Abstract
Starch, a semi-crystalline energy storage form primarily found in plant plastids plays a crucial role in various food or no-food applications. Despite the starch biosynthetic pathway's main enzymes have been characterized, their origin and evolution remained a subject of debate. In this study, we conducted the comprehensive phylogenetic and structural analysis of three types of starch biosynthetic enzymes: starch synthase (SS), starch branching enzyme (SBE) and isoamylase-type debranching enzyme (ISA) from 51,151 annotated genomes. Our findings provide valuable insights into the possible scenario for the origin and evolution of the starch biosynthetic pathway. Initially, the ancestor of SBE can be traced back to an unidentified bacterium that existed before the formation of the last eukaryotic common ancestor (LECA) via horizontal gene transfer (HGT). This transfer event likely provided the eukaryote ancestor with the ability to synthesize glycogen. Furthermore, during the emergence of Archaeplastida, one clade of SS was transferred from Deltaproteobacteria by HGT, while ISA and the other clade of SS originated from Chlamydiae through endosymbiosis gene transfer (EGT). Both these transfer events collectively contributed to the establishment of the original starch biosynthetic pathway. Subsequently, after the divergence of Viridiplantae from Rhodophyta, all three enzymes underwent multiple duplications and N-terminus extension domain modifications, resulting in the formation of functionally specialized isoforms and ultimately leading to the complete starch biosynthetic pathway. By shedding light on the evolutionary origins of key enzymes involved in the starch biosynthetic pathway, this study provides important insights into the evolutionary events of plants.
Keywords: Origin, Evolution, Starch biosynthesis, Starch synthase, Starch branching enzyme, Isoamylase-type debranching enzyme
1. Introduction
Starch is a unique α-glucan-based energy storage form found in plant plastids, possessing insoluble and semi-crystalline properties. In addition to its significance as an important and efficient natural energy storage molecule, starch finds widespread applications as an additive in various industries including paper making and textiles [[1], [2], [3]]. To date, although the starch biosynthesis pathway has been characterized in some model plants, consisting of three crucial processes, elongation mediated by starch synthase (SS), branching catalyzed by starch branching enzyme (SBE), and debranching facilitated by isoamylase-type debranching enzyme (ISA) (Fig. 1) [4,5], the origin and evolution of this pathway and its associated enzymes have remained poorly understood.
Fig. 1.
Schematic diagram of starch biosynthesis processes. (a) Sketch map illustrating the structures of amylose and amylopectin. Starch is composed of α-1,4-glucan chains with α-1,6-glucan branches. These components consist of two types: the linear amylose with minimal branching and moderately branched amylopectin, which contributes to the semi-crystalline formation of starch. Amylose, synthesized primarily by GBSS, has few branches and a high degree of polymerization. On the other hand, amylopectin synthesis involves three kinds of enzymes (SS, SBE, ISA), which result in multiple branches and the formation of a more complex starch structure. (b) Process of elongation in starch biosynthesis. GBSS or SSs utilize adenosine diphosphate glucose as a substrate to link it to the non-reducing end of α-glucan. Among the six identified classes of SS (GBSS, SSI–V) [1,3], GBSS and SSI–SSIII have been found to be chiefly responsible for elongating the α-glucan chains of starch [[17], [18], [19]]. (c) Branching process with a significant role in starch biosynthesis. Branches are formed when SBE cleave existing α-1,4-glycosidic bonds and attach α-1,6-glycosidic bond to the current or another α-glucan molecule. In plants, three isoforms of SBE (SBEI-III) have been identified [60,61]. SBEI and SBEII are responsible for generating α-1,6-glycosidic bond branches by cleaving α-1,4-glycosidic bonds [48,55,62]. (d) Debranching process in starch biosynthesis. This process involves three isoforms of ISA (ISAI-III), which are responsible for trimming excessive α-1,6-glycosidic bond branches that hinder the formation of higher structures.
Previous studies have proposed that the starch biosynthesis pathway might evolve from glycogen biosynthetic pathway, which generates a simpler α-glucan-based energy storage form found widely in most bacteria and eukaryotes [[6], [7], [8]]. Furthermore, it has been suggested that starch synthesis resulted from the integration of the bacterial and eukaryotic pathways of glycogen storage polysaccharide metabolism following the endosymbiosis event involving the acquisition of plastid from the cyanobacteria [[9], [10], [11]]. Notably, the close relationship between granule-bound starch synthase (GBSS) and its cyanobacterial counterpart has been observed in the same groups [12,13]. Additionally, the presence of SSIII-IV in SS gene family indicated their acquisition from chlamydiae [12,14], while the resemblance of SBE to other eukaryotic glycogen branching enzymes has been noted [15]. Therefore, the origin of these enzymes within the starch biosynthetic pathway appears to be diverse.
Furthermore, the evolution of starch biosynthetic enzymes has led to the emergence of multiple isoforms through gene duplications [1,7,15,16]. These isoforms not only exhibit diverse properties in terms of elongation, branching and debranching, but are also distributed within specific evolutionary lineages. For instance, within SS gene family, SSI–SSIII primarily participate in elongating the α-glucan chains of starch, whereas SSIV and SSV are believed to play a role in initiating starch granules formation [[17], [18], [19], [20]]. In the case of SBE gene family, SBEIII could differ in the branching functions compared to SBEI and SBEII, and may be involved in the synthesis of starch granules [15,21]. However, the precise details of these duplications and the potential molecular differences that lead to sub- or neo-functionalization in starch biosynthetic enzymes remain unclear.
