immunosuppression” OR “immunosuppressive” OR “immunomodulator” OR “adalimumab” OR “azathioprine” OR “biologic” OR “tumour necrosis factor” OR “infliximab” OR “anti-integrin” OR “vedolizumab”) AND (“antibody” OR “humoral” OR “immune response”) OR (“B memory cell”) OR (“T follicular helper cell”) AND (“IBD”) were used.
We have previously shown that SARS-CoV-2 antibody responses after two vaccine doses and also a booster dose were significantly lower in IBD patients treated with TNF-α inhibitors than in those receiving α4ß7-integrin antagonists or no medication. In accordance, also other studies have shown that anti-TNF treatment is associated with lower and faster waning antibody responses. There are however very limited data on the underlying mechanisms leading to this outcome, such as base line distributions of B and T cells, distributions of activated T follicular helper cell subsets and formation and maintenance of Spike-specific B memory cells over time.
Added value of this study
To our knowledge, these are new findings outlining the potential mechanisms responsible for the different vaccination outcomes in IBD patients with regard to their treatment regimens. We showed that reduced magnitude, persistence and neutralization capacity of SARS-CoV-2-specific Abs in anti-TNF-α-treated IBD patients were related to increased baseline inflammation and lack of activated cTfh 1 cell expansion. This resulted in likely extrafollicular immune responses with impaired formation and maintenance of memory B cells and diminished B cell diversification. In contrast, the kinetics and quality of vaccine responses in α4β7-integrin treated IBD patients were similar to healthy controls.
Implications of all the available evidence
Anti-TNF-α treatment inhibits the formation and diversification of memory B cells, thereby impeding long-term duration and breadth of antibody responses. Regular booster vaccinations counteract early antibody waning and loss of protection via SARS-CoV 2 specific humoral responses. Nevertheless, cellular responses with respect to IFN-γ seem unaffected by anti-TNF-α treatment which appears to provide protection from severe COVID-19.
Introduction
The approval and administration of SARS-CoV-2-mRNA vaccines were crucial steps in the worldwide efforts to combat the ongoing coronavirus pandemic. However, vaccinations in immuno-compromised individuals, such as patients with inflammatory bowel disease (IBD) under immunosuppressive/modulatory therapies, elicit lower and faster waning immune responses, a condition that can be partly overcome by application of a booster dose.1
IBDs, such as Crohn's disease (CD) and ulcerative colitis (UC), are chronic inflammatory conditions of the gastrointestinal tract with multifactorial origin and a rather high prevalence (>0.3%) in Western populations.2,3 IBD is characterized by uncontrolled activation of intestinal immune cells in a genetically susceptible host. The healthy mucosal immune system facilitates a mainly anti-inflammatory environment with intestinal macrophages producing interleukin (IL)-10 upon phagocytosis of/exposure to bacteria and dendritic cells secreting retinoic acid and transforming growth factor (TGF)-β, which leads to induction of regulatory T cells. A defective gut barrier and microbial dysbiosis in IBD induce accumulation and local activation of immune cells. Due to increased bacterial exposure, activated macrophages produce high levels of inflammatory cytokines such as tumor necrosis factor (TNF)-α or IL-12. Primed dendritic cells present bacterial antigens in Peyer's patches and mesenteric lymph nodes to naive T cells, which differentiate into Th1 and Th17 effector cells. Due to up-regulation of chemokine receptors and integrins, these effector T cells enter systemic circulation and home to intestinal tissues, where they further foster inflammation via cytokine secretion.4,5 These continuing inflammatory processes lead to complications such as fibrosis, stenosis and cancer, indicating the obvious need for effective anti-inflammatory therapies.
Therapeutic approaches with monoclonal Abs (mAbs) interfere with key factors in these pathogenic processes. TNF-α neutralising mAbs are widely used to treat IBD and inhibit its binding to tumor necrosis factor receptor (TNFR) 1 and TNFR2. TNFR1 is ubiquitously expressed and mainly facilitates the pro-inflammatory effects, while TNFR2 expression is rather restricted to lymphocytes and mediates activation and proliferation. TNF-α targeting therapy mainly inhibits interaction of TNF-α with TNFR2 on T cells.6 The α4β7-integrin expressed on effector lymphocytes is a key integrin for intestinal homing; it binds to MAdCAM-1 on the endothelium of intestinal blood vessels, facilitating lymphocyte extravasation to intestinal tissues. Biologicals targeting α4β7-integrin thus inhibit homing of T effector cells to the intestinal tissue and recent reports suggest that α4β7-integrin targeting also blocks recruitment of pro-inflammatory monocytes and dendritic cells to the intestine and leads to changes in programming of innate and acquired immune cells to limit inflammation.7 Furthermore, also Ab-secreting B cells home to the intestine via α4β7-integrin/MAdCAM-1 interaction as shown in murine chronic colitis models.8
Immunomodulatory drugs are successfully employed as treatments for IBD; however, there is evidence that immunogenicity of vaccines is altered under such conditions, and in particular TNF-α-targeting therapies are shown to reduce vaccine responses, e. g. upon influenza, hepatitis A and B, and unconjugated pneumococcal vaccine administration. In contrast, both the α4β7 integrin-antagonist vedolizumab and the IL-12/IL-23 inhibitor ustekinumab allow for intact systemic vaccine responses.9,10 With respect to SARS-CoV-2-mRNA vaccination in IBD patients, we and others report that patients treated with TNF-α inhibitors mount significantly lower SARS-CoV-2-specific Ab titers than those receiving α4β7 integrin-antagonists, IL-12/IL-23 inhibitors or no treatment, as shown both after administration of primary vaccination1,11,12 and also a booster dose.13,14
In order to elucidate the underlying mechanisms leading to these different outcomes, we investigated IBD patients receiving either TNF-α inhibitors or α4β7 integrin-antagonists and healthy individuals (controls) during the course of SARS-CoV-2-mRNA primary and booster vaccination in considerable detail. Our analysis included the quantification of Ab titers to Wuhan and Omicron, pre-vaccination distributions of B- and T-cell subsets, the induction of SARS-CoV-2 Spike-specific B memory cells, and distributions and activation of T follicular helper cell subsets. We aimed to investigate the immunologic processes and mechanisms that contribute to the magnitude of the Ab response, establishment of memory cells, and sustained immunity. Our results indicate that impaired vaccine responses in anti-TNF-α treated patients were associated with lack of cTfh activation and limited germinal center formation. This resulted in increased numbers of short lived plasmablasts and absence of Spike (S)-specific B memory cells, leading to early antibody waning and reduced B memory diversification.
Methods
Study population
Patients with inflammatory bowel disease (IBD) and controls without prior COVID-19 vaccination were invited to participate in this prospective, open-label, phase four cohort study at the outpatient vaccination clinic at the Institute of Specific Prophylaxis and Tropical Medicine and at the Department of Internal Medicine III, Division of Gastroenterology and Hepatology, of the Medical University Vienna and General Hospital of Vienna. Starting on March 11th 2021, a total of (n = 130) IBD patients and (n = 66) controls were enrolled and blood draws were performed before and after a 2-dose primary vaccination and a booster dose after six months (Figure S1).1 Inclusion criteria were age ≥18 years, diagnosis of IBD with or without immune-suppressive/immunomodulatory therapy, for controls no immunosuppression, and no previous SARS-CoV-2 vaccination (detailed eligibility in Supplementary Data 1). Of all recruited subjects only a subset of 58 IBD patients and 30 controls consented prior to vaccination to analyses of cellular immune responses (i. e. sampling of higher blood volume for PBMC isolation). Of these IBD patients 17 received α4β7 integrin-antagonist therapy, who were age and gender matched with the group of IBD patients with TNF-α inhibitor therapy (n = 19) and with healthy controls (n = 20). The subjects were not preselected according to Ab responses or other immunologic criteria, other than their IBD treatment. They had no history of SARS-CoV-2 infection or were excluded upon virus exposure. Treatment protocols and clinical disease activity of IBD patients are provided in Table S1 A–D. Evaluation of Ab responses and in-depth analyses of cellular immune responses was done at four time points: prior to 1st, one week (cellular analyses) or four weeks (Abs) post 2nd dose, six month after 2nd dose (prior to booster) and four weeks post booster (Figure S2).
Ethics
Written informed consent was obtained from the subjects according to the Declaration of Helsinki/International Conference on Harmonization Guideline for Good Clinical Practice. The study was approved by the Ethics Committee of the Medical University of Vienna (EK: 1073/2021) and international trial registration was done at EudraCT, Reg. Number: 2021-000291-11.
