Abstract
Objective
Hepatocellular carcinoma (HCC) has a high rate of postoperative recurrence and lacks an effective treatment to prevent recurrence. This study aims to investigate the efficacy and safety of anlotinib in postoperative adjuvant therapy for HCC patients with high-risk recurrence factors.
Methods
For this multicenter, retrospective study, we recruited 63 HCC patients who received either anlotinib (n=27) or transcatheter arterial chemoembolization (TACE) (n=36) from six research centers in China between March 2019 and October 2020. The primary endpoint was disease-free survival (DFS) and the secondary endpoints were overall survival (OS) and safety.
Results
In this study, the median follow-up time was 25.9 and 26.8 months in the anlotinib and TACE groups, respectively. There was no significant difference in the median DFS between the anlotinib [26.8 months, 95% confidence interval (95% CI): 6.8−NE] and TACE groups (20.6 months, 95% CI: 8.4−NE). The 12-month OS rates in the anlotinib and TACE groups were 96.3% and 97.2%, respectively. In the anlotinib group, 19 of 27 patients (70.4%) experienced treatment-emergent adverse events, with the most common events (≥10%) being hypertension (22.2%) and decreased platelet count (22.2%).
Conclusions
The results indicate that anlotinib, as a new, orally administered tyrosine kinase inhibitor, has the same efficacy as TACE, and side effects can be well controlled.
Keywords: Hepatocellular carcinoma, adjuvant therapy, anlotinib, transcatheter arterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common and third most fatal malignant tumor globally (1). More than 75% of HCC cases occur in the Asia Pacific region, mainly relating to chronic hepatitis B virus (HBV) infection (2,3). There are several therapeutic options for the treatment of HCC, including surgery, liver transplantation, locoregional therapy, and molecularly-targeted immunotherapy (4,5). To date, hepatectomy has been the main treatment option for HCC. However, due to factors such as late-stage diagnosis, the presence of multiple tumors, and limited sources of donors, only 21% of patients have the opportunity to undergo liver transplantation (6). Surgical resection can achieve a 5-year survival rate of 73.6% in patients with a limited number of tumors (i.e., one nodule with a diameter of ≤5 cm or less than or equal to three nodules with a diameter of ≤3 cm) and good liver function [a Child-Pugh score (index of liver function) of ≤6] (7,8). Unfortunately, the probability of metastasis and recurrence within 5 years after resection of HCC is 60%−70% due to high-risk recurrence factors, including more and larger tumors, vascular invasion, extrahepatic metastasis, and poor liver function (9,10). Therefore, it is imperative to improve postoperative adjuvant therapy for HCC patients with a high risk of recurrence.
In the last three decades, HCC patients have benefited from transcatheter arterial chemoembolization (TACE) therapy (11), which has become the standard postoperative adjuvant therapy for HCC (12). However, short-term adverse reactions are common in HCC patients after the TACE procedure, and some may affect the patients’ quality of life, indicating the importance of postoperative clinical observation and symptomatic treatment (13). Furthermore, in previous studies, 44.3%−70.0% of patients treated with TACE reported partial remission, and the 5-year survival rate was only 12.5%−39.3% (14,15). Relevant retrospective studies have shown that postoperative adjuvant TACE could not completely inhibit recurrence and improve prognosis as expected (16). TACE increases the levels of hypoxia-inducible factors by blocking the blood supply to the tumor, which may promote vascular endothelial growth factor release and tumor recurrence and metastasis. Incomplete embolization after TACE procedure may result in the restoration of nutrient and oxygen supply to residual tumor vessels (17,18).
In recent years, some studies have explored the role of molecularly targeted agents in the postoperative adjuvant treatment of HCC. For example, a previous study reported that the combination of lenvatinib and TACE significantly prolonged median disease-free survival (DFS) (17 months vs. 9 months, P=0.023) (19). This indicates that systematic treatment plus TACE is effective in postoperative adjuvant therapy, but better and convenient treatment options still need to be explored. However, the results of a prospective clinical study (STORM study) showed that treatment with oral sorafenib after HCC resection or ablation did not prolong the median recurrence-free survival of patients (33.3 months vs. 33.7 months, P=0.260) (19). This may be because most of the enrolled patients had a low probability of recurrence and metastasis, 91% of patients had only one tumor lesion, and 54% of patients had no microvascular invasion (MVI) (20). Therefore, the efficacy of the molecularly targeted monotherapy in postoperative adjuvant therapy after optimization of high recurrence risk factors needs to be further explored.
