Abstract
Background
Rotational thromboelastometry (ROTEM; TEM International GmbH, Munich, Germany) is a global coagulation test that guides evidence-based platelet transfusion in trauma patients. We evaluated ROTEM parameters for predicting mid-term (five days) platelet transfusion in trauma patients.
Methods
Maximum clot firmness and clot amplitudes after 5, 10, and 15 mins (A5, A10, and A15, respectively) of fibrin-specific ROTEM (FIBTEM) and extrinsically activated ROTEM (EXTEM) were retrospectively collected from 82 hospitalized, stable, non-bleeding trauma patients after successful initial resuscitation. Platelet-specific ROTEM (PLTEM) was calculated by subtracting FIBTEM from EXTEM. Platelet transfusions were reviewed for five days after ROTEM.
Results
The areas under the curve for FIBTEM, EXTEM, and PLTEM predicting platelet concentrate transfusion of >12 U at mid-term were 0.915–0.923, 0.878–0.896, and 0.551–0.735, respectively. FIBTEM and EXTEM parameters were comparable to those of fibrinogen, fibrin/fibrinogen degradation products, D-dimer, and antithrombin III. Strong correlations (r>0.7) were noted between platelet count and EXTEM (A5, A10, and A15) or PLTEM (A5), platelet function (per platelet count) and EXTEM (A10 and A15), and fibrinogen levels and all FIBTEM parameters.
Conclusions
FIBTEM and EXTEM can reliably predict mid-term platelet transfusion in trauma patients. FIBTEM, EXTEM, and PLTEM parameters correlate with conventional coagulation tests (platelets and fibrinogen).
Keywords: Fibrinogen, Platelets, Platelet transfusion, Rotational thromboelastometry, Trauma
INTRODUCTION
Trauma patients are critical patients who need massive transfusions initially to prevent shock [1]. For trauma patients, quick decisions regarding transfusions are paramount [2]. Laboratory tests are essential for evidence-based care, especially for stable trauma patients without bleeding after initial resuscitation requiring massive transfusion. The importance of patient blood management is further highlighted with the emergence of new infectious diseases and an increasingly aging population [3-6]. In times of blood shortage, platelet products are the most difficult to manage because of their short shelf-life (five days). Donor recruitment, especially for apheresis- or whole blood-derived platelets, is encouraged [6, 7]. In this context, predicting the mid-term (five days) requirements for platelet transfusion may be beneficial for the evidence-based care of trauma patients and for appropriately allocating limited healthcare resources.
A platelet count with a cutoff between 50 and 100×109/L is an indication of platelet transfusion in trauma patients [1, 2, 8, 9]. A more stringent cutoff is recommended in patients with traumatic brain injury [10]. As a sole marker in trauma patients, the platelet count may be limited in indicating transfusion [11-14]. According to the recent cell-based coagulation model [15], comprehensive evaluation of multiple components (platelets, red blood cells, and plasma coagulation factors) may account for one hemostatic state (e.g., platelet insufficiency) [13, 16, 17]. As a complementary method to conventional coagulation tests, viscoelastic tests, including thromboelastography (Haemonetics Corp., Braintree, MA, USA) and rotational thromboelastometry (ROTEM; TEM International GmbH, Munich, Germany), are global coagulation tests that provide more detailed information about clot formation and lysis [18]. The use of citrated whole blood allows multiple coagulation components to be evaluated simultaneously. With individualized goal-directed therapy, most studies using viscoelastic tests have reported an association between detection and the risk of coagulopathy [19-21] and the prediction of massive transfusion and mortality [21]. Studies on ROTEM parameters related to platelet transfusion are limited. A few studies have shown that the use of ROTEM could be helpful in the prevention of unnecessary platelet transfusion and a reduction in related transfusion reactions and hospital costs [22, 23].
We evaluated ROTEM parameters for predicting mid-term platelet transfusion in hospitalized trauma patients. We also compared ROTEM parameters with those of conventional coagulation tests.
