Abstract
Pericytes are the main cellular components of tiny arteries and capillaries. Studies have found that pericytes can undergo morphological contraction or relaxation under stimulation by cytokines, thus affecting the contraction and relaxation of microvessels and playing an essential role in regulating vascular microcirculation. Moreover, due to the characteristics of stem cells, pericytes can differentiate into a variety of inflammatory cell phenotypes, which then affect the immune function. Additionally, pericytes can also participate in angiogenesis and wound healing by interacting with endothelial cells in vascular microcirculation disorders. Here we review the origin, biological phenotype and function of pericytes, and discuss the potential mechanisms of pericytes in vascular microcirculation disorders, especially in pulmonary hypertension, so as to provide a sound basis and direction for the prevention and treatment of vascular microcirculation diseases.
Keywords: inflammation, pericytes, pluripotency, vascular microcirculatory
Pericytes are primarily found around tiny arteries or capillaries, and play a crucial role in regulating blood microcirculation. They also promote angiogenesis and immune function by communicating with other cells or their own pluripotent characteristics. Pericytes in different tissues are highly versatile, functionally pluripotent cells with phenotypic plasticity. They can differentiate into fat, muscle, cartilage, and bone cells as well as other cell types. Pericytes are versatile, which enables them to provide a strong physiological basis to maintain the stability of the internal environment as well as regulate blood vessel growth, immune function, and communication with other cells.
1. INTRODUCTION
Pericytes were discovered by the French scientist Charles Benjamin Rouget in 1873, and were originally known as “Rouget's cells”. 1 They are candidates for regulating microcirculatory blood flow because they strategically cover 90% of the surface area of capillaries and can respond quickly to neuronal stimulation. 2 In the nervous system, pericytes are one of the main components of neurovascular units and play an important role in regulating blood flow, blood–brain barrier (BBB) penetration, neuroinflammation and neuronal activity. 3 In the spinal cord microvascular system, studies have shown that platelet derived growth factor receptor β positive (PDGFRβ+) pericytes are involved in functional recovery, cicatricial fibrosis, and inflammation reduction following spinal cord injury. 4 Pericytes are a component of the pulmonary vascular unit, but compared with endothelial cells (ECs), smooth muscle cells (SMCs), and fibroblasts their function in the pulmonary system remains unknown. Pericytes play an important role in the occurrence and development of vascular homeostasis in pulmonary hypertension (PH), but this is rarely discussed in the existing literature. In the occurrence and development of vascular homeostasis in PH, but this is rarely discussed in the existing literature.
Pericytes are recognized as a type of mesenchymal stem cell or neural crest cell. 5 , 6 Because of their pluripotent stem cell characteristics, they are involved in a variety of physiological and pathological processes, such as regulating blood microcirculation, promoting angiogenesis, participating in the immune response, and communicating with other cells.
In this review, we primarily analyze the role of pericytes in vascular microcirculation, and review the morphology of pericytes in normal tissue, including their developmental sources, distribution, and phenotypic structure. We also discuss the underlying mechanisms of pericytes in vascular microcirculatory diseases.
2. ORIGIN AND DISTRIBUTION OF PERICYTES
Following their discovery, the German scientist Karl Wilhelm Zimmermann renamed Rouget cells “pericytes” 1 ; however, the origin of pericytes remains controversial. Pericytes share several characteristics with mesenchymal and adipose derived stem cells, suggesting that they are derived from mesenchymal stem cells 7 or neural crest cells. 6 The morphology of pericytes revealed by electron microscopy indicates that the nucleus is more prominent and oval in shape. There is a large amount of cytoplasm around the nucleus and several large cytoplasmic branches. 8 The cell protrusions are parallel to the long axis of the capillaries, and the protrusions gradually bifurcate and become thinner, and the distal end surrounds the capillaries. 9
