Abstract
Background
Triple‐negative breast cancer (TNBC) is an aggressive subtype of breast cancer associated with shorter survival and a higher likelihood of the cancer returning. In early TNBC, platinum‐based chemotherapy has been shown to improve pathological complete response (pCR); however, its effect on long‐term survival outcomes has not been fully elucidated and recommendations to include platinum chemotherapy are not consistent in international guidelines.
Objectives
To evaluate the benefits and harms of platinum‐based chemotherapy as adjuvant and neoadjuvant treatment in people with early triple‐negative breast cancer.
Search methods
We used standard, extensive Cochrane search methods. The latest search date was 4 April 2022.
Selection criteria
We included randomised controlled trials examining neoadjuvant or adjuvant platinum chemotherapy for early TNBC.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were disease‐free survival (DFS) and overall survival (OS). Our secondary outcomes were pCR, treatment adherence, grade III or IV toxicity related to chemotherapy, and quality of life. Prespecified subgroups included BRCA mutation status, homologous recombination deficiency (HRD) status, frequency of chemotherapy, type of platinum agent used, and the presence or absence of anthracycline chemotherapy. We assessed risk of bias using Cochrane's RoB 1 tool and certainty of evidence using the GRADE approach.
Main results
From 3972 records, we included 20 published studies involving 21 treatment comparisons, and 25 ongoing studies. For most domains, risk of bias was low across studies. There were 16 neoadjuvant chemotherapy studies (one of which combined neoadjuvant and adjuvant therapy) and four adjuvant chemotherapy trials. Most studies used carboplatin (17 studies) followed by cisplatin (two), and lobaplatin (one). Eight studies had an anthracycline‐free intervention arm, five of which had a carboplatin‐taxane intervention compared to an anthracycline‐taxane control.
All studies reporting DFS and OS used carboplatin. Inclusion of platinum chemotherapy improved DFS in neoadjuvant and adjuvant settings (neoadjuvant: hazard ratio (HR) 0.63, 95% confidence interval (CI) 0.53 to 0.75; 7 studies, 8 treatment comparisons, 1966 participants; high‐certainty evidence; adjuvant: HR 0.69, 95% CI 0.54 to 0.88; 4 studies, 1256 participants; high‐certainty evidence). Platinum chemotherapy in the regimen improved OS (neoadjuvant: HR 0.69, 95% CI 0.55 to 0.86; 7 studies, 8 treatment comparisons, 1973 participants; high‐certainty evidence; adjuvant: 0.70, 95% CI 0.50 to 0.96; 4 studies, 1256 participants; high‐certainty evidence). Median follow‐up for survival outcomes ranged from 36 to 97.6 months.
Our analysis confirmed platinum chemotherapy increased pCR rates (risk ratio (RR) 1.44, 95% CI 1.31 to 1.59; 15 studies, 16 treatment comparisons, 3083 participants; high‐certainty evidence). Subgroup analyses showed no evidence of differences in DFS according to BRCA mutation status, HRD status, lymph node status, or whether the intervention arm contained anthracycline chemotherapy or not.
Platinum chemotherapy was associated with reduced dose intensity, with participants more likely to require chemotherapy delays (RR 2.23, 95% CI 1.70 to 2.94; 4 studies, 5 treatment comparisons, 1053 participants; moderate‐certainty evidence), dose reductions (RR 1.77, 95% CI 1.56 to 2.02; 7 studies, 8 treatment comparisons, 2055 participants; moderate‐certainty evidence) and early cessation of treatment (RR 1.20, 95% CI 1.04 to 1.38; 16 studies, 17 treatment comparisons, 4178 participants; moderate‐certainty evidence). Increased haematological toxicity occurred in the platinum group who were more likely to experience grade III/IV neutropenia (RR 1.53, 95% CI 1.43 to 1.63; 19 studies, 20 treatment comparisons, 4849 participants; moderate‐certainty evidence), anaemia (RR 8.20, 95% CI 5.66 to 11.89; 18 studies, 19 treatment comparisons, 4757 participants; moderate‐certainty evidence) and thrombocytopenia (RR 7.59, 95% CI 5.10 to 11.29; 18 studies, 19 treatment comparisons, 4731 participants; moderate‐certainty evidence). There was no evidence of a difference between chemotherapy groups in febrile neutropenia (RR 1.16, 95% CI 0.89 to 1.49; 11 studies, 3771 participants; moderate‐certainty evidence). Renal impairment was very rare (0.4%, 2 events in 463 participants; note 3 studies reported 0 events in both arms; 4 studies; high‐certainty evidence). Treatment‐related death was very rare (0.2%, 7 events in 3176 participants and similar across treatment groups; RR 0.58, 95% 0.14 to 2.33; 10 studies, 11 treatment comparisons; note 8 studies reported treatment‐related deaths but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 3 studies rather than 11; 3176 participants; high‐certainty evidence). Five studies collected quality of life data but did not report them.
Authors' conclusions
Platinum‐based chemotherapy using carboplatin in the adjuvant or neoadjuvant setting improves long‐term outcomes of DFS and OS in early TNBC, with no evidence of differences by subgroup. This was at the cost of more frequent chemotherapy delays and dose reductions, and greater haematological toxicity, though serious adverse events including neuropathy, febrile neutropenia or treatment‐related death were not increased.
These findings support the use of platinum‐based chemotherapy for people with early TNBC. The optimal dose and regimen are not defined by this analysis, but there is a suggestion that similar relative benefits result from the addition of carboplatin to either anthracycline‐free regimens or those containing anthracycline agents.
Keywords: Humans; Adjuvants, Immunologic; Anthracyclines; Anthracyclines/therapeutic use; Carboplatin; Febrile Neutropenia; Platinum; Quality of Life; Triple Negative Breast Neoplasms; Triple Negative Breast Neoplasms/drug therapy
Plain language summary
Platinum‐containing chemotherapy for women before or after surgery for early triple‐negative breast cancer
Key messages
Chemotherapy including the platinum‐based medicine carboplatin improves survival and reduces the chance of cancer returning for people with early triple negative breast cancer.
However, it is also associated with increased side effects.
What is triple‐negative breast cancer?
Triple‐negative breast cancer makes up 15% of breast cancer cases. It is a type of breast cancer that does not have any of the three receptors commonly found on breast cancer cells – the oestrogen, progesterone and HER2 receptors. Early breast cancer is defined as cancer limited to the breast and lymph nodes in the armpit, and it can usually be cured.
How is early triple‐negative breast cancer treated?
Treatments for early triple‐negative breast cancer include:
– surgery to remove the cancer from the breast and lymph nodes;
– radiotherapy to the breast and lymph nodes, used to prevent the cancer from coming back in these areas;
– chemotherapy, used to prevent the cancer from coming back anywhere in the body. This can be given before surgery (called 'neoadjuvant') or after surgery (called 'adjuvant').
What did we want to find out?
There are many types of chemotherapy used in triple‐negative breast cancer. We wanted to find out if a specific class of chemotherapy called 'platinum‐based chemotherapy' increases:
– the length of time people stayed alive without cancer recurrence after diagnosis (disease‐free survival);
– the total length of life after diagnosis (overall survival);
– the likelihood that the cancer had disappeared in the removed breast and lymph node tissue when chemotherapy was given before surgery (pathological complete response).
We also wanted to find out if platinum‐based chemotherapy was associated with more unwanted outcomes like chemotherapy delays, dose reductions or side effects.
What did we do?
We searched for studies looking at chemotherapy for early triple‐negative breast cancer that compared regimens containing platinum chemotherapy to regimens without platinum chemotherapy.
We compared and summarised the results of the studies, and rated our confidence in the evidence based on factors such as study methods and size.
What did we find?
We found 20 studies that involved 4688 people with early triple‐negative breast cancer, with average follow‐up in studies ranging from three to eight years.
Platinum chemotherapy was associated with longer disease‐free survival and overall survival, and reduced the chance of disease recurrence and death by about one third. These benefits were seen with chemotherapy used before surgery (neoadjuvant) or after surgery (adjuvant). When used before surgery, it also improved the likelihood of a pathological complete response.
We did not find that any particular subgroup, such as people with a high‐risk gene mutation, had more benefit from platinum chemotherapy.
However, people receiving platinum chemotherapy were more likely to need the dose of their chemotherapy to be reduced, or to have a delay in their chemotherapy. They were also more likely to stop chemotherapy early.
Platinum chemotherapy also caused more serious side effects including low blood cell counts. It was not associated with an increase in having fevers associated with low white blood cell counts (febrile neutropenia), nerve damage symptoms (neuropathy) or death caused by treatment.
What are the limitations of the evidence?
The evidence was generally of high quality and included enough data to make judgements to answer our main questions.
However, there were many types of chemotherapy used across studies. Although we have shown that platinum chemotherapy improves long‐term outcomes, we do not know what the best chemotherapy combination is.
None of the studies reported quality of life, which we had initially set out to measure and record.
How up‐to‐date is this evidence?
This evidence is up‐to‐date to April 2022.
Summary of findings
Summary of findings 1. Platinum‐containing chemotherapy compared to chemotherapy without platinum in neoadjuvant therapy for early triple‐negative breast cancer.
| Platinum‐containing chemotherapy compared to chemotherapy without platinum in neoadjuvant therapy for early triple‐negative breast cancer | ||||||
| Patient or population: neoadjuvant therapy for early triple‐negative breast cancer Setting: outpatient Intervention: platinum‐containing chemotherapy Comparison: chemotherapy without platinum | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with chemotherapy without platinum | Risk with platinum‐containing chemotherapy | |||||
| DFS at 2 years assessed with: risk of recurrence follow‐up: range 3 years to 7.9 years | Study population | HR 0.63 (0.53 to 0.75) | 1966 (8 RCTs) | ⊕⊕⊕⊕ High | — | |
| 210 per 1000 | 138 per 1000 (117 to 162) | |||||
|
DFS at 5 years follow‐up: range 3 years to 7.9 years |
Study population | HR 0.63 (0.53 to 0.75) | 1966 (8 RCTs) | ⊕⊕⊕⊕ High | — | |
| 301 per 1000 | 202 per 1000 (173 to 235) | |||||
| OS at 2 years assessed with: risk of death follow‐up: range 1.7 years to 7.9 years | Study population | HR 0.69 (0.55 to 0.86) | 1973 (8 RCTs) | ⊕⊕⊕⊕ High | — | |
| 48 per 1000 | 33 per 1000 (27 to 41) | |||||
| OS at 5 years follow‐up: range 1.7 years to 7.9 years | Study population | HR 0.69 (0.55 to 0.86) | 1973 (8 RCTs) | ⊕⊕⊕⊕ High | — | |
| 190 per 1000 | 135 per 1000 (110 to 166) | |||||
| Pathological complete response follow‐up: range 6 weeks to 9.5 months | 305 per 1000 | 440 per 1000 (400 to 485) | RR 1.44 (1.31 to 1.59) | 3083 (15 RCTs) | ⊕⊕⊕⊕ High | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DFS: disease‐free survival; HR: hazard ratio; OS: overall survival; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_434916258784592377. | ||||||
Summary of findings 2. Platinum‐containing chemotherapy compared to chemotherapy without platinum in adjuvant therapy for early triple‐negative breast cancer.
| Platinum‐containing chemotherapy compared to chemotherapy without platinum in adjuvant therapy for early triple‐negative breast cancer | ||||||
| Patient or population: adjuvant therapy for early triple‐negative breast cancer Setting: outpatient Intervention: platinum‐containing chemotherapy Comparison: chemotherapy without platinum | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with chemotherapy without platinum | Risk with platinum‐containing chemotherapy | |||||
|
DFS at 2 years assessed with: risk of recurrence follow‐up: range 4.3 years to 8 years |
Study population | HR 0.69 (0.54 to 0.88) | 1256 (4 RCTs) | ⊕⊕⊕⊕ High | — | |
| 148 per 1000 | 105 per 1000 (83 to 131) | |||||
| DFS at 5 years follow‐up: range 4.3 years to 8 years | Study population | HR 0.69 (0.54 to 0.88) | 1256 (4 RCTs) | ⊕⊕⊕⊕ High | — | |
| 169 per 1000 | 120 per 1000 (95 to 150) | |||||
| OS at 2 years assessed with: risk of death follow‐up: range 4.3 years to 8 years | Study population | HR 0.70 (0.50 to 0.96) | 1256 (4 RCTs) | ⊕⊕⊕⊕ High | — | |
| 53 per 1000 | 37 per 1000 (27 to 50) | |||||
| OS at 5 years follow‐up: range 4.3 years to 8 years | Study population | HR 0.70 (0.50 to 0.96) | 1256 (4 RCTs) | ⊕⊕⊕⊕ High | — | |
| 81 per 1000 | 57 per 1000 (41 to 78) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DFS: disease‐free survival; HR: hazard ratio; OS: overall survival; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_434981690495911791. | ||||||
Summary of findings 3. Platinum‐containing chemotherapy compared to chemotherapy without platinum for early triple‐negative breast cancer.
| Platinum‐containing chemotherapy compared to chemotherapy without platinum for early triple‐negative breast cancer | ||||||
| Patient or population: early triple‐negative breast cancer Setting: outpatient Intervention: platinum‐containing chemotherapy Comparison: chemotherapy without platinum | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with chemotherapy without platinum | Risk with platinum‐containing chemotherapy | |||||
| Participants requiring chemotherapy delays | 154 per 1000 | 344 per 1000 (263 to 454) | RR 2.23 (1.70 to 2.94) | 1053 (5 RCTs) | ⊕⊕⊕⊕ High | — |
| Participants requiring dose reduction | 278 per 1000 | 492 per 1000 (433 to 561) | RR 1.77 (1.56 to 2.02) | 2055 (8 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
| Anaemia (grade III/IV) follow‐up: range 6 weeks to 38 weeks | 13 per 1000 | 105 per 1000 (72 to 152) | RR 8.20 (5.66 to 11.89) | 4757 (19 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
| Febrile neutropenia (grade III/IV) follow‐up: range 12 weeks to 38 weeks | 56 per 1000 | 65 per 1000 (50 to 83) | RR 1.16 (0.89 to 1.49) | 3771 (12 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
| Renal impairment (grade III/IV) follow‐up: range 6 weeks to 16 weeks | 4 studies reported renal impairment. 1 study reported 2 events in 60 people in the platinum arm (3%) and 0 events in 57 people in the non‐platinum arm. None of the other studies reported any grade III/IV events | — | 463 (4 RCTs) | ⊕⊕⊕⊕ High | — | |
| Quality of life | 4 studies collected quality of life information using validated questionnaires but none of these reported data. | — | — | — | — | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_434915765589228984. | ||||||
a Downgraded one level for inconsistency due to marked variability between trials, demonstrated by a wide range of hazard ratios and confidence intervals with minimal overlap.
Background
Description of the condition
Breast cancer is the most common type of cancer in women and the most common cause of cancer death (Ferlay 2018). Triple‐negative breast cancer (TNBC) is an aggressive subtype of breast cancer, which lacks hormone receptors and human epidermal growth factor receptor 2 (HER2) expression. It is associated with shorter survival and a higher likelihood of recurrence, and comprises about 15% of breast cancer diagnoses (Foulkes 2010; Lin 2012). Early TNBC is defined as cancer that has not spread beyond the breast or axillary lymph nodes, and is potentially curable. Surgery, radiotherapy, and chemotherapy are used to minimise the chance of relapse.
TNBC is more likely to be associated with heritable causes than other breast cancer subtypes. Over 10% of people diagnosed with TNBC under the age of 50 years, without known family history of breast or ovarian cancer, have a heritable mutation in either breast cancer gene 1 or gene 2 (BRCA1 or BRCA2) (Shimelis 2018). Whilst BRCA1 mutation is the most strongly associated, other heritable gene mutations (i.e. BRCA2; partner and localizer of BRCA2 (PALB2); RAD51 paralogue D (RAD51D) and BRCA1 associated RING domain 1 (BARD1)) have also shown associations with TNBC and higher lifetime risks of breast cancer. These mutations are implicated in DNA repair and genomic stability. A heritable mutation in either a high‐risk or moderate‐risk breast cancer gene was found in 12% of the study population with TNBC (compared to 5% for all breast cancer cases), highlighting the importance of referring women with TNBC for genetic counselling, even when there is no known family history of cancer (Shimelis 2018). Guidelines recommend genetic testing for women who are diagnosed at young ages (less than 50 years); if there is a family history of breast, ovarian, prostate, or pancreatic cancer; or if they are of Ashkenazi Jewish ancestry.
Description of the intervention
Standard chemotherapy used in the adjuvant or neoadjuvant setting for TNBC involves anthracycline and taxane chemotherapy, combined with cyclophosphamide. The role of adjuvant chemotherapy is to treat micrometastatic systemic disease, which is not detectable by standard blood tests and imaging. Chemotherapy is indicated for most women with TNBC who are in good health. The National Comprehensive Cancer Network (NCCN) Guidelines recommend offering chemotherapy to women with TNBC whose cancer size is larger than 1 cm, or any size with involvement of their lymph nodes. Chemotherapy may also be considered for women with smaller tumours.
The intervention being studied is platinum‐based chemotherapy (cisplatin or carboplatin) alone or in addition to the standard adjuvant or neoadjuvant chemotherapy, to determine whether this improves survival from early TNBC. Our primary outcomes were overall survival (OS) and disease‐free survival (DFS). Achieving a pathological complete response (pCR) has strong prognostic value, particularly in the TNBC subtype (Cortazar 2014). Because of the assumed association between survival and pCR, many trials assess pCR while either waiting for data to mature or as their primary endpoint before deciding whether larger trials are feasible. Consequently, we reported pCR, along with OS and DFS.
How the intervention might work
Platinum agents damage DNA by causing single‐strand DNA breaks, resulting in apoptosis. DNA repair deficiencies are associated with germline or somatic mutations in BRCA1, BRCA2, and PALB2, which are frequently associated with TNBC. This is a proposed mechanism for the increased efficacy of the DNA‐damaging effects of platinum chemotherapy for TNBC. With genomic profiling, women identified as having basal‐type TNBC are also seen to have DNA‐repair deficiency (Guo 2017). Besides breast cancer, an enhanced response to platinum‐based chemotherapy is seen in women with BRCA mutations who have ovarian cancer (Pennington 2014), and BRCA‐associated pancreatic cancer (O'Reilly 2020). Poly(adenosine diphosphate‐ribose) polymerase (PARP) inhibitors have shown efficacy for women with advanced BRCA breast cancer, although this treatment has not been compared to their response to platinum.
Potential adverse effects of platinum include an increase in myelosuppression, which can lead to dose omissions, interruptions or dose reduction of platinum chemotherapies, other chemotherapy drugs, or both. There are risks of additional toxicity from myelosuppression, with febrile neutropenia, anaemia or bleeding due to thrombocytopenia. Long‐term toxicities from platinum chemotherapy can include peripheral neuropathy, ototoxicity and renal impairment.
Why it is important to do this review
This review will clarify the role of platinum‐based chemotherapy in early TNBC to determine if there is a significant improvement in OS or other disease outcomes with comparable toxicity to non‐platinum‐based chemotherapy. Previous reviews on this topic suggested that the addition of platinum chemotherapy increases rates of pCR at the cost of an increase in adverse events (Pandy 2019; Poggio 2018). However, new trials have been published since these reviews.
Maximising the efficacy of treatment of early breast cancer will reduce rates of metastatic, incurable disease and premature death from this condition. However, given this is a population where the intention is long‐term survival, the prevention of permanent toxicity is also a priority.
Objectives
To evaluate the benefits and harms of platinum‐based chemotherapy as adjuvant and neoadjuvant treatment in people with early triple‐negative breast cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) examining platinum‐based chemotherapy for neoadjuvant or adjuvant treatment for people with early TNBC. This included trials which added a platinum‐based chemotherapy to another standard chemotherapy regimen, or compared a platinum regimen to a non‐platinum regimen. To be included, studies must have reported their findings for participants with TNBC separately from other participants, or only included less than 20% (a minority is less than 50%) of participants with non‐TNBC.
Types of participants
We included participants aged 18 years or older with early TNBC, defined as breast cancers with disease isolated to the breast and axillary lymph nodes that lack expression of the oestrogen receptor and progesterone receptor (as defined by the trial), and negative for human epidermal receptor 2 (HER2; negative with in situ hybridisation testing; 0 to 1+ with immunohistochemistry (IHC); or 2+ with IHC and negative with fluorescence in situ hybridisation). We included trials with all study locations, and participants of all ethnicities. We excluded trials that did not assess women for HER2 status.
Types of interventions
The intervention of interest was any chemotherapy regimen that contained platinum chemotherapy compared to regimens without platinum chemotherapy. Included studies addressed either adjuvant (postsurgery) or neoadjuvant (presurgery) delivery of chemotherapy for early TNBC. We recorded and compared the dose and duration of chemotherapy.
Types of outcome measures
Primary outcomes
Disease‐free survival (DFS), time‐to‐event outcome defined as time from surgery (in neoadjuvant setting) or randomisation (in adjuvant setting) to first date of a local, regional or distant relapse; diagnosis of a second primary cancer; or death from any cause. We included similar outcomes, such as progression‐free survival and time‐to‐progression in this section.
Overall survival (OS), time‐to‐event outcome defined as the time from randomisation or study entry until death from any cause.
Secondary outcomes
Pathological complete response (pCR) (dichotomous outcome) defined as no invasive carcinoma in the breast or axillary lymph nodes (ypT0/isypN0 TNM (tumour, node, metastasis) staging; Edge 2010) after neoadjuvant therapy.
Completion of regimens (dichotomous outcomes), assessed by absence of delay in treatment or dose reductions, or both, or early cessation of treatment.
Any grade III/IV toxicity related to chemotherapy (dichotomous outcomes).
Quality of life – quality of life information is typically not collected in these types of trials, we aimed to report any quality of life data as measured by the many validated tools available to trialists, and at all reported time points.
Search methods for identification of studies
Electronic searches
We performed a search the following databases up to the 4 April 2022.
The Cochrane Breast Cancer Group's (CBCG's) Specialised Register. Details of the search strategies used by the Group for the identification of studies and the procedure used to code references are outlined in the Group's module (breastcancer.cochrane.org/sites/breastcancer.cochrane.org/files/public/uploads/specialised_register_details.pdf). We identified and considered for inclusion any trial with the keywords: 'Cisplatin', 'cisplatinum', 'carboplatin', 'carboplatinum', 'platin', 'platinum', 'platinum diamminodichloride', 'cis‐diamminedichloroplatinum', 'cis‐dichlorodiammineplatinum', 'biocisplatinum', 'dichlorodiammineplatinum', 'nsc‐119875', 'platidiam', 'platino', 'Platinol', 'cis‐diamminedichloroplatinum', 'cis‐platinum', 'cis‐diammine (cyclobutanedicarboxylato) platinum', 'cbdca', 'jm‐8', 'nsc‐241240', 'paraplatin';
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, latest issue); see Appendix 1
MEDLINE OvidSP (top up search to complement CBCG's Specialised Register); see Appendix 2
Embase OvidSP (1947 to present); see Appendix 3
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal for all prospectively registered and ongoing trials (apps.who.int/trialsearch/Default.aspx); see Appendix 4
ClinicalTrials.gov (clinicaltrials.gov/); see Appendix 5
Searching other resources
We screened the reference lists of identified relevant trials or reviews to help identify additional studies. We obtained a full article or abstract for each reference reporting a potentially eligible trial.
We searched the abstracts of recent conference proceedings not yet included in the CBCG's Specialised Register or medical databases, including the American Society of Clinical Oncology annual meeting, European Society of Medical Oncology Congress and San Antonio Breast Cancer Symposium.
We searched systematic reviews on the topic using PubMed Clinical Queries.
We contacted the lead investigators of potentially eligible ongoing and completed trials listed in the trial registries to see if their study was complete or study results could be provided.
Data collection and analysis
Selection of studies
Two review authors (SM and AG) independently applied the selection criteria to each reference identified by the search strategy. There were no disagreements by review requiring resolution.
We included English‐language studies and studies that were translated. We recorded the selection process in the PRISMA flow diagram.
We recorded a selection of excluded studies in the Characteristics of excluded studies table.
Data extraction and management
We extracted data using standard extraction forms. We collected information on study design; randomisation methods; baseline characteristics of participants; setting; chemotherapy regimens (chemotherapy agent, dose, number of cycles); deliverability of treatment, assessed by dose intensity, dose delays or interruptions; and primary and secondary outcomes. We also collected details regarding type of toxicity for grade III or IV events (according to National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE 2017)), length of follow‐up and sources of funding.
Two review authors (SM and MW) independently extracted the data, and resolved disagreements with the support of AG and SE. For studies with more than one publication, we extracted data from all publications, and considered the most recent full‐text version of the study to be the primary reference. We combined records relating to the same study under the overall trial ID. For one included study, one colleague checked and conducted data extraction and risk of bias assessments for the translated material.
We entered data into RevMan Web 2022 for analysis.
Assessment of risk of bias in included studies
We assessed bias using Cochrane's RoB 1 tool (Higgins 2011). The domains assessed were sequence generation (selection bias), allocation sequence concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias) and other potential sources of bias. In oncology, an open‐label approach is often used as it is difficult to obscure differing treatment schedules and potential toxicities from patients and care providers. Therefore, we grouped the blinding of outcome assessment domain with outcome measures from most unlikely to most likely to be influenced by a lack of blinding. The outcomes were segregated into DFS, OS, pCR, toxicity and treatment adherence, and quality of life.
Two review authors (SM and MW) independently assessed the risk of bias, with guidance provided by two other review authors (AG and SE). We incorporated the results of this risk of bias assessment into the interpretation of results.
Measures of treatment effect
We used the following effect measures.
Time‐to‐event outcomes (DFS, OS): expressed as a hazard ratio (HR) with 95% confidence intervals (CI). For HRs and variances which were not reported in the trial publications, we calculated summary statistics indirectly using the methods outlined in Tierney 2007. In the 'Notes' section of the Characteristics of included studies table, we recorded the use of indirect methods, and whether the trial publications reported an assessment of the proportional hazards assumption. HRs less than 1.0 favour regimens with platinum chemotherapy, and HRs greater than 1.0 favour regimens without platinum chemotherapy.
Dichotomous outcomes (pCR, completion of regimens, toxicity): expressed as risk ratio (RR) with 95% CI. We reported the ratios of treatment effects for pCR (a favourable event) so that RRs greater than 1.0 favour regimens with platinum chemotherapy, and RRs less than 1.0 favour regimens without platinum chemotherapy. For completion of regimens or toxicity outcomes (unfavourable events), RRs greater than 1.0 favour regimens without platinum chemotherapy and RRs less than 1.0 favour regimens with platinum chemotherapy. Data for toxicity were the population included in the study regardless of the proportion of participants with TNBC;
Continuous data (quality of life): collected but not reported in any of the studies. If sufficient quality of life data becomes available in future review updates, the effect measure would likely be a mean difference (MD) if studies used the same scales or standardised mean difference (SMD) if studies used different scales, with 95% CI. We would interpret and report SMDs in a more easily interpreted scale for readers, considering the minimal important clinical difference (MICD) to put results into context (McGlothlin 2014). Each quality of life measurement scale may have a different MICD and we plan to review these estimates for each instrument. We would use an MICD of 0.2 to 0.5 as a guide for patient‐reported outcomes.
Two review authors (SB and MW) extracted data from each trial and discussed any data queries with two other review authors (AG and SE).
Unit of analysis issues
The trial participants were the unit of analysis in this review. One trial was a three‐arm study (Brightness: BrighTNess comparison 1 and BrighTNess comparison 2). For this study, we halved the number of women in the control group to allow for a comparison with the two different platinum‐containing arms. The Cochrane Handbook for Systematic Reviews of Interventions suggests these methods to correct for multiple intervention or control groups (Higgins 2020).
Dealing with missing data
We attempted to contact authors of included studies in writing, to request missing data (e.g. dosing or toxicity). We contacted the following authors of included studies (Ando 2014; BrighTNess: BrighTNess comparison 1 and BrighTNess comparison 2; I‐SPY2).
We also contacted studies recorded in the WHO ICTRP and ClinicalTrials.gov that have not yet published their results. We discussed the impact of any missing data.
Assessment of heterogeneity
Recommendations in the Cochrane Handbook for Systematic Reviews of Interventions have guided the assessment of heterogeneity (Deeks 2020). We examined diversity by visually inspecting the forest plots, Chi² test and I² statistic. We used a cut‐off point of P = 0.10 for the Chi² test. The I² statistic "describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance)" (Section 10.10.2, Deeks 2020). We acknowledge that there is much uncertainty in measures such as I2 statistic when there are few studies. Noting these limitations, we used it as a rough guide for interpretation, using these thresholds for the I² statistic:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity and
75% to 100%: considerable heterogeneity
The importance of the observed value of the I2 statistic depends on the magnitude and direction of effects, and strength of evidence for heterogeneity (e.g. P value from the Chi2 test, or a CI for the I2 statistic: uncertainty in the value of the I2 statistic is substantial when the number of studies is small; Deeks 2020).
Assessment of reporting biases
As there were fewer than 10 studies contributing to meta‐analyses, we were unable to investigate publication or other bias using funnel plot asymmetry.
Where possible, we reviewed the protocols of included studies to assess outcome reporting bias.
Data synthesis
We used the following methods to synthesise the data:
time‐to‐event data (DFS, OS) – we used a fixed‐effect model with an inverse‐variance model; as there was no evidence of substantial heterogeneity, a random‐effects model (DerSimonian and Laird with inverse‐variance method) was not required;
dichotomous outcomes (pCR, completion of regimens, toxicity) – we used a fixed‐effect model (Mantel‐Haenszel model (Mantel 1959)); as there was no evidence of substantial heterogeneity, a random‐effects model (DerSimonian and Laird method; (DerSimonian 1986)) was not required;
continuous data (quality of life) – no data were reported. If data are reported in future review updates, we intend to use a fixed‐effect model with an inverse variance method (Deeks 2011); or if there is evidence of substantial heterogeneity, a random‐effects model (DerSimonian and Laird with inverse‐variance method).
In the case of pCR, one study was an adaptive platform trial and reported results as an estimated rate of complete response with a 95% Bayesian probability interval. In order to include these data in the meta‐analysis, we calculated the discrete number of events in each group by using the adjusted probabilities of pCR.
Though there were occasional zero event toxicity outcomes in a single arm for dichotomous outcomes, the Mantel‐Haenszel methods requires zero‐cell corrections only if the same cell is zero in all the included studies (Deeks 2020), which was not the case in our data. In instances where there were no events in both arms of study, we followed the standard practice of excluding that study from the meta‐analysis. The rationale behind this is that these studies do not offer any insight into the direction or magnitude of the relative effect of the treatment (Deeks 2020).
Subgroup analysis and investigation of heterogeneity
We examined the following subgroups
Germline BRCA mutations
Somatic mutation of HRD
Lymph node status
Type of platinum agent used in the platinum arm
-
Types of chemotherapy
Trials where the only difference across treatment arms was the use of platinum, that is platinum plus regimen A versus regimen A, described as same backbone chemotherapy with or without platinum
Anthracycline‐containing regimens (may include taxane) versus non‐anthracycline regimen
Timing of platinum agent, that is weekly versus every two weeks versus every three weeks
We conducted subgroup analyses to assess the effects of the above factors on clinical outcomes and heterogeneity.
Sensitivity analysis
We conducted the following sensitivity analyses based on:
differences in the definition of triple negative that had a hormone receptor (oestrogen receptor (ER)/progesterone receptor (PR)) expression cut‐off other than less than 1% or was not defined;
potentially confounding extra treatments (e.g. the intervention contained a platinum as well as an additional anticancer agent) on the primary outcomes;
a high or unclear risk of bias;
considerable heterogeneity (i.e. I2 statistic between 75% and 100%). In this case, a random‐effects approach was additionally conducted.
Summary of findings and assessment of the certainty of the evidence
Teams of two review authors (from SM, MW, AG and SE) assessed the certainty of the evidence for critical outcomes using the GRADE approach. This approach uses five considerations, bias, inconsistency, indirectness, imprecision and publication bias, to provide rationale for downgrading or upgrading the evidence (Schünemann 2013). We assessed each outcome, and presented the information in summary of findings tables, using GRADEpro GDT software (GRADEpro GDT). The key outcomes assessed were:
DFS;
OS;
pCR after neoadjuvant therapy;
-
completion of regimens:
dose intensity, number of cycles completed, treatment delays;
-
any grade III/IV toxicity related to chemotherapy (stratified by haematological or non‐haematological toxicity):
non‐haematological toxicity, specifically peripheral neuropathy, renal impairment;
haematological toxicity, specifically febrile neutropenia, anaemia;
quality of life.
We reported summary of findings for time‐to‐event outcomes (DFS and OS) at two and five years (with non‐platinum group risks estimated from the mean of non‐platinum group Kaplan‐Meier probabilities at two and five years). For all other outcomes, we reported when the outcome was measured (e.g. pCR measured after surgical intervention shortly after completing neoadjuvant chemotherapy).
Results
Description of studies
Results of the search
Database and trial registry searches yielded 3972 records, and we screened the titles and abstracts of 3644 records after removing duplicates. We excluded 3468 records at title and abstract screening stage, and screened 176 full‐text articles or ongoing trial records. Of these, 114 records related to 20 included studies involving 21 treatment comparisons, and 28 records related to 25 ongoing studies. We excluded 34 records and presented the reasons for exclusion for the five studies that one may expect to find in the review the Characteristics of excluded studies table.
See PRISMA flowchart (Figure 1).
1.
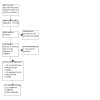
Study flow diagram.
Included studies
See Characteristics of included studies table.
The 20 included studies, involving 4468 participants, contributed to 21 treatment comparisons outlined in Table 4. Notably, the BrighTNess study has more than one intervention that was split into two treatment comparisons (BrighTNess comparison 1; BrighTNess comparison 2), which is why the number of studies and treatment comparisons included in an analysis may differ.
1. Summary of the included treatment comparisons.
| Trial | Year recruitment started | Intervention (platinum‐containing) | Control | Platinum agent | Same backbone? | Adjuvant or neoadjuvant | Hormone receptor IHC cut‐off |
| ADAPT‐TN | 2013 | Nab‐paclitaxel 125 mg/m2 + carboplatin AUC2 days 1 and 8 every 3 weeks for 4 cycles | Nab‐paclitaxel 125 mg/m2 + gemcitabine 1000 mg/m2 days 1 and 8 every 3 weeks for 4 cycles | Carboplatin AUC2 every week (days 1 and 8 every 21 days) | No | Neoadjuvant | < 1% |
| Ando 2014 | 2010 | Carboplatin AUC5 every 3 weeks for 4 cycles + paclitaxel 80 mg/m2 days 1, 8, 15 for 4 cycles, followed by 4 cycles of cyclophosphamide 500 mg/m2, epirubicin 100 mg/m2 and fluorouracil 500 mg/m2 every 3 weeks | Paclitaxel 80 mg/m2 days 1, 8, 15 for 4 cycles followed by 4 cycles of cyclophosphamide 500 mg/m2, epirubicin 100 mg/m2 and fluorouracil 500 mg/m2 every 3 weeks | Carboplatin AUC5 every 3 weeks | Yes | Neoadjuvant | < 10% |
| BrighTNess comparison 1 | 2014 | Paclitaxel 80 mg/m2 weekly + carboplatin AUC6 every 3 weeks for 12 weeks + veliparib 50 mg twice a day, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Paclitaxel 80 mg/m2 weekly for 12 weeks, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Carboplatin AUC6 every 3 weeks | Yes | Neoadjuvant | < 1% |
| BrighTNess comparison 2 | 2014 | Paclitaxel 80 mg/m2 weekly + carboplatin AUC6 every 3 weeks for 12 weeks, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Paclitaxel 80 mg/m2 weekly for 12 weeks, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Carboplatin AUC6 every 3 weeks | Yes | Neoadjuvant | < 1% |
| CALGB 40603 | 2009 | Paclitaxel 80 mg/m2 weekly + carboplatin AUC6 every 3 weeks for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles ± bevacizumab 10 mg/kg every 2 weeks for 9 cycles | Paclitaxel 80 mg/m2 weekly for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles ± bevacizumab 10 mg/kg every 2 weeks for 9 cycles | Carboplatin AUC6 every 3 weeks | Yes | Neoadjuvant | < 10% |
| GEICAM 2006‐03 | 2007 | Epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by docetaxel 75 mg/m2 + carboplatin AUC6 every 3 weeks for 4 cycles | Epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by docetaxel 75 mg/m2 every 3 weeks for 4 cycles | Carboplatin AUC6 every 3 weeks | Yes | Neoadjuvant | Not described |
| GeparOcto | 2014 | Paclitaxel 80 mg/m2 + non‐pegylated liposomal doxorubicin 20 mg/m2 + carboplatin AUC1.5 weekly for 18 weeks | Epirubicin 150 mg/m2 + paclitaxel 225 mg/m2 + cyclophosphamide 2000 mg/m2 every 2 weeks for 3 cycles | Carboplatin AUC1.5 every week | No | Neoadjuvant | < 1% |
| GeparOLA | 2016 | Paclitaxel 80 mg/m2 + carboplatin AUC2 weekly for 12 weeks followed by epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Paclitaxel 80 mg/m2 weekly + olaparib 100 mg twice a day for 12 weeks followed by epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Carboplatin AUC2 every week | No | Neoadjuvant | < 1% |
| GeparSixto | 2011 | Carboplatin AUC2 or 1.5 + paclitaxel 80 mg/m2 + non‐pegylated liposomal doxorubicin 20 mg/m2 + bevacizumab 15 mg/kg every 3 weeks for 18 weeks | Paclitaxel 80 mg/m2 + non‐pegylated liposomal doxorubicin 20 mg/m2 weekly + bevacizumab 15 mg/kg every 3 weeks for 18 weeks | Carboplatin AUC1.5 or 2 every week | Yes | Neoadjuvant | < 1% |
| Gigolaeva 2019 | NR | Doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by carboplatin AUC2 weekly + eribulin 1.4 mg/m2 or paclitaxel 175 mg/m2 every 3 weeks for 12 weeks | Doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by paclitaxel 80 mg/m2 for 12 weeks | Carboplatin AUC2 every week | No | Neoadjuvant | Not described |
| INFORM | 2012 | Cisplatin 75 mg/m2 every 3 weeks for 4 cycles | Doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2–3 weeks for 4 cycles | Cisplatin 75 mg/m2 every 3 weeks | No | Neoadjuvant | < 10% |
| I‐SPY2 | 2010 | Paclitaxel 80 mg/m2 weekly + veliparib 50 mg twice daily + carboplatin AUC6 every 3 weeks for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Paclitaxel 80 mg/m2 weekly for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Carboplatin AUC6 every 3 weeks | No | Neoadjuvant | < 5% |
| Li 2020 | 2011 | Paclitaxel 150 mg/m2 + carboplatin AUC3 every 2 weeks for 8 cycles | Epirubicin 80 mg/m2 and cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles followed by paclitaxel 175 mg/m2 every 2 weeks for 4 cycles | Carboplatin AUC3 every 2 weeks | No | Adjuvant | < 1% |
| Nasr 2015 | 2008 | 5‐fluorouracil 500 mg/m2 + epirubicin 100 mg/m2 + cyclophosphamide 500 mg/m2 every 3 weeks for 3 cycles then docetaxel 80 mg/m2 + carboplatin AUC5 every 3 weeks for 3 cycles, followed by postoperative radiotherapy, followed by cyclophosphamide 50 mg daily and methotrexate 2.5 mg twice daily on days 1, 2 of each week for 1 year | 5‐fluorouracil 500 mg/m2 + epirubicin 100 mg/m2 + cyclophosphamide 500 mg/m2 every 3 weeks for 3 cycles then docetaxel 80 mg/m2 every 3 weeks for 3 cycles | Carboplatin AUC5 every 3 weeks | No | Adjuvant | Not described |
| NeoCART | 2016 | Docetaxel 75 mg/m2 + carboplatin AUC6 every 3 weeks for 6 cycles | Epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by docetaxel 100 mg/m2 every 3 weeks for 4 cycles | Carboplatin AUC6 every 3 weeks | No | Neoadjuvant | < 1% |
| PATTERN | 2011 | Paclitaxel 80 mg/m2 + carboplatin AUC2 days 1, 8, 15, every 28 days for 6 cycles | Cyclophosphamide 500 mg/m2 + epirubicin 100 mg/m2 + fluorouracil 500 mg/m2 every 3 weeks for 3 cycles followed by docetaxel 100 mg/m2 every 3 weeks for 3 cycles | Carboplatin AUC2 every week (days 1, 8, 15 every 28 days) | No | Adjuvant | < 1% |
| TBCRC 030 | 2014 | Cisplatin 75 mg/m2 every 3 weeks for 4 cycles | Doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles | Cisplatin 75 mg/m2 every 3 weeks | No | Neoadjuvant | < 5% |
| Wu 2018 | 2014 | Lobaplatin 30 mg/m2 for 4 cycles + epirubicin 80 mg/m2 + docetaxel 75 mg/m2 every 3 weeks presurgery and 2 cycles postsurgery | Epirubicin 80 mg/m2 for 4 cycles + docetaxel 75 mg/m2 every 3 weeks presurgery and 2 cycles postsurgery | Lobaplatin 30 mg/m2 every 3 weeks | Yes | Both | < 10% |
| Zhang 2016 | 2006 | Paclitaxel 175 mg/m2 + carboplatin AUC5 every 3 weeks for 4–6 cycles | Epirubicin 75 mg/m2 + paclitaxel 175 mg/m2 every 3 weeks for 4–6 cycles | Carboplatin AUC5 every 3 weeks | No | Neoadjuvant | < 10% |
| Zhao 2014 | Not provided in translation | Paclitaxel 175 mg/m2 day 1, carboplatin AUC5 day 2, every 3 weeks for 2 cycles | Epirubicin 75 mg/m2 day 1, paclitaxel 175 mg/m2 day 2, every 3 weeks for 2 cycles | Carboplatin AUC5 every 3 weeks | No | Neoadjuvant | Not provided in translation |
| Zheng 2022 | 2009 | Docetaxel 75 mg/m2 or paclitaxel 175 mg/m2 + carboplatin AUC5 every 3 weeks for 6 cycles | Epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles, followed by docetaxel 75 mg/m2 or paclitaxel 175 mg/m2 every 3 weeks for 4 cycles | Carboplatin AUC5 every 3 weeks | No | Adjuvant | < 1% |
AUC: area under the curve; IHC: immunohistochemistry.
Table 5 details the number of treatment comparisons by subgroup and efficacy outcome.
2. Number of treatment comparisons by subgroup and efficacy outcomes.
| Outcome | ||||
| Treatment comparisons n (%) | DFS n (%) | OS n (%) | pCR n (%) | |
| Overall | 21 | 13 (62%) | 12 (57%) | 16 (76%) |
| Treatment setting | ||||
| Neoadjuvant | 16 (76%) | 8 (38%) | 8 (38%) | 16 (76%) |
| Adjuvant | 4 (19%) | 4 (19%) | 4 (19%) | 0 |
| Both | 1 (5%) | 1 (5%) | 0 | 1 (5%) |
| Subgroups | ||||
| BRCA mutation subgroup reported | 6 (29%) | 4 (19%) | 0 | 6 (29%) |
| HRD status subgroup reported | 1 (5%) | 1 (5%) | 0 | 1 (5%) |
| Lymph node positive reported | 3 (14%) | 3 (14%) | 0 | 3 (14%) |
| Type of platinum agent | ||||
| Carboplatin | 18 (%) | 12 (57%) | 12 (57%) | 13 (62%) |
| Cisplatin | 2 (10%) | 0 | 0 | 2 (10%) |
| Lobaplatin | 1 (5%) | 1 (5%) | 0 | 1 (5%) |
| Type of regimen | ||||
| Different backbone | 14 (67%) | 7 (33%) | 7 (33%) | 9 (%) |
| Same backbone | 7 (33%) | 6 (29%) | 5 (24%) | 7 (%) |
| Anthracycline content in intervention arm | ||||
| Anthracycline present | 12 (57%) | 7 (33%) | 6 (29%) | 10 (47%) |
| Anthracycline free | 9 (43%) | 6 (29%) | 6 (29%) | 6 (29%) |
| Schedule of platinum agent | ||||
| 3‐weekly | 14 (67%) | 9 (43%) | 8 (38%) | 11 (57%) |
| 2‐weekly | 1 (5%) | 1 (5%) | 1 (5%) | 0 |
| Weekly | 5 (24%) | 3 (14%) | 3 (%) | 5 (24%) |
| Hormone receptor IHC cut‐off | ||||
| > 1% or not reported | 11 (57%) | 5 (24%) | 4 (19%) | 9 (43%) |
| < 1% | 10 (47%) | 8 (38%) | 8 (38%) | 7 (33%) |
DFS: disease‐free survival; HRD: homologous recombination deficiency; IHC: immunohistochemistry; n: number; OS: overall survival; pCR: pathological complete response.
15 studies (16 treatment comparisons) involved neoadjuvant chemotherapy with one study combining neoadjuvant and adjuvant therapy, and four studies involved adjuvant chemotherapy
17 studies (18 treatment comparisons) used carboplatin, two studies used cisplatin and one study used lobaplatin
nine studies had an anthracycline‐free intervention arm
six studies stratified results for BRCA mutations, one trial for HRD status, and three by lymph node status
six studies (seven treatment comparisons) used the same chemotherapy backbone (i.e. platinum agent plus regimen A versus regimen A) and 14 trials used a different backbone (i.e. regimen A versus regimen B)
We included studies that examined other subtypes of breast cancer, provided the outcome of DFS, OS or pCR was described for the TNBC subgroup. For such studies, only efficacy analyses are reported for the TNBC group (Ando 2014; GEICAM 2006‐03; GeparOcto; GeparOLA; GeparSixto; I‐SPY2; TBCRC 030). Other outcomes including toxicity and the completion of chemotherapy regimens may be reported for the whole cohort if subgroup data were not published. This is not considered a significant change from the protocol because participants with TNBC are unlikely to have substantially different chemotherapy adverse effects compared to participants with other subtypes of breast cancer.
Notably, there were studies where participants in the intervention group received platinum agents as well as other experimental interventions. In Nasr 2015, participants randomised to the intervention received platinum chemotherapy as well as a further year of metronomic oral chemotherapy. Trialists in BrighTNess examined the effects of both carboplatin and veliparib. To compare all participants in this trial receiving platinum chemotherapy, we split this study into two analysis groups, or 'treatment comparisons' (BrighTNess comparison 1 intervention: paclitaxel, veliparib and carboplatin followed by doxorubicin and cyclophosphamide (AC), and BrighTNess comparison 2 intervention: paclitaxel and carboplatin followed by AC). Both were compared to the control group of paclitaxel alone followed by AC.
Excluded studies
We excluded 34 records on full‐text review owing to:
incorrect study population (12 records). We excluded studies where the population included people with TNBC with residual disease after chemotherapy and surgery; studies on unresectable or metastatic TNBC; studies which included hormone receptor‐positive or HER2‐positive subtypes of breast cancer, and did not separately report outcomes for TNBC; and studies where HER‐2 status was not determined or reported;
incorrect intervention (12 records). We excluded studies employing high‐dose chemotherapy requiring autologous stem cell transplant;
incorrect study design (three records). We excluded retrospective or non‐randomised study designs;
studies not reporting critical outcomes (one study). We excluded one study that did not measure DFS, OS or pCR; such studies often reported other outcomes including breast‐conserving surgery rate.
meta‐analyses (six papers). We checked meta‐analyses for eligible references, and whether our results were concordant.
The reasons for excluding five studies that may be expected in this review are provided in the Characteristics of excluded studies table.
Ongoing studies
We identified 25 eligible ongoing studies from trial records and abstract publications based on the available information (see Characteristics of ongoing studies table).
15 studies examined neoadjuvant chemotherapy, eight studies examined adjuvant therapy and two studies included both neoadjuvant and adjuvant therapy
18 studies used carboplatin, six studies used cisplatin, and one study used lobaplatin
Nine studies reported DFS or OS as a primary outcome and 18 reported DFS or OS as a secondary outcome
The results of the more recent studies are pending; however, it is considered that the inactive older trial records are unlikely to produce results despite emails requesting outcome data from the trialists (NCT03168880; NCT00919880; NCT01752686).
Risk of bias in included studies
See Figure 2 for a summary of risk of bias judgements of the included studies.
2.

