Abstract
Since autumn 2022, observed numbers of paediatric invasive group A Streptococcus infections in Portugal (n = 89) were higher than in pre-COVID-19 seasons. Between September 2022 and May 2023, the dominant diagnoses were pneumonia (25/79), mostly with empyema (20/25), and sepsis (22/79). A number of cases required admission to intensive care (27/79) and surgery (35/79), and the case fatality rate was 5.1% (4/79). Genomic sequencing (n = 55) revealed multiple genetic lineages, dominated by the M1UK sublineage (26/55) and more diverse emm12 isolates (12/55).
Keywords: Streptococcus pyogenes, group A streptococcus, paediatric infections, epidemiology, M1UK, emm type, whole genome sequencing
In line with reports from several other European countries [1], since late 2022, an increase of paediatric (aged < 18 years) invasive group A Streptococcus infections (piGAS) has been notified in the context of an ongoing prospective surveillance of piGAS in Portugal, including a high prevalence of pneumonia with empyema. Here, we report the epidemiological and clinical characteristics of the infections between September 2022 and May 2023, as well as the main molecular characteristics and antimicrobial resistance of the S. pyogenes (Lancefield group A Streptococcus (GAS)) isolates.
Prospective surveillance of paediatric invasive group A Streptococcus infections in Portugal
A nationwide prospective surveillance of piGAS in Portugal has been ongoing since January 2014. All paediatric departments are invited to notify piGAS with demographic data, clinical diagnosis and outcome, while clinical microbiology laboratories are requested to submit all GAS recovered from normally sterile sites for antimicrobial susceptibility testing and genomic sequencing. Confirmed and probable piGAS are defined according to recent guidelines [2].
After a considerable decrease recorded during the seasons of 2019/20 to 2021/22 (September to August), piGAS in Portugal started to rise again in May 2022. From December 2022 to February 2023 there was a notable increase, and numbers thereafter remained much higher than the average of pre-pandemic years (Figure 1).
Figure 1.
Monthly distribution of cases with paediatric invasive group A Streptococcus infections, Portugal, 2014/15–2022/23
The pre-pandemic average comprises the seasons from 2014/15 to 2018/19, is represented in red with 95% confidence interval error bars. The 2021/22 season is represented in green and the 2022/23 season in blue. Seasons are considered as September to August of the following year.
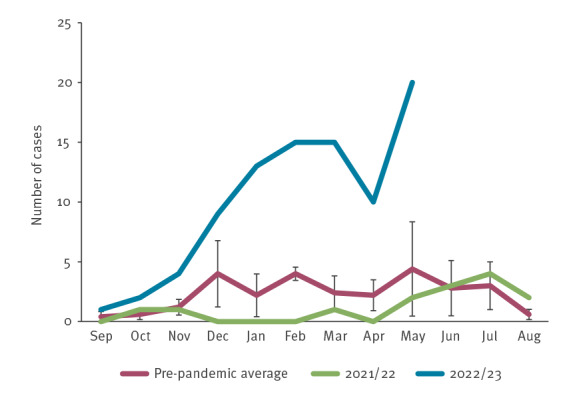
Overall, between 1 September 2022 and 31 May 2023, 89 piGAS cases were recorded (85 were confirmed and four were probable), which is 4 times higher than the average for the same period in pre-pandemic seasons (2014/15 to 2018/19, mean: 21.4 cases, range: 16–26), and at least 2.5 times the number of cases in any of the previous complete seasons (mean: 20.8 cases, range: 1–36 cases). The median age of the patients was 3 years (interquartile range (IQR): 2–6); 45 were males (50.6%) and 44 were females (49.4%).
Clinical presentations and outcomes
Clinical information was collected through a questionnaire included in the notification form, and was available for 79 cases (Table). In line with the overall cohort, the median age was 3 years (IQR: 2–6 years) and there were 43 males (54.4%) and 36 females (45.6%). Most cases occurred in children without underlying medical conditions (68/79; 86.1%), but varicella (17/69; 24.6%) or upper respiratory infection (16/65; 24.6%) within 2 weeks before admission was frequently reported, although not associated with specific clinical presentations.
