Abstract
Intravesical instillation is an efficient drug delivery route for the local treatment of various urological conditions. Nevertheless, intravesical instillation is associated with several challenges, including pain, urological infection, and frequent clinic visits for catheterization; these difficulties support the need for a simple and easy intravesical drug delivery platform. Here, we propose a novel biodegradable intravesical device capable of long-term, local drug delivery without a retrieval procedure. The intravesical device is composed of drug encapsulating biodegradable polycaprolactone (PCL) microcapsules and connected by a bioabsorbable Polydioxanone (PDS) suture with NdFeB magnets in the end. The device is easily inserted into the bladder and forms a ‘ring’ shape optimized for maximal mechanical stability as informed by finite element analysis. In this study, inserted devices were retained in a swine model for 4 weeks. Using this device, we evaluated the system’s capacity for delivery of lidocaine and resiquimod and demonstrated prolonged drug release. Moreover, a cost-effectiveness analysis supports device implementation compared to the standard of care. Our data support that this device can be a versatile drug delivery platform for urologic medications.
Keywords: Bladder disorders, Implantable device, Biodegradable, Drug delivery
1. Introduction
Urologic diseases including bladder cancer, interstitial cystitis, and overactive bladder affect a substantial fraction of the population. Bladder cancer is the sixth most common cancer in the US, with more than 81,400 patients diagnosed and an estimated 17,980 deaths in 2020 [1]. It is also estimated that 3–8 million women and 1–4 million men suffer from interstitial cystitis, a chronic bladder pain syndrome [2]. Additionally, the number of patients with overactive bladder syndrome is projected to reach 3.4 million in 2027 [3]. While these statistics demonstrate the prevalence of bladder disorders, current treatment regimens are limited. This is mainly due to the difficulty of maintaining a therapeutic level of drug in the bladder while supporting urination. For example, medications such as cisplatin and fluorouracil (5-FU) (for high-risk bladder cancer treatment), and antihistamines and tricyclic antidepressants (for interstitial cystitis treatment) are dosed systemically via intravenous or oral routes, in which only small fractions of medications reach the bladder [4]. To overcome the limitations of systemic drug delivery to the bladder, intravesical drug instillation, which is used to directly administer the medications to the bladder via catheter, is applied clinically for several medications [5]. However, intravesical drug delivery (of drugs such as gemcitabine and Bacillus Calmette–Guérin (BCG) for bladder cancer, and lidocaine for interstitial cystitis) is an inconvenient procedure that requires an uncomfortable catheter placement with each visit to the clinic, and patients often experience urinary side effects including pain, hematuria, and infection. Additionally, depending on the drug used, a harsh bladder environment, including high pressure during urination and slightly acidic urine pH, challenges drug retention and leads to sub-optimal therapeutic efficacy [6].
For these reasons, several strategies have been reported as intravesical drug delivery platforms, including hydrogels and nano/micro particles with a mucoadhesive moiety (cationic polymer, fluorinated polymer), that aim to increase the drug delivery efficiency by enhancing physical retention in the bladder [7–11]. These formulation strategies have shown promising results in the preclinical models of bladder cancer, where mucoadhesive formulation delivery of chemotherapeutics is more effective than soluble forms of drugs. In addition to preclinical efforts, several formulations were investigated in the clinical trials to deliver chemotherapeutics [12,13]. However, these formulations still require multiple intravesical instillations, which are painful and inconvenient for patients; this requirement contributes to poor treatment adherence.
To address these problems, implantable bladder drug delivery devices that avoid frequent intravesical instillations have been extensively developed in the past few decades. These devices were designed to enhance local drug concentration in the bladder and to improve medication adherence through the application of a single insertion procedure. One example is a crescent-shaped UROS pump designed by Situs Corporation for women with overactive bladder [14]. This non-degradable indwelling device, consisting of a drug container and a pressure valve, enables continuous delivery of oxybutynin directly to the bladder for a prolonged period. After Phase I/II trials, no further study was reported. One possible reason for the halt is due to the large size (i. e., 10–15 cm) and the shape of the device, causing considerable patient inconvenience when removing the non-degradable device. In another example, the lidocaine releasing intravesical system (LiRIS®) developed by TARIS Biomedical (acquired by Johnson & Johnson) has completed Phase I clinical trials [15]. This device is designed to be a tube shape for ease of insertion. Then, it turns into a “pretzel” conformation inside the bladder due to the pre-programmed shape-memory wire in the device. Patients who received LiRIS® have reported negligible urinary side effects, and local (bladder) and systemic (plasma) drug levels were detected for up to 2 weeks. Overall data showed promising potential as an indwelling device platform for bladder drug delivery. However, the non-degradable LiRIS® also requires a retrieval process, leading to patient discomfort and possible complications during removal, and therefore adversely affects patient acceptability. Since patient acceptance is the crucial point for implantable systems, the limitations and complexity of surgical procedures for implantation and removal of a device can significantly impact device acceptance [16].
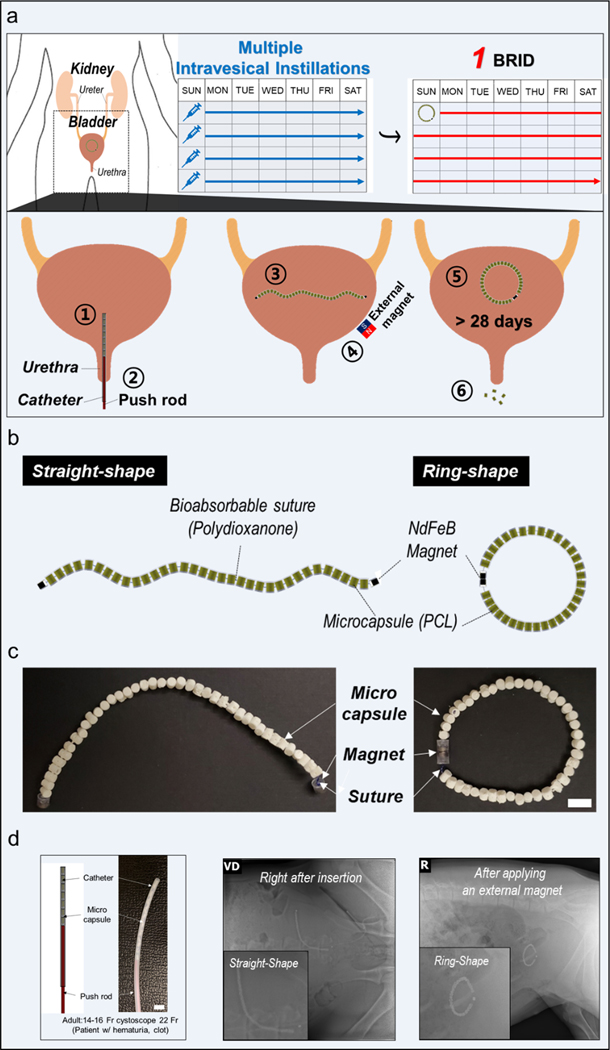
Therefore, we propose a biodegradable ring-shaped implantable device (BRID), a drug delivery platform capable of long-term and local delivery inside the bladder. Following a one-time insertion into the bladder, the BRID can release drugs in a sustained manner for a long time, which can provide advantages including higher local drug concentration and enhanced patient convenience compared to repeated intravesical instillations. Since most materials used to fabricate the BRID are made of biodegradable polymers which degrade in the bladder over time, there is no need for a retrieval procedure, further enhancing patient acceptability. The size and shape of the components of the BRID were precisely designed so that the BRID can be packed and passed via a 16-Fr catheter, the most commonly used urinary catheter in the urology clinic. Biodegradable polycaprolactone (PCL) microcapsules, which serve as drug containers, are connected by a bioabsorbable polydioxanone (PDS) suture in series with NdFeB magnets in both ends. Thus, the BRID can be easily packed in a linear shape into a 16-Fr catheter, allowing minimally invasive implantation into the bladder via the urethra. After insertion, an external magnet is applied from outside the body. Then, each magnet at the ends of the device attaches to form a firm ring-shaped device, which provides the retentive property in the bladder (Fig. 1a and Supplementary Movie.1).
