Abstract
Background and Objective
Robotic approach is used widely for paediatric upper tract urinary reconstruction. This is a narrative review looking at the current status of robotic approach in lower urinary tract reconstruction. The aim of this article is to highlight the important technical aspects of commonly performed robotic lower urinary tract reconstructive surgeries and review the current literature.
Methods
MEDLINE database search was conducted using MeSH terms and Boolean operators from Jan 2000 to Jun 2022. Abstracts were screened to exclude those in languages other than English as also articles pertaining to (I) upper urinary tract surgery, (II) only laparoscopic surgery (not robot-assisted) and (III) non-urological topics. Selected articles were then reviewed and search expanded to include their references with a focus on advanced lower urinary tract reconstruction.
Key Content and Findings
The technical aspects of robotic ureteric reimplantation, continent catheterisable channel and autoaugmentation are discussed in detail. The early outcomes are comparable to open surgery. The true advantage of robotic approach becomes apparent when performing lower urinary tract reconstruction, where space in the pelvis is limited and access is challenging. Only a few centres are currently performing bladder neck surgery and bladder augmentation.
Conclusions
Robotic lower urinary tract reconstruction in children is feasible and safe. Robotic approach offers better access, especially in the limited space within the pelvis. It reduces blood loss and post-operative pain allowing early recovery and discharge. Long-term follow-up with increasing experience could further validate these early observations.
Keywords: Robotic surgery, lower urinary tract reconstruction, paediatric urology
Introduction
Background
Robotic approach to procedures in pediatric urology has lagged behind adult urology akin to take up of laparoscopy in the 90’s (1-3). However, in recent years it has made significant progress, reflected in increased publications from an average of 12 per year before 2011 to 42 per year after 2012.
Rationale and knowledge gap
Robotic approach is mainly used for extirpative surgery in adult urology practice, whilst in children it is mostly beneficial in reconstructive procedures (4). The advantages of robotic surgery common to both adults and children include focussed approach to the target organ or area, thus minimizing operative trauma, decreasing postoperative pain, limiting the need for postoperative opioid use and reducing hospital stay (5,6). Robots were designed primarily for use in adults and therefore pediatric surgeons need to be innovative in its use, given significantly smaller patient size. The distance between the ports, as recommended by the manufacturer (8 cm apart), is also difficult to achieve in small children or neonates. Cost is another important factor particularly limiting use of robot in children. This is mainly due to lower budgets and smaller volume of patients appropriate for robotic procedures. In fact, many pediatric centres are unable to absorb the initial costs of a robotic surgery program (7). Most pediatric centres around the world share robots with adult services and this causes potential difficulties in scheduling cases pending availability of the robot and clashing commitments. Maintaining the Robotic program with ongoing training of surgeons and theatre staff is another challenge.
To overcome these difficulties, new research and development of pediatric-sized instruments would be useful. Specialised robotic centres could be created to centralise the treatment of conditions that benefit from this technology, thus maintaining a steady volume of patients.
Objective
In this narrative review we aim to provide an overview of the commonly performed lower urinary tract reconstructive procedures in children using the robot-assisted technology with an up-to-date review of the literature. We present this article in accordance with the Narrative Review reporting checklist (available at https://tp.amegroups.com/article/view/10.21037/tp-22-533/rc).
Methods
Literature search
English articles in MEDLINE database from January 2000 to June 2022 were identified using the commonly used MeSH terms and Boolean operators for (I) robotic or robotic-assisted surgical procedures, (II) lower urinary tract reconstruction and (III) in pediatric age group. Initial search was kept wide to include all relevant articles from January 2000 to June 2022. Seventy-eight articles were found. Abstracts were screened to exclude articles pertaining to (I) upper urinary tract surgery, (II) exclusive laparoscopic surgery (not robot-assisted) and (III) non-urological topics. Selected articles were then reviewed in detail along with a focus on advanced lower urinary tract reconstruction. Additional articles from the reference lists of selected articles were further included after full review, if found relevant (Table 1).
Table 1. The search strategy summary.