To unravel the origin and evolution of the starch biosynthetic pathway, we conducted a comprehensive analysis of starch synthases, starch branching enzymes, and isoamylase-type debranching enzymes. Our analysis involved the annotation of these enzymes in 46,880 Bacteria, 1647 Archaea, 1217 Fungi, 984 Metazoa, 250 Viridiplantae, and 173 other eukaryotes. Subsequently, we performed evolutionary and structural analysis to these enzymes. Finally, based on our findings, we proposed a possible scenario for the origin and evolution of the starch biosynthetic pathway.
2. Materials and Methods
2.1. Genomic data sources
For a more accurate understanding of the origin and evolution of starch biosynthetic enzymes, we used the most comprehensive genomes data up to date, sourced from NCBI [22]. Our genomic data comprised 46,880 Bacteria, 1,647 Archaea, 1,217 Fungi, 984 Metazoa, 250 Viridiplantae, and 173 other eukaryotes with annotated genomes. The Viridiplantae encompassed 31 Chlorophyta and 219 Streptophyta species (Supplement Table S1). Additionally, we included 8 red algae, which are closely related to Viridiplantae, within our dataset of 173 other eukaryotes (Supplement Table S2).
2.2. Domain features of starch biosynthesis enzymes and sequences count
With respect to the SS (EC 2.4.1.21), almost all GBSS and SS isoforms possess highly conserved GT5 for Glyco_transf_5 (PF08323) and Glycos_transf_1 (PF00534) domain in the C-terminus except for SSV (not included in this study), which are the main catalytic domains for catalyzing adenosine diphosphate glucose (ADPG) to generate α-glucan chains, and they keep variable functional domains in the N-terminus like CBM53 (PF16760) domains in SSIII. Meanwhile, similar sequences and domains are also found in the glycogen synthase (GS) of a great deal of bacteria. As for glycogen synthases, there are GT3 for Glycogen_syn (PF05693) domains type enzymes that also take action to elongate α-glucan chains, which are found in some archaea, bacteria, and almost all eukaryotes except for Archaeplastida lineages. However, the GS of almost all fungi and animal typically contain the Glycogen_syn (GT3) domains [12,23]. Despite differences in domain composition, both of them belongs to glycosyltransferases (GTs) and share similar GT-B fold, product and catalytic mechanism [[24], [25], [26]]. The schematic domains of SS and GS are supplied in Fig. S1. By detecting the specific domains of starch and glycogen synthase (GT5 and GT3) in the protein sets for the above species with annotated genomes, we got 43,279 potential starch or glycogen synthases from 29,348 species (methods seeing below).
SBEs (EC 2.4.1.18) exhibit three highly conserved domains: Alpha-amylase_C (PF02806) Alpha-amylase (PF00128), and a unique CBM48 (PF02922) domains. SBEs belong to the α-amylase superfamily and possess the ability to cleave the α-1,4-glycosidic bonds and reattach them to the C-6 positions of other chains, thereby forming branches. This function is similar to that of glycogen branching enzymes (BE). The schematic representation of BE domains is provided in Fig. S2. To investigate the evolution of starch and glycogen BEs across all kingdoms of life, we identified 31,768 potential BEs from 26,306 species using similar method.
ISAs (E.C. 3.2.1.68) have similar domains as SBEs, consisting solely of Alpha-amylase_C (PF02806) Alpha-amylase (PF00128) domains. The schematic representation of ISA and GlgX domains is supplied in Fig. S2. For ISA, we conducted a phylogenetic analysis using the same method, focusing on 23,413 potential ISAs annotated from 15,435 species.
2.3. Sequence mining and cluster
Based on 51,151 annotated genomes, we employed Hmmscan from HMMER v3.3.1 to search the sequences with corresponding Pfam IDs, including Glyco_transf_5 (PF08323), Glycogen_syn (PF05693), Alpha-amylase_C (PF02806) and Alpha-amylase (PF00128), under the cutoff E-value at 1e-4 [23,24]. Subsequently, we employed annotated KEGG orthology (KO) datasets to perform blastp analysis aiming to retrieve additional functionally reliable KO similar enzymes with a cutoff of 30% [25]. To acquire more reliable SSI-IV, GBSS and GS sequences for starch or glycogen synthases, we used K00703, K13679 and K05693 cluster sequences, respectively. For starch or glycogen branching enzymes, we relied on K00700 cluster sequences. Lastly for ISA, we used K01214 cluster sequences. However, due to large number of sequences in each cluster, conducting a phylogenetic analysis would require exponential computing power to align and construct a phylogenetic tree. Hence, we chose OrthoMCL to cluster homologous sequences, resulting in representative sequences with a 60% similarity threshold [26]. These representative sequences were served as the basis for constructing phylogenetic trees.
2.4. Multiple sequence alignment and phylogenetic tree reconstruction
Amino acid (AA) sequences of all clustered SS, GS, SBE and ISA sequences were aligned in Clustal Omega v1.2.4 with default settings [27]. Subsequently, the alignments underwent gap trimming using trimal with default parameters. Sequences more than 50 continuous AA gaps were removed, and the remaining sequences were realigned by Clustal Omega [28]. Phylogenetic trees were constructed using the maximum likelihood method implemented in RAxML v8.2.12 with the raxmlHPC-PTHREADS-SSE3. The PROTGAMMALGX substitution model was used, and a bootstrap value of 100 was set [29]. Supplementary detailed trees for the four clades were rebuilt with sequences clustered at 90% similarity from the represented sequences within each clade. This level of clustering was deemed sufficiently to depict the relationships among different species (Figs. S3b, S4, S7, S9). The resulting phylogenetic trees were visualized and colored by iTOL [30]. The domain distribution parts were scanned by Hmmscan and painted by TBtools [31]. The schematic diagrams were referenced from TimeTree and the new tree of life [32,33].
