This systematic analysis assesses the total and risk-attributable burden of lip and oral cavity cancer and other pharyngeal cancer for 204 countries and territories and by Socio-demographic Index using 2019 Global Burden of Diseases, Injuries, and Risk Factors Study estimates.
Key Points
Question
What was the burden of lip and oral cavity cancer (LOC) and other pharyngeal cancer (OPC) globally, regionally, and across Socio-demographic Index (SDI) strata between 1990 and 2019?
Findings
In this systematic analysis of estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study 2019, the global age-standardized mortality rate due to LOC and OPC in 2019 was 3.8 and 2.2 deaths per 100 000, respectively, and the age-standardized incidence rate was 7.1 and 3.2 new cases per 100 000, respectively. Low-middle and low SDI regions consistently showed the highest age-standardized mortality rates from 1990 to 2019.
Meanings
Tackling the inequities across SDI strata should be a priority to global LOC and OPC control efforts.
Abstract
Importance
Lip, oral, and pharyngeal cancers are important contributors to cancer burden worldwide, and a comprehensive evaluation of their burden globally, regionally, and nationally is crucial for effective policy planning.
Objective
To analyze the total and risk-attributable burden of lip and oral cavity cancer (LOC) and other pharyngeal cancer (OPC) for 204 countries and territories and by Socio-demographic Index (SDI) using 2019 Global Burden of Diseases, Injuries, and Risk Factors (GBD) Study estimates.
Evidence Review
The incidence, mortality, and disability-adjusted life years (DALYs) due to LOC and OPC from 1990 to 2019 were estimated using GBD 2019 methods. The GBD 2019 comparative risk assessment framework was used to estimate the proportion of deaths and DALYs for LOC and OPC attributable to smoking, tobacco, and alcohol consumption in 2019.
Findings
In 2019, 370 000 (95% uncertainty interval [UI], 338 000-401 000) cases and 199 000 (95% UI, 181 000-217 000) deaths for LOC and 167 000 (95% UI, 153 000-180 000) cases and 114 000 (95% UI, 103 000-126 000) deaths for OPC were estimated to occur globally, contributing 5.5 million (95% UI, 5.0-6.0 million) and 3.2 million (95% UI, 2.9-3.6 million) DALYs, respectively. From 1990 to 2019, low-middle and low SDI regions consistently showed the highest age-standardized mortality rates due to LOC and OPC, while the high SDI strata exhibited age-standardized incidence rates decreasing for LOC and increasing for OPC. Globally in 2019, smoking had the greatest contribution to risk-attributable OPC deaths for both sexes (55.8% [95% UI, 49.2%-62.0%] of all OPC deaths in male individuals and 17.4% [95% UI, 13.8%-21.2%] of all OPC deaths in female individuals). Smoking and alcohol both contributed to substantial LOC deaths globally among male individuals (42.3% [95% UI, 35.2%-48.6%] and 40.2% [95% UI, 33.3%-46.8%] of all risk-attributable cancer deaths, respectively), while chewing tobacco contributed to the greatest attributable LOC deaths among female individuals (27.6% [95% UI, 21.5%-33.8%]), driven by high risk-attributable burden in South and Southeast Asia.
Conclusions and Relevance
In this systematic analysis, disparities in LOC and OPC burden existed across the SDI spectrum, and a considerable percentage of burden was attributable to tobacco and alcohol use. These estimates can contribute to an understanding of the distribution and disparities in LOC and OPC burden globally and support cancer control planning efforts.
Introduction
In 2019, it was estimated that 10 million people died due to cancer worldwide.1 While a relatively small percentage of global cancer deaths were caused by lip, oral cavity, and pharyngeal cancers (3.2%), there is broad variation in survival around the world,2 and those who survive may have substantial reductions in their quality of life.1,3 Reasons for differences in outcomes are multifactorial but likely include differences in access to early-stage detection and effective therapies, as well as potential differences in risk factor exposure patterns.1 Established risk factors for oral and pharyngeal cancers are tobacco, alcohol, and betel quid consumption,4,5 all of which increase cancer risk in a dose- and time-dependent fashion.6,7 Infection with human papillomavirus (HPV) is also a known risk factor—established for oral cavity, tonsil, and oropharynx cancers4,5—which is especially relevant in certain geographic areas of the world.8,9
Monitoring the magnitude of cancer burden, as well as the demographic, spatial, and temporal variations in cancer burden, is necessary for tailoring health planning and setting priorities for future clinical care and research.10,11 Policymakers require locally relevant information on the burden of different cancers to assess the effect of cancer control programs, benchmark progress, and allocate resources in their health care systems, but some countries do not have cancer surveillance systems in place. Global cancer estimation frameworks, including the Global Burden of Diseases, Injuries, and Risk Factors (GBD) Study from the Institute for Health Metrics and Evaluation and the GLOBOCAN study from the International Agency for Research on Cancer provide estimates of cancer burden where data are scarce or do not exist. Prior studies have reported on the global incidence and mortality estimates of oral and pharyngeal cancers from previous iterations of the GBD study12,13 and GLOBOCAN.14 However, to our knowledge, there has not been a publication from the GBD Collaborator Network on these 2 cancer types, and the existing analyses do not provide a comprehensive global overview of their burden over time nor quantify the role of risk factors on their global distribution. Understanding the distribution of these 2 types of cancer worldwide and their associated risk factors is particularly relevant at present, given the adoption of an oral health resolution at the World Health Organization (WHO) 2021 World Health Assembly that includes oral cavity cancers and calls for prevention strategies among other actions.15
This systematic analysis aimed to analyze the incidence, mortality, and disability-adjusted life years (DALYs) of lip and oral cavity cancer (LOC) and other pharyngeal cancer (OPC; ie, pharyngeal other than nasopharyngeal) globally, regionally, nationally, and by Socio-demographic Index (SDI) from 1990 to 2019, as well as to assess the burden of these cancers attributable to tobacco and alcohol use in 2019 by using estimates from the GBD 2019 study.
Methods
Overview
The estimates that are presented in this report originated from the GBD 2019 study.1,10 With each new edition of the GBD, data are updated and new methods are used; thus, estimates for the entire time series supplant previously reported GBD round estimates. In this section, we provide information on the key methodological steps for the estimates reported in this study. More detailed descriptions of the methods are available in the eMethods in Supplement 1 and in the literature produced by the GBD 2019 study.10,16
The University of Washington Institutional Review Board committee approved the GBD 2019 study. Informed consent was waived because of the use of deidentified data. The GBD complies with the Guidelines for Accurate and Transparent Health Estimates Report (GATHER) statement.17 This article was produced through the GBD Collaborator Network and in accordance with the GBD Protocol.18
Definitions
All estimates are reported for adults, defined in this study as 20 years and older. The estimates are presented by sex, 5-year age groups (20-24, 25-29, 30-34, … ≥95 years), globally, and by region for the years 1990 to 2019. The estimates by region are based on 3 geographic classifications: GBD super-regions (7 total), GBD world regions (21 total), and countries or territories (204 total) (eFigures 3-5 and eTable 7 in Supplement 1).10 The countries were also classified by SDI quintiles for presentation of select results (eTable 7 in Supplement 1). All rates are presented for every 100 000 people per year, and age-standardized rates use the GBD world population standard (eMethods in Supplement 1). All estimates are reported with 95% uncertainty intervals (UIs), which are created from the 25th and 975th values of 1000 draws and propagated through each estimation step. Lip and oral cavity cancer includes International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes C00 to C08, and OPC includes ICD-10 codes C09 to C10 and C12 to C13. More detailed mapping of International Classification of Diseases, Ninth Revision (ICD-9) and ICD-10 codes to the GBD cancer causes LOC and OPC are summarized in eTable 1 in Supplement 1.