MATERIALS AND METHODS
Patients and data collection
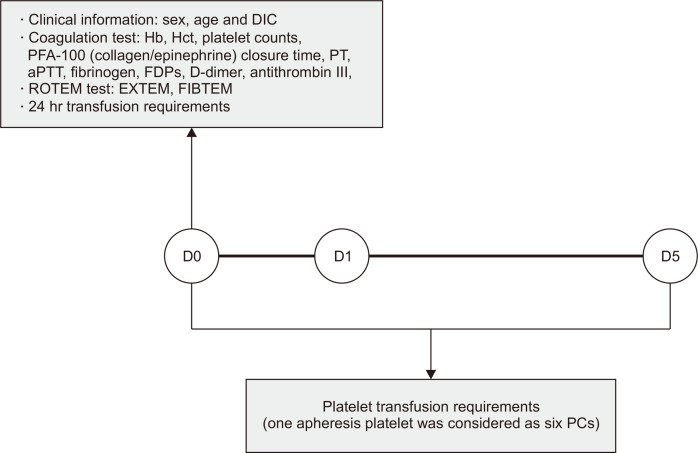
The clinical and laboratory data of patients admitted to the Trauma Center of Chonnam National University Hospital (CNUH), Gwangju, Korea, who underwent ROTEM testing from June 2021 to November 2022 were retrospectively reviewed. Patients aged <18 yrs and those who died or received blood transfusion due to surgery within five days after ROTEM testing were excluded. In total, 82 trauma patients were included. Clinical data (sex, age, disseminated intravascular coagulation [DIC], date of surgery, and 24-hr transfusion requirements) were collected at the time of conventional coagulation and ROTEM testing (Fig. 1). DIC was defined according to the International Society on Thrombosis and Hemostasis scoring system; a score of five or more was considered to indicate overt DIC [24].
Fig. 1.
Data collection of clinical information, laboratory results, and transfusion requirements in this study.
Abbreviations: DIC, disseminated intravascular coagulation; PFA-100, Platelet Function Analyzer-100; PT, prothrombin time; aPTT, activated partial thromboplastin time; FDPs, fibrin/fibrinogen degradation products; ROTEM, rotational thromboelastometry; EXTEM, extrinsically activated ROTEM; FIBTEM, fibrin-specific ROTEM; D0, day 0; D1, day 1; D5, day 5; PCs, platelet concentrates.
Platelet transfusion was determined using the following cutoffs: 50×109/L for suspected ongoing bleeding or DIC [25]; 30×109/L for minor procedures (central line insertion or radiological intervention) [26]; and 20×109/L when a continuous decrease in platelet count was expected. In patients with traumatic brain injury, the target platelet count was set between 70 and 100×109/L according to intracranial hemorrhage and antiplatelet medication history [10]. From the time of conventional coagulation and ROTEM testing, the numbers of transfused platelet concentrates (PCs) and apheresis platelets over the mid-term were reviewed. One apheresis platelet was considered to be six PCs [8].
To compare platelet and non-platelet transfusion over the mid-term, we classified 82 trauma patients based on platelet transfusion for five days from the time of conventional coagulation and ROTEM testing as follows: group A (non-platelet transfusion for mid-term) and group B (platelet transfusion for mid-term). This study was approved by the Institutional Review Board of CNUH, Gwangju, Korea (CNUH-2022-010) and was conducted in accordance with the Declaration of Helsinki.
Conventional coagulation and ROTEM test parameters
Conventional coagulation tests were performed at the Central Laboratory of CNUH using automated complete blood count analyzers XN-1000 (Sysmex, Kobe, Japan), XE-2100 (Sysmex), DxH-800 (Beckman Coulter, Brea, CA, USA), or DxH-900 (Beckman Coulter) for Hb, Hct, and platelet counts; Platelet Function Analyzer (PFA)-100 (Siemens, Deerfield, IL, USA) for platelet function tests with collagen/epinephrine; and automated coagulation analyzers CS-5100 (Sysmex) or CN-6000 (Sysmex) for prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, fibrin/fibrinogen degradation products (FDPs), D-dimer, and antithrombin III.
ROTEM was performed at the discretion of the clinician, after massive transfusion, as a point-of-care test at the Trauma Intensive Care Unit of CNUH. Clot amplitudes after 5 (A5), 10 (A10), and 15 (A15) mins of extrinsically activated ROTEM (EXTEM) and fibrin-specific ROTEM (FIBTEM) were collected. The maximum clot firmness (MCF) after EXTEM and FIBTEM was also reviewed. Platelet-specific ROTEM (PLTEM) was calculated by subtracting FIBTEM from EXTEM [27].
Statistical analysis
Continuous variables between groups A and B were compared using the Wilcoxon rank-sum test or Student’s t-test according to the Shapiro–Wilk normality test and F-test. Categorical variables were compared using Fisher’s exact test. ROC analysis was performed using conventional coagulation and ROTEM tests to predict PC transfusion of >12 U for the mid-term. The optimal cutoff was determined by maximizing the Youden index (sensitivity+ specificity−1). The sensitivity, specificity, positive predictive value, and negative predictive value were calculated. DeLong’s test was used to compare the area under the curve (AUC) [28]. For the correlation analysis between conventional coagulation and ROTEM tests, the Pearson correlation coefficient (r) was calculated. The absolute value of r was interpreted as follows: 0.00–0.30, weak correlation; 0.30–0.70, moderate correlation; 0.70–1.00, strong correlation [29]. In the correlation analysis, the PFA-100 in patients with thrombocytosis (platelet count >450 ×109/L) was excluded. All statistical analyses were conducted using RStudio (version 2022.7.1.554; RStudio Inc., Boston, MA, USA). A P<0.05 was considered statistically significant.
