Abstract
Human granulocytic ehrlichiosis (HGE) is an emerging infection caused by an Ehrlichia species closely related to Ehrlichia equi and Ehrlichia phagocytophila. Recent advances in the isolation and cultivation of this organism have allowed us to develop an immunofluorescence assay (IFA), enzyme immunoassay (EIA), and Western immunoblotting (WB) using HL-60 cell culture-derived human isolates. Antibody was detected in sera from culture-confirmed HGE patients by IFA and EIA, and these samples were reactive when analyzed by immunoblot analysis. HGE patient sera had high antibody titers and did not react with uninfected HL-60 cells. When IFA, EIA, and WB were used to analyze sera from healthy donors or those with a range of other disorders, including infections caused by Ehrlichia chaffeensis, Rickettsia rickettsii, and Coxiella burnetti, no significant cross-reactivity could be detected by EIA or immunoblot analysis with the exception of two of four serum samples from R. rickettsii-infected patients that were reactive by IFA only. Sera from HGE patients did not significantly cross-react in serologic tests for Borrelia burgdorferi. Using sera from patients previously enrolled in two clinical trials of treatment for early Lyme disease, we evaluated a two-step approach for estimation of the seroprevalence of antibodies reactive with the etiologic agent of HGE. On the basis of the immunoblot assay results for sera from culture-confirmed HGE patients, WB was used to confirm the specificity of the antibody detected by EIA and IFA. EIA was found to be superior to IFA in the ability to detect WB-confirmed antibodies to the HGE agent. When EIA and WB were used, 56 (19.9%) patients with early Lyme disease (n = 281) had either specific immunoglobulin M (IgM) or IgG antibodies; 38 patients (13.5%) had IgM only, 6 (2.1%) had IgG only, and 12 (4.3%) had both IgM and IgG. Therefore, Lyme disease patients are at high potential risk for exposure to Ehrlichia. Analysis by immunoblotting of serial samples from persons with culture-confirmed HGE or patients with Lyme disease and antibodies to the agent of HGE revealed a reproducible pattern of the immune response to specific antigens. These samples confirmed the importance of the 42- to 45-kDa antigens as early, persistent, and specific markers of HGE infection. Other significant immunogenic proteins appear at 20, 21, 28, 30, and 60 kDa. Use of the two-test method of screening by EIA and confirming the specificity by WB appears to offer a sound approach to the clinical immunodiagnosis of HGE.
The ehrlichiae are obligate intracellular bacteria which infect mammalian monocytes, granulocytes, and platelets. The human ehrlichioses are tick-borne illnesses of worldwide importance (21). Human monocytic ehrlichiosis (HME) in the United States is caused by Ehrlichia chaffeensis (2), which is thought to be transmitted by the lone star tick, Amblyomma americanum (3, 39). More than 400 cases of HME have been reported since 1987 (17).
An emerging granulocytotrophic ehrlichial infection of humans was first noted in the upper midwestern United States (6, 9). These ehrlichiae are closely related or identical to E. equi, which infects the granulocytes of horses in the United States, and Ehrlichia phagocytophila, the etiologic agent of a granulocytic infection of sheep, goats, and cattle in Europe (12, 20, 28). Human granulocytic ehrlichiosis (HGE) is thought to be transmitted by Ixodes scapularis ticks (18, 46, 50, 57), which are also the vectors of the causative agents of Lyme borreliosis and babesiosis. Although the reservoirs of human ehrlichial agents in the United States are not known, deer are the primary hosts for both I. scapularis and A. americanum and have been implicated as potentially important in the vector ecology of both HGE and HME (14, 16, 24). The white-footed mouse, Peromyscus leucopus, may be a reservoir of the HGE agent (44, 57, 58). HGE has been reported from areas where Ixodes ticks are present, with most cases reported from the midwestern and northeastern United States and California (1, 6, 10, 27, 31, 60). Evidence for the presence of HGE in Europe also exists (8, 22, 25, 52).
Infection with HGE is acute, and the clinical manifestations of infection closely resemble those of HME. Patients typically present with fever, myalgias, arthralgia, headache, and rigors (6, 9). Laboratory findings of HGE infection often include leukopenia, thrombocytopenia, and anemia with elevated hepatic transaminase and lactate dehydrogenase levels (9). HGE infection often responds to treatment with tetracyclines. For most treated patients recovery is rapid; however, some fatalities have occurred (6, 21, 31).
While diagnostic, the presence of morulae in the blood smears of infected persons is not a sensitive approach to laboratory diagnosis. Isolation of the organism and detection of granulocytic ehrlichiae in blood by PCR are possible (28), but these methods surpass the technical resources of many laboratories. Because of the difficulty of detecting HGE infection, the uncharacterized epidemiology of this disease, and the relatively protean clinical manifestations, serological testing may provide an important resource for improved diagnosis and understanding of disease epidemiology.
The primary method of detecting antibodies to Ehrlichia has been the indirect immunofluorescence assay (IFA), based upon the use of Ehrlichia equi within the neutrophils of experimentally infected horses. The recent isolation and cultivation of the agent of HGE (28, 54) have allowed us to develop and evaluate serological assays using human isolates of this organism. We used an IFA, enzyme immunoassay (EIA), and Western immunoblotting (WB) to examine sera from healthy donors and patients with culture-confirmed infection with the HGE agent and serial serum specimens from patients with physician-diagnosed Lyme borreliosis since these patients are at high risk for HGE.
MATERIALS AND METHODS
HGE patients.
HGE patients were evaluated at the University of Minnesota Academic Health Center or elsewhere by one of the authors (J.L.G.) between 1995 and 1997. Venous blood was collected and inoculated into cultures of a human promyelocytic cell line, HL-60, as described previously (28). Informed consent and Institutional Review Board approval were obtained for these studies.
Cultivation of ehrlichiae.
Human granulocytic ehrlichiae strains HGE-1 and HGE-2 were cultivated in the HL-60 cell line (CCL240; American Type Culture Collection). Both strains were isolated from patients with acute HGE and were subjected to sequencing of their entire 16S rRNA genes (28). HL-60 cells were grown in RPMI 1640 (Gibco, Grand Island, N.Y.) containing 30 mM HEPES, 20 mM sodium bicarbonate, and 10% fetal calf serum (Gibco) at 37°C with 5% CO2. HL-60 cells in 125-cm2 flasks were infected when they reached a density of ca. 5 × 105 cells per ml by the addition of a 1:100 ratio of HL-60 cells which were previously infected with ehrlichiae to a level of 90% or greater. The cells were then examined as cytospin preparations every 24 h. HL-60 cells were visualized by fixing cytospin slides in 1:1 methanol and acetone at room temperature for 10 min. The slides were then stained with 0.02% Giemsa stain (Sigma Chem., St. Louis, Mo.) for 15 min and were examined by microscopy for morulae under oil immersion at ×630.
Antigen preparation.
Ehrlichiae were harvested when greater than 95% of the HL-60 cells had visible morulae. The cultures were centrifuged in 100-ml volumes at 500 × g for 10 min at 4°C. The supernatant was discarded and the pellet was resuspended in 5 ml of ice-cold, sterile 10 mM phosphate-buffered saline (PBS; pH 7.4).
For the IFA, antigen was prepared by diluting the pellet of infected HL-60 cells in PBS to a concentration of 107 cells per ml. The cells were then applied to 18-well coated microscope slides (CelLine, Newfield, N.J.) as a volume of 5 μl per well. The slides were air dried and fixed in 1:1 methanol and acetone at ambient temperature for 10 min. Fixed slides were stored desiccated at −70°C.
For the EIA and immunoblotting, resuspended infected HL-60 cells were sonicated on ice by using a Fisher model 550 Sonic Dismembrator (Fisher Scientific, Itasca, Ill.) set at a rate of 3 A by applying three 10-s pulses interspersed with 30-s rests. The resulting material was then centrifuged at 500 × g for 10 min at 4°C to remove cellular debris. The supernatant was collected, and the bacteria were harvested by centrifugation at 10,000 × g for 20 min at 4°C.
