Abstract
Simple Summary
Performing this real-world analysis, we intended to see if postoperative (adjuvant) systemic treatment outcomes in a Polish melanoma patient population are comparable to the results from international trials based on which the treatment was registered worldwide. We intended to provide evidence on the efficacy and safety of postoperative melanoma treatment from everyday practice. We have shown that the type of surgical procedure on the lymph nodes prior to adjuvant treatment does not influence the outcome of that treatment. Our results support a de-escalation of surgery approach in melanoma patients. We support the value of adjuvant treatment for melanoma patients selected according to new guidelines implemented in parallel to the registration process. Analyzing the recorded side effects of adjuvant systemic treatment in our group of patients, we have noticed that severe complications worsen survival, giving us an indication not to treat by all means despite toxicity, particularly since it is a complementary to surgery treatment.
Abstract
Background: The landscape of melanoma management changed as randomized trials have launched adjuvant treatment. Materials and Methods: An analysis of data on 248 consecutive melanoma stage III and IV patients given adjuvant therapy in eight centers (February 2019 to January 2021) was conducted. Results: The analyzed cohort comprised 147 melanoma patients given anti-PD1 (33% nivolumab, 26% pembrolizumab), and 101 (41%) were given dabrafenib plus trametinib (DT). The 2-year overall survival (OS), relapse-free survival (RFS), and distant-metastases-free survival (DMFS) rates were 86.7%, 61.4%, and 70.2%, respectively. The disease stage affected only the RFS rate; for stage IV, it was 52.2% (95% CI: 33.4–81.5%) vs. 62.5% (95% CI: 52.3–74.8%) for IIIA-D, p = 0.0033. The type of lymph node surgery before adjuvant therapy did not influence the outcomes. Completion of lymph node dissection cessation after positive SLNB did not affect the results in terms of RFS or OS. Treatment-related adverse events (TRAE) were associated with longer 24-month RFS, with a rate of 68.7% (55.5–84.9%) for TRAE vs. 56.6% (45.8–70%) without TRAE, p = 0.0031. For TRAE of grade ≥ 3, a significant decline in OS to 60.6% (26.9–100%; p = 0.004) was observed. Conclusions: Melanoma adjuvant therapy with anti-PD1 or DT outside clinical trials appears to be effective and comparable with the results of registration studies. Our data support a de-escalating surgery approach in melanoma treatment.
Keywords: melanoma, adjuvant treatment, targeted therapy, immune check point inhibitors, early-stage melanoma, sentinel node biopsy, lymphadenectomy in melanoma, surgery de-escalation in melanoma
1. Introduction
Melanoma treatment has undergone a revolution in recent years. We have observed a shift in the early-stage treatment paradigm: de-escalation of surgery and increased use of adjuvant (and supposedly also neoadjuvant) systemic treatment. From 2016 to 2017, based on DECOG and MSLT-II randomized phase III trials, there exists strong evidence to omit completion lymphadenectomy (CLND) after positive sentinel lymph node biopsy (SLNB) [1,2]. SLNB, introduced by Morton [3], in addition to its therapeutic relevance, determines nowadays the need for adjuvant systemic therapy, which has become a standard of care for patients with high-risk resected melanoma of stage III and IV [4,5,6].
In 2017, the American Joint Committee on Cancer (AJCC) brought the updated Melanoma Staging System–Eighth Edition (AJCCv.8) to light [7]. In 2018, systemic adjuvant therapies with immune check point inhibitors (ICI): nivolumab and pembrolizumab and targeted BRAF/MEK inhibitors (BRAF/MEKi): dabrafenib plus trametinib were officially introduced in Europe based on the positive results of randomized phase III clinical trials: Checkmate-238 (for nivolumab vs. ipilimumab), Keynote-054 (for pembrolizumab vs. placebo), and Combi-AD (for dabrafenib plus trametinib vs. placebo) [8,9,10]. The registration of those therapies was based on clinical trials carried out according to inclusion criteria established before the new era; this involved patients with stage III-IV melanoma according to the AJCC v.7 (not 8) staging system with obligatory performance of CLND [9,10,11,12]. As melanoma adjuvant treatment established itself with increasing use and longer patient follow-up in everyday clinical practice, some questions have arisen, and uncertainties have emerged. Melanoma adjuvant treatment prolongs survival without relapse, thus avoiding its morbidity, but it has no proven impact on overall survival. It is potentially curative, but without an effect on survival in the entire population, it raises the topic of risk stratification for decision making. We need to search for predictive markers and weigh risks versus benefits in order to avoid overtreatment of those who can be cured only by surgery, while also having in mind possible chronic or permanent side effects and avoiding worsening quality of life for those who would benefit more from waiting for the recurrence strategy [6].
In our analysis we addressed some of those questions and uncertainties. First of all, we aimed to evaluate the effectiveness, safety and tolerability of adjuvant melanoma treatment in Polish patient population. Secondary, we intended to see if real-world results of this treatment in our patient population, not exactly represented in adjuvant registration randomized controlled trials, are comparable to the results from those studies. In addition, we took under consideration possible prognostic and predictive markers. We proposed a prognostic model and evaluated some factors as type of surgery prior to adjuvant treatment or drug used for adjuvant treatment (e.g., anti-PD1 vs. BRAF/MEKi for selected BRAF/+/ melanoma patients) which could influence adjuvant treatment results. Finally, we have investigated whether adjuvant systemic treatment outcomes correlate with occurrence of related to treatment side effects.
2. Materials and Methods
2.1. Patient Cohort and Inclusion Criteria
The subject of our analysis was a group of consecutive patients identified with stage III–IV resected melanoma who were given the adjuvant treatment between April 2019 and January 2021 in 8 Polish melanoma reference centers. The inclusion criteria were: histologically confirmed melanoma (cutaneous acral, mucosal, or melanoma of unknown primary site were accepted), >18 years of age, resected R0 stage III or IV melanoma by AJCCv.8 (CLND after positive SLNB was not mandatory), BRAF status determined (with no need for BRAF variant assessment), at least one given cycle of adjuvant systemic treatment with immune check point inhibitors (ICI), anti-PD1 antibodies (nivolumab or pembrolizumab or targeted therapy), and BRAF/MEK inhibitors (dabrafenib plus trametinib (DT)). Schedules and dosage were according to the products’ characteristics. The data cut-off point was 31 January 2022. Strict follow-up was performed according to the description of the National Drug Program.
2.2. Collected Covariates
Collected data included: patient demographics; primary melanoma characteristics including location, histologic subtype, BRAF mutation status, Breslow tumor thickness, and presence of ulceration and microsatellites; nodal characteristics including date, type, and result of nodal surgery performed (sentinel lymph node biopsy, SLNB; completion lymph node dissection, CLND; therapeutic lymph node dissection, TLND), number of nodes assessed, number of nodes positive for metastatic disease, diameter of nodal metastasis; American Joint Committee on Cancer (AJCC) version 8 staging; description of adjuvant therapy (type of drug used for systemic treatment); duration of adjuvant therapy; reason for adjuvant therapy cessation; appearance of adverse events (TRAE); grade of TRAE; treatment for TRAE; recurrence date; location of recurrence; type of additional therapy for recurrence; and date of death or last follow-up, whichever was eligible.
2.3. Analysis Plan
We determined the demographics, disease and treatment characteristics, and rate of toxicity according to adjuvant management (immunotherapy vs. targeted therapy). In different subgroups, we measured the time to event outcomes at 2 years: recurrence-free survival (RFS)—the time from the date of adjuvant treatment start to any recurrence, distant-metastasis-free survival (DMFS)—the time from adjuvant treatment commencement to distant metastasis appearance and overall survival (OS)—the time from adjuvant treatment commencement to death or last observation, regardless of disease recurrence.
2.4. Statistical Methods
The data were pseudonymized. Categorical variables were described as a proportion with the percentage, and continuous variables were described as a mean and standard deviation or a median with an interquartile range. The 2-year relapse-free survival (RFS), distant-metastases-free survival (DMFS), and overall survival (OS) were estimated using the Kaplan–Meier method. A log-rank test was used to examine differences between subgroups. Multivariate analysis was performed using the Cox Proportional-Hazards Model. Patients’ clinical data and outcomes were analyzed through the use of “R” Software v.2021, in particular with the application of “survival”, “survminer,” and “rms” packages [13].
2.5. Ethical Statement
This retrospective, noninterventional study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Maria Skłodowska-Curie National Research Institute of Oncology; ethical board approval number 27/2018 dated 19 April 2018. Prior to any given treatment, the patients signed an informed consent form for the treatment and a consent form allowing the usage of their data for scientific purposes.
3. Results
3.1. Patient and Tumor Characteristics
Overall, we identified 248 patients who met the inclusion criteria. The median follow-up was 18.9 months (95% confidence interval CI: 17.9–20.0). There were 47% females, with a median age of 57 years (IQR: 45–68; range: 20–88); in total, 54% of patients had comorbidities, and 65% were of WHO 0 performance status. The median Breslow thickness was 3.2 mm (IQR: 1.8–6.0 mm), ulceration was present in 48% of patients, and 63% (155) of patients had a positive BRAF mutational status. The stage distribution according to AJCCv.8 was as follow: IIIA 6%, IIIB 23%, IIIC 56%, IIID 4%, and IV in 9% of patients. In total, 87% of the melanomas were cutaneous with exclusion of acral subtype, 3% were cutaneous/acral, and only three cases were mucosal. Patient and tumor characteristics are summarized in Table 1.
Table 1.
Demographic and primary tumor characteristics (n = 248).
| Covariate | Group | Total | |
|---|---|---|---|
| n | % | ||
| Sex | female | 116 | 46.8 |
| male | 132 | 53.2 | |
| Age group | <45 | 62 | 25 |
| 45–54 | 41 | 16.5 | |
| 55–64 | 64 | 25.8 | |
| 65+ | 81 | 32.7 | |
| Melanoma type | skin | 215 | 86.7 |
| acral | 8 | 3.2 | |
| mucosal | 3 | 1.2 | |
| unknown | 22 | 8.9 | |
| BRAF mutational status | negative | 92 | 37.1 |
| positive | 155 | 62.5 | |
| undocumented | 1 | 0.4 | |
| AJCC8 primary tumor characteristics, pT | T0 | 21 | 8.5 |
| T1a–b | 20 | 8.0 | |
| T2a–b | 43 | 17.3 | |
| T3a–b | 62 | 25 | |
| T4a–b | 93 | 37.5 | |
| NA | 9 | 3.6 | |
3.2. Local Therapy
3.2.1. Surgical Treatment before Adjuvant Systemic Therapy
For the purpose of analysis, all patients were clustered into three groups according to the type of surgery before adjuvant treatment: surgery without recurrence (primary melanoma and nodes: positive sentinel lymph node biopsy (SLNB) +/− completion lymph node dissection (CLND)), surgery after local recurrence (therapeutic lymph node dissection (TLND) +/− in-transit resection), and surgery after distant recurrence (resected M1) (Table 2). Within the primary surgery group (resection of primary melanoma and/or nodes), there were patients who underwent TLND without SLNB (33, 25.4%), those who underwent CLND after positive SLNB (63, 48.5%), and those who had positive SLNB but no CLND afterwards (34, 26.2%).
Table 2.
Adjuvant treatment.
| Covariate | Group | Total | |
|---|---|---|---|
| n | % | ||
| WHO performance status at treatment initiation | 0 | 161 | 64.9 |
| 1 | 86 | 34.7 | |
| 2 | 1 | 0.4 | |
| AJCC8 stage at initiation of adjuvant treatment | IIIA | 16 | 6.5 |
| IIIB | 56 | 22.6 | |
| IIIC | 139 | 56 | |
| IIID | 11 | 4.4 | |
| IIIx | 3 | 1.2 | |
| IV | 23 | 9.3 | |
| Group of indications for adjuvant | Primary surgery (primary melanoma and nodes) | 130 | 52.4 |
| Resected distant metastases | 23 | 9.3 | |
| Resected local recurrence (scar/in transit/nodal) | 95 | 38.3 | |
| Node surgery in the primary surgery group | SLNB (+), no CLND | 34 | 26.2 |
| SLNB (+), CLND | 63 | 48.5 | |
| TLND | 33 | 25.4 | |
| Adjuvant type | dabrafenib plus trametinib | 101 | 40.7 |
| pembrolizumab | 65 | 26.2 | |
| nivolumab | 82 | 33.1 | |
| Adjuvant treatment ended | no | 64 | 25.8 |
| yes | 184 | 74.2 | |
| Reason for ending of adjuvant treatment | (Treatment ongoing) | 64 | 25.8 |
| As planned | 111 | 44.8 | |
| Other | 4 | 1.6 | |
| Progression | 39 | 15.7 | |
| Toxicity | 23 | 9.3 | |
| Undocumented | 7 | 2.8 | |
SLNB: sentinel lymph node biopsy; CLND: completion lymph node dissection; TLND: therapeutic lymph node dissection.
There were 174 (70%) patients with intervals longer than 13 weeks from the last operation to adjuvant treatment start.
3.2.2. Radiotherapy
Adjuvant radiotherapy was recorded in 15 (6%) analyzed patients (10 patients from ICI and 5 patients from DT subgroups).
3.3. Systemic Treatment
Applied adjuvant therapy consisted of nivolumab in 82 patients, pembrolizumab in 65 patients, and a combination of dabrafenib and trametinib (DT) in 101 patients (Table 2). In our cohort, adjuvant immunotherapy was applied in 54 (37%) melanoma BRAF-positive and 93 (63%) BRAF-negative patients. At the time of cut-off, 184 (74%) patients finished adjuvant treatment, with 107 (71%) on anti-PD1 and 77 (76%) on DT. One-year adjuvant therapy was completed as scheduled in 60% of patients (54 (50%) on immunotherapy and 57 (75%) on targeted therapy). Therapy was discontinued due to treatment-related adverse events (TRAE) in 24 patients (13%) and due to relapse in 39 patients (21%).
The overall incidence of TRAE was 46%, which was higher in DT in comparison to the anti-PD1 group at 58% and 39%, respectively (Table 3). The most common was grade two TRAE at 21%. Grade three TRAE was observed in 23 (9.3%) patients. In total, 29% of the overall TRAE led to dosage reduction or treatment discontinuation.
Table 3.
Treatment-related adverse events.
| Covariate | Group | Anti-PD1 | DT | Total | p-Value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| Toxicities after treatment | Yes | 56 (38.1) | 59 (58.4) | 115 (46.4) | 0.001 |
| No | 91 (61.9) | 40 (39.6) | 131 (52.8) | ||
| Undocumented | 0 | 2 (2.0) | 2 (0.8) | ||
| Treatment ended due to toxicity | Yes | 13 (8.8) | 11 (10.9) | 24 (9.7) | 0.302 |
| No | 91 (61.9) | 68 (67.3) | 159 (64.1) | ||
| (Treatment ongoing) | 43 (29.3) | 21 (20.8) | 64 (25.8) | ||
| Undocumented | 0 | 1 (1.0) | 1 (0.4) | ||
| Highest grade toxicity if toxicity was reported (n = 115) | 1 | 23 (41.1) | 14 (23.7) | 37 (32.2) | 0.041 |
| 2 | 25 (44.6) | 26 (44.1) | 51 (44.3) | ||
| 3 | 8 (14.3) | 15 (25.4) | 23 (20.0) | ||
| Undocumented | 0 | 4 (6.8) | 4 (3.5) |
Anti-PD1: nivolumab or pembrolizumab; DT: dabrafenib plus trametinib.
3.4. Survival Analysis
3.4.1. Registered Events
At a median follow-up of 13.9 months (average: 15.0 months; SD = 6.5), 61 (25%) patients experienced disease recurrence. There were 46 (31%) relapses in the ICI group and 15 (15%) in the DT group (Table 4). Most of the recurrences (64%) occurred during adjuvant treatment, particularly in the ICI group. Overall, 75% of all recurrences occurred in the immunotherapy arm. The most common locations of recurrence were metastases in visceral organs (34%), the skin, subcutaneous tissue, and distant nodes (25%), as well as local in-transit or scar recurrences (18%). The brain was a site of relapse in 8% of patients. Most of the relapses occurred in patients with stage IIIC melanoma. During the minimum observation period of 12.2 months (range up to 36.4 months), 11 (18%) relapses led to death.
Table 4.
