Abstract
Fingerprinting of more than 700 clinical and environmental isolates of Aspergillus fumigatus from four differential hospital settings was undertaken with a dispersed repeated DNA sequence. The analysis of the environmental isolates showed that the airborne A. fumigatus population is extremely diverse, with 85% of the strains being represented as a single genotype isolated once. The remaining 15% of the strains were isolated several times and were able to persist for several months in the same hospital environment. No strains were found to be associated with a specific location inside the hospital, and identical strains were isolated from different buildings of the hospital and outdoors. Isolation of the same strain both from patients and from the environment of the same hospital is highly suggestive of a nosocomial infection. The characteristics of the environmental fungal population explains the two main results obtained from the typing of the clinical isolates: (i) the absence of a common strain responsible for an invasive aspergillosis outbreak results from the extreme diversity of the environmental population of A. fumigatus in contact with the patients, and (ii) patients hospitalized in different wards of the same hospital can be infected with the same strain since every patient might inhale the same spore population.
Invasive aspergillosis (IA) in hospitals is becoming one of the most severe diseases among immunosuppressed patients, particularly patients in bone marrow transplant units (9, 10, 13). Most of these infections are due to Aspergillus fumigatus. This fungal species is a common inhabitant of soil, where it colonizes organic debris and releases a high number of conidia to the atmosphere. Due to its presence in the atmosphere, outbreaks of nosocomial aspergillosis have often been associated in the past with an increase in the concentration of airborne spores resulting, for example, from construction work or deficient ventilation systems (2, 5–7, 38, 40). The occurrence of such outbreaks has led to the establishment of prophylactic measures. The use of high-efficiency air filters for the capture of particulates has only reduced the level of contamination of hospital air and the risk of infection during bone marrow transplantation. It has not solved the problem of late infections (33, 34, 39). In addition, many cases of IA have occurred under laminar flow conditions. As a result, IA remains a major cause of mortality among transplant patients in the hospital setting, and the source of contamination is still unclear (21, 22).
This lack of understanding of the reservoir of the infectious inoculum has caused the supplementation of data from epidemiological studies with genomic typing data to investigate the nosocomial nature of Aspergillus infections and to identify the source of the inoculum (36). However, previous studies have not been conclusive since they have always been limited to a short period of time and to a few isolates collected from a few patients and have used molecular biology-based methods not always appropriate for strain fingerprinting (11, 17, 25, 26). Due to the extreme genetic diversity of A. fumigatus (12), a very precise method of typing must be used to perform a thorough epidemiological study of Aspergillus infections. Under these conditions, it will not be possible to use a technique such as randomly amplified polymorphic DNA analysis, which is nevertheless commonly used for the fingerprinting of strains of A. fumigatus. Randomly amplified polymorphic DNA patterns are often very difficult to reproduce and interpret objectively and are not amenable to computer-aided analysis due to the restricted range of DNA fragment sizes (4, 8, 27). Similarly, multilocus enzyme electrophoretic patterns (35) are not variable enough to define a strain and will lead to erroneous conclusions. The repeated DNA sequence that we isolated and characterized previously (16, 32) is to date the only probe that permits an efficient and precise computer-aided analysis of the DNA fingerprints of a large population of A. fumigatus strains. By using this probe, the A. fumigatus population was followed in four different hospital locations over a long period of time (1 to 2 years) to obtain an understanding of the variability and/or persistence over time of the clinical and environmental A. fumigatus population.
MATERIALS AND METHODS
Origins of the isolates of A. fumigatus.
Four hospitals (hospitals I, II, III, and IV) were monitored. The origins and total numbers of isolates fingerprinted are summarized in Table 1. The study in hospitals I and II, which were 2 miles apart, was mainly dedicated to obtaining an understanding of the behavior of A. fumigatus in the hospital environment, whereas isolates from hospitals III and IV were used for the characterization of strains responsible for pulmonary aspergillosis.
TABLE 1.