Table. Characteristics of cases with paediatric invasive group A Streptococcus infections, Portugal, 1 September 2022–31 May 2023 (n = 79).
| Characteristics | piGAS cases | |
|---|---|---|
| n | % | |
| Underlying medical condition | ||
| Yesa | 11 | 13.9 |
| No | 68 | 86.1 |
| Infection within 2 weeks before hospital admission b | ||
| Varicella | 17/69 | 24.6 |
| Respiratory infection | 16/65 | 24.6 |
| Presentation | ||
| Rash | 33 | 41.8 |
| Pharyngitis | 28 | 35.4 |
| Vomiting | 17 | 21.5 |
| Diarrhoea | 11 | 13.9 |
| Diagnosis | ||
| Pneumonia | 25 | 31.6 |
| Pneumonia with empyema | 20 | 25.3 |
| Sepsis | 22 | 27.8 |
| Bacteraemia without focus | 19 | 24.1 |
| Bone and joint infection | 17 | 21.5 |
| STSS | 14 | 17.7 |
| Meningitis | 6 | 7.6 |
| Necrotising fasciitis | 1 | 1.3 |
| Complications | ||
| Coagulopathy | 10 | 12.7 |
| Renal failure | 10 | 12.7 |
| Multiple organ dysfunction syndrome | 9 | 11.4 |
| Respiratory failure | 7 | 8.9 |
| Hepatic dysfunction | 5 | 6.3 |
| Thrombosis | 4 | 5.1 |
| Management/outcome | ||
| Adjunctive clindamycin | 55 | 69.6 |
| IVIG treatment | 8 | 10.1 |
| Surgery or drainagec | 35 | 44.3 |
| PICU admission | 27 | 34.2 |
| Sequelae at 30 days | 10 | 12.7 |
| Case fatality rate | 4 | 5.1 |
IVIG: intravenous immunoglobulin; PICU: paediatric intensive care unit; STSS: streptococcal toxic shock syndrome.
a Underlying medical conditions included chronic lung or neurological diseases, chronic skin conditions and sickle cell disease. The exact diseases or conditions were not specified in the questionnaire.
b Infections occurring within the 2 weeks preceding the hospital admission or present at admission. Information on preceding varicella infection was available for 69 cases and on preceding respiratory infection for 65 cases. No data was obtained on the detection of specific respiratory viruses.
c Mostly joint (13/35; 37.1%) and pleural fluid drainage (12/35; 34.3%).
The median overall length of hospital stay was 10 days (IQR: 5–17). Paediatric intensive care unit (PICU) admission (27/79; 34.2%) and surgical interventions or drainage (35/79; 44.3%) were frequent.
Three patients died within the first 24 h of admission with streptococcal toxic shock syndrome (STSS), and one died at day 15 with complicated meningitis. Sequelae at 30 days were noted in 10/79 cases (12.7%), although the type and severity of sequelae were not directly included in our questionnaire and were not systematically reported. The reported sequelae included foot ischaemia requiring amputation in a child with STSS who had both S. pyogenes and S. pneumoniae identified by PCR in the pleural fluid, and mild limb paresis in a child with a cerebral abscess. Four patients with pneumonia still had respiratory symptoms at 30 days follow-up.
Bacterial characteristics
Of the 85 confirmed cases, 13 were diagnosed by real-time PCR on culture-negative samples (11 from pleural fluid and 1 from a fasciitis deep tissue sample). Bacterial isolates were available for 55 of the 72 culture-positive cases.
Illumina sequencing was performed (data available in the European Nucleotide Archive (ENA); accession number PRJEB65018), and emm types and seven gene multilocus sequence types (STs) were retrieved from de novo assemblies [3] (detailed high-throughput sequencing and data analysis methods can be found in the Supplementary materials). The lineages defined by emm1-ST28/1319 (n = 30) and emm12-ST36/242 (n = 12) together accounted for 76.4% of the isolates (the emm types and STs of each isolate can be found in Supplementary Table S1). Screening for the characteristic 27 M1UK sublineage SNPs (the number of SNPs found in each isolate is available in Supplementary Table S1) [4] and core genome multilocus sequence typing (cgMLST) analysis [3] support the classification of 26/30 emm1 isolates as M1UK (Figure 2). The gene encoding superantigen SpeC was identified in two emm1 isolates (data available in Supplementary Table S1), both belonging to the M1UK sublineage and not to the recently described speC-positive M1DK sublineage [5], which was not found among our isolates (Figure 2). The cgMLST of the emm12 isolates, analysed together with a dataset of emm12 genomes selected to represent the global diversity of this emm type [6] and with the recently published emm12 genomes from Denmark [5], reveals a high genetic diversity, with no clear dominance of any sublineage (Figure 3).
