Key Points
Question
Does elexacaftor-tezacaftor-ivacaftor (ETI) therapy have any positive change in chronic rhinosinusitis as measured by changes in sinus computed tomography (CT) metrics in individuals with cystic fibrosis (CF)?
Findings
In this cohort study of 64 individuals with CF, after 1 year of ETI therapy, reduction in the median total Lund-Mackay sinus CT scoring system and the Sheikh-Lind scoring system (from 5.8 to 3.3 and from 3.8 to 2.2, respectively) was noted. There was also improvement in mean percent predicted forced expiratory volume in 1 second and body mass index, and bacterial colonization was reduced.
Meaning
Treatment of individuals with CF using ETI therapy was associated with improved chronic sinus disease and clinical outcomes; randomized trials with appropriate control groups are required to assess the efficacy of this therapy on chronic sinus disease in this population.
This cohort study quantifies the 1-year outcomes of elexacaftor-tezacaftor-ivacaftor therapy for chronic rhinosinusitis based on changes in sinus computed tomography using the Lund-Mackay and Sheikh-Lind scoring systems among individuals with cystic fibrosis.
Abstract
Importance
Cystic fibrosis (CF) is a multiorgan genetic disease with progressive upper and lower airway involvement. The effects of CF transmembrane conductance regulator (CFTR) modifier therapies on CF-related upper airway disease, specifically chronic rhinosinusitis (CRS), are not characterized.
Objective
To determine the outcome of elexacaftor-tezacaftor-ivacaftor (ETI) on CRS as measured by changes in sinus computed tomography (CT) metrics and on clinical parameters in individuals with CF.
Design, Setting, and Participants
This prospective longitudinal cohort study was conducted at the CF center of a tertiary care hospital between October 1, 2019, and July 31, 2021. A total of 64 participants with CF were included in the analysis.
Intervention
Sinus CT was obtained within 1 month of initiation of ETI therapy (baseline), and within 1 month of 1 year of ETI therapy. Images were independently analyzed by pulmonology, radiology, and otolaryngology physicians, using the Lund-Mackay and Sheikh-Lind scoring systems. Percent predicted forced expiratory volume in 1 second (ppFEV1), body mass index (BMI), and microbiologic data collected at initiation of ETI therapy and 3-month intervals for 1 year were also measured.
Main Outcomes and Measures
The study hypothesis was that ETI therapy will improve CRS as measured by changes in sinus CT at initiation and 1 year after ETI therapy and clinical parameters in individuals with CF.
Results
Among the 64 participants (39 [60.9%] female; median age, 18.5 [IQR, 16.0-28.5] years; 64 [100%] White), improvement in CRS was noted by improvements in sinus CT scans using both sinus CT scoring systems after 1 year of ETI therapy. The reduction in the median total score using the Lund-Mackay sinus CT scoring system (from 5.8 [IQR, 5.0-7.0] to 3.3 [IQR, 2.6-4.2]) and the Sheikh-Lind scoring system (from 3.8 [IQR, 3.0-5.0] to 2.2 [IQR, 2.0-2.5]) was noted. Increases in ppFEV1 and BMI were also observed by 3 months of ETI therapy with persistent improvement through 1 year of treatment. Similarly, after 1 year of ETI therapy, participants with CF had reductions in positivity for Pseudomonas aeruginosa and Staphylococcus aureus in oropharyngeal cultures.
Conclusion and Relevance
This cohort study found that use of ETI therapy was associated with improved CRS outcomes in participants with CF as quantified by improved sinus CT scans measured by 2 radiographic scoring systems and was also associated with improved clinical outcomes. Despite improvement in CT scan scores, most people with CF continue to have scores that indicate severe sinus disease.
Introduction
Chronic rhinosinusitis (CRS) with chronic upper airway infection and/or inflammation leading to symptomatic disease is seen in approximately 50% of individuals with cystic fibrosis (CF). Symptoms of CRS are caused by deficient or dysfunctional CF transmembrane conductance regulator (CFTR), a complex protein that encodes for the chloride channel present in the epithelial membrane of upper airways. The most common CFTR gene sequence variation is F508del; approximately 90% of individuals with CF who are of northern European descent have at least 1 copy of this variation, and about 50% are homozygous for F508del.1,2 The CFTR variations are associated with both processing defects and disrupted channel opening, leading to minimal CFTR channel chloride activity across the epithelial membrane.3,4 Modulator therapies for CFTR target CFTR protein dysfunction by 2 separate mechanisms: correctors for protein shape and function (such as elexacaftor and tezacaftor) and potentiators (such as ivacaftor) that increase channel opening. A combination of these 3 medications, elexacaftor-tezacaftor-ivacaftor (ETI), received US Food and Drug Administration approval for people with CF who are 12 years and older in October 2019 and for those aged 6 to 11 years in June 2021. Clinical trials have demonstrated significant improvement in respiratory function (percent predicted forced expiratory volume in 1 second [ppFEV1]), pulmonary exacerbations, weight and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), and quality of life for people with CF.5,6,7
Because CF is a multiorgan disease, the effects of CFTR modulator therapies on extrapulmonary organ systems are still being evaluated. There is correlation between pulmonary and sinus disease in CF, as both lower airways (lungs) and upper airways (sinuses) are colonized with similar organisms.8 In people with CF and progressive sinonasal involvement, sinus surgery may affect pulmonary exacerbations, though the direct relationship remains unclear.9,10,11,12,13 The effect of CFTR modulator therapy on CRS in people with CF is not well defined. The role of ivacaftor in improving clinical symptoms, increasing scores on the validated 20-item Sino-Nasal Outcome Test (SNOT-20), and improving computed tomography (CT) findings of sinus disease has been reported.14,15,16,17,18 The effect of ETI on radiographic sinus disease is less known19,20,21 and needs further investigation. The Lund-Mackay system22,23 is the most-used CF-based scoring system for CRS. Of note, the Lund-Mackay score is not CF specific and does not strongly correlate with symptom severity, quality of life measures,24,25,26,27 or progression of sinus disease.28 More recently, a CF-specific Sheikh-Lind scoring system was described for CRS in people with CF and demonstrated excellent overall interrater and intrarater reliability between physician experts in pulmonology, radiology, and otolaryngology.29
The objective of this prospective longitudinal cohort study was to quantify the 1-year outcomes of ETI therapy on CRS based on changes in sinus CT findings using both the Lund-Mackay22,23 and Sheikh-Lind29 scoring systems, including changes in the severity of CRS.30,31 Secondary objectives include outcomes in clinical parameters such as ppFEV1 and BMI and in bacterial colonization of airways. We hypothesized that ETI therapy would improve all metrics in people with CF and that both CT scoring systems would be able to quantify CRS changes with therapy.