Overall and recurrence-free survival.
| n | n Events ** | Restricted Mean * (SD) | 24-Month Survival (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|
| Recurrence-free survival (RFS) | ||||||
| Overall | 248 | 61 | 19.5 (0.5) | 61.4% (51.8–72.8%) | ||
| Indication group | LND/Primary | 130 | 24 | 20.5 (0.6) | 65.4% (50.9–83.9%) | 0.0064 |
| M1 | 23 | 10 | 15.2 (2.1) | 52.2% (33.4–81.5%) | ||
| Scar/In transit/LND | 95 | 27 | 19.2 (0.8) | 58.8% (45.3–76.5%) | ||
| Adjuvant group | Anti-PD1 | 147 | 46 | 17.7 (0.8) | 56.1% (45.6–69.1%) | <0.0001 |
| BRAF/MEKi | 101 | 15 | 21.8 (0.6) | 65.9% (48.4–89.7%) | ||
| Overall survival (OS) | ||||||
| Overall | 248 | 13 | 23.1 (0.3) | 86.7% (78.8–95.3%) | ||
| Indication group | LND/Primary | 130 | 9 | 22.6 (0.4) | 85.1% (74.6–97%) | 0.1128 |
| M1 | 23 | 2 | 22.5 (1) | 68.2% (37.6–100%) | ||
| Scar/In transit/LND | 95 | 2 | 23.7 (0.2) | 92.9% (83.3–100%) | ||
| Adjuvant group | Anti-PD1 | 147 | 8 | 22.9 (0.4) | 85.3% (74.9–97.2%) | 0.6318 |
| BRAF/MEKi | 101 | 5 | 23.2 (0.4) | 87.8% (76.2–100%) | ||
| Distant-metastases-free survival (DMFS) | ||||||
| Overall | 248 | 49 | 20.3 (0.5) | 70.2% (61.3–80.4%) | ||
| Indication group | LND/Primary | 130 | 22 | 20.7 (0.6) | 72.4% (60.5–86.7%) | 0.0525 |
| M1 | 23 | 8 | 16.7 (2.1) | 60.9% (41.8–88.7%) | ||
| Scar/In transit/LND | 95 | 19 | 20.6 (0.7) | 70.2% (56.4–87.3%) | ||
| Adjuvant group | Anti-PD1 | 147 | 37 | 18.9 (0.7) | 64.8% (54.4–77.2%) | 0.003 |
| BRAF/MEKi | 101 | 12 | 22.2 (0.5) | 76.5% (61.2–95.5%) | ||
* restricted mean calculated for the 24-month time span; ** occurrence of the following events is listed: for RFS: relapses, for OS: deaths, for DMFS: distant metastases; anti-PD1: nivolumab or pembrolizumab; BRAF/MEKi: dabrafenib plus trametinib; LND: lymph node dissection.
There were 13 (5%) deaths in total. Of these deaths, 69% of them were due to melanoma relapse, 31% were from unknown causes, and two deaths (15%) were likely due to TRAE. The distribution of deaths between the treatment groups (ICI vs. DT) was equal, with 5% in each group. The restricted mean survival during the first 24 months was 23.1 months.
We have observed relapses and fatal events to be distributed evenly between the groups split by criterion of less or equal to 13 weeks from the last operation to adjuvant start, with 19 (26%) relapses and 7 (9%) deaths in the ≤13-week group and 43 (25%) relapses and 11 (6%) deaths in the >13-week group.
3.4.2. Overall and Recurrence-Free Survival
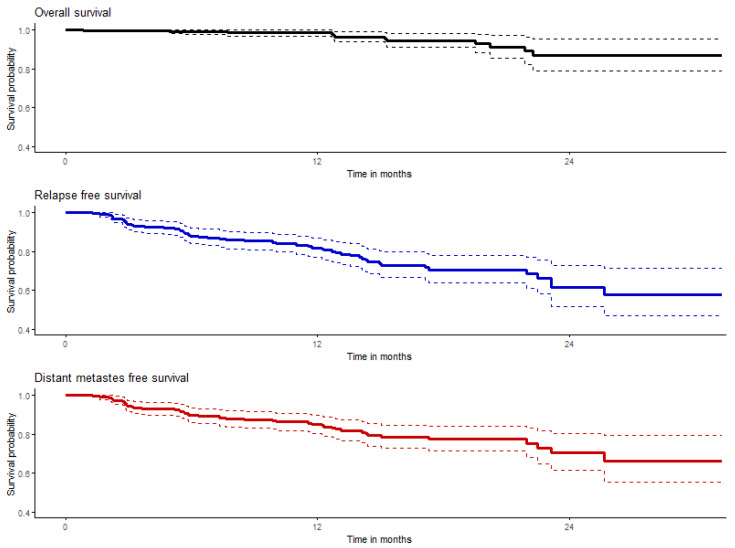
The probabilities of 2-year overall survival (OS), relapse-free survival (RFS), and distant-metastases-free survival (DMFS) were 86.7%, 61.4%, and 70.2%, respectively, in the entire study population of adjuvant-treated melanoma patients; these data are summarized in Table 4 and shown graphically in Figure 1. Medians were not reached at the time of analysis.
Figure 1.
Graphs of overall (black), relapse (blue), and distant-metastases-free survival (red); colored solid lines represent estimated probabilities of survival and dashed lines represent their confidence intervals.
3.4.3. Survival Depending on Patient and Melanoma Characteristics
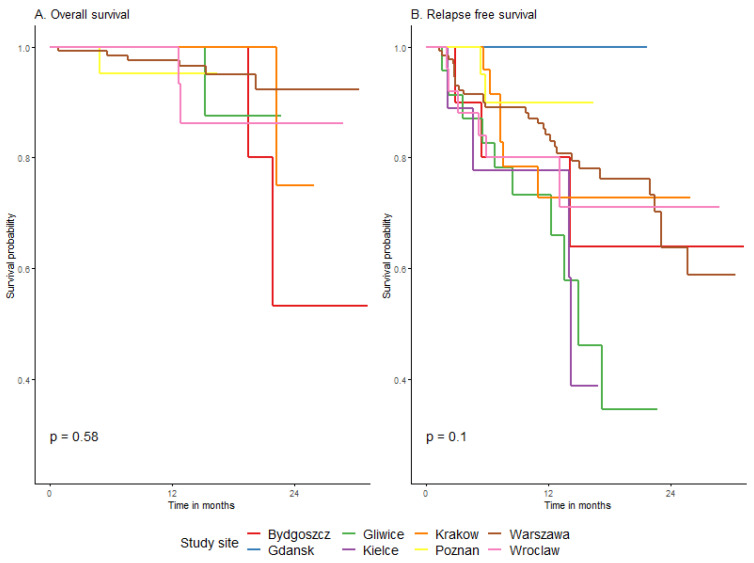
There was no significant association related to OS and RFS in the adjuvant treatment results with age, gender, comorbidities, or any other patient characteristics, as shown in Appendix B and Table A5 and Table A6. OS and RFS results of adjuvant treatment within our multicenter cohort were consistent; no significant differences in survival results were observed among eight melanoma centers (Appendix B, Figure A1).
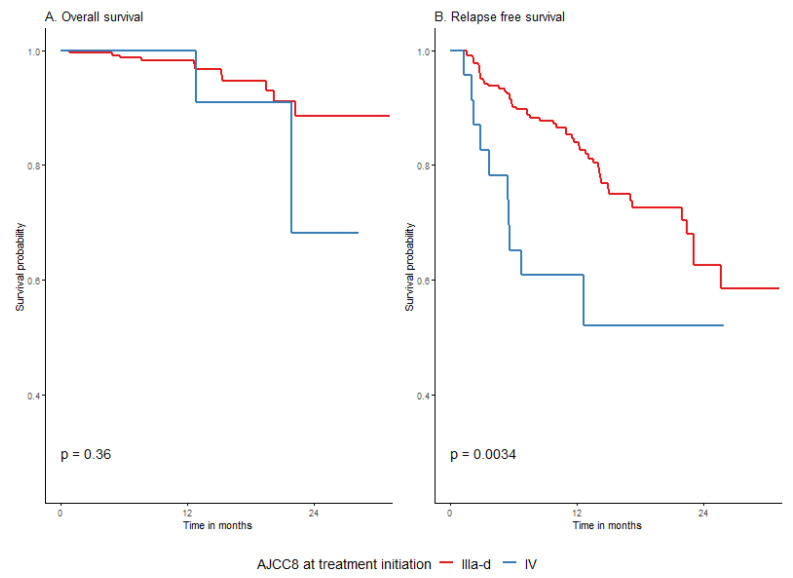
The disease stage before adjuvant treatment did not affect overall survival, but the RFS rate was significantly smaller for stage IV vs. stage IIIA-D at 52.2% (95% CI: 33.4–81.5%) vs. 62.5% (95% CI: 52.3–74.8%), p = 0.0033, respectively (Figure A2 in Appendix B).
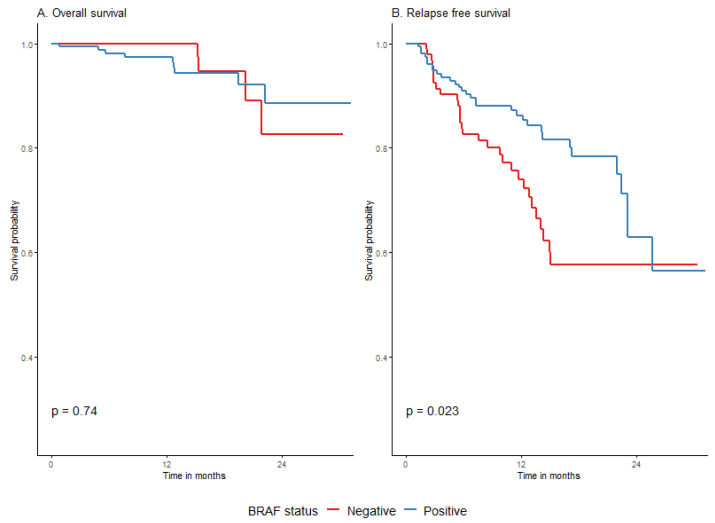
Patients with BRAF/+/melanomas had a higher probability of 2-year RFS compared to melanoma BRAF/−/patients at 62.9% (95% CI: 49.4–80%) vs. 57.7% (95% CI: 46.4–71.7%), respectively, p = 0.023. The relationship was no longer visible in OS analysis (88.6% (95% CI: 80.1–97.9%) vs. 82.7% (95% CI: 67.5–100%), p = 0.74) (Figure A3 in Appendix B).
3.4.4. Survival Association with Surgical Procedure before Adjuvant and the Adjuvant Type
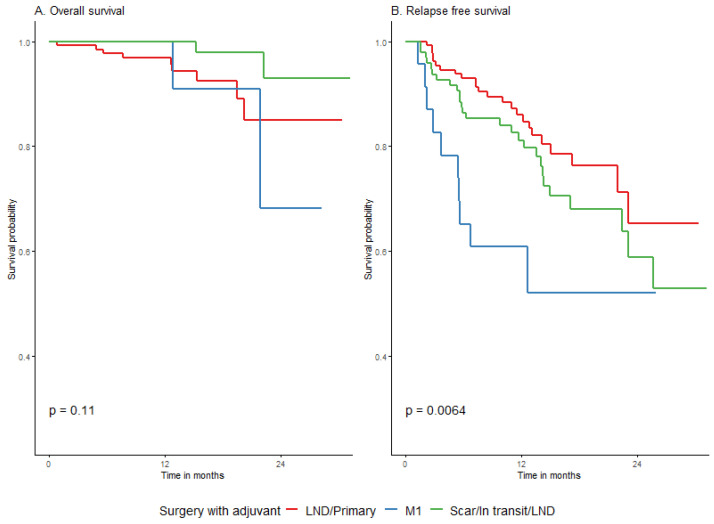
The type of surgery before adjuvant treatment of melanoma did not affect OS. Surgery of distant metastases had a negative effect only on the probability of relapse-free survival (p = 0.0062), although the difference decreased at the end of the 2-year observation period, with a 2-year RFS of 52.2% (95% CI: 33.4–81.5%) in the M1 group vs. 58.8% (95% CI: 45.3–76.5%) in the local recurrence group (Figure 2, Table 4). Of note, there was no significant difference in overall survival across the studied groups.
Figure 2.
Impact of surgery before adjuvant on overall (A) and relapse-free (B) survival.
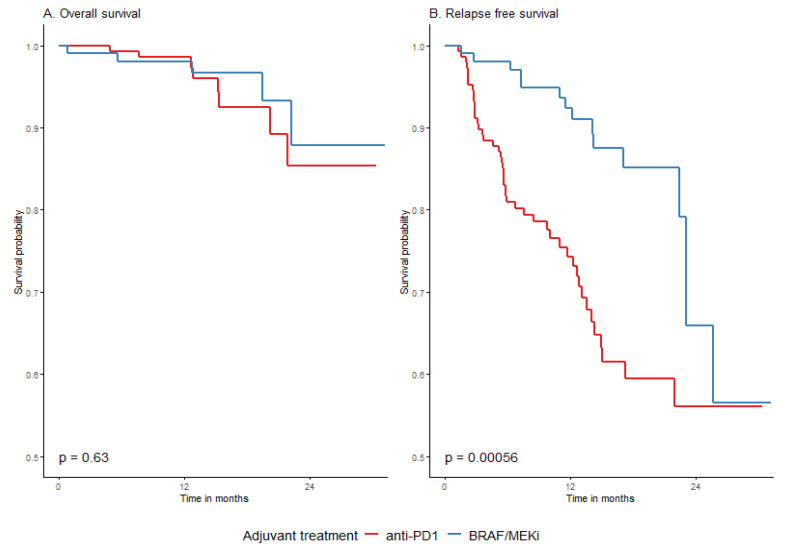
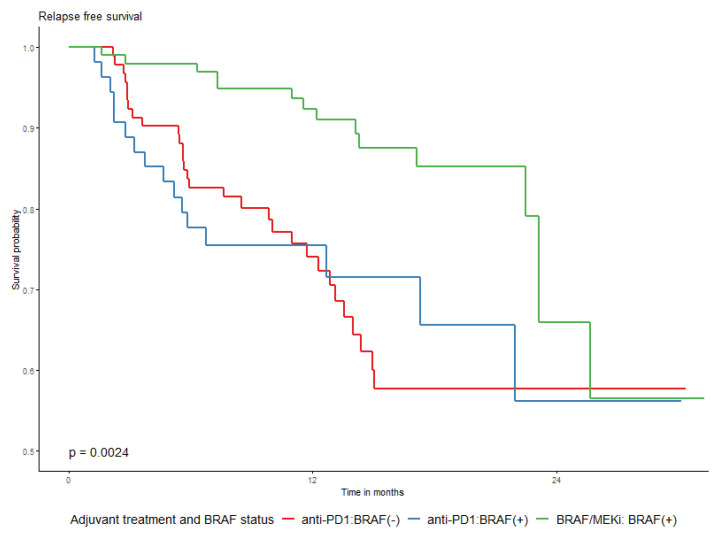
The type of drug used for adjuvant systemic therapy did not significantly influence the probability of 2-year OS in the whole group (p = 0.6318), as shown in Table 4 as well as in Table A5 and Table A6 Appendix B. A difference in RFS rates in relation to the used drugs was significantly unfavorable for anti-PD1 adjuvant treatment, especially at the beginning of the observation period, which is graphically presented in Figure 3. The 2-year RFS was 56.1% (45.6–69.1%) and 65.9% (48.4–89.7%) for anti-PD1 and BRAF/MEKi, respectively. This difference was evident both in the whole group and in the BRAF/+/group (Figure 4). The BRAF/+/melanoma patients had higher rates of 2-year recurrence-free survival (RFS) in the entire cohort, as well as in the DT-treated group, compared to those treated with anti-PD1 (Figure 4 and Figure A3 in Appendix B). The effects of TRAE on OS and RFS were similar across the subpopulations defined by the surgery before adjuvant treatment (Table A9, Appendix B).
Figure 3.
Overall (A) and relapse-free (B) survival in relation to the type of drugs used; DT vs. anti-PD1.
Figure 4.
Relapse-free survival by BRAF mutational status and adjuvant drug used; anti-PD1 or BRAF/MEKi.