RESULTS
The clinical characteristics, 24-hr transfusion requirements, and conventional coagulation and ROTEM test results are shown in Table 1. The mean number of transfused PC units within the mid-term was 17.5 in group B. There were no significant differences in age, sex, or DIC between groups A and B. Twenty-four (29.3%) and 48 (58.5%) ROTEM tests were performed pre- and postoperatively, respectively, with no significant differences between the groups. The 24-hr transfusion requirements for packed red blood cells (PRCs) and fresh frozen plasma (FFP) were significantly higher in group B. Based on the conventional coagulation test, platelet count, PFA-100 (collagen/epinephrine) closure time, PT, aPTT, fibrinogen, FDPs, D-dimer, and antithrombin III were significantly different between groups A and B. In the ROTEM test, EXTEM and FIBTEM clot amplitudes were significantly lower in group B than in group A (P<0.001). In the PLTEM test, the values at A5, A10, and A15 were significantly lower in group B than in group A (P<0.001, <0.01, and <0.05, respectively). MCF was also lower in group B than in group A, although the difference was not significant.
Table 1.
Clinical characteristics, 24-hr transfusion requirements, and conventional coagulation and ROTEM test results between groups
| Variable | Group A: No platelet transfusion for mid-term (N=51) | Group B: Platelet transfusion for mid-term (N=31) | P |
|---|---|---|---|
| Male sex, N (%) | 33 (64.7) | 23 (74.2) | 0.466 |
| Age, yrs | 58.7±19.7 | 66.5±15.4 | 0.142 |
| DIC, N (%) | 1 (2.0) | 4 (12.9) | 0.065 |
| ROTEM, N (%) | |||
| Preoperative ROTEM test | 19 (37.3) | 5 (16.1) | 0.074 |
| Postoperative ROTEM test | 30 (58.8) | 18 (58.1) | 1.000 |
| Previous 24-hr transfusion requirements | |||
| RBC, unit | 0.5±1.0 | 3.2±3.8 | <0.001 |
| FFP, unit | 0.3±1.0 | 2.2±2.9 | <0.001 |
| PC, unit | 0.9±3.1 | 2.5±4.0 | 0.022 |
| Conventional coagulation test | |||
| Hb, g/dL | 10.1±1.6 | 9.5±2.1 | 0.136 |
| Hct, % | 30.3±4.9 | 28.0±6.0 | 0.056 |
| Platelet count, ×109/L | 273.8±267.2 | 84.1±51.9 | <0.001 |
| PFA-100 (collagen/epinephrine), sec | 136.6±57.8 | 181.4±67.0 | 0.006 |
| PT, sec | 14.1±6.1 | 15.4±6.2 | 0.011 |
| aPTT, sec | 29.6±6.9 | 37.0±27.9 | 0.038 |
| Fibrinogen, mg/dL | 466.4±182.3 | 231.9±156.2 | <0.001 |
| FDPs, μg/mL | 24.4±31.3 | 123.5±164.4 | 0.003 |
| D-dimer, mg/L | 8.2±7.8 | 19.8±14.0 | <0.001 |
| Antithrombin III, % | 90.4±24.5 | 74.1±21.8 | 0.009 |
| EXTEM parameters | |||
| A5, mm | 49.6±13.9 | 29.1±10.3 | <0.001 |
| A10, mm | 59.0±12.5 | 38.6±12.1 | <0.001 |
| A15, mm | 62.6±11.4 | 43.3±12.4 | <0.001 |
| MCF, mm | 65.7±10.2 | 49.1±12.1 | <0.001 |
| FIBTEM parameters | |||
| A5, mm | 23.7±11.7 | 10.2±6.0 | <0.001 |
| A10, mm | 25.6±13.0 | 11.2±6.5 | <0.001 |
| A15, mm | 26.5±13.2 | 11.8±7.0 | <0.001 |
| MCF, mm | 27.4±13.4 | 12.5±7.9 | <0.001 |
| PLTEM parameters* | |||
| A5, mm | 25.9±7.4 | 18.9±6.5 | <0.001 |
| A10, mm | 33.4±7.6 | 27.5±8.0 | 0.001 |
| A15, mm | 36.1±7.8 | 31.5±8.1 | 0.014 |
| MCF, mm | 38.2±8.1 | 36.6±7.6 | 0.371 |
Data are expressed as number (%) or mean±SD.