Methods
This single-center, prospective longitudinal cohort study was approved by the Institutional Review Board of Nationwide Children’s Hospital, Columbus, Ohio. The study was conducted between October 1, 2019, and July 31, 2021. On diagnosis, individuals with CF were enrolled from the CF clinic at a large tertiary care hospital during visits to initiate ETI therapy. Written informed consent from adult participants and guardians and assent from pediatric participants were required for study enrollment. Seventy-nine individuals with CF were enrolled in the study; of these, 64 completed this study and their data were analyzed. Fifteen participants did not complete the study due to missing follow-up appointments and sinus CT scans during the COVID-19 pandemic. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Criteria
Inclusion criteria consisted of diagnosis of CF either by the presence of 2 disease-causing CFTR genetic variants and/or results of a quantitative sweat test (chloride level, ≥60 mEq/L [to convert to mmol/L, multiply by 1]) and the presence of CFTR variants eligible for ETI therapy with evidence of CRS on previous sinus CT scans. People with CF without radiographic evidence of CRS were excluded from the study. During the visit for initiation of ETI therapy, demographic characteristics (age and race) and clinical metrics of CF disease (lung function, BMI, bacterial colonization) were collected from the electronic medical record. Participants were followed up clinically at 3-month intervals as part of their routine CF care, with clinical data collected at each follow-up visit up to 1 year after ETI therapy initiation. Participants had sinus CT scans obtained within 1 month of ETI therapy initiation (enrollment) and also within 1 month of completing 1 year of ETI therapy. The interpreting physicians included a pediatric pulmonologist (S.S.), neuroradiologist (M.-L.H.), and otolaryngologist (M.L.) who independently assessed images while blinded to clinical history. Sinus CT scans were not obtained within 1 month of symptoms suggestive of sinopulmonary exacerbation or viral upper respiratory infection and/or within 1 month of antibiotic use for sinopulmonary symptoms or sinopulmonary exacerbation of CF.
Study End Points
The primary study end point was CF-related CRS as measured by changes in sinus CT, using the Lund-Mackay and Sheikh-Lind scoring systems. Secondary end points included changes in clinical parameters (ppFEV1, BMI, and sweat chloride level) and bacterial cultures (methicillin-resistant Staphylococcus aureus [MRSA] and Pseudomonas aeruginosa) collected at baseline and 3, 6, 9, and 12 months.
In the Lund-Mackay system,22,23 each side is scored with respect to 6 anatomical compartments: frontal sinus, anterior ethmoidal cells, posterior ethmoidal cells, maxillary sinus, sphenoid sinus, and osteomeatal complex. Each sinus is assigned a score of 0 (no abnormality), 1 (partial opacification), or 2 (complete opacification). The maximum score is 12 per side, for a maximum total score of 24. In the Sheikh-Lind scoring system,29 each side is scored using the following parameters: maxillary opacification, nasal cavity obstruction, lateral nasal wall displacement, uncinate process absence or demineralization, and mucocele. Maxillary opacification and maxillary opacification are scored in a graded fashion as none (0), mild (1; <33%), moderate (2; 33%-66%), and severe (3; >66%). Lateral nasal wall displacement, uncinate process absence or demineralization, and mucocele are scored as present (1) or absent (0). Surgical absence of the uncinate process is scored the same as demineralization. The maximum score is 9 per side, for a maximum total score of 18.