When preparing antigen for EIA, the pellet was resuspended in 1 ml of PBS (pH 7.4) per 100 ml of the original culture and was sonicated to disrupt the bacteria. This treatment consisted of three 20-s discharges at a rate of 3 A with 60-s rests. The sonicated antigen was filtered through a 0.45-μm-pore-size cellulose acetate filter. The protein concentration of the filtrate was determined by a modified biuret assay with bicinchoninic acid (Pierce Chemical, Rockford, Ill.). Antigen was then diluted to 0.1 mg per ml, aliquoted, and frozen at −70°C until needed. Antigen for immunoblotting was prepared and quantified exactly as described for EIA except that the bacterial pellet was resuspended in the smallest volume of PBS permissible and the bacteria were immediately frozen at −70°C without sonication.
Serum samples.
Sera from HGE patients were collected at the University of Minnesota Academic Health Center and at community clinics. Healthy donor sera were from the American Red Cross (St. Paul, Minn.). The specificities of the assays were assessed with sera from persons with the following physician-diagnosed disorders: rheumatoid arthritis, systemic lupus erythematosus, infectious mononucleosis, multiple sclerosis, syphilis, relapsing fever, group A streptococcal infection, Lyme disease, human monocytic ehrlichiosis (E. chaffeensis), Rocky Mountain spotted fever (Rickettsia rickettsii), and Q fever (Coxiella burnetii). Sequential serum specimens from early Lyme disease treatment studies were examined for antibody to human granulocytic ehrlichiae. The patients studied were from New York, Connecticut, New Jersey, and Minnesota and were enrolled in one of two studies (41, 48). Both groups had physician-diagnosed erythema migrans. Study group I received oral therapy consisting of 500 mg of azithromycin once daily for 7 days or 500 mg of amoxicillin three times daily for 20 days. Sera from these participants were collected at the baseline (day 0) and following the initiation of therapy at day 8, day 20, day 30, day 90, and day 180. Study group II received oral antibiotic therapy consisting of either 500 mg of cefuroxime twice daily for 20 days or 100 mg of doxycycline three times daily for 20 days. Sera were collected at the baseline and on day 8 following the initiation of therapy, 1 to 5 days after the completion of treatment, day 30 posttreatment (PT), day 60 PT, and 1 year PT.
IFA.
Details of the IFA have been described previously (25). The sera were diluted in PBS (pH 7.4) and a 5-μl volume was applied to each well of the slides. The slides were incubated for 1 h at ambient temperature, rinsed twice in PBS (pH 7.4), and submerged in PBS for 5 min. After air drying, affinity-purified fluorescein isothiocyanate-conjugated goat anti-human μ-specific immunoglobulin M (IgM) or heavy- and light-chain IgG (Kirkegaard & Perry Laboratories [KPL], Gaithersburg, Md.) were diluted to 1:40 and 1:240, respectively, and were applied to wells in a volume of 5 μl. Slides were incubated again at ambient temperature for 1 h, rinsed twice in PBS (pH 7.4), and immersed for 5 min in PBS containing 0.005% Evans blue (Sigma). Following a brief rinse with distilled water to remove excess stain, the slides were overlaid with mounting medium consisting of 10% PBS–buffered glycerol containing 0.1% (wt/vol) diazibicylooctane (Sigma). Controls were included for each slide and consisted of convalescent-phase sera from patients with culture-confirmed HGE or healthy donors with no clinical history of HGE infection. Titers were determined as the endpoint at which the fluorescence of the Ehrlichia in the cytoplasm of HL-60 cells was no longer distinct.
Cutoff values for the IFA were established on the basis of the values for sera from a sample of healthy donors (n = 32). A titer of <1:80 correlated with the presumptive absence of antibody in the sera from healthy persons. Accordingly, a titer of <1:80 was interpreted as negative, a titer of 1:80 was interpreted as equivocal, and a titer of >1:80 was interpreted as positive (21, 25, 49).
EIA.
The EIA was a solid-phase noncompetitive assay similar to that used for the detection of antibodies to Borrelia burgdorferi (33, 43). Ninety-six-well curved-bottom polystyrene plates (Nunc, Roskilde, Denmark) were coated passively by the application to each well of 50 μl of antigen diluted just before use to 10 μg per ml in Dulbecco’s PBS (DPBS; pH 7.4). The plates were then incubated uncovered at 37°C overnight. The next day, the plates were blocked with 5% nonfat dry milk (NFDM; Carnation, Los Angeles, Calif.) in DPBS by the addition of 160 μl to each well. Blocking was carried out at 37°C for 1.5 h. Human test sera were diluted to 1:200 in diluent containing 5% NFDM in DPBS, 0.1% dextran sulfate (Sigma), and 0.5% donor goat serum (Sigma). After blocking, the plates were washed three times in DPBS containing 0.5% Tween 20 (Sigma). The test sera were applied in triplicate to wells in a volume of 60 μl and were incubated at 37°C for 1 h. The plates were then washed four times in wash buffer. Affinity-purified goat anti-human μ-specific IgM or heavy- and light-chain IgG conjugated with horseradish peroxidase (KPL) were appropriately diluted in the same diluent used for human sera, and 60 μl was placed in the wells. The plates were incubated for 1 h at 37°C and washed four times in wash buffer. Sixty microliters of the substrate 2,2-azino-di-3-ethyl-benzthiazoline sulfonate (KPL) was added to each well. After 20 min at 37°C the absorbances at 405 nm were determined. The timing of the development was based upon the previously established performance of the controls. Plates were read every 10 min until the controls were within 1 to 2 standard deviations of the mean values previously obtained from at least three replicate trials for each control.
The initial equivocal and positive cutoff values were established by measuring the mean absorbances of IgM (n = 137) and IgG (n = 150) for a sample of sera from healthy donors (American Red Cross). Prior to the collection of serum, these donors were subjected to routine screening to ensure their suitability and the absence of acute disorders. Cutoff points for each isotype were based upon values 2 and 3 standard deviations above the mean absorbance for equivocal and positive, respectively (33). The determinative value of these points was later confirmed by examining the receiver operator characteristics of the assay when sensitivity and specificity were equally weighted (29). For uniformity, the EIA results were reported as a ratio of the individual sample absorbances to the established cutoff value. This resulted in a scale on which the result was negative if the IgM or IgG ratio was less than 0.7 and equivocal if the ratio was 0.7 to less than 1.0. Values of 1.0 or greater were considered positive.
EIA for the detection of antibodies to B. burgdorferi was carried out as described previously (33). A ratio of <0.9 was considered negative, a ratio of between ≥0.9 and <1.0 was considered equivocal, and a ratio of ≥1.0 was considered positive.
Immunoblotting.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis with the buffer system of Laemmli (38) was carried out as described previously (33). Proteins were separated by using a polyacrylamide gel with a linear gradient concentration of from 7.5 to 15% or with a 12% gel. Samples were diluted appropriately and were boiled for 2 min in sample buffer (63 mM Tris, 2% SDS, 15 mM dithiothreitol, 27% [wt/vol] sucrose, 0.002% bromphenol blue). Three hundred micrograms of protein was applied to a 12-cm trough in the stacking gel. The polypeptides were separated at 35 mA of constant current per gel for ca. 1.5 h. The proteins were then transferred from the gel to polyvinylidene difluoride (Millipore, Bedford, Mass.) at 1 A for 30 min by methods described previously (33). The blot was then blocked in Tris-buffered saline (TBS; 20 mM Tris, 500 mM NaCl [pH 7.5]) containing 0.5% NFDM for 1 h. Following blocking, the membranes were washed for 45 min in TBS containing 0.1% Tween-20 (TTBS) and air dried for storage at ambient temperature. At the time of the assay, the membranes were wet in TTBS and cut into 3- to 4-mm strips. The sera were diluted 1:200 in TBS with 0.5% NFDM. Following incubation of the membranes in patient sera for 1 h, the strips were washed twice for 5 min each time in TTBS. Affinity-purified goat anti-human μ-specific IgM or γ-specific IgG conjugated with alkaline phosphatase (KPL) was diluted under optimal conditions in TBS with 0.5% NFDM and was applied to the strips. Following incubation for 1 h the strips were washed twice for 5 min each time with TTBS and twice for 5 min each time with TBS and equilibrated for 5 min in 100 mM Tris–100 mM NaCl containing 5 mM Mg2+ (pH 9.5). Developing solution contained 0.4 mM Nitro Blue Tetrazolium (Sigma) and 0.4 mM 5-bromo-4-chloro-3-indolylphosphate (Sigma) and was prepared in Tris-NaCl-Mg buffer at pH 9.5. The development time was 15 to 20 min on the basis of the intensities of the first, middle, and last strips of each immunoblot which were processed with positive control sera.