Procedures
All participants were advised to receive two doses of mRNA vaccine (either BNT162b2, Comirnaty, Pfizer BioNTech or mRNA-1273, Spikevax, Moderna Biotech) with a three-to-four-week interval and a booster dose six months after the 2nd dose. Serum samples for antibody titer measurements were collected prior to first dose and four weeks after the 2nd dose, as well as prior to booster and four weeks thereafter. Samples were obtained from native venous blood and frozen and stored until analysis. Furthermore, lithium heparinized blood for isolation of peripheral blood mononuclear cells (PBMC) was taken before the first and one week after the 2nd dose, and before and four weeks after application of the booster dose. The study was conducted as an investigator-initiated, prospective, open-label, phase IV trial.
SARS-CoV-2-specific IgG antibodies against Wuhan and Omicron variants
SARS-CoV-2-specific IgG Abs directed against spike subunit 1 (S1) protein of the original (hu-1) and the omicron BA.1 variant were measured by ELISA (Quantivac® and Anti-SARS-CoV-2-Omikron-ELISA, Euroimmun, Lübeck, Germany) in diluted serum samples (1:100) according to the manufacturer's instructions. Antibody results are reported in binding antibody units/ml (BAU/ml) for hu-1 and in relative units (RU/ml) for BA.1, and were considered positive at ≥ 35.2 BAU/ml and ≥11 RU/ml respectively. IgG specific for SARS-CoV-2 Omicron BA.4/5 receptor binding domain (RBD) were measured by ELISA (OD values) and the capacity to inhibit RBD BA.4/5 binding to ACE2 (% inhibition) were assessed as previously described, using 50 ng RBD BA.4/5 and serum dilution of 1:2.15,16 OD values > 0.25 and inhibition levels of >20% were considered positive, and inhibition levels >50% as relevant.
Leukocyte and lymphocyte counts
Leukocytes and lymphocytes were measured in EDTA whole blood with SYSMEX XP-300 differential hematology analyzer.
Cellular immune responses
PBMC isolation
PBMC from lithium-heparinized blood were prepared by Ficoll density gradient centrifugation (Ficoll Paque Plus, Amersham Biosciences, NJ, USA) and restimulated with S1--specific peptide pools from hu-1 at 0.03 nmol per peptide per 5 × 105 cells (PepTivator® SARS-CoV-2 peptide pools, Milteny Biotech) for 24 h as previously described.1
Cytokine measurements
Concentrations of interleukin (IL)-2, IL-5, IL-10, GMCSF, and interferon (IFN)-γ were measured in thawed supernatants with Luminex Human High-Sensitive Cytokine Performance Assays (Bio-Techne, Minneapolis, USA) used according to the manufacturer's instruction on a Luminex® 100/200 System. Concentrations of TNF-α in culture supernatants were evaluated by enzyme-linked immunosorbent assay (Human TNF-alpha Quantikine ELISA, R&D Systems) according to the manufacturer's instructions. Serum cytokine levels were determined with a Milliplex® Human Cytokine/Chemokine/Growth Factor Panel A kit (Merck Millipore, Billerica, MA) according to the manufacturer's instructions (Detailed protocol Supplementary Data 2).
Flow cytometric lymphocyte analysis
PBMC were surface stained with fluorochrome-conjugated mAbs (Supplementary Data 3 A and B) to characterize B cell, T cell, and circulating T follicular helper cell (cTfh) subsets (Gating strategy for cTfh Figure S3). Data were acquired on a FACS Canto II flow cytometer or Beckman Coulter Navios Ex flow cytometer by gating on cells with forward/side light scatter properties of lymphocytes and analyzed with FACS Diva 8.0 software and FlowJo_v10.8.1 software (BD Biosciences, San Jose, CA, USA). Percentages of sub-populations relate to the respective parent population and absolute numbers (n/μl) were calculated based on peripheral white blood cell counts. For detection of SARS-CoV-2 S protein-specific B memory cells, biotinylated S protein (Wuhan, 1256 aa) antigen was tetramerized with streptavidin-APC or streptavidin-BV421 probes as described in Dan et al.17 (detailed protocol Supplementary Data 4). S-specific B memory cells were quantified as percentages of total memory B cells (Gating strategy Figure S4).
Statistical methods
Prior to statistical evaluation, residuals after accounting for group means were submitted to distribution analysis. Ab levels as well as B- and T-cell counts including those of subsets were best fit by a log-normal distribution and, hence, these data were log-transformed, while percentages of B- and T-cell populations were best fit by a Johnson SU distribution suggesting a logit-transformation to obtain normality. Further statistical analyses were done using transformed values. Graphically, results are presented as dot-plots with medians and interquartile ranges (IQR) or line-plots showing means and 95% confidence intervals (CI). Data were analyzed by Generalized Linear Models (GLM) with patient groups and controls as between-subjects factors and (for antibody levels) time point of blood withdrawal as within-subject factor. In this case, a Multilevel Mixed-Effect GLM was applied with subject ID as a random effect indicator. The full factorial design was used; however, hypotheses tests were done on the group × time interaction with comparisons between groups performed by linear contrasts with Bonferroni-Holm corrected p-values. Contrast were within time point as well as differences between subsequent time points comparisons of IBD groups against controls. Homogeneity of variances was tested by Brown–Forsythe tests and normality of residuals by Shapiro–Wilk tests. A multivariate discriminant analysis was performed to assess the contribution of the different subpopulations of B- and T-cells for differentiating the two IBD groups and the control group. Results are graphically presented as dot-plots presenting each participant in the plane spanned by the two canonical roots. Contribution of the different components of the B- and T-cell subsets is shown color-coded for positive and negative coefficients. Participants lost to follow-up were included up to the time point of loss. For all analyses no imputations for missing values were applied and p-values below 0.05 were considered significant. Analyses were performed using Stata 17.0 (StataCorp, College Station, TX, USA). Graphs were produced using GraphPad Prism (San Diego, CA, USA; Version 7.0).
From previous investigations, we assumed a standard deviation of the log10-Ab levels of 0.3. For three time points (4 wks after 2nd, 6 mo after 2nd, 4 wks after 3rd vaccination) and three groups (controls, IBD_TNF-α, IBD_α4β7-integrin) we defined the effect size for the group × time interaction as delta = 0.7 (square root of the ratio of the effect to the error variance) that should be detected with a power of 80% at the 5% significance level. The between time point correlation was assumed to be 0.7. Under these assumptions, a sample size of n = 10 per group is required. Since baseline as well as further B- and T-cell parameters should be assessed, it was decided to enroll about 20 patients per group. Since this is not a randomized controlled trial and only one intervention schedule was applied (COVID-19 2 + 1 vaccination) there was no randomization.
Role of funders
The study was funded by third party funding of the Institute of Specific Prophylaxis and Tropical Medicine at the Medical University Vienna. The Medical University of Vienna was the sponsor of this academic study. The funders had no role in study design, data collection, data analyses, interpretation, or writing of report.
Results
Patients and settings
Of all enrolled subjects, 114 IBD patients and 62 controls received the complete vaccination series with 2-dose primary vaccination and a booster dose after six months. Only a subgroup of IBD (n = 58), and controls (n = 30) agreed to donate also blood for cellular analyses. From these IBD patients two groups were selected according to their treatment: i) IBD patients receiving TNF-α inhibitor therapy (IBD_TNF-α; n = 19; three patients with co-administration of azathioprine) and ii) IBD patients receiving the α4β7 integrin-antagonist vedolizumab (IBD_α4β7-integrin; n = 17), which were compared to controls (n = 20) (Figure S1). The demographic parameters age, sex (self-reported by study participants), BMI and diagnosis of UC or CD are listed in Table 1. Mean age and BMI were similar in the three groups. Prior to 1st vaccination two patients in the IBD TNF-α group had moderate disease activity; all other patients were in clinical remission with only mild active disease (patient reported outcome scores for CD18 and UC19 as previously described). Detailed information on type and phase of treatment as well as disease activity is provided in Table S1 A–D.
Table 1.
Demographic data of study subjects.
| IBD (TNFα) | IBD (α4β7-integrin) | Controls | |
|---|---|---|---|
| participants (n) | 19 | 17 | 20 |
| sex (m/f) | 14/5 | 6/11 | 11/9 |
| age (y) | 45.1 (39.72–50.9) | 50.5 (40.6–60.5) | 53.5 (46.4–60.6) |
| BMI | 24.7 (22.4–27.0) | 25.5 (22.3–28.8) | 26.1 (24.2–27.9) |
| UC/CD | 7/12 | 11/6 |
Values are given as arithmetic means with 95% CI.
Abbreviations: f, female; m, male; y, year; α4β7-integrin, α4β7-integrin-antagonist therapy; BMI, body mass index; CD, Crohn's disease; IBD, inflammatory bowel disease; UC, ulcerative colitis; TNFα, TNFα-targeting therapy.