Allocation
Nine studies (10 treatment comparisons) were at low risk of bias for random sequence generation and 14 studies (15 treatment comparisons) for allocation concealment. Those deemed at unclear risk did not detail procedures for randomisation (ADAPT‐TN; CALGB 40603; INFORM; GEICAM 2006‐03; Gigolaeva 2019; Li 2020; Nasr 2015; Wu 2018; Zhang 2016; Zhao 2014; Zheng 2022), or whether allocation was performed centrally (Gigolaeva 2019; Li 2020; Nasr 2015; Wu 2018; Zhao 2014; Zhang 2016).
Blinding
Nineteen studies were described as open‐label. Performance bias due to lack of blinding of participants and personnel was not considered to be a serious concern given the objective nature of the efficacy outcomes and most toxicity outcomes. As such, these studies were deemed at unclear risk of bias. One study was double blinded throughout the course of the trial (BrighTNess), and judged at low risk of bias for all outcomes.
We assessed detection bias by outcome. For DFS, OS, pCR and toxicity, lack of blinding was perceived as unlikely to have an impact given the nature or method in which each outcome is assessed (i.e. through imaging, biochemical tests, reviewed by independent panels, or a combination of these). All studies reporting DFS or OS were perceived to be at low risk of bias. All studies reporting pCR were deemed to be at low risk of bias except for two studies at unclear risk because the papers did not provide any information on tests used or process to evaluate tumour response (Gigolaeva 2019; Zhao 2014). Similarly, studies reporting toxicities were at low risk of bias except for one study as no information was provided on how toxicity was assessed (Zhao 2014). None of the studies that collected quality of life measures reported data and no risk of bias assessment was possible.
Incomplete outcome data
Most studies did not complete a true intention‐to‐treat analysis, in that participants who were randomised but did not receive treatment were excluded from the efficacy and safety analysis. Notably, only a very small number of participants were excluded in each study after randomisation. Nine studies were at unclear risk of bias. We judged six studies at unclear risk of bias because the reasons for excluding participants were not detailed (CALGB 40603; GeparOcto; GeparSixto; Wu 2018; Zhang 2016; Zhao 2014). One study was at unclear risk of attrition bias as there were several randomised people with missing pCR data that could not be accounted for (ADAPT‐TN). Two studies did not provide a CONSORT diagram or associated information and were classified at unclear risk of bias (Gigolaeva 2019; I‐SPY2).
Selective reporting
One study was at high risk of bias as it did not report DFS or OS, despite these outcomes being listed in the trial registry records (I‐SPY2). As pCR data were reported in 2016, these important long‐term efficacy outcomes would have been expected to be reported by 2022. Four studies with more recent publications which have not yet published results on critical outcomes were at unclear risk (GeparOLA; GeparOcto; INFORM; Wu 2018). Two additional studies did not provide sufficient information for an assessment and were judged at unclear risk of bias (e.g. abstract only; Gigolaeva 2019; Nasr 2015).
Four studies identified quality of life as an outcome in their trial registry records or publications (BrighTNess; GeparOcto; I‐SPY2; Zheng 2022); however, there were no published reports of quality of life measures from these studies.
Other potential sources of bias
One study was published in abstract form only and did not have an identifiable trial registration record (Gigolaeva 2019). As such, the risk of bias assessment was limited and assessed as unclear. Another study required translation (Zhao 2014). While outcome measures were provided in the translation, we did not have sufficient translated information to make risk of bias assessments for this domain and most others.
Effects of interventions
See: Table 1; Table 2; Table 3
Neoadjuvant therapy
See Table 1.
Disease‐free survival
Ten of the 16 neoadjuvant studies collected data on DFS; however, two studies did not report data (GeparOcto; I‐SPY2). Median follow‐up ranged from 36 to 94.8 months. Platinum‐based chemotherapy improved DFS compared to non‐platinum‐containing chemotherapy (HR 0.63, 95% CI 0.53 to 0.75; P < 0.001, I2 = 30%; 7 studies, 8 treatment comparisons; high‐certainty evidence; Analysis 1.1; Figure 3). A total of 1966 people were included in the analysis with an estimated 500 DFS events.
1.1. Analysis.

Comparison 1: Neoadjuvant, Outcome 1: Disease‐free survival
3.

One other study reported on DFS following neoadjuvant and adjuvant treatment, but results could not be separated for neoadjuvant therapy alone (Wu 2018). Based on this one study, the results suggested an improvement in DFS in the platinum‐based chemotherapy group (HR 0.21, 95% CI 0.05 to 0.97; 1 study, 125 participants).
Overall survival
Ten of the 16 neoadjuvant studies collected data on OS; however, two studies collected data but did not report them (GeparOcto; I‐SPY2). Median follow‐up ranged from 36 to 94.8 months. Platinum chemotherapy reduced mortality (HR 0.69, 95% CI 0.55 to 0.86; P = 0.001, I2 = 29%; 7 studies, 8 treatment comparisons; high‐certainty evidence; Analysis 1.2; Figure 4). A total of 1973 participants were involved in these studies, with an estimated 307 deaths.
1.2. Analysis.

Comparison 1: Neoadjuvant, Outcome 2: Overall survival
4.

One other study collected "all‐cause mortality" and reported no deaths in either group and an HR was not provided or estimable (INFORM). Follow‐up time statistics for these data are unknown.
Pathological complete response
Fifteen trials (16 treatment comparisons) involving only neoadjuvant treatment reported pCR outcome data. Platinum chemotherapy was associated with a large improvement in the rate of pCR (RR 1.44, 95% CI 1.31 to 1.59, P = 0.009, I2 = 52%; 15 studies, 16 treatment comparisons, 3083 participants; high‐certainty evidence; Analysis 1.3.1; Figure 5). One study reported adjusted probabilities of pCR rather than discrete numbers and a sensitivity analysis (removing the adjusted values) gave a very similar result for pCR (RR 1.43, 95% CI 1.30 to 1.58; 3023 participants) (I‐SPY2).
1.3. Analysis.

Comparison 1: Neoadjuvant, Outcome 3: Pathological complete response
5.

One other study that combined neoadjuvant and adjuvant therapy also showed an improvement in tumour response (RR 3.05, 95% CI 1.48 to 6.26; 125 participants) (Wu 2018).
Adjuvant therapy
See Table 2.
Disease‐free survival
All four studies of adjuvant chemotherapy collected and reported DFS with median follow‐up ranging from 52 to 97.6 months. Platinum chemotherapy improved DFS (HR 0.69, 95% CI 0.54 to 0.88; P = 0.003, I2 = 38%; high‐certainty evidence; Analysis 2.1; Figure 3). These studies included 1256 participants, with an estimated 262 DFS events.
2.1. Analysis.

Comparison 2: Adjuvant, Outcome 1: Disease‐free survival
Overall survival
All four studies collected and reported OS with follow‐up ranging from 52 to 97.6 months. Adjuvant platinum chemotherapy extended OS (HR 0.70, 95% CI 0.50 to 0.96; P = 0.03, I2 = 53%; high‐certainty evidence; Analysis 2.2; Figure 4). A total of 1256 participants were included in this analysis, with an estimated 153 deaths.
2.2. Analysis.

Comparison 2: Adjuvant, Outcome 2: Overall survival
All studies
To assess the effect of platinum agents on treatment adherence and toxicity overall, we combined data from neoadjuvant and adjuvant studies. See Table 3.
Completion of regimens
Participants receiving platinum chemotherapy were more than twice as likely to have delay in starting the next cycle of chemotherapy (RR 2.23, 95% CI 1.70 to 2.94; P < 0.001, I2 = 70%; 4 studies, 5 treatment comparisons; moderate‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 1: Participants requiring chemotherapy delays
Participants receiving platinum chemotherapy were also more likely to require dose reductions (RR 1.77, 95% CI 1.56 to 2.02; P < 0.001; I2 = 91%; 7 studies, 8 treatment comparisons; moderate‐certainty evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 2: Participants requiring dose reduction
Participants receiving platinum chemotherapy were 20% more likely to require early cessation of treatment (RR 1.20, 95% CI 1.04 to 1.38; P = 0.01; I2 = 15%; 16 studies, 17 treatment comparisons; high‐certainty evidence; Analysis 3.3; Figure 6). This was not always due to toxicity, as indicated by some studies that provided reasons for early cessation (early cessation due to toxicity: ADAPT‐TN: 45% in intervention group versus 45% in control group; CALGB 40603: 40% in intervention group versus 32% in control group; I‐SPY2: 77% in intervention group versus 50% in control group). Other reasons included progression of disease, withdrawal of consent/refusal of treatment or other/unknown.
3.3. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 3: Early cessation of treatment
6.

Any grade III/IV toxicity
We collected data for grade III/IV haematological toxicity, neuropathy, nausea, renal impairment and treatment‐related death.
Haematological toxicity
Participants receiving platinum‐based chemotherapy were more likely to have grade III/IV neutropenia (RR 1.53, 95% CI 1.43 to 1.63; P < 0.001; I2 = 97%; 19 studies, 20 treatment comparisons; moderate‐certainty evidence; Analysis 3.4). Participants receiving platinum‐based chemotherapy were unlikely to have higher rates of grade III/IV febrile neutropenia (RR 1.16, 95% CI 0.89 to 1.49; P = 0.27, I2 = 69%; 11 studies, 12 treatment comparisons; moderate‐certainty evidence; Analysis 3.5).
3.4. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 4: Neutropenia
3.5. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 5: Febrile neutropenia
For platinum recipients, there were considerably higher risks of anaemia (RR 8.20, 95% CI 5.66 to 11.89; P < 0.001; I2 = 42%; 18 studies, 19 treatment comparisons; moderate‐certainty evidence; Analysis 3.6). There is likely to be a much higher risk of thrombocytopenia in participants receiving platinum chemotherapy (RR 7.59, 95% CI 5.10 to 11.29; P < 0.001, I2 = 44%; 18 studies, 19 treatment comparisons; moderate‐certainty evidence; Analysis 3.7).
3.6. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 6: Anaemia
3.7. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 7: Thrombocytopenia
Non‐haematological toxicity
There is likely little to no difference in rates of grade III/IV neuropathy associated with platinum chemotherapy (RR 1.22, 95% CI 0.95 to 1.57; P = 0.12, I2 = 0; 14 studies, 15 treatment comparisons; moderate‐certainty evidence; Analysis 3.8).
3.8. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 8: Neuropathy
Participants receiving platinum chemotherapy had a higher rate of grade III/IV nausea (RR 1.89, 95% CI 1.30 to 2.74; P < 0.001; I2 = 0; 16 studies, 17 treatment comparisons; high‐certainty evidence; Analysis 3.9).
3.9. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 9: Nausea
Four studies reported data on renal impairment (INFORM; Li 2020; Wu 2018; Zhao 2014). One study reported two events in 60 people in the platinum arm (3%) and no events in 57 people in the non‐platinum arm. None of the other studies reported any grade III/IV renal impairment (Analysis 3.10).
3.10. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 10: Renal impairment
Treatment‐related death
Treatment‐related death was a very rare event, with seven events in 3094 participants. This outcome was not different between platinum and non‐platinum intervention arms (RR 0.58, 95% CI 0.14 to 2.33; P = 0.44, I2 = 0; 10 studies, 11 treatment comparisons; note 8 studies reported treatment‐related deaths but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 3 studies rather than 11; high‐certainty evidence; Analysis 3.11).
3.11. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 11: Treatment‐related death
Quality of life
Although a prespecified outcome of four studies (1198 participants), there were no published quality of life data in the eligible studies available for this review.
Subgroup analysis
Disease‐free survival
BRCA mutation status
Four studies, with 1452 participants, reported DFS outcomes stratified by BRCA mutation status (BrighTNess; GeparSixto; PATTERN; Zheng 2022). There was no evidence of a difference in DFS outcomes based on BRCA mutation status (BRCA wild‐type: HR 0.65, 95% CI 0.50 to 0.85; BRCA mutation: HR 0.72, 95% CI 0.41 to 1.25; P = 0.76; Analysis 4.1). The number of participants in these trials with a known BRCA mutation was small, with 222 pathogenic variant carriers, of whom 118 received platinum.
4.1. Analysis.