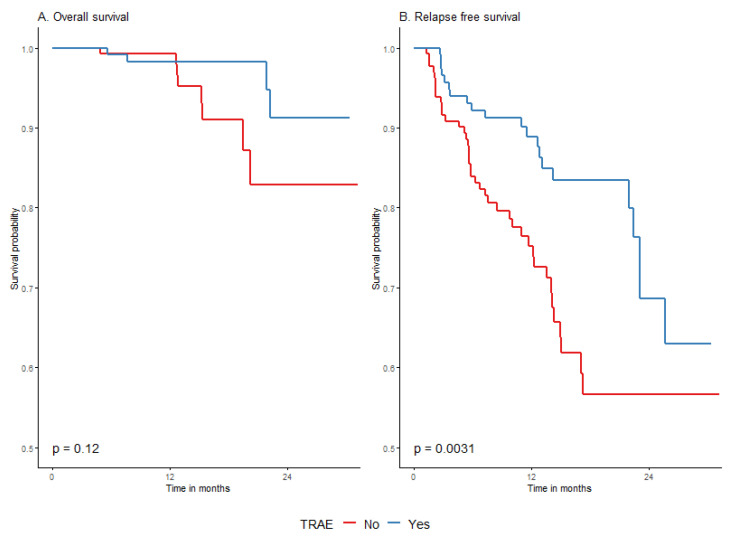
3.4.5. Survival According to Treatment-Related Adverse Events (TRAE)
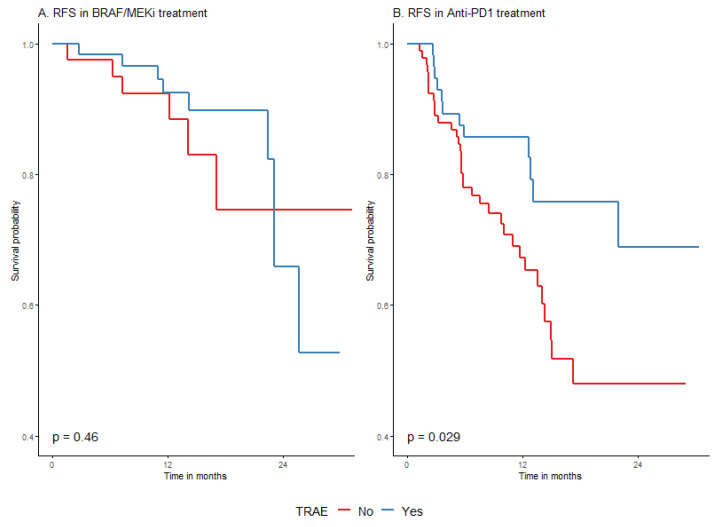
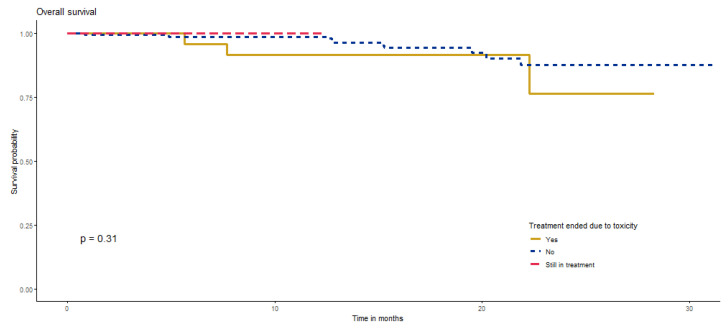
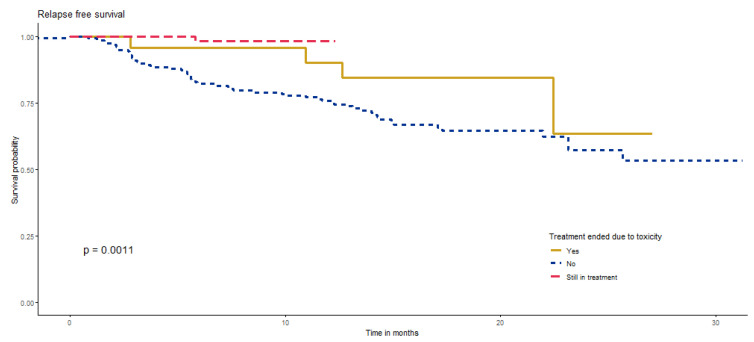
We have observed that the occurrence of TRAE was associated with significantly longer RFS in entire cohort. The 24-month RFS rate in patients with TRAE was 68.7% (55.5–84.9%) vs. 56.6% (45.8–70%) in patients without TRAE; p = 0.0031 (Figure 5). When analysis was performed in immunotherapy and targeted therapy groups separately, it appeared that the effect was basically restricted to RFS in a group treated with anti-PD1 (Figure 6 and Table A5, Appendix B). Survival probability without relapse was higher when adverse events occurred during or after anti-PD1 treatment (Figure 6). Treatment termination due to TRAE was also associated with longer RFS and did not affect OS (Figure A4 and Figure A5, Appendix B).
Figure 5.
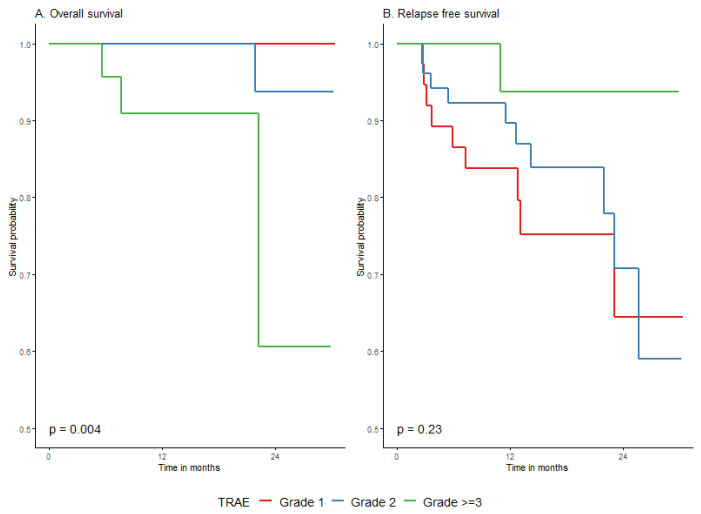
Overall survival (A) and relapse-free survival (B) by occurrence of TRAE.
Figure 6.
RFS by treatment-related adverse events (TRAE) in BRAF/MEKi (A) and anti-PD1 (B) groups.
According to our findings, grade ≥ 3 TRAE significantly worsened the overall survival prognosis (although not relapse-free survival). The 2-year OS rate decreased to 60.6% (26.9–100%), p = 0.004, as presented in Figure A6 and Table A6 in Appendix B.
Analysis of TRAE was not the focus of the current analysis, and we did not collect sufficient data to be able to investigate the topic of the differences in treatment dose intensity between the TRAE group and the non-TRAE group. We summarized the available data in Appendix C.
3.5. Prognostic Model
We have developed a prognostic model indicating that the difference in RFS between the two treatments is limited to the first months after the start of treatment, when there is a higher risk of progression for patients treated with anti-PD1 (HR 5.8, 95% CI 1.3–24.9; p = 0.0183), as shown in Table A3 in Appendix A. At longer follow-up (i.e., over 18 months), there is a reversed tendency (higher hazard for BRAF/MEK inhibitors), and the relationship disappears graphically, but it was not statistically significant, possibly due to a small number of cases with longer follow-up. To explore the possible impact of inter-site variability, a shared frailty model was fitted with the consistent results. The difference is not seen in the OS analysis in the above-mentioned groups.
3.6. Subgroup Analysis of Primary/LND Group
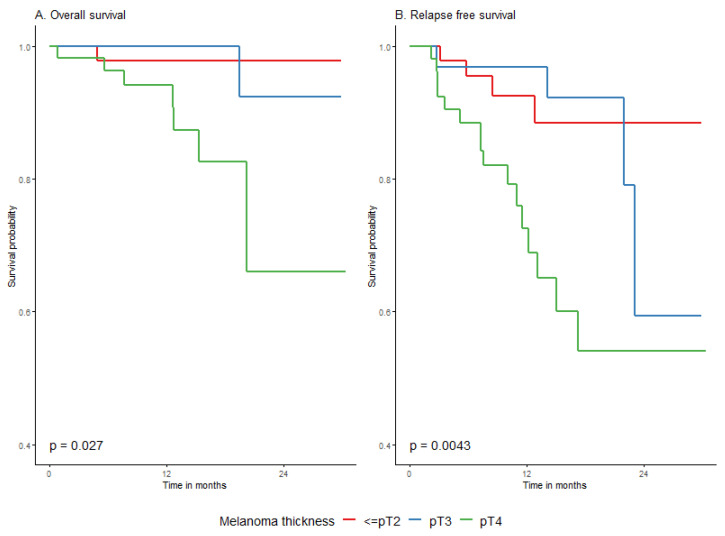
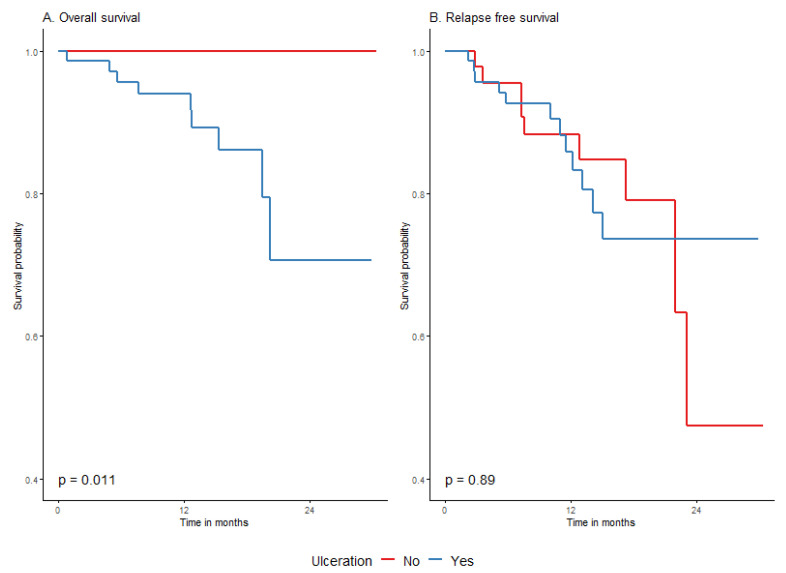
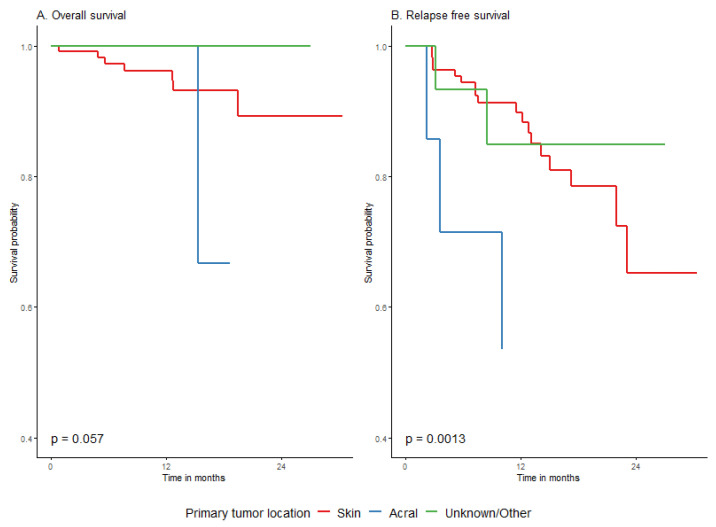
An additional subgroup analysis concerned the predictors of overall and relapse-free survival in the primary melanoma group (n = 130). We have observed a negative association of melanoma thickness above 4 mm with adjuvant treatment outcomes. Primary T4a-b melanomas had a significantly worse 2-year OS rate of 66% (41.3–100%) in comparison to 97.8% (93.6–100%) in ≤pT2 and 92.3% (78.9–100%) in pT3; p = 0.02. The respective 2-year RFS rates were 54% (37.8–77.1%), 88.4% (78–100%), and 59.3% (30.9–100%) (Table A7 and Table A8, Figure A7 in Appendix B). The absence of ulceration positively impacted the 2-year OS rate (100% vs. 70.6% (52.2–95.6%); p = 0.0114)), which is presented in Figure A8 in Appendix B. Acral melanomas had a significantly worse outcome of adjuvant treatment with respect to OS and RFS rates, as shown in Figure A9 in Appendix B.
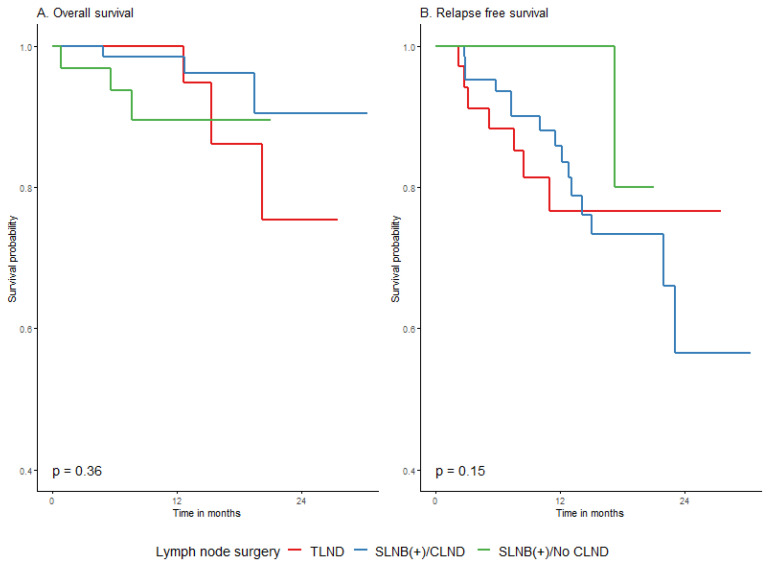
The type of lymph node surgery before adjuvant treatment (SLNB ± CLND or TLND) also did not influence the outcome with respect to OS and RFS in our cohort, as shown in Figure 7 and in Table A7 and Table A8 in Appendix B. In particular, not performing CLND after positive SLNB did not affect the results of adjuvant treatment in terms of RFS or OS. In the CLND group, compared to the no CLND group, the 2-year RFS was worse (56.5% (37.7–84.9%) vs. 80% (51.6–100%)), although the difference was not statistically significant.
Figure 7.
Impact of lymph node surgery prior to adjuvant treatment on overall (A) and relapse-free (B) survival in primary melanoma group.
4. Discussion
The locally advanced melanoma treatment paradigm is evolving. Adjuvant systemic treatment for patients with early-stage III-IV melanoma after radical resection became available worldwide since 2018 based on the results of randomized phase III clinical trials: Checkmate-238 (nivolumab vs. ipilimumab), Keynote-054 (pembrolizumab vs. placebo), and Combi-AD (dabrafenib plus trametinib vs. placebo) [8,9,10]. Additionally, melanoma adjuvant therapy’s scale of relapse risk reduction appeared to be the one of the highest among adjuvant treatments of solid tumors [6].
4.1. Approach to Adjuvant Melanoma Treatment Inclusion Criteria
In the meantime, for designing, conducting, and registering adjuvant clinical trials, a new approach to completion lymphadenectomy after positive sentinel lymph node biopsy and an actualized version of the AJCC Melanoma Staging System Eighth Edition were established. Polish patients started to receive adjuvant treatment since 2019 based on individual applications for emergency access to medical technologies. Inclusion criteria for melanoma adjuvant therapy in the Polish patient population were adjusted to those from registration trials but in adherence to the updated guidelines and to the new situation at some point. The main inclusion criteria adjustments for adjuvant therapy against all three registration trials included: staging―the AJCCv.8 classification was used (in contrast to AJCCv.7 in registration trials), non-mandatory CLND, a wider defined interval to adjuvant treatment start (restricted to 12–13 weeks from randomization in trials), and permission for radiotherapy. Patients with acral cutaneous subtype and mucosal melanoma could be enrolled (as in Keynote-054, but not in Checkmate-238 nor in Combi-AD). Resected in-transit metastases were allowed (as in Checkmate-238 and Combi-AD, but not in Keynote-054). Stage IIIA (as in Keynote-054 and Combi-AD, but not in Checkmate-238) and resected stage IV (as in Checkmate-238 but not in Keynote-054 nor in Combi-AD) were eligible. Adjuvant treatment with targeted therapy was not restricted to patients with melanoma with BRAF V600E or V600K mutations (as in the Combi-AD trial).
In light of a new approach toward melanoma staging and a lack of indications for completion of lymphadenectomy, we intended to provide evidence on the efficacy and safety of adjuvant melanoma treatment for patients not quite in line with those strictly meeting the inclusion criteria of randomized clinical trials. Since 2016, based on the Multicenter Selective Lymphadenectomy Trial II (MSLT-II), and since 2017, based on the German Dermatologic Cooperative Oncology Group trial (DeCOG-SLT), SLNB followed by CLND is no longer a standard of care for all patients with SLN-positive disease [1,2]. In those trials, no improvement in melanoma-specific survival (MSS) from CLND was demonstrated. Surgeons do not perform routine CLND after positive SNLB anymore, and they refer patients to adjuvant systemic treatment [14,15]. Omitting CLND leaves us without precise information on local disease burden. We are left with an overrepresented IIIA stage that has different risks than the former IIIA from the CLND era. Stages IIIB and IIIC might be underdiagnosed while CLND is no longer performed, and some of metastases stay occult. While AJCCv.8 staging was applied in the era of postponed CLND, prognosis estimates for IIIA and IIIB subgroups worsened due to occult disease burden in patients not having CLND after positive SLNB. Adjuvant therapy benefits across the stage III subgroups by AJCCv.8 classification may be even more profound as prognosis for the real-world patients is worse in comparison to the AJCC report, as shown in a German study based on the European population [16]. Adjuvant treatment outcomes in our dataset are in line with those of the Keynote-054 trial, where RFS benefits were confirmed for stage III subgroups adjusted according to AJCCv.8 [17].
