Key Points
Question
What are the efficacy and safety of durvalumab with concurrent curative radiotherapy followed by durvalumab for treatment of unresectable, locally advanced non–small cell lung cancer (NSCLC) without chemotherapy?
Findings
In this nonrandomized controlled trial of 35 patients with programmed cell death ligand 1–positive, unresectable, locally advanced NSCLC, the 12-month progression-free survival rate was 72.1%. Scheduled radiation therapy was completed in 97.1% of patients, and pneumonitis or radiation pneumonitis of grades 3 or 4 occurred in 11.8% of patients.
Meaning
Findings suggest that durvalumab plus concurrent radiotherapy for treatment of unresectable, locally advanced NSCLC showed promising efficacy with tolerable adverse events and warrants further investigation.
This single-arm, phase 2 nonrandomized controlled trial assesses the efficacy and safety of durvalumab with concurrent radiotherapy followed by durvalumab for treatment of patients with unresectable, locally advanced non–small cell lung cancer.
Abstract
Importance
Administration of durvalumab after concurrent chemoradiotherapy is the standard treatment of unresectable, locally advanced non–small cell lung cancer (NSCLC); however, 20% to 30% of patients do not receive durvalumab because of adverse events (AEs) during concurrent chemoradiotherapy. In addition, radiotherapy and immunotherapy have a synergistic effect.
Objective
To investigate the efficacy and safety of durvalumab immunotherapy plus concurrent radiotherapy followed by maintenance with durvalumab therapy for treatment of locally advanced NSCLC without chemotherapy.
Design, Setting, and Participants
The multicenter, single-arm DOLPHIN (Phase II Study of Durvalumab [MEDI4736] Plus Concurrent Radiation Therapy in Advanced Localized NSCLC Patients) nonrandomized controlled trial was performed by 12 institutions in Japan from September 13, 2019, to May 31, 2022. Participants in the primary registration phase included 74 patients with programmed cell death ligand 1 (PD-L1)-positive, unresectable, locally advanced NSCLC. The current analyses were conducted from June 1, 2022, to October 31, 2022.
Interventions
Patients received radiotherapy (60 Gy) in combination with concurrent and maintenance durvalumab immunotherapy, 10 mg/kg every 2 weeks, for up to 1 year.
Main Outcomes and Measures
The primary end point of the rate of 12-month progression-free survival (PFS), as assessed by an independent central review, was estimated using the Kaplan-Meier method and evaluated with 90% CIs calculated using the Greenwood formula. The key secondary end points were PFS, objective response rate, treatment completion rate, and AEs.
Results
Data from 35 patients (median [range] age, 72 [44–83] years; 31 [88.6%] men) were included in the full analysis set of the evaluable population. The 12-month PFS rate was 72.1% (90% CI, 59.1%-85.1%), and the median PFS was 25.6 months (95% CI, 13.1 months to not estimable) at a median follow-up of 22.8 months (range, 4.3-31.8 months). Scheduled radiation therapy was completed in 97.1% of patients. The confirmed objective response rate was 90.9% (95% CI, 75.7%-98.1%), and the treatment completion rate was 57.6% (95% CI, 39.2%-74.5%). Among 34 patients evaluated in the safety analysis set, AEs of grade 3 or 4 occurred in 18 patients (52.9%), and of grade 5 in 2 patients (5.9%). Pneumonitis or radiation pneumonitis of any grade occurred in 23 patients (67.6%), and of grades 3 or 4 in 4 patients (11.8%).
Conclusions and Relevance
Findings from this phase 2 nonrandomized controlled trial indicate that durvalumab immunotherapy combined with curative radiotherapy for patients with PD-L1–positive, unresectable, locally advanced NSCLC is a promising treatment with tolerable AEs and is appropriate as a study treatment for phase 3 clinical trials.
Trial Registration
Japan Registry of Clinical Trials ID: jRCT2080224763
Introduction
Lung cancer is the leading cause of cancer-related death worldwide.1 Non–small cell lung cancer (NSCLC) accounts for 76% of lung cancers, and one-third of NSCLCs are locally advanced.2,3 Unresectable, locally advanced NSCLC is considered potentially curable, but the 10-year progression-free survival (PFS) rate of patients receiving concurrent chemoradiotherapy (CRT) is only approximately 10%.4 Treatment with durvalumab, an anti–programmed cell death ligand 1 (PD-L1) antibody, after concurrent CRT improves survival in patients with unresectable, locally advanced NSCLC and became the standard treatment after the PACIFIC trial.5 However, some patients are not candidates for concurrent CRT, and 20% to 30% of patients cannot receive durvalumab due to adverse events (AEs) or poor performance status following concurrent CRT or pre-existing comorbidities.6,7 In particular, neutropenia (≥grade 3) develops in 18% to 53% of cases, febrile neutropenia in 3% to 8%, with concurrent CRT.8,9 Radiotherapy often has to be interrupted due to these AEs. New studies are needed to fulfill this enduring unmet need.
Exploratory analyses of the PACIFIC trial results indicated that administration of durvalumab immediately after completion of concurrent CRT (≤14 days) provided increased benefit in PFS compared with placebo.5 In addition, translational studies have shown that radiation enhances the antitumor immune response and that the combination of immunotherapy and radiation has a synergistic effect.10,11 Moreover, immunotherapy plays an important role in PD-L1–positive advanced NSCLC and may be able to replace platinum-doublet chemotherapy for such patients.12,13,14
Therefore, we conducted a multicenter, single-arm, phase 2 nonrandomized clinical study to investigate the efficacy and safety of durvalumab plus concurrent curative RT for PD-L1–positive, locally advanced NSCLC without chemotherapy.