Pulmonary vascular pericytes are present in precapillary arterioles (<30 μm), capillaries (about 10 μm), and postcapillary venules. 10 ECs are surrounded by the pericytes. They form a basement membrane which acts as a barrier between the microvascular and interstitial spaces. They directly interact with the microvascular endothelium. 11 Pericytes regulate vascular permeability and vascular maturity, thus taking part in the formation, maturation, and stabilization of the microvasculature. 12
Pericytes embed mainly on the surface of precapillary arterioles and postcapillary collecting venules and contribute to maintaining vascular homeostasis. 13 , 14 There may be differences in the morphology, plasticity, and function of pericytes in different organs. Even within the same organ, there are different pericyte subsets. 15 As described above, the morphology of pericytes is closely related to the barrier function of ECs. The highest density of pericytes is observed in the central nervous system (CNS) and retina, where the ratio of pericytes to ECs is as high as 1:1. 16
In the nervous system, pericytes are a major component of neurovascular units. They may be divided into three subtypes according to their different morphology and region‐specific distribution, namely ensheathing, mesh and thin‐strand pericytes, which function as regulators of blood flow, blood–brain barrier penetration, neuroinflammation, and neuronal activity. 3 Unsurprisingly, pericytes are also widely distributed around retinal capillaries, where they regulate the diameter of retinal capillaries through their vasomotor activity, and transmit vasomotor signals to neighboring pericytes along the capillaries. 17 , 18 In the microvascular system of the spinal cord, studies have shown that PDGFRβ+ pericytes break away from the vascular wall following spinal cord injury, transform into fibroblasts, and form scars. Blocking the PDGF‐BB/PDGFRβ signaling pathway with imatinib contributes to functional recovery, fibrosis scars, and reduced inflammation following spinal cord injury. 4 , 19 In mouse models involving developed fibroblasts, the primary source of scar‐forming fibroblasts is a discrete subset of perivascular cells known as type A pericytes. 20
In addition to the nervous system and retina, cardiac tissue is also rich in pericytes. Pericytes are the second most abundant cell type in cardiac tissue. 21 , 22 Cardiac pericytes are widely distributed around the microvascular in various regions of the heart. Myocardial ischemia/reperfusion injury may inhibit pericyte contraction and reduce the microvascular pericyte reflow. 9 Pericytes maintain cardiac homeostasis function by influencing vascular shape and maturation, inhibiting EC proliferation, and regulating microvascular permeability. 21 Pericytes can also secrete vascular endothelial growth factor (VEGF) and stromal cell‐derived factor‐1 (SDF‐1), which can protect cardiomyocytes. 24 , 25 Furthermore, they induce fibrin gel contraction, which increases the contractility of the heart, thereby reducing the burden on cardiomyocytes. 26 The ratio of pericytes to ECs in the lung is intermediate between that of the nervous system and that of skeletal muscle. In pulmonary vein of rat lung tissue, the ratio of ECs to pericytes is approximately 10:1. 16 The pulmonary microenvironment plays a major role in maintaining blood oxygen exchange and the homeostasis of the lung environment.
3. PLURIPOTENCY OF CELLS
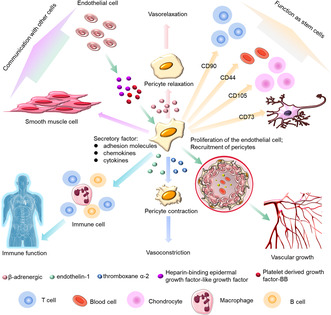
With the rapid development of science and technology, unexpected and remarkable progress has been made in understanding the functions of pericytes in many microvascular diseases, including hypoxia, hypertension, diabetic retinopathy, fibrosis, inflammation, Alzheimer's disease, multiple sclerosis, and tumor formation. 16 Pericytes in different tissues are highly versatile, functionally pluripotent cells with phenotypic plasticity (Figure 1). They can differentiate into fat, muscle, cartilage, and bone cells, as well as other cell types. 9 , 16 Pericytes are versatile, which enables them to provide a strong physiological basis to maintain the stability of the internal environment as well as regulate blood vessel growth, immune function, and communication with other cells.
FIGURE 1.
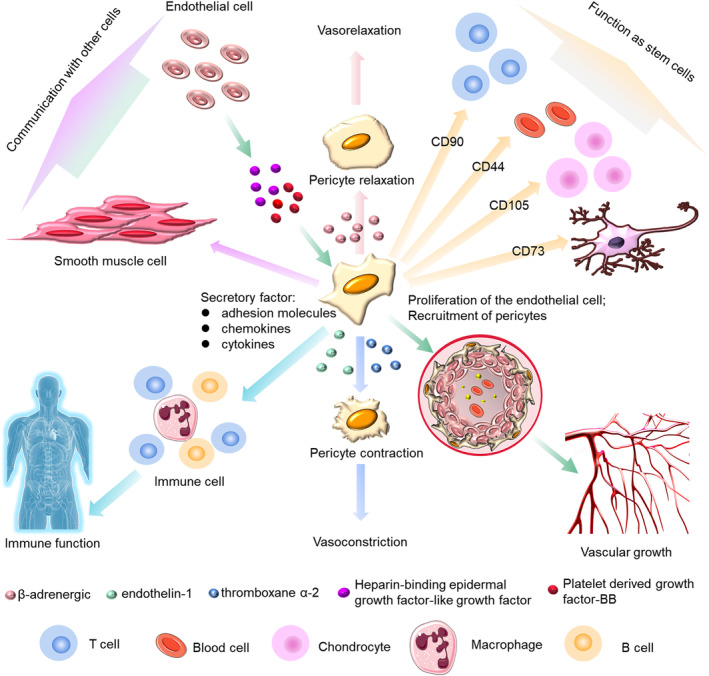
Pluripotency of pericytes. The pluripotency of pericytes includes maintaining the stability of the internal environment, regulating of blood vessel growth, influencing the immune function and communicating with other cells.