Origin and number of isolates typed
| Location | No. of sampling sites | Environment
|
Patient
|
Period surveyed (mo.day.yr) | ||||
|---|---|---|---|---|---|---|---|---|
| No. of samples
|
No. of isolates
|
No. of patients | No. of isolates | |||||
| Air | Surface | Air | Surface | |||||
| Hospital I | 1557 | 1820 | 313 | 63 | 16 | 30 | 5.19.1994 to 5.30.1996 | |
| Insidea | ||||||||
| Building A, sector A | 11 | 464 | 658 | 78 | 16 | |||
| Building A, sector M | 11 | 462 | 632 | 75 | 24 | |||
| Building A, sector G | 9 | 356 | 308 | 19 | 7 | |||
| Building B | 7 | 240 | 222 | 80 | 16 | 8.26.1994 to 5.30.1996 | ||
| Outsideb | 10 | 35 | NDh | 61 | ND | 5.7.1996 to 5.30.1996 | ||
| Hospital II | 475 | 781 | 167 | 85 | 4 | 8 | 1.16.1996 to 10.3.1996 | |
| Insidec | ||||||||
| Sector 1 | 11 | 211 | 381 | 76 | 51 | |||
| Sector 2 | 8 | 160 | 360 | 29 | 27 | |||
| Others | 13 | 84 | 40 | 16 | 7 | |||
| Outsided | 20 | 20 | ND | 46 | ND | 9.25.1996 | ||
| Hospital III, inside, sector He | 20 | NRi | NR | 3 | 12 | 22 | 31 | 1.10.1993 to 4.20.1995 |
| Hospital IV, insidef | NAg | NA | NA | NA | NA | 31 | 46 | 1.15.1994 to 12.7.1994 |
Buildings A and B of hospital I are two separate buildings of the hospital; sectors A, M, and G are three sectors of the Hematology Unit in building A. In building B, situated 200 m from building A, the outpatient clinic situated at the basement was mainly sampled.
Isolates from air samples taken twice in the garden situated between the two buildings.
Sectors 1 and 2 are the second and fourth floors, respectively, of the building with the Hematology Unit of hospital II. Others refers to samples taken on floors 1 and 3 (where extensive building activity was occurring) of the same building.
Isolates from air samples taken once outside on both sides of the building.
All samples were taken from the Hematology Unit of hospital III, mainly from IA patients.
Only clinical isolates from patients originating from four buildings of hospital IV.
NA, not appropriate; only patient isolates.
ND, not done.
NR, not recorded.
Environmental samples from hospitals I and II were always collected from the same locations on the same day of the week: on Wednesday mornings between 7 and 9 a.m., with an average 2-week interval. Aerial conidia were recovered by using a Systeme Air Surface (Bioblock, Illkirch, France) or a Bio-impactor (Sieve France, Lyon, France) apparatus, which filtered 342 liters of air per sample in 2 min or 250 liters of air per sample in 2.5 min, respectively. Spores were collected on 55- or 90-mm-diameter petri dishes containing Sabouraud medium supplemented with 0.05% (wt/vol) chloramphenicol. To isolate spores deposited on surfaces, 25 cm2 of a wall surface was swabbed with sterile dry cotton swabs. The cotton swab was used to inoculate petri dishes containing Sabouraud-chloramphenicol medium. The concentrations of spores per cubic meter of air sampled or per square meter of surface sampled were estimated (the average was calculated on the basis of 20 to 40 air samples and 30 to 50 surface samples taken per hospital at each sampling time). After incubation of the culture medium at 37°C, the vast majority of fungal colonies were A. fumigatus and Aspergillus niger. After transfer of the greenish colonies onto malt extract agar, final identification of a colony as A. fumigatus was assessed by light microscopy. Clinical isolates from patients with pulmonary aspergillosis had different origins: sputum specimens (19%), bronchoalveolar lavage specimens (21%), bronchial secretions (28%), nasal swab specimens (5%), percutaneous aspirations and biopsy specimens (23%), and not defined (4%). After inoculation of the biological samples onto media supplemented with antibiotics, colonies from positive cultures were transferred to 2% malt agar. Slants were kept at room temperature until DNA extraction. Conidia from each isolate recovered from a slant with phosphate-buffered saline–0.1% Tween 20 were also suspended in a 10% defatted milk solution and stored on dry silica gel for long-term storage.