Fig. 1.
Descriptive images of the BRID (a) Schematic description of intravesical delivery of the device and working principle of the BRID (b) Schematic description of the BRID. (c) Optical image of the BRID. Scale bar = 1 cm (d) Representative radiographs of the BRID immediately after insertion and after applying an external magnet in a swine model (n = 3). Scale bar = 1cm
Supplementary video related to this article can be found at https://doi.org/10.1016/j.biomaterials.2022.121703
Notably, we designed the diameter and height of the microcapsule and magnet based on previous literature reporting kidney stone passage rate by size (97.4% passage of stones <5 mm), so that each microcapsule can easily pass through the urethra after being released from the suture [17] (Fig. 3B and Supplementary Fig. 1). To support long-term sustained drug release, the drug release rate is precisely controlled according to the estimated diffusion flux and Fick’s law, and is modulated through a rate-controlling orifice in the microcapsule. Additionally, further tailoring of drug release can be fine-tuned by adjusting the orifice dimension and the numbers of microcapsules.
Fig. 3.
In vivo retention test. Representative radiograph images of pigs (n = 3) that were inserted with the BRID are shown. (a) When the external magnet was applied, device formed ring shape as shown in the image. (b) The BRID maintained ring shape for 24 days after implantation, followed by clearance at Day 35.
To examine the clinical translation of the device, we verified in vivo retention of the BRID using a swine model, which reflects the physiological and anatomical similarities to humans. The local anesthetic lidocaine and the immunomodulator resiquimod were selected as the active pharmaceutical ingredient (API) to monitor the pharmacokinetics of drugs after implantation. Addtionally, a preliminary cost-effective analysis was performed to explore the economic feasibility of the BRID. Combined data suggest that the BRID is a versatile intravesical drug delivery platform to treat urologic disorders.
2. Materials and methods
2.1. Fabrication of BRID
The microcapsules were designed using Solidworks (D’assault Systemes, Waltham, MA). Materials to fabricate microcapsules consisted of a 50:50 w/w blend of polycaprolactone (PCL) (Perstorp CAPA 6400, Malmo, Sweden) and barium sulfate (Sigma Aldrich 243,353). Constituents were melt-blended using a speed mixer model DAC 330–100 Pro (Flacktek Landrum, SC). Model “masters” with a casting sprue modeled into the geometry were printed with a 3D printer (Formlabs, Somerville, MA) using clear resin with 25-μm layer height. Masters were then used to form negative silicone molds using Moldstar 31T silicone (Smooth On, Macungie, PA) and centrifuging at 2500 rpm to remove bubbles. After curing, masters were removed from the mold; and the PCL composite material was inserted, melted, and centrifuged at the same settings to produce copies of the original design. After cooling, the microcapsules were removed and inserted into a jig to facilitate cutting of the sprue and adding perforations at defined positions in the side walls of the microcapsule. Lids for the microcapsules were fabricated by simple hole punching of 5/64″ circles from a 1 mm thick sheet of the same composite material made by melt-forming in a hydraulic press (Carver Wabash, IN). In this work, resiquimod and lidocaine pills were prepared using a Natoli pill press (NP-RD10A (Saint Charles, MO)). Each API was mixed with Polyethylene glycol (PEG) (BASF) and Magnesium stearate (Sigma) to obtain homogenous powder. The pills were prepared at a size of 2 mm in diameter and 2.8 mm in height. Prepared pills were weighed and stored at room temperature until further use. Drug pills and lids were inserted into microcapsules by controlled melt-forming using a hot plate and laser cut jig with aluminum foil cover. After that, microcapsules were assembled using a degradable polydioxanone suture of size 0.5 mm (PDS II, Ethicon) threaded through the perforations and securing the ends. Suture ends were retained in the microcapsules by melt-forming a wide knot. NdFeB Magnets (O.D 3.2 mm and 1.6 mm in length) were embedded on each end of the suture.
2.2. Numerical simulations
The simulations were carried out using the commercial Finite Element (FE) package Abaqus 2017 (SIMULIA, Providence, RI). The Abaqus/Standard Dynamic Implicit solver was employed for the simulations. The 3D FE models of the ring-shaped suture and microcapsules were constructed to investigate the response of the system under normal compression. The ring-shaped suture with a wire diameter of d = 0.45 mm and R = 160 mm was created using a 4-node linear tetrahedron (Abaqus element type C3D4). The material behavior of the suture was captured using a linear elastic-perfectly plastic model with elastic modulus , Poisson’s ratio of , and density of 1000 kg m−3 with yield stress (polydioxanone, PDSII, Ethicon) with directly imported uniaxial tension test data. The microcapsules are closed-end cylindrical shells of 3.8 mm height and 2 mm inner diameter with the wall thickness of 0.5 mm and a pair of 0.6 mm diameter through-hole drilled in the mid-height shown in Fig. 2a. The polycaprolactone microcapsules were considered to be relatively rigid with the density of 1125 kg m−3. The models of the system composed of the various number of microcapsules (0, 20, 30, and 40) and arrangements including equispaced (I), all in bottom (II), half-right and half-left (III), 4-quarter (IV), and half-top and half-bottom (V) were constructed. The response of these systems under normal compression was evaluated by application of normal strains using a rigid plate. The plate was meshed using a 4-node 3D bilinear rigid quadrilateral (Abaqus element type R3D4) and was initially positioned slightly above system. A simplified contact law (General Contact type interaction) was assigned to the models with frictionless tangential behavior; hard contact was assigned to those with the normal behavior. We performed a nonlinear dynamic implicit analysis with the quasi-static condition by lowering the plate in the 2-direction (Supplementary Movie. 3). No plastic deformation was observed. The reaction forces (P) of the plate were recorded as a function of , and converted to the effective engineering stress, , where A is the projected area of the system in the 1–3 plane. The stiffness was then computed as the initial slope of the stress-strain curves.
Fig. 2.
Characterization of the BRID performance (a) Various types of microcapsules used in this study. (b) In vitro cumulative drug release profiles of the Res_BRID and Lido_BRID incubated in 37 °C artificial urine (pH 6.8) for 45 and 15 days, respectively. Cumulative drug release amount represented the total drug release amount from the Res_BRID and Lido_BRID until each scheduled time for sampling. Markers represent the mean ± SD for n = 6–7 (Res_BRID groups) and n = 8 (Lido_BRID groups). (c) Uniaxial tensile testing. Black and white bars represent PDS_0 and PDS_3–0 for n = 3 samples per group (mean ± SD). (d) Stability test of resiquimod and lidocaine in artificial urine. HPLC analysis of samples incubated in artificial urine. Data represents area under the curve compared to fresh samples. (e) Numerical images showing the sequence of progressively deformed shape of the system under uniaxial compression at different levels of applied normal strain, , −0.12, −0.25, and −0.35. The 3D views (top) and side views (bottom) are illustrated for the system made of 40 microcapsules (C40) with R = 25.5 mm. The evolution of normalized stress as function of for C40 (blue) and ring-shaped suture (black) under compression obtained from FE simulations were plotted (right). (f) Relative stiffness of the system composed of 20 (C20 in green), 30 (C30 in grey), and 40 (C40 in blue) capsules versus the ring-shaped suture were computed for various arrangements of capsules denoted by I, II, III, IV, and V illustrated on the right.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.biomaterials.2022.121703
2.3. Uniaxial tensile testing
For maximum rupture force test, the following absorbable sutures were evaluated after fully immersed in pH 6.8 artificial urine at 37 °C on days 0, 14, and 28; 1) monofilament PDS*II suture (USP 0) and 2) monofilament PDS*II suture (USP 3–0). At the scheduled time points, each specimen was placed on a universal mechanical test machine (Instron 5943) connected with a load cell of 500 N. The distance between the grips was set to 10 cm with a load-displacement rate of 50 mm/min.
