Abstract
The consequences of exposure to prenatal maternal anxiety for the development of child temperament were examined in a sample of 120 healthy, 2-year-old children. Prenatal maternal state and pregnancy-specific anxiety (PSA) were measured five times during pregnancy, and maternal state anxiety was measured again at 2 years post partum. Child temperament was measured at 2 years using the Early Childhood Behavior Questionnaire. The relationship between the trajectory of maternal anxiety across gestation and negative affectivity was evaluated using hierarchical linear growth curve modeling. Higher maternal PSA between 13 and 17 weeks of gestation was associated with increased negative temperament in the children. This association could not be explained by postnatal maternal anxiety, demographic, or obstetric factors. Prenatal maternal state anxiety was not associated with child temperament. These findings demonstrate that PSA early in gestation has a distinctive influence on the developing fetus.
Keywords: Anxiety, child development, fetal programing, pregnancy, pregnancy-specific anxiety, temperament
Introduction
The prenatal period is a time of rapid development during which the fetus is especially vulnerable to both positive and negative influences that can have lasting consequences on development across the lifespan. These influences on the fetus have been described as programing: the process by which an insult or stimulus during a sensitive developmental period has a long-lasting or permanent effect (Barker 1998). Fetal organs and organ systems develop rapidly in a specific sequence during gestation and go through distinct periods of rapid cell division (Creasy and Resnik 1994; Cameron and Demerath 2002). During these periods of rapid cell division, organs are especially vulnerable to environmental influences such as stress (Kajantie 2006). Thus, the developmental consequences are dependent on both the level and timing of exposure to environmental stimuli during gestation (Sandman and Davis 2010). The goal of the current study was to explore the influence of prenatal maternal anxiety on the development of child temperament and to investigate whether there were timing effects of exposure to prenatal anxiety on child outcome.
Maternal stress during pregnancy shortens the length of gestation and threatens the developing fetal nervous system. In humans, it has been clearly documented that prenatal stress is associated with preterm delivery (Copper et al. 1996; Glynn et al. 2001, 2008; Dole et al. 2003; Hobel and Culhane 2003), but less is known about the impact of prenatal maternal psychological state on child behavioral and psychological development. A collection of evidence from animal studies demonstrates that exposure to prenatal stress can alter the neurobiology and behavior of the offspring. Prenatal exposure to maternal stress is associated with fearful and depressive-like behaviors, reduced social drive and more abnormal social behavior, increased stress reactivity, and more irritable temperament in both rodents and non-human primates (Schneider 1992; Clarke and Schneider 1993; Clarke et al. 1994; Griffin et al. 2003; Dickerson et al. 2005; Van den Hove et al. 2005; Abe et al. 2007; Lee et al. 2007). These findings indicate that prenatal stress exerts an influence on fetal neurodevelopment. However, vast interspecies differences in both developmental trajectories and maternal stress and reproductive physiology limit the ability to generalize from animals to humans.
Accumulating evidence from human studies has shown that maternal stress during pregnancy exerts persisting influences on human development. Children exposed to prenatal maternal psychological distress maybe particularly vulnerable to increases in fearful or reactive behavior and difficult temperament during infancy and toddlerhood (Huizink et al. 2002; Davis et al. 2004, 2007; Austin et al. 2005; Gutteling et al. 2005; Bergman et al. 2007). These early temperament and emotional difficulties appear to persist and result in increases in behavioral problems during childhood (O’Connor et al. 2002; Van den Bergh and Marcoen 2004). Importantly, these associations are present after adjusting for postnatal influences including postnatal maternal psychological distress. Although compelling evidence exists for long-lasting consequences of prenatal adversity (Van den Bergh and Marcoen 2004; Mennes et al. 2006; Buss et al. 2010), it is likely that aspects of the postnatal environment including quality of maternal care will moderate the consequences of prenatal exposures (Bergman et al. 2008).
Thus far, most studies have focused on general measures of prenatal maternal psychological distress such as perceived stress, state anxiety, and depression. However, more recent studies suggest that pregnancy-specific anxiety (PSA) maybe superior to general measures of distress for predicting developmental outcomes because it more accurately characterizes psychological state specific to a woman during pregnancy (Dunkel Schetter 2009; Sandman et al. 2011).
Concern or worry that is specific to pregnancy may have profound implications for both birth outcome and child development. It is only relatively recently, however, that a woman’s fears and beliefs about pregnancy, delivery, and health of the baby have been investigated. This construct has been shown to be an independent entity, separate from measures of generalized anxiety or other psychological distress measures (DiPietro et al. 2004; Huizink et al. 2004; Roesch et al. 2004; Dunkel Schetter 2009). Indeed, only a moderate amount (8–27%) of variance in PSA was found to be explained by general anxiety and depression (Huizink et al. 2004). Measures of PSA predict a wide variety of developmental outcomes including fetal behavior (DiPietro et al. 2002), birth outcome (Roesch et al. 2004; Kramer et al. 2009; Dunkel Schetter 2011), and infant and child development. Infants and toddlers exposed to elevated PSA display poorer cognitive performance (Davis and Sandman 2010), delayed motor development (Huizink et al. 2003; DiPietro et al. 2006), and poor attention regulation (Huizink et al. 2002). These early effects appear to have consequences for the developing brain with effects that persist through childhood. Buss et al. (2010) found that elevated PSA at the earliest assessment (19 weeks) was associated with focal decreases in gray matter density in several regions, primarily in the prefrontal cortex and the medial temporal lobe. Few longitudinal studies with multiple prenatal assessments of PSA exist. However, those with multiple prenatal assessments indicate that exposure to elevated PSA early in gestation exerts a stronger influence on infant and child development (Huizink et al. 2002; Buitelaar et al. 2003; Buss et al. 2010; Davis and Sandman 2010).