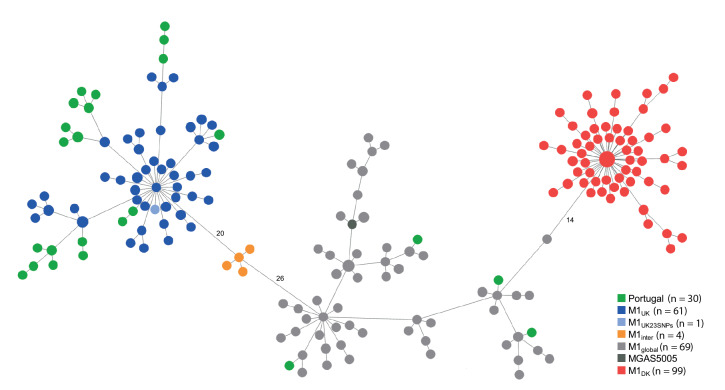
Figure 2.
Minimum spanning tree of paediatric invasive emm1 group A Streptococcus isolates, Portugal, 1 September 2022–31 May 2023 (n = 30), and of emm1 isolates from United Kingdom, 2009–2016 (n = 135) and Denmark, 2022–2023 (n = 99)
cgMLST: core genome multilocus sequence typing; UK: United Kingdom.
The tree was generated with the cgMLST profiles of paediatric invasive emm1 isolates recovered in Portugal, 1 September 2022–31 May 2023 (n = 30; green), non-invasive isolates (n = 135) recovered in London, UK [4] carrying 27 (dark blue), 23 (light blue), 13 (orange) or 0 (light grey) of 27 SNPs characteristic of the M1UK sublineage, invasive and non-invasive isolates from Denmark (n = 99) [5] carrying the 15 SNPs characteristic of the M1DK sublineage (red), and the M1 reference strain MGAS5005 (dark grey). The list of genomes used is available in Supplementary Tables S1 and S2. The size of each node is proportional to the number of isolates with that particular cgMLST profile on a logarithmic scale. Link distances separating the previously identified sublineages are labelled as the number of allelic differences between nodes (from a total of 1,249 compared loci). All isolates from Portugal carrying ≥ 26 M1UK SNPs (n = 26, found in Supplementary Table S1) grouped with the M1UK isolates, while the four isolates without M1UK SNPs grouped with M1global isolates. Link distances in the minimum spanning tree vary from 1 to 16 allelic differences between M1UK nodes, from 2 to 4 allelic differences between M1inter nodes, from 1 to 6 allelic differences between M1DK nodes, and from 1 to 40 allelic differences between M1global nodes. A maximum likelihood tree of the same isolates can be found in Supplementary Figure S1. Detailed high-throughput sequencing and data analysis methods can be found in the Supplementary materials.
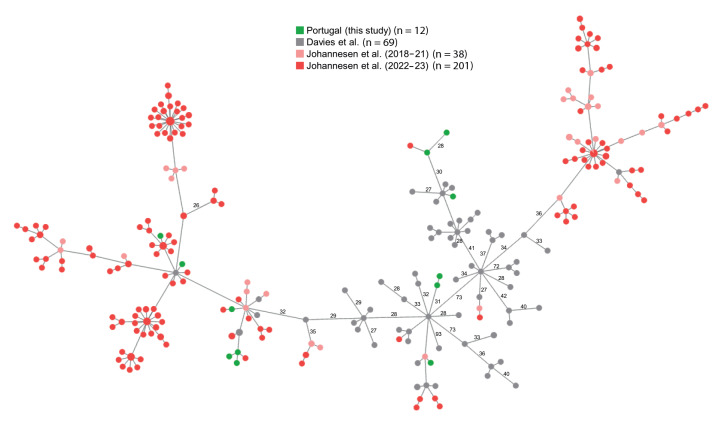
Figure 3.
