Abstract
Aim
The association between composite dietary antioxidant index (CDAI) and diabetes remains unknown. Our study was to investigate the association of CDAI with diabetes.
Methods
A total of 11,956 participants were enrolled from the National Health and Nutrition Examination Surveys (NHANES). The CDAI was calculated from the intake of six dietary antioxidants. Multivariable logistic regressions were performed to explore the associations between CDAI and the prevalence of diabetes and glycemic index. Non-linear associations were explored using restricted cubic splines.
Results
In the multivariate logistic regression model, the odds ratio (95% confidence interval) of CDAI associating with obesity was 0.98 (0.97-1.00; p = 0.033). Compared with the lowest quartile, the highest quartile was related to 0.84-fold risk of diabetes (0.71–0.99; p = 0.035). However, CDAI was not independently associated with fasting glucose and hemoglobin A1c.
Conclusion
CDAI was negatively associated with diabetes and the relationship was independent of other traditional risk factors.
Keywords: Composite dietary antioxidant index, Diabetes, Antioxidant, NHANES, Cross-sectional study
Introduction
Diabetes is a group of metabolic diseases, defined by as hyperglycemia caused by the defect of insulin secretion and action [1].The prevalence of diabetes has increased over the past several decades, with Type 2 diabetes making about 90% of the cases, which accounts for over 430 million people worldwide [2]. Diabetes is often associated with significant organ damage and failure, which leads to an increase in mortality rates. Almost all diabetes-related complications can be attributed to vascular damage, including macrovascular complications and microvascular complications [3]. Thus, investigating more risk factors and effective interventions for diabetic individuals is extremely urgent.
It is reported that dietary habit is also an important trigger of diabetes [4]. Previous studies have investigated the relationship between dietary antioxidants and diabetes [5, 6] and diabetic complications [7, 8]. Dietary total antioxidant capacity (TAC) as an indicator of diet quality, has been associated with insulin resistance and cardiometabolic risks [9]. However dietary TAC varies according to the geographic location, seasonality, water and sun availability, storage conditions, food processing, and cooking of the examined food group. The Composite Dietary Antioxidant Index (CDAI) is a valid and reliable nutritional tool to assess overall antioxidant characteristics of the diet, which is a summary score of six dietary antioxidants including vitamins A, C, and E, manganese, selenium, and zinc [10]. Previous studies have found that CDAI was associated with depression [11] and colorectal cancer [12]. However, the investigation on the association between CDAI and diabetes has been scared.
Vitamin A participates in multiple metabolic processes and has an important effect on insulin sensitivity [13]. Its concentration was lower in type 2 diabetic patients [14]. It was reported that diabetic patients have increased lipid peroxidation and decreased Vitamins C and E [15]. Plasma Vitamin C was inversely correlated to glycosylated hemoglobin and blood glucose [16]. Manganese is an essential trace metal element and deficiency or excessive Manganese exposure could increase ROS generation and result in further oxidative stress [17]. A Chinese population-based study found a U-shaped association between manganese with diabetes [18]. Selenium functions metabolically as an essential constituent of selenoproteins, and has a link with diabetes risk [19]. Zinc appears to activate key molecule in cell signaling, involved in the homeostasis of glucose and insulin receptors. Abnormal Zn may also cause diabetes complications [20]. Considering the relevance of the components of CDAI and diabetes, we aimed to examine the potential association between CDAI and the prevalence of diabetes.
Methods
Study Population
The study included participants from the National Health and Nutrition Examination Survey (NHANES), a nationwide survey conducted by the National Center of Health Statistics (NCHS). The survey was designed to assess the health and nutritional status of the non-institutionalized US population by a stratified and multistage sampling design. We combined four cycles of survey with completed data on the intake of components of CDAI from 2008 to 2014 (n = 13,116). After excluding adult participants with missing data on glucose and HbA1c (n = 1160), a total of 11,956 individuals were included in our analyses. All participants provided written informed consent and the protocol was approved by the Ethics Review Board of National Center for Health Statistics (Protocol #2011-17).