*PLTEM was calculated by subtracting FIBTEM from EXTEM.
Abbreviations: ROTEM, rotational thromboelastometry; DIC, disseminated intravascular coagulation; PRC, packed red blood cell; FFP, fresh frozen plasma; PFA-100, Platelet Function Analyzer-100; PT, prothrombin time; aPTT, activated partial thromboplastin time; FDPs, fibrin/fibrinogen degradation products; EXTEM, extrinsically activated ROTEM; A(N), clot amplitude after N mins; MCF, maximum amplitude/clot firmness; FIBTEM, fibrin-specific ROTEM; PLTEM, platelet-specific ROTEM.
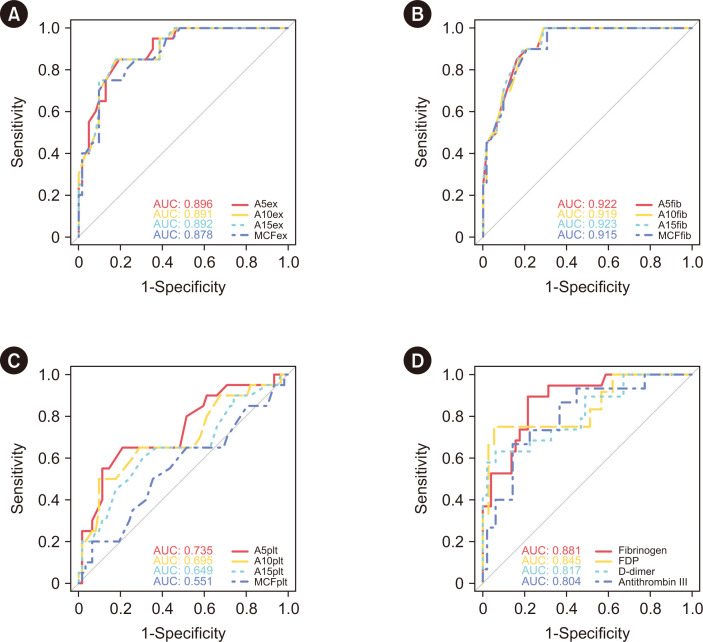
Fig. 2 shows the performance of each ROTEM parameter and the conventional coagulation test in predicting mid-term PC transfusion. The AUCs for FIBTEM, EXTEM, and PLTEM predicting PC transfusion of >12 U for the mid-term were 0.915–0.923, 0.878–0.896, and 0.551–0.735, respectively. The cutoffs of FIBTEM, EXTEM, and PLTEM for A5 were ≤15, ≤35, and ≤19 mm, respectively (Supplemental Data Table S1). In the conventional coagulation test, the AUCs for fibrinogen, FDPs, D-dimer, and antithrombin III predicting PC transfusion of >12 U for the mid-term were >0.8, with cutoffs of ≤249.3 mg/dL, >89.6 μg/mL, >22.74 mg/L fibrinogen equivalent units, and ≤67%, respectively. In predicting mid-term platelet transfusion, FIBTEM, EXTEM, and PLTEM (A5) parameters were comparable to those of fibrinogen, FDPs, D-dimer, and antithrombin III. Among ROTEM parameters, only PLTEM had significantly different AUCs between early (A5) and later (MCF) parameters for predicting mid-term platelet transfusions (P<0.001).
Fig. 2.
Prediction of mid-term platelet transfusion. (A) EXTEM parameters, (B) FIBTEM parameters, (C) PLTEM parameters, and (D) conventional coagulation tests.
Abbreviations: AUC, area under the curve. See Table 1 for definitions of the abbreviations related to the ROTEM and conventional coagulation tests.
Correlation analysis of the clot amplitude parameters of the conventional coagulation and ROTEM tests revealed a strong correlation between EXTEM (A5, A10, and A15) and platelet count (r=0.704 to 0.781), EXTEM (A10 and A15), and PFA-100 (collagen and epinephrine) closure time (per platelet count) (r=−0.712 to −0.701), and all FIBTEM parameters and fibrinogen levels (r=0.718 to 0.746) (Table 2 and Supplemental Data Fig. S1). Early parameters (EXTEM and PLTEM at A5, A10, and A15) showed a stronger correlation with platelet count than the later parameter (MCF). Conversely, the later parameter (EXTEM and FIBTEM [MCF]) showed a stronger correlation with fibrinogen levels than the early parameters (A5, A10, and A15).
Table 2.