Comparison 4: BRCA mutation status, Outcome 1: Disease‐free survival
Homologous recombination deficiency status
One study, with 521 participants, reported DFS according to HRD status, based on a multigene panel including 12 breast cancer homologous recombination repair (HRR) associated susceptibility genes (ATM, ATR, BARD1, BRCA1, BRCA2, BRIP1, CHEK2, FANCM, PALB2, RAD51C, RAD51D and RECQL) (PATTERN). There was no evidence of a difference in outcomes between HRD‐positive and HRD‐negative participants (HRD‐positive: HR 0.39, 95% CI 0.15 to 1.00; HRD negative: HR 0.70, 95% CI 0.42 to 1.15; Analysis 5.1) with no subgroup difference (P = 0.28). As there was a small number of participants with HRD‐positive tumours (120 participants), this analysis may be underpowered.
5.1. Analysis.

Comparison 5: Homologous recombination deficiency (HRD) status, Outcome 1: Disease‐free survival
Lymph node status
Three studies, with 1097 participants, reported DFS according to lymph node status (Li 2020; PATTERN; Zheng 2022). Participants were 29% lymph node‐positive and 71% lymph node negative in this analysis. There was a trend towards benefit for the addition of platinum in both subgroups (lymph node‐positive: HR 0.86, 95% CI 0.54 to 1.37; lymph node‐negative: HR 0.82, 95% CI 0.55 to 1.22; Analysis 6.1); there was no subgroup difference (P = 0.85).
6.1. Analysis.

Comparison 6: Lymph node status, Outcome 1: Disease‐free survival
Type of platinum agent used
Eleven of 12 studies reporting DFS used carboplatin, demonstrating a benefit (HR 0.65, 95% CI 0.57 to 0.75; Analysis 7.1). The remaining study reporting DFS assessed a novel platinum compound, lobaplatin, given both before and after surgery. This study also demonstrated DFS benefit albeit with wide CIs (HR 0.21, 95% CI 0.05 to 0.98).
7.1. Analysis.

Comparison 7: Type of platinum agent, Outcome 1: Disease‐free survival
Same chemotherapy backbone for intervention and control arm
There was a benefit from the addition of platinum whether this was added to an anthracycline/taxane backbone, or as another combination. There was no subgroup difference in DFS benefit between the seven studies with a different backbone (HR 0.62, 95% CI 0.51 to 0.76) and in the five studies with the same backbone (HR 0.67, 95% CI 0.55 to 0.81) with a P value for subgroup difference of 0.63 (Analysis 8.1).
8.1. Analysis.

Comparison 8: Same backbone chemotherapy with or without platinum, Outcome 1: Disease‐free survival
Anthracycline‐free platinum arm
In the 12 studies (13 treatment comparisons) reporting DFS, seven had intervention arms combining platinum chemotherapy with anthracycline chemotherapy (including doxorubicin, epirubicin and non‐pegylated liposomal doxorubicin). Six treatment comparisons had anthracycline‐free platinum intervention arms. Both subgroups had a similar impact on DFS (anthracycline‐free intervention: HR 0.59, 95% CI 0.47 to 0.73; anthracycline‐containing intervention: HR 0.69, 95% CI 0.57 to 0.83; Analysis 9.1); there was little evidence of a subgroup difference (P = 0.27).
9.1. Analysis.

Comparison 9: Anthracycline content of chemotherapy, Outcome 1: Disease‐free survival
Schedule of platinum agent
There was benefit across all schedules: three‐weekly (HR 0.71, 95% CI 0.59 to 0.85; 9 treatment comparisons), two‐weekly (HR 0.31, 95% CI 0.14 to 0.70; 1 study) and weekly groups (HR 0.58, 95% CI 0.45 to 0.74; 3 studies).
Overall survival
BRCA mutation, homologous recombination deficiency, lymph node status
No studies reported OS outcomes stratified for BRCA, HRD or lymph node status.
Type of platinum agent used
All studies reporting OS used carboplatin. Therefore, there were insufficient data to assess OS benefit in agents other than carboplatin.
Same backbone
There was improved OS in the seven studies with a different chemotherapy backbone in the platinum compared to the control arm (HR 0.62, 95% CI 0.47 to 0.81) and in the four studies with the same chemotherapy backbone (HR 0.75, 95% CI 0.57 to 0.99); there was little to no difference between groups (P = 0.85; Analysis 8.2).
8.2. Analysis.

Comparison 8: Same backbone chemotherapy with or without platinum, Outcome 2: Overall survival
Anthracycline‐free platinum arm
Eleven studies reported OS, and of these five had intervention arms adding platinum chemotherapy to anthracycline chemotherapy, and six had anthracycline‐free intervention arms with a platinum‐taxane combination. There was a survival benefit in both subgroups (anthracycline‐free studies: HR 0.57, 95% CI 0.41 to 0.78; 1607 participants; anthracycline‐containing studies: HR 0.77, 95% CI 0.61 to 0.96; 1622 participants); there was no difference between groups (P = 0.14; Analysis 9.2).
9.2. Analysis.

Comparison 9: Anthracycline content of chemotherapy, Outcome 2: Overall survival
Schedule of platinum agent
There was benefit for OS across all treatment schedules: three‐weekly (HR 0.79, 95% CI 0.64 to 0.99; 8 treatment comparisons), two‐weekly (HR 0.14, 95% CI 0.04 to 0.52; 1 study) and weekly groups (HR 0.55, 95% CI 0.39 to 0.78; 3 studies) (Analysis 10.2).
10.2. Analysis.

Comparison 10: Schedule of platinum agent, Outcome 2: Overall survival
Pathological complete response
BRCA mutation status
Five studies, with 1478 participants, were included in this analysis, including four studies (five treatment comparisons) that reported pCR stratified by BRCA mutation status (BrighTNess; GeparOcto; GeparOLA; GeparSixto), and one study that contained only participants with a BRCA mutation (INFORM). There was no evidence of a difference between groups (BRCA wild‐type: RR 1.40, 95% CI 1.21 to 1.63; BRCA mutation: RR 1.11, 95% CI 0.91 to 1.36; P = 0.07; Analysis 4.2).
4.2. Analysis.

Comparison 4: BRCA mutation status, Outcome 2: Pathological complete response
Homologous recombination deficiency status
One study with 104 participants reported rates of pCR according to HRD status (TBCRC 030). There was no evidence of a difference between subgroups (HRD‐positive: RR 0.90, 95% CI 0.28 to 2.84; HRD‐negative: RR 0.25, 95% CI 0.03 to 2.18; Analysis 5.2; P = 0.31).
5.2. Analysis.

Comparison 5: Homologous recombination deficiency (HRD) status, Outcome 2: Pathological complete response
Lymph node status
Two studies, with 721 participants, reported pCR rates according to lymph node status. There was a similar pCR benefit from addition of platinum in both subgroups (lymph node‐positive: RR 1.89, 95% CI 1.31 to 2.73; lymph node‐negative: HR 1.83, 95% CI 1.35 to 2.50; Analysis 6.2) (P = 0.91 for subgroup differences).
6.2. Analysis.

Comparison 6: Lymph node status, Outcome 2: Pathological complete response
Type of platinum agent used
In the pCR analysis, there was a clear benefit in the studies using carboplatin (HR 1.45, 95% CI 1.32 to 1.60; 12 studies, 2801 participants) and lobaplatin (RR 3.05, 95% CI 1.48 to 6.26; 1 study, 125 participants), but not in the studies using cisplatin (RR 1.00, 95% CI 0.58 to 1.75; 2 studies, 222 participants; Analysis 7.3; INFORM; TBCRC 030); there was weak evidence of a subgroup difference (P = 0.07). This supports the DFS and OS findings in that the clearest evidence for benefit in this meta‐analysis is for carboplatin, with a single study supporting the use of lobaplatin.
7.3. Analysis.

Comparison 7: Type of platinum agent, Outcome 3: Pathological complete response
Same backbone
There was weak evidence of a difference in pCR benefit between the subgroup of nine studies with a different backbone (RR 1.35, 95% CI 1.18 to 1.53; 1473 people) and in the subgroup of six studies with the same backbone (RR 1.59, 95% CI 1.38 to 1.84; 1675 people) with a P value for subgroup differences of 0.09 (Analysis 8.3).
8.3. Analysis.

Comparison 8: Same backbone chemotherapy with or without platinum, Outcome 3: Pathological complete response
Anthracycline‐free platinum arm
Outcomes for pCR were similar amongst the 10 treatment comparisons with an anthracycline‐containing intervention (RR 1.44, 95% CI 1.29 to 1.61), and the six studies with anthracycline‐free intervention (RR 1.53, 95% CI 1.24 to 1.89) with a P value for subgroup differences of 0.61 (Analysis 9.3).
9.3. Analysis.

Comparison 9: Anthracycline content of chemotherapy, Outcome 3: Pathological complete response
Schedule of platinum agent
There was an increased likelihood of pCR across the three‐weekly (11 treatment comparisons, RR 1.61, 95% CI 1.38 to 1.87) and weekly groups (5 studies, RR 1.34, 95% CI 1.19 to 1.52) in Analysis 10.3, with minimal evidence of subgroup differences (P = 0.07).
10.3. Analysis.

Comparison 10: Schedule of platinum agent, Outcome 3: Pathological complete response
Sensitivity analyses
Hormone receptor immunohistochemistry cut‐off other than less than 1%
The ideal hormone receptor IHC cut‐off varied between studies on this topic. Although less than 1% was the most commonly used definition, other cut‐offs included less than 5% and less than 10%.
When stratified by hormone receptor IHC cut‐off, improved DFS was similar in studies that defined TNBC by less than 1% hormone receptor staining (HR 0.60, 95% CI 0.50 to 0.71; ADAPT‐TN; BrighTNess; GeparSixto; Li 2020; NeoCART; PATTERN; Zheng 2022) compared to studies in this review where the cut‐off was less than 10% or not stated (HR 0.76, 95% CI 0.59 to 0.98; less than 10%: Ando 2014; CALGB 40603; Wu 2018; Zhang 2016; not stated: Nasr 2015). There was no difference between subgroups (P = 0.11).
There was improvement in OS in studies that defined TNBC by less than 1% hormone receptor staining (HR 0.62, 95% CI 0.49 to 0.79; 2468 participants; ADAPT‐TN; BrighTNess; GeparSixto; Li 2020; NeoCART; PATTERN; Zheng 2022), and in the group of studies where the cut‐off was less than 10% or not stated (HR 0.81, 95% CI 0.60 to 1.07; 761 participants; less than 10%: Ando 2014; CALGB 40603; Zhang 2016; not stated: Nasr 2015). There was no difference between subgroups (P = 0.18).
An association between platinum chemotherapy and likelihood of pCR was similar in the less than 1% cut‐off subgroup (RR 1.42, 95% CI 1.26 to 1.58; ADAPT‐TN; BrighTNess; GeparOcto; GeparOLA; GeparSixto; NeoCART), and the subgroup of studies with a cut‐off of less than 5%, 10% or not stated (RR of 1.57, 95% CI 1.30 to 1.90; less than 5%: TBCRC 030, less than 10%: Ando 2014; CALGB 40603; Wu 2018; Zhang 2016; not stated: GEICAM 2006‐03; Gigolaeva 2019; Zhao 2014). There was no difference between subgroups (P = 0.35).
Potentially confounding treatments in intervention arm
Multiple studies tested interventions combined with platinum chemotherapy. These treatments were viewed as potentially confounding in this sensitivity analysis. However, notably, all of these extra treatments have subsequently been found to be ineffective (Banys‐Paluchowski 2017; BrighTNess; Shepherd 2022). The studies of note included Nasr 2015, which provided the intervention group with a further 12 months of metronomic chemotherapy; BrighTNess comparison 1, which included veliparib; and CALGB 40603, which provided one group with bevacizumab.
The sensitivity analysis for outcome measures revealed the following.
The DFS effect was similar when studies with potentially confounding extra treatments were removed (HR 0.60, 95% CI 0.50 to 0.71). This was done by removing Nasr 2015 and BrighTNess comparison 1, and using only the bevacizumab‐free arm of CALGB‐40603.
This OS effect was similar when two studies with potentially confounding extra treatments (Nasr 2015; BrighTNess comparison 1) were removed (HR 0.64, 95% CI 0.52 to 0.79).
The likelihood of pCR was similar when one study with potentially confounding extra treatment (BrighTNess comparison 1) was removed (HR 1.49, 95% CI 1.35 to 1.63).
High or unclear risk of bias
All studies reporting DFS or OS had an overall low risk of bias so a sensitivity analysis was not performed for these outcomes.
For pCR, the effect of removing two studies with most or all domains at unclear risk (Zhao 2014; Gigolaeva 2019) was minimal on the likelihood of pCR (RR 1.44, 95% CI 1.30 to 1.59).
Outcomes with considerably heterogeneity
We applied a random‐effects model to the two outcomes with considerable heterogeneity (i.e. an I2 value of 75% to 100%). Both of these analyses resulted in a similar magnitude RR, but with wider CIs.
Participants requiring a dose reduction: RR 2.18, 95% CI 1.08 to 4.41 compared to RR 1.77, 95% CI 1.56 to 2.02 with a fixed‐effect model.
Neutropenia: RR 1.68, 95% CI 1.12 to 2.52 compared to RR 1.53, 95% CI 1.43 to 1.63 with a fixed‐effect model.
Discussion
Summary of main results
Platinum‐based chemotherapy using carboplatin in the adjuvant or neoadjuvant setting improved long‐term outcomes of DFS and OS in early TNBC, regardless of the examined subgroups. This was at the cost of more frequent chemotherapy delays and dose reductions, and greater haematological toxicity as presented in Table 3. There was benefit from platinum when platinum agents were added to both anthracycline‐containing regimens and in anthracycline‐free regimens.
Though there are certainly increased haematological toxicities associated with platinum chemotherapy, permanent toxicity such as grade III/IV neuropathy and treatment‐related death were not different between groups. These trials did not report important quality‐of‐life measures.
Attempts in this review to refine subgroups of triple‐negative biology, such as those with BRCA mutations or altered HRD status, have higher benefit from platinum therapy found no predictive role. The certainty of this evidence was low since numbers were low. Only one study assessed the role of HRD status on efficacy outcomes in our analysis. It remains unclear if more modern and focused HRD testing may offer better biomarkers for participants who will benefit from platinum chemotherapy. We were also unable to identify if there may be a subgroup of participants who might not benefit, and for whom de‐escalation therapy might be appropriate.
Overall completeness and applicability of evidence
These results were generally applicable to people with early TNBC, allowing for the trial to define hormone receptor cut‐offs which ranged from 1% to 10%. The range of ages captured in these trials was from 19 to 82 years. Outcomes based on age were not available. Information on participant gender was not collected.
While racial background of participants was not captured in our analysis, these trials took place in several countries in Europe, Asia and the US. Black and African participants are likely to be a notable ethnic gap in this meta‐analysis given the dearth of trials occurring on the African continent and the low participation rates of Black Americans in cancer clinical trials (Awidi 2021).
Recruitment of the included trials started between six and 16 years ago, and as such the standard therapy arms may not reflect current international standards. This is a shifting target, and the advent of new treatments used in early TNBC such as immunotherapy and PARP inhibitors, as well as the clinical heterogeneity of chemotherapy used in these studies, means the best regimen and timing of platinum chemotherapy remains unclear. This review also does not provide insight into the use of post‐neoadjuvant capecitabine, which is currently part of standard care for people with residual disease after neoadjuvant chemotherapy for TNBC. ECOG‐ACRIN EA1131 assessed whether platinum chemotherapy can replace capecitabine, but this study was excluded from this review given the study population included only those with residual disease. Further research into this area is warranted, particularly given the increasing number of drugs used in TNBC and increasing interest in biomarker‐directed treatment rationalisation.
Many of the studies examining neoadjuvant chemotherapy only report pCR, which is a surrogate marker for long‐term benefit. Several studies did not report on certain important toxicity outcomes such as febrile neutropenia, renal impairment and treatment‐related death. The number of participants in subgroups of BRCA mutation status, HRD status and lymph node status were relatively small and may not have provided sufficient power to sufficiently address whether there are different outcomes for these groups. Recent data suggest that a DFS and OS benefit only exists for people aged less than 50 years (Gupta 2022); however, we did not include age as a subgroup analysis, neither were there outcome data reported stratified based on age in the studies analysed.
No quality of life outcomes were reported. This is an important measure particularly when assessing outcomes which are more accurately reported by participants, such as fatigue and effects on cognition. As such, we may be missing important impacts of the addition of platinum chemotherapy on participants of these clinical trials both acutely and in the longer term.
Use of platinum chemotherapy is variable, and at the time of writing is still not routinely recommended in NCCN or European Society for Medical Oncology guidelines. A lack of DFS and OS benefit is often cited as a reservation to the routine use of platinum chemotherapy. This review presents relevant, adequately powered outcome data to support the use of platinum chemotherapy in early TNBC, acknowledging the increased rate of haematological toxicity.
Quality of the evidence
This systematic review provides evidence from 20 studies, with 4468 participants, and provides high‐certainty evidence supporting the addition of platinum chemotherapy in the neoadjuvant and adjuvant settings with an increase in DFS and OS.
An important methodological limitation in this meta‐analysis is that all but one of the included trials adopted an open‐label design. It is considered that the efficacy outcomes of DFS, OS and pCR are unlikely to be affected by a lack of blinding. However, the risk of bias may be increased for more subjective toxicity assessments such as nausea and neuropathy.
Clinical heterogeneity was present in the chemotherapy regimens, and in the type, timing and duration of the platinum agent. Some trials used platinum in addition to an existing regimen (e.g. adding carboplatin to doxorubicin‐cyclophosphamide followed by paclitaxel), while others used platinum in place of other agents (e.g. comparing carboplatin‐docetaxel to doxorubicin‐cyclophosphamide followed by paclitaxel) or used as a single agent. In other trials, extra treatments such as veliparib, bevacizumab and metronomic chemotherapy were also added.
Despite the clinical heterogeneity, this meta‐analysis demonstrated generally high‐certainty, consistent evidence for the primary outcome measures of DFS and OS. However, there was a significant degree of heterogeneity in many of the treatment delivery and toxicity outcome categories, including chemotherapy delays, dose reductions, neutropenia and febrile neutropenia. This resulted in downgrading of the certainty of evidence, as demonstrated in Table 3.
Potential biases in the review process
Using standard Cochrane search methods, we identified numerous trial records. Our final 20 trials were consistent with previous reviews and studies cited in local and international guidelines. There is a high likelihood that all relevant trials were identified.
Unfortunately, not all relevant data could be obtained. Notably, multiple studies listed outcomes including DFS, OS and quality of life which were never published. The lack of DFS and OS data reported in particular for cisplatin could indicate publication bias, given its failure to produce a benefit in pCR.
In this review, we included studies with intervention treatments in addition to carboplatin, such as veliparib, bevacizumab and metronomic chemotherapy. While these trials were potentially confounding, a sensitivity analysis showed that removing these trials had a minimal impact on the pooled outcome analysis.
Several studies recruited subgroups other than TNBC. These trials were only included if outcome measures were reported separately or only included less than 20% of participants with non‐TNBC. However, treatment completion and toxicity information were not always reported for each breast cancer subtype. As such, we presented these measures for the whole group who were receiving the same treatment, which may be a potential source of bias.
Agreements and disagreements with other studies or reviews
Early meta‐analyses of randomised, retrospective and prospective studies by Petrelli 2014, Poggio 2018, Wang 2017, and Pandy 2019 showed an improvement in pCR associated with the use of platinum chemotherapy. None of the listed studies that examined DFS or OS were able to demonstrate a difference from the addition of platinum, likely due to a lack of reporting of these outcome measures and immature follow‐up.
More recently, Saleh 2021 performed a meta‐analysis of 14 randomised or retrospective trials of platinum‐based perioperative chemotherapy. They found platinum chemotherapy was associated with an improvement in DFS but not OS. The inclusion of retrospective trials may have been a confounder in this analysis.
Our findings have aligned with those of Bian 2021, who found in seven randomised controlled trials that platinum‐based perioperative chemotherapy improved both DFS and OS for people with early TNBC. They explored subgroups including the setting of chemotherapy (adjuvant versus neoadjuvant) and lymph node status. This was the first meta‐analysis to demonstrate an OS benefit. Our review builds on the findings of this study, including more trials and adding additional subgroup analyses including BRCA, HRD, hormone receptor IHC % positive thresholds, chemotherapy content and timing.
Authors' conclusions
Implications for practice.
This review provides high‐certainty evidence that platinum‐based chemotherapy with carboplatin is associated with improved disease‐free survival (DFS), overall survival (OS) and pathological complete response in early triple‐negative breast cancer (TNBC). This is at the cost of increased grade III/IV haematological toxicity, though serious adverse events including febrile neutropenia or treatment‐related death were not increased.
These findings support the use of carboplatin, but not cisplatin, for people with early TNBC. The optimal dose and regimen are not defined by this analysis, but there is a suggestion that similar relative benefits result from the addition of carboplatin to either anthracycline‐free regimens or those containing anthracycline agents. Additionally, our analysis supports a broad rather than focused use of carboplatin based on the benefit seen across the examined subgroups.
Implications for research.
We examined a single trial using lobaplatin, a novel platinum agent which is reported to have a favourable toxicity profile in other cancers (Perabo 2007). Further testing of other platinum compounds is justified if it may alleviate some of the additive toxicity associated with combination chemotherapy, such as the haematological toxicity reported in this review.
In this review, we did not identify a subgroup of TNBC which may derive greater benefit from platinum chemotherapy. Refining groups who may benefit most would be helpful to guide treatment selection. It is currently unclear if emerging biomarkers including enhanced homologous recombination deficiency testing might be helpful in future for participant selection.
Incremental improvements in DFS and OS are being provided by new anticancer agents in the setting of early disease, including immunotherapy (Schmid 2020) and poly(adenosine diphosphate‐ribose) polymerase (PARP) inhibitors (Tutt 2021). As we add new agents, we must also consider rationalising and de‐escalating treatment for certain participants at lower risk of recurrence or who have a favourable treatment response. The use of anthracycline‐free regimens is of increasing interest (Nitz 2019; Yu 2021) given these agents are implicated in long‐term cardiotoxicity and secondary leukaemia. Further research into the optimal regimen is warranted.
Finally, this review has highlighted the need for ensuring reporting of the quality of life data collected in trials involving early breast cancer. The value of patient‐reported outcome measures is being increasingly recognised, and whilst trials are collecting data, they are not always published in a timely manner. Consideration of these outcomes from clinical trials is essential for ensuring person‐centred clinical interventions to assess objective disease control as well as more subjective health and well‐being.
History
Protocol first published: Issue 5, 2021
Acknowledgements
The following people conducted the editorial process for this review:
Sign‐off Editor (final editorial decision): Nicholas Wilcken, Cochrane Breast Cancer;
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Ava Grace Tan‐Koay, Cochrane Breast Cancer;
Copy Editor (copy editing and production): Anne Lawson, Cochrane Central Production Service;
Peer‐reviewers (provided comments and recommended an editorial decision): Dr Andrew Redfern, University of Western Australia (clinical/content review), Sara Whiting (consumer review), Ram Bajpai, Keele University (methods review).
We would like to thank the reviewers for their helpful comments on the protocol: Rebecca Seago‐Coyle (Consumer Editor), Bonner Cutting (Alamo Breast Cancer Foundation and Komen Advocate‐in‐Science; Consumer Reviewer), Alessandra Gennari (MD, PhD, University of Eastern Piedmont, Novara, Italy; Clinical Editor) and the external Clinical Peer‐reviewer who wishes to remain anonymous. The Cochrane Methods Support Unit provided methodological feedback on the protocol. We would also like to thank Alexis Lai, who translated the article by Zhao and colleagues, providing results and information related to risk of bias, and Yuan Chi who reviewed two articles published in Chinese journals to advise whether the studies met inclusion criteria.
Appendices
Appendix 1. CENTRAL
#1 MeSH descriptor: [Breast Neoplasms] explode all trees #2 triple negative breast near neoplasm* #3 triple negative breast near carcinoma* #4 triple negative breast near cancer* #5 triple negative breast near tumour* #6 triple negative breast near tumor* #7 #1 or #2 or #3 or #4 or #5 or #6 #8 (locally advanced near breast neoplasm*):ti #9 (locally advanced near breast carcinoma*):ti #10 (locally advanced near breast cancer*):ti #11 (locally advanced near breast tumour*):ti #12 (locally advanced near breast tumor*):ti #13 (metasta* near breast neoplasm*):ti #14 (metasta* near breast carcinoma*):ti #15 (metasta* near breast cancer*):ti #16 (metasta* near breast tumour*):ti #17 (metasta* near breast tumor*):ti #18 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 #19 #7 not #18 #20 platinum or cisplatin or cisplatinum or Oxaliplatin or Carboplatin or lobaplatin or nedaplatin or eptaplatin or miboplatin or sebriplatin #21 MeSH descriptor: [Platinum] explode all trees #22 MeSH descriptor: [Cisplatin] explode all trees #23 MeSH descriptor: [Platinum Compounds] explode all trees #24 MeSH descriptor: [Carboplatin] explode all trees #25 MeSH descriptor: [Oxaliplatin] explode all trees #26 #20 or #21 or #22 or #23 or #24 or #25 #27 #19 and #26 in Trials
Appendix 2. MEDLINE
| # | Searches |
| 1 | exp Breast Neoplasms/ |
| 2 | exp Triple Negative Breast Neoplasms/ |
| 3 | Triple Negative Breast cancer$.tw. |
| 4 | Triple Negative Breast neoplasm$.tw. |
| 5 | Triple Negative Breast carcinoma$.tw. |
| 6 | Triple Negative Breast tumo?r$.tw. |
| 7 | or/1‐6 |
| 8 | (local$ adj6 advance$ adj6 (breast adj6 (neoplasm$ or cancer$ or carcinoma$ or tumo?r$))).ti. |
| 9 | (metasta$ adj6 (breast adj6 (neoplasm$ or cancer$ or carcinoma$ or tumo?r$))).ti. |
| 10 | or/8‐9 |
| 11 | 7 not 10 |
| 12 | exp Cisplatin/ |
| 13 | exp Carboplatin/ |
| 14 | exp Organoplatinum Compounds/ |
| 15 | exp Platinum/ |
| 16 | exp Platinum Compounds/ |
| 17 | (cisplatinum or cisplat* or cisplatin).tw. |
| 18 | (carboplatinum or carboplat* or carboplatin).tw. |
| 19 | (platinum or platin*).tw. |
| 20 | platinum compound*.tw. |
| 21 | (platinum‐containing regime* or platinum containing regime*).tw. |
| 22 | (platinum‐based agent* or platinum based agent*).tw. |
| 23 | (Platinol or Platinol‐ AQ or CDDP or CACP or platidiam or platinum diamminodichloride or cis‐diamminedichloroplatinum or cis‐dichlorodiammineplatinum or biocisplatinum or dichlorodiammineplatinum or nsc‐119875 or nsc 119875 or cis‐platinum or Abiplatin or AI3‐62048 or AI3 62048 or Briplatin or CCRIS 221 or Cismaplat or Cisplatine or Cisplatyl or Neoplatin).tw. |
| 24 | (carboplatine or CCRIS 3404 or EINECS 255‐446‐0 or "EINECS 255 446 0" or HSDB 6957 or cbdca or jm‐8 or jm8 or nsc‐241240 or nsc 241240 or paraplatin or Paraplatin‐ AQ or NSC 201345 or NSC‐201345 or cis‐diammine cyclobutanedicarboxylato platinum).tw. |
| 25 | (lobaplatin or lobaplatinum or lobaplat* or D‐19466 or D 19466).tw. |
| 26 | (Nedaplatin or Aqupla or CCRIS 4088 or NSC 375101D or NSC‐375101D or cis‐Diammine glycolato platinum).tw. |
| 27 | (Heptaplatin or Eptaplatin or NSC D644591 or NSC‐D644591).tw. |
| 28 | (Oxaliplatin or oxalapatin or 1‐OHP or CCRIS 9143 or Dacplat or Eloxatin or Elplat or JM‐83 or JM83 or l‐OHP or Lipoxal or NSC 266046 or NSC‐266046 or Oxalatoplatin or Oxalatoplatinum or Oxaliplatin or Oxaliplatino or Oxaliplatinum or Oxalitin or Oxaloplatine or Oxaloplatino or RP‐54780 or RP54780 or SR‐96669 or SR 96669).tw. |
| 29 | (miboplatin or CCRIS 5235 or DWA 2114R).tw. |
| 30 | (sebriplatin or NK 121).tw. |
| 31 | or/12‐30 |
| 32 | 11 and 31 |
| 33 | animals/ not humans/ |
| 34 | 32 not 33 |
| 35 | randomized controlled trial.pt. |
| 36 | controlled clinical trial.pt. |
| 37 | randomized.ab. |
| 38 | placebo.ab. |
| 39 | Clinical Trials as Topic/ |
| 40 | randomly.ab. |
| 41 | trial.ti. |
| 42 | (crossover or cross‐over).tw. |
| 43 | Pragmatic Clinical Trials as Topic/ |
| 44 | pragmatic clinical trial.pt. |
| 45 | or/35‐44 |
| 46 | 34 and 45 |
| 47 | remove duplicates from 46 |
Appendix 3. Embase
| # | Searches |
| 1 | exp breast cancer/ |
| 2 | exp triple negative breast cancer/ |
| 3 | Triple Negative Breast cancer$.tw. |
| 4 | Triple Negative Breast neoplasm$.tw. |
| 5 | Triple Negative Breast carcinoma$.tw. |
| 6 | Triple Negative Breast tumo?r$.tw. |
| 7 | or/1‐6 |
| 8 | (local$ adj6 advance$ adj6 (breast adj6 (neoplasm$ or cancer$ or carcinoma$ or tumo?r$))).ti. |
| 9 | (metasta$ adj6 (breast adj6 (neoplasm$ or cancer$ or carcinoma$ or tumo?r$))).ti. |
| 10 | or/8‐9 |
| 11 | 7 not 10 |
| 12 | exp cisplatin/ |
| 13 | exp cisplatin derivative/ |
| 14 | exp carboplatin/ |
| 15 | exp platinum complex/ |
| 16 | exp platinum/ |
| 17 | exp platinum derivative/ |
| 18 | exp platinum 1,2 diaminocyclohexane bisneodecanoate/ |
| 19 | exp lobaplatin/ |
| 20 | exp nedaplatin/ |
| 21 | exp eptaplatin/ |
| 22 | exp oxaliplatin/ |
| 23 | exp miboplatin/ |
| 24 | exp sebriplatin/ |
| 25 | (cisplatinum or cisplat* or cisplatin).tw. |
| 26 | (carboplatinum or carboplat* or carboplatin).tw. |
| 27 | (platinum or platin*).tw. |
| 28 | platinum compound*.tw. |
| 29 | (platinum‐containing regime* or platinum containing regime*).tw. |
| 30 | (platinum‐based agent* or platinum based agent*).tw. |
| 31 | (Platinol or Platinol‐ AQ or CDDP or CACP or platidiam or platinum diamminodichloride or cis‐diamminedichloroplatinum or cis‐dichlorodiammineplatinum or biocisplatinum or dichlorodiammineplatinum or nsc‐119875 or nsc 119875 or cis‐platinum or Abiplatin or AI3‐62048 or AI3 62048 or Briplatin or CCRIS 221 or Cismaplat or Cisplatine or Cisplatyl or Neoplatin).tw. |
| 32 | (carboplatine or CCRIS 3404 or EINECS 255‐446‐0 or "EINECS 255 446 0" or HSDB 6957 or cbdca or jm‐8 or jm8 or nsc‐241240 or nsc 241240 or paraplatin or Paraplatin‐ AQ or NSC 201345 or NSC‐201345 or cis‐diammine cyclobutanedicarboxylato platinum).tw. |
| 33 | (lobaplatin or lobaplatinum or lobaplat* or D‐19466 or D 19466).tw. |
| 34 | (Nedaplatin or Aqupla or CCRIS 4088 or NSC 375101D or NSC‐375101D or cis‐Diammine glycolato platinum).tw. |
| 35 | (Heptaplatin or Eptaplatin or NSC D644591 or NSC‐D644591).tw. |
| 36 | (Oxaliplatin or oxalapatin or 1‐OHP pr CCRIS 9143 or Dacplat or Eloxatin or Elplat or JM‐83 or JM83 or l‐OHP or Lipoxal or NSC 266046 or NSC‐266046 or Oxalatoplatin or Oxalatoplatinum or Oxaliplatin or Oxaliplatino or Oxaliplatinum or Oxalitin or Oxaloplatine or Oxaloplatino or RP‐54780 or RP54780 or SR‐96669 or SR 96669).tw. |
| 37 | (miboplatin or CCRIS 5235 or DWA 2114R).tw. |
| 38 | (sebriplatin or NK 121).tw. |
| 39 | or/12‐38 |
| 40 | Randomized controlled trial/ |
| 41 | Controlled clinical study/ |
| 42 | Random$.ti,ab. |
| 43 | randomization/ |
| 44 | intermethod comparison/ |
| 45 | placebo.ti,ab. |
| 46 | (compare or compared or comparison).ti. |
| 47 | (open adj label).ti,ab. |
| 48 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. |
| 49 | double blind procedure/ |
| 50 | parallel group$1.ti,ab. |
| 51 | (crossover or cross over).ti,ab. |
| 52 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. |
| 53 | (assigned or allocated).ti,ab. |
| 54 | (controlled adj7 (study or design or trial)).ti,ab. |
| 55 | (volunteer or volunteers).ti,ab. |
| 56 | trial.ti. |
| 57 | or/40‐56 |
| 58 | 11 and 39 and 57 |
| 59 | remove duplicates from 58 |
| 60 | limit 59 to (human and (conference abstracts or embase)) |
Appendix 4. WHO ICTRP
Basic Search:
Triple negative breast cancer AND platinum
Advanced Searches:
1. Condition: triple negative breast cancer OR triple negative breast neoplasm
Intervention: platinum
Recruitment status: All
2. Condition: triple negative breast cancer OR triple negative breast neoplasm
Intervention: platinum‐containing regime OR platinum compound OR platinum OR cisplatin OR carboplatin OR platin OR cisplatinum OR carboplatinum OR carboplatine OR cisplatine OR lobaplatin OR nedaplatin OR heptaplatin OR oxaliplatin OR oxalapatin OR miboplatin
Recruitment status: All
3. Condition: triple negative breast cancer OR triple negative breast neoplasm
Intervention: sebriplatin OR platinol OR platinol‐ AQ OR CDDP OR CACP OR platidiam OR platinum diamminodichloride OR cis‐diamminedichloroplatinum OR cis‐dichlorodiammineplatinum OR biocisplatinum OR dichlorodiammineplatinum OR nsc‐119875
Recruitment status: All
4. Condition: triple negative breast cancer OR triple negative breast neoplasm
Intervention:abiplatin OR AI3‐62048 OR AI3 62048 OR briplatin OR CCRIS 221 OR cismaplat OR cisplatyl OR neoplatin OR CCRIS 3404 OR EINECS 255‐446‐0 OR HSDB 6957 OR cbdca OR jm‐8 OR nsc‐241240 OR paraplatin OR paraplatin‐ AQ OR NSC 201345
Recruitment status: All
5. Condition: triple negative breast cancer OR triple negative breast neoplasm
Intervention: cis‐diammine cyclobutanedicarboxylato platinum OR lobaplatinum OR D‐19466 OR Aqupla OR CCRIS 4088 OR NSC 375101D OR cis‐Diammine glycolato platinum OR Eptaplatin OR NSC D644591 OR CCRIS 9143 OR Dacplat OR Eloxatin OR Elplat OR JM‐83 OR JM83 OR l‐OHP
Recruitment status:All
6. Condition: triple negative breast cancer OR triple negative breast neoplasm
Intervention: Lipoxal OR NSC 266046 OR Oxalatoplatin OR Oxalatoplatinum OR Oxaliplatino OR Oxaliplatinum OR Oxalitin OR Oxaloplatine OR Oxaloplatino OR RP‐54780 OR SR‐96669 OR CCRIS 5235 OR DWA 2114R OR NK 121
Recruitment status: All
Appendix 5. ClinicalTrials.gov
Basic search:
Condition or disease: Triple negative breast cancer
Other terms: platinum
Advanced searches:
1. Condition or disease: Triple negative breast cancer OR triple negative breast neoplasm
Intervention/treatment: platinum‐containing regime OR platinum compound OR platinum OR cisplatin OR carboplatin OR platin OR cisplatinum OR carboplatinum OR carboplatine OR cisplatine OR lobaplatin OR nedaplatin OR heptaplatin OR oxaliplatin OR oxalapatin OR miboplatin
Study type: All studies
2. Condition or disease: Triple negative breast cancer OR triple negative breast neoplasm
Intervention/treatment: sebriplatin OR platinol OR platinol‐ AQ OR CDDP OR CACP OR platidiam OR platinum diamminodichloride OR cis‐diamminedichloroplatinum OR cis‐dichlorodiammineplatinum OR biocisplatinum OR dichlorodiammineplatinum OR nsc‐119875
Study type: All studies
3. Condition or disease: Triple negative breast cancer OR triple negative breast neoplasm
Intervention/treatment: abiplatin OR AI3‐62048 OR AI3 62048 OR briplatin OR CCRIS 221 OR cismaplat OR cisplatyl OR neoplatin OR CCRIS 3404 OR EINECS 255‐446‐0 OR HSDB 6957 OR cbdca OR jm‐8 OR nsc‐241240 OR paraplatin OR paraplatin‐ AQ OR NSC 201345
Study type: All studies
4. Condition or disease: Triple negative breast cancer OR triple negative breast neoplasm
Intervention/treatment: cis‐diammine cyclobutanedicarboxylato platinum OR lobaplatinum OR D‐19466 OR Aqupla OR CCRIS 4088 OR NSC 375101D OR cis‐Diammine glycolato platinum OR Eptaplatin OR NSC D644591 OR CCRIS 9143 OR Dacplat OR Eloxatin OR Elplat OR JM‐83 OR JM83 OR l‐OHP
Study type: All studies
5. Condition or disease: Triple negative breast cancer OR triple negative breast neoplasm
Intervention/treatment: Lipoxal OR NSC 266046 OR Oxalatoplatin OR Oxalatoplatinum OR Oxaliplatino OR Oxaliplatinum OR Oxalitin OR Oxaloplatine OR Oxaloplatino OR RP‐54780 OR SR‐96669 OR CCRIS 5235 OR DWA 2114R OR NK 121
Study type: All studies
Data and analyses
Comparison 1. Neoadjuvant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Disease‐free survival | 9 | Hazard Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1.1 Neoadjuvant | 8 | 1966 | Hazard Ratio (IV, Fixed, 95% CI) | 0.63 [0.53, 0.75] |
| 1.1.2 Includes adjuvant and neoadjuvant therapy | 1 | 125 | Hazard Ratio (IV, Fixed, 95% CI) | 0.21 [0.05, 0.97] |
| 1.2 Overall survival | 8 | 1973 | Hazard Ratio (IV, Fixed, 95% CI) | 0.69 [0.55, 0.86] |
| 1.3 Pathological complete response | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.3.1 Neoadjuvant | 16 | 3083 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.31, 1.59] |
| 1.3.2 Includes adjuvant and neoadjuvant therapy | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [1.48, 6.26] |
| 1.3.3 Neoadjuvant all | 17 | 3208 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.34, 1.62] |
Comparison 2. Adjuvant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Disease‐free survival | 5 | Hazard Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1.1 Adjuvant | 4 | 1256 | Hazard Ratio (IV, Fixed, 95% CI) | 0.69 [0.54, 0.88] |
| 2.1.2 Includes adjuvant and neoadjuvant therapy | 1 | 125 | Hazard Ratio (IV, Fixed, 95% CI) | 0.21 [0.05, 0.97] |
| 2.2 Overall survival | 4 | 1256 | Hazard Ratio (IV, Fixed, 95% CI) | 0.70 [0.50, 0.96] |
| 2.3 Disease‐free survival without Wu 2018 | 4 | Hazard Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.3.1 Adjuvant | 4 | 1256 | Hazard Ratio (IV, Fixed, 95% CI) | 0.69 [0.54, 0.88] |
2.3. Analysis.