Methods
Study Design and Patients
The DOLPHIN (Phase II Study of Durvalumab (MEDI4736) Plus Concurrent Radiation Therapy in Advanced Localized NSCLC Patients) phase 2 nonrandomized clinical study was conducted in 12 institutions in Japan from September 13, 2019, to May 31, 2022. The study protocol and all amendments were reviewed and approved by the Kobe University Hospital review board and the institutional review boards at each study site. The study was conducted according to the Declaration of Helsinki,15 the Council for International Organizations of Medical Sciences International Ethical Guidelines, and local laws and regulations of Japan. Written informed consent was obtained from all patients. The current analyses were conducted from June 1, 2022, to October 31, 2022. The Trial Protocol is provided in Supplement 1. This study followed the Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guideline.
This study had 2 stages of registration (eFigure 1 in Supplement 2). The main eligibility criteria for the primary registration included the following: age 20 years or older; Eastern Cooperative Oncology Group Performance Status Scale score of 0 or 1 (on scale of 0 to 5, with 0 and 1 indicating fully active and restricted but ambulatory, respectively, and 5 indicating dead); histologic evidence of NSCLC; suspected unresectable stage III disease or postoperative recurrent disease that was curable by radiation; adequate organ function, including hematologic, lung, hepatic, and kidney function; and written informed consent. The key eligibility criteria for the secondary registration included the following: confirmed unresectable stage IIIA-C NSCLC, defined using the Union for International Cancer Control TNM Classification of Malignant Tumours, 8th edition,16 or postoperative recurrent disease that was curable by a radiation protocol; and PD-L1 tumor proportion score (TPS) of 1% or higher (on a scale of 1% to 100%, indicating the percentage of viable tumor cells on staining) using the SP263 antibody (eTable 1 in Supplement 2). For immunohistochemistry of PD-L1, archived tumor specimens collected before enrollment were obtained. Formalin-fixed, paraffin-embedded tumor specimens were cut into 4-μm-thick sections. The expression of PD-L1 was assessed using the Ventana antibody clone SP263 immunohistochemical assay (specific antibody concentration, 1.61 mg/mL; Roche Diagnostics, Ventana Medical Systems). The PD-L1 TPS is reported as the percentage of positive tumor cells as scored by a central pathologist.
Outcomes
The primary end point was the 12-month PFS rate assessed by independent central review (ICR). The secondary end points were PFS, overall survival, objective response rate, disease control rate, time to death or distant metastasis, treatment completion rate, and AEs. Treatment completion was defined as completion of RT (60 Gy) and administration of durvalumab for 12 months.
Study Procedures
Patients received durvalumab, 10 mg/kg, every 2 weeks for up to 12 months until evidence of progressive disease or unacceptable toxic effects, whichever came first. Radiation was started on day 1 of durvalumab treatment. Planned radiation was performed by 3-dimensional conformal RT or intensity-modulated RT, either of which could be selected for each patient. Patients were scheduled to receive 60 Gy in 30 fractions 5 days per week (prescribed to the dose that covered 95% of the planning target volume) administered to involved fields without elective nodal irradiation.
Objective tumor assessments were performed by ICR and investigators (M.T., H.H., Y. Sato, T.K., S.S., Y. Shiraishi, S.T., K.A., H.D., M.Y., T. Kodaira, and M. Satouchi) in accordance with Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The assessments were conducted at baseline and every 8 weeks until progressive disease. Safety variables included symptoms; vital signs, physical examinations; clinical laboratory parameters; and the incidence, timing, and severity of AEs. The AEs were graded using the Common Terminology Criteria for Adverse Events version 5.0. An independent external data safety monitoring committee reviewed the progress of the study treatment, radiation treatment delivered, and efficacy and safety data throughout the study. All data were collected by an electronic data capture system. For quality control and assurance of RT, compliance with protocol-specified RT planning was reviewed for all patients after the completion of RT. We collected digital images and data from the following: pretreatment chest radiography, computed tomography, and positron emission tomography–computed tomography; full Digital Imaging and Communications in Medicine format data set (structure, dose, and plan) of the RT plan; RT planning reports from the treatment planning system; and dose-volume histogram data and RT medical records.
Early analysis of efficacy was planned to examine whether the study could be continued when 20 patients had completed the combination therapy of RT and durvalumab during the RT period. If 6 or more patients (30%) had progressive disease as assessed by the investigator (M.T., H.H., Y. Sato, T.K., S.S., Y. Shiraishi, S.T., K.A., H.D., M.Y., T. Kodaira, and M. Satouchi), the study would be terminated early.