2.5. Amino acids distributions analysis around catalytic pockets
We used Alphafold2 for structure prediction of starch biosynthetic enzymes [34]. Based on these predicted and crystal structures, we selected 6GNE, 3AMK and 4OKD from PDB as template sequences. Specifically, we focused on the amino acids within a 5 Å around the catalytic pockets or binding domains, and the resulting structures were plotted by PyMOL (https://pymol.org/) [[35], [36], [37]]. Next, we extracted the corresponding columns of sequences aligned with these templates. Finally, we used ggseqlogo package in R to plot their sequence logos, with the template sequence positions displayed below the sequence logo [38].
3. Results and discussion
3.1. Evolutionary and structural analysis of starch and glycogen synthases
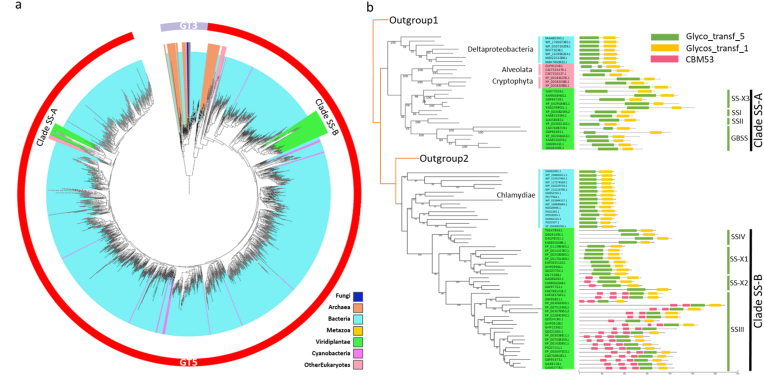
Based on the annotated domains of starch and glycogen synthase (GT5 and GT3 domains), we identified and annotated a total of 43,279 potential starch or glycogen synthases from 29,348 species, which served as the basis for constructing phylogenetic trees (see Materials and Methods). This tree revealed two distinct clades corresponding to GT5 and GT3 domains sequences (Fig. 2a). The GT5 clade predominantly comprised of starch synthases from Viridiplantae, along with some potential glycogen synthases from Bacteria and a small number of Archaea. Within GT5 clade, two distinct clades (named Clade SS-A and SS-B) were observed for starch synthases. Clade SS-A contained annotated GBSS, SSI, and SSII, while Clade SS-B included annotated SSIII and SSIV sequences. This indicates that the existing starch synthases likely have two separate origins. In contrast, the GT3 clade primarily consisted of glycogen synthases from Metazoa, Fungi, a few Bacteria and Archaea. In summary, eukaryotic glycogen synthases mainly originate from GT3 type, whereas GT5 type found only in Viridiplantae, suggesting the starch synthases of Viridiplantae may have originated by gene transfer from bacteria.
Fig. 2.
Phylogenetic tree and domain distribution analysis of characterized clades of SS. (a) Phylogenetic tree showcases clustered sequences containing GT5 and GT3 domains from annotated species genomes. The GT5 labeled red ring indicates starch or glycogen synthase-like sequences, while the GT3 labeled purple ring represents glycogen synthase-like sequences. The colored clades correspond to species from various kingdoms: green for Viridiplantae, lavender for Cyanobacteria, cyan-blue for other Bacteria, orange for Archaea, yellow for Metazoa, blue for Fungi, pink for other Eukaryotes. Other Eukaryotes includes annotated eukaryote species except for Metazoa, Fungi and Viridiplantae (Materials and Methods). (b) Phylogenetic trees and domain distributions reveal distinct clades of SS. Clade SS-A encompasses GBSS, SSI, SSII and SS-X3 from plants, while Clade SS-B comprises SSIII, SSIV, SS-X1 and SS-X2 from plants. The colored identifiers correspond to the same species mentioned above and the horizontally colored strips indicate different domains of SS.
To further investigate the origin and evolution of these two clades of starch synthases, we analyzed the phylogenetic relationship both within and outside these clades (Fig. 2b). For Clade SS-A, we observed a close relationship between the potential glycogen synthases from Deltaproteobacteria, Alveolata, and Cryptophyta. One possible explanation is that this clade was transferred to the eukaryote ancestor but subsequently lost in most other eukaryotes. Additionally, Alveolata and Cryptophyta were thought to be the species involved in a secondary plastid endosymbiotic event from red algae, acquiring plastids and some genes from red algae [11,[39], [40], [41]]. The result also showed that GBSS of Rhodophyta is closely related to Alveolata and Cryptophyte (Fig. S3a), which suggests that these genes may have been acquired from red algae. Therefore, it appears more plausible that the ancestor of Clade SS-A originated from a kind of Deltaproteobacteria. Moving on, the closest homologs of Clade SS-B were found in Chlamydiae (Fig. 2b), indicating that the Clade SS-B origin may be traced back to a kind of Chlamydiae, which is consistent with previous findings [14,42]. In summary, these results indicate that both clades SS originated from bacteria, with Deltaproteobacteria and Chlamydiae being their respective origins.