Anlotinib is a novel small molecular multi-target tyrosine kinase inhibitor that can act on many targets, including vascular endothelial growth factor receptors 1−3, platelet-derived growth factor (PDGFR) α/β, fibroblast growth factor receptor (FGFR), C-KIT, and c-Met. Anlotinib participates in the inhibition of tumor neovascularization and the process of tumor metastasis (16). A preclinical study has confirmed that anlotinib can exert an excellent inhibitory effect on HCC cells by inducing apoptosis and inhibiting their proliferation via ERK and AKT signaling pathways (21). The median overall survival (OS) of HCC patients treated with anlotinib monotherapy as a first-line treatment was 12.8 months [95% confidence interval (95% CI): 7.9−20.1, n=26). Results from several phase II studies have also demonstrated the efficacy of anti-PD-1 inhibitors plus anlotinib in HCC, with an objective response rate of 31.0%−34.6% and a PFS of 8.8−10.2 months (22,23). Multiple studies of single-agent or combination immunotherapy in patients with biliary tract cancer, non-small cell lung cancer, colorectal cancer, and esophageal cancer have demonstrated the therapeutic effects and safety of anlotinib (24,25). However, the clinical application of anlotinib in the postoperative adjuvant therapy of HCC patients remains unexplored.
In this retrospective study, we aim to evaluate the efficacy and safety of anlotinib in postoperative HCC patients with high-risk recurrent factors. Additionally, data from patients who received TACE during a contemporary period were collected as a control group.
Materials and methods
Study design and participants
For this multicenter retrospective study, we collected 63 HCC patients who received anlotinib monotherapy (n=27) or TACE (n=36) as postoperative adjuvant therapy at six research centers (the First Affiliated Hospital, Zhejiang University School of Medicine; Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine; Shulan (Hangzhou) Hospital, Zhejiang Shuren University School of Medicine; Huashan Hospital, Fudan University; the First Bethune Hospital of Jilin University; and Shanghai General Hospital) in China from March 2019 to October 2020.
The inclusion criteria were as follows: patients aged between 18 and 75 years; with a histologically or cytologically confirmed diagnosis of HCC; an Eastern Cooperative Oncology Group (ECOG) performance status of 0−2; adequate organ function and a Child-Pugh score of ≤7. Patients were also required to meet any of the following criteria: 1) with macroscopic portal vein, hepatic vein, or bile duct tumor thrombus; 2) pathologically suggestive of MVI grade II [>5 MVI, or MVI occurring in distant areas (>1 cm) of liver tissues]; 3) number of tumor lesions ≥3; and 4) preoperative rupture of liver cancer or invasion of adjacent organs by liver cancer. The exclusion criteria were as follows: 1) pregnant or lactating females; 2) patients with recurrent HCC; 3) patients with mental illness; patients treated after surgery with other targeted drugs, immunotherapy, and systemic chemotherapy; or 4) patients with 24 h urine protein >1 g.
This study was approved by the Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine (No. 2019631); Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine; Shulan (Hangzhou) Hospital, Zhejiang Shuren University School of Medicine; Huashan Hospital, Fudan University; the First Bethune Hospital of Jilin University; and Shanghai General Hospital. Written informed consent was obtained from all patients. The study was conducted following the principles of the Declaration of Helsinki.
Treatment methods
In the anlotinib group, all eligible patients received anlotinib monotherapy 2−4 weeks after hepatectomy. One treatment cycle was 3 weeks, and treatment continued for up to 6 months or until efficacy was assessed as progressive disease, intolerable by the patient, or the end of drug treatment. Anlotinib was orally administered at a dose of 12 mg/d for 2 consecutive weeks on and 1 week off. The investigator modified the dose of anlotinib (i.e., 10 mg or 8 mg) based on the extent of drug-related toxic effects and possible efficacy benefits. If adverse events (AEs) persistently occurred after dose modification, treatment was discontinued. If a patient’s dosage was downgraded to 10 mg or 8 mg, another upward dose adjustment was not allowed. Efficacy evaluation was performed every 3 months during the treatment. In the TACE group, all eligible patients received TACE around 4 weeks after hepatectomy.
Evaluation of efficacy and AEs
Computed tomography or magnetic resonance imaging scans was performed to assess the treatment effect. The primary endpoint was DFS, defined as the time from the beginning of the clinical study to disease recurrence or death. The secondary endpoints were OS and safety. OS was defined as the time from study drug administration to death. Data on AEs were collected from the time of treatment until 30 d following treatment cessation. The severity of all AEs was graded according to the National Cancer Institute Evaluation Criteria of Common Adverse Events version 5.0.