2.4. High performance liquid chromatography (HPLC) – in-vitro release analysis
High Performance Liquid Chromatography (HPLC) was used to determine the drug concentrations from all in vitro release assays. An Agilent 1260 Infinity II HPLC system equipped with a quaternary pump, autosampler, thermostat, control module, and diode array detector was utilized as described previously. Data processing and analysis was performed using OpenLab CDS ChemStation®.
Lidocaine was separated on an Agilent Zorbax Eclipse XDB C18 analytical column 4.6 × 150 mm with 5 μm particles, maintained at 40 °C. The optimized mobile phase consisted of A: 0.1% phosphoric acid in water and B: acetonitrile. Gradient elution was employed over a 3-min period beginning with 95% A and ending with 40% A at a flowrate of 1 mL/min. The total run time was 5 min, and the post run time was 2.5 min. The injection volume was 10 μL, and the selected ultraviolet (UV) detection wavelength was 230 nm at a bandwidth of 4.0, no reference wavelength, and an acquisition rate of 10 Hz.
Resiquimod was separated on an Agilent Zorbax Eclipse XDB C18 analytical column 4.6 × 150 mm with 5 μm particles, maintained at 40 °C. The optimized mobile phase consisted of A: 0.1% phosphoric acid in water and B: acetonitrile. Gradient elution was employed over a 3-min period beginning with 90% A and ending with 50% A at a flowrate of 1 mL/min. The total run time was 6 min, and the post run time was 3 min. The injection volume was 5 μL, and the selected UV detection wavelength was 242 nm at a bandwidth of 4.0, no reference wavelength, and an acquisition rate of 10 Hz.
2.5. Liquid chromatography time of flight mass spectrometry (LC-TOF) – urine analysis
Liquid Chromatography Time of Flight Mass Spectrometry (LC-TOF) was used to determine model drug concentrations from all in vivo urine samples. An Agilent 1260 Infinity II HPLC system equipped with a quaternary pump, autosampler, thermostat, and diode array detector was coupled to an Agilent 6224 accurate mass Time of Flight (TOF) mass spectrometer for the detection of model drugs in urine. Data processing and analysis was performed in Masshunter® TOF suite.
Lidocaine (API) and Procainamide (ISTD) were separated on an Agilent Poroshell 120 EC-C18 analytical column 4.6 × 150 mm with 2.7 μm particles (4.6 × 50 mm guard column with 2.7 μm particles installed), maintained at 40 °C. The optimized mobile phase consisted of A: 0.1% formic acid in water and B: acetonitrile. Gradient elution was employed over a 5-min period beginning with 95% A and ending with 5% A at a flowrate of 0.600 mL/min. The total run time was 6 min, and the post run time was 3.5 min. The injection volume was 10 μL, and the selected UV detection wavelength was 230 nm at a bandwidth of 4.0, no reference wavelength, and an acquisition rate of 10 Hz. Lidocaine and procainamide underwent electrospray ionization (positive mode) with a source gas temp of 350 °C, drying gas flow rate of 10 L/min, and nebulizer pressure of 40 psi. The capillary voltage was set to 4000 V, and the Fragmentor was set to 80 V. Reference solution containing calibration standards of 121.0508 m/z and 922.0098 m/z were continually introduced at a nebulizer pressure of 5 psi to maintain a low m z−1 deviation of less than <5 ppm. Lidocaine was quantitated at 235.1852 m/z (M + H)+, and Procainamide was quantitated at 236.1794 m/z (M + H)+ within a mass window of 20 ppm. An LLOQ of 5 ng/mL was achieved with a signal-to-noise ratio of S/N = 11 against RMS.
Resiquimod (API) and Imiquimod (ISTD) were separated on an Agilent Poroshell 120 EC-C18 analytical column 4.6 × 150 mm with 2.7 μm particles (4.6 × 50 mm guard column with 2.7 μm particles installed), maintained at 40 °C. The optimized mobile phase consisted of A: 0.1% formic acid in water and B: acetonitrile. Gradient elution was employed over a 5 min period beginning with 95% A and ending with 5% A at a flowrate of 0.500 mL/min. The total run time was 6 min, and the post run time was 3.5 min. The injection volume was 5 μL, and the selected UV detection wavelength was 242 nm at a bandwidth of 4.0, no reference wavelength, and an acquisition rate of 10 Hz. Resiquimod and Imiquimod underwent electrospray ionization (positive mode) with a source gas temp of 350 °C, drying gas flow rate of 12 L/min, and nebulizer pressure of 35 psi. The capillary voltage was set to 4000 V, and the Fragmentor was set to 110 V. Reference solution containing calibration standards of 121.0508 m/z and 922.0098 m/z were continually introduced at a nebulizer pressure of 5 psi to maintain a low m/z deviation of less than <5 ppm. Resiquimod was quantitated at 315.4517 m/z (M + H)+, and Imiquimod was quantitated at 241.3174 m/z (M + H)+ within a mass window of 20 ppm. An LLOQ of 2.5 ng/mL was achieved with a signal-to-noise ratio of S/N = 9.4 against RMS.
2.6. Liquid chromatography triple quadrupole mass spectrometry (LC-MS/MS)
Liquid Chromatography Triple Quadrupole Mass Spectrometry (LQ-MS/MS) was used to determine model drug concentrations in all in vivo serum and plasma samples. An Agilent 1200 HPLC system with a binary pump, autosampler, and thermostat was coupled to a Thermo Scientific TSQ Quantiva triple quadrupole mass spectrometer. Data sets were generated using the Xcalibur® LC-MS control suite, and data processing and analysis was performed in TraceFinder®.
Lidocaine (API) and Procainamide (ISTD) were separated on an Agilent Poroshell 120 EC-C18 analytical column 4.6 × 150 mm with 2.7 μm particles, maintained at 45 °C. The optimized mobile phase consisted of A: 0.1% formic acid and 10 mM ammonium formate in water and B: acetonitrile. Isocratic elution was employed over 4 min with 70% A and 30% B. The injection volume was 10 μL. Lidocaine and procainamide underwent electrospray ionization (positive mode) with an ion transfer tube temperature of 400 °C, vaporizer temperature of 450 °C, sheath gas flow rate of 50 (arbitrary units), auxiliary gas flow rate of 15 (arbitrary units), and sweep gas flow rate of 1 (arbitrary units). The capillary voltage was set to 4500 V, and there was no in-source fragmentation. Lidocaine and Procainamide were monitored under SRM (selective reaction monitoring) with transitions of 234.9 m/z → 86.075 m/z and 236.2 m/z → 162.9 m/z for lidocaine and procainamide, respectively. Q1 resolution was set to 0.7 Da, Q3 resolution was set to 0.4 Da, the CID gas was set to 2 mTorr, and the collision energies were set as 16 V and 18 V for lidocaine and procainamide, respectively. An LLOQ of 100 pg/mL was achieved using the sample preparatory and analytical methodologies conveyed in this report.
2.7. In vitro performance test
The in vitro drug release profiles of resiquimod and lidocaine were examined in pH 6.8 artificial urine at 37 °C with continuous stirring at 50 rpm in a shaking incubator (INNOVA44, USA). Artificial urine (pH 6.8) was prepared freshly before the use [18]. The microcapsules of different types from resiquimod and lidocaine were each fully immersed in the release media, and aliquots were collected at scheduled intervals for 35 and 14 days, respectively. Then, the collected samples were measured with HPLC to determine the amount of drug that was released. All experiments were performed in triplicate for each type of microcapsule.