In the Lund-Mackay scoring system, studies have revealed association of the mean score with disease severity and use of surgery.30,31 Hopkins et al30 suggested sinus surgery with a mean score of 4 or above, and in their study, patients with higher Lund-Mackay scores underwent more extensive surgery.30 They found no correlation between Lund-Mackay and SNOT-22 scores, and higher Lund-Mackay scores were associated with higher complication rates (odds ratio [OR], 1.08 [95% CI, 1.06-1.10]) and revision rates (OR, 1.03 [95% CI, 1.00-1.06]).30 In another study with Lund-Mackay scores,31 using a cutoff of 5 to represent true disease, the CT scans demonstrated a sensitivity and specificity of 86% and 85%, respectively. Lund-Mackay scores of 2 or less had an excellent negative predictive value, whereas Lund-Mackay scores of 5 or greater had an excellent positive predictive value (ie, strongly indicate true disease).31
Statistical Analysis
All statistical analyses were performed in R, version 4.0 (R Project for Statistical Computing), with reproducible programming in R markdown. Clinical data were summarized using frequency (percentage) for categorical variables and median (IQR) for continuous variables. Linear mixed-effects regression models were fit for the continuous clinical outcomes (ppFEV1 and BMI), and logistic mixed-effects regression models were fit for the presence of each bacterial culture to account for within-participant correlation from repeated measures, fitting separate intercepts for each participant. Logistic regression estimates were exponentiated to present ORs. Time points were used as discrete independent variables in these models, with the baseline measurement as the reference level. We estimated β coefficients representing mean estimated change from baseline scores or ORs and 95% CIs at each time point to quantify effect size. Change in sweat chloride levels from baseline to 1 month after ETI therapy initiation was analyzed using the difference in mean values and corresponding 95% CI; effect size was summarized by Cohen d with 95% CI. Cohen d was interpreted as a small effect size for values of 0.2, a medium effect size for values of 0.5, and a large effect size for values of 0.8.
For sinus CT scores, percentage interrater agreement and Fleiss κ value were computed for each outcome. We calculated mean CT scores by adding the scores of the 3 reviewers on both sides together (left and right), then dividing by 6. This method allowed us to interpret sinus CT parameter scores on their original scoring scale.22,23,29 Scores were compared before and after ETI for each scoring system using the matched-pairs rank biserial correlation coefficient (r value) with 95% CI with the R package rcompanion.32 Effect sizes for r values were interpreted as a perfect negative correlation between time point and CT score for r = −1.00, a perfect positive correlation for r = 1.00, and no relationship or a negligible correlation for r = 0. We could not compute 95% CIs for r values when the rank at 1 time point was higher for every participant.
Changes in sinus CT scores for the Lund-Mackay system at baseline and after 1 year of ETI therapy were analyzed categorically using cutoff values. Effect sizes of asymmetry for matched pairs were estimated using Cohen g with 95% CIs using the effect size package in R. Values of Cohen g range from 0 to 0.50, where less than 0.05 was interpreted as a very small effect size, 0.05 to less than 0.15 as a small effect size, 0.15 to less than 0.25 as a medium effect size, and 0.25 or greater as a large effect size.33
Results
Demographics
The cohort included 64 individuals with CF with a median age of 18.5 (IQR, 16.0-28.5) years; 39 participants [60.9%] were female and 25 [39.1%] were male). Twenty-four participants (37.5%) were younger than 18 years and the remaining 40 (62.5%) were 18 years or older. All 64 participants were White. Thirty-nine participants (60.9%) were homozygous for F508del, and 23 (35.9%) were heterozygous for F508del.
Primary Objective: Sinus CT Findings
For the Lund-Mackay scoring system, agreement between reviewers at baseline ranged from 65.6% to 87.5%, and after 1 year of ETI therapy ranged from 39.1% to 78.1%. For the Sheikh-Lind scoring system, agreement between reviewers at baseline ranged from 68.8% to 93.8%, and after 1 year of ETI therapy ranged from 64.1% to 96.9% (eTable 1 in Supplement 1).
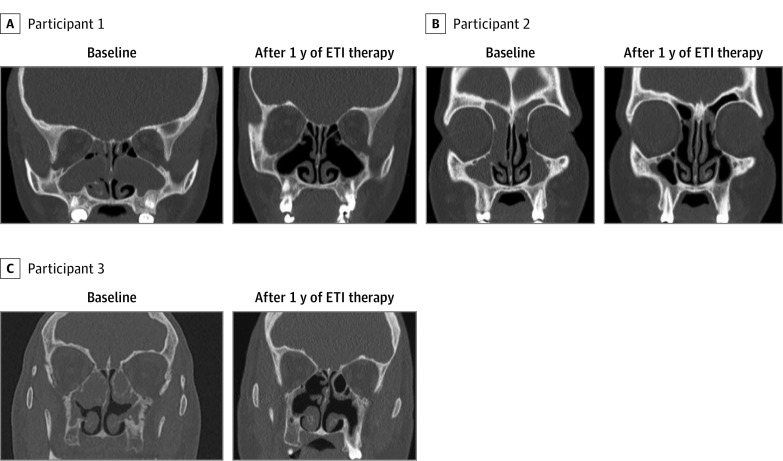
Severity of CRS disease improved after 1 year of ETI therapy compared with initiation of ETI therapy as noted by sinus CT scans in most of the participants using both scoring systems (Figure, Table 1, Table 2, and Table 3). None of the participants had an increased Lund-Mackay score from baseline to 1 year of ETI therapy, 2 (3.1%) had the same Lund-Mackay score, and 62 (96.9%) had decreased Lund-Mackay score. Using the Lund-Mackay system, there was improvement in median scores across all 6 parameters (Table 1) and in median total Lund-Mackay score from 5.8 (IQR, 5.0-7.0) at baseline to 3.3 (IQR, 2.6-4.2) (r = 1.00; 95% CI, not calculable because all scores were equal to or lower than at baseline) after 1 year of ETI therapy. Using the Sheikh-Lind scoring system, there was improvement in median scores for maxillary opacification and nasal cavity obstruction (Table 2). There was improvement in the opacification parameters (median scores for maxillary opacification, nasal cavity obstruction, and mucocele) after 1 year of ETI therapy, with total median opacification score improving from 2.8 (IQR, 2.0-3.9) to 1.2 (IQR, 1.0-1.5; r = 0.99 [95% CI, 0.97-1.00]). Median total Sheikh-Lind score (with all 5 parameters) improved from 3.8 (IQR, 3.0-5.0) at baseline to 2.2 (IQR, 2.0-2.5; r = 0.99 [95% CI, 0.97-1.00]) after 1 year of ETI therapy (Table 2).