To establish tentative criteria for the confirmation of screening test results by immunoblotting, acute- and convalescent-phase sera from six individuals with clinically diagnosed HGE (five with HGE confirmed by culture and one with HGE confirmed by examination of peripheral blood smears for morulae) were examined by WB. The presence of reactive antigens in the range of 42 to 45 kDa appeared to represent the earliest IgM and IgG responses and the most intense reactivity. These proteins were not visualized by WB in healthy donors or patients with other illnesses (see Table 2) or when patient sera were used to probe uninfected HL-60 cells. Accordingly, the presence of this complex was attributed to the presence of specific antibody and was considered the minimum criterion for interpretation of a WB result as positive.
TABLE 2.
Reactivity of sera from patients with conditions other than HGE
| Patient condition | No. of samples reactive/no. of samples examined
|
|||||
|---|---|---|---|---|---|---|
| IgM
|
IgG
|
|||||
| IFA | EIA | WB | IFA | EIA | WB | |
| Systemic lupus erythematosus | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| Rheumatoid factor | 0/5 | 1/5 | 0/5 | 0/7 | 0/7 | 0/5 |
| Chronic fatigue syndrome | 0/5 | 0/5 | 0/5 | 1/5 | 0/5 | 0/5 |
| Infectious mononucleosis | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 |
| Group A streptococcal sequelae | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| Relapsing fever | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Syphilis | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Human monocytic ehrlichiosis | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Rocky Mountain spotted fever | 2/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| Q fever | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 |
| All disorders | 2/53 | 1/31 | 0/31 | 1/53 | 0/46 | 0/31 |
| Healthy donors | 0/32 | 1/169 | 0/30 | 0/32 | 2/169 | 0/30 |
Immunoblot analysis for confirmation of infection with B. burgdorferi has been described previously (33).
RESULTS
Comparison of identically processed antigen preparations by using both infected and uninfected HL-60 cells indicated that uninfected host cell components did not contribute significantly to the reactivity of the IFA, EIA, or WB for IgM or IgG when sera from healthy donors, persons with HGE, or patients with other disorders were used. The use of two human isolates of Ehrlichia as antigen were compared. Because preliminary experiments were unable to detect significant differences between strains when the strains were used for the EIA or WB, strain HGE-2 was used throughout these studies.
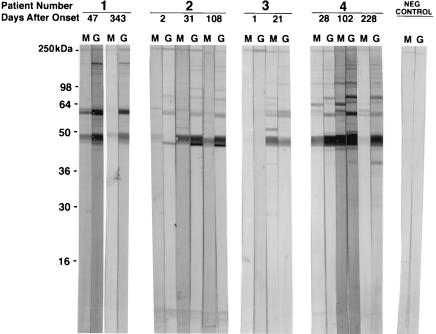
Sera from six HGE patients (five with culture-confirmed HGE and one confirmed to have morulae) were analyzed by IFA, EIA, and WB for antibodies to the HGE agent (Table 1). Culture-confirmed HGE patients 1, 2, and 3 were negative for IgM and IgG antibodies by EIA and WB at day 1 to 2 after the onset of disease symptoms. The IFA results fluctuated between negative and equivocal during this time and WB results were negative. The earliest convalescent-phase serum specimens available from patients 1, 2, and 3 were at days 47, 31, and 21 after disease onset, respectively. All these patients with the exception of patient 3 had developed strong IgM and IgG antibody responses by IFA, EIA, and WB; patient 3 remained equivocal for IgG by EIA but was WB positive for IgG. The earliest serum specimen available from patient 4 (who had culture-confirmed HGE) was at day 28. This specimen and subsequent serum specimens were positive for IgM and IgG antibodies against the HGE agent. Only a single serum specimen obtained at day 15 after the onset of disease was available from patient 5 (confirmed to have HGE by the detection of morulae), and it already contained IgM and IgG anti-HGE antibodies. We were not able to detect IgM antibodies in serum specimens obtained from patient 6 (who had culture-confirmed HGE) on either day 5 or day 150 after disease onset, while IgG antibodies were detected 150 days after the onset of disease. Sera were available from patients 1, 2, and 4 at 343, 108, and 228 days after disease onset, respectively, and both IgM and IgG antibodies were detected. The results of Western blot analysis of culture-confirmed HGE patients 1, 2, 3, and 4 are presented in Fig. 1.
TABLE 1.
Results of serology for HGE patients
| Patient no. | No. of days from onseta | IgM
|
IgG
|
||||
|---|---|---|---|---|---|---|---|
| IFA resultb | EIA resultc | WB result | IFA result | EIA result | WB result | ||
| 1 | 2 | 80 (Ed) | 0.5 (Ne) | N | 80 (E) | 0.1 (N) | N |
| 47 | 160 (Pf) | 2.9 (P) | P | 160 (P) | 2.2 (P) | P | |
| 343 | 80 (E) | 1.2 (P) | P | 80 (E) | 1.0 (P) | P | |
| 2 | 2 | 80 (E) | 0.6 (N) | N | <80 (N) | 0.2 (N) | N |
| 31 | 640 (P) | 3.9 (P) | P | 320 (P) | 2.4 (P) | P | |
| 108 | 160 (P) | 2.2 (P) | P | 320 (P) | 1.4 (P) | P | |
| 3 | 1 | <80 (N) | 0.2 (N) | N | <80 (N) | 0.1 (N) | N |
| 21 | 160 (P) | 3.6 (P) | P | 160 (P) | 0.8 (E) | P | |
| 4 | 28 | NDg | 4.3 (P) | P | ND | 2.4 (P) | P |
| 182 | 1,280 (P) | 4.2 (P) | P | 5,120 (P) | 2.8 (P) | P | |
| 228 | ND | 1.3 (P) | P | ND | 3.2 (P) | P | |
| 5 | 15 | <80 (N) | 1.4 (P) | P | 160 (P) | 1.2 (P) | P |
| 6 | 5 | <80 (N) | 0.2 (N) | N | <80 (N) | 0.1 (N) | N |
| 150 | <80 (N) | 0.3 (N) | N | <80 (N) | 1.0 (P) | P | |
Number of days following the onset of clinical ehrlichiosis.
Results are inverse of titer, with interpretation in parentheses.
Results are a ratio, with interpretation in parentheses.
E, equivocal.
N, negative.
P, positive.
ND, not determined.
FIG. 1.
Western immunoblot of acute- and convalescent-phase sera from patients with culture-confirmed HGE (Table 1) and serum from a healthy donor. Polypeptides were separated with a 12% polyacrylamide gel.
The sensitivities and specificities of IFA and EIA for the detection of IgM and IgG antibodies against the HGE agent in the culture- and morula-confirmed HGE patients were compared. IFA and EIA results were available for seven serum specimens positive for IgM and IgG by WB. The results of the two assays were very similar. EIA was positive for seven of seven serum specimens positive for IgM by WB, whereas IFA was positive for six of the seven serum specimens. The converse occurred for the detection of IgG-positive sera by WB. None of the sera negative for IgM and IgG by WB were positive or equivocal by EIA, while two of the serum specimens negative for IgM by WB and one of the serum specimens negative for IgG by WB gave equivocal IFA results (Table 1).
We next applied a two-step protocol recommended for use in the serodiagnosis of Lyme disease (11) for the serodiagnosis of HGE. The first test in this protocol is a sensitive serological screening test such as IFA or EIA. The results for all specimens found to be positive or equivocal by EIA or IFA were then confirmed by WB for the presence of the 42- to 45-kDa bands.
We first compared the sensitivities and specificities of EIA and IFA as the screening tests in this protocol (Table 2). None of the sera from 32 healthy donors analyzed by IFA and EIA possessed IgM or IgG antibodies reactive with the HGE agent. An additional 137 serum specimens from healthy donors were assayed for IgM and IgG anti-HGE antibodies by EIA. One specimen (0.7%) was IgM positive and IgG negative and two specimens (1.5%) were IgM negative and IgG positive. Western blot analyses of these sera were negative, indicating that they were false-positive reactions.
Sera from patients with a range of other disorders including rickettsial and non-HGE ehrlichial infections were examined for IgM and IgG antibodies reactive with the agent of HGE (Table 2). All of the sera were negative for IgM antibodies with the exception of two of four specimens from patients with Rocky Mountain spotted fever, which were positive by IFA but negative by both EIA and WB, and one of five serum specimens positive for rheumatoid factor, which was positive by EIA but negative by IFA and WB. IgG antibodies were detected by IFA in one of five serum specimens from chronic fatigue syndrome patients, but these sera were negative by EIA and WB. All the remaining sera were negative for IgG antibodies by IFA, EIA, and WB.