Comparison 2: Adjuvant, Outcome 3: Disease‐free survival without Wu 2018
Comparison 3. All studies: treatment adherence and toxicities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Participants requiring chemotherapy delays | 5 | 1053 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.70, 2.94] |
| 3.2 Participants requiring dose reduction | 8 | 2055 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.56, 2.02] |
| 3.3 Early cessation of treatment | 17 | 4178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.04, 1.38] |
| 3.4 Neutropenia | 20 | 4849 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.43, 1.63] |
| 3.5 Febrile neutropenia | 12 | 3771 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.89, 1.49] |
| 3.6 Anaemia | 19 | 4757 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.20 [5.66, 11.89] |
| 3.7 Thrombocytopenia | 19 | 4731 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.59 [5.10, 11.29] |
| 3.8 Neuropathy | 15 | 4312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.95, 1.57] |
| 3.9 Nausea | 17 | 4228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.30, 2.74] |
| 3.10 Renal impairment | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.11 Treatment‐related death | 11 | 3176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.14, 2.33] |
| 3.12 Participants requiring dose reduction – random effects | 8 | 2055 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [1.08, 4.41] |
| 3.13 Neutropenia – random effects | 20 | 4849 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [1.12, 2.52] |
3.12. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 12: Participants requiring dose reduction – random effects
3.13. Analysis.

Comparison 3: All studies: treatment adherence and toxicities, Outcome 13: Neutropenia – random effects
Comparison 4. BRCA mutation status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Disease‐free survival | 4 | 1452 | Hazard Ratio (IV, Fixed, 95% CI) | 0.66 [0.52, 0.84] |
| 4.1.1 BRCA wildtype | 4 | 1230 | Hazard Ratio (IV, Fixed, 95% CI) | 0.65 [0.50, 0.85] |
| 4.1.2 BRCA mutation | 4 | 222 | Hazard Ratio (IV, Fixed, 95% CI) | 0.72 [0.41, 1.25] |
| 4.2 Pathological complete response | 6 | 1478 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.17, 1.49] |
| 4.2.1 BRCA wildtype | 5 | 1145 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.21, 1.63] |
| 4.2.2 BRCA mutation | 6 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.91, 1.36] |
Comparison 5. Homologous recombination deficiency (HRD) status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Disease‐free survival | 1 | Hazard Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 HRD positive | 1 | 120 | Hazard Ratio (IV, Fixed, 95% CI) | 0.39 [0.15, 1.00] |
| 5.1.2 HRD negative | 1 | 401 | Hazard Ratio (IV, Fixed, 95% CI) | 0.70 [0.42, 1.15] |
| 5.2 Pathological complete response | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.24, 1.72] |
| 5.2.1 Homologous recombination deficiency (HRD)+ | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.28, 2.84] |
| 5.2.2 HRD− | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.18] |
Comparison 6. Lymph node status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Disease‐free survival | 3 | 1097 | Hazard Ratio (IV, Fixed, 95% CI) | 0.84 [0.62, 1.13] |
| 6.1.1 Lymph node positive | 3 | 323 | Hazard Ratio (IV, Fixed, 95% CI) | 0.86 [0.54, 1.37] |
| 6.1.2 Lymph node negative | 3 | 774 | Hazard Ratio (IV, Fixed, 95% CI) | 0.82 [0.55, 1.22] |
| 6.2 Pathological complete response | 3 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.47, 2.35] |
| 6.2.1 Lymph node positive | 3 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.31, 2.73] |
| 6.2.2 Lymph node negative | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.35, 2.50] |
Comparison 7. Type of platinum agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Disease‐free survival | 13 | 3347 | Hazard Ratio (IV, Fixed, 95% CI) | 0.65 [0.56, 0.74] |
| 7.1.1 Carboplatin | 12 | 3222 | Hazard Ratio (IV, Fixed, 95% CI) | 0.65 [0.57, 0.75] |
| 7.1.2 Lobaplatin | 1 | 125 | Hazard Ratio (IV, Fixed, 95% CI) | 0.21 [0.05, 0.98] |
| 7.2 Overall survival | 12 | Hazard Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 7.2.1 Carboplatin | 12 | 3229 | Hazard Ratio (IV, Fixed, 95% CI) | 0.69 [0.58, 0.83] |
| 7.3 Pathological complete response | 16 | 3148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.33, 1.61] |
| 7.3.1 Carboplatin | 13 | 2801 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.32, 1.60] |
| 7.3.2 Cisplatin | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.58, 1.75] |
| 7.3.3 Lobaplatin | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [1.48, 6.26] |
7.2. Analysis.

Comparison 7: Type of platinum agent, Outcome 2: Overall survival
Comparison 8. Same backbone chemotherapy with or without platinum.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Disease‐free survival | 13 | 3357 | Hazard Ratio (IV, Fixed, 95% CI) | 0.65 [0.56, 0.74] |
| 8.1.1 Same backbone | 6 | 1592 | Hazard Ratio (IV, Fixed, 95% CI) | 0.67 [0.55, 0.81] |
| 8.1.2 Different backbone | 7 | 1765 | Hazard Ratio (IV, Fixed, 95% CI) | 0.62 [0.51, 0.76] |
| 8.2 Overall survival | 12 | 3232 | Hazard Ratio (IV, Fixed, 95% CI) | 0.68 [0.56, 0.82] |
| 8.2.1 Same backbone | 5 | 1467 | Hazard Ratio (IV, Fixed, 95% CI) | 0.75 [0.57, 0.99] |
| 8.2.2 Different backbone | 7 | 1765 | Hazard Ratio (IV, Fixed, 95% CI) | 0.62 [0.47, 0.81] |
| 8.3 Pathological complete response | 16 | 3148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.33, 1.61] |
| 8.3.1 Same backbone | 7 | 1675 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.38, 1.84] |
| 8.3.2 Different backbone | 9 | 1473 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.18, 1.53] |
Comparison 9. Anthracycline content of chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Disease‐free survival | 13 | 3347 | Hazard Ratio (IV, Fixed, 95% CI) | 0.65 [0.56, 0.74] |
| 9.1.1 Anthracycline‐containing platinum | 7 | 1740 | Hazard Ratio (IV, Fixed, 95% CI) | 0.69 [0.57, 0.83] |
| 9.1.2 Anthracycline‐free platinum | 6 | 1607 | Hazard Ratio (IV, Fixed, 95% CI) | 0.59 [0.47, 0.73] |
| 9.2 Overall survival | 12 | 3229 | Hazard Ratio (IV, Fixed, 95% CI) | 0.69 [0.58, 0.83] |
| 9.2.1 Anthracycline‐containing platinum | 6 | 1622 | Hazard Ratio (IV, Fixed, 95% CI) | 0.77 [0.61, 0.96] |
| 9.2.2 Anthracycline‐free platinum | 6 | 1607 | Hazard Ratio (IV, Fixed, 95% CI) | 0.57 [0.41, 0.78] |
| 9.3 Pathological complete response | 16 | 3148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.33, 1.61] |
| 9.3.1 Anthracycline‐containing platinum | 10 | 2347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.29, 1.61] |
| 9.3.2 Anthracycline‐free platinum | 6 | 801 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.24, 1.89] |
Comparison 10. Schedule of platinum agent.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Disease‐free survival | 13 | 3347 | Hazard Ratio (IV, Fixed, 95% CI) | 0.65 [0.56, 0.74] |
| 10.1.1 3‐weekly platinum | 9 | 1906 | Hazard Ratio (IV, Fixed, 95% CI) | 0.71 [0.59, 0.85] |
| 10.1.2 2‐weekly platinum | 1 | 143 | Hazard Ratio (IV, Fixed, 95% CI) | 0.31 [0.14, 0.70] |
| 10.1.3 Weekly platinum | 3 | 1298 | Hazard Ratio (IV, Fixed, 95% CI) | 0.58 [0.45, 0.74] |
| 10.2 Overall survival | 12 | 3229 | Hazard Ratio (IV, Fixed, 95% CI) | 0.69 [0.58, 0.83] |
| 10.2.1 3‐weekly platinum | 8 | 1791 | Hazard Ratio (IV, Fixed, 95% CI) | 0.79 [0.64, 0.99] |
| 10.2.2 2‐weekly platinum | 1 | 143 | Hazard Ratio (IV, Fixed, 95% CI) | 0.14 [0.04, 0.52] |
| 10.2.3 Weekly platinum | 3 | 1295 | Hazard Ratio (IV, Fixed, 95% CI) | 0.55 [0.39, 0.78] |
| 10.3 Pathological complete response | 16 | 3148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.33, 1.61] |
| 10.3.1 3‐weekly platinum | 11 | 1837 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.38, 1.87] |
| 10.3.2 Weekly platinum | 5 | 1311 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.19, 1.52] |
10.1. Analysis.

Comparison 10: Schedule of platinum agent, Outcome 1: Disease‐free survival
Comparison 11. Triple negative definition – hormone receptor immunohistochemistry cut‐off.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Disease‐free survival | 13 | 3347 | Hazard Ratio (IV, Fixed, 95% CI) | 0.65 [0.56, 0.74] |
| 11.1.1 Hormone receptor > 1% or not defined | 5 | 876 | Hazard Ratio (IV, Fixed, 95% CI) | 0.76 [0.59, 0.98] |
| 11.1.2 Hormone receptor < 1% | 8 | 2471 | Hazard Ratio (IV, Fixed, 95% CI) | 0.60 [0.50, 0.71] |
| 11.2 Overall survival | 12 | 3229 | Hazard Ratio (IV, Fixed, 95% CI) | 0.69 [0.58, 0.83] |
| 11.2.1 Hormone receptor > 1% or not defined | 4 | 761 | Hazard Ratio (IV, Fixed, 95% CI) | 0.81 [0.60, 1.07] |
| 11.2.2 Hormone receptor < 1% | 8 | 2468 | Hazard Ratio (IV, Fixed, 95% CI) | 0.62 [0.49, 0.79] |
| 11.3 Pathological complete response | 16 | 3148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.33, 1.61] |
| 11.3.1 Hormone receptor > 1% or not defined | 9 | 1307 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.30, 1.90] |
| 11.3.2 Hormone receptor < 1% | 7 | 1841 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.26, 1.58] |
11.1. Analysis.

Comparison 11: Triple negative definition – hormone receptor immunohistochemistry cut‐off, Outcome 1: Disease‐free survival
11.2. Analysis.

Comparison 11: Triple negative definition – hormone receptor immunohistochemistry cut‐off, Outcome 2: Overall survival
11.3. Analysis.

Comparison 11: Triple negative definition – hormone receptor immunohistochemistry cut‐off, Outcome 3: Pathological complete response
Comparison 12. Platinum‐based chemotherapy versus platinum free chemotherapy without Nasr 2015 or BrigTNess comparison 1, and using bevacizumab‐free CALGB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Disease‐free survival | 11 | 2572 | Hazard Ratio (IV, Fixed, 95% CI) | 0.60 [0.50, 0.71] |
| 12.2 Overall survival | 10 | 2454 | Hazard Ratio (IV, Fixed, 95% CI) | 0.64 [0.52, 0.79] |
| 12.3 Pathological complete response | 15 | 2959 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.35, 1.63] |
12.1. Analysis.

Comparison 12: Platinum‐based chemotherapy versus platinum free chemotherapy without Nasr 2015 or BrigTNess comparison 1, and using bevacizumab‐free CALGB, Outcome 1: Disease‐free survival
12.2. Analysis.

Comparison 12: Platinum‐based chemotherapy versus platinum free chemotherapy without Nasr 2015 or BrigTNess comparison 1, and using bevacizumab‐free CALGB, Outcome 2: Overall survival
12.3. Analysis.

