Abstract
Angiosarcoma is an uncommon malignant mesenchymal neoplasm, accounting for 1–2% of all sarcomas. More than half are cutaneous, with the remainder arising in the deep soft tissue, breast, bone or viscera, particularly the liver, spleen and heart. Mediastinal angiosarcomas are exceedingly uncommon. While epithelioid morphology is sometimes a minor component in conventional angiosarcoma, tumors with a predominance of epithelioid morphologic features are designated as epithelioid angiosarcoma (EAS). This is a report of a 58-year-old woman presenting with severe chest pain, accompanied by worsening dyspnea and dysphagia. Chest computed tomography (CT) revealed a large pericardial effusion and a bulky mediastinal mass. Biopsy revealed a malignant neoplasm with vascular differentiation consistent with high-grade EAS. By immunohistochemistry, epithelioid angiosarcomas express endothelial cell markers, such as CD31, CD34, ERG and FLI-1. A variable proportion express low molecular weight cytokeratin (CK), epithelial membrane antigen (EMA) and CD30. The use of molecular techniques has proven useful in the diagnosis of this rare neoplasm. Targeted next generation sequencing showed aberrations in multiple genes including NRAS, KRAS, MYC and TP53.
Keywords: Angiosarcoma, Epithelioid Angiosarcoma, Mediastinum, Sarcoma
Introduction
Angiosarcoma is a rare malignant vascular neoplasm, which originates from the endothelial cells of blood vessels. They may arise in any part of the body and affect any organ.1 The peak age of incidence is the 7th decade, with men affected more than women. The head and neck area is the most common site of diagnosis for primary angiosarcoma. Radiation-induced angiosarcoma most commonly occurs in the breast.2
Epithelioid vascular tumors encompass a spectrum of disease that includes epithelioid hemangioma (EH; a benign neoplasm), epithelioid hemangioendothelioma (EHE; a low to intermediate grade malignancy) and epithelioid angiosarcoma (EAS; a high-grade malignancy).3
Histologically, epithelioid vascular tumors may appear similar with diagnostic challenges at the malignant end of the spectrum (ie, high grade EHE vs high-grade EAS). This distinction is aided by molecular analysis, as approximately 90% of EHE with classic morphology harbor a t(1;3) (p36; q23-25) translocation resulting in a fusion of WWTR1 (3q23-24) with CAMTA1 (1p36). This gene fusion has not been identified in other vascular tumors, and diffuse nuclear immunoreactivity for CAMTA1 protein has been reported in the majority of conventional EHE.3 The differential diagnosis includes nonvascular lesions with epithelioid morphology, including melanoma, carcinoma, and other epithelioid soft tissue tumors.
Described here is a rare case of mediastinal EAS. Targeted next generation sequencing (NGS) was performed in this case. To the authors’ knowledge, this is the first case report of this entity in the mediastinum that includes molecular findings.
Case Presentation: Clinical History and Pathology Findings
The patient is a 58-year-old female with a significant smoking history who presented with acute onset severe, non-radiating left sided chest pain along with 1 month of worsening dyspnea and dysphagia. Chest computed tomography (CT) revealed a large pericardial effusion, non-specific periportal liver edema, small left pleural effusion, and a bulky mediastinal mass. Due to concern for impending tamponade, she underwent emergent pericardiocentesis with removal of 650 mL of serosanguineous fluid and subsequent resolution of symptoms. Initial cytology/ cell block from pericardial fluid showed tumor cells with an epithelioid/plasmacytoid appearance and abundant dense cytoplasm. The tumor nuclei were large, atypical, with prominent nucleoli and multinucleated forms (Figure 1 a-b). Immunostains on the cellblock demonstrated an absence of differentiation of the tumoral cells (cytokeratin AE1/AE3, cytokeratin 7, cytokeratin 8/18, LCA, CD30, CD15, PAX-5, calretinin, TTF-1, Napsin-A, S100, SALL4 and OCT3/4).
Figure 1.

(a) Vasoformative features in angiosarcoma. Large malignant multinucleated cell with cytoplasmic vacuoles containing lymphocytes (Papanicolaou stain, 400x). (b) Cell block preparation showing dispersed malignant cells with epithelioid appearance, large, atypical nuclei with prominent nucleoli, and dense cytoplasm (Hematoxylin & Eosin stain, 400x).