Statistical analysis
The primary efficacy analysis was performed with the intent-to-treat population, which included all patients who had received anlotinib or TACE at least once. The continuous variables conforming to the normal distribution were described as 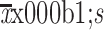 , and the discontinuous were described as median (range). Categorical variables were described as n (%). DFS and OS were calculated based on the Kaplan-Meier (KM) method, and the follow-up time was estimated using the reverse KM method. Hazard ratio (HR) and associated 95% CI were calculated using a Cox proportional hazards model. Chi-square or Fisher’s exact test was used for counting data, and P<0.05 was considered to indicate statistically significant.
, and the discontinuous were described as median (range). Categorical variables were described as n (%). DFS and OS were calculated based on the Kaplan-Meier (KM) method, and the follow-up time was estimated using the reverse KM method. Hazard ratio (HR) and associated 95% CI were calculated using a Cox proportional hazards model. Chi-square or Fisher’s exact test was used for counting data, and P<0.05 was considered to indicate statistically significant.
Results
Patients
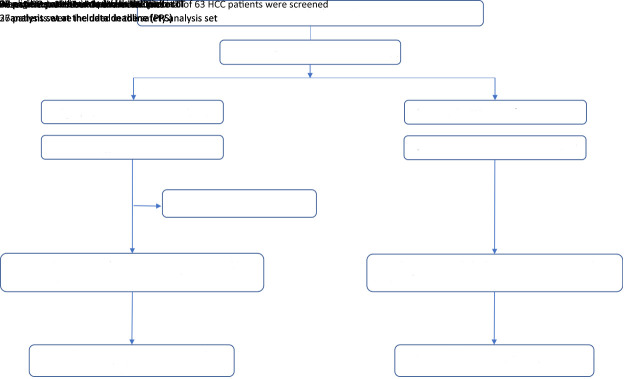
After the screening process, a total of 63 HCC patients who received either anlotinib (n=27) or TACE treatment (n=36) at six research centers in China from March 2019 to October 2020 were included in this study; all patients were finally included in the efficacy and safety analyses. Among the patients who underwent TACE in the TACE group, two patients received three treatments, eight patients received two treatments, and 26 patients received one treatment. Figure 1 presents an overview of this study.
Figure 1.
Flow diagram of study enrollment and treatment. HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization; ITT, intention-to-treat; PPS, per protocol set.
Table 1 shows the baseline characteristics of patients in the anlotinib and TACE groups. There was no significant difference in most baseline characteristics between the two groups, including sex, Child-Pugh score, Barcelona Clinic Liver Cancer (BCLC) stage, HBV infection, cirrhosis, extrahepatic metastasis, number of intrahepatic lesions, MVI and Capsule. However, there were statistical differences in some indicators between the two groups, such as ECOG performance score, portal vein tumor thrombosis (PVTT) and differentiation (P<0.05). In the anlotinib group, the ECOG performance status was 0 in 25 patients and 1 in two patients; in the TACE group, the ECOG performance status was 0 in 25 patients and 1 in 11 patients. In patients with defined PVTT grading, 22.22% of patients in the anlotinib group had VP1−2, and none in the TACE group had VP1−2. Additionally, 22.22% of patients in the TACE group had VP3, and none in the anlotinib group had VP3. For histological differentiation, 20 patients (74.07%) were identified with grade 3−4 in the anlotinib group, and 15 patients (41.67%) in the TACE group were grade 3−4.
Table 1. Baseline characteristics of patients in anlotinib group and TACE group.