Figure. Comparison of Sinus Computed Tomography Scans Before Starting Elexacaftor-Tezacaftor-Ivacaftor (ETI) Therapy (Baseline) and After 1 Year of ETI Therapy in Participants With Cystic Fibrosis.
Table 1. Comparison of Sinus Computed Tomography Parameters at Baseline and After 1 Year of Elexacaftor-Tezacaftor-Ivacaftor Therapy Using the Lund-Mackay Scoring System.
| Characteristic | Median (IQR)a | Effect size, r (95% CI)b | |
|---|---|---|---|
| Baseline (n = 64) | 1 Year (n = 64) | ||
| Frontal sinus | 1.5 (0.8-2.0) | 0.3 (0-0.8) | 1 (NC) |
| Anterior ethmoid | 1.0 (1.0-1.5) | 0.8 (0.5-1.0) | 0.97 (0.91-1.00) |
| Posterior ethmoid | 1.0 (0.8-1.0) | 0.7 (0.3-0.9) | 0.98 (0.93-1.00) |
| Maxillary sinus | 1.0 (1.0-1.3) | 1.0 (0.7-1.0) | 0.99 (0.94-1.00) |
| Sphenoid sinus | 1.0 (1.0-1.5) | 0.7 (0.3-1.0) | 0.98 (0.94-1.00) |
| Osteomeatal occlusion | 0 (0-0.2) | 0 (0-0) | 1.00 (NC) |
| Average total Lund-Mackay score | 5.8 (5.0-7.0) | 3.3 (2.6-4.2) | 1.00 (NC) |
Abbreviation: NC, noncalculable.
Calculated as the median of 3 reviewers and 2 (left and right) sides.
Matched-pairs rank biserial correlation coefficient; r = −1 is perfect negative correlation, r = 1 is perfect positive correlation, and r = 0 is no or negligible correlation. Noncalculable 95% CI indicates all score ranks at 1 year were less than or equal to baseline.
Table 2. Comparison of Sinus Computed Tomography Parameters at Baseline and After 1 Year of Elexacaftor-Tezacaftor-Ivacaftor Therapy, Using the Sheikh-Lind Scoring System.
| Characteristic | Median (IQR)a | Effect size, r (95% CI)b | |
|---|---|---|---|
| Baseline (n = 64)a | 1 Year (n = 64)a | ||
| Maxillary opacification | 2.3 (1.7 to 2.9) | 1.0 (1.0 to 1.0) | 0.99 (0.98 to 1.00) |
| Nasal cavity obstruction | 0.3 (0 to 0.7) | 0.2 (0 to 0.2) | 0.7 (0.47 to 0.87) |
| Mucocele | 0 (0 to 0.5) | 0 (0 to 0) | 0.87 (0.59 to 1.00) |
| Displacement of lateral nasal wall | 0 (0 to 0.2) | 0 (0 to 0) | 0.38 (−0.17 to 0.89) |
| Uncinate process absence | 1.0 (1.0 to 1.0) | 1.0 (1.0 to 1.0) | 0.02 (−0.82 to 0.70) |
| Total Sheikh-Lind score (opacification parameters) | 2.8 (2.0 to 3.9) | 1.2 (1.0 to 1.5) | 0.99 (0.97 to 1.00) |
| Total Sheikh-Lind score (with all 5 parameters) | 3.8 (3.0 to 5.0) | 2.2 (2.0 to 2.5) | 0.99 (0.97 to 1.00) |
Calculated as the median of 3 reviewers and 2 (left and right) sides.
Matched-pairs rank biserial correlation coefficient; r = −1 is perfect negative correlation, r = 1 is perfect positive correlation, and r = 0 is no or negligible correlation.
Table 3. Number of Participants With Severe Disease Burden at Baseline and After 1 Year of Elexacaftor-Tezacaftor-Ivacaftor Therapy, Using the Lund-Mackay Scoring System.
| Characteristic | No. (%) of participants | Effect size, Cohen g (95% CI)a | |
|---|---|---|---|
| Baseline (n = 64) | 1 Year (n = 64) | ||
| Lund-Mackay score cutoff of 4 | |||
| FESS suggested (>4) | 63 (98.4) | 52 (81.3) | 0.50 (0.24-0.50) |
| No FESS (≤ 4) | 1 (1.6) | 12 (18.8) | |
| Lund-Mackay score cutoff of 5 | |||
| High PPV for severe disease (>5) | 63 (98.4) | 48 (75.0) | 0.50 (0.30-0.50) |
| Not high PPV for severe disease (≤5) | 1 (1.6) | 16 (25.0) | |
Abbreviations: FESS, functional endoscopic sinus surgery; PPV, positive predictive value.
Less than 0.05 indicates very small; 0.05 to less than 0.15, small; 0.15 to less than 0.25, medium; and 0.25 or greater, large.