The present analysis uses the term other pharyngeal cancer (synonymous with other pharynx cancer in the GBD 2019 study and throughout Supplement 1) because nasopharyngeal cancer is separately estimated. Nasopharyngeal cancer is not included in this publication because it differs epidemiologically from cancers that occur in other pharyngeal sites, and we use the term OPC to be consistent with publicly available GBD results and visualizations.
Mortality Estimates
The GBD cancer estimation process begins with mortality (eFigure 1 in Supplement 1). The sources of these data were vital registration systems, cancer registries, and verbal autopsies. The data reported were mapped to a list of underlying causes (cancer types) in the GBD causes of death hierarchy (eTable 1 in Supplement 1).1,10 Uninformative cause of death codes (the “garbage codes”19) were redistributed among appropriate underlying causes of death.1,10 Mortality data were not captured by vital registries in some countries where cancer incidence data were available. To expand data availability informing mortality models, incidence data were transformed into mortality estimates using modeled mortality-to-incidence ratios (MIRs). The MIR modeling process used cancer registry data from locations where incidence and mortality of the same year were available. This model includes a linear-step mixed-effects model with logit link functions, with age, sex, and the Healthcare Access and Quality Index as covariates. The results of this step were smoothed over space and time and adjusted through a spatiotemporal Gaussian process regression.1,10 Death data and estimates were included in cancer cause and sex-specific Cause of Death Ensemble models (eMethods and eTables 2-4 in Supplement 1).20 Finally, cancer mortality estimates were adjusted to independently modeled all-cause mortality.10
Incidence and DALYs Estimates
Incidence estimates were obtained by dividing the final mortality estimates by their corresponding MIRs (eFigure 2 in Supplement 1). The 10-year prevalence estimate was derived from the estimated incidence and the survival modeled using MIRs.10 Years lived with disability were estimated by separating 10-year cancer prevalence into 4 sequelae with associated disability weights (eTables 5 and 6 in Supplement 1). Disability weights range from 0 to 1 and represent the magnitude of health loss (0, no health loss; 1, health loss equivalent to death). Years lived with disability were obtained by multiplying each sequela duration by the corresponding disability weight. Finally, years of life lost were estimated by multiplying the difference between the standard GBD life expectancy at the age of death and the estimated number of deaths at that age. Years lived with disability and years of life lost were summed by cause, sex, age group, location, and year to result in DALYs.10
Socio-demographic Index
The SDI incorporates 3 aspects of development: (1) total fertility rate for female individuals younger than 25 years, (2) mean education for those 15 years and older, and (3) lag-distributed income per capita.10 The eMethods in Supplement 1 present further SDI details as well as which countries make up each SDI quintile (eTable 7 in Supplement 1).
Risk Factors: Population Attributable Fraction Estimation
The GBD 2019 comparative risk assessment framework was used to estimate the proportion of deaths and DALYs for LOC and OPC in 2019 attributable to the risk factors estimated. The risk assessment used in the framework was the attributable burden, which means the discount in the current disease burden that would have been possible if past population risk exposure had changed to an alternative or counterfactual distribution of exposure. Theoretical minimum risk exposure level was the alternative distribution used in the model, which represents the level of risk exposure that minimizes risk at the population level or the level of risk that captures the maximum attributable burden. This study presents the proportion of mortality and DALYs due to LOC attributable to smoking, chewing tobacco, and alcohol consumption, as well as the proportion of mortality and DALYs due to OPC attributable to smoking and alcohol consumption; the estimates considered the population aged 20 years and older. These risk factors were chosen based on the risk-outcome pairs that the GBD 2019 study assessed as meeting the World Cancer Research Fund grades of convincing or probable evidence. The theoretical minimum risk exposure level for smoking and chewing tobacco was that all individuals were lifelong nonusers and for alcohol use was an estimated distribution of 0 to 10 g per day. Detailed methodology for risk factor estimation can be found in the eMethods in Supplement 1 and the GBD 2019 risk factors capstone publication.16
Results
The Global Burden of LOC in 2019
In 2019, 370 000 (95% UI, 338 000-401 000) new cases of LOC occurred globally, and the global age-standardized incidence rate (ASIR) was 7.1 (95% UI, 6.5-7.7) per 100 000. Deaths from LOC were estimated to be 199 000 (95% UI, 181 000-217 000) globally, with an age-standardized mortality rate (ASMR) of 3.8 (95% UI, 3.5-4.2) deaths per 100 000. Lip and oral cavity cancer was responsible for 5.45 million (95% UI, 4.95-5.97 million ) DALYs in 2019 (eTable 8 in Supplement 1).
Figure 1A shows the distribution of national-level ASMRs in 2019 due to LOC (for a map of ASIRs, see eFigure 6 in Supplement 1). Eastern Europe and South and Southeast Asia exhibited a concentration of countries with ASMRs in the highest quintile. The LOC age-specific rates for incidence, mortality, and DALYs were higher among male individuals than among female individuals in all age groups (Figure 2 and eFigure 11 in Supplement 1).
Figure 1. Global Maps of Age-Standardized Mortality Rates for Lip, Oral, and Other Pharyngeal Cancer for Both Sexes Combined in 2019.
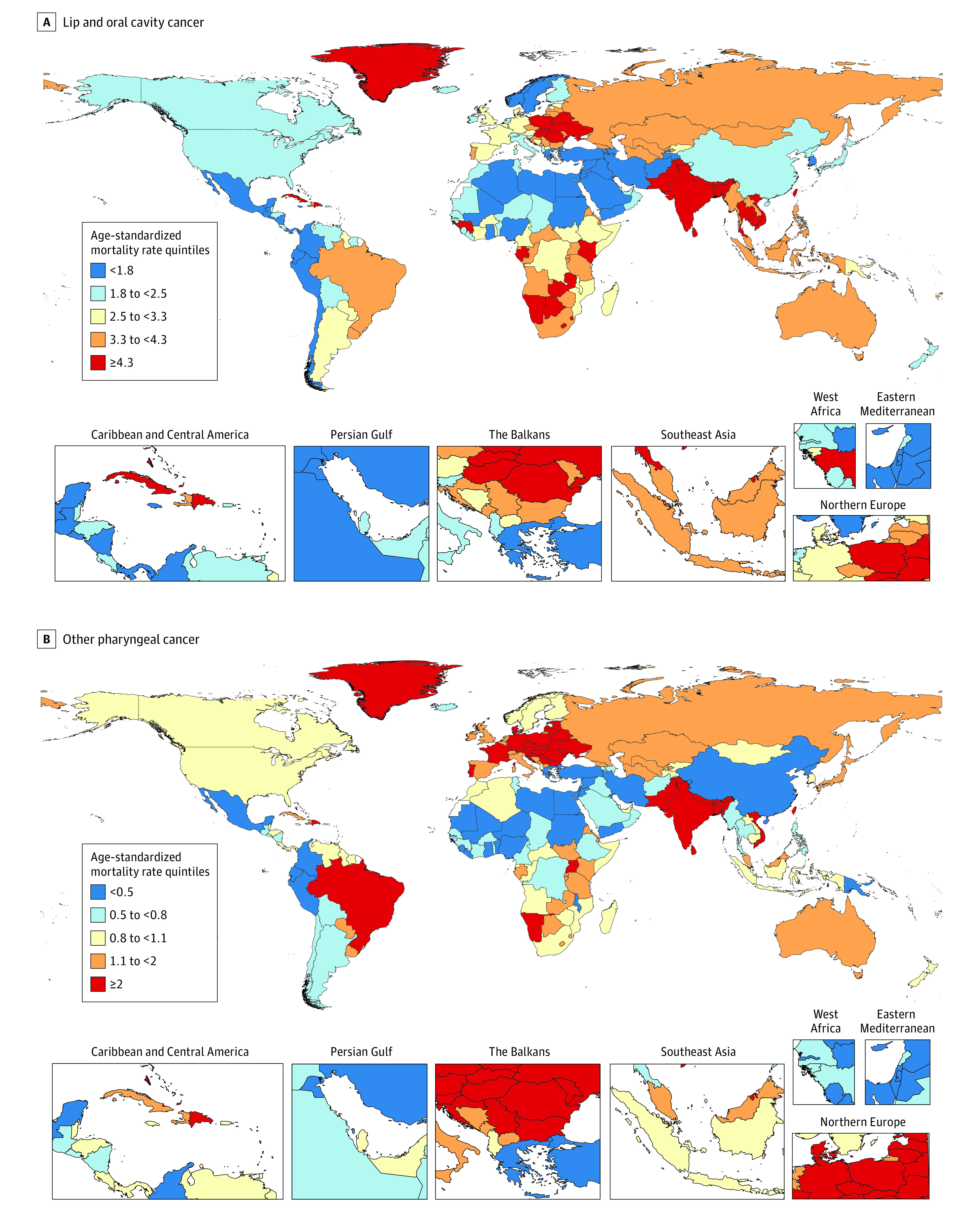
Each map represents estimates at the national level and for the age range of 20 to older than 95 years. Quintiles are based on age-standardized mortality rates per 100 000 person-years. There are several geographic locations where estimates are not available (eg, Western Sahara, French Guiana) because they were not modeled locations in the Global Burden of Diseases, Injuries, and Risk Factors Study 2019; these locations are white on the maps. eFigure 6 in Supplement 1 provides global maps showing the age-standardized incidence rate quintiles for lip and oral cavity cancer and other pharyngeal cancer among both sexes in 2019. eFigures 7 through 10 in Supplement 1 provide additional global maps of age-standardized mortality and incidence rates for lip and oral cavity cancer and other pharyngeal cancer in male and female individuals separately in 2019.