| Items | Specification |
|---|---|
| Date of Search | June 28, 22 |
| Databases and other sources searched | MEDLINE |
| Search terms used | Search: “Robotic Surgical Procedures”[Mesh] OR robotic*[tw] OR “robot-assisted”[tw] Filters: from 2000/1/1–3000/12/12 |
| (“Robotic Surgical Procedures”[MeSH Terms] OR “robotic*”[Text Word] OR “robot-assisted”[Text Word]) AND (2000/1/1:3000/12/12[pdat]) | |
| And | |
| Search: “Reconstructive Surgical Procedures”[Mesh] OR “lower urinary tract surgery”[tw] OR detrusorotomy[tw] OR “ureter* reimplant*”[tw] OR “ureter* re-implant*”[tw] OR “catheterisable channel”[tw] OR “Mitrofanoff”[tw] OR “appendico-vesicostomy”[tw] OR “appendicovesicostomy"[tw] OR “Monti”[tw] OR “Monti-Mitrofanoff”[tw] OR “bladder neck repair”[tw] OR “bladder neck reconstruction”[tw] OR “bladder augmentation”[tw] OR “ileocystoplasty”[tw] Filters: from 2000/1/1–3000/12/12 | |
| ("Reconstructive Surgical Procedures”[MeSH Terms] OR “lower urinary tract surgery”[Text Word] OR “detrusorotomy”[Text Word] OR “ureter reimplant*”[Text Word] OR “ureter re implant*”[Text Word] OR “catheterisable channel”[Text Word] OR “Mitrofanoff”[Text Word] OR “appendico-vesicostomy”[Text Word] OR “appendicovesicostomy”[Text Word] OR “Monti”[Text Word] OR “Monti-Mitrofanoff”[Text Word] OR “bladder neck repair”[Text Word] OR “bladder neck reconstruction”[Text Word] OR “bladder augmentation”[Text Word] OR “ileocystoplasty”[Text Word]) AND (2000/1/1:3000/12/12[pdat]) | |
| And | |
| Search: “Child”[Mesh] OR Child*[tw] OR Children*[tw] OR Adolescent*[tw] OR Paediatric*[tw] OR Pediatric*[tw] Filters: from 2000/1/1–3000/12/12 | |
| (“Child”[MeSH Terms] OR “child*”[Text Word] OR “children*”[Text Word] OR “adolescent*”[Text Word] OR “paediatric*”[Text Word] OR “pediatric*”[Text Word]) AND (2000/1/1:3000/12/12[pdat]) | |
| Timeframe | January 2000–June 2022 |
| Inclusion criteria | Initial search was kept wide to include all relevant articles in from January 2000 to date. 78 articles were found. Abstracts were screened to exclude those in languages other than English as also articles pertaining to (I) upper urinary tract surgery, (II) only laparoscopic surgeries and (III) non-urological topics. Rest of the articles were reviewed with a focus on advanced lower urinary tract reconstruction. Additionally relevant articles from Reference lists of above articles were included in the review |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Initial search and further selection were conducted jointly by the three authors in consensus |
| Any additional considerations, if applicable | 48 articles were included (28 original articles, 14 review articles, 6 case reports) |
The range of procedures in lower urinary tract reconstruction varies from ureteric reimplantation with or without tapering (8,9), to bladder neck procedures (5), autoaugmentation and augmentation cystoplasty (6,10). Few cases of robotic excision of utricle cyst have also been reported (11,12).
For the purpose of this review, we will focus on the following:
Ureteric reimplantation;
Continent catheterisable channel (CC);
Autoaugmentation (detrusorotomy);
Bladder neck reconstruction (BNR);
Ileocystoplasty.
Summarised below are some technical aspects regarding the above-mentioned procedures combined with experience at our institution.
General principles of port placement and docking in the pediatric population
Port placement varies, with some authors recommending supraumbilical camera port for a wider view either with docking of the robot at the side (patient supine) or in between legs (patient in lithotomy) (2). The most recent da Vinci Xi and X system have a slim body and narrow arms that enable easy docking and reduced risk of collision of arms or instruments during surgery (14-17). These characteristics have enabled the authors to achieve a degree of uniformity in terms of:
❖ Patient positioning—supine;
❖ Docking—side docking;
❖ Camera—30°.
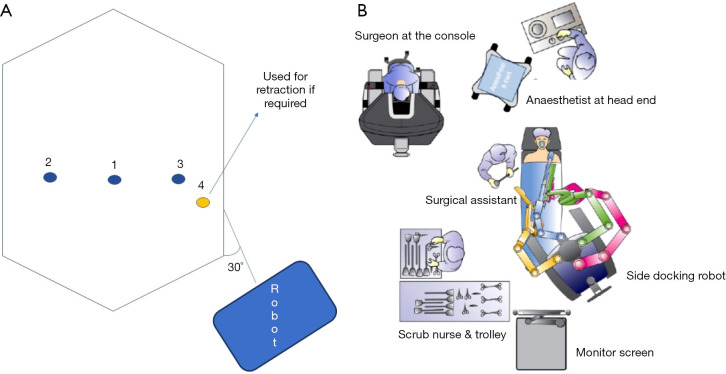
Above mentioned features are also favoured by the anaesthetic and theatre teams (Figure 1A) as they offer additional advantages in terms of position of the patient, length of operation, etc. Supine position and side docking enables better patient access and thus patient safety during the procedure. The X and Xi robots are generally placed on the left side of the patient, creating a 30° angle with the operating table because all arms are in the same plane (Figure 1B). When using the Da Vinci Si however, position of the robot varies according to the type of operation and the port placement required.
Figure 1.
Ports and theatre layout. (A) Positioning of patient, robot, console & teams in theatre room. (B) Ports and robot positioning: 1, camera port; 2 and 3, working ports; 4, accessory port.
The 30° camera provides a ‘helicopter’ (panoramic) view as compared to end-on-view with 0° camera lens.
Ports placement
We use trans-umbilical camera port and two lateral working ports in the same line on either side, minimum 4 cm apart, as the 8-cm gap generally suggested for the adult population in order to avoid arm collision is often not applicable in small patients (18). In the author’s experience, this is standard for most pediatric procedures with an additional fourth port placed in the left iliac fossa for retraction when needed (Figure 1). This is particularly useful when creating a continent CC or during other demanding procedures and it facilitates retraction of bowel or other structures away from the area of interest.
Robotic instruments—the authors use 8-mm camera and working ports.
Ureteric reimplantation
Indication for ureteric reimplantation include vesico-ureteric reflux (VUR) not suitable for endoscopic treatment or for vesico-ureteric junction (VUJ) obstruction as per clinical context. Detailed description of indications is beyond the scope of this article and readers are referred to text books (19,20).