Evaluating the link between the prenatal environment and negative or inhibited temperament in early childhood is important because these children have a higher risk of developing psychological disorders including increased anxiety and depression in later life (Kagan et al. 1999; Schwartz et al. 1999; De Pauw and Mervielde 2010; Dougherty and Klein 2010). DiPietro et al. (2006) reported that mothers who rated their pregnancies as more negative than positive had children with poorer emotional regulation and engagement/orientation as indexed by the Bayley Scales of Infant Development at 2 years of age (Bayley 1993). These data indicate that pregnancy-related anxiety or concern maybe associated with difficulty regulating emotion and behavior. However, no study has examined the consequences of pregnancy-related anxiety for negative or reactive temperament during early childhood.
The aims of the current study were to determine (i) whether prenatal maternal state anxiety and PSA were associated with child temperament at 2 years and (ii) whether there is a sensitive prenatal period during which PSA exerts effects on development. We hypothesized that psychological distress during the prenatal period would be associated with increased negative affectivity, PSA would exert a stronger influence than general anxiety, and that earlier exposures would exert a stronger influence.
Methods
Study overview
Study participants included mother–child pairs from a longitudinal study of prenatal stress and development. Women with singleton pregnancies less than 16 weeks gestational age were recruited from obstetric clinics in southern California and were followed longitudinally through 2 years post partum.
Participants
The current sample included 120 full-term children (50% first born, 45% female) and their mothers. Initial prenatal recruitment criteria were as follows: English speaking, over the age of 18 years, and no steroid medication. Exclusion criteria included: use of any recreational drugs and regular use of alcohol or nicotine during the index pregnancy. Presence of psychiatric problems was not an exclusion criterion; however, in our low-risk sample, only four women had a lifetime history of hospitalization for any emotional problem, and only three women took antidepressant medication during their pregnancy. In addition, all children included in the current investigation were full-term at birth (M gestational age = 39.6 weeks, SD = 1.1 weeks). Mothers gave written informed consent for all aspects of the protocol, which was approved by the Institutional Review Board for protection of human subjects. Descriptive information for this sample is provided in Table I.
Table I.
Description of the study sample.
| Study sample (N = 120) | |
|---|---|
| Maternal age at delivery (M, years) | 30.0* |
| Married or cohabitating (%) | 91 |
| Primiparous (%) | 50 |
| Education (%) | |
| High school or equivalent | 5 |
| Associates or vocational | 18 |
| Bachelors degree | 33 |
| Graduate degree | 21 |
| Annual household income (%) | |
| $0–$30,000 | 27 |
| $30,001–$60,000 | 20 |
| $60,001–$100,000 | 27 |
| Over $100,000 | 26 |
| Race/ethnicity (%) | |
| Non-hispanic white | 48 |
| Latina | 30 |
| Asian | 18 |
SD = 5.2, range = 18–40.
Procedures
Prenatal maternal state anxiety and PSA were collected five times during pregnancy (Time 1: 15.5 (M) ± 1.06 (SD); Time 2: 19.7 ± 1.05; Time 3: 25.8 ± 1.00; Time 4: 31.1 ± 0.82; Time 5: 36.8 ± 0.78 weeks of gestation). Maternal state anxiety and maternal report of child temperament also were collected 2 years post partum (M = 24.1 ± 0.39 months). Maternal state anxiety was measured at 2 years in order to statistically adjust both for postnatal influences on child temperament, and for reporting bias that might have occurred when mothers rated the temperament of their children. Demographic information was obtained through structured interviews at each study visit. Prenatal medical history and risk for adverse obstetric outcomes were obtained from extensive structured medical interviews conducted by a research nurse at each visit in combination with a thorough review of prenatal and hospital medical records.
Measures
Maternal psychological assessments.
The 10-item State Anxiety subscale of the State-Trait Personality Inventory (STAI; Spielberger 1983a) was used to measure state, or generalized, anxiety. The STAI assessed the degree to which participants had experienced anxiety-related symptoms or emotions. Responses were made using a 4-point Likert scale ranging from 1 (not at all) to 4 (very much), and scores could range from a minimum of 10 to a maximum of 40. The STAI has been used for research purposes with both pregnant (Rini et al. 1999; Glynn et al. 2008) and non-pregnant samples. The STAI has good internal consistency with a Cronbach’s α coefficient of 0.92 (Spielberger 1983b).