Figure 2. Global Absolute Cases and Deaths and Age-Specific Incidence and Mortality Rates for Lip, Oral, and Other Pharyngeal Cancer by Age Group and Sex in 2019.
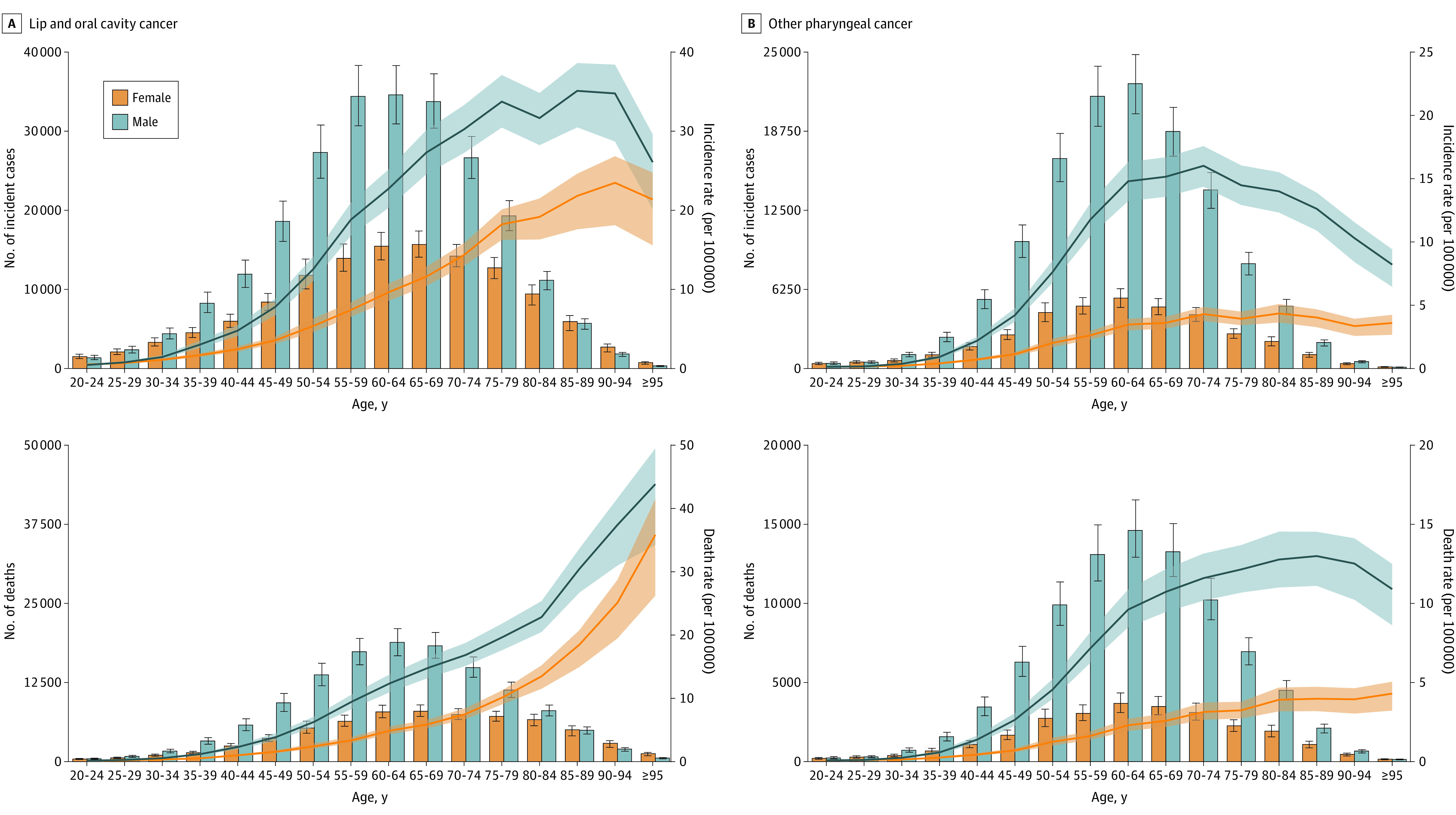
Bars indicate absolute numbers, with error bars representing 95% uncertainty intervals. Lines indicate rates, with shaded areas representing respective 95% uncertainty intervals. For an additional version of this Figure showing absolute disability-adjusted life years and age-specific disability-adjusted life year rates (per 100 000), see eFigure 11 in Supplement 1.
The South Asia and High-income super-regions had the highest ASIRs for LOC in 2019 (15.1 [95% UI, 12.8-17.5] and 7.2 [95% UI, 6.4-7.9] new cases per 100 000 inhabitants, respectively). South Asia also had the highest ASMR (10.0 [95% UI, 8.6-11.7] per 100 000); however, the second-largest ASMR occurred in Central Europe, Eastern Europe, and Central Asia (4.2 [95% UI, 3.8-4.6] per 100 000). The low-middle SDI regions had the highest ASIR and ASMR (10.4 [95% UI, 9.1-11.8] new cases and 7.0 [95% UI, 6.1-7.9] deaths per 100 000, respectively; eTable 8 in Supplement 1). The country with the highest ASIR in 2019 was Palau, with 46.6 (95% UI, 36.3-59.2) new cases per 100 000, while the highest ASMR occurred in Pakistan, with 23.2 (95% UI, 18.7-28.9) deaths per 100 000 (eTable 10 in Supplement 1).
The Burden of LOC Over Time
From 1990 to 2019, the high SDI regions showed a decreasing pattern in ASIR, ASMR, and age-standardized DALY rates (Figure 3 and eFigure 12 in Supplement 1). The high-middle SDI quintile had decreasing ASMR and age-standardized DALY rates, while the ASIR of the middle SDI regions increased (eTable 8 in Supplement 1). Low-middle and low SDI regions consistently showed the highest ASMRs due to LOC from 1990 to 2019 (Figure 3). The LOC ASIRs, ASMRs, and age-standardized DALY rates over time by sex and super-region, as well as the age-specific rates over time and by age group, are reported in eFigures 13, 15, and 14 in Supplement 1, respectively.