Exposure and outcomes
Each participant’s food and nutrient intake in the NHANES dataset was recorded via a 24-h dietary recall interview. The first dietary recall was conducted in person and then 3 to 10 days later via telephone. The Food and Nutrient Database for Dietary Studies of the United States Department of Agriculture was used to calculate the intake of antioxidants, micronutrients, and total energy[21]. Based on the questionnaire interview, we determined the intake of dietary supplements during the past month, including dosage, frequency, and duration of consumption[22].
Standardized questionnaires were administered in the home, followed by a detailed physical examination and blood specimens at a mobile examination center. Diabetes was defined as (1) self-report of a diagnosis by a physician or (2) HbA1c ≥ 6.5% or (3) fasting plasma glucose ≥ 126 mg/dL.
Covariates
To assess the influence of potential confounding factors, we selected several important covariates, including gender, age, race, education level, physical activity, and smoking status, which were collected by using standardized questionnaires. Weight and height of each participant were obtained from the physical examinations. Multiple imputation was performed for missing values.
Statistical analysis
Participants were separated into two groups based on CDAI quartiles. Baseline variables differences were tested by Student t test and Chi-Square tests. The association between CDAI and diabetes was explored with logistic regression models, while the association between CDAI and glucose and HbA1c were explored with linear regression models. The restricted cubic splines were performed to explore the nonlinear association. All statistical analyses were done in R software, version 3.6 and P < 0.05 was regarded as significant.
Results
The baseline features of participants were summarized in Table 1. The individuals with larger CDAI quartile tend to male (p < 0.001), younger (p < 0.001), and less percentage of diabetes (p < 0.001).
Table 1.
Characteristics of the study population according to CDAI quartiles
| Variable | Q1 (n = 2991) | Q2 (n = 2984) | Q3 (n = 2991) | Q4 (n = 2990) | P-value |
|---|---|---|---|---|---|
| Male (%) | 1079 (36.1) | 1274 (42.7) | 1560 (52.2) | 1989 (66.5) | < 0.001 |
| Age, years | 43.4 ± 21.9 | 44.8 ± 21.4 | 43.8 ± 20.3 | 42.2 ± 18.9 | < 0.001 |
| Race (%) | < 0.001 | ||||
| Non-Hispanic white | 1178 (39.4) | 1253 (42.0) | 1286 (43.0) | 1367 (45.7) | |
| Non-Hispanic black | 743 (24.8) | 605 (20.3) | 569 (19.0) | 526 (17.6) | |
| Mexican American | 467 (15.6) | 502 (16.8) | 533 (17.8) | 515 (17.2) | |
| Others | 603 (20.2) | 624 (20.9) | 603 (20.2) | 582 (19.5) | |
| Education | < 0.001 | ||||
| Less than high school | 1178 (39.4) | 1253 (42.0) | 1286 (43.0) | 1367 (45.7) | |