Comparison 12: Platinum‐based chemotherapy versus platinum free chemotherapy without Nasr 2015 or BrigTNess comparison 1, and using bevacizumab‐free CALGB, Outcome 3: Pathological complete response
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ADAPT‐TN.
| Study characteristics | ||
| Methods | Accrual: May 2013 to January 2015 Multicentre, 48 sites Phase of trial: 2 Study design: RCT Country or countries where the trial was conducted: Germany Median follow‐up: not reported |
|
| Participants | Age: median 50, range 26–75 years Nodal status of breast cancer: node positive 26%, node negative 74% Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: none |
|
| Interventions | Arm 1: Nab‐paclitaxel 125 mg/m2 + carboplatin AUC2 days 1 and 8, every 3 weeks for 4 cycles Arm 2: Nab‐paclitaxel 125 mg/m2 + gemcitabine 1000 mg/m2 days 1 and 8, every 3 weeks for 4 cycles |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Trial registration record: NCT01815242 Not all randomised participants were included in the analysis; 5 excluded prior to receiving treatment (3 in intervention arm, 2 in comparator arm). Safety analysis involved people receiving ≥ 1 dose of trial medication. For DFS and OS, we estimated the hazard ratio using Tierney's method. Study did not report assessing the proportional hazards assumption. Funding considerations: funded by Celgene and Teva. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation in a 1:1 ratio, method to generate random sequence was not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed centrally at West German Study group." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using the CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and, therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | pCR was assessed by a local pathologist only. It is not reported whether this pathologist was blinded to the treatment allocation; however, pCR is viewed to be an objective outcome and unlikely to be influenced by knowledge of treatment allocation. |
| Selective reporting (reporting bias) | Low risk | All prespecified primary and secondary endpoints from the trial record were reported in the manuscript and subsequent abstract publications. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | CONSORT diagram showed that 5/336 randomised participants did not receive treatment and were excluded from analysis. Reasons for exclusions were detailed, including consent withdrawn, and violation of inclusion criteria. Additionally, pCR results for 12 participants were not reported. |
| Other bias | Low risk | None identified. |
Ando 2014.
| Study characteristics | ||
| Methods | Accrual: March 2010 and September 2011 Multicentre, 10 centres Phase of trial: 2 Trial design: open‐label RCT Countries: Japan Median follow‐up: 12 months |
|
| Participants | Demographics and clinical characteristics were not reported separately for the participants of interest for this Cochrane Review topic. People with TNBC made up approximately 40% of the entire cohort. For entire cohort Age: median 47, range 30–70 years Nodal status of breast cancer: 65% node positive, 35% node negative BRCA mutation: not reported Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: none |
|
| Interventions | Arm 1: intervention: carboplatin AUC5 every 3 weeks for 4 cycles + paclitaxel 80 mg/m2 days 1, 8, 15 for 4 cycles, followed by 4 cycles of cyclophosphamide 500 mg/m2, epirubicin 100 mg/m2 and fluorouracil 500 mg/m2 every 3 weeks Arm 2: comparator: paclitaxel 80 mg/m2 days 1, 8, 15 for 4 cycles, followed by 4 cycles of cyclophosphamide 500 mg/m2, epirubicin 100 mg/m2 and fluorouracil 500 mg/m2 every 3 weeks Surgery within 8 weeks after completing neoadjuvant therapy. People who had breast‐conserving therapy received whole breast irradiation |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Trial registration record: UMIN000003355. Where reported separately, we extracted data for the TNBC cohort only (42% of entire cohort) for efficacy outcomes. As data were not presented separately for toxicity, we extracted data for the entire cohort. Authors were not contacted. All randomised patients who received ≥ 1 dose of study chemotherapy were included in the analysis. Study did not report assessing the proportional hazards assumption. Funding considerations: carboplatin provided by Bristol‐Myers Squibb. Study supported by Health and Labour Sciences Research Grants, Ministry of Health, Labour and Welfare, Cancer Research & Development and National Cancer Centre grants, Japan. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomly assigned to receive either … by the minimization method, with balancing of the treatment arms according to disease status, hormone receptor status and institution." (p. 402) |
| Allocation concealment (selection bias) | Low risk | "Central registration" listed in trial registration record. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): DFS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): OS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using the CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and, therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | Evaluated centrally by 3 breast pathologists. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in trial registration record were reported in trial publications. OS was not prespecified but collected later on and is a critical outcome for this review topic. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CONSORT diagram (p.404) showed that 2 participants randomised were excluded from analyses due to both participants refusing treatment. |
| Other bias | Low risk | None identified. |
BrighTNess comparison 1.
| Study characteristics | ||
| Methods | Accrual: April 2014 to March 2016 Multicentre, 145 sites Phase of trial: 3 Study design: quadruple‐blind (participant, care provider, investigator, outcomes assessor) RCT Country or countries where the trial was conducted: Germany, Spain, USA, Korean, France, Czech Republic, Russia, Belgium, Australia, Hungary, Italy, UK, Canada, Taiwan, Netherlands Median follow‐up: 4.5 years |
|
| Participants | Age: median 50, range 40–59 years Nodal status of breast cancer: 42% node positive, 58% node negative Proportion of people with BRCA mutations: 15% Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: none |
|
| Interventions | A 3‐arm study that has been split in this Cochrane Review into 2 separate pairwise comparisons. Arm 1 (arm named 'paclitaxel + carboplatin + veliparib' in trial publication): paclitaxel 80 mg/m2 intravenously weekly plus carboplatin AUC6 every 3 weeks for 12 weeks plus veliparib 50 mg twice a day, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles Arm 2 (named 'paclitaxel + carboplatin placebo + veliparib placebo' group in trial publication): paclitaxel 80 mg/m2 intravenously weekly for 12 weeks, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Trial registration record: NCT02032277 All randomised participants included in intention‐to‐treat analysis. Study did not report assessing the proportional hazards assumption. Funding considerations: funded by AbbVie, who participated in the design of the study, collection, analysis and interpretation of the data, as well as the writing, review and approval of the manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned (2:1:1) to one of three treatment groups by an interactive response technology system using permuted blocks (block size of four) within strata. The randomisation schedule was created by the statistics department of the study funder (AbbVie, North Chicago, IL, USA)." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation schedule … was forwarded to a third‐party vendor (Endpoint Clinical) to be implemented via the interactive response technology system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. Quote: "The study funder, members of the academic steering committee, investigators, study site personnel, and patients remained masked to each patient's treatment throughout the course of the study." |
| Blinding of outcome assessment (detection bias): DFS | Low risk | Blinding of outcome assessment occurred. |
| Blinding of outcome assessment (detection bias): OS | Low risk | Blinding of outcome assessment occurred. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using CTCAE, and double‐blind study. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | Double‐blind, local assessment with central review. Quote: "Pathological complete response was assessed by local pathology review of the resected breast specimen and lymph node tissue on haematoxylin and eosin‐stained samples. The local pathology reports were centrally reviewed by members of the steering committee to confirm the accuracy of data entry for the endpoint." |
| Selective reporting (reporting bias) | Low risk | Quality of life was a prespecified tertiary endpoint but not reported. All primary and secondary outcomes, including DFS and OS, were reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed – all participants who were randomised were included in the analysis, including those who withdrew consent or did not proceed to surgery. |
| Other bias | Low risk | None identified. |
BrighTNess comparison 2.
| Study characteristics | ||
| Methods | See BrighTNess comparison 1 | |
| Participants | See BrighTNess comparison 1 | |
| Interventions | A 3‐arm study that has been split in this Cochrane Review into 2 pair‐wise comparisons. Arm 1 (arm named 'paclitaxel + carboplatin + veliparib placebo' group in trial publication): paclitaxel 80 mg/m2 intravenously weekly plus carboplatin AUC6 every 3 weeks for 12 weeks, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles Arm 2 (named 'paclitaxel + carboplatin placebo + veliparib placebo' group in trial publication): paclitaxel 80 mg/m2 intravenously weekly for 12 weeks, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles |
|
| Outcomes | See BrighTNess comparison 1 | |
| Notes | See BrighTNess comparison 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned (2:1:1) to one of three treatment groups by an interactive response technology system using permuted blocks (block size of four) within strata. The randomisation schedule was created by the statistics department of the study funder (AbbVie, North Chicago, IL, USA)." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation schedule … was forwarded to a third‐party vendor (Endpoint Clinical) to be implemented via the interactive response technology system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Quote: "The study funder, members of the academic steering committee, investigators, study site personnel, and patients remained masked to each patient’s treatment throughout the course of the study." |
| Blinding of outcome assessment (detection bias): DFS | Low risk | Blinding of outcome assessment occurred. |
| Blinding of outcome assessment (detection bias): OS | Low risk | Blinding of outcome assessment occurred. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using CTCAE, and double‐blind study. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | Double‐blind, local assessment with central review. Quote: "Pathological complete response was assessed by local pathology review of the resected breast specimen and lymph node tissue on haematoxylin and eosin‐stained samples. The local pathology reports were centrally reviewed by members of the steering committee to confirm the accuracy of data entry for the endpoint." |
| Selective reporting (reporting bias) | Low risk | Quality of life was a prespecified tertiary endpoint but not reported. All primary and secondary outcomes, including DFS and OS, were reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed – all participants who were randomised were included in the analysis, including those who withdrew consent or did not proceed to surgery. |
| Other bias | Low risk | None identified. |
CALGB 40603.
| Study characteristics | ||
| Methods | Accrual: May 2009 to August 2012 Multicentre Phase of trial: 2–3 Study design: open‐label RCT Country or countries where the trial was conducted: USA Median follow‐up: 7.9 years for EFS and OS data |
|
| Participants | Age: mean and range not reported. 60% aged 40–59 years Nodal status of breast cancer: 52% node positive, 42% node negative, 7% missing Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: none |
|
| Interventions | 4‐arm study that has been grouped here into 2 categories according to carboplatin use. Arm 1 (listed as arms 3 and 4 in the trial publication): paclitaxel 80 mg/m2 weekly + carboplatin AUC6, 3 weekly for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles ± bevacizumab 10 mg/kg every 2 weeks for 9 cycles Arm 2 (listed as arms 1 and 2 in the trial publication): paclitaxel 80 mg/m2 weekly for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles ± bevacizumab 10 mg/kg every 2 weeks for 9 cycles Where possible, we reported pair‐wise comparisons of arm 1 vs arm 3 (see CALGB 40603 – comparison 1 (without bevacizumab)), and arm 2 vs arm 4 (see CALGB 40603 – comparison 2 (with bevacizumab)). |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Trial registration record: NCT00861705 Not all randomised participants were included in intention‐to‐treat analysis – participants who withdrew consent before completing neoadjuvant chemotherapy were excluded from pCR analyses. Study authors appeared to have assessed proportional hazards assumption. Funding considerations: funded in part by NCI grants, Genentech, the Breast Cancer Research Foundation, and the American Recovery and Research Act. These sources were not involved in data analysis or preparation of manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation in 2 × 2 format, method not described. |
| Allocation concealment (selection bias) | Low risk | Study protocol stated participant registration and randomisation occurs through CALGB web‐based system. It is most likely that randomisation was centralised. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): DFS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): OS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using the CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and, therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | Pathological response was determined locally, without central pathological review. It was not reported whether this pathologist was blinded to the treatment allocation, however pCR is viewed to be an objective outcome and unlikely to be influenced by knowledge of treatment allocation. |
| Selective reporting (reporting bias) | Low risk | All prespecified primary endpoints from the trial record were reported in the manuscript. Some secondary outcome measures, including radiographic response, clinical response, and incidence and severity of postoperative complications were not reported; however, these were not considered to be critical outcomes for this review topic. OS, a secondary outcome, was reported in a subsequent publication. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | CONSORT diagram showed that 11/454 participants who were randomised but did not receive treatment were not included in the efficacy analysis. Reasons for exclusions were not detailed. |
| Other bias | Low risk | None identified. |
CALGB 40603 – comparison 1 (without bevacizumab).
| Study characteristics | ||
| Methods | See details in CALGB 40603 | |
| Participants | See details in CALGB 40603 | |
| Interventions | 4‐arm study that has been split into 2 treatment comparisons where possible. Treatment comparison 1 includes: Arm 1 (listed as arm 3 in the trial publication): paclitaxel 80 mg/m2 weekly + carboplatin AUC6, 3 weekly for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles Arm 2 (listed as arm 1 in the trial publication): paclitaxel 80 mg/m2 weekly for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles |
|
| Outcomes | See details in CALGB 40603 | |
| Notes | See details in CALGB 40603 | |
CALGB 40603 – comparison 2 (with bevacizumab).
| Study characteristics | ||
| Methods | See details in CALGB 40603 | |
| Participants | See details in CALGB 40603 | |
| Interventions | 4‐arm study that has been split into 2 treatment comparisons where possible. Treatment comparison 2 includes: Arm 1 (listed as arm 4 in the trial publication): paclitaxel 80 mg/m2 weekly + carboplatin AUC6, 3 weekly for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles and bevacizumab 10 mg/kg every 2 weeks for 9 cycles Arm 2 (listed as arm 2 in the trial publication): paclitaxel 80 mg/m2 weekly for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles and bevacizumab 10 mg/kg every 2 weeks for 9 cycles |
|
| Outcomes | See details in CALGB 40603 | |
| Notes | See details in CALGB 40603 | |
GEICAM 2006‐03.
| Study characteristics | ||
| Methods | Accrual: April 2007 to January 2010 Multicentre Phase of trial: 2 Study design: open‐label RCT Country or countries where the trial was conducted: Spain Median follow‐up: not reported |
|
| Participants | Age: median 47, range 27–75 years Nodal status of breast cancer: 52% node positive, 48% node negative Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: none |
|
| Interventions | 4‐arm study (luminal A standard treatment, luminal A selective treatment, basal standard treatment, basal selective treatment). For this Cochrane Review, 2/4 treatment arms were relevant, i.e. data on the basal phenotype. Arm 1 ("Group 2 standard treatment" in trial registry record): epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by docetaxel 75 mg/m2 + carboplatin AUC6 every 3 weeks for 4 cycles Arm 2 ("Group 2 selective treatment" in trial registry record): epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 3 for 4 cycles followed by docetaxel 75 mg/m2 every 3 weeks for 4 cycles After neoadjuvant therapy, participants had mastectomy or conservative surgery Postoperative radiotherapy was given at physician's discretion. |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Trial registration record: NCT00432172 All randomised participants who received a dose of study treatment were included in the analysis; 1 participant never received treatment and was excluded. Funding considerations: partially supported by Pfizer and sponsored by the Spanish Breast Cancer Research Group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients randomly assigned, in a 1:1 ratio." (p.488) No details provided on method to generate random sequence. Some imbalances in baseline characteristics (i.e. ECOG, menopausal status, grade). |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization was centralized at the GEICAM headquarters." (p.488) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes were graded as per CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and, therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | pCR was viewed as an objective outcome with the study using Miller and Payne criteria. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in trial registration record reported either in trial publication or results reporting page in trial registry. All‐cause mortality added as a new outcome collected and reported on. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants who were randomised and received treatment were included in analysis. 1/94 participants did not receive treatment and were excluded from analysis. |
| Other bias | Low risk | None identified. |
GeparOcto.
| Study characteristics | ||
| Methods | Accrual: December 2014 to June 2016 Multicentre, 57 centres Phase of trial: 3 Study design: open‐label RCT Country or countries where the trial was conducted: Germany Median follow‐up: not reported |
|
| Participants |
For the triple‐negative cohort (43% of entire cohort of the trial) Age: median 48, range 21–76 years Nodal status of breast cancer: 34% node positive, 66% node negative Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: none |
|
| Interventions | Arm 1: paclitaxel 80 mg/m2 weekly + non‐pegylated liposomal doxorubicin 20 mg/m2 weekly + carboplatin AUC1.5 weekly for 18 weeks Arm 2: epirubicin 150 mg/m2 + paclitaxel 225 mg/m2 + cyclophosphamide 2000 mg/m2 every 2 weeks for 3 cycles Note: carboplatin only added for TNBC subgroup in this trial |
|
| Outcomes | Outcomes as listed in trial publication and trial registry record. Primary
Secondary
|
|
| Notes | Trial registration record: NCT02125344 All randomised participants who started treatment were included analysis, 16 were excluded who did not receive any protocol treatment and it was unclear if they were part of the TNBC cohort or not. Time‐to‐event outcomes collected but not yet reported. Funding considerations: funded by Roche, Amgen, Teva and Vifor. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by dynamic allocation using the Pocock and Simon minimisation method." (p.1, supplementary material) |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised centrally at the German Breast Group headquarters." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using the CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and involved regular laboratory tests, etc. at the end of each cycle. Therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | Local pathologists were blinded to treatment assignment. Further, all local histopathological reports were centrally evaluated by an independent pathologist blinded to treatment and not otherwise involved in the trial. |
| Selective reporting (reporting bias) | Unclear risk | The primary outcome was reported. Efficacy outcomes (including OS, distant DFS, etc.) were briefly reported in a 2020 abstract but subgroup data were not provided. Study was completed in 2017 with pCR data reported in 2019. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | CONSORT diagram indicates reasons for exclusions across both arms within a higher proportion discontinuing treatment in the platinum arm. Only those who received treatment were included in the analyses. |
| Other bias | Low risk | None identified. |
GeparOLA.
| Study characteristics | ||
| Methods | Accrual: September 2016 to July 2018 Multicentre, 27 recruiting sites Phase of trial: 2 State study design: open‐label non‐comparative RCT Country or countries where the trial was conducted: Germany Median follow‐up: not reported |
|
| Participants |
For the entire cohort (people with TNBC made up 72.6%) Age: median 47.0 years, range 25.0 to 71.0 years Nodal status of breast cancer: 32% node positive, 68% node negative. Note imbalance: carboplatin group 54% node negative, olaparib group 75% node negative Proportion of participants with BRCA mutations: 56% on central testing, 86% on local testing Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: study included only people with HRD (HRD score high, germline or somatic BRCA1/2 mutation), or both HRD score high and germline or somatic BRCA1/2 mutation |
|
| Interventions | Arm 1: paclitaxel 80 mg/m2 + carboplatin AUC2 weekly for 12 weeks followed by epirubucin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles Arm 2: paclitaxel 80 mg/m2 weekly + olaparib 100 mg twice a day for 12 weeks followed by epirubucin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | |
| Outcomes | Primary
Secondary
|
|
| Notes | Trial registration record: NCT0278933 All randomised participants who received a dose of study therapy were included in the modified intention‐to‐treat analysis. Funding considerations: funded by AstraZeneca, Germany. Role in writing and statistical analysis was not reported. Study developed by German Breast Group and Arbeitsgemeinschaft Gynakologische Onkologie Breast. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was carried out at a 1.757: 1 rate using the Pocock minimisation method." (p.51) |
| Allocation concealment (selection bias) | Low risk | No description whether randomisation was centralised, although probably done as involved randomisation across multiple sites. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using the CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and, therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | Evaluated by local pathologists and centrally reviewed by an independent pathologist blinded to treatment group. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes prespecified in the trial registry record were reported in the trial publication. Important outcomes of DFS and OS not reported but expected that information would have been collected. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/107 participants were withdrawn and study authors conducted a modified intention‐to‐treat analysis. |
| Other bias | Low risk | None identified. |
GeparSixto.
| Study characteristics | ||
| Methods | Accrual: August 2011 and December 2012 Multicentre, 54 centres Phase of trial: 2 State study design: RCT Country or countries where the trial was conducted: Germany Median follow‐up: 47.3 months |
|
| Participants | Demographic and clinical characteristics were not reported separately for the TNBC cohort (53.6% of entire cohort). For the entire cohort (i.e. people diagnosed with TNBC and HER2‐positive breast cancer). Age: median 48 (21–78) years Nodal status of breast cancer: 47% node positive, 53% node negative Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: none For TNBC cohort only BRCA mutation: 18% in carboplatin arm (26/146); 17% in comparator arm (24/145); 93% of participants had samples tested for germline mutations |
|
| Interventions | Arm 1: carboplatin AUC2 or 1.5 weekly + paclitaxel 80 mg/m2 weekly + non‐pegylated liposomal doxorubicin 20 mg/m2 weekly + bevacizumab 15 mg/kg every 3 weeks for 18 weeks Arm 2: paclitaxel 80 mg/m2 + non‐pegylated liposomal doxorubicin 20 mg/m2 weekly + bevacizumab 15 mg/kg every 3 weeks for 18 weeks |
|
| Outcomes | Primary (as listed in the trial publication and trial registry record)
Secondary
|
|
| Notes | Trial registration record: NCT01426880 Study included people with triple‐negative or HER‐2‐positive breast cancer. Data for efficacy outcomes were reported separately for TNBC cohort. However, for toxicity, we extracted data for the entire cohort. 7 randomised patients did not start treatment and were not included in the analysis. Study did not report assessing the proportional hazards assumption. For DFS BRCA1 and 2 mutation, we estimated the hazard ratio using Tierney's method. Funding considerations: funded by GlaxoSmithKline, Roche, and Teva. The authors state that the funders had no role in the collection, analysis or interpretation of the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was done … in a 1:1 ratio … and was stratified according to biological subtype and Ki67 level. The minimisation method of Pockock and Simon was used for randomisation." (p.749) |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was done centrally at the German Breast Group headquarters." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open label study. Although study investigators and participants were aware of treatment allocation and may have been associated with some performance bias, bias was not considered to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): DFS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): OS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes were graded as per CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and, therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | Quote: "Pathological response of the breast tumour and axillary lymph nodes were assessed by local pathologists. Pathological reports were reviewed by one independent board certified pathologist (KE) from whom treatment assignments were masked, and response was staged in accordance with the Union for International Cancer Control TNM system." (p.750) |
| Selective reporting (reporting bias) | Low risk | All prespecified primary and secondary outcomes were reported. Important outcomes including DFS and OS are included and updated in subsequent publications. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | CONSORT diagram showed that 7/595 patients (6 patients in the comparator arm and 1 patient in the intervention arm) did not proceed with treatment due to patient and investigator decisions. Although the numbers were not equal between groups, the reasons were stated. The trial publication stated they conducted an intention‐to‐treat analysis, however, it used a modified intention‐to‐treat analysis. Number of exclusions were small and not considered as a concern. |
| Other bias | Low risk | None identified. |
Gigolaeva 2019.
| Study characteristics | ||
| Methods | Accrual: not reported Single centre Phase of trial: not reported Study design: RCT Country or countries where the trial was conducted: Russia Median follow‐up: not reported |
|
| Participants | Age: median 47, range 32–62 years Nodal status of breast cancer: not reported Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: not reported |
|
| Interventions | Arm 1: doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by carboplatin AUC2 weekly + eribulin 1.4 mg/m2 OR paclitaxel 175 mg/m2 every 3 weeks for 12 weeks Arm 2: doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by paclitaxel 80 mg/m2 for 12 weeks |
|
| Outcomes | Primary
Secondary: none reported |
|
| Notes | Trial registration record could not be found. Abstract only available. Full article has not yet been published. Contact details for the authors could not be found to request an update on results or published works. Funding considerations: "no significant conflicts of interest" (p.S70) declared in the abstract. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A randomized prospective study" … "randomization (2:1)." No additional details were provided in the abstract. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided in the abstract. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Unclear risk | No information provided on tests used or process to evaluate response. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess from the abstract. A trial registration record could not be found. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reporting of attrition or exclusions in the available abstract. |
| Other bias | Unclear risk | Unable to assess from the abstract. |
I‐SPY2.
| Study characteristics | ||
| Methods | Accrual: May 2010 to July 2012 Multicentre, 30 sites Phase of trial: 2 Study design: open‐label adaptive/platform RCT Country or countries where the trial was conducted: USA Median follow‐up: not reported |
|
| Participants | Demographic and clinical characteristics presented below were not described separately for the hormone receptor‐negative cohort (i.e. 52% of the entire cohort). For the entire cohort Age: median 49, range 24–71 years Nodal status of breast cancer: 46% node positive, 54% node negative Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: none BRCA status collected: BRCA1/2 mutation – 17% in intervention arm, 7% in comparator arm |
|
| Interventions | Arm 1: paclitaxel 80 mg/m2 + veliparib 50 mg orally twice daily + carboplatin AUC6 every 3 weeks for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles Arm 2: paclitaxel 80 mg/m2 weekly for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles |
|
| Outcomes | Primary
Secondary listed in trial publication and trial registry record (none reported in this paper)
|
|
| Notes | Trial registration record: NCT01042379 Contacted for results for pCR in the triple negative subgroup. Only participants who received treatment were included in the analysis. Of those participants who did not receive treatment, it was unclear whether they were part of the TNBC cohort. Time‐to‐event outcomes collected but not yet reported. Funding considerations: funded by charitable donations and pharmaceutical companies including Johnson & Johnson, Genentech. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… biomarker profiles are used for randomizing each participant to a treatment arm … for every participant that is randomized, there is a 20% chance the participant will be randomized to the control arm" as part of this adaptive trial design. Trial uses web‐based randomisation system. |
| Allocation concealment (selection bias) | Low risk | Randomisation was adaptive design with drug regimens being added or dropped depending on their efficacy. The registration and randomisation process was web‐based therefore it was unlikely that those involved in the trial were aware of intended treatment allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using the CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and involved regular laboratory tests, etc. at the end of each cycle. Therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | A study‐trained pathologist evaluated pCR. The Study Lead Pathologist made the final assessment on any indeterminate or contested results. It was unclear whether the pathologists were blinded; however, pCR is generally viewed as an objective outcome and there was a minimal risk of treatment allocation affecting pCR assessment. |
| Selective reporting (reporting bias) | High risk | The primary outcome was reported; however, RFS and OS are yet to be reported. As pCR data were reported in 2016, other important long‐term efficacy outcomes would be expected to have been reported by 2022. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | CONSORT diagram was not provided for the cohort of interest in this review (i.e. TNBC); therefore, a judgement could not be made. |
| Other bias | Low risk | None identified. |
INFORM.
| Study characteristics | ||
| Methods | Accrual: January 2012 to January 2019 Multicentre, 13 centres Phase of trial: 2 Study design: open‐label RCT Country or countries where the trial was conducted: USA Median follow‐up: not reported |
|
| Participants | Demographic and clinical characteristics presented below were not described separately for the hormone receptor‐negative cohort (i.e. 64–70% of the entire cohort). For the entire cohort Age: mean 42, range 24–73 years Nodal status of breast cancer: 45% node positive, 55% node negative Adjuvant or neoadjuvant: neoadjuvant All participants were germline BRCA carriers (69% BRCA1 mutation; 30% BRCA2 mutation, 2% both) Notable exclusion criteria: none Study population included HER2 negative, hormone receptor‐positive cancers |
|
| Interventions | Arm 1: cisplatin 75 mg/m2 every 3 weeks for 4 cycles Arm 2: doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2–3 weeks for 4 cycles (every 2 weeks for TNBC cohort) Radiotherapy mandated for all participants not having a mastectomy |
|
| Outcomes | Primary (as per trial publication and trial registry record)
Secondary
|
|
| Notes | Trial registration record: NCT01670500 Trial stopped early due to slow accrual. All randomised participants who started treatment were included in the analysis, 1 was excluded who did not receive any protocol treatment. Funding considerations: funded by the Breast Cancer Research Foundation, Susan G Komen, and Myriad Genetics. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was stratified by tumor ER status (…) and by treatment site." (p. 1541) Baseline characteristics were generally well‐balanced (except for age and tumour stage). No details about how random sequence was generated. |
| Allocation concealment (selection bias) | Low risk | No description whether randomisation was centralised, although probably done as involved randomisation across multiple sites. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): OS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using the CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and involved regular laboratory tests, etc. at the end of each cycle. Therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | Pathological responses were centrally determined by the study pathologist. Central pathology review consisted of clear definitions of how to assess residual cancer following chemotherapy. It is unclear whether the central pathologist was blinded but given that pCR is an objective outcome, there was a minimal risk of treatment allocation affecting pCR assessment. |