Figure 3. Time Trends of Age-Standardized Death and Incidence Rates for Lip, Oral, and Other Pharyngeal Cancer From 1990 to 2019 by Socio-demographic Index (SDI) Quintile.
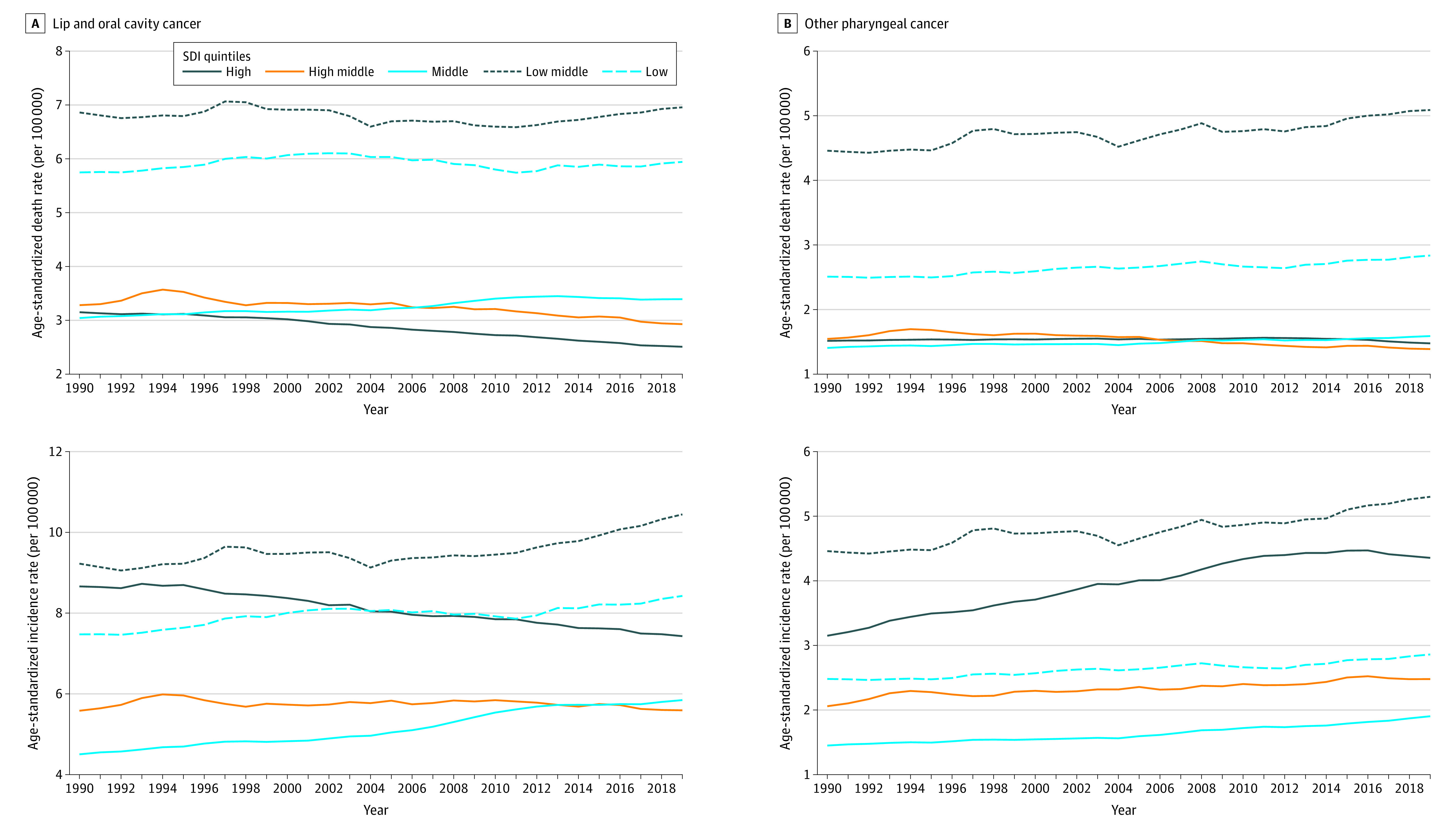
Rates represent both sexes combined and are expressed per 100 000 person-years. See eFigure 3 and eTable 7 in Supplement 1 for details and definitions of the SDI quintiles. For additional versions of this Figure, showing time trends of deaths, incidence, and disability-adjusted life years, see eFigure 12 (by SDI quintile), eFigure 13 (by sex), eFigure 14 (by 10-year age group), and eFigure 15 (by Global Burden of Diseases, Injuries, and Risk Factors Study super-region) in Supplement 1.
The Global Burden of OPC in 2019
The estimated number of new OPC cases in 2019 was 167 000 (95% UI, 153 000-180 000), with an ASIR of 3.2 (95% UI, 2.9-3.4) per 100 000 inhabitants. It was estimated that 114 000 (95% UI, 103 000-126 000) people died of OPC, revealing a global ASMR of 2.2 (95% UI, 2.0-2.4) per 100 000. Other pharyngeal cancer caused 3.23 million (95% UI, 2.90-3.57 million) DALYs in 2019 (eTable 9 in Supplement 1).
Figure 1B presents the distribution of national-level ASMRs in 2019 due to OPC (for a map of ASIRs, see eFigure 6 in Supplement 1). Most European countries were in the highest quintile of ASMRs; there was also a concentration of South Asian countries in this quintile. The OPC age-specific rates of incidence, mortality, and DALYs among male individuals were higher than female individuals in all age groups; the difference between male and female individuals in OPC age-specific rates was greater in middle age than in the older and younger age groups (Figure 2 and eFigure 11 in Supplement 1).
In 2019, South Asia had the highest ASIR and ASMR for OPC (7.7 [95% UI, 6.5-8.9] new cases and 7.4 [95% UI, 6.3-8.5] deaths per 100 000 inhabitants, respectively). Low-middle and high SDI regions had the highest ASIRs (5.3 [95% UI, 4.6-6.1] and 4.4 [95% UI, 3.9-4.9] new cases per 100 000, respectively). The low-middle SDI quintile also had the highest ASMR (5.1 [95% UI, 4.4-5.9] per 100 000), but the low SDI quintile had the second highest ASMR (2.8 [95% UI, 2.4-3.3] per 100 000) (eTable 9 in Supplement 1). Taiwan (province of China) was the country with the highest ASIR in 2019 (9.8 [95% UI, 7.4-13.0] incident cases per 100 000), while India had the highest ASMR (7.7 [95% UI, 6.4-9.2] deaths per 100 000) (eTable 11 in Supplement 1).
The Burden of OPC Over Time
The OPC ASIRs increased from 1990 to 2019 in the high, high-middle, and middle SDI quintiles. There was a general pattern of stability across all SDI settings regarding OPC ASMRs, except for the high-middle strata, which showed a reduction (eTable 9 in Supplement 1). For age-standardized DALY rates, high and high-middle SDI settings exhibited a decreasing pattern (eTable 9 in Supplement 1). From 1990 to 2019, low-middle and low SDI regions always showed the highest ASMRs due to OPC (Figure 3). The OPC ASIRs, ASMRs, and age-standardized DALY rates over time by sex and super-region, as well as the age-specific rates over time by age group, are reported in eFigures 13, 15, and 14 in Supplement 1, respectively.
Risk Factors: Population Attributable Fraction
Figure 4 shows the proportion of LOC and OPC deaths attributable to alcohol and tobacco consumption in 2019. Among male individuals, tobacco smoking and alcohol consumption were responsible for a large proportion of LOC deaths globally (42.3% [95% UI, 35.2%-48.6%] and 40.2% [95% UI, 33.3%-46.8%], respectively). For male individuals in the younger age groups (≤54 years old) and in the oldest age group (≥95 years old), alcohol consumption was a more important risk factor than smoking (Figure 4). Among female individuals, the highest proportion of risk-attributable LOC deaths globally were due to chewing tobacco (27.6% [95% UI, 21.5%-33.8%]; Figure 4), with this burden concentrated in South and Southeast Asia (eFigure 16 in Supplement 1).