| High school | 743 (24.8) | 605 (20.3) | 569 (19.0) | 526 (17.6) | |
| More than high school | 467 (15.6) | 502 (16.8) | 533 (17.8) | 515 (17.2) | |
| Activity (%) | < 0.001 | ||||
| Vigorous | 1051 (35.1) | 909 (30.5) | 840 (28.1) | 854 (28.6) | |
| Moderate | 1220 (40.8) | 1291 (43.3) | 1252 (41.9) | 1129 (37.8) | |
| Inactive | 720 (24.1) | 784 (26.3) | 899 (30.1) | 1007 (33.7) | |
| Smoker, % | < 0.001 | ||||
| Current | 2015 (67.4) | 2184 (73.2) | 2272 (76.0) | 2193 (73.3) | |
| Past | 154 (5.1) | 149 (5.0) | 132 (4.4) | 176 (5.9) | |
| Never | 822 (27.5) | 651 (21.8) | 587 (19.6) | 621 (20.8) | |
| BMI, kg/m2 | 28.3 ± 7.1 | 28.2 ± 6.9 | 28.1 ± 6.9 | 27.8 ± 6.9 | 0.074 |
| Hypertension (%) | 458 (15.3) | 449 (15.0) | 424 (14.2) | 385 (12.9) | 0.032 |
| CHD (%) | 118 (3.9) | 109 (3.7) | 96 (3.2) | 81 (2.7) | 0.046 |
| Glucose, mg/dL | 106.6 ± 32.9 | 105.4 ± 29.8 | 106.4 ± 34.8 | 105.7 ± 32.7 | 0.456 |
| HbA1c (%) | 5.68 ± 1.01 | 5.69 ± 0.96 | 5.68 ± 1.04 | 5.62 ± 1.02 | 0.027 |
| Diabetes (%) | 520 (17.4) | 485 (16.3) | 452 (15.1) | 381 (12.7) | < 0.001 |
Data are presented as mean (SD) or n (%). BMI, body mass index; HBP, hypertension; CHD, coronary heart disease; HbA1c, hemoglobin A1c
Multivariable logistic regression models were constructed to examine the relationship between CDAI and diabetes (Table 2). In Model 1, the odds ratio (OR) and 95% confidence interval (CI) was 0.97 (0.95–0.98; P < 0.001), which indicated that the risk of obesity was reduced for every unit rise in CDAI. The relationship still existed in the model 2 (OR [95% CI]: 0.97 [0.96–0.98]; P < 0.001) and model 3 (OR [95% CI]: 0.98 [0.97-1.00]; P = 0.033). Compared with the lowest quartile, the highest quartile was significantly associated with diabetes (OR [95% CI]: 0.84 [0.71–0.99]; P = 0.035). However, no dependent associations between CDAI and glucose or HbA1c were found (Table 3).
Table 2.
Association of composite dietary antioxidant index and diabetes
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR [95% CI] | P | OR [95% CI] | P | OR [95% CI] | P | |
| Q1 | Ref | - | Ref | - | Ref | - |
| Q2 | 0.92 [0.81, 1.06] | 0.242 | 0.85 [0.73, 0.98] | 0.028 | 0.92 [0.79, 1.07] | 0.256 |
| Q3 | 0.85 [0.74, 0.97] | 0.017 | 0.82 [0.70, 0.95] | 0.008 | 0.93 [0.80, 1.08] | 0.350 |
| Q4 | 0.69 [0.60, 0.80] | < 0.001 | 0.71 [0.61, 0.84] | < 0.001 | 0.84 [0.71, 0.99] | 0.035 |
| CDAI | 0.97 [0.95, 0.98] | < 0.001 | 0.97 [0.96, 0.98] | < 0.001 | 0.98 [0.97, 1.00] | 0.033 |
Model 1 was not adjusted
Model 2 was adjusted for age and gender
Model 3 was adjusted for age, gender, race, education, activity, smoking status, hypertension, and CHD. OR, odds ratio; CI, confidence interval; CHD, coronary heart diseases
Table 3.