Minimum spanning tree of paediatric invasive emm12 group A Streptococcus isolates, Portugal, 1 September 2022–31 May 2023 (n = 12), and of emm12 isolates from Denmark, 2018–2023 (n = 239) and a global collection, 2001–2015 (n = 69)
The tree was generated with the cgMLST profiles of paediatric invasive emm12 isolates recovered in Portugal, 1 September 2022–31 May 2023 (n = 12; green), genetically diverse invasive and non-invasive emm12 isolates (n = 69) from a global collection [6] (grey), and invasive and non-invasive emm12 isolates (n = 239) recovered in Denmark during 2018–21 (pink) or 2022–23 (red). The list of genomes used is available in Supplementary Tables S1 and S2. Link distances ≥ 26 allelic differences are labelled (from a total of 1,128 compared loci). A maximum likelihood tree of the same isolates can be found in Supplementary Figure S2. Detailed high-throughput sequencing and data analysis methods can be found in the Supplementary materials.
Among 48 cases with molecular and clinical information, PICU admission was more frequent among patients with emm1 (12/28 cases (43%) vs 2/20 cases with other emm types (10%), Fisher’s exact test, p = 0.02). There were no other significant associations of emm1 with clinical presentation or outcome, although all three deaths occurred in patients with this emm type.
Antimicrobial resistance was tested in all available isolates by disc diffusion according to EUCAST guidelines [7] (the antimicrobial susceptibility profile of each isolate can be found in Supplementary Table S1). Only tetracycline (n = 1) and norfloxacin (n = 2) resistance were identified; the first associated with the presence of the tet(M) gene while the latter with the S79F and A121V mutations in the parC gene.
Discussion
Following a period of very low levels of invasive GAS infections during the COVID-19 pandemic, a marked increase has been reported in multiple European countries and in some regions of the United States (US) [1,5,8-15]. In most cases, this increase was particularly striking in paediatric age groups and was associated with a shift in the dominant clinical presentations towards pulmonary infections with empyema [5,8-11,13,16]. Pneumonia, often complicated with pleural effusion, was the most frequent diagnosis among our patients. The case fatality rate (5.1%) recorded up to May 2023 is comparable to that reported in other studies [5,9,13].
Remarkably, this surge has not occurred simultaneously in all reporting countries. In the Netherlands, piGAS has increased since early 2022 [8], while in England, France, Ireland, Denmark, Spain and the US, the increase was noted in the autumn/winter of the same year [5,9-11,13,14]. In Portugal, the most dramatic increase relative to pre-pandemic seasons occurred from January 2023 onwards, with the number of cases remaining persistently high until May, despite a dip in April. This contrasts with the previous seasons, where mostly single-month peaks were observed. The reasons underlying the differences in upsurge timing between different countries remain unclear. The non-pharmaceutical interventions during the COVID-19 pandemic and their detrimental impact on child immunity, associated with the increased circulation of respiratory viruses, have been suggested as potential drivers of this multinational upsurge of piGAS [8,9]. Several countries reported a temporal coincidence between the piGAS surge and the respective respiratory viral season, in particular for respiratory syncytial virus (RSV) and influenza, or a high prevalence of preceding/concurrent viral infection among piGAS cases [9,10,13,14]. In Portugal, national data of all-age surveillance of viral respiratory infections indicate that the circulation of influenza and RSV was most intense between late October 2022 and early January 2023 [17], therefore preceding, but not coinciding, with the majority of piGAS cases. Only 24.6% of cases reported a respiratory infection within the 2 weeks preceding hospitalisation with piGAS, further questioning a major role for these infections in driving the piGAS surge in Portugal.
As observed in other countries [5,8-10,13], multiple genetic lineages were identified, despite the clear dominance of the M1UK sublineage (47.3%). The association between emm1 and PICU admission observed in Portugal and Denmark [5] supports an increased virulence of lineages expressing this emm type, which remains significantly associated with invasive disease [5,18]. The genomic analysis of our emm12 isolates (21.8%) revealed a much higher genetic diversity when compared with emm1, with no apparent expansion of any sublineage. Given that emm12 was significantly associated with non-invasive infections in Denmark [5], its abundance in piGAS during 2022/23 in Portugal may reflect its high prevalence in the population.
The absence of macrolide and lincosamide resistance among piGAS in 2022/23 follows the decreasing trend recorded among all-age invasive and pharyngeal infections in Portugal [19,20] and is in line with the low prevalence of macrolide resistance reported during the surge in England [15].