The robotic approach lends itself well to extravesical Lich-Gregoir technique which is preferred by most surgeons (8,9,21,22). A urethral catheter is inserted at the outset, clamped but accessible in the operative field. This allows bladder to fill naturally during the procedure or to be filled as needed. It is ideal to have the bladder partially full especially when it comes to detrusorotomy. The ureter is identified at the pelvic brim. In males, the peritoneal window is created just cranial to the vas. In females, the area of dissection is between the uterus and the bladder, and a window is created cranial to the round ligament. The distal ureter is identified and isolated. Further dissection is performed to expose the VUJ. The ease with which we can define the VUJ is due to the high definition provided by robotic approach unlike open or three-dimensional (3D) laparoscopy (Figure 2). The dissection is kept close to the ureter and stay well out of the pelvic wall to avoid injury to the neurovascular bundle. It is fundamental to preserve the vascularity by maintaining the integrity of the adventitial layer. A transabdominal stay stitch at an appropriate site is used to elevate the bladder and improve the exposure of the posterior bladder wall (Figure 2). Detrusorotomy is performed cranial to the VUJ, in alignment with the orientation of the ureter. Length of detrusorotomy is dependent on the diameter of the ureter, so as to achieve a ratio of 3–5:1. Detrusorotomy must be wide enough so as to allow adequate detrusor flap elevation from the mucosa and to accommodate the ureters. Adventitia of the ureter is anchored to the apex of the detrusorotomy with 5/0 polydioxanone suture (PDS). This helps to align the ureter within the tunnel. Distal ureter is then loosely wrapped within the detrusorotomy with interrupted 5/0 PDS. Peritoneum is closed and the catheter is left on free drainage for 24 hours. The patient is discharged the following day.
Figure 2.
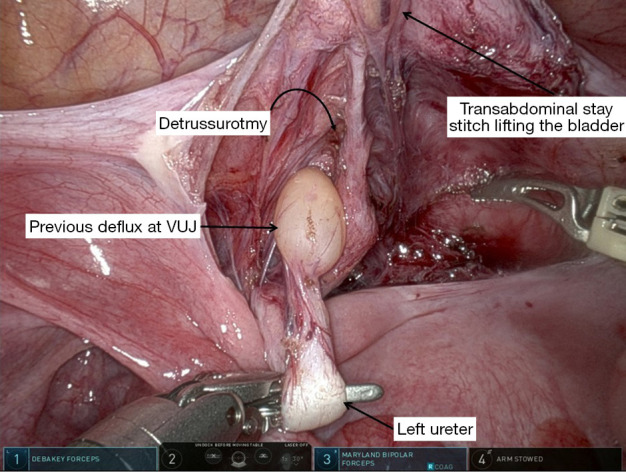
VUJ dissection and transabdominal stay stitch to improve exposure, in a patient previously treated with endoscopic correction. VUJ, vesico-ureteric junction.
In case of complete duplex system, good understanding of the anatomy of distal ureters and common sheath is essential. Occasionally, it may be necessary to dismember the ureter at the VUJ, especially when dealing with an obstructive pathology and/or when tapering is indicated.
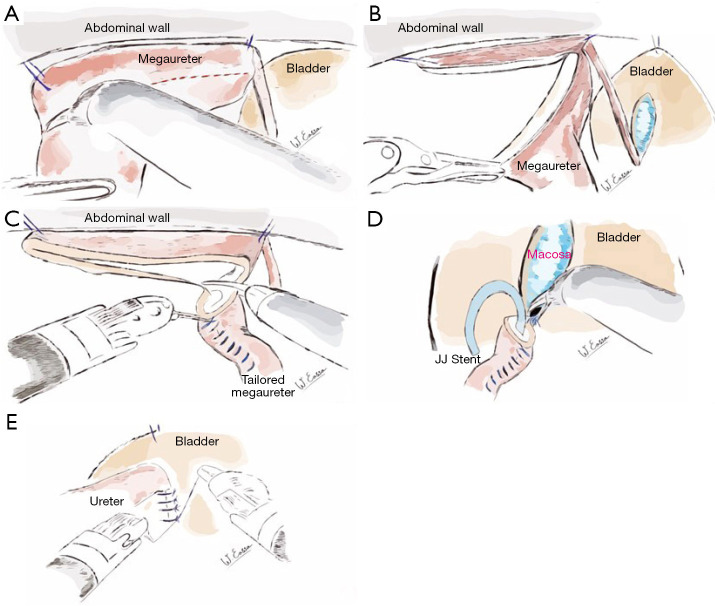
Tapering technique
The technique of excisional ureteric tapering as practiced by the senior author is as illustrated with the help of diagrams in Figure 3. Once identified the VUJ, proximal dissection of the megaureter is performed to allow distal excisional tapering. The distal ureteric segment is hitched to the anterior abdominal wall using two stay sutures (Figure 3A). The line of incision is demarcated, excised and the trimmed portion is tailored with an inverting running 5/0 PDS suture (Figure 3B,3C). The obstructed VUJ is divided and the neo-ureterocystostomy is carried out with 5/PDS (Figure 3D). The distal ureter is wrapped in the detrusorotomy as previously described. A double J stent left in situ for 6–8 weeks.
Figure 3.
Tapering technique. (A) Ureteric segment for trimming is identified, hitched to the abdominal wall and the incision line is marked. (B) Megaureter trimmed. Trim line to face bladder mucosa when laid within the detrusorotomy. (C) Tailoring performed with inverting PDS 5/0 sutures. (D) Double J stent placed percutaneously. Fashioning of the neo-ureterovesicostomy with PDS 5/0. (E) Detrusor wrap closed using PDS 5/0. PDS, polydioxanone suture.
Data from author’s unpublished personal series including patients that underwent robotic ureteric tapering for refluxing (3) or obstructive (4) megaureter, showed good outcomes at a mean follow-up of 23 months (range, 6–42 months). All patients had improvement of the hydroureteronephrosis, improved renal function or drainage at mercaptoacetyltriglycine 3 (MAG3) scan and none needed further operations. Mean console time was 113 min (range, 93–148 min), blood loss was negligible, and all patients went home the following day of the operation.
