Take Home Message
In the current era of advanced precision medicine, there is a place for better-personalized treatment strategies, especially for elderly patients. We found that low alanine aminotransferase (ALT), indicative of sarcopenia and frailty, was associated with shorter survival for prostate cancer patients and survivors. ALT measurement at baseline could guide treatment decisions in this patient population.
Keywords: Prostate cancer, Sarcopenia, Frailty, Alanine aminotransferase, Survival, Prostate-specific antigen
Abstract
Background
Sarcopenia is characterized by loss of muscle mass and function and is associated with frailty, a syndrome with higher likelihood of falls, fractures, physical disability, and mortality. Both frailty and sarcopenia are known markers of shorter survival in various cancer patient populations. Low alanine aminotransferase (ALT), reflecting loss of muscle mass (sarcopenia), may be associated with greater frailty and shorter survival in multiple cancers.
Objective
To assess the potential association between low ALT and shorter survival among prostate cancer (PCa) patients and survivors.
Design, setting, and participants
This was a retrospective analysis of a historical cohort of PCa patients and survivors. Patients were defined as those still actively receiving PCa treatment, while those no longer receiving such treatment were classified as PCa survivors.
Outcome measurements and statistical analysis
ALT data were obtained from results for basic biochemical blood testing carried out for patients on their first hospital admission. Patients were divided into two groups: those with ALT ≥17 IU/l and those with ALT <17 IU/l. Univariate and multivariable analyses were conducted for between-group survival comparisons.
Results and limitations
We identified 9489 PCa records. The final study cohort with ALT data available included 4064 patients with ALT <40 IU/l. Of this cohort, 536 patients were actively receiving medical anticancer therapy for PCa. The mean age for the entire cohort was 74.6 yr (standard deviation 9.6) and the median ALT level was 19.28 IU/l; 1676 patients (41%) had low ALT (<17 IU/l). On univariate analysis, low ALT was associated with a 78% increase in mortality risk (95% confidence interval [CI] 1.62–1.97; p < 0.001). A sensitivity analysis of the 536 patients actively receiving medical anticancer treatment revealed that low ALT was associated with a 48% increase in mortality risk (95% CI 1.19–1.85; p = 0.001). In a multivariable model controlled for age, kidney disease, history of cerebrovascular event/transient ischemic attack, and baseline prostate-specific antigen, low ALT was still associated with a 35% increase in mortality risk (95% CI 1.12–1.63; p = 0.001). Limitations include the single-center, retrospective design.
Conclusions
Low ALT, which is indicative of sarcopenia and frailty, is associated with shorter survival among PCa patients and survivors and could potentially be used for treatment personalization.
Patient summary
We compared survival for prostate cancer patients and survivors according to their blood level of the protein alanine aminotransferase (ALT). Low ALT levels in the general population are associated with loss of muscle mass. We found that in our group of prostate cancer patients and survivors, the risk of death from any cause was higher for those with low ALT levels.
1. Introduction
1.1. Sarcopenia and frailty among cancer patients and survivors
The availability of clinical tools for assessing a patient’s physiological reserves is important for treatment decision-making and consulting on prognosis for cancer patients and survivors. The Eastern Cooperative Oncology Group performance status score is an example of a widely used tool [1], [2]. In recent years, additional measures have been proposed for evaluation of patient populations in various clinical disciplines. Sarcopenia is characterized by loss of muscle mass and functions, and is associated with frailty, a syndrome associated with higher likelihood of falls, fractures, physical disability, and mortality. Both frailty status and a diagnosis of sarcopenia have been identified as markers for shorter survival in various cancer patient populations [3]. In addition, assessment of frailty and sarcopenia may improve the selection of patients eligible for therapies such as surgery [4].
1.2. Risk of sarcopenia and frailty in prostate cancer patients
Prostate cancer (PCa) is a global health problem, the second most common type of cancer among men after lung cancer, and a leading cause of death from cancer [5]. The mean age at PCa onset is 67 yr, but many cases are diagnosed at much older ages [6]. In healthy men, there is a natural decline in serum testosterone concentrations as age increases, which directly affects reductions in muscle mass and results in an increase in the risk of sarcopenia. Furthermore, one of the most common therapeutic approaches in PCa is androgen deprivation therapy (ADT), which may induce body composition changes and sarcopenia [7], [8] via several possible mechanisms [8], [9]. Therefore, the age-related effect is accentuated in these patients by the ADT.