Humoral immune response
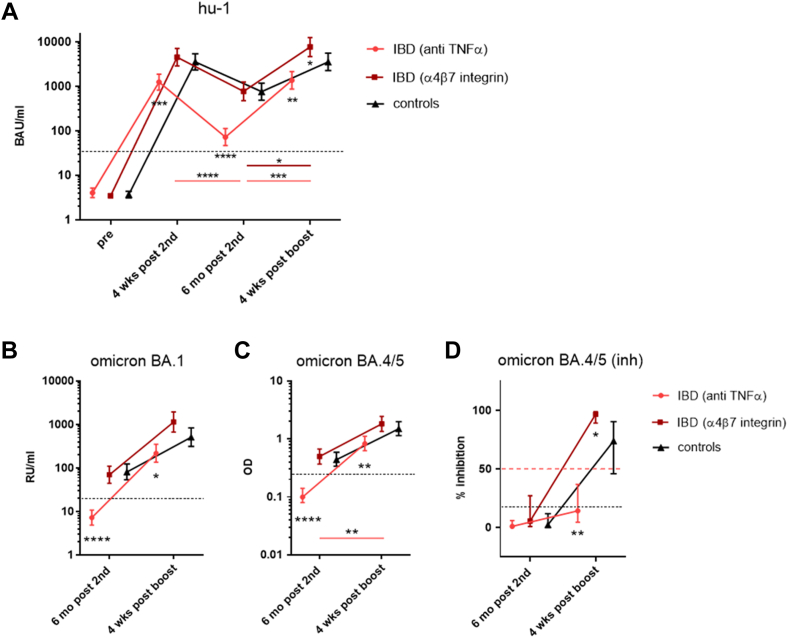
SARS-CoV-2-specific IgG Abs against hu-1 S1 protein were measured pre-vaccination and four weeks and six months after the 2nd dose, as well as four weeks after administration of a booster dose. Analysis of geometric mean concentrations (GMC) showed that four weeks after the second dose, patients treated with TNF-α inhibitors had mounted significantly lower antibody levels (GMC 1241 BAU/mL [95% CI 817–1884]) than healthy controls (GMC 3552 BAU/mL [95% CI 2339–5393]) or IBD patients receiving α4β7-integrin antagonist (GMC 4537 BAU/mL [95% CI 2881–7144]). Six months after the second dose, S1-specific IgG had declined significantly faster to significantly lower levels in TNF-α inhibitor treated IBD patients compared to controls (GMC 72 BAU/mL [95% CI 46–112] vs. GMC 757 BAU/mL [95% CI 486–1180]), and also to subjects receiving α4β7-integrin antagonist (GMC 771 BAU/mL [95% CI 476 to 1248]). Importantly, booster vaccination following six months after the second dose increased the S1-specific Ab levels in all groups four weeks thereafter. Even though fold-increase was significantly stronger in IBD_TNF-α patients vs. controls, S1-specific IgG levels after four weeks remained significantly lower than in the control group (GMC 1363 BAU/mL [95% CI 876 to 2124] vs. GMC 3557 BAU/mL [95% CI 2263 to 5589]) and also in α4β7-integrin treated IBD patients (GMC 7684 BAU/mL [95% CI 4701 to 12,559]) (Fig. 1A). The majority of IBD patients treated with TNF-α inhibitors presented with Ab levels <2000 BAU/mL after the 2nd dose which declined to <90 BAU/mL after six months and were thus classified as low-responders. High responder vaccinees in the control and IBD_α4β7-integrin group were defined by mounting S1-specific IgG >4000 BAU/mL after the 2nd dose and maintaining high Ab levels >1100 BAU/mL after six months.
Fig. 1.
Kinetics of SARS-CoV-2-Spike (S1) specific IgG antibodies. Geometric mean concentrations (GMC) with 95% CI of A) S1-specific IgG against ancestral virus hu-1 in BAU/mL in IBD patients treated with either anti-TNFα (n = 19) or α4β7-integrin-antagonists (n = 17) and controls (n = 20) measured before the first and four weeks and six months after the second dose, as well as four weeks after booster dose of SARS-CoV-2 mRNA vaccine (BNT162b2 or mRNA-1273); dashed line - positive cut-off for S1-specific IgG at 35.2 BAU/mL; B) S1-specific IgG against Omicron BA.1 (in RU/mL), dashed line—positive cut-off for BA.1 S1-specific IgG at 11.0 RU/m; C) IgG specific for RBD of Omicron BA.4/5 (as OD) and D) inhibition capacity of BA.4/0.5-specific Abs (as % inhibition) measured six months after the second dose and four weeks after booster dose; OD values > 0.25 and inhibition levels of >20% were considered positive (black dashed line), inhibition levels >50% as relevant (red dashed line). Asterisks positioned below IBD/anti-TNF-α data points represent significance of difference to controls, below IBD/integrin antagonist datapoints difference between this group and controls. Asterisks with a band indicate significant changes over time; band in light red - difference between controls and IBD/anti-TNF-α, in dark red - difference between controls and IBD/integrin antagonist. Abbreviations: BAU, binding antibody units; mo, months; RBD, receptor-binding domain; RU, relative units; S1, SARS-CoV-2 Spike protein 1. ANOVA with linear contrasts; ∗∗∗∗p ≤ 0.0001; ∗∗p ≤ 0.01; ∗p ≤ 0.05.
In addition to IgG specific for the wild-type S1 sequence we also measured concentrations of Omicron BA.1 and Omicron BA.4/5 specific IgG Abs, as well as cross neutralization capacity of Omicron BA.4/5 specific Abs at six months post 2nd dose and four weeks after booster. Similar to wild-type-specific IgG Abs, concentrations of Omicron BA.1- as well as BA.4/5-specific IgG Abs were significantly lower in anti-TNF-α treated IBD patients compared to controls and α4β7-integrin treated patients, both at six months post 2nd dose and four weeks after booster dose (Fig. 1 B, C). Cross neutralization capacity of Omicron BA.4/5-specific IgG Abs was not detectable for both treatment groups and controls six months after 2nd dose. While inhibition capacity could be significantly boosted in the integrin antagonist treated patients and the control group, no such effect could be obtained in the anti-TNF-α treated IBD group (Fig. 1 D). The individual trajectories for all antibody levels are shown in Supplementary Figure S5 A–D.
Cytokines in re-stimulated PBMC and in sera
We analyzed cell mediated immune responses (IFN-γ, IL-2, IL-10, GMSCF and IL-5) before and one week as well as six months after the 2nd dose and four weeks post booster vaccination in S1-re-stimulated PBMC. IFN-γ production after primary (2nd dose) and booster vaccination was not reduced in anti-TNF-α treated IBD patients compared to controls, while IL-2 and IL-5 levels were significantly lower one week after 2nd dose (Supplementary Figure S6 A, B, E). We also determined concentrations of IFN-γ, IL-2, IL-10, IL-12, IL-13, IL-17 A and TNF-α in sera obtained before and four weeks as well as six months after the 2nd dose, and four weeks after booster. Results showed that in neither of the investigated groups an increase of serum cytokines four weeks after primary and booster vaccination was present (Figure S7). Furthermore, some patients with elevated baseline TNF-α and IFN-γ levels in culture supernatants (Table S2) also showed elevated cytokine concentrations prior to vaccination in sera.
Leukocyte and lymphocyte counts
In comparison to the controls, both IBD groups had slightly more leukocytes (mean 6.13 × 10ˆ3/μl [95% CI 5.29–6.95] in controls vs. 7.28 × 10ˆ3/μl [95% CI 6.25–8.31] in anti-TNF-α treated and 7.67 × 10ˆ3/μl [95% CI 5.94–9.42] in α4β7-integrin treated) and lower percentages of lymphocytes (32.1% [95% CI 28.9–35.3] in controls vs. 28.5% [95% CI 22.4–34.7] in anti-TNF-α treated and 28.7% [95% CI 24.4–33.0] in α4β7-integrin treated). This resulted in similar absolute numbers of lymphocytes in the three groups (1.94 × 10ˆ3/μl [95% CI 1.70–2.19] in controls vs. 2.04 × 10ˆ3/μl [95% CI 1.60 to 2.48] in anti-TNF-α treated and 2.18 × 10ˆ3/μl [95% CI 1.59–2.76] in α4β7-integrin treated) (Figure S8.1 A–C).
Flow cytometric lymphocyte analyses
In order to investigate whether IBD treatment with different biological immuno-modulators influenced the base-line distributions of lymphocyte subsets, we analyzed PBMC samples obtained pre-vaccination by flow-cytometry.