Statistical Analysis
We set the 12-month PFS rate from secondary registration as the primary end point. In the PACIFIC trial, the 12-month PFS rates were 35.3% (95% CI, 29.0%-41.7%) in the placebo group and 55.9% (95% CI, 51.0%-60.4%) in the durvalumab group from registration after concurrent CRT.5 Although the starting point of PFS was different, considering the possibility that 20% to 30% of patients could not receive durvalumab maintenance therapy after concurrent CRT, the 12-month PFS rate under the null hypothesis was set at 28% and the alternative hypothesis was set at 50%. The number of patients needed to provide 80% power for a 1-sided 0.05 level of type I error was calculated to be 32. Considering potential ineligible patients after enrollment, the sample size was set at 35. We defined PFS as the shortest time from the date of secondary registration to either the date of all deaths, the date of imaging progression, or the date of clinical progression. Patients who had not died or showed either clinical or imaging progression at the time of analysis, or for whom it was unclear when these events occurred, were censored at the date of the last visit before becoming unavailable for follow-up. Overall survival was defined as the period from the date of secondary enrollment to the date of all-cause mortality. Patients alive or untraceable at the time of analysis were censored at the date of last known survival. The 12-month PFS rate as the primary end point was estimated using the Kaplan-Meier method and was evaluated with the 90% CI calculated using the Greenwood formula. We analyzed PFS and overall survival using the Kaplan-Meier method to estimate median survival intervals, with 95% CIs calculated using the Brookmeyer and Crowley method. For secondary end points, all analyses were 2-sided, and P < .05 was considered statistically significant. The preplanned exploratory PFS subgroup analyses were performed based on age, sex, smoking status, pathology, clinical stage, best overall response, and PD-L1 TPS. Some of those factors are commonly used in cancer trials (ie, age, sex, histopathology, stage, performance status) and were reported to influence PFS in previous immunotherapy trials (response, PD-L1 TPS).13,14 If a factor could be expressed numerically, the smaller of the 2 factors was used as the reference. A Cox proportional hazards model was used to estimate the hazard ratio and 95% CI, with statistical significance defined as a 95% CI excluding 1. The data cutoff date was September 16, 2022. All analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Patient Characteristics
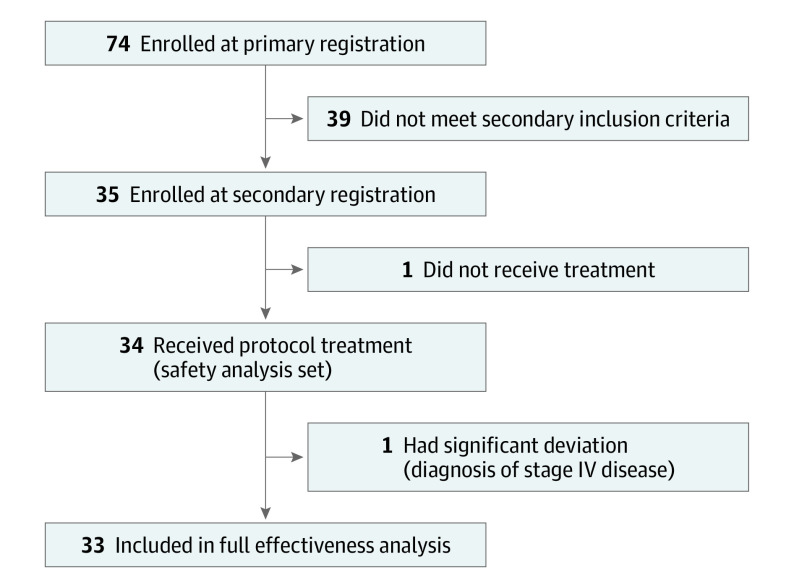
Between September 13, 2019, and November 9, 2020, 74 patients were registered at the primary registration, and 35 patients were ultimately enrolled at the secondary registration (Figure 1). One patient did not receive the protocol treatment due to an inability to undergo the radiation protocol, and 1 patient was excluded from the efficacy analysis after receiving a diagnosis of stage IV disease. Therefore, data were analyzed from 33 patients for efficacy and from 34 patients for safety. At the time of data cutoff, all patients had completed study treatment, and their median follow-up time was 22.8 months (range, 4.3-31.8 months).
Figure 1. Patient Flow Diagram.
The baseline characteristics of the 35 patients are described in Table 1. Most of the patients were men (31 men, 88.6%; 4 women [11.4%]) and current or former smokers (34 [97.1%]). The median patient age was 72 years (range, 44-83 years), and more than half of the patients had adenocarcinoma (19 [54.3%]). Most patients (26 [74.3%]) had unresectable, stage IIIA-IIIC disease, and 9 patients (25.7%) had postoperative recurrence. More than half of the patients (19 [54.3%]) had a performance status score of 0, and the median PD-L1 TPS was 60% (range, 1%-100%).
Table 1. Patient Characteristics.
| Characteristic | Participants, No. (%) |
|---|---|
| Age, median (range), y | 72 (44-83) |
| Sex, No. (%) | |
| Female | 4 (11.4) |
| Male | 31 (88.6) |
| Smoking history, No. (%) | |
| Never | 1 (2.9) |
| Former | 16 (45.7) |
| Current | 18 (51.4) |
| Pathology, No. (%) | |
| Adenocarcinoma | 19 (54.3) |
| Squamous cell carcinoma | 15 (42.9) |
| NOS | 1 (2.9) |
| Stage, No. (%) | |
| Postoperative recurrence | 9 (25.7) |
| IIIA | 16 (45.7) |
| IIIB | 7 (20.0) |
| IIIC | 3 (8.6) |
| ECOG performance status score, No. (%)a | |
| 0 | 19 (54.3) |
| 1 | 16 (45.7) |
| PD-L1 TPS, median (range)b | 60 (1-100) |
| Radiotherapy, No. (%) | |
| 3D-CRT | 24 (70.6) |
| IMRT | 11(29.4) |
Abbreviations: 3D-CRT, 3-dimensional conformal radiotherapy; ECOG, Eastern Cooperative Oncology Group; IMRT, intensity-modulated radiotherapy; NOS, not otherwise specified; PD-L1, programmed cell death ligand 1; TPS, tumor proportion score.