In the Lund-Mackay scoring system, mean score above 4 (both sides combined) is suggested as a cutoff for functional endoscopic sinus surgery.30 In our cohort at treatment initiation, 63 participants (98.4%) had scores greater than 4; after 1 year of ETI therapy this decreased to 52 (81.3%), and the number with a score less than 4 increased from 1 to 12 (18.8%) (Cohen g = 0.48 [95% CI, 0.40-0.50]). Similarly, there is no absolute cutoff in CRS, but higher scores (>5) are associated with severe disease and poorer surgical outcomes.30,31 In our cohort, 63 participants (98.4%) had mean Lund-Mackay scores of greater than 5 at baseline, which decreased to 48 (75.0%) after 1 year of ETI therapy (Cohen g = 0.48 [95% CI, 0.39-0.50]) (Table 3).
Secondary Objectives
Sweat Chloride Levels
The mean (SD) sweat chloride level at baseline (44 available for both measurements) was 93.0 (19.1) mEq/L. After 1 month this decreased to 45.2 (17.5) mEq/L (mean difference, 48 [95% CI, 41-54] mEq/L; Cohen d = 2.6 [95% CI, 2.0-3.2]).
Clinical Outcomes
The median baseline ppFEV1 of 67 (IQR, 56-85) improved at 12 months to 86 (IQR, 71-99) (28.4% change from baseline; β = 14.2 [95% CI, 12.3-16.2). Similarly, the median baseline BMI was 21.0 (IQR, 19.1-24.3), which at 12 months improved to 23.0 (IQR, 20.4-26.7) with 9.5% change from baseline (β = 1.9 [95% CI, 1.5-2.3]) (Table 4).
Table 4. Changes in Clinical Parameters for Participants With Cystic Fibrosis During the 1-Year Elexacaftor-Tezacaftor-Ivacaftor Treatment Period.
| Measurement time | |||||
|---|---|---|---|---|---|
| Baseline | 3 Months | 6 Months | 9 Months | 12 Months | |
| ppFEV1 | |||||
| Median (IQR) | 67 (56-85) | 85 (68-98) | 90 (79-101) | 87 (72-98) | 86 (71-99) |
| Change from baseline, % | NA | 26.9 | 34.3 | 29.9 | 28.4 |
| β Coefficient (95% CI)a | NA | 13.2 (11.3-15.2) | 15.1 (13.0-17.2) | 15.0 (13.0-17.0) | 14.2 (12.3-16.2) |
| BMI | |||||
| Median (IQR) | 21.0 (19.1-24.3) | 22.3 (20.4-24.8) | 23.0 (20.5-25.2) | 22.9 (20.5-26.5) | 23.0 (20.4-26.7) |
| Change from baseline, % | NA | 6.2 | 9.5 | 9.0 | 9.5 |
| β Coefficient (95% CI)a | NA | 1.1 (0.7-1.5) | 1.5 (1.1-2.0) | 1.8 (1.4-2.2) | 1.9 (1.5-2.3) |
| Positivity for Pseudomonas aeruginosa | |||||
| Total No. of participants | 64 | 56 | 60 | 60 | 62 |
| No. (%) with finding | 34 (53.1) | 15 (26.8) | 15 (25.0) | 12 (20.0) | 14 (22.3) |
| OR (95% CI)b | NA | 0.06 (0.01-0.26) | 0.04 (0.01-0.17) | 0.02 (0.01-0.12) | 0.03 (0.01-0.15) |
| Positivity for MRSA | |||||
| Total No. of participants | 64 | 56 | 60 | 60 | 62 |
| No. (%) with finding | 19 (29.7) | 11 (19.6) | 11 (18.3) | 9 (15.0) | 11 (17.7) |
| OR (95% CI)b | NA | 0.03 (0.01-0.33) | 0.06 (0.01-0.55) | 0.02 (0.01-0.25) | 0.06 (0.01-0.54) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MRSA, methicillin-resistant Staphylococcus aureus; NA, not applicable; OR, odds ratio; ppFEV1, percent predicted forced expiratory volume in 1 second.
Represents estimated mean changes from baseline using linear mixed effects models.
Compared with baseline using logistic mixed effects models.
At baseline, 34 participants with CF (53.1%) had respiratory tract cultures positive for P aeruginosa, which decreased at 12 months to 14 (22.3%; OR, 0.03 [95% CI, 0.01-0.15]) (Table 4). Nineteen participants (29.7%) had cultures positive for MRSA at baseline, which decreased at 12 months to 11 (17.7%; OR, 0.06 [95% CI, 0.01-0.54]) compared with baseline, demonstrating an overall reduction in culture positivity at 1 year after ETI (Table 4). At the start of the study, 46 of 64 participants (71.9%) were producing sputum for microbial airway cultures and the remaining 18 (28.2%) were using throat swabs. After 1 year of ETI therapy, only 15 individuals (23.4%) were still producing sputum for microbial cultures while 48 (75.0%) were using throat swabs for cultures (Cohen g = 0.47 [95% CI, 0.35-0.50]). Among those 15 individuals who were still producing sputum, 8 (53.3%) continued to have positive sputum cultures with either P aeruginosa or MRSA or both.