Figure 4. Proportion of Deaths Attributable to Risk Factors for Lip, Oral, and Other Pharyngeal Cancer by Age Group and Sex Globally in 2019.
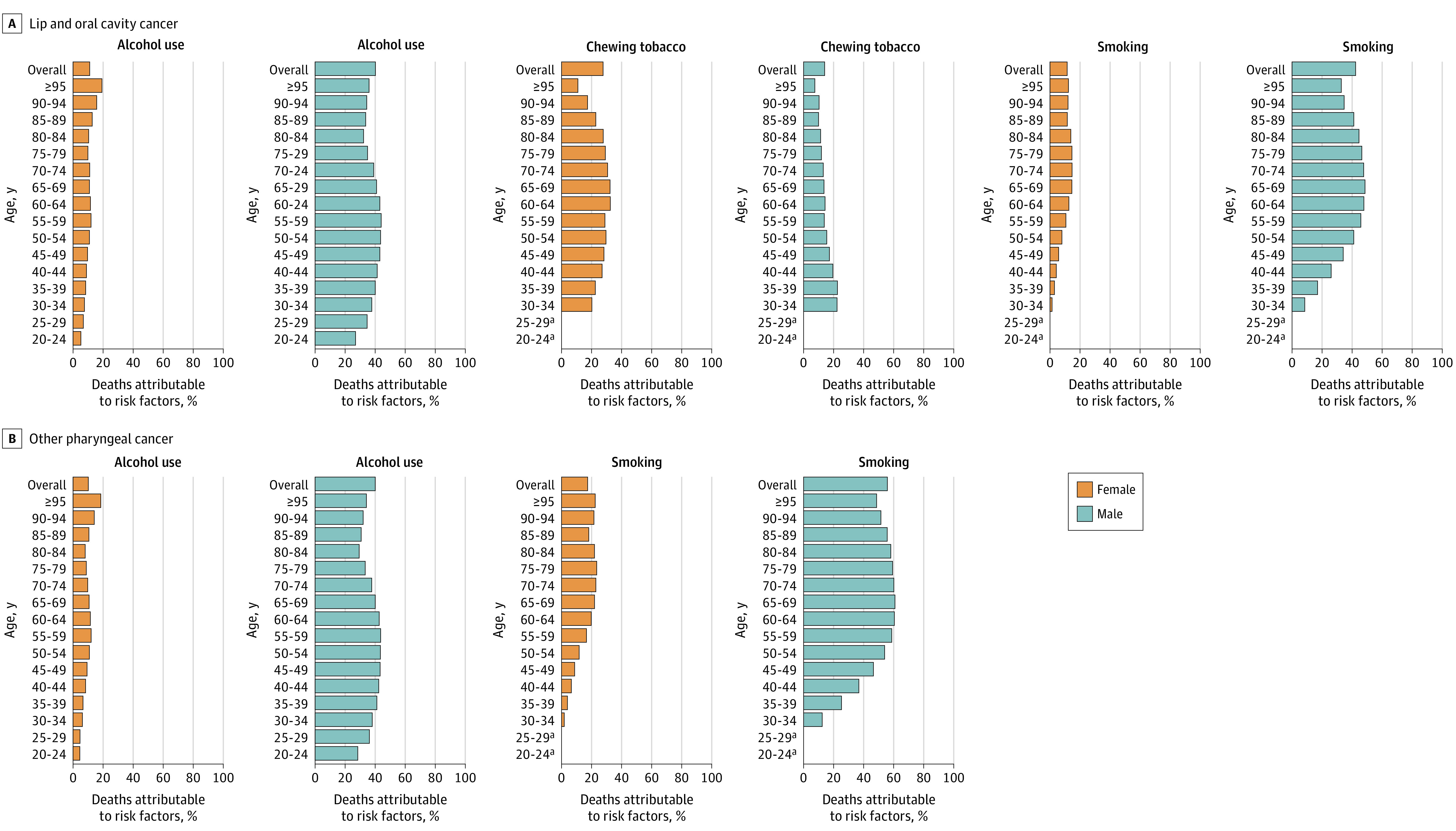
eFigure 16 in Supplement 1 provides further information on deaths attributable to risk factors for lip, oral, and other pharyngeal cancers in 2019 by Global Burden of Diseases, Injuries, and Risk Factors (GBD) Study world region. eFigure 17 in Supplement 1 provides further information on risk-attributable disability-adjusted life-years for lip, oral, and other pharyngeal cancers by 5-year age groups globally and by GBD study world region. For Tables summarizing the underlying results of risk-attributable deaths and disability-adjusted life-years by country or territory, see eTable 12 (for lip and oral cavity cancer) and eTable 13 (for other pharyngeal cancer) in Supplement 1. An additional version of this Figure showing percentage totals for each category is provided in eFigure 18 in Supplement 1.
aThe chewing tobacco and smoking risk factors were modeled with lower age restrictions of 30 years in the GBD 2019 study; thus, estimates were not produced for these risk factors in the age groups of 20 to 24 years and 25 to 29 years.
Concerning OPC, for male individuals older than 45 years and female individuals older than 50 years, the highest proportion of deaths globally was attributable to smoking tobacco; in younger age groups, however, alcohol consumption played the greater role (Figure 4). Globally, 55.8% (95% UI, 49.2%-62.0%) of OPC deaths among male individuals were attributable to tobacco smoking and 40.0% (95% UI, 31.8%-48.1%) to alcohol consumption; among female individuals, 17.4% (95% UI, 13.8%-21.2%) were due to smoking and 10.1% (95% UI, 7.1%-13.3%) to alcohol. For risk-attributable DALYs, see eFigure 17 in Supplement 1 and for results by country, eTables 12 and 13 in Supplement 1.
Discussion
This study provides an updated and comprehensive overview of lip, oral, and other pharyngeal cancer burden in the past 30 years, globally and by region using GBD 2019 estimates, including for areas where observed data are scarce. It is also, to our knowledge, the first report of the burden of LOC and OPC attributable to risk factors globally, providing important information for addressing these cancers around the world. Disparities in ASIRs and ASMRs and trends across the SDI spectrum suggested that populations from regions with a lower level of sociodemographic development have a higher chance of death when affected by LOC or OPC, with South Asia carrying substantial LOC and OPC burden. Smoking, chewing tobacco, and alcohol were substantial contributors to LOC and OPC deaths and DALYs and are crucial targets to decreasing future burden of LOC and OPC globally.