Creation of continent CC (Mitrofanoff)
Bladder is catheterised. The exit site for the channel [where the V-quadrilateral (VQ) flap is to be performed] is identified preoperatively. A “V” skin incision is made at this site in the right iliac fossa. A 10-mm laparoscopic accessory step port is inserted and later used to retrieve the appendix. The appendix is mobilized on its vascular pedicle and divided at the base. A ligature and endoloop is used to secure the caecal stump. The appendix is retrieved through the step port and anchored with a 4/0 prolene stay suture on the outside. The tip of the appendix is opened, and an appropriately sized Foley catheter (typically a 12-French) is passed. Detrusorotomy is performed in the posterior wall of the bladder. Bladder mucosa is opened at the most dependent end of the detrusorotomy. Foley catheter in the appendiceal lumen is advanced into the bladder and the balloon is inflated. The appendico-vesical anastomosis is performed using 5/0 PDS suture. The detrusor is wrapped using interrupted 4/0 PDS. Some of these stitches incorporate the appendix to maintain the tunnel length within the detrusor. The length of detrusorotomy should be a minimum of 3–5:1 ratio in comparison to the width of the appendix in order to guarantee continence (19). Galansky et al. suggested to create at least a 4-cm tunnel, regardless the size of the ureter, in order to achieve continence (23). The peritoneum is then repositioned ensuring that the CC is extraperitoneal. The readers can find the technical details of this procedure published by the senior author (24). VQ-plasty is performed to fashion a skin lined exit site for the CC (25). The CC is accessible for Clean Intermittent Catheterisation (CIC) typically after 6 weeks, once it is mature.
Autoaugmentation
In cases with refractory detrusor overactivity, therapeutic wide detrusorotomy (allowing the bladder mucosa to expand) can be safely used as an extension of medical treatment. It has the potential to delay, and in well selected cases, even avoid the need for enterocystoplasty and the related sequelae. Even if only a small group of patients with refractory detrusor overactivity can be managed in the long term without enterocystoplasty, that is still a success (6). The yield is maximum when detrusorotomy is performed prior to end-stage bladder failure (18). For details of operative steps readers are directed to the intraoperative illustration in the author’s article in the World Journal of Urology (9). Our experience suggests that robotic approach provides high magnification and allows meticulous dissection, enabling division of individual detrusor fibres. It is emphasised that bladder cycling in the post-operative period is crucial for successful outcome. Thus, in addition to comparable outcomes to open detrusorotomy, robotic approach has the advantage of reduced operative time and hospital stay (6).
Bladder neck procedures
A variety of bladder neck procedures have been reported. BNR, bladder neck sling (BNS), artificial urethral sphincter (AUS) and rarely bladder neck closure (BNC). Only few centres are currently performing robotic bladder neck procedures in children. Gargollo et al. have reported the largest series to date of 38 cases of robotic bladder neck procedures (26).
Ileocystoplasty
Ileocystoplasty is technically a very demanding procedure to be performed using robotic approach. As a result, the number of centres reporting experience of ileocystoplasty using robotic approach is sparse. Gundeti et al. from Chicago is one of the few centres performing this procedure robotically (10,27-29). In the description of their operative technique the patient is positioned semi-lithotomy with 10° Trendelenburg tilt. An 8-mm camera port is placed in supraumbilical position, two 8-mm working ports laterally at the level of the umbilicus in the mid-clavicular line, and a 5-mm assistant port in the left upper quadrant in the midclavicular line. Additional port is described in the right iliac fossa at the exit site for extra-vesical appendico-vesicostomy (AV). With this port placement, Gundeti et al. have managed to reproduce the key steps of open ileocystoplasty using robot-assisted laparoscopic approach with total intracorporeal suturing for further details of the operative steps we refer the readers to the published experience (10,27-29).
Literature review
Ureteric reimplantation
The first series of robot-assisted extravesical ureteral reimplantation performed in 27 units was published by Peters in 2004 and reported a success rate of 88% at a mean follow-up of 7 months (30). Subsequent studies confirmed a success rate varying from 77% to 98% (21,22,31-33). The most recent and large series (55 patients) confirmed a success rate of 96% after an average of 28 months (8). Rodriguez et al. reported 97% success in 16 common sheath reimplantation in complete duplex systems with a mean follow-up of 17 months (9). In a study reported by Rappaport et al. (34), 97% success rate was reported in 48 extravesical cross-trigonal detrusorotomy and reimplantation for obstructive megaureters with a follow-up of 8 months.
Limitation of these studies include the retrospective design. In studies from Esposito et al. (8) and Rappaport et al. (34) the multicenter nature and potential difference in management protocols make the comparison debatable, e.g., in the Rappaport study, post-operative voiding cystourethrogram (VCUG) was not performed routinely in all cases.
Creation of continent CC
The first robotic-assisted laparoscopic Mitrofanoff AV was performed in 2004 in a patient with posterior urethral valves and favourable outcomes (no complications and continence) were reported (35).
A multicentre study published in 2016 by Gundeti et al. reported the perioperative and functional outcomes of robotic assisted AV performed in a large cohort of 88 patients treated in 5 different centres, with a median length of follow-up was 29.5 months. They had a 29.5% of early perioperative (<90 days) complications, including ileus, urinary tract infection and surgical site infection, and 6.8% of patients had a Clavien Dindo grade >3, requiring suprapubic catheter insertion, nephrostomy, reoperation for bowel obstruction. Continence was achieved in 85.2%, the others requiring bulking agent or surgical revision. The performance of concomitant procedures did not impact the complication rate nor the continence rate (36).