During the 1 year of ETI therapy, none of the participants underwent interval sinus surgery or intravenous antibiotic therapy for acute rhinosinusitis. The median number of CF exacerbations, intravenous antibiotic courses, hospital admissions, and oral and/or nebulized antibiotic courses for sinopulmonary exacerbations during 1 year before starting ETI therapy and during 1 year of receiving ETI therapy were compared (eTable 2 in Supplement 1). In the 1-year period before starting ETI, the median number of pulmonary exacerbations was 3.5 (IQR, 1-6), which decreased to 0 (IQR, 0-1) during the 1 year of therapy with ETI (Cohen d = 1.4 [95% CI, 1.0-1.8]). The other clinical components (including number of participants requiring daily nasal corticosteroids, saline irrigations, or nebulized antibiotics) also improved from the pre-ETI to post-ETI periods (eTable 2 in Supplement 1).
Discussion
Elexacaftor-tezacaftor-ivacaftor therapy is a novel treatment for people with CF, and its effects on upper airway disease are not fully understood. Our study cohort of 64 individuals with CF and CRS revealed improvements in sinus CT metrics after 1 year of ETI therapy, confirmed using both the classic Lund-Mackay scoring system for CRS and the newer Sheikh-Lind scoring system for CF. While there were improvements in scores, most participants still had scores for both systems that indicated severe sinus disease. Therapy with ETI also improved several clinical parameters, including increased ppFEV1 and BMI, decreased sweat chloride level, and reduced respiratory culture positivity for dominant pathogens P aeruginosa and MRSA. It is unclear how well these cultures reflect the actual colonization of the lower respiratory tract.
These results are well aligned with a previous study of 12 individuals with CF and the G551D sequence variation15 who received ivacaftor and showed improvement on sinus CT results. Since ETI is indicated in individuals with the F508del sequence variation, which is present in approximately 90% of people with CF of northern European descent, larger study cohorts have become possible. A few recent studies19,20,21 have examined the role of ETI in CF-related CRS. DiMango et al,19 in their cohort of 43 adults with CF after 3 months of ETI therapy, revealed significant improvement in mean SNOT-22 scores and mean Cystic Fibrosis Questionnaire–Revised respiratory domain of quality-of-life scores, but sinus CT scans were not obtained. Beswick et al20 examined a cohort of 25 individuals with CF, in whom ETI therapy for 6 months improved sinus opacification from a mean volume of 64% to 41% (mean decrease of 22.9%), as well as increasing quality of life. Stapleton et al21 used a modified Lund-Mackay scoring system in their cohort of 28 individuals with CF to evaluate improvement with ETI therapy on CT, revealing a decrease in median score from 16 (IQR, 13.5-20.6) to 12 (IQR, 9.5-12.6). Our prospective study enrolled 64 individuals with CF, using both Lund-Mackay and Sheikh-Lind scoring systems with 3 independent and blinded expert reviewers in different specialties (pediatric pulmonology, radiology, otolaryngology) to limit single-review bias.
In people with CF, diffuse and progressive sinonasal involvement is very common. Computed tomography is a widely used, noninvasive method for assessing disease burden, though the relationship to treatment outcomes varies.24,25,26,27,34 Multiple scoring systems for rhinosinusitis severity on CT have been described in the literature. However, many proposed scoring systems are complex and difficult to use, thus limiting their application to radiologists, who often lack the crucial information for true clinical validation.22,23 The Lund-Mackay system is simple and widely used in CRS but has limited usefulness and validity in people with CF.27,30,35 It has been proposed that the broad categories used in traditional scoring systems are not granular enough to characterize the nature of disease in CF.28 The newer Sheikh-Lind scoring system has been validated as more CF specific.29 It considers changes on CT in people with CF that are not typically found in individuals with CRS due to other causes, including uncinate process demineralization, medial displacement of the lateral nasal wall in the middle meatus, maxillary and ethmoid sinus opacification, mucoceles or pseudomucoceles, and nasal polyposis.36,37,38 Therefore, the Sheikh-Lind system allows for greater differentiation in disease severity and assessment of CF-related CRS. Both the Lund-Mackay and Sheikh-Lind systems are easy to use in clinical settings and can be reproducibly implemented by clinicians, surgeons, and radiologists in different use cases.22,29
Strengths and Limitations
The strengths of this work include prospective design, larger sample size, more clinical and radiologic data collection, and longer follow-up than prior studies in the literature. We used 2 different CT scoring systems, including a CF-specific Sheikh-Lind score, with 3 independent raters from different specialties. We noted a lower agreement between reviewers in reading the sinus CT scans, which was probably secondary to the fact that scans were interpreted by physicians in 3 different specialties. Limitations also include single-center enrollment, a population that included only White individuals, lack of a control group, lack of power analysis, and lack of quality-of-life and clinical symptoms data. The study was performed during the COVID-19 pandemic with social distancing; thus, there was reduction in viral exposures that may have had some effect on outcomes. COVID-19 restrictions may also be responsible for a few participants not being able to complete the study. Our local population with CF also had high rates of MRSA positivity at baseline, probably reflective of local CF and non-CF MRSA community transmission dynamics, as MRSA positivity is not the same in all CF centers. Further, after ETI, many people with CF did not regularly produce sputum, which could cause a pathogen detection bias.