Strain fingerprinting.
A total of 643 environmental isolates and 115 patient isolates were typed. Fungal cultures, DNA extraction, Southern blot hybridization techniques with the repeated sequence λ3.9, and computerized analysis of the hybridization patterns with the Gel Compar software were performed as described previously (12, 16, 32). Two strains were considered different when the hybridization patterns of the two strains differed by at least one band (12).
RESULTS
Study of environmental isolates.
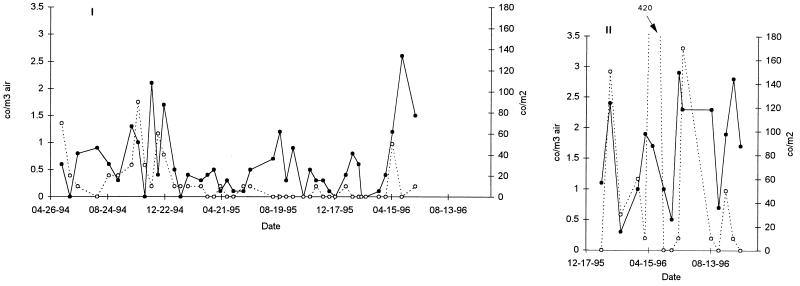
The concentrations of conidia of A. fumigatus per cubic meter of air and per square meter of surface in hospitals I and II are presented in Fig. 1. The assumption that one colony represents one conidium was verified for 5% of the randomly selected colonies for which monospore isolates from a colony always had the same hybridization pattern (data not shown). The concentration of conidia varied between 0 (i.e., no spores were isolated during the sampling time) and 3 conidia/m3. No time period or season could be associated with a higher concentration of spores. Similar results were seen with surface samples, with estimated concentrations varying from 0 to 420 conidia/m2.
FIG. 1.
Temporal evolution of the concentration of spores in the air (•) and on the surfaces (○) of hospitals I and II. The numbers of conidia per cubic meter (co/m3) of air or per square meter (co/m2) of surface were estimated.
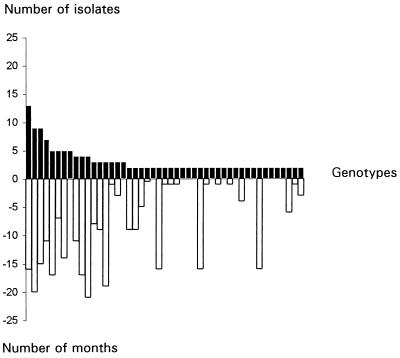
Among a total of 376 isolates from the environment of hospital I, 276 genotypes with a unique hybridization pattern were identified (Table 2). Only 47 genotypes (17%) were recovered more than once over the 2-year study, with 2 to 13 isolates of each genotype being found (Fig. 2). The maximal time interval between the recovery of two identical isolates was 21 months for isolates from two different locations and 18 months for isolates from the same sampling site. The geographical distributions of the genotypes found once or more than once in hospital I are presented in Fig. 3. There was not a location in the hospital where a single strain was continuously and repeatedly isolated (Fig. 3).
TABLE 2.