This data is consistent with other single centre series which reported a perioperative complication rate (grade 1–3) between 38% and 26% (23,37,38) to a minimal of 5.5% (Clavien Dindo 2) in the only published series considering CC formation alone, performed in 18 patients with a follow-up of 27 months (24). Continence rate from these single centre series is reported between 85% and 100% at a follow-up between 2 years (37,38) and 6 years (23).
It is difficult to compare published series due to concomitant reconstructive procedures performed along with CC formation. The main advantages reported by the senior author in previous publication are in terms of reduced operative time (less than 200 min); reduced hospital stay (2.75 days compare to 5.8 days in open procedures); reduced need for post-operative analgesia such as eliminating the need for epidural; and early feeding (same day of the operation). This all helped in reducing the costs of the procedure and hospitalisation, which is fundamental when need to demonstrate the feasibility of a robotic program (24).
Comparison between open and robotic approach by Grimsby et al. revealed no significant differences in terms of rate of acute complications or reoperations. A total of 28 open and 39 robotic AV were included. At a mean follow-up of 2.7 years there was no difference in number of complications or reoperations between groups. Time to first reoperation was shorter in the robotic group, but there was no significant difference of reoperation rate within the first 12 months postoperatively (38).
Gundeti et al. reported the results of a series of 18 patients that underwent either a posterior-wall intravesical anastomosis of the appendix (when CC creation was associated with enterocystoplasty) or an anterior-bladder wall anastomosis after cystotomy. The continence rate was 100% in the extravesical group (8 patients) and 90% in the intravesical one (10) with a median follow-up of 24 months. Postoperative complications were higher, as expected, in the intravesical cohort, given the concomitant augmentation cystoplasty performed. The author concluded that, in their hands, when performing an isolated robotic AV, anterior wall extravesical reimplantation is technically more feasible and minimizes morbidity. However, limitation of the study are the small sample size and the retrospective design (37).
Galvez et al. have reported a case of robotic Monti-Yang ileovesicostomy performed in a complexed female patient with several previous abdominal surgeries. After 3 months, the girl could perform CIC regularly and the conduit was continent (39). Despite the follow-up is very short to be able to comment on the long-term post-operative outcomes, this case shows how the use of robotic surgery can be successfully implemented in very complex patient and for complex urological procedure.
Autoaugmentation
The authors have revisited detrusorotomy as a viable option in a published a series of 10 cases (6 open, 4 robotic). Detrusorotomy was performed in the coronal plane except in one case that required a sagittal detrusorotomy due to previous Mitrofanoff channel. The median age at operation in both groups was 10 years and follow-up was 14 months in the robotic arm and 54 months in open detrusorotomy. The median operative time in the robotic group was 125 min (range, 108–152 min) as compared to 208 min (range, 186–306 min) in the open group. Median hospital stay was 2.7 days (range, 2–3 days) in the robotic group and 5.6 days (range, 4–7 days) in the open group. The increase in bladder capacity was comparable +140% (range, 90–200%) in the open group and +126% (range, 80–200%) in the robotic group. There were no intra or post-operative complications in either group (6). The limitation of this study is the small sample size and short follow-up, especially in the robotic group.
Bladder neck procedures
Gargollo et al. reported the largest series to date, with 38 cases of bladder neck procedures. Ninety percent of the patients had neuropathic bladders secondary to spina bifida. Leadbetter-Mitchell BNR was performed in combination with processed fascia lata sling in all cases. Concomitant AV was performed. Mean patient age at the time of surgery was 10 years (range, 5–16 years). Mean operative time was 5.8 hours (range, 3.6–12.25 hours). The operative time reduced significantly after the first 10 cases. Four cases required conversion to open. Mean hospital stay was 52 hours (range, 34–86 hours). At mean follow-up of 21 months (range, 5–33 months), 31 (82%) patients were completely dry during the day with regular CIC (26). The continence rates are comparable with published open series in the literature (40). Robotic approach has added advantages of better cosmesis, early recovery and reduced intra-operative blood loss, post-operative pain and post operative adhesions (5,41). One can argue that the overall continence rates from BNR are skewed because of concomitant AV. Prolonged operative time and steep learning curve are major limitations.
Ileocystoplasty
The Chicago group reported the first robotic ileocystoplasty with complete intracorporeal suturing in 2008 (10). In 2020, they published a series of 24 cases, 20 of which were completed robotically. Concomitant procedures included AV (80%), antegrade continence enema (ACE) (40%) and bladder neck procedures (30%). The median follow-up was 83.1 months. Early and late complication rate was comparable with open procedures. The mean operative time was 573 min (first case 623 min, last case 320 min) (27,42). The overall operative time, although steadily improving, remains significantly higher than open approach. This may explain why other pediatric robotic centres are still sceptical about undertaking this procedure, especially in their early phase of development.
Summary
The true advantage of robotic approach becomes apparent when performing lower urinary tract reconstruction, where space in the pelvis is limited and access is challenging (5,24,43-45). This is because robotic technology, as compared to laparoscopy, has the advantage of enhanced vision, dexterity and wider range of movements of the instruments (18). It is equipped with the fourth arm for retraction, three-dimensional (3D) visualization, 7-degree range of motion and tremor elimination. It offers a relatively shorter learning curve and improved surgeon ergonomics, but longer operative time continues to be one of the limiting factors (45,46).