Possible cross-reactivity of antibodies to the agent of HGE with B. burgdorferi was examined by the two-test protocol by using EIA and WB and sera from six patients with culture- or morula-confirmed HGE (Table 3). Patients 2, 4, 5, and 6 did not have IgM or IgG antibodies reactive with B. burgdorferi according to these criteria. Sera from patients 1 and 4 were negative for IgM antibodies to B. burgdorferi, but they were positive for IgG antibodies to B. burgdorferi by EIA and WB (Table 3).
TABLE 3.
Results of the two-test protocol for Lyme disease serology of patients with HGE infection
| Patient no. | No. of days from onseta | IgM
|
IgG
|
||||
|---|---|---|---|---|---|---|---|
| EIA resultb | WB result | Final result | EIA result | WB result | Final result | ||
| 1 | 2 | 0.5 (Nc) | NDd | N | 1.2 (Pe) | P | P |
| 47 | 0.6 (N) | ND | N | 1.3 (P) | P | P | |
| 343 | 0.7 (N) | ND | N | 0.6 (N) | ND | N | |
| 2 | 2 | 0.7 (N) | ND | N | 0.3 (N) | ND | N |
| 31 | 0.8 (N) | ND | N | 0.4 (N) | ND | N | |
| 108 | 0.8 (N) | ND | N | 0.6 (N) | ND | N | |
| 3 | 1 | 0.5 (N) | ND | N | 0.3 (N) | ND | N |
| 21 | 0.8 (N) | ND | N | 0.4 (N) | ND | N | |
| 4 | 28 | ND | ND | N | ND | ND | ND |
| 182 | 1.6 (P) | N | N | 1.1 (P) | P | P | |
| 228 | 0.7 (N) | ND | N | 1.1 (P) | N | N | |
| 5 | 15 | 0.4 (N) | ND | N | 0.1 (N) | ND | N |
| 6 | 5 | 0.5 (N) | ND | N | 0.1 (N) | ND | N |
| 150 | 0.8 (N) | ND | N | 0.1 (N) | ND | N | |
Number of days following the onset of clinical ehrlichiosis.
Results are a ratio, with interpretation in parentheses.
N, negative.
ND, not determined.
P, positive.
We next investigated the seroprevalence of antibodies reactive to the agent of HGE in patients with physician-diagnosed early Lyme disease. These patients were expected to be at high risk for HGE since they resided in areas where HGE is endemic and had at least one documented I. scapularis tick bite. Sequential serum specimens were available from 281 patients previously enrolled in two clinical treatment trials (study group I and study group II). Serum specimens collected at 21 to 25 days following the diagnosis and initiation of treatment for Lyme disease were selected for analysis. The two-step protocol was used to assay these sera. We compared the IFA and EIA as screening tests in this protocol for 139 serum specimens from those patients in study group I (Table 4). In the absence of clinical data confirming HGE in these patients, IFA and EIA were evaluated as screening tests on the basis of their ability to detect samples positive by immunoblotting. Nine serum specimens (6.5%) were IgM positive or equivocal by IFA, and five (55.6%) of these serum specimens were confirmed to be positive by WB. Forty-six serum specimens (33.1%) were EIA positive or equivocal for IgM antibodies reactive with antigens of the agent of HGE. Of those samples positive or equivocal for IgM by EIA, 23 (50.0%) were confirmed to be positive by WB. By using EIA as a first test in the two-test protocol, 16.5% of the 139 early Lyme disease patients enrolled in study group I were confirmed by WB to have IgM antibodies suggestive of exposure to the agent of HGE. In contrast, if the IFA were used to screen for IgM antibodies, only 3.6% of the patients would have been identified as having WB-confirmed IgM antibodies.
TABLE 4.
Results of HGE serology for study group I patients with physician-diagnosed early Lyme disease
| Result | No. of samples reactive for the following antibody/no. of samples examined (%):
|
|||||||
|---|---|---|---|---|---|---|---|---|
| IgM
|
IgG
|
|||||||
| IFA result | EIA result | WB result | Both tests | IFA result | EIA result | WB result | Both tests | |
| Positive | 35/139 (25.2) | 19/35 (54.2) | 19/139 (13.7) | 7/139 (5.0) | 7/7 (100) | 7/139 (5.0) | ||
| Equivocal | 11/139 (7.9) | 4/11 (36.4) | 4/139 (2.9) | 5/139 (3.6) | 5/5 (100) | 5/139 (3.6) | ||
| Positive and equivocal | 46/139 (33.1) | 23/46 (50.0) | 23/139 (16.5) | 12/139 (8.6) | 12/12 (100) | 12/139 (8.6) | ||
| Positive | 5/139 (3.6) | 4/5 (80.0) | 4/139 (2.9) | 7/139 (5.0) | 7/7 (100) | 7/139 (5.0) | ||
| Equivocal | 4/139 (2.9) | 1/4 (25.0) | 1/139 (0.7) | 1/139 (0.7) | 0/1 (0) | 0/139 (0) | ||
| Positive and equivocal | 9/139 (6.5) | 5/9 (55.6) | 5/139 (3.6) | 8/139 (5.8) | 7/8 (87.5) | 7/139 (5.0) | ||
For the detection of IgG antibodies, 8 serum samples (5.8%) were positive or equivocal by IFA and 12 (8.6%) were positive or equivocal by EIA. Seven of the 8 serum samples positive or equivocal for IgG by IFA (87.5%) and 12 serum samples (100%) positive or equivocal for IgG by EIA were confirmed to be positive by WB. By using IFA as the first test in the two-test protocol, 5.0% of the early Lyme disease patients had WB-confirmed IgG antibody evidence of past or present infection with the agent of HGE. By using EIA to screen the sera from these patients, 8.6% of the early Lyme disease patients would have been identified as having WB-confirmed IgG antibody.
The results of analysis of the study group I samples indicated that EIA was superior to IFA for screening. Accordingly, we next screened sera from early Lyme disease patients (study group II; n = 142) by EIA. Results for IgM were similar to those obtained with study group I (Table 5). Of the samples in study group II, 19.0% were WB positive for IgM, whereas 16.5% of the sera from patients in study group I were WB positive for IgM. Fewer patients in this group were found to have IgG. Analysis of samples from patients in study group II found that 4.2% were confirmed to have IgG by WB, whereas 8.6% of patients in study group I were found to have IgG by WB.
TABLE 5.
Results of HGE serology for study group II and both study group I and II patients with physician-diagnosed early Lyme disease
| Group and result | No. of samples reactive for the following antibody/no. of samples examined (%):
|
|||||
|---|---|---|---|---|---|---|
| IgM
|
IgG
|
|||||
| EIA | WB | Both tests | EIA | WB | Both tests | |
| Study group II | ||||||
| Positive | 38/142 (26.8) | 19/38 (50.0) | 19/142 (13.4) | 5/142 (3.5) | 5/5 (100) | 5/142 (3.5) |
| Equivocal | 16/142 (11.3) | 8/16 (50.0) | 8/142 (5.6) | 4/142 (2.8) | 1/4 (25.0) | 1/142 (0.7) |
| Positive and equivocal | 54/142 (38.0) | 27/54 (50.0) | 27/142 (19.0) | 9/142 (6.3) | 6/9 (66.7) | 6/142 (4.2) |
| Study groups I and II combined | ||||||
| Positive | 73/281 (26.0) | 38/73 (52.1) | 38/281 (13.5) | 12/281 (4.3) | 12/12 (100) | 12/281 (4.3) |
| Equivocal | 27/281 (9.6) | 12/27 (44.4) | 12/281 (4.3) | 9/281 (3.2) | 6/9 (66.7) | 6/281 (2.1) |
| Positive and equivocal | 100/281 (35.6) | 50/100 (50.0) | 50/281 (17.8) | 21/281 (7.5) | 18/21 (85.7) | 18/281 (6.4) |
When the results of EIA and WB for the detection of antibodies reactive with the agent of HGE in early Lyme disease patients from study Groups I and II were combined (n = 281), 50 (17.8%) of the patients had WB-confirmed IgM. IgG was confirmed by WB in 18 patients (6.4%) (Table 5). Of those samples which resulted in a positive or equivocal EIA result for IgM, antibody was confirmed by WB to be present in 50.0% of the samples. For samples which were positive or equivocal for IgG by EIA, 85.7% were confirmed to be positive by WB. Overall, 56 (19.9%) of the patients possessed either IgM or IgG antibodies reactive with the agent of HGE. Thirty-eight (13.5%) had IgM only, 6 (2.1%) had IgG only, and 12 (4.3%) had both IgM and IgG.