3.1. Maintaining homeostasis of the internal environment
Pericytes are important to the formation and integrity of vessel walls within the microcirculation. 27 They have the ability to contract and regulate microcirculatory perfusion and permeability, and to play a key role in regulating microvascular morphogenesis and permeability. 16 , 28 β‐adrenergic receptors, thromboxane α‐2, and endothelin‐1 can regulate pericyte contraction. β‐adrenergic receptors cause pericyte relaxation, whereas thromboxane α‐2 causes pericyte contraction. The adhesion of pericytes to the extraluminal surface of endothelial tubules is necessary for the formation of vascular networks. Pericytes and ECs interact and participate in angiogenesis and wound healing by synthesizing and releasing the structural substances that comprise the basement membrane and extracellular matrix, among others, and promote the maturation of overall vascular function. The abnormal contractility of pericytes is closely related to the progression of many diseases. For example, in the brains of stroke patients, ‘no recirculation’ of cerebral ischemia has been attributed to capillary constriction caused by pericytes. A similar phenomenon was observed in pericytes, in which cardiac pericyte contraction caused coronary capillary contraction and reduced microvascular blood flow after myocardial ischemia. 29
3.2. Regulation of vascular growth
The angiogenesis process can be divided into three main steps: initiation, vessel sprouting and migration, and maturation/termination. 30 During angiogenic remodeling, ECs migrate outward from the existing blood vessels. The sprouting is the basis of the vascular network. As well as the proliferation of the ECs, pericyte coverage of the vascular sprouts is required for angiogenesis and pericytes are the main regulators affecting angiogenesis. 27 The sprouting ECs can communicate with the pericytes. ECs secrete various molecular cues to facilitate the recruitment of pericytes, such as PDGF‐BB, or heparin‐binding epithelial growth factor (EGF) like growth factor (HB‐EGF), which may increase the recruitment of pericytes. 31 Vascular endothelial growth factor (VEGF) also mediates the interactions between pericytes and ECs during angiogenesis. 13 Pericytes regulate the expression of VEGFR1 by altering the expression of VEGF, which results in blood vessel instability and induces angiogenesis. 32 In addition, the contractile capacity of pericytes is similar to SMCs. Through this contractile capacity, pericytes can modulate capillary diameters in vitro 33 and regulate the size of blood flow in microvessels. 34
3.3. Impact on immune function
Pericytes can influence the immune microenvironment. Following activation by tumor cells, they can secrete a series of anti‐inflammatory cytokines such as leukemia inhibitory factor (LIF), cyclooxygenase 2 (COX‐2), and heme oxygenase 1 (HMOX‐1). 35 Pericytes can also release chemokines, such as chemokine ligand 2 (CCL2), chemokine ligand 3 (CCL3), growth‐related oncogene α (CXCL1), in response to pro‐inflammatory stimuli. 36 They also regulate the process of leukocyte differentiation. Leukocytes migrate through the vascular walls along specific pathways and pericytes affect the migration pathway of leukocytes through ECs. 37 CXCL1 secreted by pericytes drives neutrophils to crawl through the pericyte process in vivo until they reach a gap between adjacent pericytes. Pericytes are also important regulators of T‐cell activation, 38 and have a significant effect on tumor‐associated macrophages. 39 , 41 They express a large number of macrophage markers and perform functions similar to macrophages. 40 Following LPS‐induced lung inflammation, activated pericytes express proinflammatory cytokines and functional Toll‐like receptor. 41 They secrete a great many adhesion molecules and chemokines/cytokines that contribute to the recruitment and migration of monocytes, T cells, eosinophils, and neutrophils. 42 Pericytes also express pro‐inflammatory markers such as IL‐1β and TNFα, which can induce a pro‐inflammatory state in astrocytes, microglia, and ECs, and help recruit leukocytes. 43
3.4. Characteristics of stem cells
Pericytes can also behave as mesenchymal stem cells (MSCs). 44 In almost all vascularized organs, pericytes express MSC markers, including CD90, CD44, CD105, and CD73. 45 Like other stem cells, they repair the system while blood vessels remain stable. 30 HiPSC‐derived pericytes have been shown to promote revascularization of ischemic tissues, which demonstrates their direct use in cell therapy and tissue engineering.
3.5. Communication with other cells
3.5.1. Pericytes and ECs
Pericytes are present in all vascularized tissues. They surround ECs, and communicate through paracrine signaling and physical contact. ECs and pericytes are the two main cell types in microvessels. Pericytes and ECs interact and participate in angiogenesis and wound healing, as well as in the synthesis and release of structural substances constituting the basement membrane and extracellular matrix, etc. The interaction between them is important for maintaining vascular hemostasis and microvascular stability. Cytokines secreted from ECs can induce pericyte proliferation. Pericytes can also secrete cytokines to maintain hemostasis in the microenvironment. Pericytes increase microvascular stability by transforming growth factor‐β (TGF‐β) signaling pathway. One previous study indicated that changes in the shape and distribution of pericytes occurred before the initiation of capillary endothelial cell degeneration and capillary regression in diabetic retinopathy, which further confirmed the protective effect of pericytes on ECs. 17
Pericytes interact with ECs mainly through three types of intercellular junctions: gap junctions, adhesive plaques, and peg‐and‐socket junctions. Gap junctions are a form of direct connection in which ionic currents and small molecules transmit between the cytoplasm of two adjacent cells. Adherent plaques are mainly expressed on the cell membrane and mainly anchor pericytes to ECs. Peg‐and‐socket junctions are where one cell binds to a depressed site on another cell; these connections can also provide anchoring. ‘Peg‐socket’ interactions occur less frequently and seem to be concentrated in smaller areas. These three interactions of pericytes with ECs provide further evidence that pericytes play a key role in BBB stability through ECs and provides a direction for investigating how ligands and receptors naturally interact to promote intercellular signaling. It has been found that ECs may affect pericyte contraction through several contact modes, as mentioned above, and lead to a reduction in vascular lumen diameter, at least in some parts of the capillary bed. 34 , 46 , 47 Individual pericytes often contact multiple ECs through these specialized interactions, which can integrate with and coordinate signals from neighboring ECs. 11