Scored on scale of 0 to 5, with 0 and 1 indicating fully active and restricted but ambulatory, respectively, and 5 indicating dead.
Scored on a scale of 1% to 100%, indicating the percentage of viable tumor cells on staining.
Efficacy
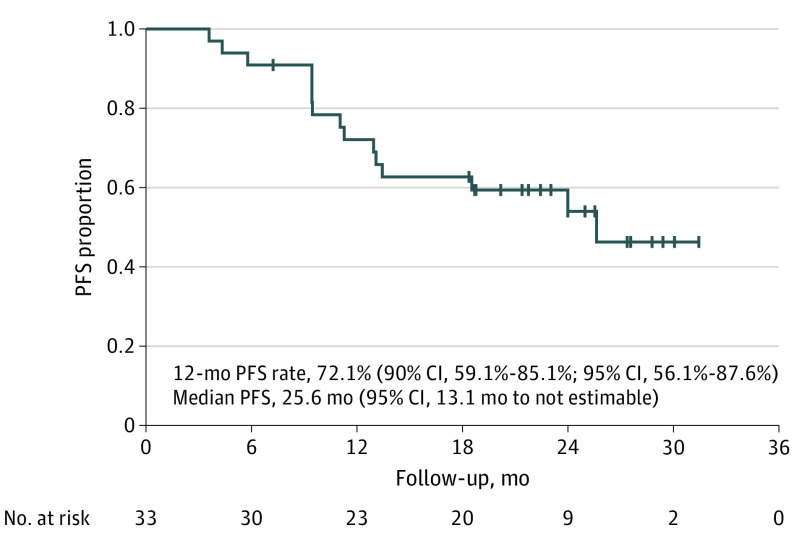
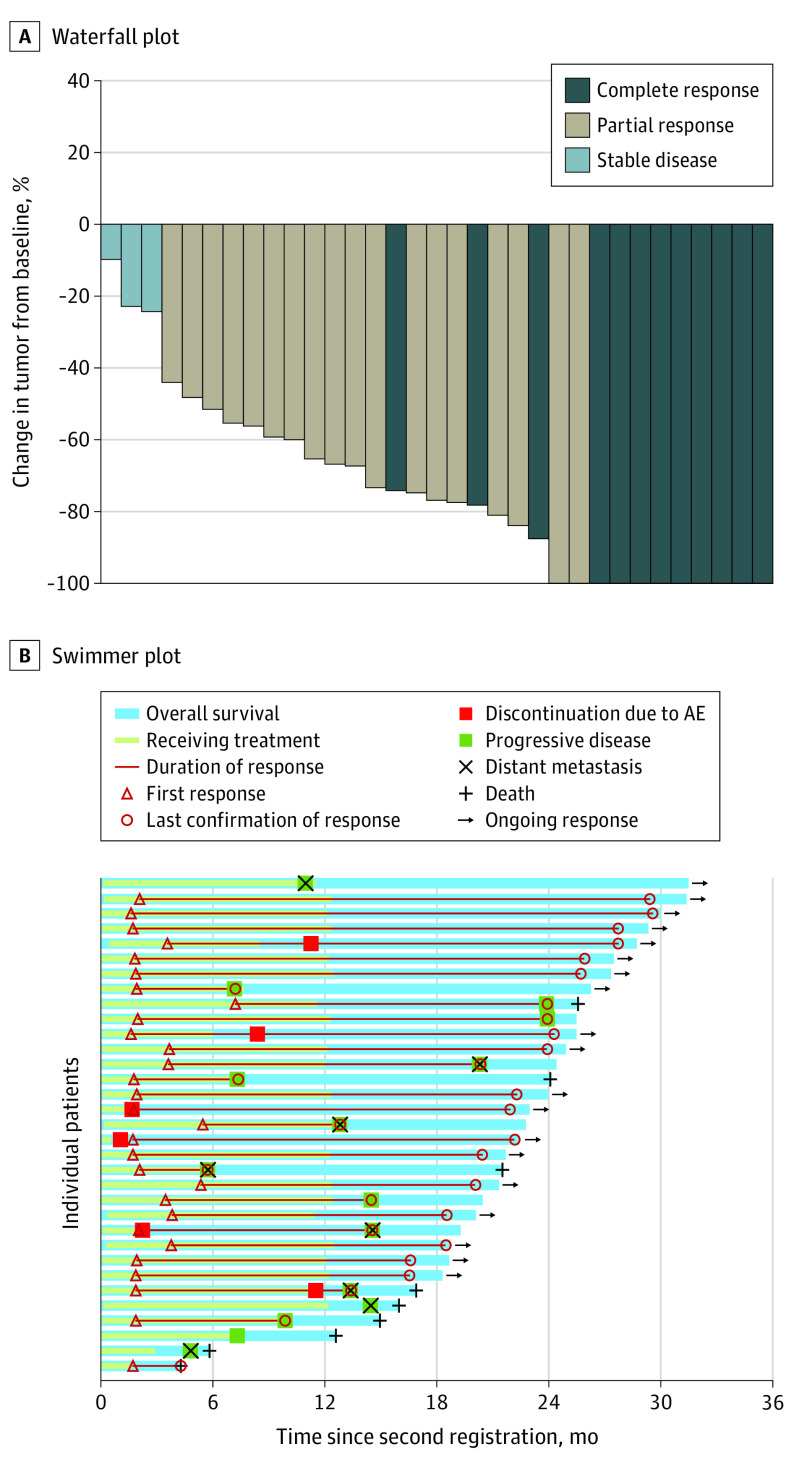
The primary end point of the 12-month PFS rate was 72.1% (90% CI, 59.1%-85.1%) (Figure 2). The median PFS was 25.6 months (95% CI, 13.1 months to not estimable) by ICR, and the median overall survival was not reached (95% CI, 25.6 months to not estimable). The confirmed objective response rate by ICR was 90.9% (95% CI, 75.5%-98.1%) (eTable 2 in Supplement 2). Among 33 patients, 11 (33.3%) had a complete response, and 19 (57.6%) had a partial response as assessed by ICR. Five patients with a complete response (45.5%) had postoperative recurrences. None of the patients had progressive disease. A waterfall plot indicated deep response, and a swimmer plot showed a short time to onset of response and a long duration of response (Figure 3). Of 33 patients, 17 (51.5%) had had no recurrence at the data cutoff date.