The impact of the CLND approach shift on melanoma adjuvant treatment results was never investigated in a prospective manner, and it was only investigated in real-world datasets of national registries or smaller multicenter analysis, such as ours. In our study, we examined a group of patients, all of whom have received adjuvant treatment, regardless of nodal resection status (SLND+ and CLND− or CLND+ were allowed). We have shown that patients without CLND have the same adjuvant treatment outcomes as patients after CLND for positive SLNB. As completion lymphadenectomy influenced only the local control, not melanoma-specific survival, a possible lack of local control is not the reason for events during adjuvant therapy (the rate of distant metastases at relapse was 82% in our cohort) [1,2]. Our data on adjuvant treatment of melanoma patients without CLND support the rationale for de-escalation of surgery. CLND can be all the more omitted as adjuvant treatment is applied. Melanoma stage III patients with positive sentinel nodes benefit from adjuvant systemic treatment and not from surgery escalation [18]. In accordance with other findings, we advocate for the principle of delayed TLND in the case of local lymphatic recurrence [19,20,21]. In our cohort, TLND after the surveillance period did not compromise melanoma adjuvant treatment results. Eventually, any type of lymph node surgery before adjuvant treatment (SLNB ± CLND or TLND) did not influence the outcome with respect to OS and RFS in our analysis; only the resection of distant metastases had a negative impact due to the worse prognosis and the anticipated, less pronounced effect of adjuvant therapy in this group of patients [22].
A modern approach to CLND has changed our attitude toward SLNB, as nowadays, it serves as a therapeutic and staging procedure, qualifying patients for adjuvant treatment [4]. SLNB also offers a cure in itself, providing the possibility of local lymphatic basin control, and some patients could be cured only with the procedure of SLNB surgery [23,24]. According to some researchers, SLNB can eradicate the regional disease in three quarters of patients [4]. In our study, not performing SLNB with late TLND did not have a negative impact on RFS or on OS while applying adjuvant treatment.
4.2. Prognostic and Predictive Tools
The largest prospective randomized trial on long-term regional basin control after removal of a positive SNLB has pointed to the positive prognostic association with basin control of such variables as younger age, thinner primary melanoma, axillary basin, and metastasis diameter less than 1 mm with area less than 5% [23]. There is a need to determine who might be cured by SLNB alone (low-risk group) and who is at high risk (at risk of nonsentinel lymph node metastases) and might benefit from adjuvant therapy [18]. There are being developed various prognostic tools and nomograms trying to determine who is at high risk of nonsentinel lymph node metastases (NSLN) to determine who needs adjuvant therapy. Most of them elaborate extensively on clinicopathological features. A recent study performed by a Dutch–American group has shown that currently available predictive models are not efficient and not validated for single positive SLNB, and they show limited performance in this subgroup of patients [25]. Their study analyzed the specific patient group with a single positive SLN, and they did not find any model that would identify patients at high risk of NSLN in a group of single positive SLN. In their research, it appeared that predictions heavily relied on the number of positive sentinel nodes after SLNB. They found out that the rule of thumb (patients at high risk have multiple positive SLN) performed as well as their newly developed model based on age, Breslow thickness, mitotic rate, largest SLN metastasis diameter, and the number of positive SLN (single vs. more than one node). Accuracy in making predictions of the rule of thumb was comparable to the best-performing Bertolli nomogram based on Breslow thickness, SLN disease burden/diameter, and number of positive SLN [26].
Patients with stage III melanoma have a high risk of distant metastases, and prognostic tools for identifying those at high risk of them are needed. The problem of survival lies within a spectrum of distant metastases, and translational research implementing information from clinical, histopathological, serological, and molecular biomarkers would be of predictive and prognostic value [27,28]. Researchers from the Dutch–American group assume that exploitation of the clinicopathological features of SLN has reached its peak [25]. Scientists are looking closely at early-stage melanoma given that the biggest rate of deaths is attributed to thin melanomas (23%), while thick or metastatic-at-presentation melanomas are responsible for 14% and 16% of deaths, respectively [29]. It is of great importance to search for predictive tools to define patients of high risk of recurrence or death who would benefit the most from additional treatment interventions. Melanoma genetics, tumor microenvironment, and serological markers are being explored with a view to identify those at high risk of distant metastases for whom more than surgery, e.g., adjuvant treatment, should be offered [30,31,32,33]. There are studies on resistance to treatment dealing with issues of minimal residual disease and its heterogeneity, drug-tolerant phenotypes, genomics, and molecular aberrations [34,35,36,37]. In our study, we have also looked at the spectrum of lower susceptibility to adjuvant therapy, and we have found that such variables associated with primary melanoma, such as thickness, ulceration, and acral cutaneous and mucosal melanoma subtypes, were negatively associated with overall survival in our cohort in univariate and multivariate analyses.
4.3. Systemic Treatment Impact
Prediction tools guiding our decisions on adjuvant treatment start and on drug selection are important in consideration of a possible significant share of chronic side effects generated by adjuvant therapy, some of them permanent [38]. In our study, there was less than 10% of treatment-related adverse events (TRAE), and 13% of patients discontinued the treatment due to TRAE; these results are comparable to those from randomized trials. Nearly thirty percent of overall TRAE led to dosage reduction or treatment discontinuation, and this gave us indirect information about the quality of life of melanoma patients during adjuvant treatment. Based on our results, we know that toxicities might be detrimental. Grade three toxicities significantly worsen OS in our real-world population, giving us a guideline not to treat by all means despite toxicity. On the other hand, the occurrence of TRAE or treatment termination due to TRAE were associated with longer RFS but not OS for the anti-PD1 treated group in our study. A similar association was observed by other researchers interpreting randomized data on nivolumab and pembrolizumab in adjuvant treatment [39]. We have not observed an association between the occurrence of TRAE and survival in the BRAF/MEKi treated group. In our cohort, patients with melanoma of BRAF-positive mutational status were treated both with BRAF/MEKi and anti-PD1 drugs. We have shown short-term better control of melanoma relapses when RAF/MEKi were used in patients with BRAF-mutated melanomas; however, beyond 2 years, long-term outcomes overlap with immunotherapy with anti-PD1 antibodies. This result probably depends mainly upon the different modes of action of available adjuvant drugs and sparks a discussion on the topic of appropriate adjuvant therapy selection for melanoma BRAF-positive patients. BRAF/MEKi provide a rapid response, while anti-PD1 antibodies act slowly and may achieve a durable response [40]. No direct comparison between adjuvant targeted therapy and immunotherapy for melanoma BRAF-positive patients has been performed. Therefore, eligibility for the adjuvant treatment and adjuvant drug selection should be carefully assessed while discussing pros and cons with the patient. Such criteria as risk versus benefit estimation, possibility of chronic and permanent toxicities, discontinuation rate, contraindications, and registration indications and disease burden should be taken into consideration [6].
4.4. Real-World Analysis in Comparison to Registration Trials
The results of our study show that a real-world cohort of melanoma patients not fully represented in phase III clinical trials benefit from adjuvant treatment. Our 2-year RFS of 61.4% is comparable to outcomes for adjuvant treatment arms in randomized trials; the estimated 2-year RFS rate in Checkmate-238 was around 62% for nivolumab, in Keynote-054 it was 68% for pembrolizumab, and in Combi-AD it was 67% for dabrafenib plus trametinib. OS at 2 years for 86.7% of our cohort is similar to the results from Combi-AD and Checkmate-238, which were estimated 91% and 87%, respectively. In all three registration trials, only RFS rates demonstrated significant benefit for experimental arms, with no OS benefit shown. The recurrence-free survival rate is a measure of all relapses, both distant and local. In registration trials, 30–60% of recurrences were distant [6]. In our study, 82% of relapses were distant, including one fourth of M1a recurrences. Improving the RFS rate limits disease spread and reduces the number of patients with advanced disease. It is important to reduce the number of patients with advanced-stage melanoma [6]. In adjuvant trials, RFS with a hazard ratio (HR) of 0.77 or less can be a surrogate for OS [41]. In randomized adjuvant trials, to which our results are comparable, the HR was around 0.6. We assume, therefore, that our adjuvant treatment outcome (of comparable RFS to that obtained in registration trials) is likely to translate into a gain in overall survival. OS benefit is hard to achieve, if only because of the subsequent lines of effective melanoma treatment nowadays.
Real-world analysis can help us better understand the details and dilemmas of novel treatment methods or approaches outside clinical trials, bring valuable information on safety and efficacy of a certain treatment, and could be used to inform decisions for new drug approvals, indications, or population expansions [42]. Our data and analysis on adjuvant treatment outcomes in melanoma patients, along with other research reports, show that adjuvant treatment of melanoma in the real-world population reduces the risk of recurrence, even outside the criteria from the registration clinical trials [43,44,45]. We have shown better short-term control of melanoma relapses when DT was used in patients with BRAF-mutated melanoma. However, after approximately two years, the long-term DT effect overlaps with immunotherapy outcomes for this group of patients.
4.5. Strengths and Limitations
The strength of our study is the analysis of real-world data outside clinical trials supporting the value of adjuvant treatment of melanoma patients with inclusion criteria tailored according to new guidelines implemented during the registration process. This is a unique and detailed study examining a group of patients, all of whom have received adjuvant treatment regardless of nodal resection status; it shows that the type of lymph node surgery before adjuvant treatment does not influence the outcome of that treatment. In light of a new classification and new approach to CLND, our study confirmed that postponing CLND was justified and fits into a new landscape of adjuvant treatment.
Limitations of our analysis are its retrospective design, the relatively small number of patients in each subgroup, and short follow-up. Longer observation is needed to gain detailed information about recurrences, their treatment, and the influence of TRAE on survival.
5. Conclusions
Multicenter data collected from across Poland confirm that a modern approach to adjuvant treatment of melanoma patients is well tolerated and offers promising early overall survival and recurrence-free survival comparable with the results of registration clinical trials. Adjuvant treatment of melanoma in the real-world environment reduces the risk of recurrence and hence reduces morbidity of stage III and IV melanoma patients in the landscape of updated AJCCv.8 staging classification and CLND evasion, while not fully in line with inclusion criteria from phase III adjuvant melanoma treatment registration trials. Adjuvant therapy for melanoma patients offers the opportunity to safely de-escalate surgical treatment without the risk of worsening prognosis. Further follow-up is needed to evaluate long-term toxicities and outcomes of melanoma adjuvant treatment in this population.
Abbreviations
| AJCC | American Joint Committee on Cancer |
| BRAF/MEKi | BRAF/MEK inhibitors |
| CLND | completion lymphadenectomy |
| DMFS | distant-metastases-free survival |
| DT | dabrafenib plus trametinib |
| HR | hazard ratio |
| ICI | immune check point inhibitors |
| NSLN | nonsentinel lymph node metastases |
| OS | overall survival |
| RFS | relapse-free survival |
| SLNB | sentinel lymph node biopsy |
| TLND | therapeutic lymph node dissection |
| TRAE | treatment-related adverse events |
| WHO | World Health Organisation |
Appendix A
Appendix A.1. Prognostic Model for Relapse-free Survival
Appendix A.1.1. Model Building Strategy
The initial full model included basic characteristics of the patient (age, sex, WHO status), known prognostic factors (stage, BRAF status), as well as factors with p-value < 0.1 in univariable analysis.
Table A1.
Full model.
| Factor | Level | HR | 95% HR-Lower | 95% HR-Upper | p-Value (for Significance of HR Not Equal 1) | p-Value (Overall Significance of the Factor) |
|---|---|---|---|---|---|---|
| Sex | M vs. F | 1.8 | 1 | 3.1 | 0.049 | 0.046 |
| Age group | 45–54 vs. <45 | 2.4 | 1 | 5.9 | 0.0607 | 0.3009 |
| 55–64 vs. <45 | 1.6 | 0.7 | 3.7 | 0.2948 | ||
| 65+ vs. <45 | 1.4 | 0.6 | 3.4 | 0.4412 | ||
| WHO performance status | 1 vs. 0 | 1.8 | 0.9 | 3.4 | 0.09 | 0.0927 |
| BRAF mutational status | Positive vs. Negative | 0.9 | 0.5 | 1.9 | 0.8785 | 0.8783 |
| AJCC8 at treatment initiation | IIIB vs. IIIA | 1.8 | 0.4 | 9.4 | 0.4606 | 0.6945 |
| IIIC vs. IIIA | 2.2 | 0.5 | 9.7 | 0.3085 | ||
| IIID vs. IIIA | 1.7 | 0.3 | 11.6 | 0.5671 | ||
| IV vs. IIIA | 7.9 | 1.5 | 40.3 | 0.0136 | ||
| Indication group for adjuvant treatment | M1 vs. Primary/LND | NA | NA | NA | NA | 0.6182 |
| Scar/In transit/LND vs. Primary/LND | 1.2 | 0.6 | 2.2 | 0.6182 | ||
| Adjuvant treatment | Anti-PD1 vs. BRAF/MEKi | 1.7 | 0.8 | 3.3 | 0.2031 | 0.2044 |
| Grade of TRAE | 1 vs. No TRAE | 0.5 | 0.2 | 1 | 0.0617 | 0.0036 |
| 2 vs. No TRAE | 0.4 | 0.2 | 0.8 | 0.0092 | ||
| 3 vs. No TRAE | 0.1 | 0 | 1 | 0.0494 |
M1 is the main factor for AJCC8 as well as the surgery group for the adjuvant. These two variables were replaced by the stage IV vs. stages III indicator for further analysis. Next, the model was reduced to exclude the variables with p > 0.1, and the proportional hazard assumption was tested, indicating that the assumption does not hold for the class of the adjuvant (Table A2).
Table A2.
Proportionality of hazard assumption test results for reduced initial model.
| Chi-Square | df | p-Value | |
|---|---|---|---|
| Sex | 0.0439 | 1 | 0.834 |
| WHO performance status | 1.7243 | 1 | 0.189 |
| AJCC8 stage | 1.8538 | 1 | 0.173 |
| Adjuvant treatment | 4.7520 | 1 | 0.029 |
| Grade of TRAE | 0.5898 | 3 | 0.899 |
| GLOBAL | 8.9276 | 7 | 0.258 |
Due to the lack of proportionality of hazards for the class of adjuvant, the time-dependent coefficient was introduced to the model, stratifying the time since the treatment start into 6-month intervals. The last interval included observation times 18 months or longer, as only one event occurred after 24 months in our dataset.
Appendix A.1.2. Final model
Table A3.
Final model.
| Factor | Level | HR | 95% HR-Lower | 95% HR-Upper | p-Value (for Significance of HR Not Equal 1) | p-Value (Overall Significance of the Factor) |
|---|---|---|---|---|---|---|
| Sex | M vs. F | 1.9 | 1.1 | 3.3 | 0.0157 | 0.0138 |
| WHO status | 1 vs. 0 | 1.9 | 1 | 3.3 | 0.0372 | 0.0407 |
| AJCC8 stage | IV vs. IIIa–IIId | 3.2 | 1.5 | 6.8 | 0.0031 | 0.0067 |
| Grade of adverse events | G1 vs. none | 0.4 | 0.2 | 1 | 0.0382 | 0.0036 |
| G2 vs. none | 0.4 | 0.2 | 0.8 | 0.0102 | ||
| ≥G3 vs. none | 0.1 | 0 | 1 | 0.0514 | ||
| Class of adjuvant (by time since the beginning of treatment) | Anti-PD1 vs. BRAF/MEKi | 0.0096 | ||||
| <6 M | 5.8 | 1.3 | 24.9 | 0.0183 | ||
| 6–<12 M | 0.8 | 0.3 | 2.6 | 0.719 | ||
| 12–<18 M | 1.8 | 0.6 | 6 | 0.3052 | ||
| 18 M+ | 0.2 | 0 | 1.5 | 0.1123 |
This model already meets proportional hazard assumptions (p > 0.05 for all variables) (Table A4).
Table A4.
Proportionality of hazard assumption test results for final model.
| Chi-Square | df | p-Value | |
|---|---|---|---|
| Sex | 0.179 | 1 | 0.67 |
| WHO performance status | 0.692 | 1 | 0.41 |
| AJCC8 stage | 1.074 | 1 | 0.30 |
| Grade of TRAE | 0.305 | 3 | 0.96 |
| Adjuvant type: Time strata | 5.567 | 4 | 0.23 |
| GLOBAL | 9.334 | 10 | 0.50 |
Appendix B
Table A5.