| Selective reporting (reporting bias) | Unclear risk | Most outcomes prespecified in trial registry record have been reported in trial publication, except for RFS. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CONSORT diagram indicated reasons for 1 exclusion in the comparator arm. Analysis of randomised and those that were allocated to treatment. |
| Other bias | Low risk | None identified. |
Li 2020.
| Study characteristics | ||
| Methods | Accrual: June 2011 to December 2015 Single centre Phase of trial: 3 Study design: open‐label RCT Country or countries where the trial was conducted: China Median follow‐up: 57.3 months |
|
| Participants | Age: median 49, range 22–64 years Nodal status of breast cancer: 37% node positive, 63% node negative Adjuvant or neoadjuvant: adjuvant Notable exclusion criteria: none |
|
| Interventions | Arm 1: paclitaxel 150 mg/m2 + carboplatin AUC3 every 2 weeks for 8 cycles Arm 2: epirubicin 80 mg/m2 and cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles followed by paclitaxel 175 mg/m2 every 2 weeks for 4 cycles |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Trial registration record: NCT01378533 All randomised participants were included in analysis. Study did not report assessing the proportional hazards assumption. Funding considerations: funded by the National Key Research and Development Program of China and the Chinese Academy of Medical Science Initiative for Innovative Medicine. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Simple randomization was conducted … using random allocation sequence" (p.487) but no details provided as to how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No information provided at single site. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): DFS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): OS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using the CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and involved regular laboratory tests, etc. at the end of each cycle. Therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in trial registration record were reported in trial publication. OS was not prespecified but collected later and was a primary outcome for this review. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were included in the analysis irrespective of outcome being measured or treatment actually received. |
| Other bias | Low risk | None identified. |
Nasr 2015.
| Study characteristics | ||
| Methods | Accrual: November 2008 to December 2014 Multicentre, 4 centres Phase of trial: 3 Study design: RCT Country or countries where the trial was conducted: Egypt Median follow‐up: 52 months |
|
| Participants | Age: mean 46, 95% confidence interval 32 to 62 years Nodal status of breast cancer: 94% node positive, 6% node negative Adjuvant or neoadjuvant: adjuvant Notable exclusion criteria: none |
|
| Interventions | Arm 1: 5‐fluorouracil 500 mg/m2 + epirubicin 100 mg/m2 + cyclophosphamide 500 mg/m2 every 3 weeks for 3 cycles then docetaxel 80 mg/m2 + carboplatin AUC5 every 3 weeks for 3 cycles, followed by postoperative radiotherapy, followed by oral cyclophosphamide 50 mg daily, and methotrexate 2.5 mg orally twice daily on days 1, 2 of each week every 28 days for 1 year Arm 2: 5‐fluorouracil 500 mg/m2 + epirubicin 100 mg/m2 + cyclophosphamide 500 mg/m2 every 3 weeks for 3 cycles then docetaxel 100 mg/m2 every 3 weeks for 3 cycles Note: intervention is addition of carboplatin AND 12 months of metronomic chemotherapy |
|
| Outcomes | Primary
Secondary
|
|
| Notes | No trial registration record identified in the World Health Organization International Clinical Trials Registry Platform. All randomised participants were included as intention‐to‐treat analysis for all outcomes. Note: none of the efficacy or safety outcome data (in tables or figures) presented the denominators. Study did not report assessing the proportional hazards assumption. For DFS and OS, we estimated the hazard ratio using Tierney's method. Funding considerations: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to one of [two] groups." (p.3) No further details were provided. |
| Allocation concealment (selection bias) | Unclear risk | No description whether randomisation was centralised, although probably done as involved randomisation across multiple sites. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): DFS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): OS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using the CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and, therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcome measures including DFS and OS were reported. However, limited data were provided and the OS Kaplan‐Meier curve was difficult to interpret. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Paper indicated that all randomised participants were included in analysis. Number of participants who did not receive treatment were equal across treatment arms (i.e. 3 participants in each). |
| Other bias | Low risk | None identified. |
NeoCART.
| Study characteristics | ||
| Methods | Accrual: September 2016 to December 2019 Multicentre, 6 centres Phase of trial: 2 Study design: open‐label RCT Country or countries where the trial was conducted: China Median follow‐up: 37 months |
|
| Participants | Age: median 50, range 19–69 years Nodal status of breast cancer: 58% node positive, 42% node negative Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: none |
|
| Interventions | Arm 1: docetaxel 75 mg/m2 + carboplatin AUC6, 3 weekly for 6 cycles Arm 2: epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by docetaxel 100 mg/m2 every 3 weeks for 4 cycles |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Trial registration record: NCT03154749 Only participants who received treatment were included in the analysis. 5 randomised participants who did not receive treatment were excluded. Study did not report assessing the proportional hazards assumption. Funding considerations: this work was supported by local public and private grants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized by means of a permuted block randomization scheme using an interactive response system (IxRS)." |
| Allocation concealment (selection bias) | Low risk | Quote: "The treatment allocation list was created, and randomization was performed centrally at the leading research center of Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias, but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): DFS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): OS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes were graded as per CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and, therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | Although there was no information regarding blinding of the local pathologist who assessed this outcome, pCR was viewed to be an objective outcome. A review by the pathologist on whether pCR had been achieved or not was unlikely to be influenced by unblinding. |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in the trial registry record were reported in trial publication. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CONSORT diagram outlined 5 participants who were randomised (3 in intervention group, 2 in control group) were excluded due to patient preference. The trial publication stated that it was an intention‐to‐treat analysis but this was not the case. Survival outcomes included the number of participants who received treatment. |
| Other bias | Low risk | None identified. |
PATTERN.
| Study characteristics | ||
| Methods | Accrual: July 2011 to April 2016 Multicentre, 9 centres Phase of trial: 3 Study design: open‐label RCT Country or countries where the trial was conducted: China Median follow‐up: 62 months |
|
| Participants | Age: mean 51, range 44–57 years Nodal status of breast cancer: 26% node positive, 74% node negative Adjuvant or neoadjuvant: adjuvant Notable exclusion criteria: none |
|
| Interventions | Arm 1: paclitaxel 80 mg/m2 + carboplatin AUC2 days 1, 8 and 15 every 28 days for 6 cycles Arm 2: cyclophosphamide 500 mg/m2 + epirubicin 100 mg/m2 + fluorouracil 500 mg/m2 every 3 weeks for 3 cycles followed by docetaxel 100 mg/m2 every 3 weeks for 3 cycles |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Trial registration record: NCT01216111 All randomised participants were included in intention‐to‐treat analysis. Study did not report assessing the proportional hazards assumption. Funding considerations: work supported by grants from the National Natural Science Foundation of China, and by local public and private grants. The manuscript stated the funders had no role in the design or conduct of the trial or the writing of the manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was performed via an interactive web‐response system … randomization was stratified according to pathological node status, age …, and tumor size." (p.1392) Baseline characteristics across groups were well‐balanced. |
| Allocation concealment (selection bias) | Low risk | Randomisation was centralised and involved randomisation across multiple sites. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): DFS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using the CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and involved regular laboratory tests, etc. at the end of each cycle. Therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in the trial registry record were reported in trial publication. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CONSORT diagram outlines reasons for exclusions and similar number of participants discontinued to similar reasons (adverse events, lost to follow‐up, etc.). Efficacy outcomes included the number of participants randomised. |
| Other bias | Low risk | None identified. |
TBCRC 030.
| Study characteristics | ||
| Methods | Accrual: April 2014 to January 2018 Multicentre Phase of trial: 2 Study design: open‐label RCT Country or countries where the trial was conducted: USA Median follow‐up: not reported |
|
| Participants | Age: median 53, range 28–82 years Nodal status of breast cancer: 37% node positive, 63% node negative Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: people with a known BRCA mutation were excluded |
|
| Interventions | Arm 1: cisplatin 75 mg/m2 every 3 weeks for 4 cycles Arm 2: doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles Both arms proceeded to surgery and further provider‐choice adjuvant chemotherapy |
|
| Outcomes | Primary outcomes recorded in trial publication and trial registry record
Secondary
|
|
| Notes | Trial registration record: NCT01982448 Study stopped early due to withdrawal of sponsor support. Randomised participants who received study treatment were included in the analysis, 7 participants who were randomised did not receive the study intervention and were excluded. Funding considerations: funded by Myriad Genetics. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated to the study in a 1:1 ratio stratified by initial lymph node assessment as well as tumour size." (p.1519) |
| Allocation concealment (selection bias) | Low risk | No description whether randomisation was centralised, although probably done as involved randomisation across multiple sites. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using the CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and involved regular laboratory tests, etc. at the end of each cycle. Therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | Although there was no information regarding blinding of the pathologist who assessed this outcome, pCR was viewed to be an objective outcome. A review by the pathologist on whether pCR had been achieved or not was unlikely to be influenced by unblinding. |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in the trial registry record were reported in the trial publication. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CONSORT diagram showed that 7/147 participants (3 in the intervention arm and 4 in the comparator arm) did not proceed with treatment due to patient and investigator decisions. For pCR analysis, only those who received treatment were included in the analysis. Justifications as to why this was the case were provided. |
| Other bias | Low risk | None identified. |
Wu 2018.
| Study characteristics | ||
| Methods | Accrual: January 2014 and February 2017 Single centre Phase of trial: 2 State study design: open‐label RCT Country: China Median follow‐up: not reported |
|
| Participants | Age: median 47, range 33–70 years Nodal status of breast cancer: 60% node positive, 40% node negative BRCA mutation: not reported Adjuvant or neoadjuvant: both (perioperative chemotherapy) Notable exclusion criteria: none |
|
| Interventions | Arm 1: before surgery: lobaplatin 30 mg/m2 + epirubicin 80 mg/m2 + docetaxel 75 mg/m2 every 3 weeks for 4 cycles; after surgery: 2 cycles Arm 2: before surgery: epirubicin 80 mg/m2 + docetaxel 75 mg/m2 every 3 weeks for 4 cycles; after surgery: 2 cycles |
|
| Outcomes | Primary
Secondary
Note: trial registration record lists additional outcomes as part of main objectives, i.e. OS and DFS |
|
| Notes | Trial registration record: ChiCTR‐TRC‐14005019 Study authors analysed participants who received chemotherapy rather than those participants who were randomised. Study did not report assessing the proportional hazards assumption. Funding considerations: funded by the Clinical Research Fund of Southwest Hospital, China and the Natural Science Fund of China (81302315). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation generated using a website (research randomizer). Baseline clinical characteristics were generally well‐balanced across treatment groups with exceptions being for participants aged ≥ 60 years, and stage II and III breast cancers. |
| Allocation concealment (selection bias) | Unclear risk | No information provided at single‐centre study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): DFS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes graded using the CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and, therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | Although there was no information regarding blinding of the pathologist who assessed this outcome, pCR was viewed to be an objective outcome. A review by the pathologist on whether pCR had been achieved or not was unlikely to be influenced by unblinding. |
| Selective reporting (reporting bias) | Unclear risk | Trial registry record indicates OS and DFS were the main objectives of this study; the trial publication stated that survival analyses were not yet possible due to short follow‐up times. No further publications have been presented with results for these important outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | CONSORT diagram (p.3) showed that a very small proportion of participants who were randomised (1 in intervention; 2 in comparator arms) were not included as part of an intention‐to‐treat analyses. Reasons for the exclusion were not provided. |
| Other bias | Low risk | None identified. |
Zhang 2016.
| Study characteristics | ||
| Methods | Accrual: May 2006 to December 2012 Single centre Phase of trial: 2 Study design: open‐label RCT Country where the trial was conducted: China Median follow‐up: 55 months |
|
| Participants | Age: median 47, range 24–73 years Nodal status of breast cancer: 77% node positive, 23% node negative Adjuvant or neoadjuvant: neoadjuvant Notable exclusion criteria: none |
|
| Interventions | Arm 1: paclitaxel 175 mg/m2, day 1 + carboplatin AUC5, day 2, every 3 weeks for 4–6 cycles Arm 2: epirubicin 75 mg/m2, day 1 + paclitaxel 175 mg/m2, day 2, every 3 weeks for 4–6 cycles |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Trial registration record: NCT01276769 Participants who underwent surgery and were not lost to follow‐up after surgery were included in analysis for efficacy outcomes. However, all randomised participants were included in toxicity assessment. Study did not report assessing the proportional hazards assumption. For relapse‐free survival and OS, we estimated the hazard ratio using Tierney's method. Funding considerations: funded by the Cancer Hospital, Chinese Academy of Medical Sciences. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "TNBC were stratified according to clinical stage, and then randomized to receive PC … or EP regimen." (p.60654) Baseline characteristics were generally well‐balanced across groups except for node involvement. No details about how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No information provided about what occurred at single site. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): DFS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): OS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes were graded as per CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and, therefore, knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Low risk | Although there was no information regarding blinding of the pathologist who assessed this outcome, pCR was viewed to be an objective outcome. A review by the pathologist on whether pCR had been achieved or not was unlikely to be influenced by unbinding. |
| Selective reporting (reporting bias) | Low risk | Outcomes prespecified in the trial registry record were reported in trial publication. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | CONSORT diagram outlined reasons for exclusions. For time‐to‐event outcomes, only those participants who had surgery were included in the analysis (not all those randomised) and for toxicity assessment, all randomised participants were included in analysis irrespective of whether they received treatment or not. There was some concern regarding the analytical approach used when reporting results. |
| Other bias | Low risk | None identified. |
Zhao 2014.
| Study characteristics | ||
| Methods | Accrual: April 2006 to February 2014 Single centre Phase of trial: not reported Trial design: RCT, no further details provided Country where the trial was conducted: China Follow‐up: not reported |
|
| Participants | Age: median 52 years Nodal status of breast cancer: 83% node positive, 17% node negative Adjuvant or neoadjuvant: neoadjuvant BRCA mutation: not reported Notable exclusion criteria: none |
|
| Interventions | Arm 1: paclitaxel 175 mg/m2 day 1, carboplatin AUC5 day 2, every 3 weeks for 2 cycles Arm 2: epirubicin 75 mg/m2 day 1, paclitaxel 175 mg/m2 day 2, every 3 weeks for 2 cycles |
|
| Outcomes | Outcomes listed in trial publication (not split by primary or secondary outcomes)
|
|
| Notes | Trial registration record could not be identified. Study authors did not appear to have analysed results based on the number of participants randomised (i.e. 2 participants were excluded from analysis with reasons not provided). Funding considerations: not collected. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients … were randomly assigned to …" Method to generate random sequence was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not reported in the journal publication. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information relating to blinding of participants or personnel was provided in the journal publication. |
| Blinding of outcome assessment (detection bias): toxicity | Unclear risk | No details were provided relating to blinding of outcome assessment for toxicities. |
| Blinding of outcome assessment (detection bias): neoadjuvant studies only: pCR | Unclear risk | No details were provided relating to blinding of outcome assessment for pCR. |
| Selective reporting (reporting bias) | Low risk | Outcomes described in the methods section in the trial publication were reported. We did not identify a trial record to cross‐check outcome reporting. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants in the 1 treatment arm dropped out with no details provided. They were not included in the analysis. No CONSORT diagram was provided. |
| Other bias | Unclear risk | Some translated material but insufficient detail to rule out any other bias. |
Zheng 2022.
| Study characteristics | ||
| Methods | Accrual: June 2009 and October 2015 Multicentre, 3 centres Phase of trial: 2 State study design: open‐label RCT Country or countries where the trial was conducted: China Median follow‐up: 97.6 months |
|
| Participants | Age: median 48.4, range 42–56 years Nodal status of breast cancer: 34% node positive, 66% node negative Adjuvant or neoadjuvant: adjuvant Notable exclusion criteria: none |
|
| Interventions | Arm 1: docetaxel 75 mg/m2 or paclitaxel 175 mg/m2 + carboplatin AUC5, every 3 weeks for 6 cycles Arm 2: epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2, every 3 weeks for 4 cycles, followed by docetaxel 75 mg/m2 or paclitaxel 175 mg/m2 every 3 weeks for 4 cycles |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Trial registration record: NCT01150513 All randomised participants were included in intention‐to‐treat analysis. The proportional hazards assumption was assessed and not met. For DFS and OS, we estimated the hazard ratio using Tierney's method. Funding considerations: this work was supported by Special Fund for breast health, Cancer Foundation of China, Capitals Funds for Health Improvement and Research (2018‐2‐4023) and National Natural Science Foundation of China (81672634). The authors stated that the funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation in a 1:1 ratio, method not described. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed 'centrally' and enroled participants at 3 institutions. It is likely that central allocation was done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were aware of treatment allocation. This may have been associated with some performance bias but it was not judged to be of serious concern given types of outcomes collected. |
| Blinding of outcome assessment (detection bias): DFS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): OS | Low risk | Lack of blinding unlikely to influence this outcome. |
| Blinding of outcome assessment (detection bias): toxicity | Low risk | Toxicity outcomes were graded as per CTCAE. Although the study was open‐label, grading symptoms using the CTCAE is standardised and therefore knowing treatment allocation may have had minimal effect on the grading of outcomes. |
| Selective reporting (reporting bias) | Low risk | All prespecified primary and secondary outcomes were reported. Important outcomes including DFS and OS are included at updated in subsequent publications. Quality of life was collected (as per conference abstract) but not yet reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed – all participants who were randomised were included in the analysis. |
| Other bias | Low risk | None identified. |
AUC: area under the curve; BRCA: breast cancer gene; CALGB: Cancer and Leukemia Group B; CTCAE: Common Terminology Criteria for Adverse Events; DFS: disease‐free survival; ECOG: Eastern Cooperative Oncology Group; EFS: event‐free survival; EORTC QLQ‐BR23: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire – Breast Cancer; EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life of Cancer Patient; EQ‐5D 5L: 5‐level Euroqol EQ‐5D; FACT‐An: Functional Assessment of Cancer Therapy – Anaemia; HRD: homologous recombination deficiency; MRI: magnetic resonance imaging; NCI CTC: National Cancer Institute Common Toxicity Criteria; NCI: National Cancer Institute; OS: overall survival; pCR: pathological complete response; RCB: residual cancer burden; RCT: randomised controlled trial; RECIST: Response Evaluation Criteria in Solid Tumors Criteria; RFS: recurrence‐free survival; TNBC: triple‐negative breast cancer.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ECOG‐ACRIN EA1131 | Study population included only people with TNBC with residual disease. |
| Jiang 2019 | Paper was translated and we were advised that this study was not an RCT. |
| NCT00004092 | It was not possible to decipher whether the cohort included people with TNBC. HER2 status was not checked. |
| SICOG 9908 | Data were not reported separately for people with TNBC. Study investigators were contacted but did not respond. |
| UMIN000030780 – jRCTs051180210 | Study population included only people with TNBC with residual disease. |
HER2: human epidermal receptor 2; RCT: randomised controlled trial; TNBC: triple‐negative breast cancer.
Characteristics of ongoing studies [ordered by study ID]
ChiCTR1800019501.
| Study name | A randomized controlled phase II clinical trial comparing neoadjuvant TP (docetaxel + cisplatin) with TAC (docetaxel + adriamycin + cyclophosphamide) regimen in the treatment of operable triple negative breast cancer |
| Methods | Accrual: recruiting Accrual target: 212 participants Single‐centre, phase 2 RCT Trial is being conducted in China Blinding: not specified |
| Participants | Clinical T2‐T4c or T1C with axillary lymph node‐positive Neoadjuvant setting |
| Interventions | Arm 1: intervention: docetaxel and cisplatin for 6 cycles Arm 2: comparator: docetaxel, doxorubicin and cyclophosphamide for 6 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 1 December 2018 Estimated completion date: 31 May 2025 |
| Contact information | Contact: Liu Zhenzhen (liuzhenzhen73@163.com) |
| Notes | Trial registration link: www.chictr.org.cn/showprojEN.html?proj=31567 Trial sponsor: He'nan Cancer Hospital Funding considerations: self‐funded |
ChiCTR1900023776.
| Study name | A randomized, controlled, single‐center clinical study for the efficacy and safety of docetaxel plus lobaplatin versus docetaxel plus epirubicin for neoadjuvant therapy in triple‐negative breast cancer |
| Methods | Accrual: recruiting Accrual target: 120 Single‐centre, phase 2 or 3 (unspecified) RCT Trial is being conducted in China Blinding: not specified |
| Participants | People with T2 and above Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | ARM 1: intervention: docetaxel and lobaplatin (no further details provided) ARM 2: comparator: docetaxel and epirubicin (no further details provided) |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 13 June 2019 Estimated completion date: 31 December 2026 |
| Contact information | Contact: Xiaowei Qi (qxw9908@foxmail.com) |
| Notes | Trial registration link: www.chictr.org.cn/showprojEN.html?proj=39908 Trial sponsor: Southwest Hospital, Army Medical University Funding considerations: self‐funded |
ChiCTR1900026499.
| Study name | Albumin‐bound paclitaxel combined with carboplatin versus epirubicin combined with docetaxel as neoadjuvant therapy for triple‐negative breast cancer: a multicenter randomized controlled phase IV clinical trial |
| Methods | Accrual: not yet recruiting Accrual target: 110 Multicentre, phase 4 RCT Trial is being conducted in China Blinding: participants, investigators, outcome assessors |
| Participants | People with stage II–III breast cancer Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | Arm 1: intervention: paclitaxel and carboplatin (no further details provided) Arm 2: comparator: epirubicin and docetaxel (no further details provided) |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 1 December 2019 Estimated completion date: 31 May 2026 |
| Contact information | Contact: Caigang Liu (liucg@sj‐hospital.org) |
| Notes | Trial registration link: www.chictr.org.cn/showprojEN.html?proj=44204 Trial sponsor: Shengjing Hospital of China Medical University Funding considerations: self‐financed |
ChiCTR2000039578.
| Study name | A prospective, open, multicenter, randomized controlled trial for the effect of albumin‐bound paclitaxel combined with cisplatin versus epirubicin combined with cyclophosphamide sequential docetaxel neoadjuvant therapy for triple negative breast cancer |
| Methods | Accrual: not yet recruiting Accrual target: 240 Multicentre, phase 4 RCT Trial is being conducted in China Blinding: not specified |
| Participants | People with early (T2‐3, N0‐1, M0) or locally advanced (T2‐3, N2‐N3, M0) breast cancer Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | Arm 1: intervention: paclitaxel and cisplatin (no further details provided) Arm 2: comparator: epirubicin, cyclophosphamide and docetaxel (no further details provided) |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 23 October 2020 Estimated completion date: 22 October 2025 |
| Contact information | Contact: Zhengkui Sun (403810956@qq.com) |
| Notes | Trial registration link: www.chictr.org.cn/showprojEN.html?proj=63610 Trial sponsor: Jiangxi Cancer Hospital Funding considerations: Jiangsu Hengrui Pharmaceutical Co, Ltd |
CTRI/2017/10/010272.
| Study name | Comparing two types of chemotherapy in breast cancer |
| Methods | Accrual: not yet recruiting Accrual target: 268 Single‐centre, phase 2 RCT Trial is being conducted in India Blinding: not specified |
| Participants | People with stage 2 and 3 breast cancer Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | Arm 1: intervention: docetaxel 75 mg/m2 day 1 and carboplatin AUC6 day 1 every 21 days for 6 cycles Arm 2: comparator: 5‐fluorouracil 500 mg/m2, epirubicin 100 mg/m2 and cyclophosphamide 500 mg/m2 day 1 every 21 days for 3 cycles followed by docetaxel 75 mg/m2 day 1 for 4 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 1 January 2017 Estimated completion date: not specified |
| Contact information | Contact: Biswajit Dubashi (drbiswajitdm@gmail.com) |
| Notes | Trial registration link: ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=20743 Trial sponsor: Jawaharlal Institute of Post Graduate Medical Education and Research Funding considerations: Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry |
CTRI/2019/05/019176.
| Study name | A trial comparing effect of addition of carboplatin to standard neoadjuvant chemotherapy for triple negative breast cancer patients |
| Methods | Accrual: not yet recruiting Accrual target: 50 Single‐centre, phase 3 RCT Trial is being conducted in India Blinding: not done, open‐label study |
| Participants | People with triple‐negative breast cancer Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | Arm 1: intervention: doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles followed by paclitaxel 80 mg/m2 (12 doses) plus carboplatin (AUC6) every 3 weeks for 4 cycles Arm 2: comparator: doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles followed by paclitaxel 80 mg/m2 (12 doses) and placebo every 3 weeks for 4 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 20 May 2019 Estimated completion date: May 2020 |
| Contact information | Contact: Bhargab Jyoti Saikia (bhargabjyotisaikia@gmail.com) |
| Notes | Trial registration link: ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=32946 Trial sponsor: Dr B Borooah Cancer Institute, Guwahati Funding considerations: Dr B Borooah Cancer Institute, Gopinath Nagar, Assam |
EUCTR2009‐015238‐31.
| Study name | Neo adjuvant chemotherapy in triple negative breast cancer (neo‐TN) |
| Methods | Accrual: active, not recruiting Accrual target: 310 Multicentre, phase 2 and 3 RCT Trial is being conducted in the Netherlands Blinding: open‐label study |
| Participants | Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | ARM 1: intervention: doxorubicin, cyclophosphamide, carboplatin and thiotepa ARM 2: comparator: doxorubicin and cyclophosphamide for non‐HRD tumours Other comparator arms existed but contained carboplatin |
| Outcomes | Primary
Secondary outcomes
|
| Starting date | Planned start date: January 2010 Estimated completion date: December 2029 |
| Contact information | Contact: S Linn (s.linn@nki.nl) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT01057069 Trial sponsor: The Netherlands Cancer Institute Funding considerations: KWF Netherlands |
EudraCT2016‐004384‐39.
| Study name | A clinical trial that examines whether the treatment with the medication olaparib in combination with the chemotherapy carboplatin is more effective than treatment with a standard chemotherapy (anthracycline/ taxane‐based) against a specific type of breast cancer |
| Methods | Accrual: active Accrual target: – Multicentre, phase 2 RCT Trial is being conducted in Australia Blinding: open‐label study |
| Participants | Early invasive triple‐negative breast cancer with positive homologous recombination deficiency (HRD) status Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | Arm 1: intervention: olioparib and carboplatin for 6 cycles Arm 2: comparator: docetaxel, doxorubicin and cyclophosphamide for 6 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: not specified Estimated completion date: not specified |
| Contact information | Contact: ABCSG (info@abcsg.at) |
| Notes | Trial registration link: clinicaltrialsregister.eu/ctr‐search/trial/2016‐004384‐39/AT Trial sponsor: Austrian Breast & Colorectal Cancer Study Group (ABCSG) Funding considerations: Astra Zeneca |
NCT00919880.
| Study name | Comparison of neo‐adjuvant weekly paclitaxel with or without carboplatin in early breast cancer |
| Methods | Accrual: completed, no results posted in ClinicalTrials.gov Accrual target: 148 Single‐centre, phase 2 RCT Trial is being conducted in China Blinding: open‐label study |
| Participants | People with medium‐ and high‐risk primary breast cancer Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | Arm 1: intervention: carboplatin AUC2 mg/mL every 3 weeks for 4 cycles and paclitaxel 80 mg/m2 every 3 weeks for 4 cycles Arm 2: comparator: paclitaxel 80 mg/m2 every 3 weeks for 4 cycles |
| Outcomes | Primary
Secondary: none listed |
| Starting date | Planned start date: July 2009 Actual completion date: December 2010 |
| Contact information | Contact: Tanfeng Wang (no contact details listed) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT00919880 Trial sponsor: Tao Ouyang Funding considerations: not provided in trial record |
NCT01752686.
| Study name | A phase III trial of carboplatin as adjuvant chemotherapy in triple negative breast cancer |
| Methods | Accrual: unknown Accrual target: 587 Phase 3 RCT Trial is being conducted in South Korea Blinding: open‐label study |
| Participants | Histologically confirmed invasive breast cancer (tumour > 2 cm, any nodal involvement) Adjuvant or neoadjuvant: adjuvant |
| Interventions | Arm 1: intervention: carboplatin (AUC6) on day 1, every 3 weeks for 6 cycles Arm 2: comparator: observation |
| Outcomes | Primary
Secondary
|
| Starting date | Estimated start date: March 2013 Estimated completion date: March 2018 |
| Contact information | Contact: Byeong Woo Park (nobellg@yuhs.ac) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT01752686 Trial sponsor: Severance Hospital Funding considerations: not specified |
NCT02041338.
| Study name | Study of optimizing neoadjuvant regimens in subtypes of breast cancer |
| Methods | Accrual: unknown Accrual target: 200 Single‐centre, phase 2 RCT Trial is being conducted in China Blinding: open‐label study |
| Participants | People with stage IIa–IIIc breast cancer Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | Arm 1: intervention: paclitaxel 175 mg/m2 and carboplatin AUC4 every 2 weeks for 4–6 cycles Arm 2: comparator: epirubicin 75 mg/m2 and paclitaxel 175 mg/m2 every 3 weeks for 4–6 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: January 2014 Estimated completion date: December 2017 |
| Contact information | Contact: Ying Fan (fanyingfy@medmail.com.cn) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT02041338 Trial sponsor: Chinese Academy of Medical Sciences Funding considerations: not specified in trial record |
NCT02455141.
| Study name | Adjuvant treatment of EC followed by taxane ± carboplatin in triple‐negative breast cancer |
| Methods | Accrual: recruiting Accrual target: 970 Multicentre, phase 3 RCT Trial is being conducted in China Blinding: open‐label study |
| Participants | Histologically confirmed triple‐negative breast cancer, with tumour removal by either modified radical mastectomy or local excision plus axillary lymph node dissection Adjuvant or neoadjuvant: adjuvant |