A CT-guided core needle biopsy (CNB) of the anterior mediastinal mass was recommended. Mediastinal mass CNB showed atypical cells in a background of lymphocytes. The cells were weakly immunopositive for epithelial membrane antigen (EMA) and negative for other markers (cytokeratin AE1/AE3, cytokeratin 8/18, cytokeratin 5/6, high molecular weight cytokeratin (HMWCK), cytokeratin 7, cytokeratin 20, PAX8, TTF1, WT1, p40, GATA3, CD45, CD5, CD30, CD138, ALK1, TdT, CD43, CD34, PAX5, MUM1, SOX10, MelanA, S100, SALL4, OCT3/4). Flow cytometry was negative for a clonal B or abnormal T-cell population. Due to the non-specific EMA positivity, which can be seen in carcinomas, subsets of hematolymphoid, mesothelial and sarcomatous neoplasias, a diagnosis of poorly differentiated malignant neoplasm was rendered, with request of additional tissue for further characterization.
The patient was subsequently admitted with reoccurrence of symptoms and imaging suggestive of persistent pericardial effusion. Repeat pericardiocentesis was performed for rapid fluid accumulation. A left video-assisted thoracoscopy with conversion to left thoracotomy was performed, with placement of a pericardial window, and biopsy of the mediastinal mass. The largest fragment of the surgical specimen measured 2.5 × 2.0 × 0.7 cm. Hematoxylin and eosin stained slides showed sheets of epithelioid cells with scattered markedly pleomorphic forms. The tumoral cells exhibited abundant eosinophilic cytoplasm with occasional intracytoplasmic vacuoles. Nuclei were large and atypical with prominent nucleoli. Mitotic figures and necrosis were present throughout (Figure 2 a-d). Immunostains were positive for vimentin, CD31, ERG (immunohistochemical stain, member of the ETS family of transcription factors, highly specific endothelial marker) (Figure 3 a-b), TLE1, CD99 and hcaldesmon. There was weak staining for EMA. Immunostains for CD34, STAT6, cytokeratin AE1/AE3, CK8/18, calretinin, SMA, desmin, CD68, CD117, HHV8 and PLAP were negative. Given the immunohistochemical evidence of vascular differentiation, the differential diagnosis included EHE and EAS, amongst others. CAMTA1 immunohistochemistry was negative. With the exclusion of EHE, the final diagnosis was high grade EAS.
Figure 2.
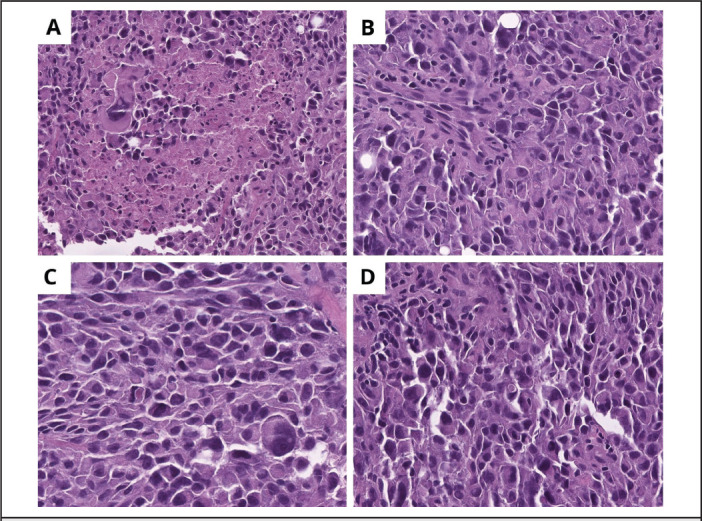
(a) Large, atypical cells with prominent nucleoli and associated necrosis. (b) Tumoral cells adopt an epithelioid/plasmacytoid shape, some with large, prominent nucleoli. (c-d) Intracytoplasmic vacuoles containing erythrocytes.
Figure 3.

Immunohistochemical staining of the tumor showing strong positivity for CD31 (immunohistochemical staining, original magnification 400x), and (b) ERG (immunohistochemical staining, original magnification 400x).
Given the patient’s diagnosis of stage IV EAS, palliative radiotherapy was recommended; however, considering the poor prognosis and estimated survival of less than 6 months, the patient declined therapy and elected to pursue hospice services.