PSA was measured using the 10-item Pregnancy-Related Anxiety scale. This measure assesses a woman’s feelings about her health during pregnancy, the health of her baby and her feelings about labor and delivery. Answers were given on a 4-point Likert scale ranging from 1 (not at all) to 4 (very much) and included items such as: I am fearful regarding the health of my baby, I am concerned or worried about losing my baby, and I am concerned or worried about developing medical problems during my pregnancy. The final score on this measure could range from 10 to 40. This reliable measure was specifically developed for use in pregnancy research and has good internal consistency (α = 0.75–0.85; Rini et al. 1999; Glynn et al. 2008).
Child temperament.
Child temperament was collected by maternal report at 2 years of age using the Early Child Behavior Questionnaire (ECBQ; Putnam et al. 2006). The ECBQ was developed specifically to reduce the possibility of maternal reporting bias by asking about specific behaviors in defined situations, rather than asking for judgments about child temperament or behavior. The ECBQ takes advantage of a mother’s ability to reflect on her child’s behavior in a wide variety of contexts. Three broad dimensions, or factors, of temperament are identified using the ECBQ, and published studies have shown good internal consistency and validity for each of these scales (Putnam et al. 2006). Because of evidence indicating that prenatal psychological distress is associated with increases in negative affect and behavioral reactivity (Huizink et al. 2002; O’Connor et al. 2002; Davis et al. 2004; Van den Bergh and Marcoen 2004; Austin et al. 2005; Gutteling et al. 2005; Bergman et al. 2007) and evidence that negative temperament at ages 2 to 3 is predictive of psychological disorders later in life (Schwartz et al. 1999; Prior et al. 2000; Dougherty and Klein 2010), we focused on the Negative Affectivity subscale of the ECBQ. The Negative Affectivity scale comprises eight subscales (discomfort, fear, motor activation, sadness, perceptual sensitivity, shyness, frustration, and loading negatively, soothability) with Cronbach’s α coefficients ranging from 0.73 to 0.88 (Putnam et al. 2006). Responses on the ECBQ ranged from 1 (never) to 7 (always) and the Negative Affectivity scale comprises items such as: when told that it was time for bed or a nap, how often did your child get irritable; when having trouble completing a task, how often did your child become sad; and during everyday activities, how often did your child startle at loud noises?
Prenatal course and birth outcome.
Based on the review of maternal medical records, a binary score assessing the presence or absence of prenatal obstetric risk was derived (Hobel 1982). This obstetric risk score includes the assessment of infection, pregnancy-induced hypertension, gestational diabetes, oligohydramnios, polyhydramnios, preterm labor, vaginal bleeding, placenta previa and anemia in the index pregnancy.
Data analysis
Preliminary analyses were carried out using correlations and t-tests to identify sociodemographic (maternal race/ethnicity, maternal age, cohabitating with child’s father, and years of maternal education), pregnancy-related (obstetric risk, gestational age at birth), and child (sex, birth order) variables that might affect maternal psychological state and/or development of child temperament. Variables that were significantly associated with negative affectivity (p < 0.05) were maternal age at delivery, number of years of education, race/ethnicity (Latina: 0 = no, 1 = yes), and cohabitating with the father of child (0 = no, 1 = yes). Higher child negative affectivity was reported by mothers who were younger (r = −0.29; p < 0.01), of Latina descent (t = −2.5; p < 0.05), had less education (r = −0.32; p < 0.01), and were not living with the father of the child at time of study enrollment (t = 2.5; p < 0.05). All variables that were associated with child negative affectivity in preliminary analyses were included as covariates in all subsequent analyses. In addition, maternal state anxiety at 2 years post partum was used as a covariate in all analyses to adjust for the effects of concurrent maternal psychological state on child temperament and/or maternal report of temperament.
Hierarchical linear modeling (HLM) growth curve analyses (Raudenbush and Bryk 2002; Singer and Willett 2003) were used to describe the trajectory of prenatal indicators of maternal anxiety (state anxiety and PSA) across pregnancy and its association with toddler temperament. HLM, when used with repeated measures, treats the data in a hierarchical fashion with observations nested within persons. This approach allows variance to be modeled at multiple levels and provides several advantages over ordinary least squares regression (Raudenbush and Bryk 2002). First, standard regression or analysis of variance models are limited to one component of variability, the deviation of the individual from the group mean. In contrast, HLM includes the within-person variability assessed over time. Second, estimates of goodness of fit in modeling each individual’s data are derived, and the most reliable data are given greater statistical weight. Third, HLM produces robust estimates despite missing values for the repeated dependent measure. There were 110 participants who had complete data at all prenatal assessments. Ten participants had missing data at one prenatal visit. In HLM models, all cases are included in the estimation of effects, but cases with complete data are weighted more heavily. Finally, HLM uses precise measures of timing (i.e. gestational week) of data collection rather than nominal estimates of assessment intervals.