2.8. Assessing drug stability
To assess the drug stability of lidocaine and resiquimod in urine, we performed chemical and functional assays using artificial urine. Briefly, lidocaine and resiquimod solutions were prepared at 0.1 mg/mL in artificial urine and aliquoted into 1.5 mL tubes. Samples were stored at 37 °C incubator with 50 RPM rotation. At pre-determined time points, samples were collected and frozen at −80 °C. Samples were analyzed by HPLC analysis to compare retention time and area to that of Day 0 samples to examine the chemical stability.
2.9. In vivo procedure on swine
All procedures conformed to the protocols approved by the Massachusetts Institute of Technology Committee on Animal Care. Female Yorkshire pigs weighting approximately 60–80 kg were fasted overnight with ad libitum access to water. Pigs were sedated with intramuscular injection of midazolam 0.25 mg/kg and dexdomitor 0.03 mg/kg and intubated and maintained on isoflurane (2–3% in oxygen) and their vital signs were monitored until end of anesthesia. Following insertion of a small speculum with surgical lubricant, a 16 Fr catheter tube with smooth rounded edge was placed into the bladder of the pig. The BRID was then placed inside the catheter and further pushed into the bladder using the push rod made from Polyethylene (PE) close to the inner diameter of the catheter (Fig. 1d). Once the BRID was inserted in the bladder, the external magnet was applied near the bladder of the pig to connect the NdFeB magnets on the BRID. The catheter was then removed once the magnet was applied, and pigs were immediately imaged via radiographs to confirm device implantation in the bladder (Fig. 3). Either a central venous catheter was inserted into the ear vein using the Seldinger technique to allow for a frequent blood sampling or mammary bleed via butterfly needle (Terumo Surflo Winged Infusion set 21 G x 3/4”) to collect a one-time blood sample. Blood was collected in EDTA tubes and centrifuged at 3202 g for 10 min at 4 °C. Plasma was aliquoted and stored at −80 °C. To collect urine samples, a 12 Fr catheter was placed in the urinary bladder while under anesthesia as described above. Once pigs were returned to their pen, they were administered intramuscularly atipamezole to reverse the dexmedetomidine and monitored until ambulatory. Once pigs were returned to their pen, they were administered intramuscularly atipamezole to reverse the dexmedetomidine and monitored until ambulatory.
2.10. Pharmacokinetic of lidocaine delivered using the BRID
For the bolus dosing groups, animals were catheterized as described above, and lidocaine solution (1 mg in 4 mL saline) was infused to the bladder followed by 20 mL saline to flush residual drug in the catheter. For the BRID-inserted groups, a total of 160 mg of lidocaine was loaded into the drug reservoirs and inserted into the animals as described above. At pre-determined time points, urine and plasma samples were collected and drug concentration was examined by LC/MS-MS method as described above. In the resiquimod study, the experimental procedure was followed identically to the lidocaine study. A bolus dose was given at 1 mg of resiqiumod per animal, and 16 mg of resiquimod was loaded into the BRID. Due to complications associated with resiquimod, the study was terminated at Day 7.
2.11. Histology and urine cytokine ELISA
At the end of the study, bladder tissue was collected from euthanized animals. Bladder tissue was fixed in 4% formalin solution and stored in 70% ethanol solution. Fixed samples were then processed for paraffin-embedding followed by H&E staining. Both luminal and cross section of bladder were analyzed. Stained samples were imaged by Aperio Digital Pathology Slide Scanners (Leica). Urine cytokine IL-6 was measured using ELISA method according to the manufacturer’s protocol (Thermo).
2.12. Cost effectiveness model design
To investigate the potential economic benefit of BRID over intravesical instillation, we performed a cost effectiveness analysis using a multi-state Markov cohort model in TreeAge Pro comparing the BRID system to standard therapy, Bacillus Calmette–Guérin (BCG) vaccination, in patients with non-muscle invasive bladder cancer (NMIBC). Model parameters are adopted from the previous studies [19–23].
The health state transition model is initiated after all patients undergo transurethral resection of a bladder tumor (TURBT), after which they either undergo a standard BCG intravesical instillation or BCG intravesical instillation via BRID. The primary outcome was the incremental cost-effectiveness ratio (ICER) with a willingness to pay of $100,000/quality adjusted life year (QALY) [24]. The cycle length of the model was 1 month over a time horizon of 3 years, which is the standard length of BCG maintenance treatment post TURBT [23]. Median survival was modeled over a three-year period in which costs and utilities were measured, limiting the model to the active treatment period only. Global discounting of 3% and the half cycle correction were applied. The model consisted of the following health transition states: 1) Tumor-free post TURBT (on maintenance BCG), 2) No recurrence (continue maintenance BCG), 3) Tumor-free post radical cystectomy (RC), 4) Metastasis, 5) Death, 6) Post-recurrence (on maintenance BCG).
3. Results
3.1. Design of the biodegradable ring-shaped implantable device (BRID)
The BRID was designed to be inserted in the bladder in a minimally invasive fashion using a catheter and to have the ability to form a ring-shaped feature, which provides the long-term retentive property in the bladder. Thus, the BRID enables the controlled release of therapeutic agents for a long-term period without burst release. As shown in Fig. 1 b, the BRID consists of three major components: microcapsules, suture, and NdFeB magnets. In this study, we prepared two different microcapsules with ratios of PCL and BaSO4, such as 60:40 and 50:50. And we finally chose a 50:50 ratio (PCL: BaSO4) microcapsule considering x-ray visibility (Supplementary Fig. 4). The microcapsules’ body was cast from 50:50 w/w blend of polycaprolactone (PCL, MW 37 K) and barium sulfate covered by a disk-shaped lid with a micro-hole as a drug-releasing outlet. We varied the cross-sectional area of the micro-hole from 0.028 mm2 to 0.523 mm2, using a computerized numerical control (CNC) milling machine with different sizes of end mills to examine the effect of the micro-hole on drug release (Fig. 2a). The microcapsules are 2.9 mm in diameter and 3.5 mm in thickness (inner diameter and height of drug containers are 2 and 2.8 mm, respectively), giving a total volume of approximately 23.1 μL. In this work, a drug container was filled with drug pills formulated with API and binder excipients. Then, lids were inserted into microcapsules and closed by heat sealing. After that, microcapsules were connected to a degradable PDS suture threaded through the perforations and securing the ends. Intervening PDS elements provided stability of the BRID macrostructure. Suture ends were retained in the microcapsules by melt-forming a wide knot. NdFeB magnets (O.D 3.2 mm and 1.6 mm in length) were attached to each end of the suture. Fig. 1c shows the optical images of the BRID prepared in this work.
3.2. In vitro performance test
For in vitro characterization, we selected resiquimod, an immune response modifier [25], and lidocaine, a local anesthetic [26] model drug, to demonstrate the versatility of the BRID as a platform. In this work, drug containers were filled with either resiquimod or lidocaine pills, which showed a reproducible drug loading amount of 3.7 ± 0.1 mg or 5.2 ± 0.2 mg, respectively.
The in vitro release profiles of resiquimod and lidocaine were examined with the microcapsules, which each contained a single micro-hole of a different cross-sectional area (Fig. 2a). To initiate drug release from the microcapsule, the water first infiltrated via the micro-hole to reach the drug formulation; then, the solubilized drug molecules diffused out via the same micro-hole.
For this reason, drug release was more sustained as the size of the micro-hole decreased, as shown in Fig. 2b. Notably, almost zero-order release was observed after the release onset for all micro-holes, and the release rate could be tuned by changing micro-holes’ cross-sectional area, according to Fick’s law and the estimated diffusion flux. Thus, depending on the dimensions of the micro-holes, the release rates from resiquimod and lidocaine could be tuned from 0.51%/day to 1.99%/day over 45 days and from 6.37%/day to 9.97%/day over 15 days, respectively (Fig. 2b). Additionally, we performed a maximum rupture test using two absorbable sutures of different thicknesses after completely immersing them in 37 °C artificial urine on days 0, 14, and 28. As a result, we confirmed that the maximum rupture force was comparable to bladder contraction force [27] and the drug delivery and device disassembly period could be programmed according to the degree of degradation of the absorbable suture (Fig. 2c).