Evidence for the development of the antibody response to the agent of HGE was extended by evaluating serial serum specimens from persons with Lyme disease who were also positive for antibody to the agent of HGE on the basis of WB results. Eleven patients determined to have antibody to the HGE agent were selected from the two clinical treatment trials on the basis of the availability of sufficient serial samples. The analyses by IFA, EIA, and WB for samples from study group I patients are presented in Table 6. The results of EIA and WB for serial samples from study group II patients are presented in Table 7. The results for these sera demonstrate that for some patients the initial increase in IgM titers was followed by a decline in this isotype coincident to an increased IgG titer. For patient I-4 (Table 6), the kinetics of the antibody response measured by EIA are presented in Fig. 2A and the WB results are presented in Fig. 2B. These results are consistent with the possibility of concurrent Lyme disease and coinfection with the agent of HGE.
TABLE 6.
Reactivities of serial serum specimens collected from early Lyme disease patients (study group I) positive by HGE serology
| Patient | State of residence | No. of days posttreatmenta | IgM
|
IgG
|
||||
|---|---|---|---|---|---|---|---|---|
| IFA resultb | EIA resultc | WB | IFA result | EIA result | WB | |||
| I-1 | Minn. | 0 | 80 | 2.5 | + | <80 | 1.4 | + |
| 8 | 160 | 2.6 | + | <80 | 1.4 | + | ||
| 20 | 160 | 2.0 | + | 80 | 2.1 | + | ||
| 30 | 160 | 1.2 | + | 160 | 2.9 | + | ||
| 180 | <80 | 0.9 | − | 80 | 1.6 | + | ||
| I-2 | Minn. | 0 | <80 | 0.9 | − | 640 | 2.7 | + |
| 20 | <80 | 0.9 | − | 320 | 2.4 | + | ||
| I-3 | Conn. | 0 | 2,560 | 3.4 | + | 2,560 | 3.1 | + |
| 8 | 1,280 | 3.0 | + | 1,280 | 3.0 | + | ||
| 20 | 1,280 | 2.4 | + | 1,280 | 4.5 | + | ||
| 30 | 640 | 1.6 | + | 640 | 4.0 | + | ||
| I-4 | Conn. | 0 | 640 | 4.8 | + | 160 | 0.7 | + |
| 8 | 640 | 4.5 | + | 160 | 1.1 | + | ||
| 20 | 320 | 4.3 | + | 320 | 1.7 | + | ||
| 30 | 160 | 4.5 | + | 320 | 3.1 | + | ||
| 60 | <80 | 2.4 | + | 320 | 3.8 | + | ||
| 180 | <80 | 2.1 | + | 320 | 4.2 | + | ||
| I-5 | N.Y. | 0 | 160 | 1.0 | − | 320 | 3.1 | + |
| 8 | 160 | 1.1 | − | 160 | 3.4 | + | ||
| 20 | 160 | 0.8 | − | 320 | 2.9 | + | ||
| 30 | 160 | 0.9 | − | 160 | 3.5 | + | ||
| 60 | 80 | 1.3 | − | 80 | 2.8 | + | ||
| 180 | 80 | 1.0 | − | 80 | 2.5 | + | ||
| I-6 | N.Y. | 0 | NDd | 2.9 | + | ND | 2.7 | + |
| 8 | ND | 2.8 | + | ND | 2.9 | + | ||
| 20 | ND | 2.3 | + | ND | 3.3 | + | ||
Number of days following the initiation of treatment for Lyme disease.
Results are inverse dilution of titer.
Results are a ratio.
ND, not determined.
TABLE 7.
Reactivities of serial serum specimens collected from early Lyme disease patients (study group II) positive for HGE serology
| Patient | State of residence | No. of days posttreatmenta | IgM
|
IgG
|
||
|---|---|---|---|---|---|---|
| EIA resultb | WB result | EIA result | WB result | |||
| II-1 | N.Y. | 0 | 1.4 | − | 3.1 | + |
| 20 | 1.3 | − | 3.4 | + | ||
| 30 | 0.9 | − | 2.9 | + | ||
| 60 | 0.9 | − | 3.5 | + | ||
| 360 | 0.7 | − | 2.8 | + | ||
| II-2 | N.Y. | 0 | 2.8 | + | 0.6 | + |
| 8 | 3.5 | + | 1.1 | + | ||
| 20 | 3.3 | + | 1.9 | + | ||
| 30 | 2.0 | − | 0.4 | + | ||
| 60 | 1.8 | − | 0.3 | − | ||
| II-3 | N.Y. | 0 | 3.5 | + | 0.8 | + |
| 8 | 2.6 | + | 0.7 | + | ||
| 20 | 2.9 | + | 0.8 | + | ||
| 30 | 2.6 | + | 0.8 | + | ||
| 60 | 2.8 | + | 0.8 | + | ||
| II-4 | Conn. | 0 | 0.6 | + | 0.3 | − |
| 8 | 1.9 | + | 0.3 | − | ||
| 20 | 1.1 | + | 0.4 | − | ||
| 30 | 0.8 | + | 0.2 | − | ||
| 60 | 0.6 | − | 0.5 | − | ||
| II-5 | Conn. | 0 | 1.8 | + | 1.1 | + |
| 8 | 1.4 | + | 1.1 | + | ||
| 20 | 0.8 | + | 0.5 | + | ||
| 30 | 0.3 | + | 0.9 | + | ||
| 60 | 0.2 | + | 1.2 | + | ||
Number of days following the initiation of treatment for Lyme disease.
Results are a ratio.
FIG. 2.
(A) Kinetics of IgM and IgG responses to the HGE agent in a patient from Connecticut measured by EIA (Table 5). (B) Western immunoblot of serum from the patient from panel A and serum from a healthy donor. Proteins were separated with a 7 to 15% linear gradient polyacrylamide gel.
Western blot analysis of these sequential serum specimens was undertaken to further characterize the specific proteins which may be important in the serodiagnosis of HGE. Initial examination of the ability of SDS-polyacrylamide gels to resolve antigens of the HGE agent revealed that gradient and nongradient gels had advantages in detecting low- and high-molecular-mass proteins, respectively. Therefore, both methods were used throughout the study. WB revealed an IgM response which was typically first generated against the 42- to 45-kDa complex often within the initial days of infection. As seen throughout this study, these polypeptides were the first to be detected, and they were consistently the most intense and enduring bands. The next antigen reactive in IgM blots was a 30-kDa polypeptide, with the response detectable at day 8, often persisting or increasing by day 30, with the response diminished by day 90. Later, at about day 30, a 28-kDa band appeared, with the response decreased by day 90. For IgG analysis, the 42- to 45-kDa complex was also evident within the earliest stages of the antibody response. This was followed by response to the 20- and 30-kDa proteins at day 20 and the 28-kDa protein by day 30. The maximum number of major bands appeared to be stable at 12 to 17 at about 60 to 90 days. Also frequently observed were minor bands in the range of ca. 21, 29, 31, 35, 37, 60, 65, 70, and 100 kDa. WB results for sera from culture-confirmed HGE patients from Minnesota and Wisconsin and Lyme disease patients with antibodies reactive with the HGE agent primarily from the eastern United States were nearly indistinguishable.
DISCUSSION
We have described the use of cell culture-derived human isolates of ehrlichiae for the development of serological assays potentially useful in the immunodiagnosis of HGE. The results of this study demonstrate the utility of an IFA and an EIA as screening tests for sera from persons potentially infected with the etiologic agent of HGE. Because of the acute nature of HGE, a presumptive diagnosis and empiric antimicrobial therapy will be made prior to serological detection for most patients. Laboratory serological testing, however, can provide a resource for the confirmation of infection and for the evaluation of clinical outcomes, as well as for epidemiological and epizootiological studies.
Previous experience with the development of serological testing for Lyme disease has demonstrated the importance of a two-test approach. This strategy uses a sensitive screening test followed by confirmation by WB (11). We found it important to use a similar approach for the serodiagnosis of HGE.