Separate two-level models were constructed to determine the association between the trajectory of maternal anxiety across gestation and toddler negative affectivity. A linear model was a better fit for state anxiety. For PSA, a quadratic function was a better fit. In both cases, predictor and outcome variables were modeled as continuous variables. Level 1 variables, or those evaluated longitudinally across the 5 prenatal assessment days, included maternal state anxiety and PSA and fetal age in weeks at the time of maternal assessment. Negative Affectivity measured at 2 years of age was the outcome of interest and was modeled continuously at level 2 along with the covariates described above. First, the overall model was tested to determine whether temperament was significantly associated with the intercept (set at the first assessment, 13 gestational weeks) or the trajectory of prenatal anxiety (linear function for state anxiety and quadratic function for PSA). If temperament was associated with the trajectory of pregnancy anxiety, the model was tested centered at each gestational week within the range of prenatal psychosocial assessments (13–39 weeks of gestation) to determine whether there was an effect of timing of exposure. The p values of less than 0.05 were considered statistically significant.
Results
Maternal psychological state
Significant changes were observed in the trajectory over gestation of maternal PSA (t = 3.25, β = 0.001, p < 0.01) but not state anxiety (p = 0.25). Average levels of PSA were highest in early pregnancy and decreased through mid-gestation. Levels of PSA (r = 0.68–0.88) and state anxiety (r = 0.49–0.71) were significantly correlated across pregnancy. Significant correlations between concurrent measures of state anxiety and PSA were additionally observed throughout pregnancy (average r = 0.52, all p < 0.001).
Women with elevated state anxiety (rs = 0.41–0.61, all p < 0.001) and PSA (rs = 0.29–0.40, all p < 0.01) during gestation were significantly more likely to have elevated state anxiety during the postnatal period. Descriptive information for pre- and postnatal maternal psychological state can be found in Table II.
Table II.
Descriptive information (mean and SD) for prenatal and postnatal measures.
| M (SD) | |
|---|---|
| Prenatal state anxiety (STAI) | |
| 15.5 ± 1.06 wks GA | 17.0(4.83) |
| 19.7 ± 1.05 wks GA | 16.7(4.48) |
| 25.8 ± 1.00 wks GA | 16.2(4.70) |
| 31.1 ± 0.82 wks GA | 16.7(5.01) |
| 36.8 ± 0.78 wks GA | 17.6(5.32) |
| Pregnancy-specific anxiety (PSA) | |
| 15.5 ± 1.06 wks GA | 18.6(5.40) |
| 19.7 ± 1.05 wks GA | 17.5(4.46) |
| 25.8 ± 1.00 wks GA | 17.5(4.60) |
| 31.1 ± 0.82 wks GA | 17.1(4.98) |
| 36.8 ± 0.78 wks GA | 17.6(5.23) |
| Postnatal maternal state anxiety (STAI; N = 120) | 17.1(5.64) |
| Child negative affectivity (N = 120) | 2.87(0.54) |
Note: wks, weeks; GA, gestational age.
Maternal prenatal psychological state and child temperament
Descriptive information for child temperament is provided in Table II. Children who displayed more negative affectivity at 2 years of age had been exposed to higher maternal PSA early in gestation. Specifically, negative affectivity was associated with both the initial intercept (t = 2.5; β = 2.4; p < 0.05) and the trajectory across gestation of PSA (t = 2.0; β = 0.001; p < 0.05). Follow-up tests to explore effects of timing indicated that children who displayed more negative affectivity had been exposed to higher maternal PSA from 13 to 17 weeks of gestation (ts = 2.0–2.4; βs = 0.16–0.25; all p < 0.05). PSA and negative affectivity were not significantly associated after 17 weeks of gestation (ts = 0.90–1.86; βs = 0.08–0.14; all p > 0.07). Furthermore, neither level nor trajectory of state anxiety was associated with child negative affectivity. Figure 1 displays PSA across gestation for children scoring in the top and bottom quartiles of negative affectivity.
Figure 1.