Conclusion
Portugal is experiencing an exceptionally pronounced and long surge of piGAS, with no signs of substantial reduction in spring of 2023, despite a brief, unexplained decrease in April. Therefore, GAS should remain one of the main suspected aetiological agents in children presenting with compatible clinical signs. The presence of pharyngitis or rash may be important clues for suspecting this agent. Together with data from other countries, our genomic analysis indicates that multiple sublineages are important in the post-COVID-19 multinational surge of piGAS.
Ethical statement
The study was approved by the Institutional Review Board of the Centro Académico de Medicina de Lisboa. Since only anonymised demographic patient information was used and the samples used were collected within the normal diagnostic procedure by the attending physician, the study was exempt from obtaining written informed consent from the patients. All methods were performed in accordance with the relevant guidelines and regulations.
Funding statement
RM was supported by the Fundação para a Ciência e Tecnologia (FCT) (grant 2020.08493.BD).
Data availability
Raw sequencing data and sample metadata for the 55 paediatric invasive GAS isolates collected in Portugal between September 2022 and May 2023 are available in the European Nucleotide Archive (ENA) under project accession number PRJEB65018.
Acknowledgements
We thank Thor Bech Johannesen and colleagues at Statens Serum Institut, Copenhagen, Denmark, for sharing information on Danish isolates.
Supplementary Data
Supplementary Data
Conflict of interest: FR has received honoraria for serving on speakers´ bureaus and as an expert in Advisory Boards for GlaxoSmithKline, MSD, Pfizer and Sanofi. JM-C received research grants administered through his university and received honoraria for serving on the speakers’ bureaus of Pfizer and MSD. MR received honoraria for serving on the speakers’ bureaus of Pfizer and MSD and for serving in expert panels of GlaxoSmithKline and MSD. All other authors declare no conflict of interest.
Authors’ contributions: CG, AIC, FR, JM-C, PGSSI and PSGPISD were involved in data collection. MPB-L, AF and PGSSI were involved in collection and laboratory manipulation of isolates. RM, MR, and AF were involved in whole genome sequencing data analysis. CG, AF and MR analysed and interpreted the data. CG, MR, JM-C and AF were involved in the conception and design of the study. CG, MR and AF drafted the manuscript. All authors read, revised, and approved the final manuscript.
References
- 1.European Centre for Disease Prevention and Control (ECDC). Increase in invasive group A streptococcal infections among children in Europe, including fatalities. Stockholm: ECDC; 2022. Available from: https://www.ecdc.europa.eu/en/news-events/increase-invasive-group-streptococcal-infections-among-children-europe-including
- 2. Miller KM, Lamagni T, Cherian T, Cannon JW, Parks T, Adegbola RA, et al. Standardization of epidemiological surveillance of invasive group A streptococcal infections. Open Forum Infect Dis. 2022;9(Suppl 1):S31-40. 10.1093/ofid/ofac281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friães A, Mamede R, Ferreira M, Melo-Cristino J, Ramirez M. Annotated whole-genome multilocus sequence typing schema for scalable high-resolution typing of Streptococcus pyogenes. J Clin Microbiol. 2022;60(6):e0031522. 10.1128/jcm.00315-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lynskey NN, Jauneikaite E, Li HK, Zhi X, Turner CE, Mosavie M, et al. Emergence of dominant toxigenic M1T1 Streptococcus pyogenes clone during increased scarlet fever activity in England: a population-based molecular epidemiological study. Lancet Infect Dis. 2019;19(11):1209-18. 10.1016/S1473-3099(19)30446-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johannesen TB, Munkstrup C, Edslev SM, Baig S, Nielsen S, Funk T, et al. Increase in invasive group A streptococcal infections and emergence of novel, rapidly expanding sub-lineage of the virulent Streptococcus pyogenes M1 clone, Denmark, 2023. Euro Surveill. 2023;28(26):2300291. 10.2807/1560-7917.ES.2023.28.26.2300291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davies MR, Keller N, Brouwer S, Jespersen MG, Cork AJ, Hayes AJ, et al. Detection of Streptococcus pyogenes M1UK in Australia and characterization of the mutation driving enhanced expression of superantigen SpeA. Nat Commun. 2023;14(1):1051. 10.1038/s41467-023-36717-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 13.0. Växjö: EUCAST; 2023. Available from: http://www.eucast.org