3.3. Drug stability data
To ensure the drug stability, we examined the chemical properties of resiquimod and lidocaine using HPLC analysis. In order to simulate the stability in the bladder, we prepared the drug stocks in artificial urine (pH 6.8) and observed up to 28 days. Compared to the HPLC measured area value of samples at Day 0, samples from the late time points showed identical values, demonstrating that both drugs maintained their chemical properties (Fig. 2d). These data supported the suitability of the model drugs for in vivo applications, and were reliable for analytical measurement, including HPLC and LC/MS.
3.4. Finite element simulations
Next, we employed finite elements (FE) simulations to characterize the mechanical response of the BRIDs under uniaxial compression. This data was used to inform the quantitative understanding of the effective stiffness of the system deployed in the lower abdomen subjected to the bladder muscle forces [27]. We developed three-dimensional (3D) FE models of systems composed of a ring-shaped polymeric wire sutured through cylindrical-shaped microcapsules, and performed dynamic implicit analysis to assess the behavior of the systems under compression using a rigid plate (see Numerical Simulations in Methods for details).
In Fig. 2e, we present snapshots obtained from the nonlinear FE simulations showing the undeformed, , and deformed configurations of the system made of 40 microcapsules (C40) positioned equispaced along the wire at different levels of applied strain, , −0.25 (III), and −0.35 (IV), demonstrating that C40 can robustly undergo an elastic deformation without any plastic effects. The evolution of normalized effective stress, , required to compress the system as a function of the corresponding was plotted for C40 (blue) and the ring-shaped suture without capsules (black), clearly showing that higher forces are required to deform C40. As Fig. 2f shows, we further numerically examined the effects of the microcapsules on the compression behavior of the systems by monitoring the relative effective stiffness of the system, , where and are the stiffness of the system and ring-shaped suture (no capsules), respectively (see Numerical Simulations in Methods for details, and Supplementary Movie. 3). In particular, we considered BRIDs composed of various numbers of the microcapsules (20, 30, and 40 denoted by C20, C30, and C40, respectively) and arrangements (I, II, III, IV, and V), as shown on the right. The results in the bar plots (left) demonstrate that the effective stiffness of all systems is higher than the ring-shaped suture (i.e., means improvement in the stiffness) with the lowest improvement for the arrangement I in which the microcapsule are equispaced positioned with , , and . Furthermore, considerably depends on the arrangement of microcapsules and loading directions, however, increasing the microcapsules enhances . Together, these results showcased the ability to quantitatively evaluate the multiple configurations of BRIDs to enhance the effective stiffness further and allow for customizations to meet specific application needs.
3.5. Long-term retention of BRID in porcine bladder
To evaluate the BRID local drug delivery platform, 60- to 80-kg female Yorkshire pigs that have similar urinary tract anatomy to humans were utilized [28,29]. The BRID, which can be easily loaded into catheter, was non-invasively inserted into the bladder via the urethra (Fig. 1d and Supplementary Fig. 2). Then, a straight shape of the device was immediately deployed, and a firm ring shape of the device was formed after an external magnet was applied (Supplementary Movie. 1). In this study, safe long-term retention in the bladder was assessed by serial radiographs obtained over a period of 1 month (Fig. 3a). In addition, since each microcapsule was designed to easily pass through the urethra after the suture was completely degraded, each microcapsule separated from the suture, and all of them passed through the urethra without causing any clinical signs (Fig. 3b and Supplementary Fig. 1a). In a separate study, we confirmed that the single microcapsules are rapidly excreted within 48 h after delivery (Supplementary Fig. 1b).
3.6. In vivo pharmacokinetic profile
As described above, animals received lidocaine via the bolus (1 mg) or the BRID (160 mg), and urine and plasma samples were collected to measure local drug concentration and systemic exposure, respectively (Fig. 4a).
Fig. 4.
In vivo pharmacokinetic and safety profiles (a) Study design is described. (b–c) Lidocaine analysis (b) Drug concentration measured by LC/MS-MS in urine are shown. (c) Drug concentration measured by LC/MS-MS in plasma are shown with the mean value and SEM. Markers represent the mean ± SEM for n = 3 (d) Histology analysis of bladder tissue collected from animals received the BRID, scale bar = 2 mm (e) Urine levels of IL-6 measured by ELISA. Markers represent the mean ± SEM for n = 3.
For the bolus-infused group, average concentration of lidocaine in the urine peaked at 2 h at concentration of 1172 ng/mL, and was not detected at 24 h. Lidocaine delivered by the BRID showed different kinetics where the highest concentration in urine was 13,447 ng/mL at Day 1. Lidocaine in urine was detected up to Day 24 (Fig. 4b). Lidocaine plasma concentration, which represents systemic exposure, is negligible in the bolus and the BRID group, where the peak concentrations were 150 ng/mL and 52 ng/mL, respectively. These concentrations are significantly lower than the reported toxic concentration of 5 mg/mL (Fig. 4c) [30].
In a separate study, we performed the identical protocol using another model drug resiquimod (Supplementary Fig. 3). Similar to the lidocaine study above, resiquimod delivered via bolus was cleared from the urine 24 h after the infusion. Animals who received resiquimod via BRID showed sustained release kinetics where peak concentration at Day 1 was 11,695 ng/mL, followed by Day 7 concentration of 116 ng/mL. Study using resiquimod was terminated at Day 7, and further repeats were discontinued due to toxicity associated with the drug.
3.7. In vivo safety
To investigate the effect of the BRID in the bladder, bladder tissue of animals that received Lidocaine loaded BRID was collected for histological analysis and urine samples were collected to measure pro-inflammatory cytokines.
Bladder tissue was collected from the BRID-inserted animal and processed with H&E staining to examine the infiltration of inflammatory cells. As shown in Fig. 4d, the urothelial lining of the bladder had direct contact with the BRID showed no signs of tissue damage or inflammation. The cross-section of the bladder also shows normal cellular structure without signs of inflammation.
The pro-inflammatory cytokine IL-6 is reported to be higher in patients with interstitial cystitis [31]. Hence, we measured urine levels of IL-6 to investigate whether long-term retention of the BRID triggers inflammation. To compare with a bladder in a pro-inflammatory state, we utilized urine samples from the animals that received a bolus dose of resiquimod, which can induce cytokine secretion in the bladder [32,33]. As shown on Fig. 4e, resiquimod-treated animals show IL-6 in urine samples, which is consistent with previous studies. However, we did not observe IL-6 from the BRID inserted animals. Combined histology and cytokine data demonstrate that implantation of the BRID does not trigger inflammation in the bladder and should be safe for patients.
3.8. Cost-effectiveness modeling comparing BRID vs. conventional bolus method
To inform the potential cost-effectiveness of implementation of BRID, we conducted a cost-effectiveness modeling analysis. The base case analysis compared standard BCG intravesical induction and maintenance treatment, which consists of 6 initial weekly instillations followed by 3 instillations at 3, 6, 12, 18, 24, and 36 months, to the BRID system requiring 2 instillations at weeks 0 and 3 followed by 1 instillation at 3, 6, 12, 18, 24, and 36 months (Supplementary Fig. 5a). The BRID system was found to be cost-saving compared to standard BCG treatment; it was less expensive and more effective (dominated standard BCG treatment) (Fig. 5).
Fig. 5.
Cost effectiveness model (a) Health state transition diagram for the simulation of BRID versus bolus instillation of BCG for patients with NMIBC (c) Results of cost-effectiveness analysis comparing BRID versus standard bolus BCG.