| Variables | n (%) | P | |
| Anlotinib (N=27) |
TACE (N=36) |
||
| TACE, transcatheter arterial chemoembolization; ECOG, Eastern Cooperative Oncology Group; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; PVTT, portal vein tumor thrombosis. | |||
| Age [median (range)] (year) | 55 (40−73) | 56 (32−84) | 0.50 |
| Sex | 1.00 | ||
| Male | 23 (85.19) | 30 (83.33) | |
| Female | 4 (14.81) | 6 (16.67) | |
| Child-Pugh score | 0.18 | ||
| A5/6 | 25 (92.59) | 36 (100) | |
| B7 | 2 (7.41) | 0 (0) | |
| ECOG performance score | 0.02 | ||
| 0 | 25 (92.59) | 25 (69.44) | |
| 1 | 2 (7.41) | 11 (30.56) | |
| BCLC stage | 0.11 | ||
| A | 23 (85.19) | 22 (61.11) | |
| B | 2 (7.41) | 5 (13.89) | |
| C | 2 (7.41) | 9 (25.00) | |
| HBV infection | 0.07 | ||
| Positive | 24 (88.89) | 25 (69.44) | |
| Negative | 3 (11.11) | 11 (30.56) | |
| Cirrhosis | 0.07 | ||
| Yes | 24 (88.89) | 25 (69.44) | |
| No | 3 (11.11) | 11 (30.56) | |
| Extrahepatic metastasis | 0.25 | ||
| Yes | 0 (0) | 3 (8.33) | |
| No | 27 (100) | 33 (91.67) | |
| No. of intrahepatic lesions | 0.37 | ||
| 1 | 20 (74.07) | 30 (83.33) | |
| >1 | 7 (25.93) | 6 (16.67) | |
| Microvascular invasion | 1.00 | ||
| Yes | 24 (88.89) | 33 (91.67) | |
| No | 3 (11.11) | 3 (8.33) | |
| PVTT | <0.01 | ||
| VP1 | 4 (14.81) | 0 (0) | |
| VP2 | 2 (7.41) | 0 (0) | |
| VP3 | 0 (0) | 8 (22.22) | |
| No | 21 (77.78) | 28 (77.78) | |
| Differentiation | 0.01 | ||
| Grade 1−2 | 7 (25.93) | 21 (58.33) | |
| Grade 3−4 | 20 (74.07) | 15 (41.67) | |
| Capsule | 1.00 | ||
| Complete | 2 (7.41) | 2 (5.56) | |
| Uncomplete | 25 (92.59) | 34 (94.44) | |
| Surgical margin | − | ||
| Positive | 0 (0) | 0 (0) | |
| Negative | 27 (100) | 36 (100) | |
Efficacy
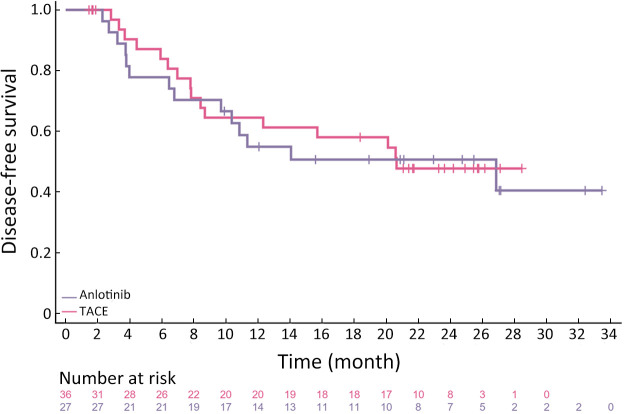
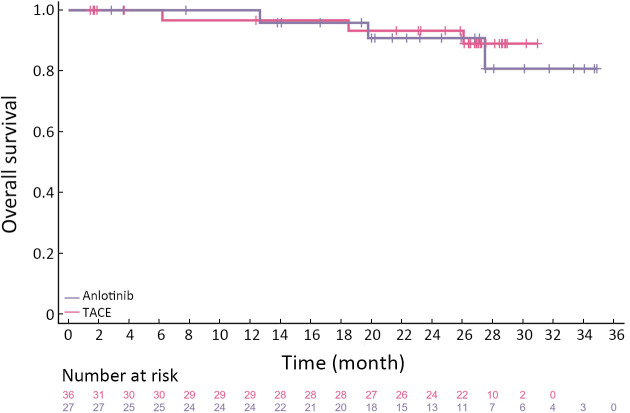
To further investigate the effect of different treatments on the prognosis of HCC patients, we followed up on all patients. The median follow-up period was 25.9 (95% CI: 20.2−31.6) months and 26.8 (95% CI: 26.1−27.5) months in the anlotinib and TACE groups, respectively. The longest DFS was 47.4 and 28.5 months in the anlotinib and TACE groups, respectively. The numbers of observed DFS events in the anlotinib and TACE groups were 14 and 16, respectively. There was no significant difference in the median DFS between the anlotinib [26.8 months (95% CI: 6.8−NE)] and TACE groups [20.6 months (95% CI: 8.4−NE)]. The median OS has not yet been reached in both groups. Four and three OS events occurred in the anlotinib and TACE groups, respectively. There was no significant difference in DFS between the two groups (HR=1.08, 95% CI: 0.5−2.2, P=0.83) (Figure 2). In the anlotinib group, the 12- and 24-month OS rates were 96.3% and 88.9%, respectively; in the TACE group, the 12- and 24-month OS rates were 97.2% and 97.2%, respectively (Figure 3).