| Interventions | Arm 1: intervention: epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 day 1, every 3 weeks for 4 cycles followed by paclitaxel 80 mg/m2 days 1, 8, 15 every 4 weeks for 4 cycles and carboplatin AUC2 for 4 cycles or, docetaxel 75 mg/m2 day 1 every 3 weeks for 4 cycles and carboplatin AUC5–6 day 1 every 3 weeks for 4 cycles Arm 2: comparator: epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 day 1, every 3 weeks for 4 cycles followed by paclitaxel 80 mg/m2 days 1 every 12 weeks or docetaxel 80–100 mg/m2 every 3 weeks for 4 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Actual start date: July 2015 Estimated completion date: December 2023 |
| Contact information | Contact: Xiaosong Chen (chenxiaosong0156@hotmail.com) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT02455141 Trial sponsor: Shanghai Jiao Tong University School of Medicine Funding considerations: not specified in trial record |
NCT02488967.
| Study name | Doxorubicin hydrochloride and cyclophosphamide followed by paclitaxel with or without carboplatin in treating patients with triple‐negative breast cancer |
| Methods | Accrual: recruiting Accrual target: 782 Multicentre, phase 3 RCT Trial is being conducted in USA Blinding: open‐label study |
| Participants | People with node positive or high‐risk node negative triple negative breast cancer Adjuvant or neoadjuvant: adjuvant |
| Interventions | Arm 1: intervention: doxorubicin then paclitaxel on days 1, 8 and 15 and carboplatin on day 1 every 3 weeks for 4 cycles Arm 2: comparator: doxorubicin and cyclophosphamide on day 3 every 2 weeks for 4 cycles, followed by paclitaxel day 1 weekly for 12 weeks |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: July 2015 Estimated completion date: November 2023 |
| Contact information | Contact: Vicente Valero (askmdanderson@mdanderson.org) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT02488967 Trial sponsor: NRG Oncology Funding considerations: not provided in trial record |
NCT02641847.
| Study name | TA(E)C‐GP versus A(E)C‐T for the high risk TNBC patients and validation of the mRNA‐lncRNA signature |
| Methods | Accrual: unknown Accrual target: 503 Single‐centre, phase 2/3 RCT Trial is being conducted in China Blinding: open‐label study |
| Participants | People with triple‐negative breast cancer confirmed by pathology Adjuvant or neoadjuvant: adjuvant |
| Interventions | Arm 1: intervention ('high risk group A' in trial record): docetaxel 75 mg/m2 and doxorubicin 50 mg/m2 or epirubicin 75 mg/m2 on day 1 for 4 cycles and cyclophosphamide 500 mg/m2 on day 1, gemcitabine 1250 mg/m2 on days 1 and 8, and cisplatin 75 mg/m2 on day 1 every 21 days for 4 cycles Arm 2: comparator 1 and 2 ('high risk group B' and 'low risk group C' in trial record): doxorubicin 60 mg/m2 or epirubicin 90 mg/m2 on day 1 and cyclophosphamide 600 mg/m2 on day 1 for 4 cycles and docetaxel 100 mg/m2 on day 1 for 4 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: July 2015 Estimated completion date: June 2021 |
| Contact information | Contact: Zhi‐min Shao (zhimingshao@yahoo.com) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT02641847 Trial sponsor: Fudan University Funding considerations: not specified in trial record |
NCT02879513.
| Study name | Trial of adjuvant chemotherapy in breast cancer patients with pathological partial response and complete response to neoadjuvant chemotherapy |
| Methods | Accrual: recruiting Accrual target: 290 Single or multicentre, phase 3 RCT Trial is being conducted in China Blinding: open‐label study |
| Participants | People with locally advanced breast cancer and had weekly paclitaxel and cisplatin as neoadjuvant chemotherapy Adjuvant or neoadjuvant: adjuvant |
| Interventions | Arm 1: intervention 1 and 2 ('continue neoadjuvant regimen' and 'pathological complete response group with chemotherapy in trial record): paclitaxel 80 mg/m2 on days 1 and 8 for 16 weeks, followed by cisplatin 25 mg/m2 weekly on days 1, 8 and 15, every 4 weeks for 4 cycles Arm 2: comparator: epirubicin 75 mg/m2 on day 1 every 3 weeks for 4 cycles, cyclophosphamide 500 mg/m2 on day 1 every 3 weeks and 5‐fluorouracil 500 mg/m2 on day 1 every 3 weeks |
| Outcomes | Primary
Second
|
| Starting date | Planned start date: January 2014 Estimated completion date: December 2022 |
| Contact information | Contact: Jinsong Lu (lujjss@163.com) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT02879513 Trial sponsor: RenJi Hospital Funding considerations: not specified in trial record |
NCT02978495.
| Study name | Neoadjuvant carboplatin in triple negative breast cancer |
| Methods | Accrual: recruiting Accrual target: 120 Single or multicentre, phase 2 RCT Trial is being conducted in Brazil Blinding: open‐label study |
| Participants | People with stage II or III triple‐negative breast cancer Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | Arm 1: intervention ('A‐BRCA mutation' and 'C‐BRCA wild‐type' in trial record): doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by paclitaxel 80 mg/m2 every week for 12 weeks and carboplatin AUC1.5 once a week for 12 weeks Arm 2: comparator ('B‐BRCA mutation' and 'D‐BRCA wild‐type' in trial record): doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by paclitaxel 80 mg/m2 once a week for 12 weeks |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 17 May 2017 Estimated completion date: December 2022 |
| Contact information | Contact: Cristiano Souza (crispadua10@gmail.com) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT02978495 Trial sponsor: Barretos Cancer Hospital Funding considerations: not specified in trial record |
NCT03168880.
| Study name | A randomized controlled trial of neoadjuvant weekly paclitaxel versus weekly paclitaxel plus weekly carboplatin in women with large operable or locally advanced, triple negative breast cancer (TNBC) |
| Methods | Accrual: active, not recruiting Accrual target: 720 Single‐centre, phase 3 RCT Trial is being conducted in India Blinding: open‐label study |
| Participants | People with clinical staging T4, N0‐3, M0 or T1‐4, N2‐3, M0 and T3, N1, M0 with triple negative hormone status Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | Arm 1: intervention: paclitaxel 100 mg/m2 weekly for 8 weeks followed by carboplatin AUC2 weekly and doxorubicin 60 mg/mg2 or epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks Arm 2: comparator: paclitaxel 100 mg/m2 weekly for 8 weeks followed by doxorubicin 60 mg/mg2 or epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: April 2010 Estimated completion date: 30 November 2024 |
| Contact information | Contact: Rajendra A Badwe (no contact details provided) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT03168880 Trial sponsor: Tata Memorial Hospital Funding considerations: not specified in trial record |
NCT03201861.
| Study name | Addition of cisplatin to adjuvant chemotherapy for early stage breast cancer in high‐risk women |
| Methods | Accrual: recruiting Accrual target: 762 Single‐centre, phase 3 RCT Trial is being conducted in China Blinding: open‐label study |
| Participants | People with triple‐negative breast cancer confirmed by pathology Adjuvant or neoadjuvant: adjuvant |
| Interventions | Arm 1: intervention: paclitaxel 80 mg/m2 on days 1 and 8 for 12 weeks and cisplatin 25 mg/m2 on days 1, 8 and 15 every 4 weeks for 3 cycles followed by epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 day 1 for 4 cycles Arm 2: comparator: epirubicin 90 mg/m2 and cyclophosphamide 600 mg/m2 on day 1 every 3 weeks for 4 cycles followed by paclitaxel 80 mg/m2 for 12 weeks or docetaxel 75 mg/m2 on day 1 every 21 days for 4 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 27 July 2017 Estimated completion date: 31 December 2022 |
| Contact information | Contact: Yueyao Du (jessicayy8629@126.com) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT03201861 Trial sponsor: RenJi Hospital Funding considerations: not specified in trial record |
NCT03876886.
| Study name | The trial comparing dose‐dense AC‐T with TP as adjuvant therapy for TNBC with homologous recombination repair deficiency |
| Methods | Accrual: recruiting Accrual target: 200 Single‐centre, phase 3 RCT Trial is being conducted in China Blinding: open‐label study |
| Participants | People with histologically confirmed triple‐negative breast cancer, complete tumour removal by either modified radical mastectomy or local excision plus axillary lymph node dissection Adjuvant or neoadjuvant: adjuvant |
| Interventions | Arm 1: intervention: paclitaxel 175 mg/m2 on day 1 every 2 weeks for 8 cycles and carboplatin AUC3 day 2 every 2 weeks for 8 cycles Arm 2: comparator: epirubicin 90 mg/m2 on day 1, cyclophosphamide 600 mg/m2 on day 1 every 2 weeks for 4 cycles and paclitaxel 175 mg/m2 on day 1 every 2 weeks for 4 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 22 February 2019 Estimated completion date: December 2024 |
| Contact information | Contact: Binghe Xu (xubinghe@medmail.com.cn) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT03876886 Trial sponsor: Chinese Academy of Medical Sciences Funding considerations: not specified in trial record |
NCT04136782.
| Study name | Albumin‐bound paclitaxel and carboplatin versus epirubicin and docetaxel for triple‐negative breast cancer |
| Methods | Accrual: recruiting Accrual target: 110 Multicentre, phase 4 RCT Trial is being conducted in China Blinding: participant, investigator and outcome assessor |
| Participants | People with stage II–III triple‐negative breast cancer Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | Arm 1: intervention: paclitaxel 125 mg/m2 on days 1 and 8 every 21 days for 6 cycles and carboplatin AUC2 on days 1 and 8 every 21 days Arm 2: comparator: epirubicin 90 mg/m2 on day 1 every 21 days and docetaxel 75 mg/m2 every 3 weeks for 4 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 19 July 2021 Estimated completion date: 30 November 2026 |
| Contact information | Contact: Xi Gu (jadegx@163.com) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT04136782 Trial sponsor: Shengjing Hospital Funding considerations: not specified in trial record |
NCT04138719.
| Study name | Nab‐paclitaxel plus carboplatin versus nab‐paclitaxel plus epirubicin in the neoadjuvant therapy for breast cancer |
| Methods | Accrual: unknown Accrual target: 520 Multicentre, phase 2 RCT Trial is being conducted in China Blinding: open‐label study |
| Participants | People with histologically confirmed primary invasive triple‐negative breast cancer Adjuvant or neoadjuvant: neoadjuvant |
| Interventions | Arm 1: intervention: paclitaxel 125 mg/m2 on days 1 and 8 and carboplatin AUC5 on day 1 every 3 weeks for 6 cycles Arm 2: comparator: paclitaxel 125 mg/m2 on days 1 and 8 and epirubicin 75 mg/m2 on day 1 every 3 weeks for 6 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 20 November 2019 Estimated completion date: 20 June 2021 |
| Contact information | Contact: Cuizhi Geng (gengcuizhi@hotmail.com) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT04138719 Trial sponsor: Hebei Medical University Fourth Hospital Funding considerations: not specified in trial record |
NCT04296175.
| Study name | Carboplatin intensified chemotherapy for triple negative breast cancer (CITRINE) |
| Methods | Accrual: recruiting Accrual target: 808 Single‐centre, phase 3 RCT Trial is being conducted in China Blinding: open‐label study |
| Participants | People with high‐risk, triple‐negative breast cancer. High risk defined as positive lymph nodes or negative lymph nodes but ki‐67 is not less than 50% |
| Interventions | Arm 1: intervention: epirubicin 90 mg/m2 on day 1 and cyclophosphamide 600 mg/m2 on day 1 every 2 weeks followed by paclitaxel 80 mg/m2 and carboplatin AUC2 on days 1, 8 and 15 every 4 weeks Arm 2: comparator: epirubicin 90 mg/m2 on day 1 and cyclophosphamide 600 mg/m2 on day 1 every 2 or 3 weeks followed by paclitaxel 80 mg/m2 on days 1, 8 and 15 every 3 weeks |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 18 September 2018 Estimated completion date: June 2025 |
| Contact information | Contact: Zhimin Shao (zhimingshao@yahoo.com) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT04296175 Trial sponsor: Fudan University Funding considerations: not specified in trial record |
NCT04664972.
| Study name | The TP regimen in the treatment of early triple negative breast cancer |
| Methods | Accrual: recruiting Accrual target: 166 Single or multicentre, phase 2 RCT Trial is being conducted in China Blinding: open‐label study |
| Participants | People with clinical T2‐T4c, or T1c with axillary lymph node‐positive, triple‐negative breast cancer |
| Interventions | Arm 1: intervention: docetaxel 75 mg/m2 on day 1 and cisplatin 25 mg/m2 on days 1, 2 and 3 every 3 weeks for 6 cycles Arm 2: comparator: docetaxel 75 mg/m2, adriamycin 50 mg/m2 and cyclophosphamide 500 mg/m2 every 3 weeks for 6 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: 23 November 2018 Estimated completion date: 23 November 2022 |
| Contact information | Contact: Dechuang Jiao (jiaodechuang@163.com) |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT04664972 Trial sponsor: Henan Cancer Hospital Funding considerations: not specified in trial record |
Nordic Trip.
| Study name | Nordic trip, a randomized phase 3 study in early triple negative breast cancer |
| Methods | Accrual: recruiting Accrual target: 1800 Multicentre, phase 3 RCT Trial is being conducted in Sweden, Denmark, Finland and Iceland Blinding: open‐label study |
| Participants | People with early triple‐negative breast cancer stage I (> 10 mm) to III Adjuvant or neoadjuvant: both |
| Interventions | Arm 1: intervention: carboplatin followed by epirubicin and cyclophosphamide (further details not provided) Arm 2: comparator(s): paclitaxel followed epirubicin and cyclophosphamide or capecitabine followed by epirubicin and cyclophosphamide |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: not specified Estimated completion date: not specified |
| Contact information | Contact: Niklas Loman (niklas.loman@med.lu.se) |
| Notes | Trial registration link: not specified Trial sponsor: not specified Funding considerations: not specified |
PEARLY.
| Study name | Carboplatin in EARLY triple negative breast cancer trial (PEARLY Trial) |
| Methods | Accrual: recruiting Accrual target: 840 Multicentre, phase 3 RCT Trial is being conducted in South Korea Blinding: open‐label study |
| Participants | People with stage II/III operable triple‐negative breast cancer Adjuvant or neoadjuvant: both |
| Interventions | Arm 1: intervention: doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by taxane (docetaxel 75 mg/m2 every 3 weeks or paclitaxel 80 mg/m2 weekly for 12 doses) plus carboplatin AUC5 every 3 weeks for 4 cycles |
| Outcomes | Primary
Secondary
|
| Starting date | Planned start date: December 2015 Estimated completion date: March 2023 |
| Contact information | Contact: Joo Hyuk Sohn, Yonsei University |
| Notes | Trial registration link: clinicaltrials.gov/ct2/show/NCT02441933 Trial sponsor: Yonsei University Funding considerations: not specified in trial record |
AUC: area under the curve; RCT: randomised controlled trial.
Differences between protocol and review
Risk of bias assessment: we decided to employ the original Cochrane risk of bias tool (RoB 1) rather than the second version (RoB 2).
Subgroup analyses: two proposed subgroup analyses were not undertaken for the following reasons.
BRCA was the only high‐risk gene mutation stratified in the reported trials. Therefore, it was not possible to review any other 'high‐risk genes' as planned.
Most studies, except one, included a taxane in the chemotherapy regimen. Therefore, we did not conduct the planned subgroup analysis of 'platinum and taxane‐containing regimen versus taxane‐containing regimen'.
We added one new subgroup analysis on lymph node status as several trials stratified outcomes by this subgroup.
Sensitivity analyses: we added two sensitivity analyses that were not prespecified in the protocol. This included:
assessing the impact of different immunohistochemical definitions for triple‐negative breast cancer because the definition varied slightly across trials and
removing adjusted estimates when a meta‐analysis included both unadjusted and adjusted values. In this review, we did not use any adjusted estimates.
We did not conduct a sensitivity analysis based on risk of bias because all studies reporting on DFS and OS were at low risk of bias for most if not all domains.
Contributions of authors
Draft the protocol: SM, MW, AG with support of all authors
Study selection: MW, AG, SM, the Cochrane Breast Cancer Group's Information Specialist
Extract data from studies: SM, MW with support of AG
Enter data into Review Manager Web: MW, SM
Carry out the analysis: SM, MW, AG, SE with support of all authors
Interpret the analysis: SM, AG, MW, SE, JB, RD with support of all authors
Draft the final review: SM, MW, AG with support of all authors
Disagreement resolution: JB, RD
Update the review: SM, AG
Sources of support
Internal sources
NHMRC Clinical Trials Centre, University of Sydney, Australia
External sources
No sources of support provided
Declarations of interest
SM: none.
MW: none. Melina is a member of the Cochrane Breast Cancer editorial team but was not involved in the editorial process for this review.
SE: none.
JB: none related to this review. Jane has received funding to travel to conferences from Novartis. Jane has served on advisory boards for Pfizer and Lilly for unrelated matters and payment has been made to her institution.
RD: none.
AG: none related to this review. Annabel reports consultancy/expert witness or advisory roles for Pfizer (2018, 2019) and AstraZeneca (2018) for matters unrelated to this review topic. There is no competing interest associated with funding of travel to attend an educational meeting, or to provide expertise regarding Cancer Genetic counselling in Australia. Annabel also receives ongoing funds for editorial support for manuscript writing for the EMBRACA trial (not related to this review). Annabel is also a member of the Cochrane Breast Cancer editorial team but was not involved in the editorial process for this review.
New
References
References to studies included in this review
ADAPT‐TN {published data only}
- Gluz O, Liedtke C, Prat A, Christgen M, Gebauer D, Kates RE, et al. Association of molecular subtype, proliferation, and immune genes with efficacy of carboplatin versus gemcitabine addition to taxane-based, anthracycline-free neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC): results of the randomized WSG ADAPT-TN trial. Journal of Clinical Oncology 2017;35(15 Suppl):573. [Google Scholar]
- Gluz O, Nitz U, Christgen M, Grischke EM, Forstbauer H, Braun MW, et al. Efficacy of 12 weeks neoadjuvant nab-paclitaxel combined with carboplatinum vs. gemcitabine in triple-negative breast cancer: WSG-ADAPT TN randomized phase II trial. Journal of Clinical Oncology 2015;33(15 Suppl):1032. [Google Scholar]
- Gluz O, Nitz U, Liedtke C, Christgen M, Grischke EM, Forstbauer H, et al. Comparison of neoadjuvant nab-paclitaxel+carboplatin vs nab-paclitaxel+gemcitabine in triple-negative breast cancer: randomized WSG-ADAPT-TN trial results. Journal of the National Cancer Institute 2018;110(6):628-37. [DOI] [PubMed] [Google Scholar]
- Gluz O, Nitz U, Liedtke C, Christgen M, Grischke EM, Forstbauer H, et al. Impact of 12 weeks nab-paclitaxel + carboplatin or gemcitabine followed by anthracycline administration according to pCR in triple-negative early breast cancer: survival results of WSG-ADAPT-TN phase II trial. Journal of Clinical Oncology 2018;36(15 Suppl):573. [Google Scholar]
- Gluz O, Nitz U, Liedtke C, Christgen M, Grischke EM, Forstbauer H, et al. Prognostic impact of anthracyclines and immune/proliferation markers in TNBC according to pCR after de-escalated neoadjuvant chemotherapy with 12 weeks of nab-paclitaxel/carboplatin or gemcitabine: Survival results of WSG-ADAPT-TN phase II trial. Annals of Oncology 2018;29(Suppl 8):viii703. [Google Scholar]
- Gluz O, Nitz U, Liedtke C, Christgen M, Sotlar K, Grischke EM, et al. Comparison of 12 weeks neoadjuvant Nab-paclitaxel combined with carboplatinum vs. gemcitabine in triple-negative breast cancer: WSG – ADAPT TN randomized phase II trial. Cancer Research 2016;76(4 Suppl):S6-07. [Google Scholar]
- Gluz O, Nitz U, Liedtke C, Prat A, Christgen M, Feuerhake F, et al. No survival benefit of chemotherapy escalation in patients with pCR and "high-immune" triple-negative early breast cancer in the neoadjuvant WSG-ADAPT-TN trial. Cancer Research 2019;79(4 Suppl):GS5-06. [Google Scholar]
- Harbeck N, Hofmann D, Gluz O, Kates RE, Kummel S, Nuding B, et al. ADAPT: Adjuvant Dynamic marker-Adjusted Personalized Therapy trial optimizing risk assessment and therapy response prediction in early breast cancer. Journal of Clinical Oncology 2013;31(15 Suppl):TPS655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke C, Gluz O, Nitz U, Christgen M, Sotlar K, Grischke EM, et al. Comparison of 12 weeks neoadjuvant nab-paclitaxel combined with carboplatinum vs. gemcitabine in triple negative breast cancer: WSG-ADAPT TN randomized phase II trial. Oncology Research and Treatment 2016;1:53. [Google Scholar]
- NCT01815242. Adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer - triple negative breast cancer (ADAPT). clinicaltrials.gov/show/NCT01815242 (first received 21 March 2013).
- Nitz U, Gluz O, Von Schumann R, Hofmann D, Kates RE, Kuemmel S, et al. ADAPT – Adjuvant Dynamic marker-Adjusted Personalized Therapy trial optimizing risk assessment and therapy response prediction in early breast cancer. Cancer Research. 2015;75(9 Suppl):OT3-2-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ando 2014 {published data only}
- Ando M, Yamauchi H, Aogi K, Shimizu S, Iwata H, Masuda N, et al. Randomized phase II study of weekly paclitaxel with and without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA breast cancer without HER2 overexpression. Breast Cancer Research and Treatment 2014;145(2):401-9. [DOI] [PubMed] [Google Scholar]
- Iwase M, Ando M, Aogi K, Aruga T, Inoue K, Shimomura A, et al. Long-term survival analysis of addition of carboplatin to neoadjuvant chemotherapy in HER2-negative breast cancer. Breast Cancer Research and Treatment 2020;180(3):687-94. [DOI] [PubMed] [Google Scholar]
- Tamura K, Hashimoto J, Tsuda H, Yoshida M, Yamauchi H, Aogi K, et al. Randomized phase II study of weekly paclitaxel with or without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA HER2-negative breast cancer. Journal of Clinical Oncology 2014;32(15 Suppl):1017. [Google Scholar]
- UMIN000003355. Randomized phase II study of preoperative systemic chemotherapy of carboplatin/weekly paclitaxel followed by CEF versus weekly paclitaxel followed by CEF for operable HER2-negative breast cancer. Center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000004074 (first received 22 March 2010).
BrighTNess comparison 1 {published data only}
- EudraCT2013-002377-21. A study evaluating safety and efficacy of the addition of ABT-888 plus carboplatin versus the addition of carboplatin to standard chemotherapy versus standard chemotherapy in subjects with early stage triple negative breast cancer. clinicaltrialsregister.eu/ctr-search/search?query=2013-002377-21 (first received 17 November 2014).
- Filho OM, Stover DG, Asad S, Ansell PJ, Watson M, Loibl S, et al. Association of immunophenotype with pathologic complete response to neoadjuvant chemotherapy for triple-negative breast cancer: a secondary analysis of the BrighTNess phase 3 randomized clinical trial. JAMA Oncology 2021;7(4):603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer CE, Sikov WM, Huober J, Rugo HS, Wolmark N, O'Shaughnessy J, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Annals of Oncology 2022;33(4):384-94. [DOI] [PubMed] [Google Scholar]
- Golshan M, Loibl S, Huober JB, O'Shaughnessy J, Rugo HS, Wolmark N, et al. Breast conservation after neoadjuvant chemotherapy for triple-negative breast cancer: surgical results from an international randomized trial (BrighTNess). Journal of Clinical Oncology 2017;35(15 Suppl):514. [Google Scholar]
- Golshan M, Loibl S, Wong SM, Houber JB, O'Shaughnessy J, Rugo HS, et al. Breast conservation after neoadjuvant chemotherapy for triple-negative breast cancer: surgical results from the BrighTNess randomized clinical trial. JAMA Surgery 2020;155(3):e195410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshan M, Wong SM, Loibl S, Huober JB, O'Shaughnessy J, Rugo HS, et al. Early assessment with magnetic resonance imaging for prediction of pathologic response to neoadjuvant chemotherapy in triple-negative breast cancer: results from the phase III BrighTNess trial. European Journal of Surgical Oncology 2020;46(2):223-8. [DOI] [PubMed] [Google Scholar]
- Loibl S, O'Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncology 2018;19(4):497-509. [DOI] [PubMed] [Google Scholar]
- Loibl S, Sikov W, Huober J, Rugo HS, Wolmark N, O'Shaughnessy J, et al. Event-free survival (EFS), overall survival (OS), and safety of adding veliparib (V) plus carboplatin (Cb) or carboplatin alone to neoadjuvant chemotherapy in triple-negative breast cancer (TNBC) after >=4 years of follow-up: BrighTNess, a randomized phase III trial. Annals of Oncology 2021;32(Suppl 5):S408. [Google Scholar]
- Metzger-Filho O, Collier K, Asad S, Ansell PJ, Watson M, Bae J, et al. Matched cohort study of germline BRCA mutation carriers with triple negative breast cancer in BrighTNess. NPJ Breast Cancer 2021;7(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02032277. A study evaluating safety and efficacy of the addition of ABT-888 plus carboplatin versus the addition of carboplatin to standard chemotherapy versus standard chemotherapy in subjects with early stage triple negative breast cancer. clinicaltrials.gov/show/NCT02032277 (first received 10 January 2014).
- Telli ML, Metzger O, Timms K, Evans B, Vogel D, Wei H, et al. Evaluation of homologous recombination deficiency (HRD) status with pathological response to carboplatin +/- veliparib in BrighTNess, a randomized phase 3 study in early stage TNBC. Journal of Clinical Oncology 2018;36(15 Suppl):519. [Google Scholar]
BrighTNess comparison 2 {published data only}
- EudraCT2013-002377-21. A study evaluating safety and efficacy of the addition of ABT-888 plus carboplatin versus the addition of carboplatin to standard chemotherapy versus standard chemotherapy in subjects with early stage triple negative breast cancer. clinicaltrialsregister.eu/ctr-search/search?query=2013-002377-21 (first received 17 November 2014).
- Filho OM, Stover DG, Asad S, Ansell PJ, Watson M, Loibl S, et al. Association of immunophenotype with pathologic complete response to neoadjuvant chemotherapy for triple-negative breast cancer: a secondary analysis of the BrighTNess phase 3 randomized clinical trial. JAMA Oncology 2021;7(4):603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer CE, Sikov WM, Huober J, Rugo HS, Wolmark N, O'Shaughnessy J, et al. Long-term efficacy and safety of addition of carboplatin with or without veliparib to standard neoadjuvant chemotherapy in triple-negative breast cancer: 4-year follow-up data from BrighTNess, a randomized phase III trial. Annals of Oncology 2022;33(4):384-94. [DOI] [PubMed] [Google Scholar]
- Golshan M, Loibl S, Huober JB, O'Shaughnessy J, Rugo HS, Wolmark N, et al. Breast conservation after neoadjuvant chemotherapy for triple-negative breast cancer: surgical results from an international randomized trial (BrighTNess). Journal of Clinical Oncology 2017;35(15 Suppl):514. [Google Scholar]
- Golshan M, Loibl S, Wong SM, Houber JB, O'Shaughnessy J, Rugo HS, et al. Breast conservation after neoadjuvant chemotherapy for triple-negative breast cancer: surgical results from the BrighTNess randomized clinical trial. JAMA Surgery 2020;155(3):e195410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshan M, Wong SM, Loibl S, Huober JB, O'Shaughnessy J, Rugo HS, et al. Early assessment with magnetic resonance imaging for prediction of pathologic response to neoadjuvant chemotherapy in triple-negative breast cancer: results from the phase III BrighTNess trial. European Journal of Surgical Oncology 2020;46(2):223-8. [DOI] [PubMed] [Google Scholar]
- Loibl S, O'Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncology 2018;19(4):497-509. [DOI] [PubMed] [Google Scholar]
- Loibl S, Sikov W, Huober J, Rugo HS, Wolmark N, O'Shaughnessy J, et al. Event-free survival (EFS), overall survival (OS), and safety of adding veliparib (V) plus carboplatin (Cb) or carboplatin alone to neoadjuvant chemotherapy in triple-negative breast cancer (TNBC) after >=4 years of follow-up: BrighTNess, a randomized phase III trial. Annals of Oncology 2021;32(Suppl 5):S408. [Google Scholar]
- Metzger-Filho O, Collier K, Asad S, Ansell PJ, Watson M, Bae J, et al. Matched cohort study of germline BRCA mutation carriers with triple negative breast cancer in BrighTNess. NPJ Breast Cancer 2021;7(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02032277. A study evaluating safety and efficacy of the addition of ABT-888 plus carboplatin versus the addition of carboplatin to standard chemotherapy versus standard chemotherapy in subjects with early stage triple negative breast cancer. clinicaltrials.gov/show/NCT02032277 (first received 10 January 2014).
- Telli ML, Metzger O, Timms K, Evans B, Vogel D, Wei H, et al. Evaluation of homologous recombination deficiency (HRD) status with pathological response to carboplatin +/- veliparib in BrighTNess, a randomized phase 3 study in early stage TNBC. Journal of Clinical Oncology 2018;35(15 Suppl):519. [Google Scholar]
CALGB 40603 {published data only}
- Golshan M, Cirrincione CT, Carey LA, Sikov WM, Berry DA, Burstein HJ, et al. Impact of neoadjuvant therapy on breast conservation rates in triple negative and HER2-positive breast cancer: combined results of CALGB 40603 and 40601 (Alliance). Journal of Clinical Oncology 2015;33(15 Suppl):1007. [Google Scholar]
- Golshan M, Cirrincione CT, Sikov WM, Berry DA, Jasinski S, Weisberg TF, et al. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Annals of Surgery 2015;262(3):434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00861705. Paclitaxel with or without carboplatin and/or bevacizumab followed by doxorubicin and cyclophosphamide in treating patients with breast cancer that can be removed by surgery. clinicaltrials.gov/show/NCT00861705 (first received 13 March 2009).
- Shepherd JH, Ballman K, Polley MC, Campbell JD, Fan C, Selitsky S, et al. CALGB 40603 (Alliance): long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. Journal of Clinical Oncology 2022;40(12):1323-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JH, Hyslop T, Fan C, Martinez AF, Parker J, Hoadley K, et al. Genomic analysis of the CALGB 40603 (Alliance) neoadjuvant trial in TNBC identifies immune features associated with pathological complete response and event-free survival. Cancer Research 2021;81(4 Suppl):PD9-03. [Google Scholar]
- Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione C, Tolaney S, et al. Impact of the addition of carboplatin (Cb) and/or bevacizumab (B) to neoadjuvant weekly paclitaxel (P) followed by dose-dense AC on pathologic complete response (pCR) rates in triple-negative breast cancer (TNBC): CALGB 40603 (Alliance). Cancer Research 2013;73(24 Suppl):S5-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Event-free and overall survival following neoadjuvant weekly paclitaxel and dose-dense AC +/- carboplatin and/or bevacizumab in triple-negative breast cancer: outcomes from CALGB 40603 (Alliance). Cancer Research 2016;76(4 Suppl):S2-05. [Google Scholar]
- Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603. Journal of Clinical Oncology 2015;33(1):13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikov WM, Perou CM, Golshan M, Collyar D, Berry DA, Hahn OM, et al. Randomized phase II trial of adding carboplatin and/or bevacizumab to neoadjuvant weekly paclitaxel and dose-dense AC in triple-negative breast cancer. Journal of Clinical Oncology 2010;28(15 Suppl):TPS110. [Google Scholar]
- Sikov WM, Polley MY, Twohy E, Perou CM, Singh B, Berry DA, et al. CALGB (Alliance) 40603: Long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT) +/- carboplatin (Cb) and bevacizumab (Bev) in triple negative breast cancer (TNBC). Journal of Clinical Oncology 2019;37(15 Suppl):591. [Google Scholar]
CALGB 40603 – comparison 1 (without bevacizumab) {published data only}
- Shepherd JH, Ballman K, Polley MC, Campbell JD, Fan C, Selitsky S, et al. CALGB 40603 (Alliance): long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. Journal of Clinical Oncology 2022;40(12):1323-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603. Journal of Clinical Oncology 2015;33(1):13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
CALGB 40603 – comparison 2 (with bevacizumab) {published data only}
- Shepherd JH, Ballman K, Polley MC, Campbell JD, Fan C, Selitsky S, et al. CALGB 40603 (Alliance): long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. Journal of Clinical Oncology 2022;40(12):1323-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603. Journal of Clinical Oncology 2015;33(7):13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
GEICAM 2006‐03 {published data only}
- Alba E, Chacon JI, Lluch A, Anton A, Estevez L, Cirauqui B, et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting. Results from the GEICAM/2006-03, multicenter study. Breast Cancer Research and Treatment 2012;136(2):487-93. [DOI] [PubMed] [Google Scholar]
- Alba E, Chacon JI, Lluch A, Garcia-Estevez L, Anton A, Cirauqui B, et al. Chemotherapy (CT) with or without carboplatin as neoadjuvant treatment in patients with basal-like breast cancer: GEICAM 2006-03 – a multicenter, randomized phase II study. Journal of Clinical Oncology 2011;29(15 Suppl):1015. [Google Scholar]
- NCT00432172. Selective neoadjuvant treatment according to immunohistochemical subtype for HER2 negative breast cancer patients. clinicaltrials.gov/show/NCT00432172 (first received 7 February 2007).
- Santonja A, Albanell J, Chacon JI, Lluch A, Sanchez-Munoz A, Rojo F, et al. Triple-negative breast cancer subtypes and pathologic complete-response rate to neoadjuvant chemotherapy: results from the GEICAM/2006-2003 study. Journal of Clinical Oncology 2014;32(15 Suppl):1024. [Google Scholar]
GeparOcto {published data only}
- NCT02125344. A phase III trial comparing two dose-dense, dose-intensified approaches (ETC and PM(Cb)) for neoadjuvant treatment of patients with high-risk early breast cancer (GeparOcto). clinicaltrials.gov/show/NCT02125344. (first received 29 April 2014).
- Pohl E, Schneeweiss A, Hauke J, Moebus V, Furlanetto J, Denkert C, et al. Germline mutation status and therapy response in patients with triple negative breast cancer (TNBC): results of the GeparOcto study. Annals of Oncology 2018;29(8 Suppl):viii77. [Google Scholar]
- Pohl-Rescigno E, Hauke J, Loibl S, Mobus V, Denkert C, Fasching PA, et al. Association of germline variant status with therapy response in high-risk early-stage breast cancer: a secondary analysis of the GeparOcto randomized clinical trial. JAMA Oncology 2020;6(5):744-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeweiss A, Mobus V, Tesch H, Hanusch C, Denkert C, Lubbe K, et al. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto-GBG 84): a randomised phase III trial. European Journal of Cancer 2019;106:181-92. [DOI] [PubMed] [Google Scholar]
- Schneeweiss A, Mobus V, Tesch H, Klare P, Denkert C, Kast K, et al. Survival analysis of the randomized phase III GeparOcto trial comparing neoadjuvant chemotherapy (NACT) of iddEPC versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer, TNBC) (PM(Cb)) for patients (pts) with high-risk early breast cancer (BC). Annals of Oncology 2020;31(4 Suppl):S303. [Google Scholar]
- Schneeweiss A, Moebus V, Blohmer JU, Dan Costa S, Denkert C, Eidtmann H, et al. A randomized phase III trial comparing two dose-dense dose-intensified approaches (EPC and PM(Cb)) for neoadjuvant treatment of patients with high-risk early breast cancer (GeparOcto). Journal of Clinical Oncology 2015;33(15 Suppl):TPS1101. [Google Scholar]