Variations in the surgical techniques are apparent as surgeons try to adapt experience and instruments from adult to the pediatric population. Table 2 summarises the key advantages of robotic surgery during lower urinary tract reconstructive procedures.
Table 2. Advantages of robotic surgery.
| Advantages aiding surgical technique |
| Higher magnification (18,45) |
| Wider range of movements and dexterity (18,44,45) |
| Better stability and control of camera & working instruments resulting in greater precision (44,45) |
| Reduced collateral damage (e.g., neurovascular bundles) (47) |
| Better ergonomics (45) |
| Advantages aiding patient recovery |
| Reduced post-operative pain (5) |
| Reduced blood loss (5) |
| Reduced length of hospital stay (5) |
| Better cosmesis (48,49) |
| Comparable outcomes to conventional open surgery (38,42,50) |
Enhanced vision, stability, manoeuvrability and precision during robotic surgery implies reduced blood loss and collateral damage to important neurovascular structures especially when working within the constraints of the pelvis (18,45). Improved cosmesis, reduced post-operative pain and early discharge from the hospital are welcome adjuncts from a patient perspective (5,48,49).
Table 3 summarises the practical key points in authors experience to help during robotic-assisted lower urinary tract reconstructive procedures.
Table 3. Practical key points.
| Technical points | Rationale |
|---|---|
| Port size—use of 8-mm camera/working ports | Permits all the advantages of robotic instruments without compromising magnification or range of movements, unlike the smaller 5-mm ports |
| Linear port placement | Reduces clashing of instruments |
| Allows easy swap between working and retracting instruments | |
| Additional 4th port | Helps operator-controlled retraction |
| Side docking of the robot | Allows uniformity. Easy for theatre staff to reproduce. Preferred by anaesthetist as provides easy access to airway throughout the procedure |
| Bladder catheterisation and Transabdominal traction suture | Allows intraoperative optimum bladder filling and retraction with good access to deeper areas in the pelvis |
With increasing experience, robotic approach has been used successfully for complex lower urinary tract reconstruction even with previous history of multiple open procedures (43).
Literature review as presented above suggests, robotic surgery in pediatric urology has progressed, especially in the last decade. Limited pediatric centres across the globe are currently performing advanced lower urinary tract reconstruction. Small sample size, short follow-up, retrospective design, prolonged operative times and steep learning curves are the salient limitations (8,10,33,36,38,40,42). From a clinical perspective although initial outcomes are safe and promising further long-term outcomes and comparative data is awaited. From a technical perspective small size ports and instruments without compromising the magnification and manoeuvrability are needed. Senior author recommends use of fourth arm for additional retraction and accessing difficult areas. Routine use of side docking helps facilitate uniformity and is preferred by anaesthetist due to easy access to airway and lines intraoperatively. With development of new robotic platforms one can expect healthy competition and technological advancement better suited for pediatric application (46). From a financial perspective, measures to reduce the cost include centralising cases to maintain adequate workload, sharing of robots between specialties and a robust robotic training program for surgeons and the theatre staff.
Conclusions
Robotic lower urinary tract reconstruction in children is feasible and safe. Robotic approach offers better access especially in the limited space within the pelvis. It reduces blood loss and post-operative pain allowing early recovery and discharge. Long-term follow-up with increasing experience could further validate these early observations.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ciro Esposito) for the series “Pediatric Robotic Surgery” published in Translational Pediatrics. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tp.amegroups.com/article/view/10.21037/tp-22-533/rc
Peer Review File: Available at https://tp.amegroups.com/article/view/10.21037/tp-22-533/prf
Conflicts of interest: All authors have completed the ICMJE uniform disclosure form (available at https://tp.amegroups.com/article/view/10.21037/tp-22-533/coif). The series “Pediatric Robotic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.Losty PD. Recent advances: paediatric surgery. BMJ 1999;318:1668-72. 10.1136/bmj.318.7199.1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tam PK. Laparoscopic surgery in children. Arch Dis Child 2000;82:240-3. 10.1136/adc.82.3.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport M. Laparoscopic surgery in children. Ann R Coll Surg Engl 2003;85:324-30. 10.1308/003588403769162440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramaniam R. Current Use of and Indications for Robot-assisted Surgery in Paediatric Urology. Eur Urol Focus 2018;4:662-4. 10.1016/j.euf.2018.08.020 [DOI] [PubMed] [Google Scholar]