CD19+ B cells and CD3+ T cells
Flow-cytometric characterization of lymphocytes showed that mean levels of CD19+ B cells and CD3+ T cells as percentages of total lymphocytes and absolute numbers (n/μl) were normal. The relative percentage of CD19+ B cells was significantly reduced in the IBD_TNF-α group and but not absolute numbers (Figure S8.2 A, B). Four B cell subsets were distinguished: IgD+/CD27− naive B cells, IgD+/CD27+ un-switched B memory cells, IgD−/CD27+ switched B memory cells, and a double-negative population, consisting of B memory and Ab secreting cell (APC) precursors lacking CD27 expression.20 Respective analysis showed by trend reduced absolute naïve B cells (0.98 × 10ˆ3/μl [95% CI 0.68–1.28]) in the IBD_TNF-α group compared to controls (1.28 × 10ˆ3/μl [95% CI 1.02–1.54]) and a similar pattern for un-switched B cells (IBD TNF-α group 0.21 × 10ˆ3/μl [95% CI 0.15–0.26] vs. controls 0.32 × 10ˆ3/μl [95% CI 0.21–0.44]). (Figure S9 A, B, E, and F). Of note, we also observed significantly more plasmablasts (PB) (CD19+/CD27++/CD38high) as % of B cells at base line in this group compared to controls and to α4β7-integrin antagonist treated IBD patients (Figure S8.2C and D).
CD3+ T cells as percentages of lymphocytes were significantly increased in IBD_TNF-α group compared to controls, yet not in absolute numbers (Figure S8.2E and F). The CD4+ and CD8+ T cell subsets did not differ significantly between IBD groups and controls, but NK-T cells were significantly increased as % and absolute numbers in the IBD_TNF-α group compared to α4β7-integrin antagonist treated patients (Figure S8.2 I and J). Four memory subsets of CD4+ and CD8+ T cells were quantified based on their CD45RA and CCR7 expression: naive T cells, lymph node homing central memory T cells, peripheral tissue homing effector memory T cells, and CD45RA re-expressing effector memory T cells.21,22 IBD patients receiving α4β7-integrin antagonists had significantly more naïve T cells as relative % of CD4 T cells compared to controls (Figure S8.2 G and H). Naïve and memory compartments of CD8 T cells did not differ significantly between the groups (data not shown).
Multivariate analysis of respective B and T cell subsets (in absolute numbers) discriminated between controls and IBD patients, and further between anti-TNF-α and α4β7-integrin antagonist treatment in these patients (Figure S10).
Distributions, shifts and activation status of T follicular helper cell subsets
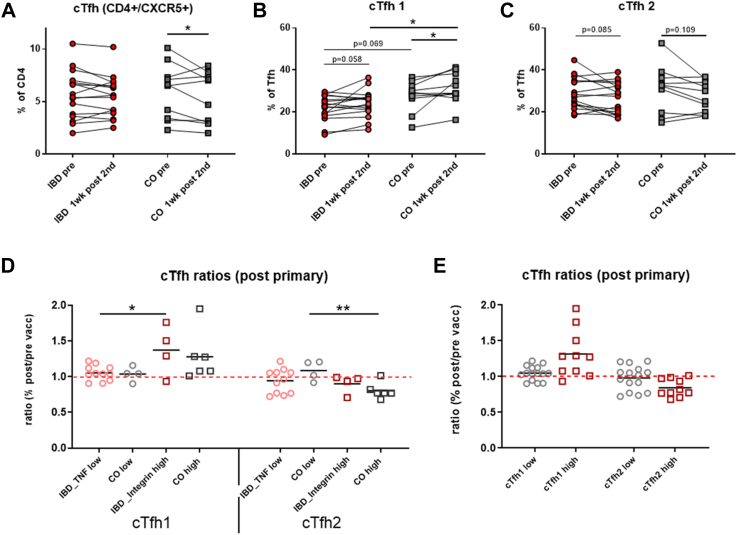
We quantified circulating T follicular helper cells (cTfh) as percent of CD4+ T cells in IBD patients and controls by expression of CXCR5. No differences in cTfh cells before and one week after the 2nd vaccine dose were observed between 16 IBD patients, ([n = 12] with anti-TNF α treatment and [n = 4] with integrin antagonists) and 10 controls ([n = 6] high- and [n = 4] low-responder controls. A significant decrease of total cTfh cells was only observed in controls, which we further investigated in the subgroup analysis (Fig. 2A). Similar to classical T helper cells, cTfh cells can be divided into cTfh1, cTfh2, and cTfh17 subsets according to expression of the chemokine receptors CXCR3 and CCR6 (Figure S3). Generation of CXCR3+ cTfh1 cells is reported in response to viral infections,23, 24, 25 while cTfh2 and cTfh17 cells are associated with human auto-immune diseases.26 Subset analysis indicated increased cTfh1 cells in IBD patients (p = 0.058, ANOVA with linear contrasts) and controls (p < 0.05, ANOVA with linear contrasts) one week post 2nd compared to pre-vaccination. This resulted in significantly more cTfh1 cells in the control vs. IBD group after vaccination (Fig. 2B). When grouping IBD patients into integrin antagonist-treated high responders and anti-TNF-α treated low responders, integrin antagonist-treated IBD high responders showed increased cTfh1 subsets after vaccination (post/pre ratio >1) which was not the case in anti-TNF treated IBD low responders. In control high responders cTfh1 cells were increased non-significantly, while decrease of the Tfh2 subset (post/pre ratio <1) was significant (Fig. 2D). Fig. 2E summarizes that high- and low-responder vaccinees differed with regard to their cTfh1 and cTfh2 ratios.
Fig. 2.
Quantification of circulating T follicular helper (cTfh) cells, cTfh1 and cTfh2 subset, and post/pre ratios of cTfh1 and cTfh2. PBMC were stained with fluorochrome-labeled mAbs and analyzed on a BD FACS Canto II flow cytometer. Quantification of A) cTfh (CD3+/CD4+/CD45RA-/CXCR5+) in 16 IBD patients (of these [n = 12] with anti-TNF α treatment and [n = 4] receiving integrin antagonists) and 10 controls (of these [n = 6] high responders and [n = 4] low responders before (pre) and one week after 2nd dose as % of total CD4+ T cells; B) cTfh1 and C) cTfh2 cells as % of total cTfh before (pre) and one week after 2nd dose; D) Ratios (% post 2nd/% pre vaccination) of cTfh1 and cTfh2 subsets shown in low-responder IBD patients with anti-TNF α treatment (n = 12) and low-responder controls (n = 4) as well as high-responders IBD patients treated with integrin antagonists (n = 4) and high-responder controls (n = 6), black line is arithmetic mean; and E) Ratios of cTfh1 and cTfh2 subsets combined in low-responders and high-responders of all groups, black line is arithmetic mean. ANOVA with linear contrasts; ∗p ≤ 0.05.
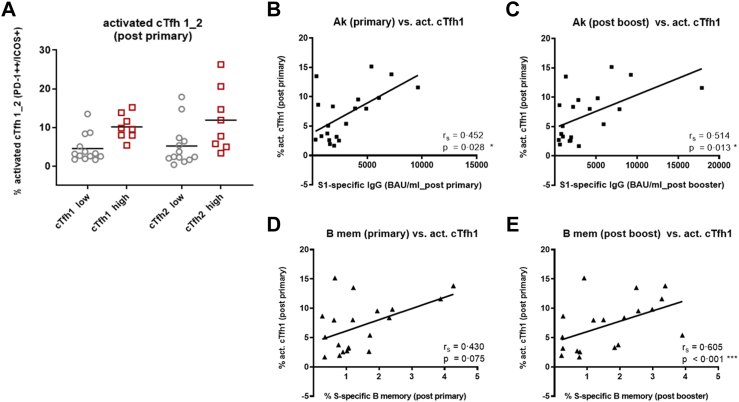
Quantification of activated cTfh1 and cTfh2 cells (PD-1++/ICOS+)27 showed that high responder vaccinees (integrin antagonist-treated IBD patients and high responder controls) had more activated cTfh1 and also Tfh2 cells after primary vaccination than low responders (anti-TNF-α treated IBD patients and low-responder controls) (Fig. 3A). After booster vaccination no differences in numbers of activated cTfh1 and cTfh2 cells (Figure S11) and in cTfh1 and cTfh2 ratios between high and low responders were observed (data not shown).
Fig. 3.