3.5.2. Pericytes and SMCs
SMCs are highly located in arteries and veins. They regulate blood flow through vasoconstriction and vasodilation. The unique location of the pericytes makes them a focal point between ECs and SMCs or between the intima and media of higher vascular branches. Pericytes may differentiate into a series of specialized cells. 48 They can also differentiate into SMCs during coronary artery development. 49 During vascular development after the beginning of blood flow, Notch3 is upregulated in pericytes at the site of arterial remodeling. If Notch3 is deficient, the differentiation process of pericytes into coronary SMCs is disrupted. 50 Pericytes isolated from idiopathic pulmonary arterial hypertension (IPAH) exhibit high expression of C‐X‐C motif chemokine receptor (CXCR)‐7 and TGF‐β receptors. Overexpression of the TGF‐β receptor promotes the differentiation of pulmonary vascular pericytes into SMCs, displaying a higher capacity to migrate and proliferate. 51
3.5.3. Pericytes and immune cells
Pericytes are important in assisting immune cell migration. They can secrete a variety of adhesion molecules and chemokines and cytokines, and promote the recruitment and migration of monocytes, T‐cells, eosinophils and neutrophils. 42 Pericytes also promote macrophage recruitment and polarization towards an M2‐like phenotype through the secretion of CXCL14. In the central nervous system, pericytes regulate the immune system. Elimination of CD4+ T‐cells reduces pericyte coverage. In addition, retinal pericytes suppress the activation of CD4+ T‐cells.
4. PERICYTES IN VASCULAR CIRCULATION DISEASES
4.1. Pericytes in pulmonary circulation disease
PH is a common and serious pulmonary circulation disease. Therefore, here we mainly describe the impact of pericytes on PH. PH is a major global health problem and the prevalence of PH ranges from 1–3/million to 15–25/million. 52 The 5‐year overall survival rates remain low for some age groups. The survival rates were 63%, 56%, and 36% for patients in the age groups 46–64, 65–74, and ≥75 years, respectively. 53 The pathogenesis of PH results from the excessive proliferation of vascular wall cells (including ECs, SMCs, and fibroblasts) leading to pulmonary endothelial dysfunction and smooth muscle hypertrophy, and inflammation infiltration resulting in progressive remodeling of the peripheral pulmonary artery. Vasoconstriction and in situ thrombosis, which impair lung flexibility and the hemodynamics of the vascular system, result in right ventricular dysfunction, myocardial cell injury, and death. 54 , 55 , 56 , 57 , 58 , 59 , 60
Pericytes are known to influence the progression of PH through multiple mechanisms and play an important role in regulating baseline cellular activities involved in maintaining homeostasis. 61 Also, pericytes are supportive mesenchymal cells, which can differentiate into numerous cell types, including SMCs. 62 In pathological states, the morphology and number of pericytes can change. The accumulation of pericytes is one of the hallmarks of PH. 63 Compared with healthy subjects, the number of pericytes in the distal pulmonary artery was significantly increased by two times in PH. 64 An increase in the number of pericytes was detected prior to detecting changes in arterial pressure, suggesting that changes in pericytes occur before hemodynamic disturbances. 62 Coordination between pericytes and ECs is critical for a properly organized microvascular network. 61 EC dysfunction is the most obvious manifestation of PH. ECs release paracrine signals to communicate with the surrounding cells, and the closest communication is with the pericytes, which collaborate to maintain the integrity of the vascular walls. 63 , 65
Different cell lineages can arise when pericytes are exposed to certain types of environmental stimulation. Several signaling pathways are involved in the pathogenesis of PH (Figure 2), mainly focused on the functions of cell differentiation, proliferation, migration, growth and communication. Firstly, TGF‐β and ALK pathways may induce cell differentiation of pericytes. In PH, pericytes undergo a unique differentiation to generate rich SMC‐like clusters, in which TGF‐β promotes the differentiation of pericytes into contractile‐SMA cells. 63 Another study indicated that TGF‐β could induce the differentiation of CD34+/PDGGFR+ pericytes into SMCs via ALK‐5‐induced phosphorylation of Smad2/3 by acting on the TGF‐β RII receptors. 66 Therefore, pericytes from IPAH patients are more responsive to TGF‐β due to increased TGF‐β RII receptors. 51 An increase in the number of pericytes and differentiation into a threshold number of SMCs further promotes pulmonary vascular remodeling. 65 , 69 Secondly, FGF‐2 and stromal cell‐derived factor‐1 (SDF‐1) pathways promote the proliferation and migration of pericytes. A previous study indicated that FGF‐2 neutralizing beneficial antibody could have an effect on PH‐induced regeneration of musle by decreasing recruitment of pericytes. 63 Meanwhile, they found that IL‐6 could stimulate pericyte migration but not proliferation nor differentiation towards contractile‐SMA+ cells. 63 Moreover, SDF‐1 also induces CXCR7+ pericyte proliferation and migration in PH. 51 Thirdly, the Wnt pathway mediates communication between pericytes and other cells. Wnt5a as a key mediator in the establishment of pulmonary vascular EC‐pericyte interactions, and Wnt signaling can inhibit the expressions of coiled‐coil like receptor 7 (FZD7) and cell division cycle 42 (CDC42) in lung tissues, which could contribute to PH by reducing the viability of newly formed vessels. 67 , 68 Finally, VEGF and slit guide ligand 3 (SLIT3) pathways have been shown to play a central role in cell growth and angiogenesis. SLIT3, integrin subunit α8 (ITGA8) and their homologues nephronectin (NPNT) and PDGF‐BB were found to be upregulated in pericytes of patients with IPAH, suggesting that they may play an autocrine role in pericyte hypertrophy. 69 , 70 , 71 , 72 In addition, VEGF‐A or PDGF‐BB stimulates the coverage area of pericytes increases, and affects the angiogenesis process. 63 , 65 Taken together, numerous studies have shown the important role of the above pathways in controlling the function of pericytes. However, more studies of the underlying mechanisms of pericytes contributing to the pathogenesis of PH are needed.