Relapse-free survival in univariable analysis.
| n | n Events | Restricted Mean (se) | Median (95% CI) | 12 M Survival (95% CI) | 18 M Survival (95% CI) | 24 M Survival (95% CI) | HR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | female | 116 | 23 | 24.8 (1.2) | NA (23.1–NA) | 84.8% (78.1–92.1%) | 76.9% (68.3–86.7%) | 62.7% (45.9–85.5%) | Ref. | 0.1696 |
| male | 132 | 38 | 22.7 (1.1) | NA (23.1–NA) | 79.2% (72.3–86.8%) | 65.9% (56.6–76.8%) | 59.3% (48–73.3%) | 1.4 (0.9–2.4) | ||
| Age group | <45 | 62 | 10 | 26.4 (1.4) | NA (NA–NA) | 90.3% (83.2–98%) | 81% (69.8–93.9%) | 73.6% (58–93.4%) | Ref. | 0.3518 |
| 45–54 | 41 | 12 | 22.6 (2) | 25.7 (25.7–NA) | 79.6% (67.8–93.4%) | 68.4% (53.9–86.7%) | 68.4% (53.9–86.7%) | 1.9 (0.8–4.4) | ||
| 55–64 | 64 | 18 | 22.2 (1.7) | NA (17.1–NA) | 80.1% (69.9–91.8%) | 58.8% (44–78.4%) | 50.4% (33.2–76.5%) | 1.9 (0.9–4.2) | ||
| 65+ | 81 | 21 | 23.7 (1.4) | NA (23.1–NA) | 77.8% (68.9–87.8%) | 74% (64.4–85%) | 59.2% (42.1–83.1%) | 1.7 (0.8–3.6) | ||
| Comorbidities | No | 114 | 27 | 24.4 (1.1) | NA (NA–NA) | 84.5% (77.9–91.6%) | 72.5% (63.4–82.9%) | 64.7% (52.5–79.6%) | Ref. | 0.5045 |
| Yes | 134 | 34 | 23.1 (1.2) | NA (23.1–NA) | 79.4% (72.3–87.1%) | 68.9% (59.3–80.1%) | 58.3% (44.2–76.9%) | 1.2 (0.7–2) | ||
| BRAF mutational status | negative | 92 | 30 | 21.9 (1.4) | NA (14.9–NA) | 74% (65–84.2%) | 57.7% (46.4–71.7%) | 57.7% (46.4–71.7%) | Ref. | 0.0237 |
| positive | 155 | 31 | 24.7 (1) | NA (23.1–NA) | 86.3% (80.8–92.1%) | 78.4% (70.8–86.8%) | 62.9% (49.4–80%) | 0.6 (0.3–0.9) | ||
| WHO performance status | 0 | 161 | 37 | 24.4 (1) | NA (25.7–NA) | 85.5% (80–91.4%) | 71.9% (63.7–81.1%) | 65.5% (54.9–78.2%) | Ref. | 0.1753 |
| 1 | 87 | 24 | 22.4 (1.5) | NA (22.5–NA) | 74.7% (65.5–85.2%) | 69.3% (58.5–82%) | 52% (33.7–80.2%) | 1.4 (0.9–2.4) | ||
| AJCC8 stage at initiation of adjuvant treatment | IIIA | 16 | 2 | 27.6 (2.4) | NA (NA–NA) | 93.8% (82.6–100%) | 83.3% (64–100%) | 36.2% (15.7–83.7%) | Ref. | 0.0483 |
| IIIB | 56 | 11 | 23.5 (1.7) | 23.1 (22.5–NA) | 91.8% (84.2–100%) | 80.4% (67.6–95.7%) | 69.4% (60.6–79.5%) | 1.4 (0.3–6.4) | ||
| IIIC | 139 | 34 | 24.2 (1) | NA (NA–NA) | 81.5% (75–88.5%) | 69.4% (60.6–79.5%) | 66.3% (41–100%) | 1.8 (0.4–7.3) | ||
| IIID | 11 | 3 | 24 (3.5) | NA (11.5–NA) | 66.3% (41–100%) | 66.3% (41–100%) | 52.2% (33.4–81.5%) | 1.9 (0.3–11.7) | ||
| IV | 23 | 10 | 19 (2.9) | NA (5.6–NA) | 60.9% (43.9–84.5%) | 52.2% (33.4–81.5%) | 36.2% (15.7–83.7%) | 4.4 (1–20) | ||
| AJCC8 stage primary tumor, pT | T0-T2 | 84 | 16 | 25 (1.4) | NA (25.7–NA) | 87.1% (79.8–95%) | 78% (67.8–89.7%) | 71.5% (57.3–89.2%) | Ref. | 0.012 |
| T3a-b | 62 | 12 | 25.2 (1.5) | NA (23.1–NA) | 91.9% (85.4–99%) | 82.4% (72.3–93.9%) | 58.7% (39–88.5%) | 0.9 (0.4–1.9) | ||
| T4a-b | 93 | 31 | 21.4 (1.4) | NA (17.1–NA) | 68.7% (59.1–79.8%) | 55.4% (43.3–70.9%) | 55.4% (43.3–70.9%) | 2 (1.1–3.7) | ||
| Group of indications for adjuvant | LND/Primary | 130 | 24 | 25.2 (1.1) | NA (NA–NA) | 85.9% (79.7–92.7%) | 76.4% (67.6–86.4%) | 65.4% (50.9–83.9%) | Ref. | 0.0065 |
| M1 | 23 | 10 | 19 (2.9) | NA (5.6–NA) | 60.9% (43.9–84.5%) | 52.2% (33.4–81.5%) | 52.2% (33.4–81.5%) | 3.2 (1.5–6.6) | ||
| Scar/In transit/LND | 95 | 27 | 23.1 (1.3) | NA (23.1–NA) | 81.1% (73.4–89.7%) | 68% (57.5–80.4%) | 58.8% (45.3–76.5%) | 1.4 (0.8–2.5) | ||
| Adjuvant type | Anti-PD1 | 147 | 46 | 21.7 (1.1) | NA (17.3–NA) | 74.3% (67.1–82.2%) | 59.4% (49.9–70.8%) | 56.1% (45.6–69.1%) | Ref. | <0.0001 |
| BRAF/MEKi | 101 | 15 | 26.1 (1.2) | NA (23.1–NA) | 92.3% (87–98%) | 85.2% (77.1–94.2%) | 65.9% (48.4–89.7%) | 0.4 (0.2–0.7) | ||
| TRAE | no | 131 | 40 | 21.8 (1.2) | NA (17.1–NA) | 75.2% (67.7–83.5%) | 56.6% (45.8–70%) | 56.6% (45.8–70%) | Ref. | 0.0031 |
| yes | 115 | 21 | 25.8 (1) | NA (25.7–NA) | 88.9% (83.1–95.1%) | 83.4% (76.1–91.4%) | 68.7% (55.5–84.9%) | 0.5 (0.3–0.8) | ||
| Highest grade toxicity | 1 | 37 | 9 | 23.9 (1.9) | NA (23.1–NA) | 83.8% (72.7–96.5%) | 75.2% (61.1–92.4%) | 64.4% (44.7–92.9%) | Ref. | 0.2286 |
| 2 | 52 | 10 | 25.3 (1.4) | NA (25.7–NA) | 89.7% (81.4–98.8%) | 83.8% (73.3–95.9%) | 70.8% (53.9–92.9%) | 0.7 (0.3–1.8) | ||
| 3 | 23 | 1 | 29.2 (1.2) | NA (NA–NA) | 93.8% (82.6–100%) | 93.8% (82.6–100%) | 93.8% (82.6–100%) | 0.2 (0–1.5) | ||
| Toxicities after immuno | no | 91 | 34 | 19.3 (1.4) | 17.3 (14–NA) | 67.2% (57.5–78.6%) | 48% (35.5–65%) | 48% (35.5–65%) | Ref. | 0.0286 |
| yes | 56 | 12 | 24.3 (1.5) | NA (NA–NA) | 85.7% (77–95.4%) | 75.8% (63.6–90.4%) | 68.9% (53.3–89.1%) | 0.5 (0.3–0.9) | ||
| Toxicities after BRAF/MEKi | no | 40 | 6 | 26.4 (1.8) | NA (NA–NA) | 92.3% (84.3–100%) | 74.6% (56.9–97.9%) | 74.6% (56.9–97.9%) | Ref. | 0.4709 |
| yes | 59 | 9 | 26.4 (1.3) | NA (23.1–NA) | 92.4% (85.5–99.9%) | 89.8% (81.5–98.9%) | 65.8% (45.6–95%) | 0.7 (0.2–2) | ||
| Primary melanoma type | cutaneous | 215 | 49 | 24.1 (0.9) | NA (25.7–NA) | 84.7% (79.8–89.9%) | 72.7% (65.5–80.6%) | 61.3% (50.4–74.7%) | Ref. | <0.0001 |
| acral | 8 | 5 | 15.0 (4.1) | 10.0 (9.8–NA) | 30% (9.5–94.3%) | NA | NA | 4.2 (1.7–10.8) | ||
| mucosal | 3 | 3 | 4.1 (0.7) | 3.6 (2.9–NA) | NA | NA | NA | 13.6 (4–45.5) | ||
| FPI | 22 | 4 | 26.0 (2.3) | NA (NA–NA) | 85.2% (71–100%) | 78.1% (60.8–100%) | 78.1% (60.8–100%) | 0.7 (0.3–2.1) |
Table A6.
Overall survival in univariable analysis.
| n | n Events | Restricted Mean (se) | Median (95% CI) | 12 M Survival (95% CI) | 18 M Survival (95% CI) | 24 M Survival (95% CI) | HR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | female | 116 | 6 | 29.1 (0.9) | NA (NA–NA) | 98.3% (95.9–100%) | 94.7% (89.5–100%) | 84.2% (70.7–100%) | Ref. (-) | 0.8392 |
| male | 132 | 7 | 29.5 (0.6) | NA (NA–NA) | 98.4% (96.3–100%) | 94.2% (89.3–99.5%) | 88.1% (78.9–98.3%) | 0.9 (0.3–2.7) | ||
| Age group | <45 | 62 | 2 | 30.2 (0.7) | NA (NA–NA) | 98.1% (94.5–100%) | 98.1% (94.5–100%) | 93.2% (83.7–100%) | Ref. (-) | 0.6666 |
| 45–54 | 41 | 2 | 29.1 (1.5) | NA (NA–NA) | 100% (100–100%) | 96.3% (89.4–100%) | 80.2% (55.7–100%) | 1.6 (0.2–11.7) | ||
| 55–64 | 64 | 5 | 28 (1.3) | NA (NA–NA) | 98.4% (95.4–100%) | 91.4% (82.1–100%) | 74.8% (55.5–100%) | 2.7 (0.5–13.8) | ||
| 65+ | 81 | 4 | 29.9 (0.7) | NA (NA–NA) | 97.5% (94.2–100%) | 93.7% (87.8–100%) | 93.7% (87.8–100%) | 1.7 (0.3–9.1) | ||
| Comorbidities | No | 114 | 4 | 29.9 (0.6) | NA (NA–NA) | 100% (100–100%) | 98.6% (95.8–100%) | 88.4% (77.7–100%) | Ref. (-) | 0.1566 |
| Yes | 134 | 9 | 29 (0.8) | NA (NA–NA) | 97% (94.1–99.9%) | 90.5% (83.9–97.7%) | 86% (75.8–97.5%) | 2.3 (0.7–7.5) | ||
| BRAF mutational status | negative | 92 | 4 | 29.2 (1) | NA (NA–NA) | 100% (100–100%) | 94.6% (87.6–100%) | 82.7% (67.5–100%) | Ref. (-) | 0.7446 |
| positive | 155 | 9 | 29.4 (0.6) | NA (NA–NA) | 97.4% (94.8–99.9%) | 94.4% (90.3–98.6%) | 88.6% (80.1–97.9%) | 1.2 (0.4–4) | ||
| WHO performance status | 0 | 161 | 7 | 29.6 (0.6) | NA (NA–NA) | 99.3% (97.9–100%) | 97% (93.5–100%) | 86.5% (76.7–97.5%) | Ref. (-) | 0.2101 |
| 1 | 87 | 6 | 29.1 (0.9) | NA (NA–NA) | 96.6% (92.8–100%) | 89.5% (81.2–98.7%) | 89.5% (81.2–98.7%) | 2 (0.7–6) | ||
| AJCC8 stage at initiation of adjuvant treatment | IIIA | 16 | 0 | 31.3 (0) | NA (NA–NA) | 100% (100–100%) | 100% (100–100%) | 100% (100–100%) | Ref. (-) | 0.5055 |
| IIIB | 56 | 1 | 30.8 (0.5) | NA (NA–NA) | 98.2% (94.8–100%) | 98.2% (94.8–100%) | 98.2% (94.8–100%) | 9074122.1 (0-Inf) | ||
| IIIC | 139 | 9 | 29 (0.7) | NA (NA–NA) | 97.8% (95.3–100%) | 94% (89.3–99%) | 84.2% (73.5–96.6%) | 32889264.9 (0-Inf) | ||
| IIID | 11 | 1 | 28.1 (2.9) | NA (NA–NA) | 100% (100–100%) | 80% (51.6–100%) | 80% (51.6–100%) | 48063018.4 (0-Inf) | ||
| IV | 23 | 2 | 27.5 (2.3) | NA (21.9–NA) | 100% (100–100%) | 90.9% (75.4–100%) | 68.2% (37.6–100%) | 50160099.4 (0-Inf) | ||
| AJCC8 stage primary tumor, pT | T0-T2 | 84 | 2 | 30.6 (0.5) | NA (NA–NA) | 98.8% (96.5–100%) | 96.7% (92.2–100%) | 96.7% (92.2–100%) | Ref. (-) | 0.0204 |
| T3a-b | 62 | 2 | 30.3 (0.7) | NA (NA–NA) | 100% (100–100%) | 97.2% (92–100%) | 92.6% (82.9–100%) | 1.1 (0.2–8.1) | ||
| T4a-b | 93 | 9 | 27.1 (1.3) | NA (22.3–NA) | 96.6% (93–100%) | 90% (82.2–98.6%) | 68% (48.8–94.8%) | 5 (1.1–23.1) | ||
| Group of indications for adjuvant | LND/Primary | 130 | 9 | 28.8 (0.8) | NA (NA–NA) | 96.8% (93.8–99.9%) | 92.4% (86.8–98.4%) | 85.1% (74.6–97%) | Ref. (-) | 0.1128 |
| M1 | 23 | 2 | 27.5 (2.3) | NA (21.9–NA) | 100% (100–100%) | 90.9% (75.4–100%) | 68.2% (37.6–100%) | 1.3 (0.3–5.9) | ||
| Scar/In transit/LND | 95 | 2 | 30.5 (0.5) | NA (NA–NA) | 100% (100–100%) | 97.8% (93.7–100%) | 92.9% (83.3–100%) | 0.2 (0.1–1.1) | ||
| Adjuvant type | Anti-PD1 | 147 | 8 | 29.1 (0.7) | NA (NA–NA) | 98.5% (96.6–100%) | 92.5% (86.6–98.8%) | 85.3% (74.9–97.2%) | Ref. (-) | 0.6318 |
| Inh BRAF/MEK | 101 | 5 | 29.6 (0.7) | NA (NA–NA) | 98% (95.3–100%) | 96.7% (93–100%) | 87.8% (76.2–100%) | 0.8 (0.2–2.3) | ||
| TRAE | no | 131 | 8 | 28.7 (0.9) | NA (NA–NA) | 99.2% (97.8–100%) | 91% (84–98.6%) | 82.8% (71.1–96.5%) | Ref. (-) | 0.1207 |
| yes | 115 | 4 | 30.2 (0.5) | NA (NA–NA) | 98.2% (95.8–100%) | 98.2% (95.8–100%) | 91.2% (82–100%) | 0.4 (0.1–1.3) | ||
| Highest grade toxicity | 1 | 37 | 0 | 30.4 (0) | NA (NA–NA) | 100% (100–100%) | 100% (100–100%) | 100% (100–100%) | Ref. (-) | 0.004 |
| 2 | 52 | 1 | 29.8 (0.5) | NA (NA–NA) | 100% (100–100%) | 100% (100–100%) | 93.8% (82.6–100%) | 186760662.3 (0-Inf) | ||
| 3 | 23 | 3 | 25.8 (2.4) | NA (22.3–NA) | 90.9% (79.5–100%) | 90.9% (79.5–100%) | 60.6% (26.9–100%) | 2022430985.8 (0-Inf) | ||
| Toxicities after immuno | no | 91 | 6 | 27.7 (1) | NA (NA–NA) | 98.9% (96.8–100%) | 88.6% (79.2–99.1%) | 81.8% (67.4–99.2%) | Ref. (-) | 0.253 |
| yes | 56 | 2 | 29.3 (0.7) | NA (NA–NA) | 98% (94.2–100%) | 98% (94.2–100%) | 91% (78.3–100%) | 0.4 (0.1–2) | ||
| Toxicities after BRAF/MEKi | no | 40 | 2 | 29.3 (1.3) | NA (NA–NA) | 100% (100–100%) | 96.3% (89.4–100%) | 85.6% (67.2–100%) | Ref. (-) | 0.4335 |
| yes | 59 | 2 | 30.2 (0.7) | NA (NA–NA) | 98.3% (95.1–100%) | 98.3% (95.1–100%) | 91.3% (78.6–100%) | 0.5 (0.1–3.4) | ||
| Primary melanoma type | cutaneous | 215 | 10 | 29.6 (0.5) | NA (NA–NA) | 98.1% (96.2–100%) | 95.5% (92.1–99.0%) | 88.2% (80.0–97.3%) | Ref. (-) | 0.0396 |
| acral | 8 | 2 | 18.6 (1.3) | 20.2 (15.3–NA) | 100% (100–100%) | 66.7% (30.0–100%) | NA | 6.8 (1.4–31.9) | ||
| mucosal | 3 | 0 | 31.3 (NA) | NA (NA–NA) | NA | NA | NA | 0 (0-Inf) | ||
| FPI | 22 | 1 | 29.8 (1.4) | NA (NA–NA) | 100% (100–100%) | 92.3% (78.9–100%) | 92.3% (78.9–100%) | 0.8 (0.1–6.5) |
Figure A1.