Association of composite dietary antioxidant index and glycemic index
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β [95% CI] | P | β [95% CI] | P | β [95% CI] | P | |
| Glucose | ||||||
| Q1 | Ref | - | Ref | - | Ref | - |
| Q2 | -1.16 [-2.81, 0.50] | 0.171 | -2.13 [-3.72, -0.54] | 0.009 | -1.49 [-3.07, 0.09] | 0.065 |
| Q3 | -0.13 [-1.79, 1.52] | 0.874 | -1.28 [-2.87, 0.32] | 0.117 | -0.25 [-1.85, 1.36] | 0.764 |
| Q4 | -0.84 [-2.50, 0.81] | 0.317 | -2.20 [-3.82, -0.57] | 0.008 | -0.82 [-2.45, 0.82] | 0.329 |
| HbA1c | ||||||
| Q1 | Ref | - | Ref | - | Ref | - |
| Q2 | 0.00 [-0.05, 0.05] | 0.914 | -0.02 [-0.07, 0.02] | 0.331 | 0.01 [-0.04, 0.05] | 0.794 |
| Q3 | -0.01 [-0.06, 0.04] | 0.816 | -0.02 [-0.07, 0.02] | 0.321 | 0.02 [-0.03, 0.07] | 0.359 |
| Q4 | -0.06 [-0.12, -0.01] | 0.012 | -0.07 [-0.12, -0.02] | 0.006 | -0.00 [-0.05, 0.05] | 0.870 |
Model 1 was not adjusted
Model 2 was adjusted for age and gender
Model 3 was adjusted for age, gender, race, education, activity, smoking status, hypertension, and CHD. OR, odds ratio; CI, confidence interval; CHD, coronary heart diseases
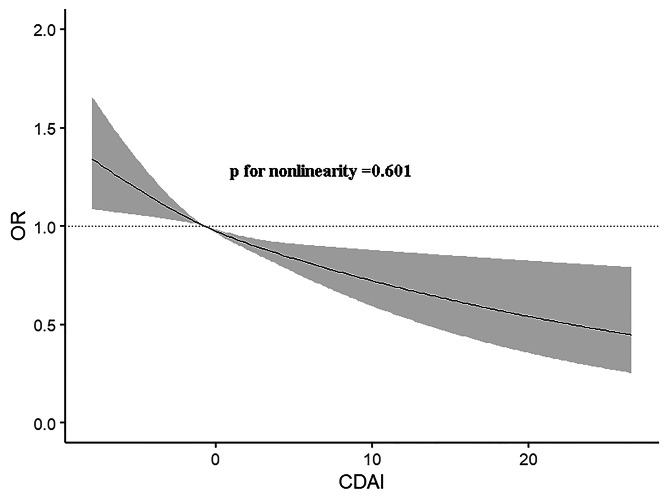
Furthermore, restricted cubic spline suggested the relationship between CDAI and obesity was linear (P for nonlinearity = 0.601; Fig. 1).
Fig. 1.
The dose-response relationship between CDAI and the prevalence of diabetes
Discussion
In our study, we found that CDAI was negatively associated with diabetes. And the relationship remained even after adjusted other covariates, which indicated that CDAI was a protective factor for the development of diabetes. A dose-response analysis found that this negative relationship was linear.
A meta-analysis estimated antioxidant intake was associated with a 13% reduction of diabetes risk, mainly attributed to vitamin E and carotenoids. However, the reduction was not found to be substantial[23]. In addition to other fruits and vegetables contain antioxidant vitamins[24], some review demonstrated that the intake of magnesium [25], zinc [26] and selenium [27] are able to regulate inflammatory and oxidation cascade, which could mediate the effect of a healthy dietary pattern to diabetes. In consistent with these results, our study also found that CDAI, consisting of vitamins A, C, and E, manganese, selenium, and zinc, was inversely correlated with diabetes.
The mechanism is closely related to oxidative stress. Because multiple antioxidants may have synergistic effects. Many dietary antioxidants use their bioactive molecules to reduce oxidative stress and exert antioxidant effects [28]. So, antioxidant nutrients may be able to reduce the risk of diabetes caused by oxidative stress. However, the exact molecular mechanisms are not well-understood, and more research is needed.
There are several limitations to this study. Firstly, the diet assessment might involve measurement errors and inaccuracies. Secondly, bias is inevitable in cross-sectional studies.
Conclusion
Our study found a negative association between CDAI and diabetes after adjusting for potential confounders.
Acknowledgements
None.
Author contributions
C XJ and L H designed the study; C YW and S HQ performed the statistical analysis; T Y and X YF wrote the manuscript. All authors approved the final manuscript.
Funding
None.
Data Availability
All data could be available upon request from the corresponding author.
Declarations
Ethics approval and consent to participate
The protocol was approved by the Institutional Review Board of National Center for Health Statistics and no new data was added.
Consent for publication
Not Applicable.
Conflict of interest
The authors have nothing to disclose regarding conflict of interest with respect to this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaojie Chen and He Lu contributed equally.