Figure 2.
Kaplan-Meier curve of DFS for FAS population. Testing between the two treatment arms was carried out by log-rank test, DFS of the anlotinib and the TACE groups were 26.8 (95% CI: 6.8−NE) months and 20.6 (95% CI: 8.4−NE) months, respectively (P=0.83). DFS, disease-free survival; FAS, full analysis set; 95% CI, 95% confidence interval; NE, not evaluated; TACE, transcatheter arterial chemoembolization
Figure 3.
Kaplan-Meier curve of OS for FAS population. Testing between the two treatment arms was carried out by log-rank test. In the anlotinib group and the TACE group, the 12-month OS rates were 96.3% and 97.2%, respectively (P=0.44). OS, overall survival; FAS, full analysis set; TACE, transcatheter arterial chemoembolization.
Safety
In the present study, among all patients who received anlotinib after HCC, 19 patients (70.4%) experienced treatment-emergent adverse events (TEAEs), with the most common events being hypertension (22.2%), decreased platelet count (22.2%), hand-foot syndrome (14.8%) and sphagitis (11.1%). AE grade 3 or higher included hypertension (7.4%) and hand-foot syndrome (3.7%) (Table 2). Grade 1 and 2 AEs of patients were relieved after symptomatic treatment. Two patients had a dose reduction of anlotinib due to hand-foot syndrome, and the treatment was suspended in one patient due to AEs and then resumed with the normal dose after the alleviation of AEs (i.e., after a week).
Table 2. All AEs resulting from anlotinib treatment in patients with HCC (N=27).
| AEs | n (%) | |
| Any grade | Grade 3 | |
| AE, adverse event; HCC, hepatocellular carcinoma. | ||
| Hypertension | 6 (22.2) | 2 (7.4) |
| Platelet count decreased | 6 (22.2) | 0 (0) |
| Hand-foot syndrome | 4 (14.8) | 1 (3.7) |
| Sphagitis | 3 (11.1) | 0 (0) |
| Fever | 1 (3.7) | 0 (0) |
| Abdominal distension | 1 (3.7) | 0 (0) |
| Diarrhea | 1 (3.7) | 0 (0) |
| Vomiting | 1 (3.7) | 0 (0) |
Table 3 summarizes the toxicity data of patients who received adjuvant TACE. Overall, the adjuvant TACE was well tolerated. The most common TEAEs associated with adjuvant TACE were abdominal pain (75.0%), liver function injury (25.0%) and nausea (11.1%). In this study, grade 4 and 5 AEs never occurred.
Table 3. All AEs resulting from TACE treatment in patients with HCC (N=36).
| AEs | n (%) | |
| Any grade | Grade 3 | |
| AE, adverse event; TACE, transcatheter arterial chemoembolization; HCC, hepatocellular carcinoma. *, included elevation of transaminase, bilirubin and g-glutamyltranspeptidase. | ||
| Abdominal pain | 27 (75.0) | 4 (11.1) |
| Liver function injury* | 9 (25.0) | 0 (0) |
| Nausea | 4 (11.1) | 0 (0) |
| Abdominal distension | 3 (8.3) | 0 (0) |
| Vomiting | 1 (2.8) | 0 (0) |
| Appetite loss | 1 (2.8) | 0 (0) |
Discussion
This multicenter retrospective study investigated the efficacy and safety of anlotinib monotherapy, an antiangiogenic agent, as postoperative adjuvant therapy for HCC patients with high recurrence risk. The results showed that the median DFS reached 26.8 (95% CI: 6.8−NE) months with a good safety profile when anlotinib was used as postoperative adjuvant therapy for HCC patients. Patients treated with TACE had a shorter median DFS (20.6 months) at a median follow-up of 26.8 months than those treated with anlotinib (25.9 months).
Different inclusion criteria of current studies on antiangiogenic agents or adjuvant therapy with TACE for HCC result in wide variation in the reported efficacy of adjuvant therapy. The primary goal of adjuvant therapy is to reduce recurrence risk for HCC patients with hepatectomy, but there are still no standard criteria to characterize these populations. In addition to basic indicators such as viral hepatitis infection (26), preoperative alpha-fetoprotein level (27), cirrhosis, MVI, tumor size, PVTT, multiple tumors or accompanying satellite nodules, differentiation grade, and envelope integrity, other factors have also been considered risk factors for postoperative recurrence (28). How to effectively integrate these risk factors and define the population with a high risk of recurrence after HCC surgery still needs further exploration.