Quantification of activated cTfh1 and cTfh2 cells and correlation with S1-specific IgG Abs and S-specific B memory cells. A) Quantification of activated cTfh1 and cTfh2 (as % of total cTfh1 and cTfh2 cells) pre and post primary vaccination in 8 high-responders (squares) (of these [n = 3] IBD patients with integrin antagonists and [n = 5] high-responder controls) and 13 low-responders (circles) (of these [n = 9] IBD patients with anti-TNF α treatment and [n = 4] low responder controls), the shown percentages are [% activation post 2nd dose] minus [% activation pre vaccination], black line is arithmetic mean. Correlation of activated cTfh1 cells present after primary vaccination with B) hu-1 S1-specific IgG Ab levels after primary vaccination and C) after booster vaccination; with D) percentages of S-specific B memory cells after primary vaccination and E) after booster vaccination. Spearman rank correlation coefficients (rs) with p-values obtained by bootstrapping; ∗∗∗p ≤ 0.001, ∗∗p ≤ 0.01, ∗p ≤ 0.05.
SARS-CoV-2 spike (S) protein-specific memory B cells
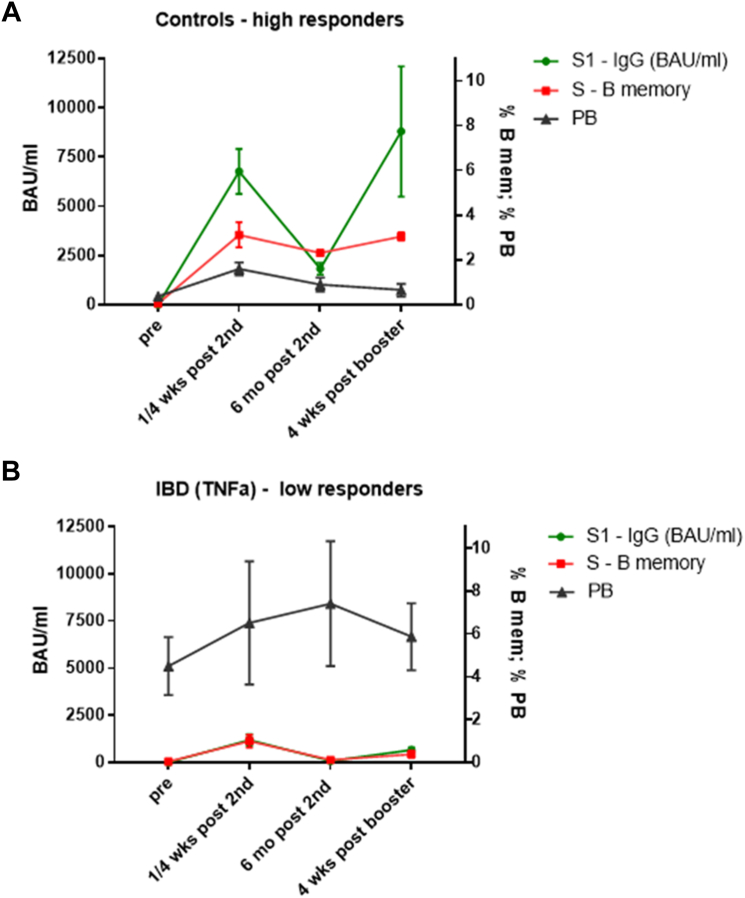
High responder controls (n = 4) with hu-1 S1-specific IgG >4000 BAU/mL one month after 2nd dose and high Ab levels after six months (>1100 BAU/mL) formed high numbers of S-specific B memory cells one week after the 2nd dose. These S-specific B memory cells remained stable or further expanded until six months post 2nd dose and further increased after booster vaccination. These high-responders showed a normal pattern of PB induction, i. e. low frequencies of PB pre-vaccination and increase of PB one week after primary vaccination (Fig. 4A). In contrast, anti-TNF-α treated IBD low-responders (n = 4; Ab levels <2000 BAU/mL after 2nd dose which declined <90 BAU/mL after six months) established low numbers of S-specific B memory cells, which were not maintained until six months after 2nd dose and hardly expanded upon booster. In parallel, increased total PB populations were present before vaccination. This kinetic was most prominent in association with elevated IFN-γ/TNF-α baselines (Fig. 4 B) and not so clearly seen in anti-TNF-α treated IBD low-responders without inflammatory cytokines (n = 9, Figure S12A). Levels of S1-specific IgG Abs and S-specific B memory cells in α4β7-integrin antagonist treated IBD high responders (n = 4) were comparable to controls (Figure S12B). In accordance, the percentages of S-specific B memory cells in all analyzed subjects were positively correlated with SARS-CoV-2 S1-specific Ab levels at all time points (four weeks and six months post 2nd and four weeks post booster), (Figure S13 A–C).
Fig. 4.
Kinetics of S1-specific IgG, S-specific memory B cells and plasmablasts in high- and low-responder vaccinees. Kinetic of S1-specific IgG (green line, in BAU/mL), S protein-specific memory B cells (as % of total B memory cells, red line) and plasmablasts (PB; as % of total CD19+ B cells, black line) determined at four time points: pre-vaccination, either one week (S-specific B memory, PB) or four weeks (S1-specific IgG) post 2nd dose, six months post 2nd dose and four weeks post booster vaccination; A) in control high-responders (n = 4; S1-specific IgG >4000 BAU/mL 4 wks after 2nd dose and >1100 BAU/mL after 6 months), and B) in anti-TNF-α treated IBD low-responders with elevated IFN-γ/TNF-α baseline levels (n = 4; S1-specific IgG <2000 BAU/mL after 2nd dose and <90 BAU/mL after six months). Data points represent arithmetic mean with SEM.
Correlations between cTfh, S1-specific Abs and B memory cells
The numbers of activated cTfh1 cells present after primary vaccination correlated with hu-1 S1-specific Ab levels after primary and after booster vaccination (Fig. 3 B, C); the correlation with presence of S-specific B memory cells was highly significant after booster but not after primary vaccination. (Fig. 3 D, E).
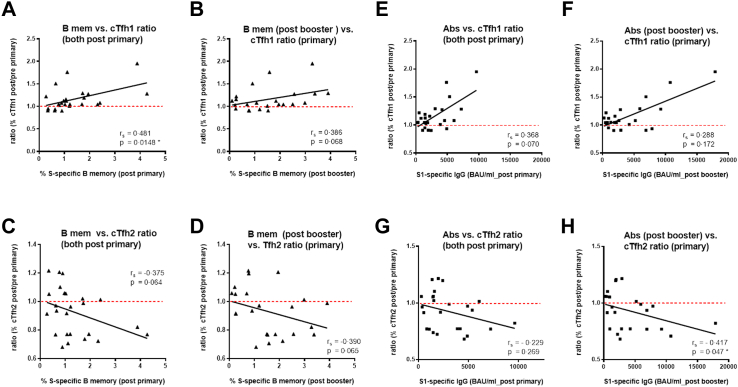
Furthermore, we correlated the ratios of cTfh1 and cTfh2 cells (percentages post/pre vaccination) with S-specific memory B cells and hu-1 S1-specific IgG levels after primary and booster vaccination. This indicated that the increase of cTfh1 cells correlated significantly with S-specific memory B cells after primary (p = 0.01) but less after booster vaccination (p = 0.06, Spearman rank correlation). Circulating Tfh2 ratios showed negative correlations though not statistically significant (Fig. 5A–D). Similar associations were observed between hu-1 S1-specific IgG Ab levels and cTfh1 and cTfh2 ratios, yet statistically significant only between cTfh2 decrease and Abs post booster (Fig. 5E–H).
Fig. 5.
Correlationsof cTfh1 and cTfh2 cells ratios post primary vaccination with S1-specific IgG Abs and S-specific B memory cells post primary and booster vaccination. Correlation of cTfh1 cell ratio after primary vaccination (% post 2nd/% pre vaccination) with S-specific B memory cells A) after primary vaccination and B) after booster vaccination; correlation of cTfh2 cell ratio after primary vaccination with S-specific B memory cells C) after primary vaccination and D) after booster vaccination. Correlation of cTfh1 cell ratio present after primary vaccination with hu-1S1-specificIgG Ab levels E) after primary vaccination and F) after booster vaccination; correlation of cTfh2 cell ratio present after primary vaccination with hu-1S1-specificIgG Ab levels G) after primary vaccination and H) after booster vaccination. Spearman rank correlation coefficients (rs), ∗p ≤ 0.05.