Pearson’s correlation coefficients between the clot amplitude parameters of ROTEM and conventional coagulation tests
| ROTEM parameters | Platelet count | PFA-100 (collagen/epinephrine) closure time per platelet count | Fibrinogen | |||||
|---|---|---|---|---|---|---|---|---|
| r | 95% CI | r | 95% CI | r | 95% CI | |||
| EXTEM parameters | ||||||||
| A5ex | 0.781 | 0.672; 0.856 | –0.688 | –0.802; –0.526 | 0.573 | 0.391; 0.712 | ||
| A10ex | 0.733 | 0.606; 0.824 | –0.701 | –0.810; –0.543 | 0.603 | 0.429; 0.734 | ||
| A15ex | 0.704 | 0.567; 0.803 | –0.712 | –0.818; –0.559 | 0.612 | 0.440; 0.740 | ||
| MCFex | 0.667 | 0.517; 0.777 | –0.695 | –0.806; –0.535 | 0.626 | 0.459; 0.751 | ||
| FIBTEM parameters | ||||||||
| A5fib | - | - | - | - | 0.719 | 0.583; 0.816 | ||
| A10fib | - | - | - | - | 0.718 | 0.581; 0.815 | ||
| A15fib | - | - | - | - | 0.725 | 0.590; 0.820 | ||
| MCFfib | - | - | - | - | 0.746 | 0.620; 0.835 | ||
| PLTEM parameters | ||||||||
| A5plt | 0.757 | 0.640; 0.841 | –0.608 | –0.747; –0.419 | - | - | ||
| A10plt | 0.661 | 0.509; 0.772 | –0.606 | –0.745; –0.416 | - | - | ||
| A15plt | 0.583 | 0.409; 0.716 | –0.577 | –0.725; –0.379 | - | - | ||
| MCFplt | 0.490 | 0.295; 0.646 | –0.475 | –0.651; –0.252 | - | - | ||
Abbreviations: ROTEM, rotational thromboelastometry; CI, confidence interval; PFA-100, Platelet Function Analyzer-100. See Table 1 for the abbreviations related to ROTEM parameters and the conventional coagulation tests.
DISCUSSION
Using ROTEM results of 82 hospitalized trauma patients, we demonstrated the reliability of clot amplitude parameters in predicting mid-term platelet transfusion. FIBTEM, EXTEM, and PLTEM (A5) parameters were comparable to those of fibrinogen, FDPs, D-dimer, and antithrombin III. EXTEM (A5, A10, and A15) and PLTEM (A5) parameters were highly correlated with platelet count. All FIBTEM parameters were highly correlated with fibrinogen levels. EXTEM (A10 and A15) parameters were highly correlated with platelet function (per platelet count).
This is the first study to predict mid-term platelet transfusion using ROTEM parameters. With the blood shortage owing to the recent pandemic, the importance of predicting transfusion has increased, particularly for platelet transfusion due to the short shelf-life (five days) [3, 4, 6, 30]. The prediction of mid-term transfusion is difficult because transfusion depends on the coagulation state of the patient. To address this issue, we selected a stable study population hospitalized after successful initial resuscitation. Patients who died within the mid-term period or who received transfusion during surgery were excluded. In contrast to unstable trauma patients who require rapid resuscitation with massive transfusion [1], there are few guidelines for mid-term platelet transfusion in stable non-bleeding trauma patients after successful initial resuscitation [31]. Most PCs of 497 U (95.0%) were transfused at mid-term for preventive purposes (Supplemental Data Table S2). A low platelet count (or a tendency toward a decreased platelet count) and intracranial hemorrhage or antiplatelet medication history were the most common indications for preventive platelet transfusions. The target platelet count was set to 50, 80, or 100×109/L, according to the clinical state of the patient [10]. Clinicians primarily consider bleeding risk and clinical condition when deciding on preventive platelet transfusions for stable trauma patients. Our results may help to guide evidence-based decisions on mid-term platelet transfusion in stable non-bleeding trauma patients after comprehensively evaluating the coagulation status using ROTEM.
Most studies using ROTEM reported an association between thrombocytopenia and the prediction of massive platelet transfusion [21]. However, studies on ROTEM parameters directly related to platelet transfusion are limited. Decreased clot amplitudes in viscoelastic tests are associated with thrombocytopenia [27, 32] and transfusion, including other blood products [33, 34]. In this study, decreased FIBTEM clot amplitudes were especially good predictors of mid-term platelet transfusion (AUCs >0.9). Clot amplitude reflects clot strength based on fibrin formation [18]. The performance of FIBTEM parameters in predicting mid-term platelet transfusion was comparable with that of low fibrinogen levels, increased FDP and D-dimer levels, and decreased antithrombin III levels. Fibrinogen plays a critical role in clot formation and platelet aggregation during hemostasis [35, 36]. Fibrinogen depletion is observed in trauma-induced coagulopathy and supplementation is recommended when it reaches a specific level [1]. In coagulopathy, when PRC and FFP transfusion for the mid-term were compared between groups A and B, more units of PRC and FFP were transfused in group B than in group A (Supplemental Data Table S3). A previous study [37] reported that timely and aggressive ROTEM-guided correction of coagulopathy—including fibrinogen concentrate-based therapy, which contributes to fibrin clot firmness—can reduce the number of platelet transfusions. Because impaired clot formation due to platelet insufficiency can be compensated for by fibrinogen supplementation [38], fibrinogen depletion also increases the number of platelet transfusions, especially during the mid-term, as shown in this study.