One-way deterministic sensitivity analyses were performed to measure the effects of parameter uncertainties on the outcome of the model. Ranges for parameters were taken from the published literature. Where published values were not available, parameters were varied by ± 25% (costs) and from 0 to 1 (utilities) as in other studies [34–36]. No parameter variation was found to change the model outcome, and the BRID system remained cost-saving (Supplementary Fig. 5b).
4. Discussion
Intravesical therapy has transformed the ability to effectively treat urologic disorders such as interstitial cystitis, overactive bladder syndrome, and, most extensively, bladder cancer. Multiple intravesical instillations can provide a high drug concentration in the bladder and minimize systemic toxicity and side effects compared to systemic administration routes. However, urinary catheterization procedures are painful and inconvenient for patients and can cause infections associated with repeat clinic visits, resulting in poor medication adherence. Importantly, due to the rapid washout from urination, frequent treatments are often required, especially when considering the maintenance regimen of BCG in bladder cancer.
Therefore, we propose the biodegradable ring-shaped implantable device (BRID) capable of long-term, sustained localized drug delivery, which does not require a retrieval procedure. With a one-time insertion, the indwelling BRID can reside in the bladder over 28 days while releasing drugs in a sustained manner, thereby replacing repeated intravesical instillations to obtain a constant therapeutic drug level. In particular, the BRID, in which microcapsules are connected by a suture in series with NdFeB magnets at both ends, is designed to be a linear shape for easy insertion. Thus, the device can be easily packaged into a catheter, enabling minimally invasive insertion through the urinary tract. After that, the device conforms to a ring-shaped device, preventing unwanted device clearance from the bladder during urination. As most materials described here are made of biodegradable polymers (PCL and PDS), there is no need for a retrieval procedure, further increasing patient acceptability. We observed the suture degradation induced device disassembly around day 28 (Fig. 3). Once the device becomes a collection of individual capsules, they are eliminated from the bladder without any surgical procedure. As described above, we designed the diameter and height of the microcapsule based on previous literature reporting kidney stone passage rates by size [17]. Hence, we expect that each microcapsule to easily pass through the urethra (avg diameter 6 mm) without pain after being released from the suture. As shown in Supplementary Movie. 2 and Supplementary Fig. 1, we demonstrated that single microcapsules easily pass through the urinary tract when dissociated from the device.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.biomaterials.2022.121703
Furthermore, the BRID loaded with lidocaine was well tolerated in the animals over a long period of time without any apparent changes in urinary behaviors and was observed to be BAR. As shown in Fig. 4F, negligible inflammation (and an insignificant number of polymorphonuclear cells) was examined around the BRID regardless of the tissue location. Urine cytokine data further supports that the BRID residency in the bladder does not elicit an inflammatory response. Considering the clinical application of BRID for the treatment of bladder disorders, more consideration may be required regarding safety and patient comfort.
In addition to the preclinical data, cost-effectiveness analysis further supports the benefit of the BRID compared to conventional bolus infusion. Our preliminary cost-effectiveness analysis found that the BRID could be cost-saving compared to standard maintenance BCG treatment. This is likely due to improved quality of life and reduced healthcare costs from fewer clinic visits and procedures. Our cost-effectiveness model assumes that BCG administration via the BRID system is as effective as BCG delivered via standard intravesical administration. While our in vivo data suggests this could be true, a clinical trial comparing the two administration methods is needed to test this assumption, and updated cost-effectiveness analysis should be performed. Additionally, our model assumes that patients prefer an implantable system due to its ability to reduce clinic visits and procedures. This may not be true for all patients, and further studies are needed to understand the utility of implantable devices in this patient population [37].
The composition of the microcapsules and sutures can also be modified with other biodegradable polymers [38,39] such as PLA, PGA, PLGA, or Zn/Mg alloy, depending on the indication and physiology of patients’ bladder. The recommended intravesical lidocaine dose for a human is 200 mg per bolus dose, which is similar to the amount that a single BRID can deliver [40]. By changing the orifice dimension and the number of microcapsules, we can optimize the drug release according to the dosage regimen. Interestingly, we only loaded 16 mg of resiquimod on the BRID, compared to 160 mg of lidocaine on the loaded BRID. However, drug concentration profiles were relatively similar in both model drugs. As shown in Fig. 2b, in vitro drug release kinetics from the BRID was different in the two drugs, where resiquimod shows a slow, sustained release while lidocaine shows an initial burst release, which may be explained by their chemical properties. Hence, combined data demonstrate that in vitro release kinetics of the BRID can serve as an indicator for in vivo drug release. In that respect, our device could also be applied to other drugs used for intravesical delivery that require long-term sustained release. For example, BRID can be loaded with chemotherapeutics, including gemcitabine and mitomycin C after the transurethral resection of bladder tumor (TURBT) to target small residual tumors that were not detected or treated during the surgery [41]. Clinical evaluation of a gemcitabine-loaded intravesical device reported that patients have not experienced treatment-related side effects and showed promising therapeutic efficacy against the bladder cancer, which further supports that long term intra-vesical resident delivery of chemotherapeutics can provide sufficient drug concentration to effectively kill tumor without causing critical side effects [42]. One critical aspect of long-term resident systems is their capacity to mitigate the risk of rapid drug release given the total drug load. This critical aspect can be mitigated through the use of controlled deliver with solid formulations [43,44]. Additionally, each microcapsule can load any drug pill, which allows for combination drug therapy depending on the disease and its therapeutic regimen. Future development of intra-vesical degradable and excretable systems will require larger sample sizes to support further validation of the pharmacokinetics. Moreover, pharmacodynamics in disease states will have to be established given the recognized variable tissue drug absorption.
In conclusion, we describe the development of the BRID as a device platform for intravesical therapy of bladder disorders that provides several advantages: (1) it requires only a one-time minimally invasive intravesical instillation without a retrieval procedure, which could increase patient acceptance; (2) its ring-shape provides long-term retentive features in the bladder; (3) it degrades in a predictable manner to exit the bladder with minimal risk of potential obstruction (biodegradable materials and small size (<5 mm)); and (4) it allows sustained localized drug delivery for long time periods that can provide high drug concentration at the bladder, thereby avoiding multiple intravesical instillations. Notably, our in vivo results showed that urine drug concentration could be maintained at a steady level until the drug release is completed.
Therefore, we conclude that the BRID for intravesical therapy is an ideal platform to administer long-term localized delivery of therapeutic agents for the treatment of bladder disorders.
Supplementary Material
Acknowledgments
We are grateful to all of the other members of the Langer and Traverso laboratories for their expertise and discussions around drug delivery. We thank the Histology Core at the Koch Institute for Integrative Cancer Research, MIT.
Funding
This work was supported in part by the Karl van Tassel (1925) Career Development Professorship, Department of Mechanical Engineering, MIT. J.C was supported by supported by NIH 5T32DK007191-45
Footnotes
Credit author
H. K., S.L. and G.T. designed the study. H. K., and S.L. performed the experiments, analyzed and interpreted the data and wrote the manuscript. S.L. designed, fabricated, and evaluated the prototypes of the system with H.K., A. W., C. E., R. M., S. P., and Q. W. S. B analyzed finite element (FE) simulations of the BRID and J. E. C. and J. C. performed cost-effectiveness model design of the BRID. H.K., and S. L. performed and helped to analyze the in vivo evaluation of the system with A. H., K. I., J. K., and J. J. A.L., K. H., J. M., N. K., and H. K., analyzed drugs. D. W. reviewed and edited the manuscript. R.L., and G.T. supervised the research and reviewed the data, and edited the manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: www.dropbox.com/sh/szi7vnr4a2ajb56/AABs5N5i0q9AfT1IqIJAE-T5a?dl=0www.dropbox.com/s/yc3xqb5s8s94v7x/Rev Complete details of all relationships for profit and not for profit for G.T. can be found at the following link: https://www.dropbox.com/sh/szi7vnr4a2ajb56/AABs5N5i0q9AfT1IqIJAE-T5a?dl=0. Complete details for R.L. can be found at the following link: https://www.dropbox.com/s/yc3xqb5s8s94v7x/Rev%20Langer%20COI.pdf?dl=0.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.biomaterials.2022.121703.