Molecular Findings
Targeted next generation sequencing (NGS) detected a missense NRAS mutation c.182A>G(p.Gln61Arg) and a splice donor site in TP53 c.375+2T>A. Amplification of AKT2, ATM, BRAF, CCND1, CDK6, CHEK1, EGFR, ERCC1, ERCC2, FGF19, FGF3, FGF4, FGFR1, MDM4, MET, MYC, MYCL, NRAS and NRG1 genes were observed. The tumor was microsatellite stable, with a low tumor mutation burden (TMB) and homologous recombination proficient.
Discussion
Angiosarcoma is a rare soft tissue sarcoma that may arise from any organ, though the origin is frequently localized to the skin, liver, spleen, or heart.1 These lesions account for 1-2% of all soft tissue malignancies.4,5 A subset of angiosarcomas, epithelioid angiosarcoma (EAS) often presents in deep soft tissue but may arise in any location. EAS of the skin appears to affect patients from a broader age range than conventional cutaneous angiosarcoma, which typically occurs on sun-damaged skin of the head and neck in elderly patients. EASs often present with early nodal and solid organ metastases, especially to the lungs, bone, soft tissue, and skin. In the first 2 to 3 years following diagnosis, more than 50% of patients are dead of disease, but 20% to 30% of people remain disease free.6–8
A PubMed search from 1970 to 2022 revealed 4 case reports of mediastinal angiosarcoma.9–12 Adult case series of malignant thoracic vascular tumors have also included cases of EAS. One study described 13 cases (of 52) of EAS in the thorax (lung, pleura, mediastinum, heart, and great vessels)3; another series described 1 case (of 16) of an EAS involving the posterior mediastinum13; a final series of primary angiosarcomas of the mediastinum reported 2 cases (of 9) to have epithelioid features.14
Malignant thoracic epithelioid vascular tumors are an uncommon and heterogenous group of tumors that include low to intermediate grade EHE and high-grade EAS.3 The diagnosis can be challenging due to morphologic overlap, particularly on small biopsies.3 Variants of EAS display a range of features, from inter-anastomosing vessels lined by epithelioid endothelial cells to solid sheets of epithelioid cells where it is difficult to identify vascular morphologic features.15 EAS is variably positive for immunohistochemical markers of vascular differentiation, including CD31, CD34, and ERG. Immunoreactivity for cytokeratin, neuroendocrine markers and CD30 have been described and represent diagnostic pitfalls.15 According to Anderson et al,3 CD31 and ERG are the most reliable markers of vascular differentiation in these lesions, seen in 96% and 100% of cases of EHE and EAS, respectively. This case exhibited immunoreactivity for CD31, ERG and EMA.
This report is the first describing molecular findings of mediastinal EAS. A targeted massive parallel sequencing study of angiosarcomas published by Murali et al16 identified MAPK pathway alterations in 18/34 (53%) of cases, involving mutations in KRAS, HRAS, NRAS, BRAF, MAPK1, and NF1, as well as amplifications in MAPK1/CRKL, CRAF or BRAF. In addition, mutations in TP53 (35%), PTPRB (29%), PLCG1 R707Q (3%), and losses of CDKN2A (26%), were also identified. Similarly, this case demonstrated NRAS and BRAF amplifications and a mutation in TP53. MYC gene amplification and overexpression has been identified in secondary angiosarcomas as well as certain sporadic angiosarcoma subtypes.17 These findings were replicated in this case, which also showed MYC amplification; however, to the authors’ knowledge the patient did not possess these risk factors.
The differential diagnosis of EAS in the mediastinum includes EHE, epithelioid carcinoma, epithelioid mesothelioma, and spindle cell melanoma. EHE expresses vascular markers and occasionally epithelial markers,3 with up to 29% of EHEs exhibiting keratin expression. Most EHE cases (90%) exhibit CAMTA1-WWTR1 gene fusions while a subset exhibits YAP1- TFE3 gene fusions.18 This case was CAMTA1-WWTR1 negative. Epithelioid mesothelioma is an important diagnostic consideration; an immunostain for WT1 aids this differential diagnosis.19 In this case, WT1 was negative. Spindle cell melanoma may display pseudovascular spaces resembling angiosarcoma.19 Stains for SOX10, MelanA, and S100 were negative in this case. Finally, a mediastinal epithelioid malignancy is statistically more likely to be carcinoma than EAS, with the added pitfall of both tumors demonstrating immunoreactivity cytokeratin stains19; while this case was keratin negative, the tumor cells were positive for EMA.