- 5.Gargollo PC, White LA. Robotic-assisted bladder neck procedures in children with neurogenic bladder. World J Urol 2020;38:1855-64. 10.1007/s00345-019-02912-6 [DOI] [PubMed] [Google Scholar]
- 6.Subramaniam R. Experience with detrusorotomy in children by open and robotic approach. World J Urol 2020;38:1869-74. 10.1007/s00345-019-02777-9 [DOI] [PubMed] [Google Scholar]
- 7.Denning NL, Kallis MP, Prince JM. Pediatric Robotic Surgery. Surg Clin North Am 2020;100:431-43. 10.1016/j.suc.2019.12.004 [DOI] [PubMed] [Google Scholar]
- 8.Esposito C, Masieri L, Steyaert H, et al. Robot-assisted extravesical ureteral reimplantation (revur) for unilateral vesico-ureteral reflux in children: results of a multicentric international survey. World J Urol 2018;36:481-8. 10.1007/s00345-017-2155-9 [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez MV, Boysen WR, Gundeti MS. Robot-assisted laparoscopic common sheath ureteral reimplantation in duplex ureters: LUAA technique tips for optimal outcomes. J Pediatr Urol 2018;14:353-5. 10.1016/j.jpurol.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 10.Gundeti MS, Eng MK, Reynolds WS, et al. Pediatric robotic-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff appendicovesicostomy: complete intracorporeal--initial case report. Urology 2008;72:1144-7; discussion 1147. 10.1016/j.urology.2008.06.070 [DOI] [PubMed] [Google Scholar]
- 11.Macedo A, Jr, Del Debbio Di Migueli R, Ottoni SL, et al. Robotic-assisted excision of a prostatic utricle cyst in a 12-month boy with proximal hypospadia and 45X0/ 46XY karyotype. J Pediatr Urol 2020;16:725-6. 10.1016/j.jpurol.2020.08.020 [DOI] [PubMed] [Google Scholar]
- 12.Nguyen A, Arora H, Reese J, et al. Robot-assisted laparoscopic excision of prostatic utricle in a 3-year old. J Pediatr Urol 2018;14:343-4. 10.1016/j.jpurol.2018.07.015 [DOI] [PubMed] [Google Scholar]
- 13.Gargollo PC, White LA. Robotic-Assisted Bladder Neck Procedures for Incontinence in Pediatric Patients. Front Pediatr 2019;7:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Bergen P, Kunert W, Buess GF. The effect of high-definition imaging on surgical task efficiency in minimally invasive surgery: an experimental comparison between three-dimensional imaging and direct vision through a stereoscopic TEM rectoscope. Surg Endosc 2000;14:71-4. 10.1007/s004649900015 [DOI] [PubMed] [Google Scholar]
- 15.Freschi C, Ferrari V, Melfi F, et al. Technical review of the da Vinci surgical telemanipulator. Int J Med Robot 2013;9:396-406. 10.1002/rcs.1468 [DOI] [PubMed] [Google Scholar]
- 16.Yu DY, Chang YW, Lee HY, et al. Detailed comparison of the da Vinci Xi and S surgical systems for transaxillary thyroidectomy. Medicine (Baltimore) 2021;100:e24370. 10.1097/MD.0000000000024370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel MN, Aboumohamed A, Hemal A. Does transition from the da Vinci Si to Xi robotic platform impact single-docking technique for robot-assisted laparoscopic nephroureterectomy? BJU Int 2015;116:990-4. 10.1111/bju.13210 [DOI] [PubMed] [Google Scholar]
- 18.Spinoit AF, Nguyen H, Subramaniam R. Role of Robotics in Children: A brave New World! Eur Urol Focus 2017;3:172-80. 10.1016/j.euf.2017.08.011 [DOI] [PubMed] [Google Scholar]
- 19.Gearhart JP, Rink RC, Mouriquand PDE. editors. Pediatric Urology. 2nd ed. Elsevier; 2010. [Google Scholar]
- 20.Partin AW, Dmochowski RR, Kavoussi LR, et al. editors. Campbell Walsh Wein Urology. 12th ed. Elsevier; 2020. [Google Scholar]
- 21.Chalmers D, Herbst K, Kim C. Robotic-assisted laparoscopic extravesical ureteral reimplantation: an initial experience. J Pediatr Urol 2012;8:268-71. 10.1016/j.jpurol.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 22.Grimsby GM, Dwyer ME, Jacobs MA, et al. Multi-institutional review of outcomes of robot-assisted laparoscopic extravesical ureteral reimplantation. J Urol 2015;193:1791-5. 10.1016/j.juro.2014.07.128 [DOI] [PubMed] [Google Scholar]
- 23.Galansky L, Andolfi C, Adamic B, et al. Continent Cutaneous Catheterizable Channels in Pediatric Patients: A Decade of Experience with Open and Robotic Approaches in a Single Center. Eur Urol 2021;79:866-78. 10.1016/j.eururo.2020.08.013 [DOI] [PubMed] [Google Scholar]
- 24.Subramaniam R. Robotic Approach to Creation of Continent Catheterisable Channels—Technical Steps, Current Status, and Review of Outcomes. Front Pediatr 2019;7:1. 10.3389/fped.2019.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.England RJ, Subramaniam R. Functional and cosmetic outcome of the VQ plasty for Mitrofanoff stomas. J Urol 2007;178:2607-10; discussion 2610. 10.1016/j.juro.2007.08.030 [DOI] [PubMed] [Google Scholar]
- 26.Gargollo PC. Robotic-assisted bladder neck repair: feasibility and outcomes. Urol Clin North Am 2015;42:111-20. 10.1016/j.ucl.2014.09.013 [DOI] [PubMed] [Google Scholar]
- 27.Adamic B, Kirkire L, Andolfi C, et al. Robot-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff appendicovesicostomy in children: Step-by-step and modifications to UChicago technique. BJUI Compass 2020;1:32-40. 10.1002/bco2.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gundeti MS, Acharya SS, Zagaja GP. The University of Chicago technique of complete intracorporeal pediatric robotic-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff appendicovesicostomy. J Robot Surg 2009;3:89-93. 10.1007/s11701-009-0137-7 [DOI] [PubMed] [Google Scholar]