Data availability
Data will be made available on request.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, CA, Cancer J. Clin 70 (2020) 7–30, 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- [2].Konkle KS, Berry SH, Elliott MN, Hilton L, Suttorp MJ, Clauw DJ, Clemens JQ, Comparison of an interstitial cystitis/bladder pain syndrome clinical cohort with symptomatic community women from the RAND Interstitial Cystitis Epidemiology study, J. Urol 187 (2012) 508–512, 10.1016/j.juro.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Puckrein G, Walker D, Xu L, Congdon P, Gooch K, The prevalence and forecast prevalence of overactive bladder in the medicare population, Clin. Med. Insights Urol 12 (2019), 1179561119847464, 10.1177/1179561119847464. [DOI] [Google Scholar]
- [4].Patel VG, Oh WK, Galsky MD, Treatment of muscle-invasive and advanced bladder cancer in 2020, CA, Cancer J. Clin 70 (2020) 404–423, 10.3322/caac.21631. [DOI] [PubMed] [Google Scholar]
- [5].Witjes JA, Debruyne FMJ, Intravesical chemotherapy for superficial bladder cancer, Nat. Clin. Pract. Urol 1 (2004) 56–57, 10.1038/ncpuro0009. [DOI] [PubMed] [Google Scholar]
- [6].Joice GA, Bivalacqua TJ, Kates M, Optimizing pharmacokinetics of intravesical chemotherapy for bladder cancer, Nat. Rev. Urol 16 (2019) 599–612, 10.1038/s41585-019-0220-4. [DOI] [PubMed] [Google Scholar]
- [7].Guo H, Li F, Xu W, Chen J, Hou Y, Wang C, Ding J, Chen X, Mucoadhesive cationic polypeptide nanogel with enhanced penetration for efficient intravesical chemotherapy of bladder cancer, Adv. Sci 5 (2018), 10.1002/advs.201800004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li G, Lei Q, Wang F, Deng D, Wang S, Tian L, Shen W, Cheng Y, Liu Z, Wu S, Fluorinated polymer mediated transmucosal peptide delivery for intravesical instillation therapy of bladder cancer, Small 15 (2019) 1–11, 10.1002/smll.201900936. [DOI] [PubMed] [Google Scholar]
- [9].Xu X, Liu K, Jiao B, Luo K, Ren J, Zhang G, Yu Q, Gan Z, Mucoadhesive nanoparticles based on ROS activated gambogic acid prodrug for safe and efficient intravesical instillation chemotherapy of bladder cancer, J. Contr. Release 324 (2020) 493–504, 10.1016/j.jconrel.2020.03.028. [DOI] [PubMed] [Google Scholar]
- [10].Donin NM, Duarte S, Lenis AT, Caliliw R, Torres C, Smithson A, Strauss-Ayali D, Agmon-Gerstein Y, Malchi N, Said J, Raman SS, Holden S, Pantuck A, Belldegrun AS, Chamie K, Sustained-release formulation of mitomycin C to the upper urinary tract using a thermosensitive polymer: a preclinical study, Urology 99 (2017) 270–277, 10.1016/j.urology.2016.09.039. [DOI] [PubMed] [Google Scholar]
- [11].Lin T, Wu J, Zhao X, Lian H, Yuan A, Tang X, Zhao S, Guo H, Hu Y, In situ floating hydrogel for intravesical delivery of adriamycin without blocking urinary tract, J. Pharmacol. Sci 103 (2014) 927–936, 10.1002/jps.23854. [DOI] [PubMed] [Google Scholar]
- [12].Bassi PF, Volpe A, D’Agostino D, Palermo G, Renier D, Franchini S, Rosato A, Racioppi M, Paclitaxel-hyaluronic acid for intravesical therapy of bacillus Calmette-Guérin refractory carcinoma in situ of the bladder: results of a phase I study, J. Urol 185 (2011) 445–449, 10.1016/j.juro.2010.09.073. [DOI] [PubMed] [Google Scholar]
- [13].McKiernan JM, Barlow LJ, Laudano MA, Mann MJ, Petrylak DP, Benson MC, A phase I trial of intravesical nanoparticle albumin-bound paclitaxel in the treatment of bacillus Calmette-Guérin refractory nonmuscle invasive bladder cancer, J. Urol 186 (2011) 448–451, 10.1016/j.juro.2011.03.129. [DOI] [PubMed] [Google Scholar]
- [14].Fraser MO, Lavelle JP, Sacks MS, Chancellor MB, The future of bladder control-intravesical drug delivery, a pinch of pepper, and gene therapy, Rev. Urol 4 (2002) 1–11. [PMC free article] [PubMed] [Google Scholar]
- [15].Nickel JC, Jain P, Shore N, Anderson J, Giesing D, Lee H, Kim G, Daniel K, White S, Larrivee-Elkins C, Lekstrom-Himes J, Cima M, Continuous intravesical lidocaine treatment for interstitial cystitis/bladder pain syndrome: safety and efficacy of a new drug delivery device, Sci. Transl. Med 4 (2012), 3003804, 10.1126/scitranslmed.3003804. [DOI] [PubMed] [Google Scholar]
- [16].Pons-Faudoa FP, Ballerini A, Sakamoto J, Grattoni A, Advanced implantable drug delivery technologies: transforming the clinical landscape of therapeutics for chronic diseases, Biomed. Microdevices 21 (2019), 10.1007/s10544-019-0389-6s10544-019-0389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ahmed AF, Gabr AH, Emara AA, Ali M, Abdel-Aziz AS, Alshahrani S, Factors predicting the spontaneous passage of a ureteric calculus of ≤10 mm, Arab J. Urol 13 (2015) 84–90, 10.1016/j.aju.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang Q, Neoh KG, Xu L, Lu S, Kang ET, Mahendran R, Chiong E, Functionalized mesoporous silica nanoparticles with mucoadhesive and sustained drug release properties for potential bladder cancer therapy, Langmuir 30 (2014) 6151–6161, 10.1021/la500746e. [DOI] [PubMed] [Google Scholar]
- [19].Bachir BG, Dragomir A, Aprikian AG, Tanguay S, Fairey A, Kulkarni GS, Breau RH, Black PC, Kassouf W, Contemporary cost-effectiveness analysis comparing sequential bacillus Calmette-Guerin and electromotive mitomycin versus bacillus Calmette-Guerin alone for patients with high-risk non–muscle-invasive bladder cancer, Cancer 120 (2014) 2424–2431. [DOI] [PubMed] [Google Scholar]
- [20].Kulkarni GS, Alibhai SMH, Finelli A, Fleshner NE, Jewett MAS, Lopushinsky SR, Bayoumi AM, Cost-effectiveness analysis of immediate radical cystectomy versus intravesical Bacillus Calmette-Guerin therapy for high-risk, high-grade (T1G3) bladder cancer, Cancer 115 (2009) 5450–5459. [DOI] [PubMed] [Google Scholar]
- [21].Sharma V, Wymer KM, Borah BJ, Saigal CS, Litwin MS, Packiam VT, Thompson RH, Tollefson MK, Karnes RJ, Boorjian SA, Cost-effectiveness of maintenance bacillus calmette-guérin for intermediate and high risk nonmuscle invasive bladder cancer, J. Urol 204 (2020) 442–449. [DOI] [PubMed] [Google Scholar]