FIGURE 2.
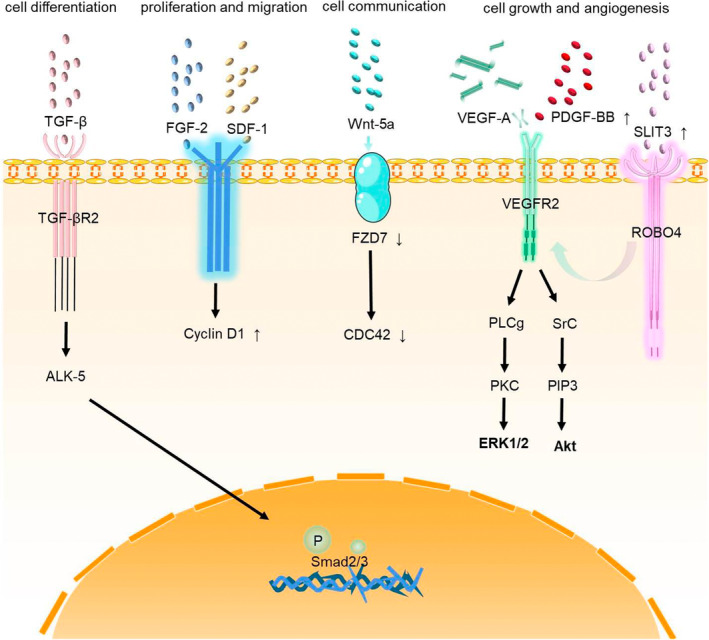
The major cell signaling pathways related to pericytes in PH. (1) Cell differentiation: TGF‐β and ALK pathways; (2) proliferation and migration: FGF‐2 and SDF‐1 pathways; (3) cell communication: Wnt pathway; (4) cell growth and angiogenesis: VEGF and SLIT3 pathways.
4.2. Pericytes in systemic circulatory diseases
There have been many reviews on the role of pericytes in systemic circulation diseases, such as cardiocerebrovascular diseases or other organ microvascular diseases. In our review, we just briefly summarize current research. Through a succession of studies, it has been found that pericytes play an indispensable role in various vascular diseases. For example, in the heart, pericytes, an abundant cell population, not only possess intrinsic plasticity, but can also be modulated by drugs to promote vascular repair in the ischemic heart. 73 In neurological diseases mediated by neuroinflammation such as Parkinson's disease, multiple system atrophy, and dementia with Lewy bodies, pericytes communicate with glial cells to affect the aggregation and diffusion of α‐synuclein, thereby affecting the development of diseases. 74 In diabetic retinopathy (DR), vascular pericyte degeneration is the main clinical manifestation. 75 Nevertheless, several functions of pericytes such as capillary contractility, neuroinflammation, and multipotent stem cell activity remain still to be fully characterized. It is also unclear how each pericyte subtype contributes to pericyte function. 76
5. OUTLOOK
In summary, pericytes are multifunctional cells that have been proven to act as a key element in vascular microcirculation, regulation of blood vessel growth, and communication with ECs and SMCs, in the pathogenesis of vascular diseases. However, there are relatively few studies on pericytes and further studies on their function is warranted.
AUTHOR CONTRIBUTIONS
YW, JF and YH conceived and drafted the manuscript. RD, WZ, CW, SW, XH and HZ designed and made the graphics. LW, JL, GG and PY helped structural adjustment and language modification.
FUNDING INFORMATION
Program of Fundamental Research Funds for the Central Universities (22120220562), Program of Natural Science Foundation of Shanghai (21ZR1453800 and 201409004100), National Natural Science Foundation of China (81870042 and 81900050), Three Year Action Plan to Promote Clinical Skills and Clinical Innovation in Municipal Hospitals (SHDC2020CR4021 and SHDC2020CR6016‐002), and Program of Shanghai Pulmonary Hospital (FKLY20005 and fkzr2320).
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest to declare.
ETHICS STATEMENT
None.
ACKNOWLEDGMENTS
This work was supported by funding from the Program of Fundamental Research Funds for the Central Universities (22120220562), Program of Natural Science Foundation of Shanghai (21ZR1453800 and 201409004100), National Natural Science Foundation of China (81870042 and 81900050), Three Year Action Plan to Promote Clinical Skills and Clinical Innovation in Municipal Hospitals (SHDC2020CR4021 and SHDC2020CR6016‐002), and Program of Shanghai Pulmonary Hospital (FKLY20005 and fkzr2320).