Suspicion of angiosarcoma and its variants in cytology/CNB cases is essential, as this is often the first sample received from a patient with a mediastinal mass. Based on published literature, CNB and incisional biopsies do not differ significantly in terms of accuracy rate. Diagnostic yields depend on the tumor’s histologic architecture.20–22 There is a significantly lower diagnostic yield for tumors with heterogeneous architecture, such as angiosarcomas and synovial sarcomas. It has been observed in some studies that incisional biopsy following non-diagnostic CNB increases the overall pathology work-up yield.23
EAS may demonstrate cytologic features similar to adenocarcinoma, including tridimensional and papillary clusters, micro-acini, and singly dispersed epithelioid or plasmacytoid cells, with cytoplasmic vacuoles, mimicking mucin.24 While the presence of pencillate nucleoli, erythrophagocytosis, and other vasoformative features are highly suggestive of vascular origin, these features are not specific, and they overlap with nonvascular neoplasms.24
The prognosis of any type of angiosarcoma is relatively poor. Two and 5-year disease-free survival rates are 44% and 24%, respectively.9 Surgical resection with or without adjuvant radiation or chemotherapy is the main treatment modality for angiosarcoma.9 In unresectable cases, the median survival time is 7.3 months.25
Conclusion
Although rare, EAS should be considered in patients presenting with a mediastinal mass. CNB is a reliable and effective method for soft tissue sarcoma diagnosis. Angiosarcomas tend to be more heterogeneous than other soft tissue tumors. A thoracoscopic or open biopsy can be considered in cases of non-diagnostic CNB in order to obtain adequate tissue for histopathology, immunohistochemistry, and molecular diagnosis.
Abbreviations
- CK
cytokeratin
- CNB
core needle biopsy
- CT
chest computed tomography
- EAS
epithelioid angiosarcoma
- EHE
epithelioid hemangioendothelioma
- EMA
epithelial membrane antigen
- ERG
immunohistochemical stain, member of the ETS family of transcription factors, highly specific endothelial marker
- FNA
fine needle aspiration
- NGS
next generation sequencing
- TMB
tumor mutation burden
Conflict of Interest
None of the authors identify a conflict of interest.
References
- 1.Durani U, Gallo de Moraes A, Beachey J, Nelson D, Robinson S, Anavekar NS. Epithelioid angiosarcoma: A rare cause of pericarditis and pleural effusion. Respir Med Case Rep. 2018;24:77–80. doi: 10.1016/j.rmcr.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonescu C. Malignant vascular tumors-an update. Mod Pathol. 2014;27(Suppl 1):S30–S38. doi: 10.1038/modpathol.2013.176. [DOI] [PubMed] [Google Scholar]
- 3.Anderson T, Zhang L, Hameed M, Rusch V, Travis WD, Antonescu CR. Thoracic epithelioid malignant vascular tumors: A clinicopathologic study of 52 cases with emphasis on pathologic grading and molecular studies of WWTR1-CAMTA1 fusions. Am J Surg Path. 2015;39(1):132–139. doi: 10.1097/PAS.0000000000000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dainese E, Pozzi B, Milani M, et al. Primary pleural epithelioid angiosarcoma. A case report and review of the literature. Pathol Res Pract. 2010;206(6):415–419. doi: 10.1016/j.prp.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Zhang S, Zheng Y, Liu W, Yu X. Primary epithelioid angiosarcoma of the pleura: a case report and review of literature. Int J Clin Exp Pathol. 2015;8(2):2153–2158. [PMC free article] [PubMed] [Google Scholar]
- 6.Sakamoto A, Takahashi Y, Oda Y, Iwamoto Y. Aggressive clinical course of epithelioid angiosarcoma in the femur: A case report. World J Surg Onc. 2014;12(281) doi: 10.1186/1477-7819-12-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart J, Mandavilli S. Epithelioid angiosarcoma A brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135(2):268–272. doi: 10.5858/135.2.268. [DOI] [PubMed] [Google Scholar]