There is controversy about the prognostic role of sarcopenia in PCa. Numerous studies have investigated sarcopenia and frailty among PCa patients, and most showed adverse outcomes, including shorter survival [10], [11], [12], [13], [14], [15]. Recent meta-analyses yielded differing conclusions: there was positive correlation in three publications [16], [17], [18], borderline correlation in report [19], and no correlation in another [3].
1.3. Alanine aminotransferase as a biomarker of sarcopenia and frailty
Alanine aminotransferase (ALT) is a liver enzyme commonly monitored in routine blood tests. ALT catalyzes pyruvate to alanine in skeletal muscle and alanine to pyruvate in the liver [20]. Elevated blood ALT levels are commonly used as a biomarker of hepatocellular injury, but little was known regarding the clinical significance of lower-than-normal ALT. However, several studies have demonstrated that low serum ALT (<17 IU/l) is associated with sarcopenia [21] and shorter survival for middle-aged healthy adults and patients hospitalized for various conditions [22]. Low ALT, reflecting low muscle mass (sarcopenia), may be associated with greater frailty and shorter survival in multiple cancers.
The aim of the current study was to assess the potential association between low ALT, indicative of sarcopenia and frailty, and shorter survival among PCa patients and survivors.
2. Patients and methods
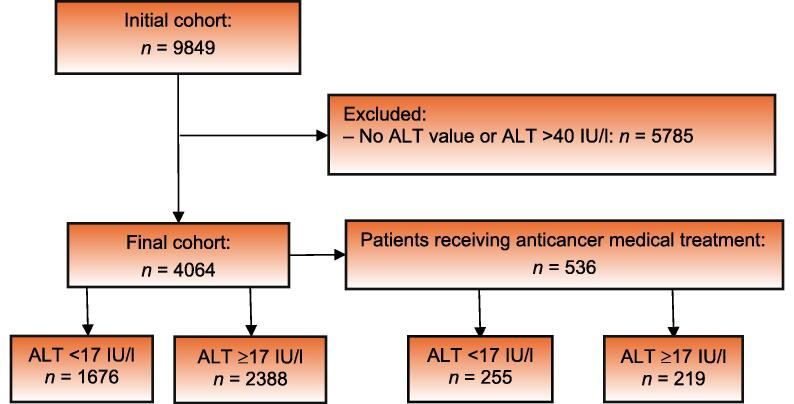
The study population included men diagnosed with PCa treated in a large, tertiary medical center as outpatients or inpatients. Following approval from the local ethics committee (reference SMC-23-0216), data for patient characteristics were extracted from electronic medical records. We excluded patients with ALT >40 IU/l, as levels this high are primarily associated with ALT originating from liver damage (various types of acute and chronic hepatitis) rather than serving as a reliable marker for striated muscle mass. The final cohort included patients with ALT levels established at the time of PCa diagnosis. In this cohort, we identified patients actively receiving medical anticancer therapy for PCa; the remainder were free from such treatment, and therefore represented PCa survivors. Figure 1 shows a Consolidated Standards of Reporting Trials (CONSORT) flow diagram of patient inclusion and exclusion.
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of patient inclusion in the study.
The final data analysis was performed on two cohorts: (1) patients whose complete data were available and (2) patients actively receiving medical therapy for PCa. Baseline demographic and clinical data were retrieved from electronic medical records. The primary outcome of the study was all-cause mortality. Survival data were available for all subjects from the Israeli Population National Registry. Continuous variables are expressed as the mean ± standard deviation (SD) if normally distributed, or median with interquartile range if the distribution was skewed. Normality was determined using Anderson-Darling and Shapiro-Wilk tests. Categorical variables are presented as the frequency and percentage. Continuous data were compared using Student’s t test, and categorical data were compared using a χ2 or Fisher exact test. The log-rank test was used to analyze survival estimated using the Kaplan-Meier method. A univariate regression model was used to determine the unadjusted hazard ratio (HR) for the primary outcome, and a multivariate model was constructed to examine the correlation and to control for possible confounders. We then performed a sensitivity analysis for PCa patients actively receiving anticancer therapy. An association was considered statistically significant for a two-sided p value of <0.05. All analyses were performed using R v4.3.0 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Records were identified for 9489 patients with PCa. The final study population included 4064 patients (Fig. 1), of whom 536 were actively receiving medical therapy for PCa. The mean age for the entire cohort was 74.6 ± 9.6 yr. The median ALT level for the study population was 19.28 U/l, and 1676 patients (41%) had low ALT, defined as <17 IU/l. Patient demographics and characteristics stratified by ALT level (<17 vs ≥17 IU/l) are presented in Table 1.