Raza et al. found that the median DFS of TACE in the adjuvant treatment of HCC ranged from 13.0 to 17.5 months (29-31), while the median DFS in this study was 20.6 months. The main reason for the discrepancy may be due to the use of different inclusion criteria for HCC patients. Among these studies using TACE, Peng’s study used the following inclusion criteria: the number of monocentric HCCs or nodules ≤3 and extension of thrombi to the main portal vein or opposite branch by ≤3 cm (31), which were different from those used in our study. However, their study gave us improved directions and inspiration for our follow-up research in this study. In addition, high-risk recurrence factors were one of the reasons that contributed to the difference in the median DFS of adjuvant TACE for HCC patients. In their study, Kudo et al. (30) excluded patients with extrahepatic metastases, and the median survival time was significantly longer (i.e., 13.5 months vs. 9.0 months) than that reported in the study by Peng et al. (31) in which the study participants were all patients with PVTT. Similarly, in our study, the proportion of patients with PVTT was high (i.e., 22.2%). Bruix et al. showed that there was no difference in median recurrence-free survival between the sorafenib and placebo groups (33.3 months vs. 33.7 months; HR=0.94; 95% CI: 0.8−1.1; P=0.26) (20), and the main reason was that the patients included in the study were all mildly patients. In this study, the study population primarily comprised patients with MVI, PVTT, and BCLC stage C. This may be one of the reasons for the shorter median DFS in this study. Overall, differences in baseline patient characteristics could lead to differences in the median DFS. In the study of AEs with anlotinib, AE grade 3 or higher mainly included hypertension and hypothyroidism, fatigue, hand-foot syndrome, elevated levels of bilirubin, and diarrhea (32). Serious AEs related to sorafenib in the STORM study mainly included hand-foot skin reaction (10, <2%), abnormal liver function (4, <1%), and fatigue (3, <1%) (20). AE grade 3 or higher in TACE treatment mainly included fever, hyperbilirubinemia, and elevated levels of alanine aminotransferase (ALT), while AE grade 3 or higher was mainly fever, elevated levels of ALT, and thrombocytopenia. Most treatment-related AEs fell into grade 1 or 2 (33). The safety profiles of both anlotinib and TACE were still within the scope of the existing studies, with no new safety issues identified.
In addition to targeted agents alone or TACE alone, other combined strategies have been explored, such as TACE combined with an antiangiogenic drug, in which the median PFS of TACE combined with sorafenib was significantly improved compared with that of TACE alone (25.2 vs. 13.5 months; P=0.006) (30). The median OS was significantly longer in lenvatinib plus TACE treatment than in TACE alone treatment (12.0 months vs. 8.0 months, P=0.036) (19). A prospective study of anlotinib combined with TACE in the adjuvant setting for HCC showed that the median DFS of 25 HCC patients was 15.2 months and that both the 6- and 12-month DFS rates were 77.5% (95% CI: 53.8−90.0) (34).
In addition, with the breakthrough of first-line treatment of HCC with atezolizumab combined with bevacizumab (35), immunotherapy has been explored a few times in the postoperative adjuvant treatment (36-38). Several large prospective studies of immunotherapy as monotherapy or in combination with targeted therapies are currently underway, including studies of CheckMate-9DX, KEYNOTE-937, IMbrave050, and EMERALD-2. Systemic pharmacotherapy in the future will likely provide more effective options for postoperative adjuvant therapy in HCC.
This study has some limitations. First, this study had limited sample sizes, and so the results could not be considered representative of the population. A study with a larger sample size should be conducted to validate these efficacy and safety data. Second, OS data were incomplete. In the future, OS data will be analyzed to investigate the survival benefit of anlotinib monotherapy as an adjuvant treatment for HCC patients with high recurrence risk.
Conclusions
The results indicate that anlotinib, as a new, orally administered tyrosine kinase inhibitor, has the same efficacy as the standard postoperative adjuvant therapy (TACE) in reducing the risk of postoperative recurrence in HCC patients and complications related to TACE.
Acknowledgements
This study was supported by Key Program, National Natural Science Foundation of China (No. 81930016); Natural Science Foundation of Zhejiang Province (No. LY22H160046) and Key Research & Development Plan of Zhejiang Province (No. 2019C03050).