Following the acquisition of these genes by the ancestors, both the clades of SS underwent further evolution, leading to the formation of four sub-clades through multiple duplications. SS-A consists of GBSS, SSI, SSII and a Chlorophyta-specific sub-clade (named SS-X3). Clade SS-B comprises SSIII, SSIV and two uncharacterized sub-clades (named SS-X1 and SS-X2). All Viridiplantae species possess six sub-clades, whereas Chlorophyta lost SSIV clade but gained an additional SS-X3 and an extra duplication of SSIII (Figs. S3b and S4). This suggests that the additional duplications may serve as substitutes for the lost clades. In terms of the evolution of these copies, the protein domains analysis revealed that most starch synthases exhibit variations in their N-terminus regions compared to bacterial glycogen synthases [20,43]. Particularly, the CBM53 domains in N-terminus region have evolved from one to three in SS-X2 and SSIII, which may be responsible for protein-protein interactions or non-catalytic glucan binding function [44,45]. Despite the variations in the N-terminus regions, all sub-clades displayed conserved residue distributions and structural scaffolds in the glucan and adenosine diphosphate glucose binding domains, which may indicate that the catalytic function of SSs has been largely conserved by strong purifying selection, and their functional differences are likely attributed to other regions (Fig. S5).
3.2. Evolutionary and structural analysis of starch branching enzymes
SBEs play a crucial role in determining the structure of starch granules [15]. As glycoside hydrolases (GHs), plant SBEs share similar sequences and structures with glycogen branching enzyme (BE) found in bacteria, fungi, and animals [12]. To investigate the origin and evolution of plant SBEs, a total of 31,768 potential SBEs and BEs were annotated from 26,306 genome-sequenced species (Figs. S6 and S7) (Materials and Methods). The phylogenetic tree revealed that all eukaryotic BEs and SBEs originated from a single clade, with the closest outgroup being Bacteria from multiple phylum rather than Archaea (Fig. 3a), suggesting that an unknown bacterium may have contributed BE to LECA.
Fig. 3.
Phylogenetic and structural analysis of SBE and ISA clades. (a) Phylogenetic tree and domain distribution analysis involves clustered sequences derived from SBE and its outgroup of BE. The colored identifiers represent species as mentioned earlier, while the horizontally colored strips represent different domains of SBE and its outgroup of BE. (b, c) Structures of amino acids within a 5 Å region surrounding the catalytic domains along with their sequence logos of SBE clades. The existing SBEs belong to the Retaining GHs, characterized by double-displacement mechanism, whose general acid or base and nucleophile (red mark) are typically spatially close, specifically at positions (415D) and (470E) within the catalytic pocket, respectively [49,54]. (d) Phylogenetic tree and domain distribution analysis pertain to clustered sequences of ISA and its outgroup. (e, f) Structures of amino acids within a 5 Å region surrounding the catalytic domains and their sequence logos of ISA clades. Similar to the GHs, ISAs share similar essential AAs (yellow mark): (410D) and (466E) in the catalytic domains.
Following the transfer, the ancestor of BEs diverged into two branches within eukaryotes (Clade BE-A and BE-B). The BE-A is present in almost all eukaryotes and further evolved into SBEI and SBEII in plants through duplication events. Both SBEI and SBEII are widely distributed in Viridiplantae but absent in Rhodophyta, indicating that the duplication likely occurred prior to the divergence of Viridiplantae [36,46]. The BE-B has been lost in most eukaryotic lineages but gives rise to Streptophyta-specific subgroup SBEIII [1,47,48]. The result demonstrates that SBEIII may contribute to the specificity of starch granules synthesis [16,21,36,46]. Comparative analysis of the domain and amino acids of SBEIII with SBEI-II showed that the two key catalytic residues (415D, 470E of NP_001105370.1 from Zea mays as template) involved in the double-displacement mechanism have been replaced by His and Tyr in SBEIII [49], meaning that the SBEIII with its altered function is not a redundant feature for starch synthesis in plants (Fig. 3b and c).
3.3. Evolutionary and structural analysis of isoamylase-type debranching enzyme
ISA is similar with glycogen debranching enzyme (GlgX) in bacteria, which plays a crucial role in hydrolyzing α-1,6-linkage chains to generate the proper structure of amylopectin [48,50]. Using similar methods (Materials and Methods), we annotated a total of 23,413 potential ISAs from 15,435 species. Phylogenetic analysis revealed that the closest homologous sequences of ISAs were from Chlamydiae (Fig. 3d, S8), which is similar to the relationship observed in Clade SS-B. The result demonstrates that Clade ISA and Clade SS-B may have been simultaneously transferred into the ancestor of Viridiplantae from Chlamydiae [14]. Besides, the Clade SS-B is exclusively found in Viridiplantae, not in Rhodophyta, indicating that Rhodophyta might have lost the Clade SS-B.
ISA has also evolved into three sub-clades in Viridiplantae (Fig. S9). Among them, ISAIII were involved in the degradation process of starch and is widely present in Viridiplantae [51,52]. On the other hand, ISAI and ISAII were inferred to form the heteromultimeric complex [16,46]. In this complex, ISAI was thought to be the main catalytic subunit for debranching function, while ISAII contributes mainly to the higher structure crystallization of starch [1,8]. We found that ISAI exists in almost Rhodophyta and Viridiplantae, whereas ISAII is exclusively found in Streptophyta, which may be related to Streptophyta with more complex starch structure. Furthermore, apart from using H2O as the acceptor substrate for hydrolyzing α-1,6-glycosidic bonds [49,53,54], we discovered changes within the catalytic pockets of the key catalytic residues (410D and 466E of NP_181522.1 from Arabidopsis thaliana as template) of ISAII, as well as longer regions of N-terminus compared to other isoforms were found [49,55]. These differences may explain the functional shift of ISAII into a non-catalytic subunit within the heteromultimeric complex (Fig. 3e and f).