Correlation of Ab decline with the demographic parameters age and BMI
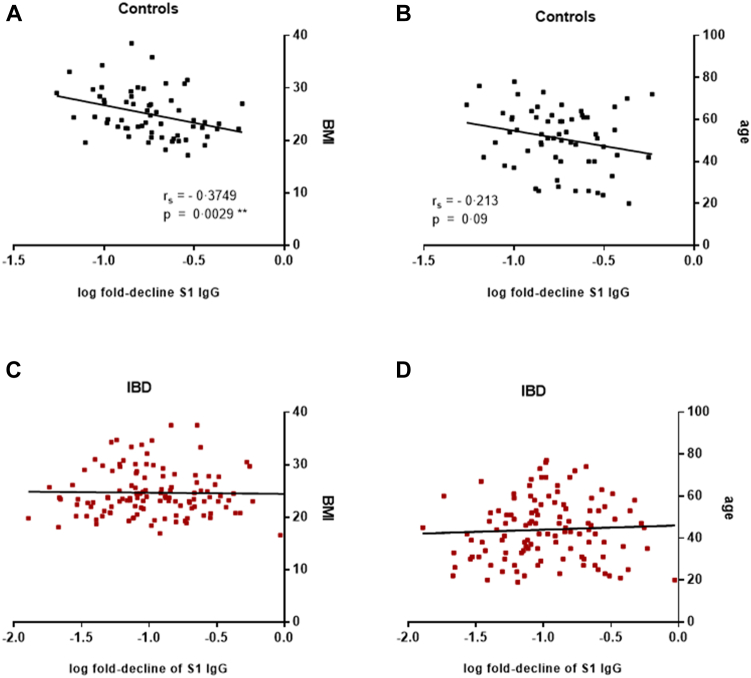
In order to assess the relative influence of demographic parameters on the decline of vaccine-induced Abs, we investigated whether the decline rates of SARS-CoV-2 hu-1 S1-specific IgG (BAU/mL) between four weeks and six months after the 2nd dose correlated with age and body mass index (BMI) of the subjects. This was done with data from the entire cohorts of IBD patients (n = 130) and controls (n = 66) that were recruited for our study on SARS-CoV-2 vaccine responses in immune-compromised patients.1 Spearman rank correlations showed that the Ab decline correlated significantly with BMI (r = −0.374, p = 0.003) and by trend also with age (r = −0.213, p = 0.09) in healthy controls, but not in the IBD patients (Fig. 6A–D).
Fig. 6.
Correlation of S1-specific IgG decline with demographic parameters. Correlation of log transformed decline rates of SARS-CoV-2 S1-specific IgG concentrations between four weeks and six months after the 2nd dose with A) BMI and B) age of healthy control subjects (n = 66), and C) BMI and D) age of IBD patients (n = 130); Spearman rank correlation coefficient (rs) for log transformed S1-specific IgG decline rates and BMI (rs = −0.374, p = 0.003), and age (rs = −0.213, p = 0.09) in healthy controls. Abbreviations: BMI, body mass index; IBD, inflammatory bowel disease. ∗∗p ≤ 0.01.
Discussion
Patients undergoing immunosuppressive treatment represent a risk group for developing severe COVID-19 disease and were therefore prioritized in SARS-CoV-2 vaccination campaigns. In order to monitor vaccine-responsiveness in such cohorts we recruited a total of 263 immunocompromised patients, of these 130 with IBD. The majority of IBD patients (54%) received anti-TNF-α treatment, and of those treated with other or non-biologicals, the largest group (17%) were patients receiving α4β7-integrin antagonists. The data obtained in that study show that humoral (Ab) non/low-responsiveness to SARS-CoV-2 vaccination in IBD patients occurred in association with anti-TNF-α but not α4β7-integrin-antagonist treatment.1 Our goal of the current study was to identify potential alterations in immune cell distributions in the respective treatment cohorts and to elucidate immunologic processes and mechanisms that contributed to the observed differences in magnitude and persistence of vaccine-induced humoral responses.
IBD patients receiving anti-TNF-α blockers responded with a lower magnitude and faster waning of SARS-CoV-2 Abs against both Wuhan and Omicron variants after primary vaccination. Booster vaccination could not restore antibody levels as well as inhibition capacity to the responses in healthy controls and integrin-antagonist treated IBD patients. These observations were associated with reduced B cells but expanded plasmablast populations at baseline.
The diminished Ab production in anti-TNF-α treated IBD vaccinees might be associated with their limited total and naïve B cell pool. Reduced circulating CD19+ B cells and un-switched B memory cells in TNF-α treated IBD patients are also described by others28 and both, the crucial role of TNF-α in formation of proper lymphoid organ structure29 and its function in B cell activation and proliferation might be causative.30 Furthermore, we hypothesized that lack of expansion of cTfh1 cells after primary vaccination in low responders might be impairing Ab production. Indeed, we here show the importance of activated cTfh1 cells for induction of long-term humoral responses, because particularly these cells strongly correlated with antibodies and memory B cells after booster. Similarly, also others described that expansion of cTfh1 cells correlated with the magnitude of the induced antibody response after yellow fever and influenza vaccination31,32 and HCV and SARS-CoV-2 infection.23,24 In addition, data from studies with H1N1 influenza vaccination show that high responsiveness is associated with cTfh activation in healthy controls and HIV patients.33
Despite the faster waning of antibody responses in anti-TNF treated patients, cytokine/IFN-γ production by S1 stimulated PBMC was comparable to healthy controls as also described by others.34, 35, 36 Vaccine-specific cellular responses - particularly Th1 cytokines - may prevent severe COVID-19 disease even in the absence/lack of specific antibodies. This is supported by the fact that in case of breakthrough infections (with Omicron variants) only mild disease without increased rates of hospitalization occurred in our cohorts (data not shown) as well as in other studies.37,38
Regarding the function of TNF-α in humoral immune responses, previous murine research shows that TNF-α and its receptor are required for the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers (GC),29,39 and a similar phenotype and reduced B memory cells are shown in rheumatoid arthritis patients with anti-TNF-α therapy.40 However, more recent preclinical models show that actually increased TNF-α levels contribute to GC B cell suppression during intracellular bacterial infection41 and that TNF-α and IFN-γ limit GC responses in malaria infection by inhibiting Tfh cell differentiation.42 Furthermore, patients who succumbed to severe COVID-19 infection show high TNF-α production and loss of Bcl-6-expressing Tfh cells and GCs in post-mortem LN analyses.43,44
By definition, mAbs targeting TNF-α bind to membrane bound TNF-α on macrophages and disrupt the costimulatory interaction with TNFR2 on mucosal T cells, leading to T cell apoptosis, while migration of the different proinflammatory cell subsets remains unimpaired in this treatment collective.45 Accordingly in our study we observed increased base-line/systemic IFN-γ levels in several patients receiving TNF-α blockers. Interestingly, TNF-α baselines were also elevated, indicating that, notwithstanding the treatment, TNF-α levels fluctuated probably because of drug pharmacokinetics or drug resistance and treatment failures.46 We suggest that the increased inflammatory cytokine levels in IBD patients treated with anti-TNF-α blockers led to mostly extrafollicular (EF) vaccine responses by limiting cTfh cell entry to GC, and in consequence to impaired GC formation, lack of long-lived S-specific B memory and plasma cells and diminished B memory cell diversification. The expanded plasmablast populations prior to vaccination might furthermore suggest that EF immune responses to other antigens were continuously ongoing in these patients. In addition, we detected expanded NK-T cell populations in TNF-α treated IBD patients, which might also have contributed to the elevated IFN-γ baselines, as it was shown that Type I NK-T cells increase IFN-γ production upon contact with activated macrophages.47
IBD patients treated with α4β7-integrin antagonists exhibited expanded naive CD4 T cell populations. Treatment targeting α4β7-integrin by definition reduces the circulation of inflammatory innate and CD4 Th1 effector cells in peripheral blood and tissues, and accordingly no elevated base lines of inflammatory cytokines were measured. Expanded naive T cells and normal B cell populations prior to vaccination might have allowed for sufficient T and B cell priming, adequate type 1 cTfh cell activation and differentiation and subsequent follicular GC responses with formation of long-lived B memory and plasma cells in these patients, similar to healthy controls.
Our data clearly demonstrate that vaccine responsiveness in IBD patients depends on the type of anti-inflammatory treatment and that respective sub-analysis is essential. Along these lines, a previous study–not stratifying according to treatment–described that receptor binding domain–specific B memory cells were generated at similar rates in controls and IBD patients.48 Another recent study also did not show any difference between anti-TNF-α and α4β7-integrin antagonist treatment, in all likelihood because SARS-CoV-2-specific B memory cells were quantified only in the switched (CD19+/IgD-/CD27+) B memory compartment and maintenance of B memory cells up to six months was not investigated.49 In contrast to these studies, we succeeded to show by treatment-subgroup analysis that activation of cTfh1 cells after primary vaccination strongly correlated with formation of B memory cells and S1 specific antibody levels, indicating the critical role of activated cTfh1 cells for establishment of long-term humoral immunity and breadth of memory B cell clones.