Table 1.
Patient characteristics in the overall cohort and by ALT group
| Parameter a | Overall (n = 4064) |
ALT ≥17 IU/l (n = 2388) |
ALT <17 IU/l (n = 1676) |
p value |
|---|---|---|---|---|
| ALT at baseline (IU/l) | 19.28 ± 7.87 | 24.39 ± 6 | 12 ± 2.93 | <0.001 |
| Demographic data | ||||
| Age (yr) | 74.6 ± 9.6 | 73 ± 9.5 | 77 ± 9.1 | <0.001 |
| Body mass index (kg/m2) | 26.8 ± 4.3 | 27.4 ± 4.2 | 26 ± 4.2 | <0.001 |
| Comorbidity | ||||
| Arterial hypertension, n (%) | 2,282 (56) | 1,321 (55) | 961 (57) | 0.213 |
| Diabetes mellitus, n (%) | 1,116 (28) | 646 (27) | 470 (28) | 0.509 |
| Dyslipidemia, n (%) | 1,682 (41) | 1,035 (43) | 647 (39) | 0.003 |
| Ischemic heart disease, n (%) | 1,359 (33) | 801 (34) | 558 (33) | 0.895 |
| Chronic kidney disease, n (%) | 834 (20) | 408 (17) | 426 (25) | <0.001 |
| COPD, n (%) | 274 (4) | 136 (6) | 138 (8) | 0.002 |
| Atrial fibrillation/flutter, n (%) | 476 (12) | 257 (11) | 219 (13) | 0.028 |
| Stroke, n (%) | 397 (10) | 189 (8) | 208 (12) | <0.001 |
| Laboratory parameters | ||||
| Baseline PSA (ng/l) | 85.6 ± 398.7 | 44.2 ± 266 | 148 ± 535 | <0.001 |
| Testosterone (ng/ml) | 8.71 ± 5.78 | 9 ± 5.91 | 8.21 ± 5.52 | 0.285 |
| Hemoglobin (g/dl) | 12.69 ± 2.19 | 13.09 ± 2.12 | 12.12 ± 2.15 | <0.001 |
| Albumin (g/dl) | 3.93 ± 2.62 | 3.99 ± 2.31 | 3.85 ± 3.01 | 0.086 |
ALT = alanine aminotransferase; COPD = chronic obstructive pulmonary disease; PSA = prostate-specific antigen.
Data for continuous variables are presented as the mean ± standard deviation.
3.1. Univariate analysis
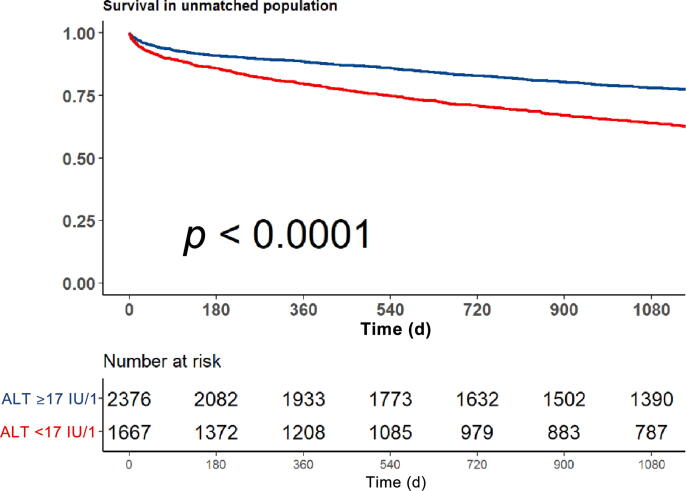
On univariate analysis, low ALT was associated with a 78% increase in mortality risk (95% confidence interval [CI] 1.62–1.97; p < 0.001). Figure 2 shows a Kaplan-Meir curve for the crude survival analysis according to ALT levels.
Fig. 2.
Kaplan Meir survival analysis according to alanine aminotransferase (ALT) level (<17 IU/l vs ≥17 IU/l).