Throughout the entire study period, the low and low-middle SDI strata had the highest LOC and OPC ASMRs and age-standardized DALY rates, even though they did not always have the highest ASIRs. The differences between ASMRs and ASIRs were smaller in the low and low-middle SDI regions, particularly for OPC. The ASMRs and age-standardized DALY rates for LOC and OPC showed a pattern of stability across time for almost all SDI groups, but the high and high-middle SDI groups exhibited a decline in these rates for LOC in the past 2 decades. These results may reflect different regional patterns of exposure to the risk factors estimated in this study (tobacco and alcohol), as well as other risk factors not estimated herein, such as oncogenic HPV infection, which is responsible for higher incidence rates of HPV-associated oropharynx cancer in wealthier countries9 and is reported to carry lower mortality.21,22 The latter may also be one factor contributing to the increasing trend of ASIRs in the high SDI quintile noted in this analysis.9 Smoking may lead to poorer survival in HPV-associated oropharyngeal cancer and was found to contribute to a large proportion of OPC deaths and DALYs in High-income regions in this analysis, highlighting the critical role of this risk factor even in settings where HPV-associated oropharyngeal cancer may be more prevalent.9,23 The differences in OPC ASMRs in the setting of similar ASIRs that were identified in some SDI strata (eg, high-middle and low SDI) could suggest that fewer patients survive their OPC diagnoses in the most impoverished parts of the world, potentially due to later-stage diagnoses and/or less access to cancer treatment.24,25
At the World Health Assembly in 2021, the WHO adopted a resolution on oral health—the WHA74.5 resolution15—which was a considerable step forward for the oral health agenda. This resolution enabled the development of a global strategy on oral health, a global action plan adopted by the member states in the 2022 World Health Assembly, which has the vision to reach universal health coverage for oral health for all individuals and communities by 2030.26 In addition, the WHO global noncommunicable diseases action plan for 2013 to 2030 advocates for specific interventions for oral cancer, including screening in high-risk groups linked with timely diagnostic and comprehensive cancer treatment.27 These efforts provide a collective opportunity to improve patient outcomes in a historically neglected area of health care.28,29
The present results suggest that inequities exist in LOC and OPC burden worldwide, which should be considered in oral health planning and implementation initiatives. Public health strategies to control exposure to major risk factors and promote access to early diagnosis and treatment in low- and low-middle–income regions are urgently needed, given the higher mortality rates in lower SDI countries. Including oral health care as part of the universal health care agenda can support early diagnosis and access to timely treatment of LOC and OPC; oral health care is unaffordable for many individuals around the world, and most health systems with universal coverage do not currently include it.30 Delay in diagnosis and treatment of these cancers negatively affects survival, so referral pathways capable of quick diagnosis of suspected LOC and OPC cases and treatment of those confirmed are crucial.24 Finally, monitoring actions should include creating and expanding population-based registration systems that include information on the staging of these cancers at the time of diagnosis and their subsequent outcomes.
This study showed that smoking tobacco remains an important risk factor for oral cavity and other pharyngeal cancers globally. Tobacco control efforts have been mobilized over the past several decades, including the WHO Framework Convention on Tobacco Control in 2003,31 the first global health treaty aimed at reducing tobacco consumption in member states. Evidence indicates that tobacco control measures adopted by the countries participating in the Framework Convention on Tobacco Control effectively reduced the prevalence of smoking. However, the pace of implementation of these measures has been heterogeneous around the world, and the tobacco epidemic is still far from over.32,33 The high percentage of LOC and OPC deaths and DALYs attributable to smoking tobacco in 2019 reinforces the need to strengthen the implementation of tobacco control measures.
The percentage of DALYs and deaths for LOC attributable to chewing tobacco is concentrated in certain world regions, mainly in South and Southeast Asia. The largest ASMRs and ASIRs for LOC and OPC in 2019 occurred in South Asia, and for LOC these rates were more than double the next highest region rates. The GBD 2019 study estimated that 83.3% (95% UI, 82.2%-84.2%) of the chewing tobacco users in 2019 lived in South Asia,34 with the present study suggesting that chewing tobacco in South and Southeast Asia is especially relevant among female individuals. The critical contribution of chewing tobacco to oral cavity cancer burden among female individuals is concerning because the prevalence of this risk factor in female individuals has been constant in recent decades, unlike smoking tobacco, which has been decreasing.34 These results imply that tobacco control initiatives need to be expanded, intensified, or better able to address chewing tobacco in some world regions.35 Because chewing tobacco is a habit related to local culture and beliefs,7 these initiatives should be planned in close collaboration with groups and institutions that are aware of the local challenges and opportunities.
The present results indicate that a large proportion of LOC and OPC deaths in 2019 were attributable to alcohol, particularly in young men. A previous GBD study indicated that the prevalence of alcohol consumption varied considerably worldwide, being higher in high SDI areas, and revealed that alcohol and its effects on health may become an increasing challenge as regions advance across the SDI spectrum.36 This suggests that low and low-middle SDI regions could benefit from policies aimed at reducing population-level exposure to alcohol while this habit is not so widespread, in addition to addressing this risk factor in higher SDI countries. While the synergistic effect of alcohol and tobacco on the risk of developing LOC and OPC is well recognized in the literature,37 the importance of alcohol alone is less explored; however, the present results on the critical role of alcohol as a risk factor for LOC and OPC are in line with previous studies.6,38
Limitations
While the GBD 2019 study provided useful estimates of global LOC and OPC burden, there are several limitations. Locations where observed data on disease burden were unavailable are reported with appropriate uncertainty, but their estimation is limited by the data available across space and time, highlighting the importance of expanding cancer and vital registration systems. The availability and quality of primary data are also a limitation in the estimation of risk-attributable cancer burden.16 In addition, there are limitations more specific to the estimation of LOC and OPC. Currently, it is not possible in the GBD study to separately analyze cancers at a more granular level than the LOC and OPC categories reported herein, and the current GBD classification groups include some anatomical cancer sites that do not share the same pathophysiological determinants, such as lip cancer with other sites of the oral cavity. Potential improvements to the GBD categorization of these cancers would include, depending on data availability, (1) the separation of lip, salivary gland, oropharynx, and hypopharynx cancers into individual categories and (2) the inclusion of cancer of the base of the tongue in the oropharyngeal cancer category, instead of analyzing it as part of oral cavity cancers.39,40 Finally, the current study did not estimate the burden of LOC and OPC attributable to other risk factors for these diseases, such as HPV infection, an important and growing risk factor for OPC,22,41 and betel quid without tobacco consumption for LOC.4
Conclusions
In this systematic analysis, disparities in LOC and OPC incidence, mortality, and DALY rates across the SDI spectrum were evident in 2019. Actions to reduce the burden of LOC and OPC through improvements in early diagnosis, access to treatment, and reduction of exposure to risk factors should consider the inequities in the global distribution of these diseases. Including oral health into universal health coverage is one crucial strategy to reach this goal, as part of comprehensive cancer control efforts. Tobacco and alcohol use are important targets to decreasing the future burden of LOC and OPC globally.
eMethods.