Conclusion
In this cohort study, ETI therapy in individuals with CF was associated with a decrease in CRS based on sinus CT scans using 2 scoring systems and was also associated with improvements in clinical outcomes and reductions in bacterial colonization. While there were improvements in scores, most participants still had scores for both systems that indicated severe sinus disease.
eTable 1. Agreement Percentage and κ Values Among 3 Sinus CT Reviewers
eTable 2. Comparison of Clinical Outcomes 1 Year vs 1 Year of Receiving ETI and Number of Participants Receiving Therapies at the Start Compared With 1 Year of Receiving ETI
Data Sharing Statement
References
- 1.US CF Foundation, Johns Hopkins University, the Hospital for Sick Children . The clinical and functional translation of CFTR (CFTR2). Updated April 7, 2023. Accessed September 8, 2022. http://cftr2.org
- 2.Cystic Fibrosis Foundation . Patient registry: annual data report, 2020. Updated September 2021. Accessed April 10, 2022. https://www.cff.org/medical-professionals/patient-registry
- 3.Farinha CM, Amaral MD. Most F508del-CFTR is targeted to degradation at an early folding checkpoint and independently of calnexin. Mol Cell Biol. 2005;25(12):5242-5252. doi: 10.1128/MCB.25.12.5242-5252.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma M, Benharouga M, Hu W, Lukacs GL. Conformational and temperature-sensitive stability defects of the delta F508 cystic fibrosis transmembrane conductance regulator in post-endoplasmic reticulum compartments. J Biol Chem. 2001;276(12):8942-8950. doi: 10.1074/jbc.M009172200 [DOI] [PubMed] [Google Scholar]
- 5.Habib A-RR, Kajbafzadeh M, Desai S, Yang CL, Skolnik K, Quon BS. A systematic review of the clinical efficacy and safety of CFTR modulators in cystic fibrosis. Sci Rep. 2019;9(1):7234. doi: 10.1038/s41598-019-43652-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Middleton PG, Mall MA, Dřevínek P, et al. ; VX17-445-102 Study Group . Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809-1819. doi: 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heijerman HGM, McKone EF, Downey DG, et al. ; VX17-445-103 Trial Group . Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940-1948. doi: 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armbruster CR, Li K, Kiedrowski MR, et al. Low diversity and instability of the sinus microbiota over time in adults with cystic fibrosis. Microbiol Spectr. 2022;10(5):e0125122. doi: 10.1128/spectrum.01251-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin M, Gao X, Di L, et al. Effect of endoscope sinus surgery on pulmonary function in cystic fibrosis patients: a meta-analysis. Laryngoscope. 2021;131(4):720-725. doi: 10.1002/lary.29066 [DOI] [PubMed] [Google Scholar]
- 10.Cheng TZ, Choi KJ, Honeybrook AL, et al. Decreased antibiotic utilization after sinus surgery in cystic fibrosis patients with lung transplantation. Am J Rhinol Allergy. 2019;33(4):354-358. doi: 10.1177/1945892419830624 [DOI] [PubMed] [Google Scholar]
- 11.Roby BB, McNamara J, Finkelstein M, Sidman J. Sinus surgery in cystic fibrosis patients: comparison of sinus and lower airway cultures. Int J Pediatr Otorhinolaryngol. 2008;72(9):1365-1369. doi: 10.1016/j.ijporl.2008.05.011 [DOI] [PubMed] [Google Scholar]
- 12.Khalfoun S, Tumin D, Ghossein M, Lind M, Hayes D Jr, Kirkby S. Improved lung function after sinus surgery in cystic fibrosis patients with moderate obstruction. Otolaryngol Head Neck Surg. 2018;158(2):381-385. doi: 10.1177/0194599817739284 [DOI] [PubMed] [Google Scholar]
- 13.Choi KJ, Cheng TZ, Honeybrook AL, et al. Correlation between sinus and lung cultures in lung transplant patients with cystic fibrosis. Int Forum Allergy Rhinol. 2018;8(3):389-393. doi: 10.1002/alr.22067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormick J, Cho DY, Lampkin B, et al. Ivacaftor improves rhinologic, psychologic, and sleep-related quality of life in G551D cystic fibrosis patients. Int Forum Allergy Rhinol. 2019;9(3):292-297. doi: 10.1002/alr.22251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheikh SI, Long FR, McCoy KS, Johnson T, Ryan-Wenger NA, Hayes D Jr. Ivacaftor improves appearance of sinus disease on computerised tomography in cystic fibrosis patients with G551D mutation. Clin Otolaryngol. 2015;40(1):16-21. doi: 10.1111/coa.12310 [DOI] [PubMed] [Google Scholar]
- 16.Chang EH, Tang XX, Shah VS, et al. Medical reversal of chronic sinusitis in a cystic fibrosis patient with ivacaftor. Int Forum Allergy Rhinol. 2015;5(2):178-181. doi: 10.1002/alr.21440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vreede CL, Berkhout MC, Sprij AJ, Fokkens WJ, Heijerman HGM. Ivacaftor and sinonasal pathology in a cystic fibrosis patient with genotype deltaF508/S1215N. J Cyst Fibros. 2015;14(3):412-413. doi: 10.1016/j.jcf.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 18.Hayes D Jr, McCoy KS, Sheikh SI. Improvement of sinus disease in cystic fibrosis with ivacaftor therapy. Am J Respir Crit Care Med. 2014;190(4):468. doi: 10.1164/rccm.201403-0595IM [DOI] [PubMed] [Google Scholar]