Figure 2. Progression-Free Survival (PFS) Since Second Registration by Independent Central Review.
Median follow-up was 22.8 months; range, 4.3-31.8 months.
Figure 3. Waterfall Plot and Swimmer Plot for Individual Patients in the Full Analysis Set.
Median follow-up was 22.8 months (range, 4.3-31.8 months). AE indicates adverse event.
In the preplanned exploratory subgroup analyses for PFS, patients with a complete response or a partial response had better PFS (hazard ratio, 10.4; 95% CI, 2.4-44.4) (eFigure 2 in Supplement 2). Younger patients (<75 years) and patients with postoperative recurrence had better PFS. With regard to PD-L1 expression, there was no difference in PFS between patients with a PD-L1 TPS lower or higher than 25%, but patients with a PD-L1 TPS higher than 50% had better PFS than those with a PD-L1 TPS lower than 50%.
Safety
A summary of the safety analysis is given in Table 2. Adverse events of any grade occurred in 34 of 34 patients (100%). Adverse events of all grades occurring in at least 10% of patients are given in eTable 3 in Supplement 2. Grade 3 or 4 AEs occurred in 18 patients (52.9%), and grade 5 AEs occurred in 2 patients (5.9%). Grade 3 or 4 AEs were decreased lymphocyte count (5 patients [14.7%]), pneumonitis (4 patients [11.8%]), lung infection (3 patients [8.8%]), hyperglycemia (3 patients [8.8%]) and increased aspartate aminotransferase levels (2 patients [5.9%]). Thrombocytopenia and increased amylase and lipase levels occurred in 1 patient for each AE (2.9%). One patient developed grade 5 lung infection due to steroid therapy for thrombocytopenia, an immune-related AE (irAE), and another patient developed grade 5 bronchoesophageal fistula at the site of irradiation due to disease progression during follow-up. The treatment completion rate of the protocol was 57.6% (95% CI, 39.2%-74.5%). Adverse events leading to discontinuation of protocol treatment occurred in 7 patients (20.6%), and RT was completed in all but 1 patient (97.1%).
Table 2. Safety Summary.
| AE | Participants, No. (%) |
|---|---|
| Any grade AEs | 34 (100) |
| Grade 3 or 4 | 18 (52.9) |
| Grade 5 | 2 (5.9) |
| Leading to discontinuation of protocol treatment | 6 (17.6) |
| Leading to discontinuation of durvalumab | 7 (20.6) |
| Leading to discontinuation of radiotherapy | 1 (2.9) |
| Any grade study drug–related AE | 31 (91.2) |
| Grade 3 or 4 | 10 (29.4) |
| Grade 5 | 1 (2.9) |
| AEs of special interest | 25 (73.5) |
| Grade 3 or 4 | 6 (17.6) |
| Grade 5 | 0 |
| Corticosteroid required | 7 (20.6) |
| Pneumonitis or radiation pneumonitis | 23 (67.6) |
| Grade 3 or 4 | 4 (11.8) |
| Grade 5 | 0 |
| Leading to discontinuation of durvalumab | 3 (8.8) |
| Leading to discontinuation of radiotherapy | 1 (2.9) |
Abbreviation: AE, adverse event.
Adverse events of special interest (AESIs), or irAEs due to durvalumab, occurred in 25 patients (73.5%), and AESIs requiring corticosteroid treatment were observed in 7 patients (20.6%). Those included 4 cases of pneumonitis and 1 case each of thrombocytopenia, pneumonia, and rheumatoid arthritis. Pneumonitis or radiation pneumonitis of any grade occurred in 23 patients (67.6%), and grade 3 or 4 pneumonitis or radiation pneumonitis was observed in 4 patients (11.8%). No patient had grade 5 pneumonitis, and pneumonitis peaked 8 to 12 weeks after initiation of treatment.