Overall (A) and relapse-free (B) survival curves by study site.
Figure A2.
Overall (A) and relapse-free (B) survival curves by AJCC8 stage at treatment initiation.
Figure A3.
Overall (A) and relapse-free (B) survival curves by BRAF mutational status.
Figure A4.
OS by whether treatment was interrupted due to toxicity.
Figure A5.
RFS by whether treatment was interrupted due to toxicity.
Figure A6.
Overall (A) and relapse-free (B) survival curves among patients who experienced treatment adverse events, by the grade of event.
Table A7.
Relapse-free survival in primary surgery group.
| n | n Events | Restricted Mean (se) | Median (95% CI) | 12 M Survival (95% CI) | 18 M Survival (95% CI) | 24 M Survival (95% CI) | HR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | female | 54 | 6 | 27.2 (1.2) | (−) | 86% (75.9–97.4%) | 86% (75.9–97.4%) | 86% (75.9–97.4%) | Ref. (-) | 0.1303 |
| male | 76 | 18 | 23.3 (1.4) | (22−) | 85.9% (78.1–94.5%) | 70.2% (57.9–85%) | 56.1% (39–80.8%) | 2 (0.8–5.1) | ||
| Age group | <45 | 36 | 3 | 27.1 (1.7) | (22−) | 100% (100–100%) | 87.7% (73–100%) | 73.1% (48.9–100%) | Ref. (-) | 0.0765 |
| 45–54 | 27 | 4 | 26.5 (1.8) | (−) | 92.3% (82.5–100%) | 80.7% (64.8–100%) | 80.7% (64.8–100%) | 1.7 (0.4–7.8) | ||
| 55–64 | 30 | 5 | 24.9 (2.2) | (17.3−) | 82.3% (67.4–100%) | 70.5% (49.1–100%) | 70.5% (49.1–100%) | 2.3 (0.5–9.7) | ||
| 65+ | 37 | 12 | 21.5 (2.1) | (23.1−) | 70.6% (56.7–87.9%) | 66.4% (51.7–85.2%) | 53.1% (32.1–88%) | 4.2 (1.2–14.9) | ||
| Comorbidities | No | 64 | 11 | 25.1 (1.4) | (22−) | 92.6% (85.8–99.9%) | 76% (63.5–90.8%) | 65.1% (45.8–92.5%) | Ref. (-) | 0.4491 |
| Yes | 66 | 13 | 24.4 (1.5) | (23.1−) | 79.1% (69–90.8%) | 79.1% (69–90.8%) | 65.9% (44.9–96.7%) | 1.4 (0.6–3.1) | ||
| BRAF mutational status | negative | 35 | 13 | 20.6 (2.1) | (13.1−) | 69.1% (54.6–87.3%) | 56% (40.1–78.4%) | 56% (40.1–78.4%) | Ref. (-) | 0.0041 |
| positive | 94 | 11 | 25.7 (1.4) | (23.1−) | 92.5% (86.8–98.6%) | 84.9% (75.4–95.6%) | 61.9% (39.1–98%) | 0.3 (0.1–0.7) | ||
| WHO performance status | 0 | 90 | 14 | 25.4 (1.2) | (−) | 91.5% (85.7–97.8%) | 79.9% (69.6–91.9%) | 67.1% (50.7–88.8%) | Ref. (-) | 0.0964 |
| 1 | 40 | 10 | 23.3 (1.9) | (−) | 73% (58.9–90.4%) | 68.4% (53.4–87.7%) | 68.4% (53.4–87.7%) | 2 (0.9–4.5) | ||
| AJCC8 stage at initiation of adjuvant treatment | IIIA | 15 | 1 | 28.4 (1.8) | (−) | 100% (100–100%) | 88.9% (70.6–100%) | 88.9% (70.6–100%) | Ref. (-) | 0.2252 |
| IIIB | 23 | 2 | 25.2 (2.1) | 23.1 (22−) | 100% (100–100%) | 100% (100–100%) | 33.3% (6.7–100%) | 1.2 (0.1–13.3) | ||
| IIIC | 87 | 19 | 24.2 (1.2) | (−) | 82.5% (74.5–91.4%) | 70.9% (59.8–84%) | 70.9% (59.8–84%) | 3.3 (0.4–24.4) | ||
| IIID | 5 | 2 | 17.6 (5.2) | 11.5 (11−) | 33.3% (6.7–100%) | 33.3% (6.7–100%) | 33.3% (6.7–100%) | 5.9 (0.5–66.2) | ||
| AJCC8 stage primary tumor, pT | T0-T2 | 45 | 4 | 27.8 (1.2) | (−) | 92.4% (84.4–100%) | 88.4% (78–100%) | 88.4% (78–100%) | Ref. (-) | 0.0043 |
| T3a-b | 32 | 4 | 26.2 (1.8) | (23.1−) | 96.9% (91–100%) | 92.3% (82.3–100%) | 59.3% (30.9–100%) | 1.1 (0.3–4.3) | ||
| T4a-b | 53 | 16 | 21 (1.8) | (15−) | 72.5% (59.8–87.8%) | 54% (37.8–77.1%) | 54% (37.8–77.1%) | 3.9 (1.3–11.8) | ||
| Primary melanoma type | cutaneous | 108 | 18 | 25 (1.1) | (23.1−) | 89.7% (83.8–96.1%) | 78.5% (69–89.3%) | 65.2% (48.8–87.1%) | Ref. (-) | <0.0001 |
| acral | 6 | 3 | 17.4 (5) | 11 (10−) | 41.7% (14.7–100%) | 41.7% (14.7–100%) | 41.7% (14.7–100%) | 4.7 (1.3–16.3) | ||
| mucosal | 1 | 1 | 3.6 (0) | 3.6 (−) | 0% (NA–NA%) | 0% (NA–NA%) | 0% (NA–NA%) | 23.5 (2.8–198.2) | ||
| FPI | 15 | 2 | 26.7 (2.4) | (−) | 84.8% (67.4–100%) | 84.8% (67.4–100%) | 84.8% (67.4–100%) | 0.8 (0.2–3.4) | ||
| Ulceration | No | 44 | 9 | 23.6 (1.8) | 23.1 (22−) | 88.2% (79–98.5%) | 79.1% (65.3–95.8%) | 47.4% (22.6–99.5%) | Ref. (-) | 0.8919 |
| Yes | 69 | 12 | 25 (1.4) | (−) | 85.8% (76.8–95.7%) | 73.6% (60.9–88.8%) | 73.6% (60.9–88.8%) | 0.9 (0.4–2.2) | ||
| LND group | No SLNB/TLND | 34 | 7 | 24.7 (1.9) | (−) | 76.6% (62.4–94%) | 76.6% (62.4–94%) | 76.6% (62.4–94%) | Ref. (-) | 0.1489 |
| SLNB(+)/CLND | 62 | 15 | 23.6 (1.5) | (23.1−) | 85.8% (77.1–95.5%) | 73.3% (61.5–87.4%) | 56.5% (37.7–84.9%) | 1 (0.4–2.5) | ||
| SLNB(+)/No CLND | 32 | 1 | 27.8 (2.3) | (−) | 100% (100–100%) | 80% (51.6–100%) | 80% (51.6–100%) | 0.2 (0–1.4) | ||
| Adjuvant type | Anti-PD1 | 67 | 18 | 22.1 (1.6) | (17.3−) | 77.7% (67.3–89.6%) | 63.5% (49.5–81.3%) | 56.4% (40.2–79.2%) | Ref. (-) | 0.0056 |
| Inh BRAF/MEK | 63 | 6 | 26.9 (1.4) | (23.1−) | 94.3% (88.2–100%) | 88.9% (79.9–98.9%) | 71.1% (45.3–100%) | 0.3 (0.1–0.7) | ||
| TRAE | no | 67 | 15 | 23 (1.6) | (17.3−) | 80.3% (70.3–91.9%) | 63% (47.5–83.6%) | 63% (47.5–83.6%) | Ref. (-) | 0.0599 |
| yes | 61 | 9 | 26.2 (1.2) | (−) | 91.2% (84–99%) | 86.4% (77.3–96.5%) | 71.2% (53.3–95.2%) | 0.5 (0.2–1.1) | ||
| Highest grade toxicity | 1 | 21 | 5 | 24.7 (2.2) | (23.1−) | 90.5% (78.8–100%) | 77% (59.2–100%) | 64.2% (41.1–100%) | Ref. (-) | 0.2152 |
| 2 | 22 | 4 | 24.2 (2.6) | (22−) | 83.9% (68.4–100%) | 83.9% (68.4–100%) | 55.9% (24.5–100%) | 1.1 (0.3–4.1) | ||
| 3 | 15 | 0 | 30.4 (0) | (−) | 100% (100–100%) | 100% (100–100%) | 100% (100–100%) | 0 (0-Inf) | ||
| Toxicities after immuno | no | 43 | 12 | 21.5 (2.1) | (15−) | 71.5% (57.3–89.1%) | 57.2% (39.1–83.6%) | 57.2% (39.1–83.6%) | Ref. (-) | 0.5184 |
| yes | 24 | 6 | 23.4 (2.4) | (22−) | 87.5% (75.2–100%) | 72.9% (54.3–97.9%) | 60.8% (38.2–96.6%) | 0.7 (0.3–1.9) | ||
| Toxicities after BRAF/MEKi | no | 24 | 3 | 25.8 (2.2) | (−) | 95.5% (87.1–100%) | 76.6% (55.3–100%) | 76.6% (55.3–100%) | Ref. (-) | 0.2003 |
| yes | 37 | 3 | 27.4 (1.4) | (23.1−) | 93.8% (85.7–100%) | 93.8% (85.7–100%) | 75% (47.9–100%) | 0.3 (0.1–2) |
Table A8.
Overall survival in primary surgery group.
| n | n Events | Restricted Mean (se) | Median (95% CI) | 12 M Survival (95% CI) | 18 M Survival (95% CI) | 24 M Survival (95% CI) | HR (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | female | 116 | 6 | 29.1 (0.9) | NA (NA-NA) | 98.3% (95.9–100%) | 94.7% (89.5–100%) | 84.2% (70.7–100%) | Ref. (-) | 0.8392 |
| male | 132 | 7 | 29.5 (0.6) | NA (NA-NA) | 98.4% (96.3–100%) | 94.2% (89.3–99.5%) | 88.1% (78.9–98.3%) | 0.9 (0.3–2.7) | ||
| Age group | <45 | 62 | 2 | 30.2 (0.7) | NA (NA-NA) | 98.1% (94.5–100%) | 98.1% (94.5–100%) | 93.2% (83.7–100%) | Ref. (-) | 0.6666 |
| 45–54 | 41 | 2 | 29.1 (1.5) | NA (NA-NA) | 100% (100–100%) | 96.3% (89.4–100%) | 80.2% (55.7–100%) | 1.6 (0.2–11.7) | ||
| 55–64 | 64 | 5 | 28 (1.3) | NA (NA-NA) | 98.4% (95.4–100%) | 91.4% (82.1–100%) | 74.8% (55.5–100%) | 2.7 (0.5–13.8) | ||
| 65+ | 81 | 4 | 29.9 (0.7) | NA (NA-NA) | 97.5% (94.2–100%) | 93.7% (87.8–100%) | 93.7% (87.8–100%) | 1.7 (0.3–9.1) | ||
| Comorbidities | No | 114 | 4 | 29.9 (0.6) | NA (NA-NA) | 100% (100–100%) | 98.6% (95.8–100%) | 88.4% (77.7–100%) | Ref. (-) | 0.1566 |
| Yes | 134 | 9 | 29 (0.8) | NA (NA-NA) | 97% (94.1–99.9%) | 90.5% (83.9–97.7%) | 86% (75.8–97.5%) | 2.3 (0.7–7.5) | ||
| BRAF mutational status | negative | 92 | 4 | 29.2 (1) | NA (NA-NA) | 100% (100–100%) | 94.6% (87.6–100%) | 82.7% (67.5–100%) | Ref. (-) | 0.7446 |
| positive | 155 | 9 | 29.4 (0.6) | NA (NA-NA) | 97.4% (94.8–99.9%) | 94.4% (90.3–98.6%) | 88.6% (80.1–97.9%) | 1.2 (0.4–4) | ||
| WHO performance status | 0 | 161 | 7 | 29.6 (0.6) | NA (NA-NA) | 99.3% (97.9–100%) | 97% (93.5–100%) | 86.5% (76.7–97.5%) | Ref. (-) | 0.2101 |
| 1 | 87 | 6 | 29.1 (0.9) | NA (NA-NA) | 96.6% (92.8–100%) | 89.5% (81.2–98.7%) | 89.5% (81.2–98.7%) | 2 (0.7–6) | ||
| AJCC8 stage at initiation of adjuvant treatment | IIIA | 16 | 0 | 31.3 (0) | NA (NA-NA) | 100% (100–100%) | 100% (100–100%) | 100% (100–100%) | Ref. (-) | 0.5055 |
| IIIB | 56 | 1 | 30.8 (0.5) | NA (NA-NA) | 98.2% (94.8–100%) | 98.2% (94.8–100%) | 98.2% (94.8–100%) | 9074122.1 (0-Inf) | ||
| IIIC | 139 | 9 | 29 (0.7) | NA (NA-NA) | 97.8% (95.3–100%) | 94% (89.3–99%) | 84.2% (73.5–96.6%) | 32889264.9 (0-Inf) | ||
| IIID | 11 | 1 | 28.1 (2.9) | NA (NA-NA) | 100% (100–100%) | 80% (51.6–100%) | 80% (51.6–100%) | 48063018.4 (0-Inf) | ||
| IV | 23 | 2 | 27.5 (2.3) | NA (21.9-NA) | 100% (100–100%) | 90.9% (75.4–100%) | 68.2% (37.6–100%) | 50160099.4 (0-Inf) | ||
| AJCC8 stage primary tumor, pT | T0-T2 | 84 | 2 | 30.6 (0.5) | NA (NA-NA) | 98.8% (96.5–100%) | 96.7% (92.2–100%) | 96.7% (92.2–100%) | Ref. (-) | 0.0204 |
| T3a-b | 62 | 2 | 30.3 (0.7) | NA (NA-NA) | 100% (100–100%) | 97.2% (92–100%) | 92.6% (82.9–100%) | 1.1 (0.2–8.1) | ||
| T4a-b | 93 | 9 | 27.1 (1.3) | NA (22.3-NA) | 96.6% (93–100%) | 90% (82.2–98.6%) | 68% (48.8–94.8%) | 5 (1.1–23.1) | ||
| Group of indications for adjuvant | LND/Primary | 130 | 9 | 28.8 (0.8) | NA (NA-NA) | 96.8% (93.8–99.9%) | 92.4% (86.8–98.4%) | 85.1% (74.6–97%) | Ref. (-) | 0.1128 |
| M1 | 23 | 2 | 27.5 (2.3) | NA (21.9-NA) | 100% (100–100%) | 90.9% (75.4–100%) | 68.2% (37.6–100%) | 1.3 (0.3–5.9) | ||
| Scar/In transit/LND | 95 | 2 | 30.5 (0.5) | NA (NA-NA) | 100% (100–100%) | 97.8% (93.7–100%) | 92.9% (83.3–100%) | 0.2 (0.1–1.1) | ||
| Adjuvant type | Anti-PD1 | 147 | 8 | 29.1 (0.7) | NA (NA-NA) | 98.5% (96.6–100%) | 92.5% (86.6–98.8%) | 85.3% (74.9–97.2%) | Ref. (-) | 0.6318 |
| Inh BRAF/MEK | 101 | 5 | 29.6 (0.7) | NA (NA-NA) | 98% (95.3–100%) | 96.7% (93–100%) | 87.8% (76.2–100%) | 0.8 (0.2–2.3) | ||
| TRAE | no | 131 | 8 | 28.7 (0.9) | NA (NA-NA) | 99.2% (97.8–100%) | 91% (84–98.6%) | 82.8% (71.1–96.5%) | Ref. (-) | 0.1207 |
| yes | 115 | 4 | 30.2 (0.5) | NA (NA-NA) | 98.2% (95.8–100%) | 98.2% (95.8–100%) | 91.2% (82–100%) | 0.4 (0.1–1.3) | ||
| Highest grade toxicity | 1 | 37 | 0 | 30.4 (0) | NA (NA-NA) | 100% (100–100%) | 100% (100–100%) | 100% (100–100%) | Ref. (-) | 0.004 |
| 2 | 52 | 1 | 29.8 (0.5) | NA (NA-NA) | 100% (100–100%) | 100% (100–100%) | 93.8% (82.6–100%) | 186760662.3 (0-Inf) | ||
| 3 | 23 | 3 | 25.8 (2.4) | NA (22.3-NA) | 90.9% (79.5–100%) | 90.9% (79.5–100%) | 60.6% (26.9–100%) | 2022430985.8 (0-Inf) | ||
| Toxicities after immuno | no | 91 | 6 | 27.7 (1) | NA (NA-NA) | 98.9% (96.8–100%) | 88.6% (79.2–99.1%) | 81.8% (67.4–99.2%) | Ref. (-) | 0.253 |
| yes | 56 | 2 | 29.3 (0.7) | NA (NA-NA) | 98% (94.2–100%) | 98% (94.2–100%) | 91% (78.3–100%) | 0.4 (0.1–2) | ||
| Toxicities after BRAF/MEKi | no | 40 | 2 | 29.3 (1.3) | NA (NA-NA) | 100% (100–100%) | 96.3% (89.4–100%) | 85.6% (67.2–100%) | Ref. (-) | 0.4335 |
| yes | 59 | 2 | 30.2 (0.7) | NA (NA-NA) | 98.3% (95.1–100%) | 98.3% (95.1–100%) | 91.3% (78.6–100%) | 0.5 (0.1–3.4) | ||
| Primary melanoma type | cutaneous | 215 | 10 | 29.6 (0.5) | NA (NA-NA) | 98.1% (96.2–100%) | 95.5% (92.1–99.0%) | 88.2% (80.0–97.3%) | Ref. (-) | 0.0396 |
| acral | 8 | 2 | 18.6 (1.3) | 20.2 (15.3-NA) | 100% (100–100%) | 66.7% (30.0–100%) | NA | 6.8 (1.4–31.9) | ||
| mucosal | 3 | 0 | 31.3 (NA) | NA (NA-NA) | NA | NA | NA | 0 (0-Inf) | ||
| FPI | 22 | 1 | 29.8 (1.4) | NA (NA-NA) | 100% (100–100%) | 92.3% (78.9–100%) | 92.3% (78.9–100%) | 0.8 (0.1–6.5) |
Figure A7.