Most current applications of IFA for HGE use E. equi prepared from the buffy coat of blood from infected horses. There are many obvious advantages to the use of continuous cultures of human isolates, including the cost, elimination of the need for animals, and the ability to maintain quality assurance standards. In this regard, isolates from ticks or humans cultivated in HL-60 cells have been shown to be useful for IFA (49, 54). The use of IFA based on host cells infected with rickettsial organisms has sometimes resulted in high rates of background fluorescence, and false-positive results may be associated with this assay (15, 34, 35, 40, 42).
EIA offers several advantages over IFA for the detection and quantification of antibodies reactive with the HGE agent. EIA is also more efficient, less laborious, and less subjective than IFA, and it can be more sensitive. For sera from patients in study group I, EIA was capable of detecting 12.9% additional samples positive for IgM by WB and 3.6% additional samples positive for IgG by WB than IFA. When EIA-positive sera which were negative by IFA were analyzed, 43.2% of IgM-positive samples and 10.5% of IgG-positive samples were WB positive. These sera would have been excluded by the use of IFA as the screening method.
With the cutoff values used in this study, EIA detected more samples which were WB positive. Of those samples found to be positive by IFA, a larger proportion were positive by WB than by EIA. A total of 52.1 and 100% of the samples found to be positive for IgM and IgG by EIA were confirmed to be positive by WB, respectively. A total of 80 and 100% of samples found to be positive for IgM and IgG by IFA were confirmed, to be positive by WB, respectively. In the equivocal range, 36.4% of the EIA samples equivocal for IgM and 100% of the EIA samples equivocal for IgG were confirmed to be positive by WB. A significant number of samples, positive by EIA were positive for IgM, but this result could not be confirmed by WB. Ideally, the screening step of the two-test protocol should be highly sensitive. Furthermore, the rate of positivity for IgM by EIA may be high in this study because the day 21 to 25 serum samples were from patients who already had an active infection with B. burgdorferi. These patients may be dissimilar to persons who would have been healthy prior to or at the time of exposure to the HGE agent.
Examination of serial serum specimens from persons infected with the agent of HGE has provided a preliminary characterization of the use of immunoblotting for the serodiagnosis of HGE. These findings suggest that the presence of the 42- to 45-kDa complex may have a strong predictive value. The 42- to 45-kDa bands have been shown to be specific for the detection of antibodies to the HGE agent and do not appear to react with antibodies to a protein of a similar size from E. chaffeensis (20, 59). These bands were not reactive for the sera from healthy donors and persons with other disorders that we examined and were always the strongest and earliest reactive bands for sera from HGE patients. These were also found to represent the earliest reactive proteins in sera from IgM-positive patients. These results are in agreement with those of previous studies which demonstrated the presence of specific antibodies to proteins with similar migration patterns when E. equi or human granulocytic Ehrlichia sp. antigen was probed with HGE patient sera (4, 20, 32, 59, 62).
Studies have shown that polymorphisms exist for the 42- to 45-kDa immunodominant proteins in the agent of HGE as well as other Ehrlichia (4, 13, 62). The interpretation of immunoblots in which proteins may have variable migration patterns can be aided by the use of monoclonal antibodies and by the use of the appropriate antigen. The antibody response to these proteins appears to be similar among the polymorphic variants. The 42- to 45-kDa antigens expressed in the strain used in our study were able to detect antibody in sera from patients from New York, New Jersey, Connecticut, Rhode Island, Minnesota, and Wisconsin. In the absence of sequential samples, the presence of the 42- to 45-kDa proteins is likely to be significant in the interpretation of the HGE immunoblotting result. The criteria for an immunodiagnostic WB assay for HGE, however, must await further analysis of the specific antibody response to antigens from various human isolates and with sera from persons with culture-confirmed HGE.
Cross-reactivity did not occur when EIA was used to examine samples from persons with other rickettsial infections, nor did we find evidence that coinfection with B. burgdorferi commonly resulted in cross-reactive samples (Table 1). The cross-reactivities of antibodies to E. chaffeensis with the antigens of E. equi or the HGE agent have been demonstrated to be infrequent when IFA and WB are used (20, 21, 45, 46, 49, 52, 54, 59). However, there are reports of the occurrence of cross-reactivity, especially when titers are high (54, 60). Two of four serum samples from patients with Rocky Mountain spotted fever were positive for IgM by IFA but not by EIA. The use of the two-step test approach eliminated most concern for cross-reactivity with the specimens examined. This strategy has been shown to improve specificity for the serodiagnosis of B. burgdorferi, human immunodeficiency virus, and other infections (9, 30, 33).
The degree of cross-reactivity associated with HGE patients and Lyme disease serology was minimal. The reactivity of sera from two of six patients is consistent with predicted rates of exposure to both of these pathogens (61). That patient 1 (Table 3) had a Lyme disease serology indicating positivity only for IgG at the acute stage of HGE suggests that the patient may have previously been exposed to B. burgdorferi. Such cross-reactivity has been reported to be much higher (61). Cross-reactivity between antibodies to heat shock proteins of Ehrlichia and antigens of B. burgdorferi may also result in a loss of specificity (37, 56, 59). While a wider range of patients will need to be examined prospectively, our results suggest that for most patients serology will be a valid method of differentiating between these infections.
Some of the early Lyme disease patients were strongly positive for IgG at the time that the treatment study was initiated (Tables 6 and 7). The epidemiological risk factors which led to the initial exposure to Ixodes ticks and B. burgdorferi would presumably set the stage for previous exposure to the agent of HGE. In some of the areas where patients resided, the prevalence of the HGE agent in ticks has been reported to range from 10 to 50% (46, 55, 57).
The observation of persistent IgM antibodies against the agent of HGE was unexpected, and its significance is not yet clear. Treatment of early Lyme disease patients with antimicrobial agents other than tetracyclines may not have been effective against Ehrlichia in these treatment trials (36). However, it is notable that culture-confirmed HGE patients also had high levels of IgM antibody for prolonged periods following appropriate chemotherapy and the resolution of clinical symptoms. Some rickettsial and ehrlichial pathogens are capable of establishing persistent infections (5, 19, 23, 51, 53). Such infections may result in IgM production. Persistent antibody levels have been detected in serial samples from HGE patients for up to 30 months by IFA with E. equi antigen (7). Further studies are needed to determine the significance of the observation of persistent IgM antibody in HGE patients and whether this signifies a response to recurrent infection.
While the true prevalence of HGE in the Lyme disease patient groups is unknown, the WB results appear to offer reasonable confirmatory evidence that IFA and EIA detected specific antibody in some patients. The kinetics of the isotype present in these samples and the progression of polypeptides from the HGE agent with which these antibodies reacted further support that assertion (Tables 6 and 7; Fig. 2). The exact sensitivities and specificities of these tests, however, cannot be definitively determined until a larger sample of sera from patients with culture- or PCR-confirmed HGE are available.
This study offers evidence that a significant number of patients with Lyme borreliosis may also be exposed to or coinfected with the agent of HGE. The application of a sensitive EIA followed by immunoblotting, as used in this investigation, suggests that the prevalence of HGE exposure in Lyme borreliosis patients is higher than that previously detected by seroprevalence studies by IFA alone (8, 22, 26, 45, 47). In light of the prevalence of antibody observed in this and other studies as well as the potential severity of HGE, the possibility of coinfection with both pathogens is worthy of clinical consideration. At present, doxycycline remains the only antibiotic shown to have both in vitro and in vivo activities against B. burgdorferi and the HGE agent (36).
ACKNOWLEDGMENTS
This research was supported in part by Public Health Service grant AI40952-01 from the National Institutes of Health (to J.L.G. and R.C.J.) and AR34744 (to R.C.J.).
We acknowledge the technical assistance of Trelawney Grenfell and Mi-Ky Lowe. Tim Leonard prepared the artwork for the manuscript. We thank Jacqueline Dawson and James Olson of the Centers for Disease Control and Prevention for providing sera from patients with rickettsial infections. We are grateful to Marina Klein for critically reviewing the manuscript.
Footnotes
This paper is dedicated to Mary C. Panghorn, the discoverer of cardiolipin, at the Division of Laboratories and Research, New York State Department of Health, Albany, New York, for her 90th birthday.