Discussion
The DOLPHIN phase 2 nonrandomized controlled trial was the first, to our knowledge, to show the efficacy and safety of immunotherapy combined with curative RT for treatment of patients with PD-L1–positive, unresectable, locally advanced NSCLC with good performance status. This treatment strategy may help 20% to 30% of patients who cannot receive durvalumab after concurrent CRT. Although the concurrent immunotherapy and RT (ie, immunoradiotherapy) used in this study may resolve an unmet need with an additional clinical benefit, this protocol was not designed for patients who did not meet the criteria for platinum-doublet chemotherapy or for patients with poor performance status scores.
The 12-month PFS rate for patients recruited in the secondary registration was 72.1%, and the lower limit of the 90% CI was 59.1%. Therefore, the 12-month PFS rate not only far exceeded the threshold set under the null hypothesis of 28% but also exceeded the expected value for the alternative hypothesis of 50%. In the PACIFIC trial, the 12-month PFS rate after concurrent CRT was 55.9%.5 The 12-month PFS rate in our study compares favorably with that of the PACIFIC trial, although the definition of PFS and patient characteristics were different. DOLPHIN included more older patients (≥75 years) and patients with postoperative recurrence compared with the PACIFIC trial. In other previous phase 3 studies assessing concurrent CRT, the 12-month PFS was 49.2% in the NRG Oncology RTOG 0617 trial17 and approximately 40% in PROCLAIM.8 Compared with those data, the 12-month PFS in the present study was much higher. The favorable efficacy obtained despite the absence of chemotherapy may be because of the simultaneous administration of immunotherapy and RT. This hypothesis is supported by findings from preclinical studies. For instance, in tumor-bearing mice, concurrent administration of a PD-L1 inhibitor and radiation increased immune activation compared with other treatment timing.11 Concurrent immunoradiotherapy is expected to have a synergistic effect.18
Despite the lack of chemotherapy, this protocol treatment had a high and deep response and a short time to response. Thirty patients (90.9%) achieved complete or partial response at the first efficacy assessment after 2 months of treatment. The reason for the high complete response rate is thought to be that patients with postoperative recurrence have multiple mediastinal lymph node metastases and are more likely to be judged as having achieved complete response based on RECIST. That there were no cases of progressive disease in the concurrent radiation phase is indicative of a synergistic effect of immunoradiotherapy.
Subgroup analysis indicated that younger patients (<75 years), patients with partial or complete response, and patients with postoperative recurrence had better PFS than patients without these characteristics. Postoperative recurrence often presents with mediastinal lymph node metastases, which indicates low tumor volume. Radiotherapy is typically more likely to be effective when tumor volume is low. In addition, baseline tumor size has emerged as an independent negative prognostic factor for the survival of patients treated with immune checkpoint inhibitor monotherapy.19 It is easy to speculate that immunoradiotherapy is likely to have a greater effect when the tumor volume is low. The PFS benefit was observed irrespective of PD-L1 TPS at a cutoff of 25% in the present study as well as in the PACIFIC trial.5 However, when a cutoff of 50% or higher was used for the PD-L1 TPS, patients with a higher TPS had better PFS than those with a lower TPS.
With regard to safety, AEs of grade 3 or 4 were more common in the present study (52.9%) than in the PACIFIC trial (29.9%).5 The most common AE in the present study was decreased lymphocyte count (14.7%). Decreased lymphocyte count also occurs with RT. Atagi et al20 reported 53% lymphocyte depletion with RT alone for locally advanced NSCLC, most of which was grade 1 or 2. However, the possibility of a higher grade of lymphocyte depletion with RT in combination with immunotherapy cannot be excluded. Most of the remaining AEs were pneumonitis and lung infection. The frequency of grade 3 pneumonitis or radiation pneumonitis (11.8%) was higher than that in the PACIFIC trial (3.4%).5 The PACIFIC trial excluded patients with grade 2 or higher pneumonitis after concurrent CRT, which may explain the differences in the rates of severe pneumonitis between that study and DOLPHIN. In addition, in the PACIFIC study, the incidence of any grade and grade 2 or higher of pneumonitis for Japanese vs all patients was 73.6% vs 33.9%, and 30.6% vs 19.9%, respectively. This finding suggests that the incidence of pneumonitis may be higher among Japanese patients. In the present study, pneumonitis peaked 8 to 12 weeks after initiation of treatment, which may be earlier than that in the PACIFIC trial. It is possible that concurrent immunoradiotherapy may increase severe lung toxic effects and may hasten the onset of pneumonitis. The frequencies of grade 5 AEs and those leading to discontinuation of durvalumab in the present study were equivalent to those in the PACIFIC trial. One patient developed grade 5 bronchoesophageal fistula at the site of irradiation during follow-up in the present study. Acquired bronchoesophageal fistula is a known complication of radiation for lung cancer and esophageal cancer. This patient had tumor recurrence at the site of irradiation, and it was thought that the fistula formed for both reasons. However, the possibility that immunotherapy may have enhanced the effects of RT in this regard cannot be completely ruled out, and attention should be paid to the occurrence of fistula during large clinical trials assessing immunoradiotherapy.
The scheduled RT was completed in all but 1 patient (97.1%). In previous reports,8,9 5.7% to 8.8% of patients receiving concurrent CRT did not complete RT (60 Gy). Due to the small sample size in the present study, it is not possible to say definitively, but it is likely that the completion rate was at least as high as or higher than the completion rate for concurrent CRT. The completion of RT is important in the treatment of unresectable, locally advanced NSCLC, and this immunoradiotherapy protocol was shown to be tolerated in this study.