Overall (A) and relapse-free (B) survival curves by melanoma thickness.
Figure A8.
Overall (A) and relapse-free (B) survival curves by presence of ulceration in primary melanoma.
Figure A9.
Overall (A) and relapse-free (B) survival curves by tumor subtype.
Table A9.
Overall and relapse-free survival in each surgery group by TRAE.
| Operation Group | Occurrence of TRAE | n | n Events | Restricted Mean (SD) | Median (95% CI) | 12 M Survival (95% CI) | 18 M Survival (95% CI) | 24 M Survival (95% CI) | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Relapse-free survival | |||||||||
| LND/Primary | Overall | 130 | 24 | 20.5 (0.6) | NA (NA-NA) | 85.9% (79.7–92.7%) | 76.4% (67.6–86.4%) | 65.4% (50.9–83.9%) | |
| No TRAE | 67 | 15 | 19 (1.1) | NA (17.3-NA) | 80.3% (70.3–91.9%) | 63% (47.5–83.6%) | 63% (47.5–83.6%) | 0.0598 | |
| TRAE | 61 | 9 | 21.7 (0.8) | NA (NA-NA) | 91.2% (84–99%) | 86.4% (77.3–96.5%) | 71.2% (53.3–95.2%) | ||
| Scar/In transit/LND | Overall | 95 | 27 | 19.2 (0.8) | NA (23.1-NA) | 81.1% (73.4–89.7%) | 68% (57.5–80.4%) | 58.8% (45.3–76.5%) | |
| No TRAE | 53 | 19 | 17.4 (1.2) | NA (14.4-NA) | 74.7% (63.6–87.7%) | 54.2% (39.6–74.2%) | 54.2% (39.6–74.2%) | 0.05 | |
| TRAE | 42 | 8 | 21.5 (1) | NA (23.1-NA) | 89.4% (80–100%) | 85.4% (73.9–98.6%) | 67.2% (46.7–96.7%) | ||
| M1 | Overall | 23 | 10 | 15.2 (2.1) | NA (5.6-NA) | 60.9% (43.9–84.5%) | 52.2% (33.4–81.5%) | 52.2% (33.4–81.5%) | |
| No TRAE | 11 | 6 | 13 (3.1) | 6.7 (5.6-NA) | 45.5% (23.8–86.8%) | 45.5% (23.8–86.8%) | 45.5% (23.8–86.8%) | 0.208 | |
| TRAE | 12 | 4 | 17.6 (2.6) | NA (12.7-NA) | 75% (54.1–100%) | 62.5% (38.5–100%) | 62.5% (38.5–100%) | ||
| Overall survival | |||||||||
| LND/Primary | Overall | 130 | 9 | 22.6 (0.4) | NA (NA-NA) | 96.8% (93.8–99.9%) | 92.4% (86.8–98.4%) | 85.1% (74.6–97%) | |
| No TRAE | 67 | 6 | 21.8 (0.8) | NA (20.2-NA) | 98.5% (95.6–100%) | 88.3% (77.5–100%) | 66.2% (42.9–100%) | 0.0735 | |
| TRAE | 61 | 2 | 23.4 (0.4) | NA (NA-NA) | 96.6% (92.1–100%) | 96.6% (92.1–100%) | 96.6% (92.1–100%) | ||
| Scar/In transit/LND | Overall | 95 | 2 | 23.7 (0.2) | NA (NA-NA) | 100% (100–100%) | 97.8% (93.7–100%) | 92.9% (83.3–100%) | |
| No TRAE | 53 | 1 | 23.6 (0.4) | NA (NA-NA) | 100% (100–100%) | 95.7% (87.7–100%) | 95.7% (87.7–100%) | 0.9435 | |
| TRAE | 42 | 1 | 23.8 (0.2) | NA (NA-NA) | 100% (100–100%) | 100% (100–100%) | 90.9% (75.4–100%) | ||
| M1 | Overall | 23 | 2 | 22.5 (1) | NA (21.9-NA) | 100% (100–100%) | 90.9% (75.4–100%) | 68.2% (37.6–100%) | |
| No TRAE | 11 | 1 | 20.3 (3) | NA (12.8-NA) | 100% (100–100%) | 66.7% (30–100%) | 66.7% (30–100%) | 0.1025 | |
| TRAE | 12 | 1 | 23.5 (0.5) | NA (21.9-NA) | 100% (100–100%) | 100% (100–100%) | 75% (42.6–100%) | ||
Appendix C
Table A10.
Treatment intensity and occurrence of TRAE.
| No TRAE | TRAE | Total | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Overall | Dt * | 31 | 24.6 | 55 | 47.8 | 86 | 35.7 | <0.001 |
| q14 | 14 | 11.1 | 18 | 15.7 | 32 | 13.3 | ||
| q21 | 27 | 21.4 | 22 | 19.1 | 49 | 20.3 | ||
| q28 | 42 | 33.3 | 17 | 14.8 | 59 | 24.5 | ||
| q42 | 12 | 9.5 | 3 | 2.6 | 15 | 6.2 | ||
| Anti-PD1 group | q14 | 14 | 16.1 | 18 | 32.1 | 32 | 22.4 | 0.023 |
| q21 | 27 | 31 | 22 | 39.3 | 49 | 34.3 | ||
| q28 | 34 | 39.1 | 13 | 23.2 | 47 | 32.9 | ||
| q42 | 12 | 13.8 | 3 | 5.4 | 15 | 10.5 | ||
| BRAF/MEK inhibitor group | dt | 31 | 79.5 | 55 | 93.2 | 86 | 87.8 | 0.086 |
| q28 | 8 | 20.5 | 4 | 6.8 | 12 | 12.2 |
* Table summarizes the initial planned intensity.
Table A11.
Dose reduction and treatment termination by occurrence of TRAE.
| No TRAE | TRAE | Total | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Overall | ||||||||
| Treatment termination as intended | Yes | 53 | 42.1 | 56 | 48.7 | 109 | 45.2 | 0.306 |
| No | 36 | 28.6 | 35 | 30.4 | 71 | 29.5 | ||
| (Treatment ongoing) | 37 | 29.4 | 24 | 20.9 | 61 | 25.3 | ||
| Dose reduction | No | 119 | 94.4 | 43 | 37.4 | 162 | 67.2 | <0.001 |
| Yes | 7 | 5.6 | 72 | 62.6 | 79 | 32.8 | ||
| Percent of intended dose received | (Treatment ongoing) | 36 | 28.6 | 24 | 20.9 | 60 | 24.9 | 0.014 |
| <50% | 21 | 16.7 | 28 | 24.3 | 49 | 20.3 | ||
| 50 – <100% | 24 | 19 | 34 | 29.6 | 58 | 24.1 | ||
| ≥100% | 40 | 31.7 | 20 | 17.4 | 60 | 24.9 | ||
| 5 | 4 | 9 | 7.8 | 14 | 5.8 | |||
| Anti-PD1 group | ||||||||
| Treatment termination as intended | Yes | 31 | 35.6 | 19 | 33.9 | 50 | 35 | 0.97 |
| No | 31 | 35.6 | 21 | 37.5 | 52 | 36.4 | ||
| (Treatment ongoing) | 25 | 28.7 | 16 | 28.6 | 41 | 28.7 | ||
| Dose reduction | No | 83 | 95.4 | 34 | 60.7 | 117 | 81.8 | <0.001 |
| Yes | 4 | 4.6 | 22 | 39.3 | 26 | 18.2 | ||
| Percent of intended dose received | (Treatment ongoing) | 25 | 28.7 | 16 | 28.6 | 41 | 28.7 | 0.857 |
| <50% | 19 | 21.8 | 14 | 25 | 33 | 23.1 | ||
| 50–<100% | 17 | 19.5 | 10 | 17.9 | 27 | 18.9 | ||
| ≥100% | 25 | 28.7 | 14 | 25 | 39 | 27.3 | ||
| 1 | 1.1 | 2 | 3.6 | 3 | 2.1 | |||
| BRAF/MEK inhibitor group | ||||||||
| Treatment termination as intended | Yes | 22 | 56.4 | 37 | 62.7 | 59 | 60.2 | 0.082 |
| No | 5 | 12.8 | 14 | 23.7 | 19 | 19.4 | ||
| (Treatment ongoing) | 12 | 30.8 | 8 | 13.6 | 20 | 20.4 | ||
| Dose reduction | No | 36 | 92.3 | 9 | 15.3 | 45 | 45.9 | <0.001 |
| Yes | 3 | 7.7 | 50 | 84.7 | 53 | 54.1 | ||
| Percent of intended dose received | (Treatment ongoing) | 11 | 28.2 | 8 | 13.6 | 19 | 19.4 | <0.001 |
| <50% | 2 | 5.1 | 14 | 23.7 | 16 | 16.3 | ||
| 50–<100% | 7 | 17.9 | 24 | 40.7 | 31 | 31.6 | ||
| ≥100% | 15 | 38.5 | 6 | 10.2 | 21 | 21.4 | ||
| 4 | 10.3 | 7 | 11.9 | 11 | 11.2 |
Author Contributions
Conceptualization, J.P., M.R., P.R. (Piotr Rutkowski) and H.K.-P.; data curation, J.P., M.R. and A.B.; formal analysis, M.R., A.B. and J.P.; investigation, J.P., B.C.-S., T.Ś., A.B., M.Z., N.K.-K., G.K.-W., W.B., Ł.G., M.L.-J., R.D. and K.D.; methodology, J.P., M.R. and P.R. (Piotr Rutkowski); project administration, J.P., M.R. and P.R. (Piotr Rutkowski); resources, J.P., M.R., P.S., M.Z., N.K.-K., B.C.-S., G.K.-W., W.B., J.M., Ł.G., M.L.-J., M.J., R.D., K.D., A.B., T.Ś., P.R. (Paweł Rogala), K.K., A.K., P.J.-M., A.S.-C., H.K.-P. and P.R. (Piotr Rutkowski); software, M.R. and A.B.; supervision, H.K.-P., P.S. and P.R. (Piotr Rutkowski); validation, P.S. and M.R.; visualization, M.R.; writing—original draft, J.P. and M.R.; writing—review and editing, J.P., P.S., M.R. and P.R. (Piotr Rutkowski); senior authors—H.K.-P. and P.R. (Piotr Rutkowski). All authors have contributed substantially to the work’s data acquisition, analysis or interpretation, intellectual content development or critical review, and integrity. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of the Maria Sklodowska-Curie National Research Institute of Oncology; ethical board approval number: 27/2018 dated 19 April 2018.