- [22].Wang Z, Xiao H, Wei G, Zhang N, Wei M, Chen Z, Peng Z, Peng S, Qiu S, Li H, Low-dose Bacillus Calmette-Guerin versus full-dose for intermediate and high-risk of non-muscle invasive bladder cancer: a Markov model, BMC Cancer 18 (2018) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Welk B, Isaranuwatchai W, Krassioukov A, Husted Torp L, Elterman D, Cost-effectiveness of hydrophilic-coated intermittent catheters compared with uncoated catheters in Canada: a public payer perspective, J. Med. Econ 21 (2018) 639–648. [DOI] [PubMed] [Google Scholar]
- [24].Neumann PJ, Cohen JT, Weinstein MC, Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold, N. Engl. J. Med 371 (2014) 796–797. [DOI] [PubMed] [Google Scholar]
- [25].Tomai MA, Miller RL, Lipson KE, Kieper WC, Zarraga IE, Vasilakos JP, Resiquimod and other immune response modifiers as vaccine adjuvants, Expert Rev. Vaccines 6 (2007) 835–847, 10.1586/14760584.6.5.835. [DOI] [PubMed] [Google Scholar]
- [26].Parsons CL, Successful downregulation of bladder sensory nerves with combination of heparin and alkalinized lidocaine in patients with interstitial cystitis, Urology 65 (2005) 45–48. [DOI] [PubMed] [Google Scholar]
- [27].Griffiths DJ, Constantinou CE, Van Mastrigt R, Urinary bladder function and its control in healthy females, Am. J. Physiol. Regul. Integr. Comp. Physiol 251 (1986) 225–230, 10.1152/ajpregu.1986.251.2.r225. [DOI] [PubMed] [Google Scholar]
- [28].Soebadi MA, Bakula M, Hakim L, Puers R, De Ridder D, Wireless intravesical device for real-time bladder pressure measurement: study of consecutive voiding in awake minipigs, PLoS One 14 (2019), e0225821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Oliveira C, Barros AA, Reis RL, Correia-Pinto J, Lima E, New endoscopic procedure for bladder wall closure: results from the porcine model, Sci. Rep 9 (2019) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brosh-Nissimov T, Ingbir M, Weintal I, Fried M, Porat R, Central nervous system toxicity following topical skin application of lidocaine, Eur. J. Clin. Pharmacol 60 (2004) 683–684, 10.1007/s00228-004-0814-4. [DOI] [PubMed] [Google Scholar]
- [31].Gonzalez EJ, Arms L, Vizzard MA, The role(s) of cytokines/chemokines in urinary bladder inflammation and dysfunction, BioMed Res. Int 2014 (2014), 120525, 10.1155/2014/120525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Camargo JA, Passos GR, Ferrari KL, Billis A, Saad MJA, Reis LO, Intravesical immunomodulatory Imiquimod enhances Bacillus calmette-guérin downregulation of nonmuscle-invasive bladder cancer, Clin. Genitourin. Cancer 16 (2018), 10.1016/j.clgc.2017.10.019 e587–e593. [DOI] [PubMed] [Google Scholar]
- [33].Azevedo R, Ferreira JA, Peixoto A, Neves M, Sousa N, Lima A, Santos LL, Emerging antibody-based therapeutic strategies for bladder cancer: a systematic review, J. Contr. Release 214 (2015) 40–61, 10.1016/j.jconrel.2015.07.002. [DOI] [PubMed] [Google Scholar]
- [34].Verma M, Vishwanath K, Eweje F, Roxhed N, Grant T, Castaneda M, Steiger C, Mazdiyasni H, Bensel T, Minahan D, Soares V, Salama JAF, Lopes A, Hess K, Cleveland C, Fulop DJ, Hayward A, Collins J, Tamang SM, Hua T, Ikeanyi C, Zeidman G, Mule E, Boominathan S, Popova E, Miller JB, Bellinger AM, Collins D, Leibowitz D, Batra S, Ahuja S, Bajiya M, Batra S, Sarin R, Agarwal U, Khaparde SD, Gupta NK, Gupta D, Bhatnagar AK, Chopra KK, Sharma N, Khanna A, Chowdhury J, Stoner R, Slocum AH, Cima MJ, Furin J, Langer R, Traverso G, A gastric resident drug delivery system for prolonged gram-level dosing of tuberculosis treatment, Sci. Transl. Med 11 (2019) aau6267, 10.1126/scitranslmed.aau6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chu JN, Choi J, Ostvar S, Torchia JA, Reynolds KL, Tramontano A, Gainor JF, Chung DC, Clark JW, Hur C, Cost-effectiveness of immune checkpoint inhibitors for microsatellite instability–high/mismatch repair–deficient metastatic colorectal cancer, Cancer 125 (2019) 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Verma M, Chu JN, Salama JAF, Faiz MT, Eweje F, Gwynne D, Lopes A, Hess K, Soares V, Steiger C, McManus R, Koeppen R, Hua T, Hayward A, Collins J, Tamang SM, Ishida K, Miller JB, Katz S, Slocum AH, Sulkowski MS, Thomas DL, Langer R, Traverso G, Development of a long-acting direct-acting antiviral system for hepatitis C virus treatment in swine, Proc. Natl. Acad. Sci. U. S. A 117 (2020) 11987–11994, 10.1073/pnas.2004746117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Whaley NS, Burke AE, Intrauterine contraception, Wom. Health 11 (2015) 759–767. [DOI] [PubMed] [Google Scholar]
- [38].Nair LS, Laurencin CT, Biodegradable polymers as biomaterials, Prog. Polym. Sci 32 (2007) 762–798, 10.1016/j.progpolymsci.2007.05.017. [DOI] [Google Scholar]
- [39].Zheng YF, Gu XN, Witte F, Biodegradable metals, Mater. Sci. Eng. R Rep 77 (2014) 1–34, 10.1016/j.mser.2014.01.001. [DOI] [Google Scholar]
- [40].Patterson LJ, Henry R, Hunter DJW, Morales A, Nickel J, Emmerson L, Dose-finding study of intravesical lidocaine in healthy volunteers, Eur. J. Anaesthesiol 17 (2000). [Google Scholar]
- [41].Grimm M-O, Steinhoff C, Simon X, Spiegelhalder P, Ackermann R, Vögeli TA, Effect of routine repeat transurethral resection for superficial bladder cancer: a long-term observational study, J. Urol 170 (2003) 433–437. [DOI] [PubMed] [Google Scholar]
- [42].Daneshmand S, Pohar KS, Steinberg GD, Aron M, Cutie C, Effect of GemRIS (gemcitabine-releasing intravesical system, TAR-200) on antitumor activity in muscle-invasive bladder cancer (MIBC), J. Clin. Oncol 35 (2017), 10.1200/JCO.2017.35.15_suppl.e16000 e16000–e16000. [DOI] [Google Scholar]
- [43].Kirtane AR, Hua T, Hayward A, Bajpayee A, Wahane A, Lopes A, Bensel T, Ma L, Stanczyk FZ, Brooks S, Gwynne D, Wainer J, Collins J, Tamang SM, Langer R, Traverso G, A once-a-month oral contraceptive, Sci. Transl. Med 11 (2019), eaay2602, 10.1126/scitranslmed.aay2602. [DOI] [PubMed] [Google Scholar]
- [44].Bellinger AM, Jafari M, Grant TM, Zhang S, Slater HC, Wenger EA, Mo S, Lee YAL, Mazdiyasni H, Kogan L, Barman R, Cleveland C, Booth L, Bensel T, Minahan D, Hurowitz HM, Tai T, Daily J, Nikolic B, Wood L, Eckhoff PA, Langer R, Traverso G, Oral, ultra-long-lasting drug delivery: application toward malaria elimination goals, Sci. Transl. Med 8 (2016), 10.1126/scitranslmed.aag2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.