The percentage of AESIs or irAEs that required treatment with glucocorticoids was 15.2% in the PACIFIC trial and 20.6% in DOLPHIN. Therefore, immunoradiotherapy did not enhance the severity of irAEs. No unexpected AEs or increased toxic effects were observed. These data suggest that the use of concurrent durvalumab treatment with RT has manageable AEs.
Several treatment options have been examined to decrease disease progression. There are 2 major strategies: concurrent CRT plus immunotherapy, and concurrent CRT followed by new immunotherapy. Several phase 1 and 2 clinical trials21,22,23 have examined combined immunotherapy and concurrent CRT. In the NICOLAS study, which was a single-arm phase 2 trial of concurrent CRT and the anti–PD-1 antibody nivolumab, grade 3 or higher pneumonitis occurred in 11.7% of patients.21 The treatment success rate of their protocol was 39.2%, and 43.5% of patients discontinued treatment due to AEs. The treatment completion rate of the present protocol was 57.6%. That the regimen in the present study did not include concurrent chemotherapy may have led to this higher treatment completion rate. The 1-year PFS (53.7%) and median PFS (12.7 months) in the NICOLAS trial were lower than expected.21 Compared with those trials, the present study had a high completion rate of 57.6% for protocol treatment and a low discontinuation rate due to an AE rate of 21.2%, and it led to a good effectiveness. Other phase 1 and 2 studies showed similar results.24,25 This treatment strategy may be compelling, but there are some concerns. Chemotherapy causes bone marrow suppression and organ damage. In addition, RT disruption occurs in some patients because of these AEs. Moreover, we must consider that chemotherapeutic agents and their premedications, such as glucocorticoids, can affect the immune system. A phase 3 study to evaluate the efficacy and safety of concurrent CRT plus concurrent durvalumab compared with conventional concurrent CRT is ongoing (PACIFIC-2).26 Even if the PACIFIC-2 regimen results in better outcomes and feasibility, it will not answer whether concurrent CRT plus concurrent treatment with an immune checkpoint inhibitor, or concurrent CRT followed by treatment with an immune checkpoint inhibitor is better.
The COAST study (Durvalumab Alone or in Combination With Novel Agents in Locally Advanced, Unresectable, Stage III NSCLC)27 demonstrated improved clinical outcomes by combining immunomodulation (the anti-CD73 monoclonal antibody oleclumab or the anti-NKG2A monoclonal antibody monalizumab) with durvalumab compared with durvalumab alone. Combining immune checkpoint inhibitors with new immunomodulation therapy is an expected therapeutic option, but it can be administered only to patients who can complete concurrent CRT.
Limitations
This study has limitations. First, it was a single-arm study with a small sample size. Second, many of the patients experienced postoperative recurrence, and the inclusion of these patients may have contributed to the high rate of response. However, in clinical practice, this population is treated similarly to patients with unresectable stage III NSCLC, accounting for 8% of cases with concurrent CRT followed by treatment with durvalumab.7 Third, there was a lack of long-term follow-up. Our median follow-up was approximately 2 years. A 5-year PFS would be a better end point to determine whether the disease had been cured. We plan to continue to follow-up the participants in this study and determine the 5-year PFS rate. Despite these limitations, this trial is the first prospective study, to our knowledge, assessing unresectable, locally advanced NSCLC, and the PACIFIC and ongoing PACIFIC-2 trials were not designed to confirm our findings.
Conclusions
The DOLPHIN phase 2 nonrandomized controlled trial suggests the efficacy and safety of definitive RT in combination with concurrent and maintenance durvalumab therapy in patients with PD-L1–positive, unresectable, locally advanced NSCLC. This treatment strategy is promising with tolerable AEs and warrants further investigation.
Trial Protocol
eTable 1. Inclusion and Key Exclusion Criteria
eTable 2. Antitumor Efficacy
eTable 3. Adverse Events of Any Cause in ≥10% of Patients
eFigure 1. Study Schema
eFigure 2. Progression-Free Survival Subgroup Analyses by Characteristics
Data Sharing Statement
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute Surveillance, Epidemiology and End Results Program. SEER cancer statistics review (CRS) 1975-2016. Updated April 9, 2020. Accessed July 14, 2023. (https://seer.cancer.gov/csr/1975_2016/.