- Schneeweiss A, Moebus V, Tesch H, Hanusch C, Denkert C, Luebbe K, et al. A randomised phase III trial comparing two dose-dense, dose-intensified approaches (EPC and PM(Cb)) for neoadjuvant treatment of patients with high risk early breast cancer (GeparOcto). Journal of Clinical Oncology 2017;35(15 Suppl):518. [Google Scholar]
GeparOLA {published data only}
- EUDRACT2015-003509-41-DE. A randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel / carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients with HER2-negative early breast cancer and homologous recombination deficiency (HRD) (GeparOla). clinicaltrialsregister.eu/ctr-search/search?query=2015-003509-41 (first received 1 August 2016).
- Fasching PA, Blohmer JU, Burchardi N, Costa SD, Denkert C, Hanusch C, et al. A randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel/carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients with HER2-negative early breast cancer and homologous recombination deficiency (HRD): GeparOLA. Journal of Clinical Oncology 2016;34(15 Suppl):TPS1096. [Google Scholar]
- Fasching PA, Jackisch C, Rhiem K, Schneeweiss A, Klare P, Hanusch C, et al. GeparOLA: a randomized phase II trial to assess the efficacy of paclitaxel and olaparib in comparison to paclitaxel/carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients (pts) with HER2-negative early breast cancer. Journal of Clinical Oncology 2019;37(15 Suppl):506. [Google Scholar]
- Fasching PA, Link T, Hauke J, Seither F, Jackisch C, Klare P, et al. Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study). Annals of Oncology 2021;32(1):49-57. [DOI] [PubMed] [Google Scholar]
- Hauke J, Ernst C, Fasching PA, Jackisch C, Seither F, Klare P, et al. Germline mutation status and therapy response in patients with homologous recombination deficient, HER2-negative early breast cancer: results of the GeparOLA study (NCT02789332). Annals of Oncology 2020;31(4 Suppl):S313. [Google Scholar]
- NCT02789332. Assessing the efficacy of paclitaxel and olaparib in comparison to paclitaxel / carboplatin followed by epirubicin/cyclophosphamide as neoadjuvant chemotherapy in patients with HER2-negative early breast cancer and homologous recombination deficiency. clinicaltrials.gov/show/NCT02789332 (first received 3 June 2016).
GeparSixto {published data only}
- Darb-Esfahani S, Weichert W, Denkert C, Minckwitz G, Nekljudova V, Lindner J, et al. P53 mutations in HER2 positive and triple negative breast cancer treated with neoadjuvant chemotherapy – a translational subproject of the GeparSixto trial. Annals of Oncology 2015;26(Suppl 3):III15. [Google Scholar]
- Denkert C, Loibl S, Salat C, Sinn BV, Schem C, Endris V, et al. Increased tumor-associated lymphocytes predict benefit from addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer in the GeparSixto trial (GBG 66). Cancer Research 2013;73(24 Suppl):S1-06. [Google Scholar]
- Denkert C, Minckwitz G, Brase JC, Darb-Esfahani S, Gade S, Kronenwett R, et al. Expression of immunologic genes in triple-negative and HER2-positive breast cancer in the neoadjuvant GEPARSIXTO trial: prediction of response to carboplatin-based chemotherapy. Journal of Clinical Oncology 2014;32(15 Suppl):510. [Google Scholar]
- Denkert C, Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. Journal of Clinical Oncology 2015;33(9):983-91. [DOI] [PubMed] [Google Scholar]
- EUDRAC2011-000553-23-DE. A randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer – GeparSixto. clinicaltrialsregister.eu/ctr-search/search?query=2011-000553-23 (first received 29 September 2011).
- Guo S, Loibl S, Minckwitz G, Darb-Esfahani S, Lederer B, Denkert C. PIK3CA H1047R mutation associated with a lower pathological complete response rate in triple-negative breast cancer patients treated with anthracycline-taxane-based neoadjuvant chemotherapy. Cancer Research 2020;52(3):689-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnen E, Lederer B, Hauke J, Loibl S, Krober S, Schneeweiss A, et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: secondary analysis of the GeparSixto randomized clinical trial. JAMA Oncology 2017;3(10):1378-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llop-Guevara A, Loibl S, Villacampa G, Vladimirova V, Schneeweiss A, Karn T, et al. Association of RAD51 with homologous recombination deficiency (HRD) and clinical outcomes in untreated triple-negative breast cancer (TNBC): analysis of the GeparSixto randomized clinical trial. Annals of Oncology 2021;32(12):1590-6. [DOI] [PubMed] [Google Scholar]
- Loibl S, Denkert C, Loi S, Andre F, Mueller B, Schneeweiss A, et al. PIK3CA mutations in primary HER2-positive and triple negative breast cancer. Journal of Clinical Oncology 2013;31(15 Suppl):11061. [Google Scholar]
- Loibl S, Denkert C, Schneeweis A, Paepke S, Lehmann A, Rezai M, et al. PIK3CA mutation predicts resistance to anti-HER2/chemotherapy in primary HER2-positive/hormone-receptor-positive breast cancer-prospective analysis of 737 participants of the GeparSixto and GeparQuinto studies. Cancer Research 2013;73(24 Suppl):S4-06. [Google Scholar]
- Loibl S, Weber KE, Timms KM, Elkin EP, Hahnen E, Fasching PA, et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Annals of Oncology 2018;29(12):2341-7. [DOI] [PubMed] [Google Scholar]
- NCT01426880. Addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). clinicaltrials.gov/show/NCT01426880 (first received 1 September 2001).
- Stevic I, Muller V, Weber K, Fasching PA, Karn T, Marme F, et al. Specific microRNA signatures in exosomes of triple-negative and HER2-positive breast cancer patients undergoing neoadjuvant therapy within the GeparSixto trial. BMC Medicine 2018;16(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untch M, Schneeweiss A, Salat C, Rezai M, Zahm DM, Klare P, et al. Long-term survival analysis of the randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative (TNBC) and HER2-positive early breast cancer (GeparSixto). Annals of Oncology 2017;28(Suppl 5):V49. [Google Scholar]
- Minckwitz G, Hahnen E, Fasching PA, Hauke J, Schneeweis A, Salat C, et al. Pathological complete response (pCR) rates after carboplatin-containing neoadjuvant chemotherapy in patients with germline BRCA (gBRCA) mutation and triple-negative breast cancer (TNBC): results from GeparSixto. Journal of Clinical Oncology 2014;32(15 Suppl):1005. [Google Scholar]
- Minckwitz G, Loibl S, Schneeweiss A, Salat CT, Rezai M, Zahm DM, et al. Early survival analysis of the randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). Cancer Research 2016;76(4 Suppl):S2-04. [Google Scholar]
- Minckwitz G, O'Shaughnessy J, Winer EP, Wolmark N, Geyer CE, Huober JB, et al. Phase III study evaluating safety and efficacy of the addition of veliparib plus carboplatin versus the addition of carboplatin to standard neoadjuvant chemotherapy in subjects with early-stage triple-negative breast cancer (TNBC). Journal of Clinical Oncology 2014;32(15 Suppl):TPS1149. [Google Scholar]
- Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncology 2014;15(7):747-56. [DOI] [PubMed] [Google Scholar]
- Minckwitz G, Schneeweiss A, Salat C, Rezai M, Zahm DM, Klare P, et al. A randomized phase II trial investigating the addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer (GeparSixto). Journal of Clinical Oncology 2013;31(15 Suppl):1004. [Google Scholar]
- Minckwitz G, Timms K, Untch M, Elkin EP, Fasching PA, Schneeweiss A, et al. Prediction of pathological complete response (pCR) by homologous recombination deficiency (HRD) after carboplatin-containing neoadjuvant chemotherapy in patients with TNBC: results from GeparSixto. Journal of Clinical Oncology 2015;33(15 Suppl):1004. [Google Scholar]
- Minckwitz G, Timms K, Untch M, Elkin EP, Hahnen E, Fasching PA, et al. Homologous repair deficiency (HRD) as measure to predict the effect of carboplatin on survival in the neoadjuvant phase II trial GeparSixto in triple-negative early breast cancer. Cancer Research 2017;77(4 Suppl):P1-09-02. [Google Scholar]
Gigolaeva 2019 {published data only}
- Gigolaeva L, Krivorotko P, Zhiltsova E, Dashyan G, Chadjimatova S, Pesotckiy R, et al. Neoadjuvant chemotherapy regimens for triple negative breast cancer patients. Breast 2019;44(Suppl 1):S70. [Google Scholar]
INFORM {published data only}
- NCT01670500. Cisplatin vs. doxorubicin/cyclophosphamide in BRCA. clinicaltrials.gov/show/NCT01670500 (first received 22 August 2012).
- Tung N, Arun B, Hacker MR, Hofstatter E, Toppmeyer DL, Isakoff SJ, et al. TBCRC 031: randomized phase II study of neoadjuvant cisplatin versus doxorubicin-cyclophosphamide in germline BRCA carriers with HER2-negative breast cancer (the INFORM trial). Journal of Clinical Oncology 2020;38(14):1539-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung N, Arun B, Hofstatter E, Hacker MR, Toppmeyer DL, Isakoff SJ, et al. Cisplatin versus doxorubicin/cyclophosphamide as neoadjuvant treatment in germline BRCA mutation carriers (BRCA carriers) with HER2-negative breast cancer: results from the INFORM trial (TBCRC 031). Cancer Research 2019;80(4 Suppl):GS6-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
I‐SPY2 {published data only}
- Liu MC, Symmans WF, Yau C, Chen YY, Rugo HS, Olopade OF, et al. Residual cancer burden (RCB) with veliparib/carboplatin in the I-SPY2 trial. Cancer Research 2016;76(4 Suppl):P3-07-49. [Google Scholar]
- NCT01042379. I-SPY TRIAL: neoadjuvant and personalized adaptive novel agents to treat breast cancer (I-SPY). clinicaltrials.org/show/NCT01042379 (first received 5 January 2010).
- Printz C. I-SPY2 trial yields first results on combination therapy for triple-negative breast cancer. Cancer 2014;120(6):773. [DOI] [PubMed] [Google Scholar]
- Rugo HS, Olopade OI, DeMichele A, Yau C, van't Veer LJ, Buxton MB, et al. Adaptive randomization of veliparib-carboplatin treatment in breast cancer. New England Journal of Medicine 2016;375(1):23-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Veer L, Esserman L, Sanil A, Glas A, Severson T, Linn SC, et al. DNA repair deficiency biomarkers and identification of ER positive breast cancer patients who may benefit from veliparib/carboplatin: results from the I-SPY 2 trial. Journal of Clinical Oncology 2015;33(15 Suppl):521. [Google Scholar]
- Wolf DM, Yau C, Sanil A, Brown-Swigart L, Hirst G, Buxton M, et al. Combining sensitivity markers to identify triple-negative breast cancer patients most responsive to veliparib/carboplatin: results from the I-SPY 2 TRIAL. Cancer Research 2016;76(14 Suppl):858. [Google Scholar]
- Wolf DM, Yau C, Sanil A, Daemen A, Heiser L, Gray J, et al. Evaluation of an in vitro derived signature of olaparib response (PARPi-7) as a predictive biomarker of response to veliparib/carboplatin plus standard neoadjuvant therapy in high-risk breast cancer: results from the I-SPY 2 TRIAL. Cancer Research 2015;75(9 Suppl):P3-06-05. [Google Scholar]
- Wolf DM, Yau C, Sanil A, Glas A, Petricoin C, Wulfkuhle J, et al. DNA repair deficiency biomarkers and MammaPrint high/(ultra)high risk as predictors of veliparib/carboplatin response: results from the neoadjuvant I-SPY 2 trial for high risk breast cancer. Cancer Research 2017;77(4 Suppl):S2-06. [Google Scholar]
Li 2020 {published data only}
- Li Q, Wang J, Mu Y, Zhang T, Han Y, Luo Y, et al. Dose-dense paclitaxel plus carboplatin vs. epirubicin and cyclophosphamide with paclitaxel as adjuvant chemotherapy for high-risk triple-negative breast cancer. Chinese Journal of Cancer Research 2020;32(4):485-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Zhang T, Han Y, Wang J, Li Q, Luo Y, et al. A randomized phase III trial comparing dose-dense epirubicin and cyclophosphamide (ECdd) followed by paclitaxel (T) with paclitaxel plus carboplatin (PCdd) as adjuvant chemotherapy for early triple negative breast cancer patients with high recurrence risk. Journal of Clinical Oncology 2019;37(15 Suppl):528. [Google Scholar]
- NCT01378533. The trial comparing dose-dense AC-T with PC as adjuvant therapy for TNBC. clinicaltrials.gov/show/NCT01378533 (first received 22 June 2011).
- Wang J, Li Q, Mu Y, Zhang T, Han Y, Wang J, et al. A randomized phase III trial comparing dose-dense epirubicin and cyclophosphamide (ECdd) followed by paclitaxel (T) with paclitaxel plus carboplatin (PCdd) as adjuvant chemotherapy for early triple-negative breast cancer patients with high-recurrence risk. Journal of Clinical Oncology 2019;37(15 Suppl):528. [Google Scholar]
Nasr 2015 {published data only}
- El Kady MS, El Nasr K, Moneam Osman MA, Ellithy M. Metronomic methotrexate and cyclophosphamide after carboplatin included adjuvant chemotherapy in triple-negative breast cancer: a phase III study. Journal of Clinical Oncology 2015;33(15 Suppl):e12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr KE, Osman MA, Elkady MS, Ellithy MA. Metronomic methotrexate and cyclophosphamide after carboplatin included adjuvant chemotherapy in triple negative breast cancer: a phase III study. Annals of Translational Medicine 2015;3(19):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
NeoCART {published data only}
- NCT03154749. TC (docetaxel/carboplatin) versus EC-T (epirubicin/cyclophosphamide followed by docetaxe) as neoadjuvant chemotherapy for triple-negative breast cancer. clinicaltrials.gov/show/NCT03154749 (first received 16 May 2017).
- Zhang L, Wu Z, Lin Y, Li J, Liu Z, Cao Y, et al. Neoadjuvant docetaxel + carboplatin versus epirubicin+cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): results from a multicenter, randomized controlled, open-label, phase II trial. Journal of Clinical Oncology 2022;38(15 Suppl):586. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wu ZY, Li J, Lin Y, Liu Z, Cao Y, et al. Neoadjuvant docetaxel plus carboplatin vs epirubicin plus cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): results from a multicenter, randomized controlled, open-label phase II trial. International Journal of Cancer 2022;150(4):654-62. [DOI] [PubMed] [Google Scholar]
PATTERN {published data only}
- NCT01216111. Comparison study of adjuvant chemotherapy for Chinese triple negative breast cancer. clinicaltrials.gov/show/NCT01216111 (first received 7 October 2010).
- NCT04031703. Study comparing paclitaxel plus carboplatin versus anthracyclines followed by docetaxel as adjuvant chemotherapy for triple negative breast cancer (PATTERN). clinicaltrials.gov/show/NCT04031703 (first received 24 July 2019).
- Yu KD, Ye FG, He M, Fan L, Ma D, Mo M, et al. Effect of adjuvant paclitaxel and carboplatin on survival in women with triple-negative breast cancer: a phase 3 randomized clinical trial. JAMA Oncology 2020;6(9):1390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
TBCRC 030 {published data only}
- Mayer EL, Abramson V, Jankowitz R, Falkson C, Marcom PK, Traina T, et al. TBCRC 030: a phase II study of preoperative cisplatin versus paclitaxel in triple-negative breast cancer: evaluating the homologous recombination deficiency (HRD) biomarker. Annals of Oncology 2020;31(11):1518-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EL, Abramson VG, Jankowitz RC, Falkson CI, Marcom PK, Traina TA, et al. TBCRC 030: a randomized phase II study of preoperative cisplatin versus paclitaxel in TNBC- evaluating the homologous recombination deficiency (HRD) biomarker. Journal of Clinical Oncology 2019;37(15 Suppl):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EL, Vaz-Luis I, Richardson AL, Perou CM, Tung NM, Abramson VG, et al. TBCRC030: a randomized, phase II study of preoperative cisplatin versus paclitaxel in patients (pts) with BRCA1/2-proficient triple-negative breast cancer (TNBC) – evaluating the homologous recombination deficiency (HRD) biomarker. Journal of Clinical Oncology 2014;32(15 Suppl):TPS1145. [Google Scholar]
- NCT01982448. Cisplatin vs paclitaxel for triple negative breast cancer. clinicaltrials.gov/show/NCT01982448 (first received 13 November 2013).
Wu 2018 {published data only}
- ChiCTR-TRC-14005019. Docetaxel combined with epirubicin and lobaplatin (TEL) in neoadjuvant chemotherapy for triple negative breast cancer: a prospective randomized controlled clinical study. www.chictr.org.cn/showprojEN.html?proj=4555 (first received 29 May 2014).
- Wu X, Tang P, Li S, Wang S, Liang Y, Zhong L, et al. A randomized and open-label phase II trial reports the efficacy of neoadjuvant lobaplatin in breast cancer. Nature Communications 2018;9(1):832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2016 {published data only}
- NCT01276769. Comparison study of neoadjuvant paclitaxel plus carboplatin/epirubicin treatment in triple-negative breast cancer. clinicaltrials.gov/show/NCT01276769 (first received 13 January 2011).
- Zhang P, Yin Y, Mo H, Zhang B, Wang X, Li Q, et al. Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial. Oncotarget 2016;7(37):60647-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Yin Y, Xu B, Wang X, Zhang B, Li Q, et al. Carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for triple-negative breast cancer – a phase II clinical trial. Cancer Research 2013;73(24 Suppl):P3-14-07. [Google Scholar]
Zhao 2014 {published data only}
- Zhao Y, Li JF, Chu GW. Neoadjuvant chemotherapy regimens for patients with triple-negative breast cancer: TE versus TC. Journal of Practical Oncology 2014;29(6):576-8. [Google Scholar]
Zheng 2022 {published data only}
- Du F, Wang W, Wang Y, Li M, Zhu A, Wang J, et al. Carboplatin plus taxanes are non-inferior to epirubicin plus cyclophosphamide followed by taxanes as adjuvant chemotherapy for early triple-negative breast cancer. Breast Cancer Research and Treatment 2020;182(1):67-77. [DOI] [PubMed] [Google Scholar]
- NCT01150513. Docetaxel+carboplatin vs epirubicin+cyclophosphamide followed by docetaxel as adjuvant treatment in triple-negative breast cancer. clinicaltrials.gov/show/NCT01150513 (first received 25 June 2010).
- Yuan P, Du F, Wang J, Ma F, Fan Y, Xu B. Comparison of four cycles epirubicin and cyclophosphamide (EC) followed by four cycles docetaxel (T) versus six cycles docetaxel and carboplatin (TP) as adjuvant chemotherapy for women with operable triple negative breast cancer. Journal of Clinical Oncology 2016;34(Suppl 15):1068. [Google Scholar]
- Yuan P, Xu B. A phase III, randomized trial of docetaxel plus carboplatin (TP) versus epirubicin plus cyclophosphamide followed by docetaxel (EC-T) as adjuvant treatment for triple-negative, early-stage breast cancer in Chinese patients. Journal of Clinical Oncology 2012;30(15 Suppl):TPS1135. [Google Scholar]
- Yuan P, Xu BH, Wang JY, Ma F, Li Q, Zhang P, et al. Docetaxel plus carboplatin versus EC-T as adjuvant chemotherapy for triple-negative breast cancer: safety data from a phase III randomized open-label trial. Chung Hua Chung Liu Tsa Chih 2012;34(6):465-8. [PubMed] [Google Scholar]
- Zheng F, Du F, Wang W, Wang Y, Li M, Zhao J, et al. Updated efficacy of adjuvant epirubicin plus cyclophosphamide followed by taxanes versus carboplatin plus taxanes in early triple-negative breast cancer in phase 2 trial: 8.1-year median follow-up. Breast Cancer Research and Treatment 2022;191(1):97-105. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ECOG‐ACRIN EA1131 {published data only}
- Mayer IA, Zhao F, Arteaga CL, Symmans WF, Park BH, Burnette BL, et al. A randomized phase III postoperative trial of platinum-based chemotherapy (P) versus capecitabine (C) in patients (pts) with residual triple-negative breast cancer (TNBC) following neoadjuvant chemotherapy (NAC): ECOG-ACRIN EA1131. Journal of Clinical Oncology 2021;39(15 Suppl):605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer IA, Zhao F, Arteaga CL, Symmans WF, Park BH, Burnette BL, et al. Randomized phase III postoperative trial of platinum-based chemotherapy versus capecitabine in patients with residual triple-negative breast cancer following neoadjuvant chemotherapy: ECOG-ACRIN EA1131. Journal of Clinical Oncology 2021;39(23):2539-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02445391. Platinum based chemotherapy or capecitabine in treating patients with residual triple-negative basal-like breast cancer following neoadjuvant chemotherapy. clinicaltrials.gov/show/NCT02445391 (first received 15 May 2015).
Jiang 2019 {published data only}
- Jiang W, Wang L, Yongtao L, Zhang C, Yi L, Ou J. Clinical application of docetaxel combined with lobaplatin in adjuvant chemotherapy of elderly triple-negative breast cancer. Anti-tumor Pharmacy 2019;9(6):897-901. [Google Scholar]
NCT00004092 {published data only}
- NCT00004092. Combination chemotherapy in treating patients with high-risk breast cancer. clinicaltrials.gov/ct2/show/NCT00004092 (first received 27 January 2003).
SICOG 9908 {published data only}
- Frasci G, D'Aiuto G, Comella P, D'Aiuto M, Di Bonito M, Ruffolo P, et al. Preoperative weekly cisplatin, epirubicin, and paclitaxel (PET) improves prognosis in locally advanced breast cancer patients: an update of the Southern Italy Cooperative Oncology Group (SICOG) randomised trial 9908. Annals of Oncology 2010;21:707-16. [DOI] [PubMed] [Google Scholar]
- Frasci G, D'Aiuto G, Comella P, Thomas R, Botti G, Di Bonito M, et al. Weekly cisplatin, epirubicin, and paclitaxel with granulocyte colony-stimulating factor support vs triweekly epirubicin and paclitaxel in locally advanced breast cancer: final analysis of a SICOG phase II study. British Journal of Cancer 2006;95:1005-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
UMIN000030780 – jRCTs051180210 {published data only}
- UMIN000030780. Phase 3 trial of carboplatin in triple negative breast cancer patient with residual invasive carcinoma after neoadjuvant chemotherapy. center6.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000034530 (first received 1 March 2018).
- jRCTs051180210. Phase 3 trial of carboplatin in triple negative breast cancer patient with residual invasive carcinoma after neoadjuvant chemotherapy. jrct.niph.go.jp/latest-detail/jRCTs051180210 (first received 27 March 2019).
References to ongoing studies
ChiCTR1800019501 {published data only}
- ChiCTR1800019501. A randomized controlled phase II clinical trial comparing neoadjuvant TP (docetaxel + cisplatin) with TAC (docetaxel + adriamycin + cyclophosphamide) regimen in the treatment of operable triple negative breast cancer. www.chictr.org.cn/showprojEN.html?proj=31567 (first received 14 November 2018).
ChiCTR1900023776 {published data only}
- ChiCTR1900023776. A randomized, controlled, single-center clinical study for the efficacy and safety of docetaxel plus lobaplatin versus docetaxel plus epirubicin for neoadjuvant therapy in triple-negative breast cancer. www.chictr.org.cn/showprojEN.html?proj=39908 (first received 11 June 2019).
ChiCTR1900026499 {published data only}
- ChiCTR1900026499. Albumin-bound paclitaxel combined with carboplatin versus epirubicin combined with docetaxel as neoadjuvant therapy for triple-negative breast cancer: a multicenter randomized controlled phase IV clinical trial. www.chictr.org.cn/showprojEN.html?proj=44204 (first received 12 October 2019).
ChiCTR2000039578 {published data only}
- ChiCTR2000039578. A prospective, open, multicenter, randomized controlled trial for the effect of albumin-bound paclitaxel combined with cisplatin versus epirubicin combined with cyclophosphamide sequential docetaxel neoadjuvant therapy for triple negative breast cancer. www.chictr.org.cn/showprojEN.html?proj=63610 (first received 1 November 2020).
CTRI/2017/10/010272 {published data only}
- CTRI/2017/10/010272. Comparing two types of chemotherapy in breast cancer. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=20743 (first received 31 October 2017).
CTRI/2019/05/019176 {published data only}
- CTRI/2019/05/019176. A trial comparing effect of addition of carboplatin to standard neoadjuvant chemotherapy for triple negative breast cancer patients. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=32946 (first received 16 May 2019).
EUCTR2009‐015238‐31 {published data only}
- EUCTR2009-015238-31. Randomized phase II/III study of individualized neoadjuvant chemotherapy in 'triple-negative' breast tumors. www.clinicaltrialsregister.eu/ctr-search/search?query=2009-015238-31 (first received 16 December 2009).
- NCT01057069. Neo adjuvant chemotherapy in triple negative breast cancer (neo-TN). clinicaltrials.gov/ct2/show/NCT01057069 (first received 27 January 2010).
EudraCT2016‐004384‐39 {published data only}
- EudraCT2016-004384-39. A clinical trial that examines whether the treatment with the medication olaparib in combination with the chemotherapy carboplatin is more effective than treatment with a standard chemotherapy (anthracycline/ taxane-based) against a specific type of breast cancer. www.clinicaltrialsregister.eu/ctr-search/trial/2016-004384-39/AT (first received 6 November 2018).
NCT00919880 {published data only}
- NCT00919880. Comparison of neo-adjuvant weekly paclitaxel with or without carboplatin in early breast cancer. clinicaltrials.gov/show/NCT00919880 (first received 12 June 2009).
NCT01752686 {published data only}
- NCT01752686. A phase III trial of carboplatin as adjuvant chemotherapy in triple negative breast cancer. clinicaltrials.gov/show/NCT01752686 (first received 19 December 2012).
NCT02041338 {published data only}
- NCT02041338. Study of optimizing neoadjuvant regimens in subtypes of breast cancer. clinicaltrials.gov/show/NCT02041338 (first received 22 January 2014).
NCT02455141 {published data only}
- NCT02455141. Adjuvant treatment of EC followed by taxane +/- carboplatin in triple-negative breast cancer. clinicaltrials.gov/show/NCT02455141 (first received 27 May 2015).
NCT02488967 {published data only}
- NCT02488967. Doxorubicin hydrochloride and cyclophosphamide followed by paclitaxel with or without carboplatin in treating patients with triple-negative breast cancer. clinicaltrials.gov/show/NCT02488967 (first received 2 July 2015).
NCT02641847 {published data only}
- NCT02641847. TA(E)C-GP versus A(E)C-T for the high risk TNBC patients and validation of the mRNA-lncRNA signature. clinicaltrials.gov/show/NCT02641847 (first received 30 December 2015).
NCT02879513 {published data only}
- NCT02879513. Trial of adjuvant chemotherapy in breast cancer patients with pathological partial response and complete response to neoadjuvant chemotherapy. clinicaltrials.gov/show/NCT02879513 (first received 25 August 2016).
NCT02978495 {published data only}
- NCT02978495. Neoadjuvant carboplatin in triple negative breast cancer. clinicaltrials.gov/show/NCT02978495 (first received 1 December 2016).
NCT03168880 {published data only}
- NCT03168880. A randomized controlled trial of neoadjuvant weekly paclitaxel versus weekly paclitaxel plus weekly carboplatin in women with large operable or locally advanced, triple negative breast cancer (TNBC). clinicaltrials.gov/ct2/show/NCT03168880 (first received 30 May 2017).
NCT03201861 {published data only}
- NCT03201861. Addition of cisplatin to adjuvant chemotherapy for early stage breast cancer in high-risk women. clinicaltrials.gov/show/NCT03201861 (first received 28 June 2017).
NCT03876886 {published data only}
- NCT03876886. The trial comparing dose-dense AC-T with TP as adjuvant therapy for TNBC with homologous recombination repair deficiency. clinicaltrials.gov/show/NCT03876886 (first received 15 March 2019).
NCT04136782 {published data only}
- NCT04136782. Albumin-bound paclitaxel and carboplatin versus epirubicin and docetaxel for triple-negative breast cancer. clinicaltrials.gov/show/NCT04136782 (first received 23 October 2019).
NCT04138719 {published data only}
- NCT04138719. Nab-paclitaxel plus carboplatin versus nab-paclitaxel plus epirubicin in the neoadjuvant therapy for breast cancer. clinicaltrials.gov/show/NCT04138719 (first received 24 October 2019).
NCT04296175 {published data only}
- NCT04296175. Carboplatin intensified chemotherapy for triple negative breast cancer (CITRINE). clinicaltrials.gov/show/NCT04296175 (first received 5 March 2020).
NCT04664972 {published data only}
- NCT04664972. The TP regimen in the treatment of early triple negative breast cancer. clinicaltrials.gov/show/NCT04664972 (first received 11 December 2020).
Nordic Trip {published data only}
- Loman N, Linderholm B, Joensuu H, Ejlertsen B, Johannsson OT, Geisler J, et al. Abstract OT3-02-10: Nordic trip, a randomized phase 3 study in early triple negative breast cancer. Cancer Research 2016;76(4 Suppl 1):OT3-02-10. [Google Scholar]
PEARLY {published data only}
- KCT0003529. A randomized, multicenter, open-label, phase III trial comparing anthracyclines followed by taxane versus anthracyclines followed by taxane plus carboplatin as (neo)adjuvant therapy in patients with triple-negative breast cancer. cris.nih.go.kr/cris/search/detailSearch.do?seq=14516&search_page=L&search_lang=E&lang=E&latest=Y (first received 23 November 2018).
- Kim GM, Jeung HC, Jung KH, Kim SH, Kim HJ, Lee KH, et al. PEARLY: a randomized, multicenter, open-label, phase III trial comparing anthracyclines followed by taxane versus anthracyclines followed by taxane plus carboplatin as (neo)adjuvant therapy in patients with early triple-negative breast cancer. Journal of Clinical Oncology 2017;35(15 Suppl 1):TPS587. [Google Scholar]
- NCT02441933. Carboplatin in EARLY triple negative breast cancer trial (PEARLY trial). clinicaltrials.gov/show/NCT02441933 (first received 12 May 2015).
Additional references
Awidi 2021
- Awidi M, Al Hadidi S. Participation of Black Americans in cancer clinical trials: current challenges and proposed solutions. JCO Oncology Practice 2021;17(5):265-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banys‐Paluchowski 2017
- Banys-Paluchowski M, Ruckhäberle E, Schütz F, Krawczyk N, Fehm T. Metronomic chemotherapy for primary non-metastatic breast cancer – a systematic review of the literature. Geburtshilfe Frauenheilkd 2017;77(2):142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bian 2021
- Bian L, Yu P, Wen J, Li N, Huang W, Xie X, et al. Survival benefit of platinum-based regimen in early stage triple negative breast cancer: a meta-analysis of randomized controlled trials. NPJ Breast Cancer 2021;7(1):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
BrighTNess
- Loibl S, O'Shaughnessy J, Untch M, Sikov WM, Rugo HS, McKee MD, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncology 2018;19(4):497-509. [DOI] [PubMed] [Google Scholar]
Cortazar 2014
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384(9938):164-72. [DOI] [PubMed] [Google Scholar]
CTCAE 2017
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed 25 March 2021).
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Edge 2010
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of Surgical Oncology 2010;17(6):1471-4. [DOI] [PubMed] [Google Scholar]
Ferlay 2018
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al, editor(s). Cancer Today (powered by GLOBOCAN 2018) IARC CancerBase No. 15. publications.iarc.fr/Databases/Iarc-Cancerbases/Cancer-Today-Powered-By-GLOBOCAN-2018--2018 (accessed 17 July 2020).
Foulkes 2010
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. New England Journal of Medicine 2010;363(20):1938-48. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed prior to 4 September 2023. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guo 2017
- Guo W, Lin L, He X, He F, Wang C, Chen N, et al. Biomarkers of DNA repair and related pathways: significance of treatment in triple-negative breast cancer. Critical Reviews in Oncogenesis 2017;22(5-6):427-37. [DOI] [PubMed] [Google Scholar]
Gupta 2022
- Gupta S, Nair NS, Hawaldar RW, Vanmali V, Parmar V, Gulia S, et al. Addition of platinum to sequential taxane-anthracycline neoadjuvant chemotherapy in patients with triple-negative breast cancer: a phase III randomized controlled trial. San Antonio Breast Cancer Symposium; 2022 Dec 6-10; San Antonio, Texas.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2020
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook/archive/v6.1.
Lin 2012
- Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN, et al. Clinicopathological features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012;118(22):5463-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719-48. [PubMed] [Google Scholar]
McGlothlin 2014
- McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA 2014;312(13):1342-3. [DOI] [PubMed] [Google Scholar]
Nitz 2019
- Nitz U, Gluz O, Clemens M, Malter W, Reimer T, Nuding B, et al. West German Study PlanB trial: adjuvant four cycles of epirubicin and cyclophosphamide plus docetaxel versus six cycles of docetaxel and cyclophosphamide in HER2-negative early breast cancer. Journal of Clinical Oncology 2019;37(10):799-808. [DOI] [PubMed] [Google Scholar]
O'Reilly 2020
- O'Reilly EM, Lee JW, Zalupski M, Capanu M, Park J, Golan T, et al. Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation. Journal of Clinical Oncology 2020;38(13):1378-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pandy 2019
- Pandy JG, Balolong-Garcia JC, Cruz-Ordinario MV, Que FV. Triple negative breast cancer and platinum-based systemic treatment: a meta-analysis and systematic review. BMC Cancer 2019;19(1):1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pennington 2014
- Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clinical Cancer Research 2014;20(3):764-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perabo 2007
- Perabo FG, Müller SC. New agents for treatment of advanced transitional cell carcinoma. Annals of Oncology 2007;18:835-43. [DOI] [PubMed] [Google Scholar]
Petrelli 2014
- Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V, et al. The value of platinum agents as neoadjuvant chemotherapy in triple-negative breast cancers: a systematic review and meta-analysis. Breast Cancer Research and Treatment 2014;144(2):223-32. [DOI] [PubMed] [Google Scholar]
Poggio 2018
- Poggio F, Bruzzone M, Ceppi M, Ponde NF, La Valle G, Del Mastro L, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Annals of Oncology 2018;29(7):1497-508. [DOI] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 2.4.2. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Saleh 2021
- Saleh RR, Nadler MB, Desnoyers A, Meti N, Fazelzad R, Amir E. Platinum-based chemotherapy in early-stage triple negative breast cancer: a meta-analysis. Cancer Treatment Reviews 2021;100:102283. [DOI] [PubMed] [Google Scholar]
Schmid 2020
- Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. New England Journal of Medicine 2020;382(9):810-21. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shepherd 2022
- Shepherd JH, Ballman K, Polley MC, Campbell JD, Fan C, Selitsky S, et al. CALGB 40603 (Alliance): long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. Journal of Clinical Oncology 2022;40(12):1323-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shimelis 2018
- Shimelis H, LaDuca H, Hu C, Hart SN, Na J, Thomas A, et al. Triple-negative breast cancer risk genes identified by multigene hereditary cancer panel testing. JNCI: Journal of the National Cancer Institute 2018;110(8):855-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney J, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tutt 2021
- Tutt AN, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. New England Journal of Medicine 2021;384(25):2394-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2017
- Wang LY, Xie H, Zhou H, Yao WX, Zhao X, Wang Y. Efficacy of carboplatin-based preoperative chemotherapy for triple-negative breast cancer. A meta-analysis of randomized controlled trials. Saudi Medical Journal 2017;38(1):18-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2021
- Yu K, Liu X, Chen L, Mo M, Jiong W, Guang-Yu L, et al. Anthracycline-free or short-term regimen as adjuvant chemotherapy for operable breast cancer: a phase III randomized non-inferiority trial. Lancet Regional Health Western Pacific 2021;11:100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Mason 2021
- Mason S, Beith J, Willson ML, Egger SJ, Dear RF, Goodwin A. Platinum-based chemotherapy for early triple-negative breast cancer. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD014805. [DOI: 10.1002/14651858.CD014805] [DOI] [PMC free article] [PubMed] [Google Scholar]