Additionally, we also observed that the decline rate of SARS-CoV-2-specific IgG correlated with BMI and age in healthy controls, indicating that baseline inflammation due to obesity (associated with metabolic processes) or age (inflammaging) in otherwise healthy individuals was associated with faster Ab decline, a finding also observed in our previous work with such cohorts.50,51 We suggest that the here described pathway responsible for faster antibody waning may also account for other pro-inflammatory situations, including obesity and increased age.
The observed decline of memory B cells and antibody levels in anti-TNF treated patients suggest a need for repeated booster vaccinations. However, their T cell responses with particular regard to IFN-γ production comparable to healthy controls indicate that protection against severe disease might be established even in the absence of antibody production. A limitation of the study is the rather small sample size; however, the clear-cut results seem representative for the selected groups.
Thus, our results have implications for patient-tailored vaccines/vaccination schedules in anti-TNF treated patients, irrespective of their underlying disease.
Contributors
Literature search: EGS, AW, GN, HS, WR, UW; figures: EGS, MK; study design: EGS, AW, GN, WR, UW; experiments and data collection: EGS, MOT, AS, VG, RK, PM, UW; data analysis: EGS, MOT, AS, MK, HS, PG, BK, ANAS, UW; data interpretation: EGS, AW, MOT, HS, GN, WR, RC, UW; writing: EGS, UW; revising the manuscript: EGS, AW, VG, AS, MOT, MK, RK, PM, JH, HS, PG, RV, BK, ANAS, WFP, GN, WR, RC, UW.
All authors read and approved the final version of the manuscript. EGS and UW have verified the underlying data of this study.
Data sharing statement
Ethics approval does not allow data sharing of person related data. Upon reasonable request and depending on a positive ethics vote data can be provided by the principal investigator.
Declaration of interests
EGS: none; AW: received fees from AbbVie and Astra Zeneca; VG: none; AS: none; MOT: none; MK: received an investigator initiated research contract from Pfizer outside the present work; RK: none; PM: none; JBH: Boehringer Ingelheim; HS: none; GN has received fees from AbbVie, MSD, Takeda, Janssen, Sandoz, Pfizer, Astro Pharma, Falk Pharma, Ferring Galapagos, Bristol-Myers Squibb, and Vifor; PG: none; RV: has received research grants from Worg Pharmaceuticals, Hangzhou, China, HVD Biotech, Vienna, Austria and Viravaxx, Vienna, Austria. He serves as consultant for Viravaxx and Worg; BK: none; ANAS: none; WFP: has received fees from Novartis, Astra Zeneca, Medical Dialogue, BMS; WR has served as speaker for AbbVie, Celltrion, Falk Pharma GmbH, Ferring, Janssen, Galapagos Medice, MSD, Roche, Pfizer, Pharmacosmos, Shire, Takeda, Therakos, as consultant for AbbVie, Amgen, AOP Orphan, Arena Pharmaceuticals, Astellas, Astra Zeneca, Bioclinica, Boehringer Ingelheim, Bristol Myers Squibb, Calyx, Celgene, Celltrion, Eli Lilly, Falk Pharma GmbH, Ferring, Galapagos, Gatehouse Bio Inc., Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Janssen, Landos Biopharma, Medahead, MedImmune, Microbiotica, Mitsubishi Tanabe Pharma Corporation, MSD, Novartis, OMass, Otsuka, Parexel, Periconsulting, Pharmacosmos, Pfizer, Protagonist, Provention, Quell Therapeutics, Sandoz, Seres Therapeutics, Setpointmedical, Sigmoid, Sublimity, Takeda, Teva Pharma, Therakos, Theravance, Zealand, as advisory board member for AbbVie, Amgen, Astra Zeneca, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Galapagos, Janssen, Mitsubishi Tanabe Pharma Corporation, MSD, Pharmacosmos, Pfizer, Sandoz, Takeda, and has received research funding from AbbVie, Janssen, MSD, Sandoz, Sanofi, Takeda; RC: none; UW is PI of clinical studies sponsored by GSK, Novartis and Pfizer. No conflict of interest regarding the presented clinical study.
Acknowledgements
We would like to thank the clinical study team Ines Zwazl, Andrea Wessely, Dooa Al-Mamoori, Lisa Dohr-Loufouma, Peter Pichler, Melita Poturica, Andrea Schagerl and Claudia Seidl-Friedrich for their efforts in conducting the study at the Institute of Specific Prophylaxis and Topical Medicine. Furthermore, we thank Petra Waidhofer-Söllner for her expertise in measuring cytokines at the Institute of Immunology and Maximillian Kutschera and Jurij Maurer at the Division of Gastroenterology and Hepatology, Department of Internal Medicine III, for clinical data collection. We sincerely appreciate the commitment of the serology team Tatjana Matschi, Vanessa Maurer, Barbara Schaar, Karin Schoiswohl, and Andrea Wendl at the Institute for Hygiene and Applied Immunology of the Medical University of Vienna to perform antibody measurements. We thank all participating patients.
Funding: The study was sponsored and financed by the Medical University of Vienna—third party funding by the Institute of Specific Prophylaxis and Tropical Medicine. VG and JBH were supported by the innovative Medicines initiative 2 Joint Undertaking under grant agreement No 945393 of the European Union’s Horizon 2020 research and innovation program and EFPIA. WFP, ANAS and BK received funding from the Danube Allergy Research Cluster (Danube ARC) supported by the State of Lower Austria.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2023.104788.
Contributor Information
Erika Garner-Spitzer, Email: erika.garner-spitzer@meduniwien.ac.at.
Ursula Wiedermann, Email: ursula.wiedermann@meduniwien.ac.at.
Appendix A. Supplementary data
References
- 1.Wagner A., Garner-Spitzer E., Schotta A.M., et al. SARS-CoV-2-mRNA booster vaccination reverses non-responsiveness and early antibody waning in immunocompromised patients - a phase four study comparing immune responses in patients with solid cancers, multiple myeloma and inflammatory bowel disease. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.889138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng S.C., Shi H.Y., Hamidi N., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 3.Alatab S., Sepanlou S.G., Ikuta K., et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. 2020;5(1):17–30. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang J.T. Pathophysiology of inflammatory bowel diseases. N Engl J Med. 2020;383(27):2652–2664. doi: 10.1056/NEJMra2002697. [DOI] [PubMed] [Google Scholar]
- 5.Neurath M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. 2019;20(8):970–979. doi: 10.1038/s41590-019-0415-0. [DOI] [PubMed] [Google Scholar]
- 6.Gareb B., Otten A.T., Frijlink H.W., Dijkstra G., Kosterink J.G.W. Review: local tumor necrosis factor-alpha inhibition in inflammatory bowel disease. Pharmaceutics. 2020;12(6):539. doi: 10.3390/pharmaceutics12060539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luzentales-Simpson M., Pang Y.C.F., Zhang A., Sousa J.A., Sly L.M. Vedolizumab: potential mechanisms of action for reducing pathological inflammation in inflammatory bowel diseases. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.612830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyler C.J., Guzman M., Lundborg L.R., et al. Antibody secreting cells are critically dependent on integrin alpha 4 beta 7/MAdCAM-1 for intestinal recruitment and control of the microbiota during chronic colitis. Mucosal Immunol. 2022;15(1):109–119. doi: 10.1038/s41385-021-00445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wellens J., Colombel J.F., Satsangi J.J., Wong S.Y. SARS-CoV-2 vaccination in IBD: past lessons, current evidence, and future challenges. J Crohns Colitis. 2021;15(8):1376–1386. doi: 10.1093/ecco-jcc/jjab046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty J., Fennessy S., Stack R., et al. Review Article: vaccination for patients with inflammatory bowel disease during the COVID-19 pandemic. Aliment Pharmacol Ther. 2021;54(9):1110–1123. doi: 10.1111/apt.16590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy N.A., Lin S.M., Goodhand J.R., et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut. 2021;70(10):1884–1893. doi: 10.1136/gutjnl-2021-324789. [DOI] [PubMed] [Google Scholar]
- 12.Alexander J.L., Kennedy N.A., Ibraheim H., et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatology. 2022;7(4):342–352. doi: 10.1016/S2468-1253(22)00005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy N.A., Janjua M., Chanchlani N., et al. Vaccine escape, increased breakthrough and reinfection in infliximab-treated patients with IBD during the Omicron wave of the SARS-CoV-2 pandemic. Gut. 2023;72(2):295–305. doi: 10.1136/gutjnl-2022-327570. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z., Le K., Zhou X., et al. Neutralising antibody potency against SARS-CoV-2 wild-type and omicron BA.1 and BA.4/5 variants in patients with inflammatory bowel disease treated with infliximab and vedolizumab after three doses of COVID-19 vaccine (CLARITY IBD): an analysis of a prospective multicentre cohort study. Lancet Gastroenterol Hepatology. 2023;8(2):145–156. doi: 10.1016/S2468-1253(22)00389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gattinger P., Tulaeva I., Borochova K., et al. Omicron: a SARS-CoV-2 variant of real concern. Allergy. 2022;77(5):1616–1620. doi: 10.1111/all.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gattinger P., Borochova K., Dorofeeva Y., et al. Antibodies in serum of convalescent patients following mild COVID-19 do not always prevent virus-receptor binding. Allergy. 2021;76(3):878–883. doi: 10.1111/all.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dan J.M., Mateus J., Kato Y., et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529) doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna R., Zou G., D'Haens G., et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn's disease activity. Aliment Pharmacol Ther. 2015;41(1):77–86. doi: 10.1111/apt.13001. [DOI] [PubMed] [Google Scholar]