- 19.DiMango E, Overdevest J, Keating C, Francis SF, Dansky D, Gudis D. Effect of highly effective modulator treatment on sinonasal symptoms in cystic fibrosis. J Cyst Fibros. 2021;20(3):460-463. doi: 10.1016/j.jcf.2020.07.002 [DOI] [PubMed] [Google Scholar]
- 20.Beswick DM, Humphries SM, Balkissoon CD, et al. Impact of cystic fibrosis transmembrane conductance regulator therapy on chronic rhinosinusitis and health status: deep learning CT analysis and patient-reported outcomes. Ann Am Thorac Soc. 2022;19(1):12-19. doi: 10.1513/AnnalsATS.202101-057OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stapleton AL, Kimple AJ, Goralski JL, et al. Elexacaftor-Tezacaftor- Ivacaftor improves sinonasal outcomes in cystic fibrosis. J Cyst Fibros. 2022;21(5):792-799. doi: 10.1016/j.jcf.2022.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183-184. [PubMed] [Google Scholar]
- 23.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3, pt 2):S35-S40. doi: 10.1016/S0194-5998(97)70005-6 [DOI] [PubMed] [Google Scholar]
- 24.Basu S, Georgalas C, Kumar BN, Desai S. Correlation between symptoms and radiological findings in patients with chronic rhinosinusitis: an evaluation study using the Sinonasal Assessment Questionnaire and Lund-Mackay grading system. Eur Arch Otorhinolaryngol. 2005;262(9):751-754. doi: 10.1007/s00405-004-0891-0 [DOI] [PubMed] [Google Scholar]
- 25.Holbrook EH, Brown CL, Lyden ER, Leopold DA. Lack of significant correlation between rhinosinusitis symptoms and specific regions of sinus computer tomography scans. Am J Rhinol. 2005;19(4):382-387. doi: 10.1177/194589240501900411 [DOI] [PubMed] [Google Scholar]
- 26.Zheng Y, Zhao Y, Lv D, et al. Correlation between computed tomography staging and quality of life instruments in patients with chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(1):e41-e45. doi: 10.2500/ajra.2010.24.3430 [DOI] [PubMed] [Google Scholar]
- 27.Carter JM, Johnson BT, Patel A, Palacios E, Rodriguez KH. Lund-Mackay staging system in cystic fibrosis: a prognostic factor for revision surgery? Ochsner J. 2014;14(2):184-187. [PMC free article] [PubMed] [Google Scholar]
- 28.Zinreich SJ. Imaging for staging of rhinosinusitis. Ann Otol Rhinol Laryngol Suppl. 2004;193:19-23. doi: 10.1177/00034894041130S506 [DOI] [PubMed] [Google Scholar]
- 29.Sheikh SI, Handly B, Ryan-Wenger NA, et al. Novel computed tomography scoring system for sinus disease in adults with cystic fibrosis. Ann Otol Rhinol Laryngol. 2016;125(10):838-843. doi: 10.1177/0003489416656645 [DOI] [PubMed] [Google Scholar]
- 30.Hopkins C, Browne JP, Slack R, Lund V, Brown P. The Lund-Mackay staging system for chronic rhinosinusitis: how is it used and what does it predict? Otolaryngol Head Neck Surg. 2007;137(4):555-561. doi: 10.1016/j.otohns.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 31.Bhattacharyya N, Jones DT, Hill M, Shapiro NL. The diagnostic accuracy of computed tomography in pediatric chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg. 2004;130(9):1029-1032. doi: 10.1001/archotol.130.9.1029 [DOI] [PubMed] [Google Scholar]
- 32.Mangiafico SS. rcompanion, Version 2.4.30: functions to support extension education program evaluation. Accessed May 5, 2023. https://CRAN.R-project.org/package=rcompanion/
- 33.Ben-Shachar MS, Lüdecke D, Makowski D. effectsize: Estimation of effect size indices and standardized parameters. J Open Source Software. 2020;5(56):815. doi: 10.21105/joss.02815 [DOI] [Google Scholar]
- 34.Nair S. Correlation between symptoms and radiological findings in patients of chronic rhinosinusitis: a modified radiological typing system. Rhinology. 2009;47(2):181-186. [PubMed] [Google Scholar]
- 35.McMurphy AB, Morriss C, Roberts DB, Friedman EM. The usefulness of computed tomography scans in cystic fibrosis patients with chronic sinusitis. Am J Rhinol. 2007;21(6):706-710. doi: 10.2500/ajr.2007.21.3104 [DOI] [PubMed] [Google Scholar]
- 36.Nishioka GJ, Cook PR, McKinsey JP, Rodriguez FJ. Paranasal sinus computed tomography scan findings in patients with cystic fibrosis. Otolaryngol Head Neck Surg. 1996;114(3):394-399. doi: 10.1016/S0194-59989670208-5 [DOI] [PubMed] [Google Scholar]
- 37.Kim HJ, Friedman EM, Sulek M, Duncan NO, McCluggage C. Paranasal sinus development in chronic sinusitis, cystic fibrosis, and normal comparison population: a computerized tomography correlation study. Am J Rhinol. 1997;11(4):275-281. doi: 10.2500/105065897781446676 [DOI] [PubMed] [Google Scholar]
- 38.Sakano E, Ribeiro AF, Barth L, Condino Neto A, Ribeiro JD. Nasal and paranasal sinus endoscopy, computed tomography and microbiology of upper airways and the correlations with genotype and severity of cystic fibrosis. Int J Pediatr Otorhinolaryngol. 2007;71(1):41-50. doi: 10.1016/j.ijporl.2006.08.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Agreement Percentage and κ Values Among 3 Sinus CT Reviewers
eTable 2. Comparison of Clinical Outcomes 1 Year vs 1 Year of Receiving ETI and Number of Participants Receiving Therapies at the Start Compared With 1 Year of Receiving ETI
Data Sharing Statement