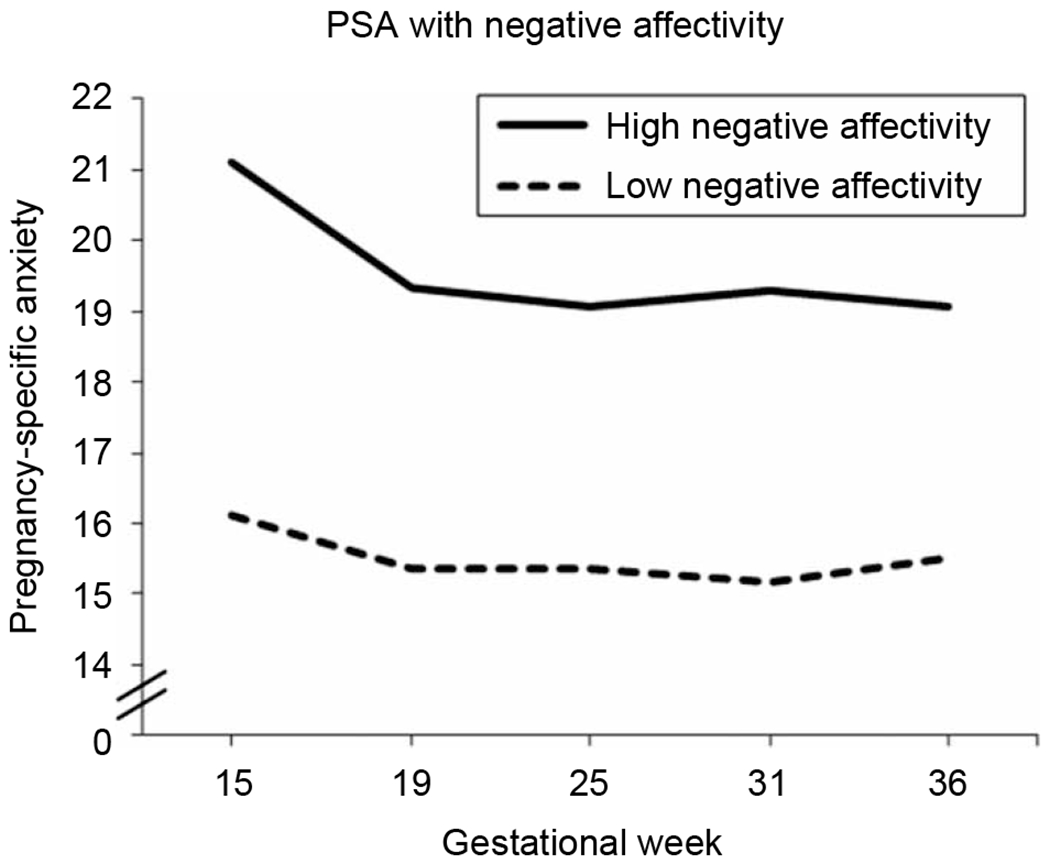
Elevated PSA early in gestation is associated with increased negative affectivity at 2 years of age after adjusting for concurrent maternal anxiety and prenatal obstetric risk (all t = 2.0–2.4, β = 0.16–0.25, p < 0.05). Note: Both maternal PSA and child negative affectivity were analyzed as continuous variables in the complete sample of 120 participants. For graphing purposes only, we have displayed the top (N = 29) and bottom (N = 28) quartiles of negative affectivity.
Discussion
During early gestation, a mother’s fears and concerns about her pregnancy, the health of her fetus, and her delivery are associated with the temperament of her child at 2 years of age. Importantly, prenatal maternal PSA predicts child negative affectivity after adjusting for the effects of sociodemographic factors, obstetric risk, and postnatal maternal psychological state. Our findings are consistent with prior studies which found that PSA exerted significant and persisting influences on development that can be detected in the fetus (DiPietro et al. 2002), infant, and child (Huizink et al. 2003; DiPietro et al. 2006; Buss et al. 2010; Davis and Sandman 2010). These data suggest that (i) signals of maternal psychosocial distress during the prenatal period maybe transmitted to the fetus and have persisting consequences for development, (ii) the fetus appears to be more vulnerable to the effects of these signals early in gestation, and (iii) maternal anxiety related to pregnancy may have more profound implications for the fetus as compared with generalized maternal anxiety.
The consequences of maternal PSA based on the timing of exposure is consistent with prior study evaluating the consequences of this type of psychological distress (Buitelaar et al. 2003; Huizink et al. 2003; Buss et al. 2010; Davis and Sandman 2010) and may result from changes in PSA over gestation (Dunkel Schetter and Glynn 2011; Sandman et al. 2011), changes in maternal and placental physiology (Brown et al. 1996; Sandman and Davis 2010), and variations in the sensitive periods of development of different fetal physiological systems (Seckl and Meaney 2006). Between the gestational ages of 8 and 16 weeks, migrating neurons form the subplate zone, in which they arborize and branch to establish appropriate connections with afferent neurons from the thalamus, basal forebrain, and brainstem (Sidman and Rakic 1973). Exposure to elevated maternal PSA early in gestation may alter these early developmental processes with consequences for child functioning. The mechanism by which maternal psychological distress signals are transduced to the fetus is not known, but an interaction among the maternal environment, placental changes, and epigenetic programming of the fetus is likely. Variation in concentrations of stress-sensitive hormones (placental corticotropin-releasing hormone (CRH), cortisol) during pregnancy predicts fetal as well as infant behavioral neurodevelopmental outcomes (de Weerth et al. 2003; Class et al. 2008; Ellman et al. 2008; Bergman et al. 2010; Davis and Sandman 2010; Davis et al. 2011). Potential mechanisms by which maternal stress and associated increases in placental CRH and cortisol may produce long-lasting changes in brain function have been suggested from animal models and may include changes in gene expression (Korosi and Baram 2009), neurotransmitter levels (Roceri et al. 2002), adult neurogenesis (Coe et al. 2003; Fujioka et al. 2006) as well as cell growth and survival (Roceri et al. 2002; Van den Hove et al. 2006).