In contrast, decreased EXTEM clot amplitudes, in addition to low platelet counts and platelet dysfunction, were good predictors of platelet transfusion within 24 hrs (Supplemental Data Table S4). As the sole marker in trauma patients, platelet count may be limited in predicting transfusion because the decrease in peripheral blood platelet count may be delayed during early traumatic coagulopathy [11, 12]. Platelet dysfunction and increased platelet consumption were observed in patients at risk for early traumatic coagulopathy and in patients with platelet refractoriness, respectively [13, 14]. These conditions may increase the need for additional platelet transfusions. In addition to low platelet counts, numerical or functional platelet and fibrinogen defects, possibly detected by ROTEM, are also associated with platelet transfusion in trauma patients.
Among ROTEM parameters, clot amplitude reflects the FIBTEM clot strength based on fibrin formation and reflects the EXTEM platelet count, platelet function, and fibrin formation [18]. To guide platelet transfusion in trauma patients using ROTEM, previous studies proposed algorithms with arbitrary cutoffs using a combination of EXTEM and FIBTEM clot amplitudes (A5: <35 [EXTEM] and ≥9 mm [FIBTEM]; A10: <40 or 45 [EXTEM] and ≥10 or 12 mm [FIBTEM]) [18, 39, 40]. Similarly, we used PLTEM, which was calculated by subtracting FIBTEM clot amplitudes from those of EXTEM. Early PLTEM parameters (A5, A10, and A15) showed moderate to strong correlations with platelet count or platelet function (per platelet count), whereas the later PLTEM parameter (MCF) showed only a moderate correlation with conventional coagulation tests. This dependence on the measurement time in PLTEM is consistent with the results of other studies [27, 41]. In ROC analysis, the performance of PLTEM parameters showed significant differences between A5 (AUC: 0.735) and MCF (AUC: 0.551). In contrast, EXTEM and FIBTEM showed no significant differences in the time dependence in predicting platelet transfusion. In this study, MCF was determined by the mean of 30.3 mins for EXTEM (range: 11.1–51.7 mins) and 23.9 mins for FIBTEM (range: 2.4–52.4 mins). The better performance of the early PLTEM parameters is advantageous.
Although this was a single-center retrospective study with a small sample size, our results in well-defined patients support the use of ROTEM in predicting mid-term platelet transfusion in hospitalized trauma patients after successful initial resuscitation. One limitation is that the results can only be applied to stable trauma patients and not to all trauma patients due to the selected population for this study. Larger prospective randomized studies are required to confirm our results.
In conclusion, EXTEM and FIBTEM reliably predicted mid-term platelet transfusion in stable non-bleeding trauma patients after successful initial resuscitation. Low clot amplitudes for EXTEM, FIBTEM, and early PLTEM parameters, which mainly reflect fibrinogen or platelet defects, are good predictors of mid-term platelet transfusion. EXTEM, FIBTEM, and PLTEM parameters correlated with conventional coagulation tests (platelets and fibrinogen).
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.3343/alm.2024.44.1.74
ACKNOWLEDGMENTS
None.
Funding Statement
RESEARCH FUNDING None declared.
Footnotes
AUTHOR CONTRIBUTIONS
Lim HJ and Jang H curated the data, performed the experiments, wrote the manuscript, and designed the study; Lim HJ, Jang H, Lee YE, and Shin MG edited the manuscript and participated in the review; Lim HJ, Jang H, Lee YE, Choi HJ, Kee SJ, Shin JH, and Shin MG participated in discussions; Jang H, Lee N, Jeong E, Park Y, Jo Y, and Kim J provided resources; Jang H and Shin MG supervised the study. All authors have accepted their responsibility for the entire content of this manuscript and approved the submission.
CONFLICTS OF INTEREST
None declared.