eFigure 1. Input data and methodological summary for mortality and Years of Life Lost (YLLs) for all cancers, including LOC and OPC
eTable 1. List of International Classification of Diseases (ICD) codes mapped to the Global Burden of Disease causes Lip and oral cavity cancer and Other pharynx cancer for cancer incidence data
eTable 2. Age restrictions for LOC and OPC in GBD 2019 modeling
eTable 3. Covariates for lip and oral cavity cancer
eTable 4. Covariates for other pharynx cancer
eFigure 2. Flowchart of GBD cancer incidence and Years Lived with Disability (YLDs) estimation, including LOC and OPC
eTable 5. Duration (months) of each sequalae for LOC and OPC
eTable 6. Lay description of cancer states and corresponding disability weights
eReferences
eFigure 3. Socio-demographic Index quintiles for the Global Burden of Disease Study 2019
eTable 7. Socio-demographic Index (SDI) quintiles for countries and territories estimated in GBD 2019
eFigure 4. Map of GBD world super-regions, 2019
eFigure 5. Map of GBD world regions, 2019
eTable 8. Global and regional deaths, incidence, and DALYs counts and age-standardized rates (per 100 000) for Lip and oral cavity, both sexes combined, in 2019, and change in age-standardized rates from 1990 to 2019
eTable 9. Global and regional deaths, incidence, and DALYs counts and age-standardized rates (per 100 000) for Other pharynx cancer, both sexes combined, in 2019, and change in age-standardized rates from 1990 to 2019
eTable 10. Deaths, incidence, and DALYs counts and age-standardized rates (per 100 000) for Lip and oral cavity cancer (LOC), both sexes combined, by country or territory, in 2019, and change in age-standardized rates from 1990 to 2019
eTable 11. Deaths, incidence, and DALYs counts and age-standardized rates (per 100 000) for Other pharynx cancer (OPC), both sexes combined, by country or territory, in 2019, and change in age-standardized rates from 1990 to 2019
eFigure 6. Global map of age-standardized incidence rate quintiles for A) lip and oral cavity cancer, and B) other pharynx cancer, both sexes combined, 2019
eFigure 7. Global map of A) age-standardized mortality rate quintiles, and B) age-standardized incidence rate quintiles for lip and oral cavity cancer, males, 2019
eFigure 8. Global map of A) age-standardized mortality rate quintiles, and B) age-standardized incidence rate quintiles for other pharynx cancer, males, 2019
eFigure 9. Global map of A) age-standardized mortality rate quintiles, and B) age-standardized incidence rate quintiles for lip and oral cavity cancer, females, 2019
eFigure 10. Global map of A) age-standardized mortality rate quintiles, and B) age-standardized incidence rate quintiles for other pharynx cancer, females, 2019
eFigure 11. Global absolute DALYs and age-specific DALY rates (per 100,000) for Lip and oral cavity cancer, and Other pharynx cancer by 5-year age group and sex in 2019
eFigure 12. Time trends of age-standardized DALY rates for Lip and oral cavity cancer and Other pharynx cancer from 1990 to 2019, by SDI quintile
eFigure 13. Time trends of age-standardized deaths, incidence, and DALY rates for Lip and oral cavity cancer and Other pharynx cancer from 1990 to 2019, by sex
eFigure 14. Time trends of age-specific deaths, incidence, and DALY rates for Lip and oral cavity cancer and Other pharynx cancer from 1990 to 2019, by ten-year age group globally
eFigure 15. Time trends of age-standardized deaths, incidence, and DALY rates for Lip and oral cavity cancer and Other pharynx cancer from 1990 to 2019, by GBD super-region
eFigure 16. Proportion of deaths attributable to risk factors for Lip and oral cavity cancer and Other pharynx cancer for males and females in 2019 by GBD world region
eFigure 17. Proportion of DALYs attributable to risk factors for Lip and oral cavity cancer and Other pharynx cancer A) by five-year age group globally, and B) by GBD world region, for males and females in 2019
eTable 12. Proportion of Lip and oral cavity cancer (LOC) deaths and DALYs attributable to risk factors, in 2019, by country or territory, both sexes combined
eTable 13. Proportion of Other pharynx cancer (OPC) deaths and DALYs attributable to risk factors, in 2019, by country or territory, both sexes combined
Data Sharing Statement
References
- 1.Kocarnik JM, Compton K, Dean FE, et al. ; Global Burden of Disease 2019 Cancer Collaboration . Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8(3):420-444. doi: 10.1001/jamaoncol.2021.6987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer survival in Africa, Asia, the Caribbean and Central America (SurvCan). Accessed July 17, 2023. https://survcan.iarc.fr/
- 3.Høxbroe Michaelsen S, Grønhøj C, Høxbroe Michaelsen J, Friborg J, von Buchwald C. Quality of life in survivors of oropharyngeal cancer: a systematic review and meta-analysis of 1366 patients. Eur J Cancer. 2017;78:91-102. doi: 10.1016/j.ejca.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 4.Agents classified by the IARC monographs, volumes 1-132. International Agency for Research on Cancer . Accessed July 17, 2023. https://monographs.iarc.who.int/agents-classified-by-the-iarc/
- 5.Cogliano VJ, Baan R, Straif K, et al. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103(24):1827-1839. doi: 10.1093/jnci/djr483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gormley M, Dudding T, Sanderson E, et al. A multivariable Mendelian randomization analysis investigating smoking and alcohol consumption in oral and oropharyngeal cancer. Nat Commun. 2020;11(1):6071. doi: 10.1038/s41467-020-19822-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niaz K, Maqbool F, Khan F, Bahadar H, Ismail Hassan F, Abdollahi M. Smokeless tobacco (paan and gutkha) consumption, prevalence, and contribution to oral cancer. Epidemiol Health. 2017;39:e2017009. doi: 10.4178/epih.e2017009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi AK. Epidemiology and clinical aspects of HPV in head and neck cancers. Head Neck Pathol. 2012;6(suppl 1):S16-S24. doi: 10.1007/s12105-012-0377-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anantharaman D, Abedi-Ardekani B, Beachler DC, et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int J Cancer. 2017;140(9):1968-1975. doi: 10.1002/ijc.30608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vos T, Lim SS, Abbafati C, et al. ; GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204-1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO methods and data sources for country-level causes of death 2000-2016. World Health Organization . March 2018. Accessed July 17, 2023. https://terrance.who.int/mediacentre/data/ghe/GlobalCOD_method_2000_2016.pdf
- 12.Ren ZH, Hu CY, He HR, Li YJ, Lyu J. Global and regional burdens of oral cancer from 1990 to 2017: results from the Global Burden of Disease Study. Cancer Commun (Lond). 2020;40(2-3):81-92. doi: 10.1002/cac2.12009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du M, Nair R, Jamieson L, Liu Z, Bi P. Incidence trends of lip, oral cavity, and pharyngeal cancers: Global Burden of Disease 1990-2017. J Dent Res. 2020;99(2):143-151. doi: 10.1177/0022034519894963 [DOI] [PubMed] [Google Scholar]
- 14.Miranda-Filho A, Bray F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020;102:104551. doi: 10.1016/j.oraloncology.2019.104551 [DOI] [PubMed] [Google Scholar]
- 15.Seventy-fourth World Health Assembly: Geneva, 24 May-1 June 2021: resolutions and decisions, Annexes. World Health Organization . Accessed July 17, 2023. https://apps.who.int/iris/handle/10665/352594.
- 16.Murray CJL, Aravkin AY, Zheng P, et al. ; GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223-1249. doi: 10.1016/S0140-6736(20)30752-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens GA, Alkema L, Black RE, et al. ; (The GATHER Working Group ). Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet. 2016;388(10062):e19-e23. doi: 10.1016/S0140-6736(16)30388-9 [DOI] [PubMed] [Google Scholar]
- 18.Protocol for the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD). Institute for Health Metrics and Evaluation . March 2020. Accessed July 24, 2023. https://www.healthdata.org/research-analysis/about-gbd/protocol
- 19.Naghavi M, Makela S, Foreman K, O’Brien J, Pourmalek F, Lozano R. Algorithms for enhancing public health utility of national causes-of-death data. Popul Health Metr. 2010;8(1):9. doi: 10.1186/1478-7954-8-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foreman KJ, Lozano R, Lopez AD, Murray CJL. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. 2012;10(1):1-23. doi: 10.1186/1478-7954-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121(8):1813-1820. doi: 10.1002/ijc.22851 [DOI] [PubMed] [Google Scholar]
- 22.Lechner M, Liu J, Masterson L, Fenton TR. HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat Rev Clin Oncol. 2022;19(5):306-327. doi: 10.1038/s41571-022-00603-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen SY, Massa S, Mazul AL, et al. The association of smoking and outcomes in HPV-positive oropharyngeal cancer: a systematic review. Am J Otolaryngol. 2020;41(5):102592. doi: 10.1016/j.amjoto.2020.102592 [DOI] [PubMed] [Google Scholar]
- 24.Graboyes EM, Kompelli AR, Neskey DM, et al. Association of treatment delays with survival for patients with head and neck cancer: a systematic review. JAMA Otolaryngol Head Neck Surg. 2019;145(2):166-177. doi: 10.1001/jamaoto.2018.2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seoane J, Alvarez-Novoa P, Gomez I, et al. Early oral cancer diagnosis: the Aarhus statement perspective: a systematic review and meta-analysis. Head Neck. 2016;38(1)(suppl 1):E2182-E2189. doi: 10.1002/hed.24050 [DOI] [PubMed] [Google Scholar]
- 26.Seventy-fifth World Health Assembly: provisional agenda item 14.1: follow-up to the political declaration of the third high-level meeting of the General Assembly on the prevention and control of non-communicable diseases. World Health Organization . April 27, 2022. Accessed July 17, 2023. https://apps.who.int/gb/ebwha/pdf_files/WHA75/A75_10Add1-en.pdf
- 27.WHO Discussion Paper: draft updated appendix 3 of the WHO Global NCDs action plan 2013-2030. World Health Organization . June 8, 2022. Accessed July 17, 2023. https://cdn.who.int/media/docs/default-source/ncds/mnd/2022_discussion_paper_final.pdf?sfvrsn=78343686_7.