3.4. The origin and evolution schematic diagram of starch biosynthetic pathway
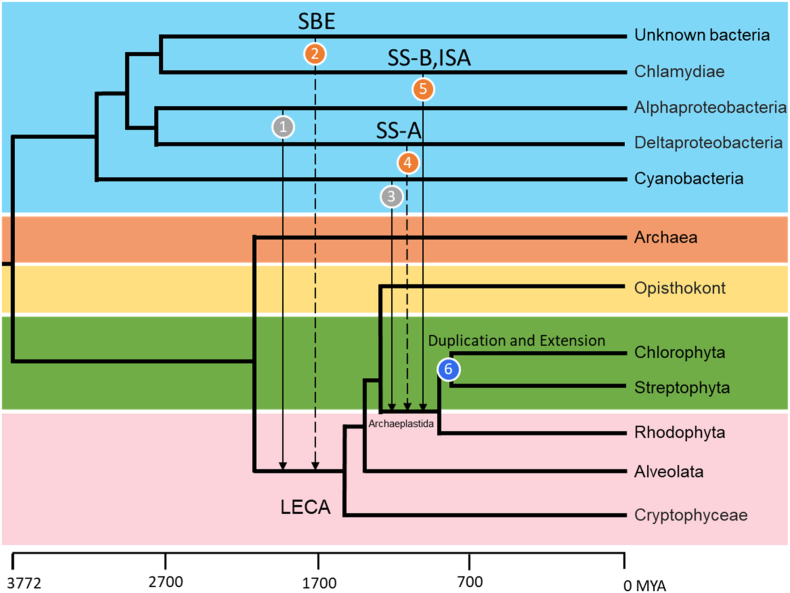
Taking into consideration the origin and evolution of the starch biosynthetic enzymes discussed above, we propose a comprehensive scenario for the starch biosynthetic pathway (Fig. 4). Firstly, given that SBE and BE were found in most Viridiplantae and many other eukaryotes, we suggest that the ancestor of SBE and BE was transferred to the eukaryote ancestor approximately 2 billion years ago from an unknown bacterium (Fig. 4: point2). This transfer event might give the eukaryotic ancestor the ability to synthesize glycogen, considering BE is the key enzyme in bacterial glycogen synthesis, which is distinct from the endosymbiosis of Alphaproteobacteria (Fig. 4: point1), thought to be associated with the origin of mitochondria and the formation of LECA [56].
Fig. 4.
Evolutionary events schematic diagram of starch biosynthetic enzymes and other organelles. Background colors in the diagram represent species from different kingdoms, while solid and dashed lines represent EGT and HGT events. Point 1 represents the origin of mitochondria in LECA through endosymbiosis of Alphaproteobacteria; Point 2 represents the transfer of BE from an unknown bacterium to LECA; Point 3 represents the endosymbiosis event where Archaeplastida emerged from a cyanobacterium, which is thought to be related to the origin of Archaeplastida lineage; Point 4 represents the origin of Clade SS-A through HGT from Deltaproteobacteria; Point 5 represents another endosymbiosis event of Archaeplastida from Chlamydiae, which provided Clade SS-B and ISA; Point 6 denotes the duplication and subsequent evolution of these three enzymes during the formation and differentiation of Viridiplantae.
During the origin of the Archaeplastida lineage, which underwent a single endosymbiosis event by feeding on a cyanobacterium approximately 1.4 billion years ago (Fig. 4: point3) [6,14,57], two additional independent transfer events contributed to the formation of the starch biosynthesis pathway. One is that Clade SS-A likely originated from Deltaproteobacteria by HGT (Fig. 4: point4), whose isoforms within this clade were found to chiefly elongate the α-glucan chains of starch. Furthermore, we discovered that not only Clade SS-B but also ISA showed a high degree of relation to Chlamydial counterparts. Meanwhile, other researchers have also identified Chlamydiae as contributors of numerous genes to Viridiplantae approximately one billion years ago through EGT and inferred that Chlamydiae may have also gone through endosymbiosis during the formation of Archaeplastida (Fig. 4: point5) [14,58,59]. As a result, the original starch biosynthetic pathway was established, likely producing glycogen-like structure by the action of these enzymes.
After the incorporation of these enzymes into this pathway, the formation and differentiation of Viridiplantae led to the generation of many new isoforms including 6 SSs, 2 SBEs, and 2 ISAs (Fig. 4: point 6). Throughout evolution, these isoforms acquired diverse functions, such as SSI-III were found to elongate amylopectin with varying degrees of polymerization, while the heteromultimeric complex of ISAI-II contributed to the removal of excess branches, resulting in higher structural crystallization of starch. Furthermore, most isoforms underwent evolution in their N-terminus regions, which potentially serves functions such as binding functions of SSIII or acting as plastid localization signal. However, the structural characteristics of these regions remain unpredictable based on Alphafold2 due to scarce crystal structures and research available on them (Figs. S10–12) [34]. In conclusion, the evolution of these isoforms not only confer functional specialization, but also gradually facilitated the development of complex crystallization processes in the complete starch biosynthetic pathway.