3.2. Sensitivity analysis
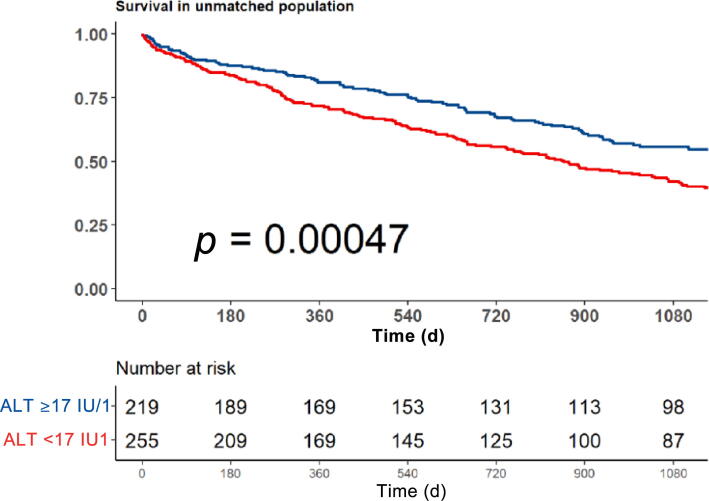
We performed a sensitivity analysis of 536 patients actively receiving medical anticancer treatment. In this subgroup, low ALT was associated with a 48% increase in mortality risk (95% CI 1.19–1.85; p = 0.001). Figure 3 shows a Kaplan-Meir curve for the crude survival analysis according to ALT levels for the subgroup actively receiving anticancer treatment.
Fig. 3.
Sensitivity analysis for patients actively receiving medical anticancer treatment. Kaplan Meir survival by alanine aminotransferase (ALT) level (<17 IU/l vs ≥17 IU/l).
3.3. Multivariable analysis
A multivariable model (Table 2) controlled for age, kidney disease, history of CVA/TIA, baseline PSA, hemoglobin concentration, and history of chronic obstructive pulmonary disease, low ALT was still associated with a 35% increase in mortality risk (95% CI 1.12–1.63; p = 0.001).
Table 2.
Multivariate analysis of survival
| Parameter | HR (95% CI) | p value |
|---|---|---|
| Alanine aminotransferase <17 IU/l (categorical variable) | 1.35 (1.12–1.63) | 0.001 |
| Baseline prostate-specific antigen >5 ng/ml (categorical variable) | 1.81 (1.51–2.17) | <0.001 |
| Age (continuous variable) | 1.07 (1.06–1.09) | <0.001 |
| Chronic kidney disease (categorical variable) | 1.22 (1.00–1.49) | 0.047 |
| Chronic obstructive pulmonary disease (categorical variable) | 1.27 (0.94–1.72) | 0.126 |
| Hemoglobin <10 g/dl (categorical variable) | 2.21 (1.77–2.75) | <0.001 |
| Cerebrovascular event/transient ischemic attack (categorical value) | 1.65 (1.26–2.17) | <0.001 |
4. Discussion
Clinical tools for evaluating a patient’s physiological reserves are essential for decision-making regarding medical and surgical treatment. Those tools may also help in consultations on prognosis for cancer patients and survivors. Sarcopenia is a condition involving loss of muscle mass and function, while frailty refers to a state characterized by a deterioration in physical function associated with various adverse outcomes and worse survival. Frailty and sarcopenia have been identified as markers of shorter patient survival in multiple cancer types. Sarcopenia is one of the most important hallmarks of cachexia [23]. Approximately 100 studies have evaluated the association between lean muscle mass and cancer mortality. The overall pooled HR on cancer mortality was 1.69 (95% CI 1.56–1.83) for patients with sarcopenia [3]. Multiple methods have been proposed for detection and classification of sarcopenia, but none has shown clear superiority. For instance, body mass index, a commonly used marker of general health and body surface area, is often misleading and cannot directly reflect lean muscle mass [24]. Measurement of the L3 skeletal muscle mass index on computed tomography is recommended for identification of sarcopenia [25], [26], [27]. The L3 psoas index has been proposed as a simplified alternative [28], but this approach has yet to be validated.
Frailty is a more elusive term and corresponds to a state of vulnerability with poor return to homeostasis following a stressor event such as surgery or chemotherapy [29]. Frail cancer patients are at higher risk of postoperative complications, chemotherapy intolerance, disease, and death [30]. There are more than 70 tools for measurement of frailty; they are primarily unvalidated and range from a single-item assessment tool, such as gait speed or sarcopenia, to comprehensive questionnaires comprising more than 90 items [30].