- 3.Goldstraw P, Chansky K, Crowley J, et al. ; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions . The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51. doi: 10.1016/j.jtho.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 4.Zenke Y, Tsuboi M, Chiba Y, et al. Effect of second-generation vs third-generation chemotherapy regimens with thoracic radiotherapy on unresectable stage III non–small-cell lung cancer: 10-year follow-up of a WJTOG0105 phase 3 randomized clinical trial. JAMA Oncol. 2021;7(6):904-909. doi: 10.1001/jamaoncol.2021.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 6.Lau SCM, Ryan M, Weiss J, et al. Concurrent chemoradiation with or without durvalumab in elderly patients with unresectable stage III NSCLC: safety and efficacy. JTO Clin Res Rep. 2021;2(12):100251. doi: 10.1016/j.jtocrr.2021.100251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito G, Oya Y, Taniguchi Y, et al. Real-world survey of pneumonitis and its impact on durvalumab consolidation therapy in patients with non–small cell lung cancer who received chemoradiotherapy after durvalumab approval (HOPE-005/CRIMSON). Lung Cancer. 2021;161:86-93. doi: 10.1016/j.lungcan.2021.08.019 [DOI] [PubMed] [Google Scholar]
- 8.Senan S, Brade A, Wang LH, et al. PROCLAIM: randomized phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non–small-cell lung cancer. J Clin Oncol. 2016;34(9):953-962. doi: 10.1200/JCO.2015.64.8824 [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto N, Nakagawa K, Nishimura Y, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non–small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol. 2010;28(23):3739-3745. doi: 10.1200/JCO.2009.24.5050 [DOI] [PubMed] [Google Scholar]
- 10.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687-695. doi: 10.1172/JCI67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74(19):5458-5468. doi: 10.1158/0008-5472.CAN-14-1258 [DOI] [PubMed] [Google Scholar]
- 12.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1-positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 13.Mok TSK, Wu YL, Kudaba I, et al. ; KEYNOTE-042 Investigators . Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non–small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830. doi: 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 14.Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328-1339. doi: 10.1056/NEJMoa1917346 [DOI] [PubMed] [Google Scholar]
- 15.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Union for International Cancer Control. In: Brierley JD, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours. 8th ed. Wiley-Blackwell; 2017. [Google Scholar]
- 17.Bradley JD, Hu C, Komaki RR, et al. Long-term results of NRG Oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable stage III non–small-cell lung cancer. J Clin Oncol. 2020;38(7):706-714. doi: 10.1200/JCO.19.01162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theelen WSME, Chen D, Verma V, et al. Pembrolizumab with or without radiotherapy for metastatic non–small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Respir Med. 2021;9(5):467-475. doi: 10.1016/S2213-2600(20)30391-X [DOI] [PubMed] [Google Scholar]
- 19.Katsurada M, Nagano T, Tachihara M, et al. Baseline tumor size as a predictive and prognostic factor of immune checkpoint inhibitor therapy for non–small cell lung cancer. Anticancer Res. 2019;39(2):815-825. doi: 10.21873/anticanres.13180 [DOI] [PubMed] [Google Scholar]
- 20.Atagi S, Kawahara M, Yokoyama A, et al. ; Japan Clinical Oncology Group Lung Cancer Study Group . Thoracic radiotherapy with or without daily low-dose carboplatin in elderly patients with non–small-cell lung cancer: a randomised, controlled, phase 3 trial by the Japan Clinical Oncology Group (JCOG0301). Lancet Oncol. 2012;13(7):671-678. doi: 10.1016/S1470-2045(12)70139-0 [DOI] [PubMed] [Google Scholar]
- 21.Peters S, Felip E, Dafni U, et al. Progression-free and overall survival for concurrent nivolumab with standard concurrent chemoradiotherapy in locally advanced stage IIIA-B NSCLC: results from the European Thoracic Oncology Platform NICOLAS Phase II Trial (European Thoracic Oncology Platform 6-14). J Thorac Oncol. 2021;16(2):278-288. doi: 10.1016/j.jtho.2020.10.129 [DOI] [PubMed] [Google Scholar]
- 22.Lin SH, Lin Y, Yao L, et al. Phase II trial of concurrent atezolizumab with chemoradiation for unresectable NSCLC. J Thorac Oncol. 2020;15(2):248-257. doi: 10.1016/j.jtho.2019.10.024 [DOI] [PubMed] [Google Scholar]
- 23.Jabbour SK, Berman AT, Decker RH, et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non–small cell lung cancer: a nonrandomized controlled trial. JAMA Oncol. 2020;6(6):848-855. doi: 10.1001/jamaoncol.2019.6731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin SH, Lin Y, Yao L, et al. Phase II trial of concurrent atezolizumab with chemoradiation for unresectable NSCLC. J Thorac Oncol. 2020;15(2):248–257. doi: 10.1016/j.jtho.2019.10.024 [DOI] [PubMed] [Google Scholar]
- 25.Jabbour SK, Berman AT, Decker RH, et al. Phase 1 trial of pembrolizumab administered concurrently with chemoradiotherapy for locally advanced non–small cell lung cancer: a nonrandomized controlled trial. JAMA Oncol. 2020;6(6):848–855. doi: 10.1001/jamaoncol.2019.6731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradley JD, Nishio M, Okamoto I, et al. PACIFIC-2: phase 3 study of concurrent durvalumab and platinum-based chemoradiotherapy in patients with unresectable, stage III NSCLC. J Clin Oncol. 2019;37(15):suppl. doi: 10.1200/JCO.2019.37.15_suppl.TPS8573 [DOI] [Google Scholar]
- 27.Herbst RS, Majem M, Barlesi F, et al. COAST: an open-label, phase II, multidrug platform study of durvalumab alone or in combination with oleclumab or monalizumab in patients with unresectable, stage III non–small-cell lung cancer. J Clin Oncol. 2022;40(29):3383-3393. doi: 10.1200/JCO.22.00227 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Inclusion and Key Exclusion Criteria
eTable 2. Antitumor Efficacy
eTable 3. Adverse Events of Any Cause in ≥10% of Patients
eFigure 1. Study Schema
eFigure 2. Progression-Free Survival Subgroup Analyses by Characteristics
Data Sharing Statement