REFERENCES
- 1.Aguero-Rosenfeld M E, Horowitz H W, Wormser G P, McKenna D F, Nowakowski J, Munoz J, Dumler J S. Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann Intern Med. 1996;125:904–908. doi: 10.7326/0003-4819-125-11-199612010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Anderson B E, Dawson J E, Jones D C, Wilson K H. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J Clin Microbiol. 1991;29:2838–2842. doi: 10.1128/jcm.29.12.2838-2842.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson B E, Sims K G, Olson J G, Childs J E, Piesman J F, Happ C M, Maupin G O, Johnson B J B. Amblyomma americanum: a potential vector of human ehrlichiosis. Am J Trop Med Hyg. 1993;49:239–244. doi: 10.4269/ajtmh.1993.49.239. [DOI] [PubMed] [Google Scholar]
- 4.Asanovich K M, Bakken J S, Madigan J E, Aguero-Rosenfeld M, Wormser G P, Dumler J S. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J Infect Dis. 1997;176:1029–1034. doi: 10.1086/516529. [DOI] [PubMed] [Google Scholar]
- 5.Austin S M, Smith S M, Co B, Coppel I G, Johnson J E. Case report: serologic evidence of acute murine typhus infection in a patient with culture-negative endocarditis. Am J Med Sci. 1987;293:320–323. doi: 10.1097/00000441-198705000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Bakken J S, Dumler J S, Chen S M, Eckman M R, Van Etta L L, Walker D H. Human granulocytic ehrlichiosis in the upper midwest United States: a new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 7.Bakken J S, Krueth J, Tilden R L, Asanovich K, Walls J, Dumler J S. Abstracts of the Infectious Disease Society of America 35th Annual Meeting. 1997. Duration of IFA serologic response in humans infected with the agent of human granulocytic ehrlichiosis (HGE), abstr. 73. [Google Scholar]
- 8.Bakken J S, Krueth J, Tilden R L, Dumler J S, Kristiansen B E. Serological evidence of human granulocytic ehrlichiosis in Norway. Eur J Clin Microbiol Infect Dis. 1996;15:829–832. doi: 10.1007/BF01701530. [DOI] [PubMed] [Google Scholar]
- 9.Bakken J S, Krueth J, Wilson-Nordskog C, Tilden R L, Asanovich K, Dumler J S. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 10.Barlough J E, Madigan J E, Kramer V L, Clover J R, Hui L T, Webb J P, Vredevoe L K. Ehrlichia phagocytophila genogroup rickettsiae in ixodid ticks from California collected in 1995 and 1996. J Clin Microbiol. 1997;35:2018–2021. doi: 10.1128/jcm.35.8.2018-2021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morbid Mortal Weekly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 12.Chen S-M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotrophic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen S-M, Popov V L, Feng H M, Walker D H. Analysis and ultrastructural localization of Ehrlichia chaffeensis proteins with monoclonal antibodies Am. J Trop Med Hyg. 1996;54:405–412. doi: 10.4269/ajtmh.1996.54.405. [DOI] [PubMed] [Google Scholar]
- 14.Dawson J E, Childs J E, Biggie K L, Moore C, Stallknecht D, Shaddock J, Bouseman J, Hofmeister E, Olson J G. White-tailed deer as a potential reservoir of Ehrlichia spp. J Wildl Dis. 1994;30:162–168. doi: 10.7589/0090-3558-30.2.162. [DOI] [PubMed] [Google Scholar]
- 15.Dawson J E, Fishbein D B, Eng T R, Redus M A, Greene N R. Diagnosis of human ehrlichiosis with the indirect fluorescent antibody test: kinetics and specificity. J Infect Dis. 1990;162:91–95. doi: 10.1093/infdis/162.1.91. [DOI] [PubMed] [Google Scholar]
- 16.Dawson J E, Warner C K, Baker V, Ewing S A, Stallknecht D E, Davidson W R, Kocan A A, Lockhart J M, Olson J G. Ehrlichia-like 16s rDNA sequence from wild white-tailed deer (Odocoileus virginianus) J Parasitol. 1996;82:52–58. [PubMed] [Google Scholar]
- 17.Dawson J E, Warner C K, Standaert S, Olson J G. The interface between research and diagnosis of an emerging tick-borne disease, human ehrlichiosis due to Ehrlichia chaffeensis. Arch Intern Med. 1996;156:137–142. [PubMed] [Google Scholar]
- 18.Des Vignes F, Fish D. Transmission of the agent of human granulocytic ehrlichiosis by host-seeking Ixodes scapularis (Acari:Ixodidae) in southern New York state. J Med Entomol. 1997;34:379–382. doi: 10.1093/jmedent/34.4.379. [DOI] [PubMed] [Google Scholar]
- 19.Duffy J, Pittlekow M R, Kolbert C P, Rutledge B J, Persing D H. Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis. Lancet. 1997;349:399. doi: 10.1016/S0140-6736(97)80017-7. [DOI] [PubMed] [Google Scholar]
- 20.Dumler J S, Asanovich K M, Bakken J S, Richter P, Kimsey R, Madigan J E. Serologic cross-reactions among Ehrlichia equi, Ehrlichia phagocytophila, and human granulocytic ehrlichia. J Clin Microbiol. 1995;33:1098–1103. doi: 10.1128/jcm.33.5.1098-1103.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dumler J S, Bakken J S. Ehrlichial diseases of humans: emerging tick-borne infections. Clin Infect Dis. 1995;20:1102–1110. doi: 10.1093/clinids/20.5.1102. [DOI] [PubMed] [Google Scholar]
- 22.Dumler J S, Dotevall L, Gustafson R, Granstrom M. A population-based seroepidemiologic study of human granulocytic ehrlichiosis and Lyme borreliosis on the west coast of Sweden. J Infect Dis. 1997;175:720–722. doi: 10.1093/infdis/175.3.720. [DOI] [PubMed] [Google Scholar]
- 23.Dumler J S, Sutker W L, Walker D H. Persistent infection with Ehrlichia chaffeensis. Clin Infect Dis. 1993;17:903–905. doi: 10.1093/clinids/17.5.903. [DOI] [PubMed] [Google Scholar]
- 24.Ewing S A, Dawson J E, Kocan A A, Barker R W, Warner C K, Panciera R J, Fox J C, Kocan K M, Blouin E F. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales:Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari:Ixodidae) J Med Entomol. 1995;32:368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- 25.Fingerle V, Goodman J L, Johnson R C, Kurtti T J, Munderloh U G, Wilske B. Human granulocytic ehrlichiosis in southern Germany: increased seroprevalence in high-risk groups. J Clin Microbiol. 1997;35:3244–3247. doi: 10.1128/jcm.35.12.3244-3247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritz C L, Kjemtrup A M, Conrad P A, Flores G R, Campbell G L, Schriefer M E, Gallo D, Vugia D J. Seroepidemiology of emerging tickborne infectious diseases in a northern California community. J Infect Dis. 1997;175:1432–1439. doi: 10.1086/516476. [DOI] [PubMed] [Google Scholar]
- 27.Gewirtz A S, Cornbleet P J, Vugia D J, Traver C, Niederhuber J, Kolbert C P, Persing D H. Human granulocytic ehrlichiosis: report of a case in Northern California. Clin Infect Dis. 1996;23:653–654. doi: 10.1093/clinids/23.3.653. [DOI] [PubMed] [Google Scholar]
- 28.Goodman J L, Nelson C, Vitale B, Madigan J E, Dumler J S, Kurtti T J, Munderloh U G. Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med. 1996;334:209–215. doi: 10.1056/NEJM199601253340401. [DOI] [PubMed] [Google Scholar]
- 29.Greiner M, Sohr D, Gobel P. A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J Immunol Methods. 1995;185:123–132. doi: 10.1016/0022-1759(95)00121-p. [DOI] [PubMed] [Google Scholar]
- 30.Gurtler L. Difficulties and strategies of HIV diagnosis. Lancet. 1996;348:176–179. doi: 10.1016/s0140-6736(96)01036-7. [DOI] [PubMed] [Google Scholar]
- 31.Hardalo C J, Quagliarello V, Dumler J S. Human granulocytic ehrlichiosis in Connecticut: report of a fatal case. Clin Infect Dis. 1995;21:910–914. doi: 10.1093/clinids/21.4.910. [DOI] [PubMed] [Google Scholar]
- 32.IJdo J W, Zhang Y, Hodzic E, Magnarelli L A, Wilson M L, Telford III S R, Barthold S W, Fikrig E. The early humoral response in human granulocytic ehrlichiosis. J Infect Dis. 1997;176:687–692. doi: 10.1086/514091. [DOI] [PubMed] [Google Scholar]