Low serum ALT has been suggested as readily available surrogate marker of sarcopenia and frailty. Several studies have demonstrated that low ALT is associated with shorter survival in otherwise healthy adults [31]. Low ALT, reflecting low muscle mass (sarcopenia), may be associated with greater frailty and consequently shorter survival in various cancers [32].
Sarcopenia is common among PCa patients. Most men with PCa are in their seventh decade of life or older and are already at greater risk of sarcopenia and frailty. In addition, the mainstay of PCa medical treatment is ADT, with long periods of testosterone at castrate levels, a well-known risk factor for sarcopenia [7], [8]. Even PCa survivors with no evidence of disease may continue ADT injections for extended periods or indefinitely. Treatment with ADT may also lead to frailty [33]. The incidence of sarcopenia among patients with advanced PCa and survivors after ADT is as high as 60% [18]. Studies on sarcopenia as a prognostic factor in PCa were usually positive. Mason et al [12] found no correlation between sarcopenia and all-cause mortality among patients undergoing radical prostatectomy. Another group showed that ≥5% muscle loss with ADT was associated with a 5.6-fold increase in noncancer mortality in a cohort of patients with nonmetastatic high-risk PCa who received radiation to the prostate [13]. In a pooled analysis of two large RCTs with radiation therapy, psoas area, comorbidity score, baseline prostate-specific antigen (PSA), and age were significantly associated with survival in the final multivariable model [16]. Patients with a subcutaneous fat index of ≥39.9 cm2/m2 on diagnosis of castration-resistant PCa had better cancer-specific survival than those with a subcutaneous fat index of <39.9 cm2/m2 at diagnosis [10]. In a cohort with castration-resistant PCa treated with chemotherapy [11], patients with sarcopenia experienced significantly shorter survival after docetaxel treatment. Several meta-analyses or systematic reviews on this subject showed more varied results. Meyer et al [18] found that computed tomography–defined low skeletal muscle mass among 1221 patients was associated with overall survival (HR = 1.4). A comprehensive review from Spain [19] that included 1600 patients in eight studies, mainly with advanced-stage disease, revealed an association between sarcopenia and progression-free survival, a possible surrogate for survival. There was only a weak association in the few studies that included a survival analysis. Jahrreiss et al [17] included more patients with early-stage PCa as well as survivors in their systematic review. Most studies found that sarcopenia was a predictor for survival for patients treated with radical surgery, radiotherapy, or ADT. However, there is significant heterogeneity across these studies regarding the definition of sarcopenia. By contrast, another meta-analysis comprising 100 studies across many cancer types showed that mortality was significantly associated with sarcopenia in many cancers, except for hematopoietic, breast, ovarian, and endometrial cancers and PCa [3].
In the current era of personalized medicine, we propose that sarcopenia and frailty should be included in decision-making in PCa using low ALT as a marker. ALT measurement could be included as a decision support tool when considering medical treatment for advanced PCa and possibly curative treatment for early-stage disease. A few other studies have addressed this issue. Using standard sarcopenia metrics in hormone-sensitive metastatic PCa, Lee et al. [14] found an inferior response to cancer therapy in patients with sarcopenia. In the cohort with castration-resistant PCa receiving chemotherapy mentioned above [11], response to and compliance with docetaxel chemotherapy were significantly lower in the subgroup with sarcopenia. For surgical candidates, Lascano et al. [34] reported that the 15-point Frailty Index and other factors such as sarcopenia may help in identifying which patients have a higher risk of surgical complications that may outweigh the benefit of surgery.
4.1. Study limitations
This was a single-center, retrospective study. Future prospective studies are required to establish the role of sarcopenia and frailty among PCa patients via measurement of their baseline ALT level.
5. Conclusions
In an era of advanced precision medicine that allows disease-centered therapy, there is a place for better-personalized treatment strategies, especially for elderly patients. We demonstrated that low ALT, indicative of sarcopenia and frailty, was associated with shorter survival among PCa patients and survivors. Therefore, ALT measurement at baseline could guide treatment decisions in this patient population.
Author contributions: Gad Segal had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Laufer, Perelman, Sarfaty, Itelman, Segal.
Acquisition of data: Laufer, Perelman, Sarfaty, Itelman, Segal.