The finding that PSA predicts child outcomes when generalized measures of distress do not maybe because stress related to events, beliefs, or fears proximal to pregnancy is more relevant to the pregnant woman and, therefore, more consequential to the fetus. Furthermore, it is plausible that pregnant women are able to more accurately report anxiety related to pregnancy than to more generalized concerns or worry. In contrast to other distress measures which evaluate stress related to generalized worry, daily hassles, and negative mood, the PSA scale evaluates maternal stress that is directly related to her pregnancy including concern about the health of her baby, fear of miscarriage, and worry about the development of pregnancy-related medical problems. It may also be the case that PSA is more consequential than general anxiety because it is more dynamic over the course of gestation. Levels of PSA are highest early and then decline through mid-gestation.
The current study is the first to examine the association between PSA and the development of child temperament beyond the infancy period. The link between negative or difficult temperament in early childhood and adverse outcomes in later life has been well established, underscoring the importance of this research agenda. Children classified as having a difficult temperament during infancy and early childhood are at risk of developing behavioral problems (Earls and Jung 1987; Sanson et al. 1993) as well as psychiatric conditions including eating disorders, depression, and anxiety (Kagan et al. 1999; Schwartz et al. 1999; Martin et al. 2000; Prior et al. 2000; De Pauw and Mervielde 2010; Dougherty and Klein 2010) during later childhood and adolescence.
This study relied on naturally occurring variations in maternal anxiety, rather than experimental manipulations of anxiety. It is difficult, therefore, to separate the effects of prenatal maternal psychological state from the consequences of other factors that might contribute to this association such as shared genes. However, the current findings are consistent with studies evaluating the developmental consequences of prenatal stress among children conceived by in vitro fertilization who were not genetically related to their mothers (Rice et al. 2010). In addition, the present study relied on maternal report of both maternal psychological state and child temperament. To reduce the effects of biased reporting of child behavior, post partum maternal psychological state was taken into account in all analyses. Moreover, this study used the Early Childhood Behavior Questionnaire which reduces maternal bias by asking mothers to report on specific behaviors in clearly defined situations as opposed to making evaluative judgments about their child’s temperament. The strength of the study is that mother–child pairs were assessed using a prospective longitudinal design beginning early in pregnancy which allowed for greater resolution when examining the programing effects of prenatal maternal psychological state on development of negative temperament in the offspring.
Clinical and research implications
Child and adult functioning, including vulnerability to disease, appears to be determined, in part, by the prenatal environment (Barker 1998). The current findings indicate that prenatal maternal psychological state exerts programing influences on the developing fetus with implications for more negative affective behaviors such as sadness, frustration, and perceptual sensitivity in early childhood. Associations were observed in a healthy, low-risk sample of mother–child pairs. It is plausible that more profound effects would be observed among clinically anxious or depressed women. Because children who have high negative affectivity in early childhood are more likely to develop a variety of psychological disorders later in life, it would be beneficial for clinicians to consider PSA when working with pregnant women. These data are encouraging because the strongest effects on child outcome were observed with a woman’s concerns and fears about pregnancy, and not about generalized state anxiety. Thus, approaches focused on cognitive reassessment of the risks associated with pregnancy maybe most effective at promoting the psychological health of both mother and child. Specifically, reducing PSA may benefit a wide range of developmental outcomes including childhood temperament and emotional regulation, resulting in more optimal psychological functioning and decreased risk for psychopathology.
Acknowledgments
The work of Cheryl Crippen and Christina Canino is gratefully acknowledged.
Declaration of interest:
This research was supported by the NIH (R01 HD050662 to EPD and NS041298 to CAS). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abe H, Hidaka N, Kawagoe C, Odagiri K, Watanabe Y, Ikeda T, Ishizuka Y, Hashiguchi H, Takeda R, Nishimori T, Ishida Y. 2007. Prenatal psychological stress causes higher emotionality, depression-like behavior, and elevated activity in the hypothalamo–pituitary–adrenal axis. Neurosci Res 59:145–151. [DOI] [PubMed] [Google Scholar]
- Austin MP, Hadzi-Pavlovic D, Leader L, Saint K, Parker G. 2005. Maternal trait anxiety, depression and life event stress in pregnancy: Relationships with infant temperament. Early Hum Dev 81:183–190. [DOI] [PubMed] [Google Scholar]
- Barker DJ. 1998. In utero programming of chronic disease. Clin Sci 95:115–128. [PubMed] [Google Scholar]