- 8.Meis-Kindblom JM, Kindblom LG. Angiosarcoma of soft tissue. Study of 80 cases. Am J Surg Pathol. 1998;22(6):683–697. doi: 10.1097/00000478-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Tane S, Tanaka Y, Tauchi S, Uchino K, Nakai R, Yoshimura M. Radically resected epithelioid angiosarcoma that originated in the mediastinum. Gen Thorac Cardiovasc Surg. 2011;59(7):503–506. doi: 10.1007/s11748-010-0710-z. [DOI] [PubMed] [Google Scholar]
- 10.Demiröz M, Findik G, Aydoğdu K, et al. Mediastinal epithelioid angiosarcoma arising in schwannoma: The first case in the literature. Turk Gogus Kalp Damar Cerrahisi Derg. 2018;26(2):305–308. doi: 10.5606/tgkdc.dergisi.2018.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vats K, Al-Nourhji O, Wang H, Wang C. Primary epithelioid angiosarcoma of the mediastinum, cytomorphologic features of a rare entity—A case report and literature review. Diagn Cytopathol. 2022:1–7. doi: 10.1002/dc.24946. Published online. [DOI] [PubMed] [Google Scholar]
- 12.Xiang Y, Yan L, Lin X, Zhang X, Zhang F, Wu Z. Posterior mediastinal epithelioid angiosarcoma arising in schwannoma: A case report and review of the literature. Front Surg. 2021;8 doi: 10.3389/fsurg.2021.666389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Li X, Liu X. 2015 Epithelioid angiosarcoma clinicopathological study of 16 Chinese cases. Int J Clin Exp Pathol. 2015;8(4):3901–3909. [PMC free article] [PubMed] [Google Scholar]
- 14.Weissferdt A, Kalhor N, Suster S, Moran CA. Primary angiosarcomas of the anterior mediastinum: A clinicopathologic and immunohistochemical study of 9 cases. Hum Pathol. 2010;41(12):1711–1717. doi: 10.1016/j.humpath.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Ko JS, Billings SD. Diagnostically challenging epithelioid vascular tumors. Surg Pathol Clin. 2015;8(3):331–351. doi: 10.1016/j.path.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Murali R, Chandramohan R, Möller I, et al. Targeted massively parallel sequencing of angiosarcomas reveals frequent activation of the mitogen activated protein kinase pathway. Oncotarget. 2015;6(34):36041–36052. doi: 10.18632/oncotarget.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shon W, Billings SD. Epithelioid vascular tumors: A review. Adv Anat Pathol. 2019;26(3):186–197. doi: 10.1097/PAP.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 18.Lamar JM, Nehru VM, Weinberg G. Epithelioid hemangioendothelioma as a model of YAP/ TAZ-driven cancer: Insights from a rare fusion sarcoma. Cancers (Basel) 2018;10(7):229. doi: 10.3390/cancers10070229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paral K, Krausz T. Vascular tumors of the mediastinum. Mediastinum. 2020;4:25. doi: 10.21037/med-20-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein A, Fell T, Birkenmaier C, et al. Relative sensitivity of core-needle biopsy and incisional biopsy in the diagnosis of musculoskeletal sarcomas. Cancers (Basel) 2021;13(6):1–10. doi: 10.3390/cancers13061393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heslin MJ, Lewis JJ, Woodruff JM, Brennan MF. Core needle biopsy for diagnosis of extremity soft tissue sarcoma. Ann Surg Oncol. 1997;4(5):425–431. doi: 10.1007/BF02305557. [DOI] [PubMed] [Google Scholar]
- 22.Kasraeian S, Allison DC, Ahlmann ER, Fedenko AN, Menendez LR. A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin Orthop Relat Res. 2010;468(11):2992–3002. doi: 10.1007/s11999-010-1401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verheijen P, Witjes H, van Gorp J, Hennipman A, van >Dalen T. Current pathology work-up of extremity soft tissue sarcomas, evaluation of the validity of different techniques. Eur J Surg Oncol. 2010;36(1):95–99. doi: 10.1016/j.ejso.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Geller RL, Hookim K, Sullivan HC, Stuart LN, Edgar MA, Reid MD. Cytologic features of angiosarcoma: A review of 26 cases diagnosed on FNA. Cancer Cytopathol. 2016;124(9):659–668. doi: 10.1002/cncy.21726. [DOI] [PubMed] [Google Scholar]
- 25.Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14(6):1953–1967. doi: 10.1245/s10434-006-9335-y. [DOI] [PubMed] [Google Scholar]