Analysis and interpretation of data: Laufer, Perelman, Sarfaty, Itelman, Segal.
Drafting of the manuscript: Laufer, Perelman, Sarfaty, Itelman, Segal.
Critical revision of the manuscript for important intellectual content: Laufer, Perelman, Sarfaty, Itelman, Segal.
Statistical analysis: Laufer, Perelman, Sarfaty, Itelman, Segal.
Obtaining funding: None.
Administrative, technical, or material support: Laufer, Perelman, Sarfaty, Itelman, Segal.
Supervision: Laufer, Segal.
Other: None.
Financial disclosures: Gad Segal certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: Roderick van den Bergh
References
- 1.Al-Ezzi E.M., Alqaisi H.A., Iafolla M.A.J., et al. Clinicopathologic factors that influence prognosis and survival outcomes in men with metastatic castration-resistant prostate cancer treated with radium-223. Cancer Med. 2021;10:5775–5782. doi: 10.1002/cam4.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azad A.A., Eigl B.J., Leibowitz-Amit R., et al. Outcomes with abiraterone acetate in metastatic castration-resistant prostate cancer patients who have poor performance status. Eur Urol. 2015;67:441–447. doi: 10.1016/j.eururo.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Au P.C.M., Li H.L., Lee G.K.Y., et al. Sarcopenia and mortality in cancer: a meta-analysis. Osteoporos Sarcopenia. 2021;7:S28–S33. doi: 10.1016/j.afos.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korc-Grodzicki B., Downey R.J., Shahrokni A., Kingham T.P., Patel S.G., Audisio R.A. Surgical considerations in older adults with cancer. J Clin Oncol. 2014;32:2647–2653. doi: 10.1200/jco.2014.55.0962. [DOI] [PubMed] [Google Scholar]
- 5.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 6.Culp M.B.B., Soerjomataram I., Efstathiou J.A., Bray F., Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77:38–52. doi: 10.1016/j.eururo.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Smith M.R., Saad F., Egerdie B., et al. Sarcopenia during androgen-deprivation therapy for prostate cancer. J Clin Oncol. 2012;30:3271–3276. doi: 10.1200/jco.2011.38.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara N., Ishizaki F., Saito T., Nishiyama T., Kawasaki T., Takahashi K. Decrease in lean body mass in men with prostate cancer receiving androgen deprivation therapy: mechanism and biomarkers. Urology. 2013;81:376–380. doi: 10.1016/j.urology.2012.10.050. [DOI] [PubMed] [Google Scholar]
- 9.Pan C., Agrawal N.J., Zulia Y., et al. Prostate tumor-derived GDF11 accelerates androgen deprivation therapy-induced sarcopenia. JCI Insight. 2020;5:e127018. doi: 10.1172/jci.insight.127018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J.S., Lee H.S., Ha J.S., et al. Subcutaneous fat distribution is a prognostic biomarker for men with castration resistant prostate cancer. J Urol. 2018;200:114–120. doi: 10.1016/j.juro.2018.01.069. [DOI] [PubMed] [Google Scholar]
- 11.Ohtaka A., Aoki H., Nagata M., et al. Sarcopenia is a poor prognostic factor of castration-resistant prostate cancer treated with docetaxel therapy. Prostate Int. 2019;7:9–14. doi: 10.1016/j.prnil.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason R.J., Boorjian S.A., Bhindi B., et al. The association between sarcopenia and oncologic outcomes after radical prostatectomy. Clin Genitourin Cancer. 2018;16:e629–e636. doi: 10.1016/j.clgc.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Chiang P.K., Tsai W.K., Chiu A.W.H., Bin L.J., Yang F.Y., Lee J. Muscle loss during androgen deprivation therapy is associated with higher risk of non-cancer mortality in high-risk prostate cancer. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.722652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J.H., Jee B.A., Kim J.H., et al. Prognostic impact of sarcopenia in patients with metastatic hormone-sensitive prostate cancer. Cancers. 2021;13:6345. doi: 10.3390/cancers13246345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zakaria H.M., Llaniguez J.T., Telemi E., et al. Sarcopenia predicts overall survival in patients with lung, breast, prostate, or myeloma spine metastases undergoing stereotactic body radiation therapy (SBRT), independent of histology. Neurosurgery. 2020;86:705–716. doi: 10.1093/neuros/nyz216. [DOI] [PubMed] [Google Scholar]