- 29.Gundeti MS, Acharya SS, Zagaja GP, et al. Paediatric robotic-assisted laparoscopic augmentation ileocystoplasty and Mitrofanoff appendicovesicostomy (RALIMA): feasibility of and initial experience with the University of Chicago technique. BJU Int 2011;107:962-9. 10.1111/j.1464-410X.2010.09706.x [DOI] [PubMed] [Google Scholar]
- 30.Peters CA. Robotically assisted surgery in pediatric urology. Urol Clin North Am 2004;31:743-52. [DOI] [PubMed] [Google Scholar]
- 31.Smith RP, Oliver JL, Peters CA. Pediatric robotic extravesical ureteral reimplantation: comparison with open surgery. J Urol 2011;185:1876-81. 10.1016/j.juro.2010.12.072 [DOI] [PubMed] [Google Scholar]
- 32.Casale P, Patel RP, Kolon TF. Nerve sparing robotic extravesical ureteral reimplantation. J Urol 2008;179:1987-9; discussion 1990. [DOI] [PubMed] [Google Scholar]
- 33.Akhavan A, Avery D, Lendvay TS. Robot-assisted extravesical ureteral reimplantation: outcomes and conclusions from 78 ureters. J Pediatr Urol 2014;10:864-8. 10.1016/j.jpurol.2014.01.028 [DOI] [PubMed] [Google Scholar]
- 34.Rappaport YH, Kord E, Noh PH, et al. Minimally Invasive Dismembered Extravesical Cross-Trigonal Ureteral Reimplantation for Obstructed Megaureter: A Multi-Institutional Study Comparing Robotic and Laparoscopic Approaches. Urology 2021;149:211-5. 10.1016/j.urology.2020.10.018 [DOI] [PubMed] [Google Scholar]
- 35.Pedraza R, Weiser A, Franco I. Laparoscopic appendicovesicostomy (Mitrofanoff procedure) in a child using the da Vinci robotic system. J Urol 2004;171:1652-3. 10.1097/01.ju.0000116066.72132.9a [DOI] [PubMed] [Google Scholar]
- 36.Gundeti MS, Petravick ME, Pariser JJ, et al. A multi-institutional study of perioperative and functional outcomes for pediatric robotic-assisted laparoscopic Mitrofanoff appendicovesicostomy. J Pediatr Urol 2016;12:386.e1-5. [DOI] [PubMed] [Google Scholar]
- 37.Famakinwa OJ, Rosen AM, Gundeti MS. Robot-assisted laparoscopic Mitrofanoff appendicovesicostomy technique and outcomes of extravesical and intravesical approaches. Eur Urol 2013;64:831-6. 10.1016/j.eururo.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 38.Grimsby GM, Jacobs MA, Gargollo PC. Comparison of Complications of Robot-Assisted Laparoscopic and Open Appendicovesicostomy in Children. J Urol 2015;194:772-6. 10.1016/j.juro.2015.02.2942 [DOI] [PubMed] [Google Scholar]
- 39.Galvez C, Lopategui DM, Horodyski L, et al. Totally robotic intracorporeal Monti-Yang continent ileovesicostomy in patient with previous robotic surgery-Technique description. J Pediatr Urol 2021;17:579-80. 10.1016/j.jpurol.2021.05.002 [DOI] [PubMed] [Google Scholar]
- 40.Grimsby GM, Jacobs MA, Menon V, et al. Perioperative and Short-Term Outcomes of Robotic vs Open Bladder Neck Procedures for Neurogenic Incontinence. J Urol 2016;195:1088-92. 10.1016/j.juro.2015.11.043 [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez MV, Wallace A, Gundeti MS. Robotic Bladder Neck Reconstruction With Mitrofanoff Appendicovesicostomy in a Neurogenic Bladder Patient. Urology 2020;137:206-7. 10.1016/j.urology.2019.11.023 [DOI] [PubMed] [Google Scholar]
- 42.Cohen AJ, Brodie K, Murthy P, et al. Comparative Outcomes and Perioperative Complications of Robotic Vs Open Cystoplasty and Complex Reconstructions. Urology 2016;97:172-8. 10.1016/j.urology.2016.06.053 [DOI] [PubMed] [Google Scholar]
- 43.Gargollo PC, Granberg C, Gong E, et al. Complex Robotic Lower Urinary Tract Surgery in Patients with History of Open Surgery. J Urol 2019;201:162-8. 10.1016/j.juro.2018.06.017 [DOI] [PubMed] [Google Scholar]
- 44.Gundeti MS, Kojima Y, Haga N, et al. Robotic-assisted laparoscopic reconstructive surgery in the lower urinary tract. Curr Urol Rep 2013;14:333-41. 10.1007/s11934-013-0328-7 [DOI] [PubMed] [Google Scholar]
- 45.Esposito C, Autorino G, Castagnetti M, et al. Robotics and future technical developments in pediatric urology. Semin Pediatr Surg 2021;30:151082. [DOI] [PubMed] [Google Scholar]
- 46.Sheth KR, Koh CJ. The Future of Robotic Surgery in Pediatric Urology: Upcoming Technology and Evolution Within the Field. Front Pediatr 2019;7:259. 10.3389/fped.2019.00259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mittal S, Srinivasan A. Robotics in Pediatric Urology: Evolution and the Future. Urol Clin North Am 2021;48:113-25. 10.1016/j.ucl.2020.09.008 [DOI] [PubMed] [Google Scholar]
- 48.Wang MK, Li Y, Selekman RE, et al. Scar acceptance after pediatric urologic surgery. J Pediatr Urol 2018;14:175.e1-6. 10.1016/j.jpurol.2017.11.018 [DOI] [PubMed] [Google Scholar]
- 49.Barbosa JA, Barayan G, Gridley CM, et al. Parent and patient perceptions of robotic vs open urological surgery scars in children. J Urol 2013;190:244-50. 10.1016/j.juro.2012.12.060 [DOI] [PubMed] [Google Scholar]
- 50.Trevisani LF, Nguyen HT. Current controversies in pediatric urologic robotic surgery. Curr Opin Urol 2013;23:72-7. 10.1097/MOU.0b013e32835b0ad2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as