Wu Y, Fu J, Huang Y, et al. Biology and function of pericytes in the vascular microcirculation. Anim Models Exp Med. 2023;6:337‐345. doi: 10.1002/ame2.12334
Yue Wu and Jiaqi Fu contributed equally to this work.
Contributor Information
Jinming Liu, Email: jinmingliu@tonji.edu.cn.
Guosheng Gao, Email: yukisa26@126.com.
Ping Yuan, Email: pandyyuan@tongji.edu.cn.
REFERENCES
- 1. Cao L, Zhou Y, Chen M, Li L, Zhang W. Pericytes for therapeutic approaches to ischemic stroke. Front Neurosci. 2021;15:629297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alarcon‐Martinez L, Villafranca‐Baughman D, Quintero H, et al. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature. 2020;585(7823):91‐95. [DOI] [PubMed] [Google Scholar]
- 3. Zhang H, Zhang X, Hong X, Tong X. Homogeneity or heterogeneity, the paradox of neurovascular pericytes in the brain. Glia. 2021;69(10):2474‐2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Z, Zheng M, Yu S, et al. M2 macrophages promote PDGFRβ(+) pericytes migration after spinal cord injury in mice via PDGFB/PDGFRβ pathway. Front Pharmacol. 2021;12:670813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meijer EM, van Dijk CGM, Kramann R, Verhaar MC, Cheng C. Implementation of pericytes in vascular regeneration strategies. Tissue Eng Part B Rev. 2022;28(1):1‐21. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto S, Muramatsu M, Azuma E, et al. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci Rep. 2017;7(1):3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geevarghese A, Herman IM. Pericyte‐endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl Res. 2014;163(4):296‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergers G, Song S. The role of pericytes in blood‐vessel formation and maintenance. Neuro Oncol. 2005;7(4):452‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Su X, Huang L, Qu Y, Xiao D, Mu D. Pericytes in cerebrovascular diseases: an emerging therapeutic target. Front Cell Neurosci. 2019;. 13:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yuan K, Orcholski ME, Panaroni C, et al. Activation of the Wnt/planar cell polarity pathway is required for pericyte recruitment during pulmonary angiogenesis. Am J Pathol. 2015;185(1):69‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512‐523. [DOI] [PubMed] [Google Scholar]
- 12. Huang H. Pericyte‐endothelial interactions in the retinal microvasculature. Int J Mol Sci. 2020;21(19):7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raza A, Franklin MJ, Dudek AZ. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol. 2010;85(8):593‐598. [DOI] [PubMed] [Google Scholar]
- 14. Ago T. Why are pericytes important for brain functions? Rinsho Shinkeigaku. 2019;59(11):707‐715. [DOI] [PubMed] [Google Scholar]
- 15. Birbrair A. Pericyte biology: development, homeostasis, and disease. Adv Exp Med Biol. 2018;1109:1‐3. [DOI] [PubMed] [Google Scholar]
- 16. Zhang ZS, Zhou HN, He SS, Xue MY, Li T, Liu LM. Research advances in pericyte function and their roles in diseases. Chin J Traumatol. 2020;23(2):89‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kamiya E, Morita A, Mori A, Sakamoto K, Nakahara T. The process of methylglyoxal‐induced retinal capillary endothelial cell degeneration in rats. Microvasc Res. 2023;146:104455. [DOI] [PubMed] [Google Scholar]
- 18. Yao F, Luo Y, Liu YC, et al. Imatinib inhibits pericyte‐fibroblast transition and inflammation and promotes axon regeneration by blocking the PDGF‐BB/PDGFRβ pathway in spinal cord injury. Inflamm Regener. 2022;42(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu K, Cheng T, Zhai Z, Jiang C, Zhou X. Alpha 7‐nicotinic cholinergic regulation of pericyte‐containing retinal capillaries. Br J Pharmacol. 2023;16067. doi: 10.1111/bph.16067 [DOI] [PubMed] [Google Scholar]
- 20. Dias DO, Kalkitsas J, Kelahmetoglu Y, et al. Pericyte‐derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun. 2021;12(1):5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Su H, Cantrell AC, Zeng H, Zhu SH, Chen JX. Emerging role of pericytes and their secretome in the heart. Cell. 2021;10(3):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee LL, Chintalgattu V. Pericytes in the heart. Adv Exp Med Biol. 2019;1122:187‐210. [DOI] [PubMed] [Google Scholar]
- 23. Li Q, Guo Z, Wu C, et al. Ischemia preconditioning alleviates ischemia/reperfusion injury‐induced coronary no‐reflow and contraction of microvascular pericytes in rats. Microvasc Res. 2022;142:104349. [DOI] [PubMed] [Google Scholar]
- 24. Seo J, Kim YO, Jo I. Differential expression of stromal cell‐derived factor 1 in human brain microvascular endothelial cells and pericytes involves histone modifications. Biochem Biophys Res Commun. 2009;382(3):519‐524. [DOI] [PubMed] [Google Scholar]