Contributor Information
Shusen Zheng, Email: zyzss@zju.edu.cn.
Xiao Xu, Email: zjxu@zju.edu.cn.
References
- 1.Sung H, Ferlay J, Siegel RL, et al Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Kelley RK, Villanueva A, et al Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 3.Li Q, Cao M, Lei L, et al Burden of liver cancer: From epidemiology to prevention. Chin J Cancer Res. 2022;34:554–66. doi: 10.21147/j.issn.1000-9604.2022.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang JD, Hainaut P, Gores GJ, et al A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Liu J, Wu H, et al All-trans retinoic acid (ATRA) inhibits insufficient radiofrequency ablation (IRFA)-induced enrichment of tumor-initiating cells in hepatocellular carcinoma. Chin J Cancer Res. 2021;33:694–707. doi: 10.21147/j.issn.1000-9604.2021.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zane KE, Nagib PB, Jalil S, et al Emerging curative-intent minimally-invasive therapies for hepatocellular carcinoma. World J Hepatol. 2022;14:885–95. doi: 10.4254/wjh.v14.i5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelli M, Sebagh M, Porcher R, et al Liver resection for early hepatocellular carcinoma: Preoperative predictors of non transplantable recurrence and implications for treatment allocation. Ann Surg. 2020;272:820–6. doi: 10.1097/SLA.0000000000004259. [DOI] [PubMed] [Google Scholar]
- 8.Romano F, Chiarelli M, Garancini M, et al Rethinking the Barcelona clinic liver cancer guidelines: Intermediate stage and Child-Pugh B patients are suitable for surgery. World J Gastroenterol. 2021;27:2784–94. doi: 10.3748/wjg.v27.i21.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordan JD, Kennedy EB, Abou-Alfa GK, et al Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38:4317–45. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 10.Bureau of Medical Administration, National Health Commission of the People’s Republic of China Standardization for diagnosis and treatment of hepatocellular carcinoma (2022 edition) Zhonghua Gan Zang Bing Za Zhi. 2022;30:367–88. doi: 10.3760/cma.j.cn501113-20220413-00193. [DOI] [PubMed] [Google Scholar]
- 11.Habib A, Desai K, Hickey R, et al Transarterial approaches to primary and secondary hepatic malignancies. Nat Rev Clin Oncol. 2015;12:481–9. doi: 10.1038/nrclinonc.2015.78. [DOI] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Guo W, Chen S, Wu Z, et al. Efficacy and safety of transarterial chemoembolization combined with anlotinib for unresectable hepatocellular carcinoma: A retrospective study. Technol Cancer Res Treat 2020;19:1533033820965587.
- 14.Yu MQ, An TZ, Li JX, et al Integrated liver inflammatory score predicts the therapeutic outcome of patients with hepatocellular carcinoma after transarterial chemoembolization. J Vasc Interv Radiol. 2021;32:1194–202. doi: 10.1016/j.jvir.2021.03.540. [DOI] [PubMed] [Google Scholar]
- 15.Yamada R, Bassaco B, Bracewell S, et al Long-term follow-up after conventional transarterial chemoembolization (c-TACE) with mitomycin for hepatocellular carcinoma (HCC) J Gastrointest Oncol. 2019;10:348–53. doi: 10.21037/jgo.2019.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen G, Zheng F, Ren D, et al Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:120. doi: 10.1186/s13045-018-0664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M, Shu G, Lv X, et al HIF-2α-targeted interventional chemoembolization multifunctional microspheres for effective elimination of hepatocellular carcinoma. Biomaterials. 2022;284:121512. doi: 10.1016/j.biomaterials.2022.121512. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YJ, Chen MS, Chen Y, et al Long-term outcomes of transcatheter arterial chemoembolization combined with radiofrequency ablation as an initial treatment for early-stage hepatocellular carcinoma. JAMA Netw Open. 2021;4:e2126992. doi: 10.1001/jamanetworkopen.2021.26992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Lu L, Wen T, et al Adjuvant lenvatinib in combination with TACE for hepatocellular carcinoma patients with high risk of postoperative relapse (LANCE): Interim results from a muticenter prospective cohort study. J Clin Oncol. 2020;38(suppl):4580. doi: 10.1200/JCO.2020.38.15_suppl.4580. [DOI] [Google Scholar]