- Bayley N. 1993. Bayley scales of infant development. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V. 2007. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry 46:1454–1463. [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, Glover V, O’Connor TG. 2008. Quality of child–parent attachment moderates the impact of antenatal stress on child fearfulness. J Child Psychol Psychiatry 49:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, Glover V, O’Connor TG. 2010. Maternal prenatal cortisol and infant cognitive development: Moderation by infant–mother attachment. Biol Psychiatry 67:1026–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW, Diaz R, Robson AC, Kotelevtsev Y, Mullins JJ, Kaufman MH, Seckl JR. 1996. The ontogeny of 11β-hydroxysteroid dehydrogenase type 2 and mineralicorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology 137:794–797. [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, Huizink AC, Mulder EJ, de Medina PG, Visser GH. 2003. Prenatal stress and cognitive development and temperament in infants. Neurobiol Aging 24(Suppl 1):S53–S60, discussion S67−58. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. 2010. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology 35:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N, Demerath EW. 2002. Critical periods in human growth and their relationship to diseases of aging. Am J Phys Anthropol 35(Suppl):159–184. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Schneider ML. 1993. Prenatal stress has long-term effects on behavioral responses to stress in juvenile rhesus monkeys. Dev Psychobiol 26:293–304. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Wittwer DJ, Abbott DH, Schneider ML. 1994. Long-term effects of prenatal stress on HPA axis activity in juvenile rhesus monkeys. Dev Psychobiol 27:257–269. [DOI] [PubMed] [Google Scholar]
- Class QA, Buss C, Davis EP, Gierczak M, Pattillo C, Chicz-DeMet A, Sandman CA. 2008. Low levels of corticotropin-releasing hormone during early pregnancy are associated with precocious maturation of the human fetus. Dev Neurosci 30:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. 2003. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry 54:1025–1034. [DOI] [PubMed] [Google Scholar]
- Copper RL, Goldenberg RL, Das A, Elder N, Swain M, Norman G, Ramsey R, Cotroneo P, Collins BA, Johnson F, Jones P, Meier AM. 1996. The preterm prediction study: Maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 175:1286–1292. [DOI] [PubMed] [Google Scholar]
- Creasy R, Resnik R. 1994. Maternal-fetal medicine: Principles and practice. Philadelphia, PA: Saunders. [Google Scholar]
- Davis EP, Sandman CA. 2010. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev 81:131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, Sandman CA. 2004. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy 6:319–331. [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. 2007. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry 46:737–746. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Waffarn F, Sandman CA. 2011. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry 52:119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pauw SS, Mervielde I. 2010. Temperament, personality and developmental psychopathology: A review based on the conceptual dimensions underlying childhood traits. Child Psychiatry Hum Dev 41:313–329. [DOI] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, Buitelaar JK. 2003. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev 74:139–151. [DOI] [PubMed] [Google Scholar]
- Dickerson PA, Lally BE, Gunnel E, Birkle DL, Salm AK. 2005. Early emergence of increased fearful behavior in prenatally stressed rats. Physiol Behav 86:586–593. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Hilton SC, Hawkins M, Costigan KA, Pressman EK. 2002. Maternal stress and affect influence fetal neurobehavioral development. Dev Psychol 38:659–668. [PubMed] [Google Scholar]
- DiPietro JA, Ghera MM, Costigan K, Hawkins M. 2004. Measuring the ups and downs of pregnancy stress. J Psychosom Obstet Gynaecol 25:189–201. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP. 2006. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev 77:573–587. [DOI] [PubMed] [Google Scholar]
- Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. 2003. Maternal stress and preterm birth. Am J Epidemiol 157:14–24. [DOI] [PubMed] [Google Scholar]
- Dougherty L, Klein D. 2010. Temperamental positive and negative emotionality and children’s depressive symptoms: A longitudinal prospective study from age three to age ten. J Soc Clin Psychol 29:462–488. [Google Scholar]
- Dunkel Schetter C. 2009. Stress processes in pregnancy and preterm birth. Curr Dir in Psychol Sci 18:205–209. [Google Scholar]
- Dunkel Schetter C. 2011. Psychological science on pregnancy: Stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol 62:531–558. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C, Glynn LM. 2011. Stress in pregnancy: Empirical evidence and theoretical issues to guide interdisciplinary research. In: Contrada RJ, Baum A, editors. The handbook of stress science. New York: Springer Publishing Company, LLC. p.321–343. [Google Scholar]
- Earls F, Jung KG. 1987. Temperament and home environment characteristics as causal factors in the early development of childhood psychopathology. J Am Acad Child Adolesc Psychiatry 26:491–498. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. 2008. Timing of fetal exposure to stress hormones: Effects on newborn physical and neuromuscular maturation. Dev Psychobiol 50:232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka A, Fujioka T, Ishida Y, Maekawa T, Nakamura S. 2006. Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience 141:907–915. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. 2001. When stress happens matters: Effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol 184:637–642. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Dunkel Schetter C, Hobel C, Sandman CA. 2008. Pattern of perceived stress and anxiety in pregnancy predict preterm birth. Health Psychol 27:42–51. [DOI] [PubMed] [Google Scholar]
- Griffin WC 3rd, Skinner HD, Salm AK, Birkle DL. 2003. Mild prenatal stress in rats is associated with enhanced conditioned fear. Physiol Behav 79:209–215. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Willemsen-Swinkels SH, Huizink AC, Mulder EJ, Visser GH, Buitelaar JK. 2005. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. Eur Child Adolesc Psychiatry 14:41–51. [DOI] [PubMed] [Google Scholar]