References
- 1.Pasquel FJ, Lansang MC, Dhatariya K, Umpierrez GE. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol. 2021;9(3):174–88. doi: 10.1016/S2213-8587(20)30381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magkos F, Hjorth MF, Astrup A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2020;16(10):545–55. doi: 10.1038/s41574-020-0381-5. [DOI] [PubMed] [Google Scholar]
- 3.Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18(9):525–39. doi: 10.1038/s41574-022-00690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton-Freeman B, Brzezinski M, Park E, Sandhu A, Xiao D, Edirisinghe I. A selective role of Dietary Anthocyanins and Flavan-3-ols in reducing the risk of type 2 diabetes Mellitus: a review of recent evidence. Nutrients. 2019;11(4). 10.3390/nu11040841. [DOI] [PMC free article] [PubMed]
- 5.Tan LJ, Hwang SB, Jun S, Joung H, Shin S. Dietary antioxidant consumption and the risk of type 2 diabetes in south korean adults: a prospective cohort study based on the Health Examinees study. BMJ Open. 2022;12(7):e065073. doi: 10.1136/bmjopen-2022-065073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancini FR, Affret A, Dow C, Balkau B, Bonnet F, Boutron-Ruault MC, Fagherazzi G. Dietary antioxidant capacity and risk of type 2 diabetes in the large prospective E3N-EPIC cohort. Diabetologia. 2018;61(2):308–16. doi: 10.1007/s00125-017-4489-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Chen Y, Zou L, Jin L, Yang B, Shu Y, Gong R. Dose-response relationship between dietary antioxidant intake and diabetic kidney disease in the US adults with diabetes. Acta Diabetol. 2023 doi: 10.1007/s00592-023-02125-9. [DOI] [PubMed] [Google Scholar]
- 8.Baharirad N, Pasdar Y, Nachvak M, Ghavamzadeh S, Soroush A, Saber A, Mostafai S, Naghipour A, Abdollahzad H. The relationship of dietary total antioxidant capacity with sarcopenia and cardiometabolic biomarkers in type 2 diabetes patients. Physiol Rep. 2022;10(3):e15190. doi: 10.14814/phy2.15190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galarregui C, Zulet MA, Cantero I, Marin-Alejandre BA, Monreal JI, Elorz M, Benito-Boillos A, Herrero JI, Tur JA, Abete I, Martinez JA. Interplay of Glycemic Index, Glycemic load, and dietary antioxidant capacity with insulin resistance in subjects with a Cardiometabolic Risk Profile. Int J Mol Sci. 2018;19(11). 10.3390/ijms19113662. [DOI] [PMC free article] [PubMed]
- 10.Wright ME, Mayne ST, Stolzenberg-Solomon RZ, Li Z, Pietinen P, Taylor PR, Virtamo J, Albanes D. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. 2004;160(1):68–76. doi: 10.1093/aje/kwh173. [DOI] [PubMed] [Google Scholar]
- 11.Zhao L, Sun Y, Cao R, Wu X, Huang T, Peng W. Non-linear association between composite dietary antioxidant index and depression. Front Public Health. 2022;10:988727. doi: 10.3389/fpubh.2022.988727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu YC, Paragomi P, Wang R, Jin A, Schoen RE, Sheng LT, Pan A, Koh WP, Yuan JM, Luu HN. Composite dietary antioxidant index and the risk of colorectal cancer: findings from the Singapore Chinese Health Study. Int J Cancer. 2022;150(10):1599–608. doi: 10.1002/ijc.33925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brun PJ, Yang KJ, Lee SA, Yuen JJ, Blaner WS. Retinoids: potent regulators of metabolism. BioFactors. 2013;39(2):151–63. doi: 10.1002/biof.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polidori MC, Mecocci P, Stahl W, Parente B, Cecchetti R, Cherubini A, Cao P, Sies H, Senin U. Plasma levels of lipophilic antioxidants in very old patients with type 2 diabetes. Diabetes Metab Res Rev. 2000;16(1):15–9. doi: 10.1002/(sici)1520-7560(200001/02)16:1<15::aid-dmrr71>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Odum EP, Ejilemele AA, Wakwe VC. Antioxidant status of type 2 diabetic patients in Port Harcourt, Nigeria. Niger J Clin Pract. 2012;15(1):55–8. doi: 10.4103/1119-3077.94099. [DOI] [PubMed] [Google Scholar]