- 20.Bruix J, Takayama T, Mazzaferro V, et al Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–54. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 21.He C, Wu T, Hao Y Anlotinib induces hepatocellular carcinoma apoptosis and inhibits proliferation via Erk and Akt pathway. Biochem Biophys Res Commun. 2018;503:3093–9. doi: 10.1016/j.bbrc.2018.08.098. [DOI] [PubMed] [Google Scholar]
- 22.Han C, Ye S, Hu C, et al Clinical activity and safety of penpulimab (anti-PD-1) with anlotinib as first-line therapy for unresectable hepatocellular carcinoma: An open-label, multicenter, phase Ib/II trial (AK105-203) Front Oncol. 2021;11:684867. doi: 10.3389/fonc.2021.684867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Li W, Wu X, et al Safety and efficacy of sintilimab and anlotinib as first line treatment for advanced hepatocellular carcinoma (KEEP-G04): A single-arm phase 2 study. Front Oncol. 2022;12:909035. doi: 10.3389/fonc.2022.909035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J, Sun Y, Zhang W, et al Phase Ib study of anlotinib combined with TQB2450 in pretreated advanced biliary tract cancer and biomarker analysis. Hepatology. 2023;77:65–76. doi: 10.1002/hep.32548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Y, Lu J, Hu F, et al Effect and outcomes analysis of anlotinib in non-small cell lung cancer patients with liver metastasis: results from the ALTER 0303 phase 3 randomized clinical trial. J Cancer Res Clin Oncol. 2023;149:1417–24. doi: 10.1007/s00432-022-03964-9. [DOI] [PubMed] [Google Scholar]
- 26.Tsai IT, Wang CP, Yu TH, et al Circulating visfatin level is associated with hepatocellular carcinoma in chronic hepatitis B or C virus infection. Cytokine. 2017;90:54–9. doi: 10.1016/j.cyto.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Kim MN, Kim BK, Kim SU, et al Longitudinal assessment of alpha-fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis. Scand J Gastroenterol. 2019;54:1283–90. doi: 10.1080/00365521.2019.1673478. [DOI] [PubMed] [Google Scholar]
- 28.Huang M, Liao B, Xu P, et al Prediction of microvascular invasion in hepatocellular carcinoma: Preoperative Gd-EOB-DTPA-dynamic enhanced MRI and histopathological correlation. Contrast Media Mol Imaging. 2018;2018:9674565. doi: 10.1155/2018/9674565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raza A, Sood GK Hepatocellular carcinoma review: current treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:4115–27. doi: 10.3748/wjg.v20.i15.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudo M, Ueshima K, Ikeda M, et al Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492–501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng BG, He Q, Li JP, et al Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg. 2009;198:313–8. doi: 10.1016/j.amjsurg.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Zhou A, Zhang W, et al Anlotinib in the treatment of advanced hepatocellular carcinoma: an open-label phase II study (ALTER-0802 study) Hepatol Int. 2021;15:621–9. doi: 10.1007/s12072-021-10171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu L, Shen Z, Ji LL, et al High-intensity focused ultrasound alone or combined with transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with unsuitable indications for hepatectomy and radiofrequency ablation: a phase II clinical trial. Surg Endosc. 2022;36:1857–67. doi: 10.1007/s00464-021-08465-3. [DOI] [PubMed] [Google Scholar]
- 34.Wu Z, Wang Z, Zhang L, et al Updated results of the phase II ALTER-H004: Anlotinib combined with TACE as adjuvant therapy in hepatocellular carcinoma patients at high risk of recurrence after surgery. J Clin Oncol. 2022;40(suppl):445. doi: 10.1200/JCO.2022.40.4_suppl.445. [DOI] [Google Scholar]
- 35.Galle PR, Finn RS, Qin S, et al Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:991–1001. doi: 10.1016/S1470-2045(21)00151-0. [DOI] [PubMed] [Google Scholar]
- 36.Xiang X, Wang J, Lu D, et al Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6:75. doi: 10.1038/s41392-021-00484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ling S, Shan Q, Zhan Q, et al USP22 promotes hypoxia-induced hepatocellular carcinoma stemness by a HIF1alpha/USP22 positive feedback loop upon TP53 inactivation. Gut. 2020;69:1322–34. doi: 10.1136/gutjnl-2019-319616. [DOI] [PubMed] [Google Scholar]
- 38.Lin Z, Lu D, Wei X, et al Heterogeneous responses in hepatocellular carcinoma: the achilles heel of immune checkpoint inhibitors. Am J Cancer Res. 2020;10:1085–102. [PMC free article] [PubMed] [Google Scholar]