- Hobel CJ. 1982. Identifying the patient at risk. In: Bolognese RJ, Schwartz RH, Schneider J, editors. Perinatal medicine: Management of the high risk fetus and neonate. Baltimore, MA: Williams & Wilkins. p.3–28. [Google Scholar]
- Hobel C, Culhane J. 2003. Role of psychosocial and nutritional stress on poor pregnancy outcome. J Nutr 133:1709S–1717S. [DOI] [PubMed] [Google Scholar]
- Huizink AC, de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. 2002. Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry 41:1078–1085. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. 2003. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry 44:810–818. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ, Robles de Medina PG, Visser GH, Buitelaar JK. 2004. Is pregnancy anxiety a distinctive syndrome? Early Hum Dev 79:81–91. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N, Zentner M, Peterson E. 1999. Infant temperament and anxious symptoms in school age children. Dev Psychopathol 11:209–224. [DOI] [PubMed] [Google Scholar]
- Kajantie E. 2006. Fetal origins of stress-related adult disease. Ann N Y Acad Sci 1083:11–27. [DOI] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. 2009. The pathways from mother’s love to baby’s future. Front Behav Neurosci 3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Seguin L, Goulet L, Kahn SR, McNamara H, Genest J, Dassa C, Chen MF, Sharma S, Meaney MJ, Thomson S, Van Uum S, Koren G, Dahhou M, Lamoureux J, Platt RW. 2009. Stress pathways to spontaneous preterm birth: The role of stressors, psychological distress, and stress hormones. Am J Epidemiol 169:1319–1326. [DOI] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. 2007. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Res 1156:152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GC, Wertheim EH, Prior M, Smart D, Sanson A, Oberklaid F. 2000. A longitudinal study of the role of childhood temperament in the later development of eating concerns. Int J Eat Disord 27:150–162. [DOI] [PubMed] [Google Scholar]
- Mennes M, Stiers P, Lagae L, Van den Bergh B. 2006. Long-term cognitive sequelae of antenatal maternal anxiety: Involvement of the orbitofrontal cortex. Neurosci Biobehav Rev 30:1078–1086. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. 2002. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry 180:502–508. [DOI] [PubMed] [Google Scholar]
- Prior M, Smart D, Sanson A, Oberklaid F. 2000. Does shy-inhibited temperament in childhood lead to anxiety problems in adolescence? J Am Acad Child Adolesc Psychiatry 39:461–468. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Gartstein MA, Rothbart MK. 2006. Measurement of fine-grained aspects of toddler temperament: The early childhood behavior questionnaire. Infant Behav Dev 29:386–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. 2002. Hierarchical linear models: Application and data anylysis methods. Thousand Oaks, CA: Sage. [Google Scholar]
- Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. 2010. The links between prenatal stress and offspring development and psychopathology: Disentangling environmental and inherited influences. Psychol Med 40:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. 1999. Psychological adaptation and birth outcomes: The role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol 18:333–345. [DOI] [PubMed] [Google Scholar]
- Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. 2002. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry 7:609–616. [DOI] [PubMed] [Google Scholar]
- Roesch SC, Dunkel-Schetter C, Woo G, Hobel C. 2004. Modeling the types and timing of stress in pregnancy. Anxiety Stress Coping 17:87–102. [Google Scholar]
- Sandman CA, Davis EP. 2010. Gestational stress influences cognition and behavior. Future Neurol 5:675–690. [Google Scholar]
- Sandman CA, Davis EP, Buss C, Glynn LM. 2011. Prenatal programming of the mother and her fetus. Neuroendocrinology DOI: 10.1159/000327017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanson A, Smart D, Prior M, Oberklaid F. 1993. Precursors of hyperactivity and aggression. J Am Acad Child Adolesc Psychiatry 32:1207–1216. [DOI] [PubMed] [Google Scholar]
- Schneider ML. 1992. Prenatal stress exposure alters postnatal behavioral expression under conditions of novelty challenge in rhesus monkey infants. Dev Psychobiol 25:529–540. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. 1999. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry 38:1008–1015. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Meaney MJ. 2006. Glucocorticoid “programming” and PTSD risk. Ann N Y Acad Sci 1071:351–378. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. 1973. Neuronal migration, with special reference to developing human brain: A review. Brain Res 62:1–35. [DOI] [PubMed] [Google Scholar]
- Singer J, Willett J. 2003. Applied longitudinal data analysis: Modeling change and event occurance. New York: Oxford University Press. [Google Scholar]
- Spielberger C. 1983a. Manual for the state-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press, Inc. [Google Scholar]
- Spielberger C. 1983b. State-trait anxiety inventory. Redwood City, CA: Mind Garden. [Google Scholar]
- Van den Bergh BR, Marcoen A. 2004. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev 75:1085–1097. [DOI] [PubMed] [Google Scholar]
- Van den Hove DL, Blanco CE, Aendekerk B, Desbonnet L, Bruschettini M, Steinbusch HP, Prickaerts J, Steinbusch HW. 2005. Prenatal restraint stress and long-term affective consequences. Dev Neurosci 27:313–320. [DOI] [PubMed] [Google Scholar]
- Van den Hove DL, Steinbusch HW, Scheepens A, Van de Berg WD, Kooiman LA, Boosten BJ, Prickaerts J, Blanco CE. 2006. Prenatal stress and neonatal rat brain development. Neuroscience 137:145–155. [DOI] [PubMed] [Google Scholar]