- 16.McDonald A.M., DeMora L., Yang E.S., et al. Body composition and mortality in men receiving prostate radiotherapy: a pooled analysis of NRG/RTOG 9406 and NRG/RTOG 0126. Cancer. 2023;129:685–696. doi: 10.1002/cncr.34596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahrreiss V., Laukhtina E., D’Andrea D., Shariat S.F. The prognostic value of sarcopenia in patients with prostate cancer: a systematic review. Curr Opin Urol. 2021;31:315–323. doi: 10.1097/mou.0000000000000885. [DOI] [PubMed] [Google Scholar]
- 18.Meyer H.J., Wienke A., Surov A. CT-defined low-skeletal muscle mass as a prognostic marker for survival in prostate cancer: a systematic review and meta-analysis. Urol Oncol. 2022;40:103.e9–103.e16. doi: 10.1016/j.urolonc.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 19.de Pablos-Rodríguez P., del Pino-Sedeño T., Infante-Ventura D., et al. Prognostic impact of sarcopenia in patients with advanced prostate carcinoma: a systematic review. J Clin Med. 2022;12:57. doi: 10.3390/jcm12010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z., Que S., Xu J., Peng T. Alanine aminotransferase—old biomarker and new concept: a review. Int J Med Sci. 2014;11:925–935. doi: 10.7150/ijms.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anani S., Goldhaber G., Brom A., et al. Frailty and sarcopenia assessment upon hospital admission to internal medicine predicts length of hospital stay and re-admission: a prospective study of 980 patients. J Clin Med. 2020;9:2659. doi: 10.3390/jcm9082659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruhl C.E., Everhart J.E. The association of low serum alanine aminotransferase activity with mortality in the US population. Am J Epidemiol. 2013;178:1702–1711. doi: 10.1093/aje/kwt209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fearon K., Strasser F., Anker S.D., et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/s1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 24.Iyengar N.M., Arthur R., Manson J.E., et al. Association of body fat and risk of breast cancer in postmenopausal women with normal body mass index: a secondary analysis of a randomized clinical trial and observational study. JAMA Oncol. 2019;5:155–163. doi: 10.1001/jamaoncol.2018.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prado C.M., Lieffers J.R., McCargar L.J., et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/s1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 26.Mitsiopoulos N., Baumgartner R.N., Heymsfield S.B., Lyons W., Gallagher D., Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 27.Portal D., Hofstetter L., Eshed I., et al. L3 skeletal muscle index (L3SMI) is a surrogate marker of sarcopenia and frailty in non-small cell lung cancer patients. Cancer Manag Res. 2019;11:2579–2588. doi: 10.2147/cmar.s195869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanaoka M., Yasuno M., Ishiguro M., et al. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int J Colorectal Dis. 2017;32:847–856. doi: 10.1007/s00384-017-2773-0. [DOI] [PubMed] [Google Scholar]
- 29.Handforth C., Clegg A., Young C., et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26:1091–1101. doi: 10.1093/annonc/mdu540. [DOI] [PubMed] [Google Scholar]
- 30.Ethun C.G., Bilen M.A., Jani A.B., Maithel S.K., Ogan K., Master V.A. Frailty and cancer: implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J Clin. 2017;67:362–377. doi: 10.3322/caac.21406. [DOI] [PubMed] [Google Scholar]
- 31.Ramaty E., Maor E., Peltz-Sinvani N., et al. Low ALT blood levels predict long-term all-cause mortality among adults. A historical prospective cohort study. Eur J Intern Med. 2014;25:919–921. doi: 10.1016/j.ejim.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Uliel N., Segal G., Perri A., Turpashvili N., Kassif Lerner R., Itelman E. Low ALT, a marker of sarcopenia and frailty, is associated with shortened survival amongst myelodysplastic syndrome patients: a retrospective study. Medicine. 2023;102:e33659. doi: 10.1097/MD.0000000000033659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bylow K., Mohile S.G., Stadler W.M., Dale W. Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer?: a conceptual review. Cancer. 2007;110:2604–2613. doi: 10.1002/cncr.23084. [DOI] [PubMed] [Google Scholar]
- 34.Lascano D., Pak J.S., Kates M., et al. Validation of a frailty index in patients undergoing curative surgery for urologic malignancy and comparison with other risk stratification tools. Urol Oncol. 2015;33:426.e1–426.e12. doi: 10.1016/j.urolonc.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]