4. Conclusion
Our study contributes to a clearer understanding of the evolutionary origins of these starch biosynthetic enzymes. Initially, the eukaryotic BE originated from an unknown bacterium before the LECA through HGT, while SBE were inherited from eukaryote ancestor. Furthermore, Clade SS-A were transferred from Deltaproteobacteria via HGT, and Clade SS-B and ISAs were originated from Chlamydiae before the formation of Archaeplastida through an EGT event. These findings, in conjunction with previous research, indicate that the original enzymes of polysaccharide storage pathway involved more than a simple combination of eukaryotic and prokaryotic enzymes. Subsequently, after these enzymes were redirected to plastid in Viridiplantae, multiple duplications occurred prior to differentiation, conferring it to sub-functionalization or neo-functionalization. Meanwhile, these enzymes underwent evolutionary changes such as extensions of the N-terminus and the formation of multiple isoforms, which endowed them with additional properties within the starch biosynthetic pathway.
CRediT authorship contribution statement
Hong Chang: Conceptualization, Methodology, Software, Data curation, Formal analysis, Writing – original draft. Jie Bai: Data curation, Formal analysis. Hejian Zhang: Writing – review & editing. Rong Huang: Writing – review & editing. Huanyu Chu: Writing – review & editing. Qian Wang: Writing – review & editing. Hao Liu: Writing – review & editing. Jian Cheng: Supervision, Writing – review & editing. Huifeng Jiang: Conceptualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
We thank professor Bhupal Govinda Shrestha from Kathmandu University and Miss Nida Ahmed for words editing. This work was supported by the National Key R&D Program of China (No. 2021YFC2103500), and the Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (No. TSBICIP-KJGG-009-02 and No. TSBICIP-CXRC-003).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.synbio.2023.05.006.
Contributor Information
Jian Cheng, Email: cheng_j@tib.cas.cn.
Huifeng Jiang, Email: jiang_hf@tib.cas.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Qu J.Z., Xu S.T., Zhang Z.Q., Chen G.Z., Zhong Y.Y., Liu L.S., et al. Evolutionary, structural and expression analysis of core genes involved in starch synthesis. Sci Rep. 2018;8 doi: 10.1038/s41598-018-30411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister B., Zeeman S.C. Formation of starch in plant cells. Cell Mol Life Sci. 2016;73:2781–2807. doi: 10.1007/s00018-016-2250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang L., Tan H., Zhang C., Li Q., Liu Q. Starch biosynthesis in cereal endosperms: an updated review over the last decade. Plant Commun. 2021;2 doi: 10.1016/j.xplc.2021.100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irshad A., Guo H., Rehman S.U., Wang X., Wang C., Raza A., et al. Soluble starch synthase enzymes in cereals: an updated review. Agronomy. 2021;11:1983. [Google Scholar]
- 5.Li C., Powell P.O., Gilbert R.G. Recent progress toward understanding the role of starch biosynthetic enzymes in the cereal endosperm. Amylase. 2017;1:59–74. [Google Scholar]
- 6.Ball S., Colleoni C., Cenci U., Raj J.N., Tirtiaux C. The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J Exp Bot. 2011;62:1775–1801. doi: 10.1093/jxb/erq411. [DOI] [PubMed] [Google Scholar]
- 7.Cenci U., Nitschke F., Steup M., Minassian B.A., Colleoni C., Ball S.G. Transition from glycogen to starch metabolism in Archaeplastida. Trends Plant Sci. 2014;19:18–28. doi: 10.1016/j.tplants.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Zeeman S.C., Kossmann J., Smith A.M. Starch: its metabolism, evolution, and biotechnological modification in plants. Annu Rev Plant Biol. 2010;61:209–234. doi: 10.1146/annurev-arplant-042809-112301. [DOI] [PubMed] [Google Scholar]
- 9.Zimorski V., Ku C., Martin W.F., Gould S.B. Endosymbiotic theory for organelle origins. Curr Opin Microbiol. 2014;22:38–48. doi: 10.1016/j.mib.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Fettke J., Hejazi M., Smirnova J., Höchel E., Stage M., Steup M. Eukaryotic starch degradation: integration of plastidial and cytosolic pathways. J Exp Bot. 2009;60:2907–2922. doi: 10.1093/jxb/erp054. [DOI] [PubMed] [Google Scholar]
- 11.Gould S.B., Waller R.F., McFadden G.I. Plastid evolution. Annu Rev Plant Biol. 2008;59:491–517. doi: 10.1146/annurev.arplant.59.032607.092915. [DOI] [PubMed] [Google Scholar]
- 12.Deschamps P., Colleoni C., Nakamura Y., Suzuki E., Putaux J.L., Buleon A., et al. Metabolic symbiosis and the birth of the plant kingdom. Mol Biol Evol. 2008;25:536–548. doi: 10.1093/molbev/msm280. [DOI] [PubMed] [Google Scholar]
- 13.Liu H., Yu G., Wei B., Wang Y., Zhang J., Hu Y., et al. Identification and phylogenetic analysis of a novel starch synthase in maize. Front Plant Sci. 2015;6:1013. doi: 10.3389/fpls.2015.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ball S.G., Subtil A., Bhattacharya D., Moustafa A., Weber A.P.M., Gehre L., et al. Metabolic effectors secreted by bacterial pathogens: essential facilitators of plastid endosymbiosis? Plant Cell. 2013;25:7–21. doi: 10.1105/tpc.112.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tetlow I.J., Emes M.J. A review of starch-branching enzymes and their role in amylopectin biosynthesis: starch-Branching Enzymes in Amylopectin Biosynthesis. IUBMB Life. 2014;66:546–558. doi: 10.1002/iub.1297. [DOI] [PubMed] [Google Scholar]