- 25. Eilken HM, Diéguez‐Hurtado R, Schmidt I, et al. Pericytes regulate VEGF induced endothelial sprouting through VEGFR1. Nat Commun. 2017;8(1):1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaul S, Methner C, Mishra A. The role of pericytes in hyperemia‐induced capillary de‐recruitment following stenosis. Curr Tissue Microenviron Rep. 2020;1(4):163‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Payne LB, Zhao H, James CC, et al. The pericyte microenvironment during vascular development. Microcirculation. 2019;26(8):e12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dabravolski SA, Andreeva ER, Eremin II, et al. The role of pericytes in regulation of innate and adaptive immunity. Biomedicine. 2023;11(2):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Farrell FM, Mastitskaya S, Hammond‐Haley M, Freitas F, Wah WR, Attwell D. Capillary pericytes mediate coronary no‐reflow after myocardial ischaemia. Elife. 2017;6:e29280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schrimpf C, Teebken OE, Wilhelmi M, Duffield JS. The role of pericyte detachment in vascular rarefaction. J Vasc Res. 2014;51(4):247‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xiang DN, Feng YF, Wang J, et al. Platelet‐derived growth factor‐BB promotes proliferation and migration of retinal microvascular pericytes by up‐regulating the expression of C‐X‐C chemokine receptor types 4. Exp Ther Med. 2019;18(5):4022‐4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dabravolski SA, Markin AM, Andreeva ER, Eremin II, Orekhov AN, Melnichenko AA. Emerging role of pericytes in therapy of cardiovascular diseases. Biomed Pharmacother. 2022;156:113928. [DOI] [PubMed] [Google Scholar]
- 33. Hamilton NB, Attwell D, Hall CN. Pericyte‐mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rucker HK, Wynder HJ, Thomas WE. Cellular mechanisms of CNS pericytes. Brain Res Bull. 2000;51(5):363‐369. [DOI] [PubMed] [Google Scholar]
- 35. Chen CW, Okada M, Proto JD, et al. Human pericytes for ischemic heart repair. Stem Cells. 2013;31(2):305‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun R, Kong X, Qiu X, Huang C, Wong PP. The emerging roles of pericytes in modulating tumor microenvironment. Front Cell Dev Biol. 2021;9:676342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rudziak P, Ellis CG, Kowalewska PM. Role and molecular mechanisms of pericytes in regulation of leukocyte diapedesis in inflamed tissues. Mediators Inflamm. 2019;2019:4123605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Navarro R, Compte M, Álvarez‐Vallina L, Sanz L. Immune regulation by pericytes: modulating innate and adaptive immunity. Front Immunol. 2016;7:480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou J, Tang Z, Gao S, Li C, Feng Y, Zhou X. Tumor‐associated macrophages: recent insights and therapies. Front Oncol. 2020;10:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kittikulsuth W, Nakano D, Kitada K, Suzuki N, Yamamoto M, Nishiyama A. Renal NG2‐expressing cells have a macrophage‐like phenotype and facilitate renal recovery after ischemic injury. Am J Physiol Renal Physiol. 2021;321(2):F170‐f178. [DOI] [PubMed] [Google Scholar]
- 41. Liu X, Yin S, Chen Y, et al. LPS‐induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF‐κB, STAT3 or AP‐1 activation. Mol Med Rep. 2018;17(4):5484‐5491. [DOI] [PubMed] [Google Scholar]
- 42. Kaushik DK, Bhattacharya A, Lozinski BM, Wee Yong V. Pericytes as mediators of infiltration of macrophages in multiple sclerosis. J Neuroinflammation. 2021;18(1):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Carroll SJ et al. Pro‐inflammatory TNFα and IL‐1β differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J Neuroinflammation. 2015;12:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mills SJ, Cowin AJ, Kaur P. Pericytes, mesenchymal stem cells and the wound healing process. Cell. 2013;2(3):621‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen WC et al. Human myocardial pericytes: multipotent mesodermal precursors exhibiting cardiac specificity. Stem Cells. 2015;33(2):557‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Procter TV, Williams A, Montagne A. Interplay between brain pericytes and endothelial cells in dementia. Am J Pathol. 2021;191(11):1917‐1931. [DOI] [PubMed] [Google Scholar]
- 47. Pfau SJ, Langen UH, Fisher TM, et al. Vascular and perivascular cell profiling reveals the molecular and cellular bases of blood‐brain barrier heterogeneity. bioRxiv. 2021. doi: 10.1101/2021.04.26.441465 [DOI] [Google Scholar]
- 48. Zhu S, Chen M, Ying Y, et al. Versatile subtypes of pericytes and their roles in spinal cord injury repair, bone development and repair. Bone Res. 2022;10(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kumar A, D'Souza SS, Moskvin OV, et al. Specification and diversification of pericytes and smooth muscle cells from mesenchymoangioblasts. Cell Rep. 2017;19(9):1902‐1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kofler NM, Cuervo H, Uh MK, Murtomäki A, Kitajewski J. Combined deficiency of Notch1 and Notch3 causes pericyte dysfunction, models CADASIL, and results in arteriovenous malformations. Sci Rep. 2015;5:16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bordenave J, Tu L, Berrebeh N, et al. Lineage tracing reveals the dynamic contribution of pericytes to the blood vessel remodeling in pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2020;40(3):766‐782. [DOI] [PubMed] [Google Scholar]