- 19.Jairath V., Khanna R., Zou G.Y., et al. Development of interim patient-reported outcome measures for the assessment of ulcerative colitis disease activity in clinical trials. Aliment Pharmacol Ther. 2015;42(10):1200–1210. doi: 10.1111/apt.13408. [DOI] [PubMed] [Google Scholar]
- 20.Sanz I., Wei C.W., Jenks S.A., et al. Challenges and opportunities for consistent classification of human B cell and plasma cell populations. Front Immunol. 2019;10:2458. doi: 10.3389/fimmu.2019.02458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanzavecchia A., Sallusto F. Understanding the generation and function of memory T cell subsets. Curr Opin Immunol. 2005;17(3):326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Woodland D.L., Kohlmeier J.E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9(3):153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J., Liu W., Hu Y., Xie T., Qu X. Circulating CXCR3+ Tfh cells positively correlate with neutralizing antibodies responses during HCV infection. Eur J Immunol. 2019;49:1007. doi: 10.1038/s41598-019-46533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J., Wu Q., Liu Z., et al. Spike-specific circulating T follicular helper cell and cross-neutralizing antibody responses in COVID-19-convalescent individuals. Nat Microbiol. 2021;6(1):51–58. doi: 10.1038/s41564-020-00824-5. [DOI] [PubMed] [Google Scholar]
- 25.Baumjohann D., Fazilleau N. Antigen-dependent multistep differentiation of T-follicular helper cells and its role in SARS-CoV-2 infection and vaccination. Eur J Immunol. 2021;51(6):1325–1333. doi: 10.1002/eji.202049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueno H. Human circulating T follicular helper cell subsets in health and disease. J Clin Immunol. 2016;36(Suppl 1):34–39. doi: 10.1007/s10875-016-0268-3. [DOI] [PubMed] [Google Scholar]
- 27.Bentebibel S.E., Jacquemin C., Schmitt N., Ueno H. Analysis of human blood memory T follicular helper subsets. Methods Mol Biol. 2015;1291:187–197. doi: 10.1007/978-1-4939-2498-1_16. [DOI] [PubMed] [Google Scholar]
- 28.Li Z., Vermeire S., Bullens D., et al. Anti-tumor necrosis factor therapy restores peripheral blood B-cell subsets and CD40 expression in inflammatory bowel diseases. Inflamm Bowel Dis. 2015;21(12):2787–2796. doi: 10.1097/MIB.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 29.Pasparakis M., Alexopoulou L., Episkopou V., Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184(4):1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Figgett W.A., Vincent F.B., Saulep-Easton D., Mackay F. Roles of ligands from the TNF superfamily in B cell development, function, and regulation. Semin Immunol. 2014;26(3):191–202. doi: 10.1016/j.smim.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Huber J.E., Ahlfeld J., Scheck M.K., et al. Dynamic changes in circulating T follicular helper cell composition predict neutralising antibody responses after yellow fever vaccination. Clin Transl Immunol. 2020;9(5) doi: 10.1002/cti2.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koutsakos M., Wheatley A.K., Loh L., et al. Circulating T-FH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci Transl Med. 2018;10(428) doi: 10.1126/scitranslmed.aan8405. [DOI] [PubMed] [Google Scholar]
- 33.Pallikkuth S., de Armas L.R., Rinaldi S., et al. Dysfunctional peripheral T follicular helper cells dominate in people with impaired influenza vaccine responses: results from the FLORAH study. PLoS Biol. 2019;17(5) doi: 10.1371/journal.pbio.3000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin S., Kennedy N.A., Saifuddin A., et al. Antibody decay, T cell immunity and breakthrough infections following two SARS-CoV-2 vaccine doses in inflammatory bowel disease patients treated with infliximab and vedolizumab. Nat Commun. 2022;13(1):1379. doi: 10.1038/s41467-022-28517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cerna K., Duricova D., Hindos M., et al. Cellular and humoral immune responses to SARS-CoV-2 vaccination in inflammatory bowel disease patients. J Crohns Colitis. 2022;16(9):1347–1353. doi: 10.1093/ecco-jcc/jjac048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li D., Xu A., Mengesha E., et al. The T-cell response to SARS-CoV-2 vaccination in inflammatory bowel disease is augmented with anti-TNF therapy. Inflamm Bowel Dis. 2022;28(7):1130–1133. doi: 10.1093/ibd/izac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lev-Tzion R., Focht G., Lujan R., et al. COVID-19 vaccine is effective in inflammatory bowel disease patients and is not associated with disease exacerbation. Clin Gastroenterol Hepatol. 2022;20(6):e1263–e1282. doi: 10.1016/j.cgh.2021.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin S., Lau L.H., Chanchlani N., Kennedy N.A., Ng S.C. Recent advances in clinical practice: management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2022;71(7):1426–1439. doi: 10.1136/gutjnl-2021-326784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto M., Fu Y.X., Molina H., Chaplin D.D. Lymphotoxin-alpha-deficient and TNF receptor-I-deficient mice define developmental and functional characteristics of germinal centers. Immunol Rev. 1997;156:137–144. doi: 10.1111/j.1600-065x.1997.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 40.Anolik J.H., Ravikumar R., Barnard J., et al. Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J Immunol. 2008;180(2):688–692. doi: 10.4049/jimmunol.180.2.688. [DOI] [PubMed] [Google Scholar]
- 41.Popescu M., Cabrera-Martinez B., Winslow G.M. TNF-Alpha contributes to lymphoid tissue disorganization and germinal center B cell suppression during intracellular bacterial infection. J Immunol. 2019;203(9):2415–2424. doi: 10.4049/jimmunol.1900484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryg-Cornejo V., Ioannidis L.J., Ly A., et al. Severe malaria infections impair germinal center responses by inhibiting T follicular helper cell differentiation. Cell Rep. 2016;14(1):68–81. doi: 10.1016/j.celrep.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Kaneko N., Kuo H.H., Boucau J., et al. Loss of bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell. 2020;183(1):143–+. doi: 10.1016/j.cell.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roltgen K., Nielsen S.C.A., Silva O., et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell. 2022;185(6):1025–+. doi: 10.1016/j.cell.2022.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atreya R., Neurath M.F., Siegmund B. Personalizing treatment in IBD: hype or reality in 2020? Can we predict response to anti-TNF? Front Med. 2020;7:517. doi: 10.3389/fmed.2020.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vande Casteele N., Gils A. Pharmacokinetics of anti-TNF monoclonal antibodies in inflammatory bowel disease: adding value to current practice. J Clin Pharmacol. 2015;55(3):S39–S50. doi: 10.1002/jcph.374. [DOI] [PubMed] [Google Scholar]
- 47.Brailey P.M., Lebrusant-Fernandez M., Barral P. NKT cells and the regulation of intestinal immunity: a two-way street. FEBS J. 2020;287(9):1686–1699. doi: 10.1111/febs.15238. [DOI] [PubMed] [Google Scholar]
- 48.Pape K.A., Dileepan T., Matchett W.E., et al. Boosting corrects a memory B cell defect in SARS-CoV-2 mRNA-vaccinated with bowel disease. JCI Insight. 2022;7(12) doi: 10.1172/jci.insight.159618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boland B.S., Goodwin B., Zhang Z.L., et al. Preserved SARS-CoV-2 vaccine cell-mediated immunogenicity in patients with inflammatory bowel disease on immune-modulating therapies. Clin Transl Gastroen. 2022;13(4):e00484. doi: 10.14309/ctg.0000000000000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garner-Spitzer E., Poellabauer E.M., Wagner A., et al. Obesity and sex affect the immune responses to tick-borne encephalitis booster vaccination. Front Immunol. 2020;11:860. doi: 10.3389/fimmu.2020.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner A., Garner-Spitzer E., Jasinska J., et al. Age-related differences in humoral and cellular immune responses after primary immunisation: indications for stratified vaccination schedules. Sci Rep. 2018;8(1):9825. doi: 10.1038/s41598-018-28111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.