- 16.Abt M.R., Zeeman S.C. Evolutionary innovations in starch metabolism. Curr Opin Plant Biol. 2020;55:109–117. doi: 10.1016/j.pbi.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Sano Y. Differential regulation of waxy gene expression in rice endosperm. TAG Theoretical and applied genetics Theoretische und angewandte Genetik. 1984;68:467–473. doi: 10.1007/BF00254822. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z.Y., Wu Z.L., Xing Y.Y., Zheng F.G., Guo X.L., Zhang W.G., et al. Nucleotide sequence of rice waxy gene. Nucleic Acids Res. 1990;18:5898. doi: 10.1093/nar/18.19.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denyer K., Waite D., Motawia S., Moller B.L., Smith A.M. Granule-bound starch synthase I in isolated starch granules elongates malto-oligosaccharides processively. Biochem J. 1999;340:183–191. [PMC free article] [PubMed] [Google Scholar]
- 20.Leterrier M., Holappa L.D., Broglie K.E., Beckles D.M. Cloning, characterisation and comparative analysis of a starch synthase IV gene in wheat: functional and evolutionary implications. BMC Plant Biol. 2008;8:98. doi: 10.1186/1471-2229-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang G., Li S., Zhang M., Peng H., Wang C., Zhu Y., et al. Molecular cloning and expression analysis of the starch-branching enzyme III gene from common wheat (Triticum aestivum) Biochem Genet. 2013;51:377–386. doi: 10.1007/s10528-013-9570-4. [DOI] [PubMed] [Google Scholar]
- 22.O'Leary N.A., Wright M.W., Brister J.R., Ciufo S., Haddad D., McVeigh R., et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44:D733–D745. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finn R.D., Bateman A., Clements J., Coggill P., Eberhardt R.Y., Eddy S.R., et al. Pfam: the protein families database. Nucleic Acids Res. 2014;42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mistry J., Chuguransky S., Williams L., Qureshi M., Salazar G.A., Sonnhammer E.L.L., et al. Pfam: the protein families database in 2021. Nucleic Acids Res. 2021;49:D412–D419. doi: 10.1093/nar/gkaa913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanehisa M., Goto S., Sato Y., Furumichi M., Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer S., Brunk B.P., Chen F., Gao X., Harb O.S., Iodice J.B., et al. Using OrthoMCL to assign proteins to OrthoMCL-DB groups or to cluster proteomes into new ortholog groups. Curr Protoc Bioinformatics. 2011;35:6(12):1–9. doi: 10.1002/0471250953.bi0612s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sievers F., Higgins D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018;27:135–145. doi: 10.1002/pro.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capella-Gutierrez S., Silla-Martinez J.M., Gabaldon T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Letunic I., Bork P. Interactive Tree of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Hug L.A., Baker B.J., Anantharaman K., Brown C.T., Probst A.J., Castelle C.J., et al. A new view of the tree of life. Nat Microbiol. 2016;1 doi: 10.1038/nmicrobiol.2016.48. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S., Suleski M., Craig J.M., Kasprowicz A.E., Sanderford M., Li M., et al. TimeTree 5: an expanded resource for species divergence times. Mol Biol Evol. 2022;39:msac174. doi: 10.1093/molbev/msac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen M.M., Ruzanski C., Krucewicz K., Alexander S., Cenci U., Ball S.G., et al. Crystal structures of the catalytic domain of Arabidopsis thaliana starch synthase IV, of granule bound starch synthase from CLg1 and of granule bound starch synthase I of cyanophora paradoxa illustrate substrate recognition in starch synthases. Front Plant Sci. 2018;9:1138. doi: 10.3389/fpls.2018.01138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noguchi J., Chaen K., Vu N.T., Akasaka T., Shimada H., Nakashima T., et al. Crystal structure of the branching enzyme I (BEI) from Oryza sativa L with implications for catalysis and substrate binding. Glycobiology. 2011;21:1108–1116. doi: 10.1093/glycob/cwr049. [DOI] [PubMed] [Google Scholar]
- 37.Sim L., Beeren S.R., Findinier J., Dauvillée D., Ball S.G., Henriksen A., et al. Crystal structure of the chlamydomonas starch debranching enzyme isoamylase ISA1 reveals insights into the mechanism of branch trimming and complex assembly. J Biol Chem. 2014;289:22991–23003. doi: 10.1074/jbc.M114.565044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagih O. ggseqlogo: a versatile R package for drawing sequence logos. Bioinformatics. 2017;33:3645–3647. doi: 10.1093/bioinformatics/btx469. [DOI] [PubMed] [Google Scholar]
- 39.Patron N.J., Keeling P.J. Common evolutionary origin of starch biosynthetic enzymes in green and red algae. J Phycol. 2005;41:1131–1141. [Google Scholar]