- 52. Berra G, Noble S, Soccal PM, Beghetti M, Lador F. Pulmonary hypertension in the elderly: a different disease? Breathe (Sheff). 2016;12(1):43‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hjalmarsson C, Rådegran G, Kylhammar D, et al. Impact of age and comorbidity on risk stratification in idiopathic pulmonary arterial hypertension. Eur Respir J. 2018;51(5):1702310. [DOI] [PubMed] [Google Scholar]
- 54. Zhou J. Synemin promotes pulmonary artery smooth muscle cell phenotypic switch in shunt‐induced pulmonary arterial hypertension. ESC Heart Fail. 2022;9(5):3221‐3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang D, Mo Y, Zhang D, Bai Y. Analysis of m(7)G methylation modification patterns and pulmonary vascular immune microenvironment in pulmonary arterial hypertension. Front Immunol. 2022;13:1014509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shah AJ, Vorla M, Kalra DK. Molecular pathways in pulmonary arterial hypertension. Int J Mol Sci. 2022;23(17):10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Santos‐Gomes J, le Ribeuz H, Brás‐Silva C, Antigny F, Adão R. Role of ion channel remodeling in endothelial dysfunction induced by pulmonary arterial hypertension. Biomolecules. 2022;12(4):484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhou JJ, Yang J, Li L, et al. Transgelin exacerbates pulmonary artery smooth muscle cell dysfunction in shunt‐related pulmonary arterial hypertension. ESC Heart Fail. 2022;9(5):3407‐3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cuthbertson I, Morrell NW, Caruso P. BMPR2 mutation and metabolic reprogramming in pulmonary arterial hypertension. Circ Res. 2023;132(1):109‐126. [DOI] [PubMed] [Google Scholar]
- 60. Yeo Y, Jeong H, Kim M, Choi Y, Kim KL, Suh W. Crosstalk between BMP signaling and KCNK3 in phenotypic switching of pulmonary vascular smooth muscle cells. BMB Rep. 2022;55(11):565‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nwadozi E, Rudnicki M, Haas TL. Metabolic coordination of pericyte phenotypes: therapeutic implications. Front Cell Dev Biol. 2020;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rowley JE, Johnson JR. Pericytes in chronic lung disease. Int Arch Allergy Immunol. 2014;164(3):178‐188. [DOI] [PubMed] [Google Scholar]
- 63. Ricard N, Tu L, le Hiress M, et al. Increased pericyte coverage mediated by endothelial‐derived fibroblast growth factor‐2 and interleukin‐6 is a source of smooth muscle‐like cells in pulmonary hypertension. Circulation. 2014;129(15):1586‐1597. [DOI] [PubMed] [Google Scholar]
- 64. Summerhill V, Orekhov A. Pericytes in atherosclerosis. Adv Exp Med Biol. 2019;1147:279‐297. [DOI] [PubMed] [Google Scholar]
- 65. Kato K, Diéguez‐Hurtado R, Park DY, et al. Pulmonary pericytes regulate lung morphogenesis. Nat Commun. 2018;9(1):2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dierick F, Solinc J, Bignard J, Soubrier F, Nadaud S. Progenitor/stem cells in vascular remodeling during pulmonary arterial hypertension. Cell. 2021;10:1338. doi: 10.3390/cells10061338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang Y, Wang Y. Cell‐to‐cell crosstalk: a new insight into pulmonary hypertension. Rev Physiol Biochem Pharmacol. 2023;184:159‐179. [DOI] [PubMed] [Google Scholar]
- 68. Yuan K, Shamskhou EA, Orcholski ME, et al. Loss of endothelium‐derived Wnt5a is associated with reduced pericyte recruitment and small vessel loss in pulmonary arterial hypertension. Circulation. 2019;139(14):1710‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vladar EK, Königshoff M. Noncanonical Wnt planar cell polarity signaling in lung development and disease. Biochem Soc Trans. 2020;48(1):231‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Saygin D, Tabib T, Bittar HET, et al. Transcriptional profiling of lung cell populations in idiopathic pulmonary arterial hypertension. Pulm Circ. 2020;10(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yuan K, Liu Y, Zhang Y, et al. Mural cell SDF1 signaling is associated with the pathogenesis of pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2020;62(6):747‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hosaka K, Yang Y, Nakamura M, et al. Dual roles of endothelial FGF‐2‐FGFR1‐PDGF‐BB and perivascular FGF‐2‐FGFR2‐PDGFRβ signaling pathways in tumor vascular remodeling. Cell Discov. 2018;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Avolio E, Katare R, Thomas AC, et al. Cardiac pericyte reprogramming by MEK inhibition promotes arteriologenesis and angiogenesis of the ischemic heart. J Clin Invest. 2022;132:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang Q, Zheng J, Pettersson S, Reynolds R, Tan EK. The link between neuroinflammation and the neurovascular unit in synucleinopathies. Sci Adv. 2023;9:eabq1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jiang Q, Liu C, Li CP, et al. Circular RNA‐ZNF532 regulates diabetes‐induced retinal pericyte degeneration and vascular dysfunction. J Clin Invest. 2020;130:3833‐3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. 2016;19:771‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]