REFERENCES
- 1.Spahn DR, Bouillon B, Cerny V, Duranteau J, Filipescu D, Hunt BJ, et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit Care. 2019;23:98. doi: 10.1186/s13054-019-2347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slichter SJ. Evidence-based platelet transfusion guidelines. Hematology Am Soc Hematol Educ Program. 2007;2007:172–8. doi: 10.1182/asheducation-2007.1.172. [DOI] [PubMed] [Google Scholar]
- 3.Shander A, Goobie SM, Warner MA, Aapro M, Bisbe E, Perez-Calatayud AA, et al. Essential role of patient blood management in a pandemic: a call for action. Anesth Analg. 2020;131:74–85. doi: 10.1213/ANE.0000000000004844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae HJ, Ahn BS, Youn MA, Park DY. Survey on blood donation recognition and Korean Red Cross' response during COVID-19 pandemic. Korean J Blood Transfus. 2021;32:191–200. doi: 10.17945/kjbt.2021.32.3.191. [DOI] [Google Scholar]
- 5.Greinacher A, Fendrich K, Hoffmann W. Demographic changes: the impact for safe blood supply. Transfus Med Hemother. 2010;37:141–8. doi: 10.1159/000313949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gammon RR, Rosenbaum L, Cooke R, Friedman M, Rockwood L, Nichols T, et al. Maintaining adequate donations and a sustainable blood supply: lessons learned. Transfusion. 2021;61:294–302. doi: 10.1111/trf.16145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanworth SJ, New HV, Apelseth TO, Brunskill S, Cardigan R, Doree C, et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020;7:e756–64. doi: 10.1016/S2352-3026(20)30186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205–13. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 9.Estcourt LJ, Birchall J, Allard S, Bassey SJ, Hersey P, Kerr JP, et al. Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176:365–94. doi: 10.1111/bjh.14423. [DOI] [PubMed] [Google Scholar]
- 10.Joseph B, Pandit V, Meyer D, Butvidas L, Kulvatunyou N, Khalil M, et al. The significance of platelet count in traumatic brain injury patients on antiplatelet therapy. J Trauma Acute Care Surg. 2014;77:417–21. doi: 10.1097/TA.0000000000000372. [DOI] [PubMed] [Google Scholar]
- 11.Hess JR, Lindell AL, Stansbury LG, Dutton RP, Scalea TM. The prevalence of abnormal results of conventional coagulation tests on admission to a trauma center. Transfusion. 2009;49:34–9. doi: 10.1111/j.1537-2995.2008.01944.x. [DOI] [PubMed] [Google Scholar]
- 12.Stansbury LG, Hess AS, Thompson K, Kramer B, Scalea TM, Hess JR. The clinical significance of platelet counts in the first 24 hours after severe injury. Transfusion. 2013;53:783–9. doi: 10.1111/j.1537-2995.2012.03828.x. [DOI] [PubMed] [Google Scholar]
- 13.Wohlauer MV, Moore EE, Thomas S, Sauaia A, Evans E, Harr J, et al. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214:739–46. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohn CS. Platelet transfusion refractoriness: how do I diagnose and manage? Hematology Am Soc Hematol Educ Program. 2020;2020:527–32. doi: 10.1182/hematology.2020000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman M, Monroe DM., 3rd A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–65. doi: 10.1055/s-0037-1615947. [DOI] [PubMed] [Google Scholar]
- 16.Valles J, Santos MT, Aznar J, Marcus AJ, Martinez-Sales V, Portoles M, et al. Erythrocytes metabolically enhance collagen-induced platelet responsiveness via increased thromboxane production, adenosine diphosphate release, and recruitment. Blood. 1991;78:154–62. doi: 10.1182/blood.V78.1.154.154. [DOI] [PubMed] [Google Scholar]
- 17.Bennett JS. Platelet-fibrinogen interactions. Ann N Y Acad Sci. 2001;936:340–54. doi: 10.1111/j.1749-6632.2001.tb03521.x. [DOI] [PubMed] [Google Scholar]
- 18.Görlinger K, Dirkmann D, et al. Rotational thromboelastometry (ROTEM®) In: Gonzalez E, Moore HB, et al., editors. Trauma induced coagulopathy. Springer International Publishing; Cham: 2016. pp. 267–98. [DOI] [Google Scholar]