- 28.Benzian H, Guarnizo-Herreño CC, Kearns C, Muriithi MW, Watt RG. The WHO global strategy for oral health: an opportunity for bold action. Lancet. 2021;398(10296):192-194. doi: 10.1016/S0140-6736(21)01404-5 [DOI] [PubMed] [Google Scholar]
- 29.Varenne B, Fox CH. The role of research in the WHO oral health resolution. JDR Clin Trans Res. 2021;6(2):112-114. doi: 10.1177/2380084421997095 [DOI] [PubMed] [Google Scholar]
- 30.Wang TT, Mathur MR, Schmidt H. Universal health coverage, oral health, equity and personal responsibility. Bull World Health Organ. 2020;98(10):719-721. doi: 10.2471/BLT.19.247288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO Framework Convention on Tobacco Control. World Health Organization; 2003. [Google Scholar]
- 32.Flor LS, Reitsma MB, Gupta V, Ng M, Gakidou E. The effects of tobacco control policies on global smoking prevalence. Nat Med. 2021;27(2):239-243. doi: 10.1038/s41591-020-01210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reitsma MB, Kendrick PJ, Ababneh E, et al. ; GBD 2019 Tobacco Collaborators . Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. 2021;397(10292):2337-2360. doi: 10.1016/S0140-6736(21)01169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendrick PJ, Reitsma MB, Abbasi-Kangevari M, et al. ; GBD 2019 Chewing Tobacco Collaborators . Spatial, temporal, and demographic patterns in prevalence of chewing tobacco use in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet Public Health. 2021;6(7):e482-e499. doi: 10.1016/S2468-2667(21)00065-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehrotra R, Yadav A, Sinha DN, et al. Smokeless tobacco control in 180 countries across the globe: call to action for full implementation of WHO FCTC measures. Lancet Oncol. 2019;20(4):e208-e217. doi: 10.1016/S1470-2045(19)30084-1 [DOI] [PubMed] [Google Scholar]
- 36.Griswold MG, Fullman N, Hawley C, et al. ; GBD 2016 Alcohol Collaborators . Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015-1035. doi: 10.1016/S0140-6736(18)31310-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mello FW, Melo G, Pasetto JJ, Silva CAB, Warnakulasuriya S, Rivero ERC. The synergistic effect of tobacco and alcohol consumption on oral squamous cell carcinoma: a systematic review and meta-analysis. Clin Oral Investig. 2019;23(7):2849-2859. doi: 10.1007/s00784-019-02958-1 [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Mao Y, Zhang Y, et al. Alcohol drinking and upper aerodigestive tract cancer mortality: a systematic review and meta-analysis. Oral Oncol. 2014;50(4):269-275. doi: 10.1016/j.oraloncology.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 39.Conway DI, Purkayastha M, Chestnutt IG. The changing epidemiology of oral cancer: definitions, trends, and risk factors. Br Dent J. 2018;225(9):867-873. doi: 10.1038/sj.bdj.2018.922 [DOI] [PubMed] [Google Scholar]
- 40.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612-619. doi: 10.1200/JCO.2007.14.1713 [DOI] [PubMed] [Google Scholar]
- 41.Stein AP, Saha S, Kraninger JL, et al. Prevalence of human papillomavirus in oropharyngeal cancer: a systematic review. Cancer J. 2015;21(3):138-146. doi: 10.1097/PPO.0000000000000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Input data and methodological summary for mortality and Years of Life Lost (YLLs) for all cancers, including LOC and OPC
eTable 1. List of International Classification of Diseases (ICD) codes mapped to the Global Burden of Disease causes Lip and oral cavity cancer and Other pharynx cancer for cancer incidence data
eTable 2. Age restrictions for LOC and OPC in GBD 2019 modeling
eTable 3. Covariates for lip and oral cavity cancer
eTable 4. Covariates for other pharynx cancer
eFigure 2. Flowchart of GBD cancer incidence and Years Lived with Disability (YLDs) estimation, including LOC and OPC
eTable 5. Duration (months) of each sequalae for LOC and OPC
eTable 6. Lay description of cancer states and corresponding disability weights
eReferences
eFigure 3. Socio-demographic Index quintiles for the Global Burden of Disease Study 2019
eTable 7. Socio-demographic Index (SDI) quintiles for countries and territories estimated in GBD 2019
eFigure 4. Map of GBD world super-regions, 2019
eFigure 5. Map of GBD world regions, 2019
eTable 8. Global and regional deaths, incidence, and DALYs counts and age-standardized rates (per 100 000) for Lip and oral cavity, both sexes combined, in 2019, and change in age-standardized rates from 1990 to 2019
eTable 9. Global and regional deaths, incidence, and DALYs counts and age-standardized rates (per 100 000) for Other pharynx cancer, both sexes combined, in 2019, and change in age-standardized rates from 1990 to 2019
eTable 10. Deaths, incidence, and DALYs counts and age-standardized rates (per 100 000) for Lip and oral cavity cancer (LOC), both sexes combined, by country or territory, in 2019, and change in age-standardized rates from 1990 to 2019
eTable 11. Deaths, incidence, and DALYs counts and age-standardized rates (per 100 000) for Other pharynx cancer (OPC), both sexes combined, by country or territory, in 2019, and change in age-standardized rates from 1990 to 2019
eFigure 6. Global map of age-standardized incidence rate quintiles for A) lip and oral cavity cancer, and B) other pharynx cancer, both sexes combined, 2019
eFigure 7. Global map of A) age-standardized mortality rate quintiles, and B) age-standardized incidence rate quintiles for lip and oral cavity cancer, males, 2019
eFigure 8. Global map of A) age-standardized mortality rate quintiles, and B) age-standardized incidence rate quintiles for other pharynx cancer, males, 2019
eFigure 9. Global map of A) age-standardized mortality rate quintiles, and B) age-standardized incidence rate quintiles for lip and oral cavity cancer, females, 2019
eFigure 10. Global map of A) age-standardized mortality rate quintiles, and B) age-standardized incidence rate quintiles for other pharynx cancer, females, 2019
eFigure 11. Global absolute DALYs and age-specific DALY rates (per 100,000) for Lip and oral cavity cancer, and Other pharynx cancer by 5-year age group and sex in 2019
eFigure 12. Time trends of age-standardized DALY rates for Lip and oral cavity cancer and Other pharynx cancer from 1990 to 2019, by SDI quintile
eFigure 13. Time trends of age-standardized deaths, incidence, and DALY rates for Lip and oral cavity cancer and Other pharynx cancer from 1990 to 2019, by sex
eFigure 14. Time trends of age-specific deaths, incidence, and DALY rates for Lip and oral cavity cancer and Other pharynx cancer from 1990 to 2019, by ten-year age group globally
eFigure 15. Time trends of age-standardized deaths, incidence, and DALY rates for Lip and oral cavity cancer and Other pharynx cancer from 1990 to 2019, by GBD super-region
eFigure 16. Proportion of deaths attributable to risk factors for Lip and oral cavity cancer and Other pharynx cancer for males and females in 2019 by GBD world region
eFigure 17. Proportion of DALYs attributable to risk factors for Lip and oral cavity cancer and Other pharynx cancer A) by five-year age group globally, and B) by GBD world region, for males and females in 2019
eTable 12. Proportion of Lip and oral cavity cancer (LOC) deaths and DALYs attributable to risk factors, in 2019, by country or territory, both sexes combined
eTable 13. Proportion of Other pharynx cancer (OPC) deaths and DALYs attributable to risk factors, in 2019, by country or territory, both sexes combined
Data Sharing Statement