Informed Consent Statement
Prior to any given treatment, the patients signed an informed consent form for the treatment and a consent form allowing the usage of their data for scientific purposes.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
J.P.: Travel: Accommodations, expenses—Bristol-Myers Squibb, Roche, MSD, Novartis, Pierre Fabre; P.S.: Has received travel grants from MSD, Roche, Novartis, and Pierre Fabre and honoraria for lectures from Swixx BioPharma and BMS. Non-financial interests: European Society of Medical Oncology—Officer; Polish Society of Clinical Oncology—Member of the Board of Directors; M.R.: Received consulting fees from MSD; M.Z.: Honoraria—Novartis; Travel, accommodations, expenses–Novartis; N.K.-K.: Travel, accommodations, expenses—Bristol-Myers Squibb, Roche, MSD, Novartis, Pierre Fabre; B.C.-S.: Honoraria—Bristol-Myers Squibb; MSD; Pierre Fabre; Roche, Speakers’ Bureau—Bristol-Myers Squibb; MSD; Novartis; Pierre Fabre; Roche, travel, accommodations, expenses—Astellas Pharma; Pierre Fabre; G.K.-W.: Honoraria—Bristol-Myers Squibb; Novartis, Speakers’ Bureau—Bristol-Myers Squibb; Novartis, travel, accommodations, expenses—Novartis; Takeda; W.B.: Consulting or advisory role—Astellas, Bayer, Janssen; J.M.: Consulting or advisory role—Bristol-Myers Squibb, MSD, Speakers’ Bureau—Bristol-Myers Squibb, GlaxoSmithKline, Roche, MSD, Novartis; Travel, accommodations, expenses—Bristol-Myers Squibb, GlaxoSmithKline, Roche, MSD, Novartis, Pierre Fabre; L.G.: Travel, accommodations, expenses—Bristol-Myers Squibb, Roche, MSD, Novartis, Pierre Fabre; R.D.: Travel, accommodations, expenses—Bristol-Myers Squibb, Roche, MSD, Pierre Fabre; A.B.: Honoraria—Nanobiotix, Phillogen, Amgen; T.S.: Honoraria—Bristol-Myers Squibb; Novartis; Roche, Research funding—Amgen; P.Rogala.: Honoraria—Bristol-Myers Squibb; MSD; Novartis; Roche; K.K.: Honoraria—Bristol-Myers Squibb; MSD; Novartis; Pierre Fabre, Speakers’ Bureau—Bristol-Myers Squibb; MSD; Novartis; Pierre Fabre; P.J.-M.: Honoraria from Roche, Agenus, MSD, Amgen, Janssen Oncology, AstraZeneca, Genentech/Roche, Novartis, Takeda, Bristol-Myers Squibb, Genmab, Merck, Incyte, Pfizer, Pierre Fabre; A.S.-C.: Honoraria—Merck, consulting or advisory role—Merck; H.K.-P.: Honoraria from Roche, Agenus, MSD, Amgen, Janssen Oncology, AstraZeneca, Genentech/Roche, Novartis, Takeda, Bristol-Myers Squibb, Genmab, Merck, Incyte, Pfizer, Pierre Fabre; P. Rutkowski: Honoraria—Bristol-Myers Squibb; Lilly; Merck; MSD; Novartis; Pfizer; Pierre Fabre; Roche; Sanofi; Sanofi, consulting or advisory role—Amgen; Blueprint Medicines; Bristol-Myers Squibb; MSD; Novartis; Pierre Fabre, Speakers’ Bureau—Lilly; Novartis; Pfizer, research funding—Bristol-Myers Squibb (Inst); Novartis (Inst); Roche (Inst), travel, accommodations, expenses—Orphan Europe; Pierre Fabre. All declared disclosures are beyond the scope of this study.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Faries M.B., Thompson J.F., Cochran A.J., Andtbacka R.H., Mozzillo N., Zager J.S., Jahkola T., Bowles T.L., Testori A., Beitsch P.D., et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N. Engl. J. Med. 2017;376:2211–2222. doi: 10.1056/NEJMoa1613210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leiter U., Stadler R., Mauch C., Hohenberger W., Brockmeyer N., Berking C., Sunderkötter C., Kaatz M., Schulte K.-W., Lehmann P., et al. Complete Lymph Node Dissection versus No Dissection in Patients with Sentinel Lymph Node Biopsy Positive Melanoma (DeCOG-SLT): A Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2016;17:757–767. doi: 10.1016/S1470-2045(16)00141-8. [DOI] [PubMed] [Google Scholar]
- 3.Morton D.L., Wen D.R., Wong J.H., Economou J.S., Cagle L.A., Storm F.K., Foshag L.J., Cochran A.J. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch. Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 4.Faries M.B., Cochran A.J., Thompson J.F. Re: “Time to Reconsider the Role of Sentinel Lymph Node Biopsy in Melanoma”. J. Am. Acad. Dermatol. 2023;88:e25–e26. doi: 10.1016/j.jaad.2019.03.064. [DOI] [PubMed] [Google Scholar]
- 5.Michielin O., van Akkooi A., Lorigan P., Ascierto P.A., Dummer R., Robert C., Arance A., Blank C.U., Chiarion Sileni V., Donia M., et al. ESMO Consensus Conference Recommendations on the Management of Locoregional Melanoma: Under the Auspices of the ESMO Guidelines Committee. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020;31:1449–1461. doi: 10.1016/j.annonc.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Lao C.D., Khushalani N.I., Angeles C., Petrella T.M. Current State of Adjuvant Therapy for Melanoma: Less Is More, or More Is Better? Am. Soc. Clin. Oncol. Educ. Book. 2022;42:738–744. doi: 10.1200/EDBK_351153. [DOI] [PubMed] [Google Scholar]
- 7.Gershenwald J.E., Scolyer R.A., Hess K.R., Sondak V.K., Long G.V., Ross M.I., Lazar A.J., Faries M.B., Kirkwood J.M., McArthur G.A., et al. Melanoma Staging: Evidence-Based Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J. Clin. 2017;67:472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggermont A.M.M., Blank C.U., Mandalà M., Long G.V., Atkinson V.G., Dalle S., Haydon A.M., Meshcheryakov A., Khattak A., Carlino M.S., et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma (EORTC 1325-MG/KEYNOTE-054): Distant Metastasis-Free Survival Results from a Double-Blind, Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2021;22:643–654. doi: 10.1016/S1470-2045(21)00065-6. [DOI] [PubMed] [Google Scholar]
- 9.Long G.V., Hauschild A., Santinami M., Atkinson V., Mandalà M., Chiarion-Sileni V., Larkin J., Nyakas M., Dutriaux C., Haydon A., et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017;377:1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 10.Weber J., Mandala M., Del Vecchio M., Gogas H.J., Arance A.M., Cowey C.L., Dalle S., Schenker M., Chiarion-Sileni V., Marquez-Rodas I., et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 11.Edge S.B., Compton C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 12.Eggermont A.M.M., Blank C.U., Mandala M., Long G.V., Atkinson V., Dalle S., Haydon A., Lichinitser M., Khattak A., Carlino M.S., et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 13.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2021. [Google Scholar]
- 14.Downs J.S., Subramaniam S., Henderson M.A., Paton E., Spillane A.J., Mathy J.A., Gyorki D.E. A Survey of Surgical Management of the Sentinel Node Positive Melanoma Patient in the Post-MSLT2 Era. J. Surg. Oncol. 2021;124:1544–1550. doi: 10.1002/jso.26641. [DOI] [PubMed] [Google Scholar]
- 15.Nijhuis A.A.G., Spillane A.J., Stretch J.R., Saw R.P.M., Menzies A.M., Uren R.F., Thompson J.F., Nieweg O.E. Current Management of Patients with Melanoma Who Are Found to Be Sentinel Node-positive. ANZ J. Surg. 2020;90:491–496. doi: 10.1111/ans.15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garbe C., Keim U., Suciu S., Amaral T., Eigentler T.K., Gesierich A., Hauschild A., Heinzerling L., Kiecker F., Schadendorf D., et al. Prognosis of Patients With Stage III Melanoma According to American Joint Committee on Cancer Version 8: A Reassessment on the Basis of 3 Independent Stage III Melanoma Cohorts. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:2543–2551. doi: 10.1200/JCO.19.03034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eggermont A.M.M., Blank C.U., Mandala M., Long G.V., Atkinson V.G., Dalle S., Haydon A.M., Meshcheryakov A., Khattak A., Carlino M.S., et al. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results From the EORTC 1325-MG/KEYNOTE-054 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:3925–3936. doi: 10.1200/JCO.20.02110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M.E. The Role of Clinical Prediction Tools to Risk Stratify Patients with Melanoma After a Positive Sentinel Lymph Node Biopsy. Ann. Surg. Oncol. 2021;28:4082–4083. doi: 10.1245/s10434-018-07099-5. [DOI] [PubMed] [Google Scholar]
- 19.Broman K.K., Bettampadi D., Pérez-Morales J., Sun J., Kirichenko D., Carr M.J., Eroglu Z., Tarhini A.A., Khushalani N., Schabath M.B., et al. Surveillance of Sentinel Node Positive Melanoma Patients Who Receive Adjuvant Therapy without Undergoing Completion Lymph Node Dissection. Ann. Surg. Oncol. 2021;28:6978–6985. doi: 10.1245/s10434-021-10570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eroglu Z., Broman K.K., Thompson J.F., Nijhuis A., Hieken T.J., Kottschade L., Farma J.M., Hotz M., Deneve J., Fleming M., et al. Outcomes with Adjuvant Anti-PD-1 Therapy in Patients with Sentinel Lymph Node-Positive Melanoma without Completion Lymph Node Dissection. J. Immunother. Cancer. 2022;10:e004417. doi: 10.1136/jitc-2021-004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitra D., Ologun G., Keung E.Z., Goepfert R.P., Amaria R.N., Ross M.I., Gershenwald J.E., Lucci A., Fisher S.B., Davies M.A., et al. Nodal Recurrence Is a Primary Driver of Early Relapse for Patients with Sentinel Lymph Node Positive Melanoma in the Modern Therapeutic Era. Ann. Surg. Oncol. 2021;28:3480–3489. doi: 10.1245/s10434-021-09804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livingstone E., Zimmer L., Hassel J.C., Fluck M., Eigentler T.K., Loquai C., Haferkamp S., Gutzmer R., Meier F., Mohr P., et al. Adjuvant Nivolumab plus Ipilimumab or Nivolumab Alone versus Placebo in Patients with Resected Stage IV Melanoma with No Evidence of Disease (IMMUNED): Final Results of a Randomised, Double-Blind, Phase 2 Trial. Lancet. 2022;400:1117–1129. doi: 10.1016/S0140-6736(22)01654-3. [DOI] [PubMed] [Google Scholar]
- 23.Multicenter Selective Lymphadenectomy Trials Study Group Therapeutic Value of Sentinel Lymph Node Biopsy in Patients With Melanoma: A Randomized Clinical Trial. JAMA Surg. 2022;157:835–842. doi: 10.1001/jamasurg.2022.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhodin K.E., Beasley G.M., Tyler D.S. Prognostic or Therapeutic—The Role of Sentinel Lymph Node Biopsy in Contemporary Practice. JAMA Surg. 2022;157:843. doi: 10.1001/jamasurg.2022.2054. [DOI] [PubMed] [Google Scholar]
- 25.Rentroia-Pacheco B., Tjien-Fooh F.J., Quattrocchi E., Kobic A., Wever R., Bellomo D., Meves A., Hieken T.J. Clinicopathologic Models Predicting Non-Sentinel Lymph Node Metastasis in Cutaneous Melanoma Patients: Are They Useful for Patients with a Single Positive Sentinel Node? J. Surg. Oncol. 2022;125:516–524. doi: 10.1002/jso.26736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertolli E., Franke V., Calsavara V.F., de Macedo M.P., Pinto C.A.L., van Houdt W.J., Wouters M.W.J.M., Duprat Neto J.P., van Akkooi A.C.J. Validation of a Nomogram for Non-Sentinel Node Positivity in Melanoma Patients, and Its Clinical Implications: A Brazilian–Dutch Study. Ann. Surg. Oncol. 2019;26:395–405. doi: 10.1245/s10434-018-7038-9. [DOI] [PubMed] [Google Scholar]
- 27.Isaksson K., Katsarelias D., Mikiver R., Carneiro A., Ny L., Olofsson Bagge R. A Population-Based Comparison of the AJCC 7th and AJCC 8th Editions for Patients Diagnosed with Stage III Cutaneous Malignant Melanoma in Sweden. Ann. Surg. Oncol. 2019;26:2839–2845. doi: 10.1245/s10434-019-07448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tonella L., Pala V., Ponti R., Rubatto M., Gallo G., Mastorino L., Avallone G., Merli M., Agostini A., Fava P., et al. Prognostic and Predictive Biomarkers in Stage III Melanoma: Current Insights and Clinical Implications. Int. J. Mol. Sci. 2021;22:4561. doi: 10.3390/ijms22094561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiteman D.C., Baade P.D., Olsen C.M. More People Die from Thin Melanomas (≤1 Mm) than from Thick Melanomas (>4 Mm) in Queensland, Australia. J. Investig. Dermatol. 2015;135:1190–1193. doi: 10.1038/jid.2014.452. [DOI] [PubMed] [Google Scholar]
- 30.Dizier B., Callegaro A., Debois M., Dreno B., Hersey P., Gogas H.J., Kirkwood J.M., Vansteenkiste J.F., Sequist L.V., Atanackovic D., et al. A Th1/IFNγ Gene Signature Is Prognostic in the Adjuvant Setting of Resectable High-Risk Melanoma but Not in Non–Small Cell Lung Cancer. Clin. Cancer Res. 2020;26:1725–1735. doi: 10.1158/1078-0432.CCR-18-3717. [DOI] [PubMed] [Google Scholar]
- 31.Gastman B.R., Gerami P., Kurley S.J., Cook R.W., Leachman S., Vetto J.T. Identification of Patients at Risk of Metastasis Using a Prognostic 31-Gene Expression Profile in Subpopulations of Melanoma Patients with Favorable Outcomes by Standard Criteria. J. Am. Acad. Dermatol. 2019;80:149–157.e4. doi: 10.1016/j.jaad.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 32.Hsueh E.C., DeBloom J.R., Lee J., Sussman J.J., Covington K.R., Middlebrook B., Johnson C., Cook R.W., Slingluff C.L., McMasters K.M. Interim Analysis of Survival in a Prospective, Multi-Center Registry Cohort of Cutaneous Melanoma Tested with a Prognostic 31-Gene Expression Profile Test. J. Hematol. Oncol. 2017;10:152. doi: 10.1186/s13045-017-0520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diaz-Ramón J.L., Gardeazabal J., Izu R.M., Garrote E., Rasero J., Apraiz A., Penas C., Seijo S., Lopez-Saratxaga C., de la Peña P.M., et al. Melanoma Clinical Decision Support System: An Artificial Intelligence-Based Tool to Diagnose and Predict Disease Outcome in Early-Stage Melanoma Patients. Cancers. 2023;15:2174. doi: 10.3390/cancers15072174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luskin M.R., Murakami M.A., Manalis S.R., Weinstock D.M. Targeting Minimal Residual Disease: A Path to Cure? Nat. Rev. Cancer. 2018;18:255–263. doi: 10.1038/nrc.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menon D.R., Das S., Krepler C., Vultur A., Rinner B., Schauer S., Kashofer K., Wagner K., Zhang G., Rad E.B., et al. A Stress-Induced Early Innate Response Causes Multidrug Tolerance in Melanoma. Oncogene. 2015;34:4545. doi: 10.1038/onc.2014.432. [DOI] [PubMed] [Google Scholar]
- 36.Rabbie R., Ferguson P., Molina-Aguilar C., Adams D.J., Robles-Espinoza C.D. Melanoma Subtypes: Genomic Profiles, Prognostic Molecular Markers and Therapeutic Possibilities. J. Pathol. 2019;247:539–551. doi: 10.1002/path.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rambow F., Rogiers A., Marin-Bejar O., Aibar S., Femel J., Dewaele M., Karras P., Brown D., Chang Y.H., Debiec-Rychter M., et al. Toward Minimal Residual Disease-Directed Therapy in Melanoma. Cell. 2018;174:843–855.e19. doi: 10.1016/j.cell.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 38.Goodman R.S., Lawless A., Woodford R., Fa’ak F., Tipirneni A., Patrinely J.R., Yeoh H.L., Rapisuwon S., Haydon A., Osman I., et al. Extended Follow-Up of Chronic Immune-Related Adverse Events Following Adjuvant Anti–PD-1 Therapy for High-Risk Resected Melanoma. JAMA Netw. Open. 2023;6:e2327145. doi: 10.1001/jamanetworkopen.2023.27145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eggermont A., Kicinski M., Suciu S. Association of Selected (Immune-Related) Adverse Events and Outcome in Two Adjuvant Phase III Trials, Checkmate-238 and EORTC1325/KEYNOTE-054. J. Immunother. Cancer. 2022;10:e004272. doi: 10.1136/jitc-2021-004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Funck-Brentano E., Malissen N., Roger A., Lebbé C., Deilhes F., Frénard C., Dréno B., Meyer N., Grob J.-J., Tétu P., et al. Which Adjuvant Treatment for Patients with BRAF(V600)-Mutant Cutaneous Melanoma? Ann. Dermatol. Venereol. 2021;148:145–155. doi: 10.1016/j.annder.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Suciu S., Eggermont A.M.M., Lorigan P., Kirkwood J.M., Markovic S.N., Garbe C., Cameron D., Kotapati S., Chen T.-T., Wheatley K., et al. Relapse-Free Survival as a Surrogate for Overall Survival in the Evaluation of Stage II-III Melanoma Adjuvant Therapy. J. Natl. Cancer Inst. 2018;110:87–96. doi: 10.1093/jnci/djx133. [DOI] [PubMed] [Google Scholar]
- 42.Signorovitch J., Moshyk A., Zhao J., Le T.K., Burns L., Gooden K., Hamilton M. Overall Survival in the Real-World and Clinical Trials: A Case Study Validating External Controls in Advanced Melanoma. Future Oncol. 2022;18:1321–1331. doi: 10.2217/fon-2021-1054. [DOI] [PubMed] [Google Scholar]
- 43.de Meza M.M., Ismail R.K., Rauwerdink D., van Not O.J., van Breeschoten J., Blokx W.A.M., de Boer A., van Dartel M., Hilarius D.L., Ellebaek E., et al. Adjuvant Treatment for Melanoma in Clinical Practice-Trial versus Reality. Eur. J. Cancer. 2021;158:234–245. doi: 10.1016/j.ejca.2021.08.044. [DOI] [PubMed] [Google Scholar]
- 44.Farrow N.E., Raman V., Williams T.P., Nguyen K.Y., Tyler D.S., Beasley G.M. Adjuvant Therapy Is Effective for Melanoma Patients with a Positive Sentinel Lymph Node Biopsy Who Forego Completion Lymphadenectomy. Ann. Surg. Oncol. 2020;27:5121–5125. doi: 10.1245/s10434-020-08478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torphy R.J., Friedman C., Ho F., Leonard L.D., Thieu D., Lewis K.D., Medina T.M., Robinson W.A., Gonzalez R.C., Stewart C.L., et al. Adjuvant Therapy for Stage III Melanoma Without Immediate Completion Lymph Node Dissection. Ann. Surg. Oncol. 2022;29:806–815. doi: 10.1245/s10434-021-10775-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.