- 19.Shin KH, Kim IS, Lee HJ, Kim HH, Chang CL, Hong YM, et al. Thromboelastographic evaluation of coagulation in patients with liver disease. Ann Lab Med. 2017;37:204–12. doi: 10.3343/alm.2017.37.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SY, Gu JY, Yoo HJ, Kim JE, Jang S, Choe S, et al. Benefits of thromboelastography and thrombin generation assay for bleeding prediction in patients with thrombocytopenia or hematologic malignancies. Ann Lab Med. 2017;37:484–93. doi: 10.3343/alm.2017.37.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veigas PV, Callum J, Rizoli S, Nascimento B, da Luz LT. A systematic review on the rotational thrombelastometry (ROTEM®) values for the diagnosis of coagulopathy, prediction and guidance of blood transfusion and prediction of mortality in trauma patients. Scand J Trauma Resusc Emerg Med. 2016;24:114. doi: 10.1186/s13049-016-0308-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Görlinger K, Saner FH. Prophylactic plasma and platelet transfusion in the critically Ill patient: just useless and expensive or even harmful? BMC Anesthesiol. 2015;15:86. doi: 10.1186/s12871-015-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klages M, Zacharowski K, Weber CF. Coagulation management in trauma-associated coagulopathy: allogenic blood products versus coagulation factor concentrates in trauma care. Curr Opin Anaesthesiol. 2016;29:245–9. doi: 10.1097/ACO.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 24.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH), author Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–30. doi: 10.1055/s-0037-1616068. [DOI] [PubMed] [Google Scholar]
- 25.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 26.Zeidler K, Arn K, Senn O, Schanz U, Stussi G. Optimal preprocedural platelet transfusion threshold for central venous catheter insertions in patients with thrombocytopenia. Transfusion. 2011;51:2269–76. doi: 10.1111/j.1537-2995.2011.03147.x. [DOI] [PubMed] [Google Scholar]
- 27.Olde Engberink RH, Kuiper GJ, Wetzels RJ, Nelemans PJ, Lance MD, Beckers EA, et al. Rapid and correct prediction of thrombocytopenia and hypofibrinogenemia with rotational thromboelastometry in cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28:210–6. doi: 10.1053/j.jvca.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 28.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 29.Ratner B. The correlation coefficient: Its values range between +1/−1, or do they? J Target Meas Anal Mark. 2009;17:139–42. doi: 10.1057/jt.2009.5. [DOI] [Google Scholar]
- 30.Kim HO. Current State of Blood Management Services in Korea. Ann Lab Med. 2022;42:306–13. doi: 10.3343/alm.2022.42.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanworth SJ, Shah A. How I use platelet transfusions. Blood. 2022;140:1925–36. doi: 10.1182/blood.2022016558. [DOI] [PubMed] [Google Scholar]
- 32.Song JG, Jeong SM, Jun IG, Lee HM, Hwang GS. Five-minute parameter of thromboelastometry is sufficient to detect thrombocytopenia and hypofibrinogenaemia in patients undergoing liver transplantation. Br J Anaesth. 2014;112:290–7. doi: 10.1093/bja/aet325. [DOI] [PubMed] [Google Scholar]
- 33.Plotkin AJ, Wade CE, Jenkins DH, Smith KA, Noe JC, Park MS, et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma. 2008;64:S64–8. doi: 10.1097/TA.0b013e318160772d. [DOI] [PubMed] [Google Scholar]
- 34.Meyer AS, Meyer MA, Sørensen AM, Rasmussen LS, Hansen MB, Holcomb JB, et al. Thrombelastography and rotational thromboelastometry early amplitudes in 182 trauma patients with clinical suspicion of severe injury. J Trauma Acute Care Surg. 2014;76:682–90. doi: 10.1097/TA.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 35.Bombeli T, Spahn DR. Updates in perioperative coagulation: physiology and management of thromboembolism and haemorrhage. Br J Anaesth. 2004;93:275–87. doi: 10.1093/bja/aeh174. [DOI] [PubMed] [Google Scholar]
- 36.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 37.Schöchl H, Nienaber U, Maegele M, Hochleitner G, Primavesi F, Steitz B, et al. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15:R83. doi: 10.1186/cc10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velik-Salchner C, Haas T, Innerhofer P, Streif W, Nussbaumer W, Klingler A, et al. The effect of fibrinogen concentrate on thrombocytopenia. J Thromb Haemost. 2007;5:1019–25. doi: 10.1111/j.1538-7836.2007.02481.x. [DOI] [PubMed] [Google Scholar]
- 39.Schöchl H, Maegele M, Solomon C, Görlinger K, Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med. 2012;20:15. doi: 10.1186/1757-7241-20-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Görlinger K, Pérez-Ferrer A, Dirkmann D, Saner F, Maegele M, Calatayud ÁAP, et al. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J Anesthesiol. 2019;72:297–322. doi: 10.4097/kja.19169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ji SM, Kim SH, Nam JS, Yun HJ, Choi JH, Lee EH, et al. Predictive value of rotational thromboelastometry during cardiopulmonary bypass for thrombocytopenia and hypofibrinogenemia after weaning of cardiopulmonary bypass. Korean J Anesthesiol. 2015;68:241–8. doi: 10.4097/kjae.2015.68.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.