- 16.Carter P, Gray LJ, Talbot D, Morris DH, Khunti K, Davies MJ. Fruit and vegetable intake and the association with glucose parameters: a cross-sectional analysis of the Let’s Prevent Diabetes Study. Eur J Clin Nutr. 2013;67(1):12–7. doi: 10.1038/ejcn.2012.174. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Yang X. The essential element Manganese, oxidative stress, and metabolic Diseases: links and interactions. Oxid Med Cell Longev. 2018;2018:7580707. doi: 10.1155/2018/7580707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Cui Z, Lu W, Wang P, Wang J, Zhou Z, Zhang N, Wang Z, Lin T, Song Y, Liu L, Huang X, Chen P, Tang G, Duan Y, Wang B, Zhang H, Xu X, Yang Y, Qin X, Song F. Association between serum manganese levels and diabetes in chinese adults with hypertension. J Clin Hypertens (Greenwich) 2022;24(7):918–27. doi: 10.1111/jch.14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang YC, Combs GF, Jr, Wu TL, Zeng H, Cheng WH. Selenium status and type 2 diabetes risk. Arch Biochem Biophys. 2022;730:109400. doi: 10.1016/j.abb.2022.109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barman S, Srinivasan K. Diabetes and zinc dyshomeostasis: can zinc supplementation mitigate diabetic complications? Crit Rev Food Sci Nutr. 2022;62(4):1046–61. doi: 10.1080/10408398.2020.1833178. [DOI] [PubMed] [Google Scholar]
- 21.Ahuja JK, Moshfegh AJ, Holden JM, Harris E. USDA food and nutrient databases provide the infrastructure for food and nutrition research, policy, and practice. J Nutr. 2013;143(2):241S–9S. doi: 10.3945/jn.112.170043. [DOI] [PubMed] [Google Scholar]
- 22.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in Dietary supplement use among US adults from 1999–2012. JAMA. 2016;316(14):1464–74. doi: 10.1001/jama.2016.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamer M, Chida Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: systematic review and meta-analysis. J Hypertens. 2007;25(12):2361–9. doi: 10.1097/HJH.0b013e3282efc214. [DOI] [PubMed] [Google Scholar]
- 24.Valdes-Ramos R, Guadarrama-Lopez AL, Martinez-Carrillo BE, Benitez-Arciniega AD. Vitamins and type 2 diabetes mellitus. Endocr Metab Immune Disord Drug Targets. 2015;15(1):54–63. doi: 10.2174/1871530314666141111103217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WA EL, Naser IA, Taleb MH, Abutair AS. The Effects of oral magnesium supplementation on glycemic response among type 2 diabetes patients. Nutrients. 2018;11(1). 10.3390/nu11010044. [DOI] [PMC free article] [PubMed]
- 26.Fernandez-Cao JC, Warthon-Medina M, V HM, Arija V, Doepking C, Serra-Majem L, Lowe NM. Zinc intake and status and risk of type 2 diabetes Mellitus: a systematic review and Meta-analysis. Nutrients. 2019;11(5). 10.3390/nu11051027. [DOI] [PMC free article] [PubMed]
- 27.Kohler LN, Foote J, Kelley CP, Florea A, Shelly C, Chow HS, Hsu P, Batai K, Ellis N, Saboda K, Lance P, Jacobs ET. Selenium and type 2 diabetes. Syst Rev Nutrients. 2018;10(12). 10.3390/nu10121924. [DOI] [PMC free article] [PubMed]
- 28.Schiffrin EL. Antioxidants in hypertension and cardiovascular disease. Mol Interv. 2010;10(6):354–62. doi: 10.1124/mi.10.6.4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data could be available upon request from the corresponding author.