- 8. de Gier B, Marchal N, de Beer-Schuurman I, Te Wierik M, Hooiveld M, de Melker HE, et al. Increase in invasive group A streptococcal (Streptococcus pyogenes) infections (iGAS) in young children in the Netherlands, 2022. Euro Surveill. 2023;28(1):2200941. 10.2807/1560-7917.ES.2023.28.1.2200941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guy R, Henderson KL, Coelho J, Hughes H, Mason EL, Gerver SM, et al. Increase in invasive group A streptococcal infection notifications, England, 2022. Euro Surveill. 2023;28(1):2200942. 10.2807/1560-7917.ES.2023.28.1.2200942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santé publique France. Situation des infections invasives à streptocoque A en France au 26 mars 2023. [Status of invasive streptococcal A infections in France as of March 26, 2023]. Paris: Santé publique France; 2023. French. Available from: https://www.santepubliquefrance.fr/docs/situation-des-infections-invasives-a-streptocoque-a-en-france-au-26-mars-2023
- 11.Health Protection Surveillance Centre (HPSC). Update on Group A Streptococcus. Dublin: HPSC. [Accessed: 29 May 2023]. Available from: https://www.hpsc.ie/news/title-22663-en.html
- 12.European Centre for Disease Prevention and Control (ECDC). Communicable disease threats report, 7-13 May 2023, week 19. 2023. Stockholm: ECDC; 2023. Available from: https://www.ecdc.europa.eu/en/publications-data/communicable-disease-threats-report-7-13-may-2023-week-19
- 13. Barnes M, Youngkin E, Zipprich J, Bilski K, Gregory CJ, Dominguez SR, et al. Notes from the field: increase in pediatric invasive Group A Streptococcus Infections — Colorado and Minnesota, October–December 2022. MMWR Morb Mortal Wkly Rep. 2023;72(10):265-7. 10.15585/mmwr.mm7210a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cobo-Vázquez E, Aguilera-Alonso D, Carrasco-Colom J, Calvo C, Saavedra-Lozano J, Calvo C, et al. Increasing incidence and severity of invasive group A streptococcal disease in Spanish children in 2019-2022. Lancet Reg Health Eur. 2023;27:100597. 10.1016/j.lanepe.2023.100597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United Kingdom Health Security Agency. Group A streptococcal infections: 14th update on seasonal activity in England. London: Gov.uk. [Accessed: 29 May 2023]. Available from: https://www.gov.uk/government/publications/group-a-streptococcal-infections-activity-during-the-2022-to-2023-season/group-a-streptococcal-infections-14th-update-on-seasonal-activity-in-england
- 16. van Kempen EB, Bruijning-Verhagen PCJ, Borensztajn D, Vermont CL, Quaak MSW, Janson JA, et al. Increase in invasive group A streptococcal infections in children in the Netherlands, a survey among 7 hospitals in 2022. Pediatr Infect Dis J. 2023;42(4):e122-4. 10.1097/INF.0000000000003810 [DOI] [PubMed] [Google Scholar]
- 17.Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA). Boletim de vigilância epidemiológica da gripe e outros vírus respiratórios (Semana 30 – 24 a 30 jul). [Bulletin of Influenza Epidemiological Surveillance (Week 30 – 24 to 30 Jul)]. Lisbon: INSA; 2023. Available from: https://www.insa.min-saude.pt/category/informacao-e-cultura-cientifica/publicacoes/atividade-gripal
- 18. Friães A, Pinto FR, Silva-Costa C, Ramirez M, Melo-Cristino J, Portuguese Group for the Study of Streptococcal Infections . Group A streptococci clones associated with invasive infections and pharyngitis in Portugal present differences in emm types, superantigen gene content and antimicrobial resistance. BMC Microbiol. 2012;12(1):280. 10.1186/1471-2180-12-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friães A, Melo-Cristino J, Ramirez M, Vaz T, Gião M, Ferreira R, et al. Changes in emm types and superantigen gene content of Streptococcus pyogenes causing invasive infections in Portugal. Sci Rep. 2019;9(1):18051. 10.1038/s41598-019-54409-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silva-Costa C, Ramirez M, Melo-Cristino J, Portuguese Group for Study of Streptococcal Infections . Declining macrolide resistance in Streptococcus pyogenes in Portugal (2007-13) was accompanied by continuous clonal changes. J Antimicrob Chemother. 2015;70(10):2729-33. 10.1093/jac/dkv182 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.