- 40.Keeling P.J. The endosymbiotic origin, diversification and fate of plastids. Philos Trans R Soc Lond Ser B Biol Sci. 2010;365:729–748. doi: 10.1098/rstb.2009.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strassert J.F.H., Irisarri I., Williams T.A., Burki F. A molecular timescale for eukaryote evolution with implications for the origin of red algal-derived plastids. Nat Commun. 2021;12:1879. doi: 10.1038/s41467-021-22044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reyes-Prieto A., Hackett J.D., Soares M.B., Bonaldo M.F., Bhattacharya D. Cyanobacterial contribution to algal nuclear genomes is primarily limited to plastid functions. Curr Biol. 2006;16:2320–2325. doi: 10.1016/j.cub.2006.09.063. [DOI] [PubMed] [Google Scholar]
- 43.Roldan I., Wattebled F., Lucas M.M., Delvalle D., Planchot V., Jimenez S., et al. The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant J. 2007;49:492–504. doi: 10.1111/j.1365-313X.2006.02968.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X.L., Szydlowski N., Delvalle D., D'Hulst C., James M.G., Myers A.M. Overlapping functions of the starch synthases SSII and SSIII in amylopectin biosynthesis in Arabidopsis. BMC Plant Biol. 2008;8:96. doi: 10.1186/1471-2229-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujita N., Yoshida M., Kondo T., Saito K., Utsumi Y., Tokunaga T., et al. Characterization of SSIIIa-Deficient mutants of rice: the function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol. 2007;144:2009–2023. doi: 10.1104/pp.107.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaen K., Noguchi J., Omori T., Kakuta Y., Kimura M. Crystal structure of the rice branching enzyme I (BEI) in complex with maltopentaose. Biochem Biophys Res Commun. 2012;424:508–511. doi: 10.1016/j.bbrc.2012.06.145. [DOI] [PubMed] [Google Scholar]
- 47.Colleoni C., Ball S.G. Bound substrate in the structure of cyanobacterial branching enzyme supports a new mechanistic model. J Biol Chem. 2017;292:5465–5475. doi: 10.1074/jbc.M116.755629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zmasek C.M., Godzik A. Phylogenomic analysis of glycogen branching and debranching enzymatic duo. BMC Evol Biol. 2014;14:183. doi: 10.1186/s12862-014-0183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ardèvol A., Rovira C. Reaction mechanisms in carbohydrate-active enzymes: glycoside hydrolases and glycosyltransferases. Insights from ab initio quantum mechanics/molecular mechanics dynamic simulations. J Am Chem Soc. 2015;137:7528–7547. doi: 10.1021/jacs.5b01156. [DOI] [PubMed] [Google Scholar]
- 50.Facon M., Lin Q., Azzaz A.M., Hennen-Bierwagen T.A., Myers A.M., Putaux J.-L., et al. Distinct functional properties of isoamylase-type starch debranching enzymes in monocot and dicot leaves. Plant Physiol. 2013;163:1363–1375. doi: 10.1104/pp.113.225565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goren A., Ashlock D., Tetlow I.J. Starch formation inside plastids of higher plants. Protoplasma. 2018;255:1855–1876. doi: 10.1007/s00709-018-1259-4. [DOI] [PubMed] [Google Scholar]
- 52.Streb S., Eicke S., Zeeman S.C. The simultaneous abolition of three starch hydrolases blocks transient starch breakdown in Arabidopsis. J Biol Chem. 2012;287:41745–41756. doi: 10.1074/jbc.M112.395244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Møller M.S., Henriksen A., Svensson B. Structure and function of α-glucan debranching enzymes. Cell Mol Life Sci. 2016;73:2619–2641. doi: 10.1007/s00018-016-2241-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee S.S., Hong S.Y., Errey J.C., Izumi A., Davies G.J., Davis B.G. Mechanistic evidence for a front-side, SNi-type reaction in a retaining glycosyltransferase. Nat Chem Biol. 2011;7:631–638. doi: 10.1038/nchembio.628. [DOI] [PubMed] [Google Scholar]
- 55.The reaction mechanism of retaining glycosyltransferases. Biochem Soc Trans. 2016;44:51–60. doi: 10.1042/BST20150177. [DOI] [PubMed] [Google Scholar]
- 56.Fan L., Wu D., Goremykin V., Xiao J., Xu Y., Garg S., et al. Phylogenetic analyses with systematic taxon sampling show that mitochondria branch within Alphaproteobacteria. Nat Ecol Evol. 2020;4:1213–1219. doi: 10.1038/s41559-020-1239-x. [DOI] [PubMed] [Google Scholar]
- 57.Evanovich E., de Souza Mendonça-Mattos P.J., Guerreiro J F J b. A timescale for the radiation of photosynthetic eukaryotes. bioRxiv. 2020 2020.04.18. [Google Scholar]
- 58.Moustafa A., Reyes-Prieto A., Bhattacharya D. Chlamydiae has contributed at least 55 genes to plantae with predominantly plastid functions. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Becker B., Hoef-Emden K., Melkonian M. Chlamydial genes shed light on the evolution of photoautotrophic eukaryotes. Mol Biol Evol. 2008;8:203. doi: 10.1186/1471-2148-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Y., Sun F.-J., Rosales-Mendoza S., Korban S.S. Three orthologs in rice, Arabidopsis, and Populus encoding starch branching enzymes (SBEs) are different from other SBE gene families in plants. Gene. 2007;401:123–130. doi: 10.1016/j.gene.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 61.Nougue O., Corbi J., Ball S.G., Manicacci D., Tenaillon M.I. Molecular evolution accompanying functional divergence of duplicated genes along the plant starch biosynthesis pathway. BMC Evol Biol. 2014;14:103. doi: 10.1186/1471-2148-14-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nishi A., Nakamura Y., Tanaka N., Satoh H. Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol. 2001;127:459–472. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.