- 33.Johnson R C, Johnson B J B. Lyme disease: serodiagnosis of Borrelia burgdorferi sensu lato infection. In: Rose H R, Conway de Macario E, Folds J D, Lane H C, Nakamura R M, editors. Manual of clinical laboratory immunology. 5th ed. Washington, D.C: American Society for Microbiology; 1997. pp. 526–533. [Google Scholar]
- 34.Jongejan F, Wassink L A, Thielemans M J C, Perie N M, Uilenberg G. Serotypes in Cowdria ruminatium and their relationship with Ehrlichia phagocytophila determined by immunofluorescence. Vet Microbiol. 1989;21:31–40. doi: 10.1016/0378-1135(89)90016-3. [DOI] [PubMed] [Google Scholar]
- 35.Jongejan F, De Vries N, Nieuwenhuijs J, Van Vliet A M H, Wassink L A. The immunodominant 32-kilodalton protein of Cowdria ruminatium is conserved within the genus Ehrlichia. Rev Elev Med Vet Pays Trop. 1993;46:145–152. [PubMed] [Google Scholar]
- 36.Klein M B, Nelson C M, Goodman J L. Antibiotic susceptibility of the newly cultivated agent of human granulocytic ehrlichiosis: promising activity of quinolones and rifamycins. Antimicrob Agents Chemother. 1997;41:76–79. doi: 10.1128/aac.41.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolbert C P, Bruinsma E S, Abdulkarim A S, Hofmeister E K, Tompkins R B, Telford III S R, Mitchell P D, Adams-Stich J, Persing D H. Characterization of an immunoreactive protein from the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:1172–1178. doi: 10.1128/jcm.35.5.1172-1178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.Lockhart J M, Davidson W R, Stallknecht D E, Dawson J E, Howerth E W. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681–1686. doi: 10.1128/jcm.35.7.1681-1686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Logan L L, Holland C J, Mebus C A, Ristic M. Serological relationship between Cowdria ruminatium and certain Ehrlichia species. Vet Rec. 1986;119:458–459. doi: 10.1136/vr.119.18.458. [DOI] [PubMed] [Google Scholar]
- 41.Luft B J, Dattwyler R J, Johnson R C, Luger S W, Bosler E M, Rahn D W, Masters E J, Grunwaldt E, Gadgil S D. Azithromycin compared with amoxicillin in the treatment of erythema migrans. Ann Intern Med. 1996;124:785–791. doi: 10.7326/0003-4819-124-9-199605010-00002. [DOI] [PubMed] [Google Scholar]
- 42.Madigan J E, Rikihisa Y, Palmer J E, DeRock E, Mott J. Evidence for a high rate of false positive results with the indirect fluorescent antibody test for Ehrlichia risticii antibody in horses. J Am Vet Med Assoc. 1995;207:1448–1453. [PubMed] [Google Scholar]
- 43.Magnarelli L A, Anderson J F, Johnson R C, Nadelman R B, Wormser G P. Comparison of different strains of Borrelia burgdorferi sensu lato used as antigens in enzyme-linked immunosorbent assays. J Clin Microbiol. 1994;32:1154–1158. doi: 10.1128/jcm.32.5.1154-1158.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magnarelli L A, Anderson J F, Stafford III K C, Dumler J S. Antibodies to multiple tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in white-footed mice. J Wildl Dis. 1997;33:466–473. doi: 10.7589/0090-3558-33.3.466. [DOI] [PubMed] [Google Scholar]
- 45.Magnarelli L A, Dumler J S, Anderson J F, Johnson R C, Fikrig E. Coexistence of antibodies to tick-borne pathogens of babesiosis, ehrlichiosis, and Lyme borreliosis in human sera. J Clin Microbiol. 1995;33:3054–3057. doi: 10.1128/jcm.33.11.3054-3057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magnarelli L A, Stafford III K C, Mather T N, Yeh M-T, Horn K D, Dumler J S. Hemocytic rickettsia-like organisms in ticks: serologic reactivity with antisera to ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710–2714. doi: 10.1128/jcm.33.10.2710-2714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell P D, Reed K D, Hofkes J M. Immunoserologic evidence of coinfection with Borrelia burgdorferi, Babesia microti, and human granulocytic Ehrlichia species in residents of Wisconsin and Minnesota. J Clin Microbiol. 1996;34:724–727. doi: 10.1128/jcm.34.3.724-727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nadelman R B, Luger S W, Frank E, Wisniewski M, Collins J J, Wormser G P. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273–280. doi: 10.7326/0003-4819-117-4-273. [DOI] [PubMed] [Google Scholar]
- 49.Nicholson W L, Comer J A, Sumner J W, Gingrich-Baker C, Coughlin R T, Magnarelli L A, Olson J G, Childs J E. An indirect immunofluorescence assay using a cell culture-derived antigen for the detection of antibodies to the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:1510–1516. doi: 10.1128/jcm.35.6.1510-1516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pancholi P, Kolbert C P, Mitchell P D, Reed K D, Dumler J S, Bakken J S, Telford III S R, Persing D H. Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. Clin Infect Dis. 1995;172:1007–1012. doi: 10.1093/infdis/172.4.1007. [DOI] [PubMed] [Google Scholar]
- 51.Parker R T, Menon P G, Merideth A M, Snyder M J, Woodward T E. Persistence of Rickettsia rickettsii in a patient recovered from Rocky Mountain spotted fever. J Immunol. 1954;73:383–386. [PubMed] [Google Scholar]
- 52.Petrovec M, Furlan S L, Zupanc T A, Strle F, Brouqui P, Roux V, Dumler J S. Human disease in Europe caused by a granulocytic Ehrlichia species. J Clin Microbiol. 1997;35:1556–1559. doi: 10.1128/jcm.35.6.1556-1559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price W H. Studies on the interepidemic survival of endemic typhus fever. J Bacteriol. 1955;69:106–107. doi: 10.1128/jb.69.1.106-107.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rikihisa Y, Zhi N, Wormser G, Wen B, Horowitz H W, Hechemy K E. Direct isolation and cultivation of human granulocytic Ehrlichia from a human patient. J Infect Dis. 1997;175:210–213. doi: 10.1093/infdis/175.1.210. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz I, Fish D, Daniels T J. Prevalence of the rickettsial agent of human granulocytic ehrlichiosis in ticks from a hyperendemic focus of Lyme disease. N Engl J Med. 1997;337:49–50. doi: 10.1056/NEJM199707033370111. [DOI] [PubMed] [Google Scholar]
- 56.Sumner J W, Nicholson W L, Massung R F. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J Clin Microbiol. 1997;35:2087–2092. doi: 10.1128/jcm.35.8.2087-2092.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Telford S R, III, Dawson J E, Katavolos P, Warner C K, Kolbert C P, Persing D H. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walls J J, Greig B, Neitzel D F, Dumler J S. Natural infection of small mammal species in Minnesota with the agent of human granulocytic ehrlichiosis. J Clin Microbiol. 1997;35:853–855. doi: 10.1128/jcm.35.4.853-855.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong S J, Brady G S, Dumler J S. Serological responses to Ehrlichia equi, Ehrlichia chaffeensis, and Borrelia burgdorferi in patients from New York State. J Clin Microbiol. 1997;35:2198–2205. doi: 10.1128/jcm.35.9.2198-2205.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wormser G, McKenna D, Aguero-Rosenfeld M, Horowitz H, Monoz J, et al. Human granulocytic ehrlichiosis-New York, 1995. Morbid Mortal Weekly Rep. 1995;44:593–595. [PubMed] [Google Scholar]
- 61.Wormser G P, Horowitz H W, Nowakowski J, McKenna D, Dumler J S, Varde S, Schwartz I, Carbonaro C, Aguero-Rosenfeld M. Positive Lyme disease serology in patients with clinical and laboratory evidence of human granulocytic ehrlichiosis. Am J Clin Pathol. 1997;107:142–147. doi: 10.1093/ajcp/107.2.142. [DOI] [PubMed] [Google Scholar]
- 62.Zhi N, Rikihisa Y, Kim H Y, Wormser G P, Horowitz H W. Comparison of major antigen proteins of six strains of the human granulocytic ehrlichiosis agent by Western immunoblot analysis. J Clin Microbiol. 1997;35:2606–2611. doi: 10.1128/jcm.35.10.2606-2611.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]