Analysis of genotypes of A. fumigatus iolated from the environments of hospitals I and II
| Location | No. of isolates | No. (%) of genotypes
|
No. (%) of common genotypesa | |
|---|---|---|---|---|
| Total | Repeatedb | |||
| Hospital I | 376 | 276 | 47 (17) | |
| Inside | 315 | 220 | 34 (15.4) | |
| Building A | 212 | 163 | 15 (9.2) | |
| Sector A | 94 | 79 | 7 (8.9) | |
| Sector M | 89 | 78 | 9 (11.5) | |
| Building B | 96 | 79 | 13 (16.4) | |
| Outside | 61 | 56 | 5 (8.9) | |
| Hospital II | 252 | 157 | 19 (12.1) | |
| Comparison | ||||
| Sector A and sector M (hospital I) | 157 | 9 (5.7) | ||
| Building A and building B (hospital I) | 242 | 22 (9.1) | ||
| Inside and outside (hospital I) | 276 | 8 (2.9) | ||
| Hospital I and hospital II | 433 | 21 (4.8) | ||
| Hospital I + hospital II and non-Parisian environment | 589 | 5 (0.8)* | ||
Genotypes common to the two populations identified in the two locations compared. The proportion that was significantly different by the chi-square test is indicated by an asterisk.
Repeated genotypes are genotypes found more than once in the location surveyed.
FIG. 2.
Number of isolates per genotype isolated at least twice in hospital I (closed bars) and maximal time interval (in months) separating the isolation of two isolates of the same genotype (open bars).
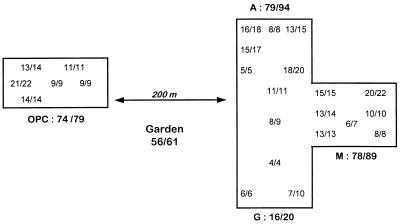
FIG. 3.
Map of hospital I showing the number of isolates typed and the different strains encountered in the different locations sampled. For each location, the ratio x/y indicates the number of unique genotypes (x)/the total number of genotypes (y) collected at the spot. A, M, and G, three sectors of the Hematology Unit situated at the third floor of building A; OPC, outpatient clinic at the basement of building B.
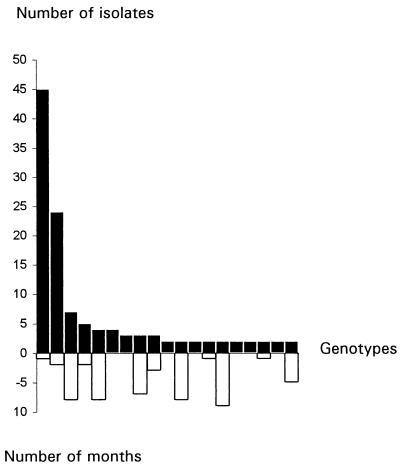
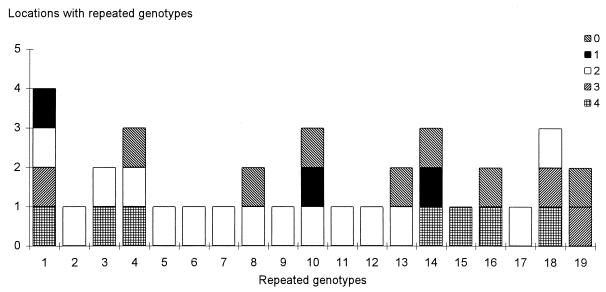
In hospital II, the fingerprinting of 252 isolates resulted in the identification of 157 distinct genotypes (Table 2). Only 19 genotypes (12%) included several isolates. These results were slightly different from those for hospital I. In hospital II, two genotypes accounted for 58% of the isolates recovered more than once (Fig. 4). Most of the isolates belonging to these two genotypes were isolated on the same day. For example, the genotype most repeatedly isolated contained 45 isolates, among which 35 were isolated on the same day. As for hospital I, identical genotypes were found in different locations both inside and outside the building, suggesting that no one genotype is specific to a single location (Fig. 5).
FIG. 4.
Number of isolates per genotype isolated at least twice in hospital II (closed bars) and maximal time interval between the isolation of two identical isolates within the same genotype (open bars).
FIG. 5.
Location of the identical isolates from the 19 genotypes found at least twice in hospital II. The numbers 1 to 4 indicate the first, second, third, and fourth floors of the building surveyed, respectively; 0 indicates outside the building.
Table 2 indicates the number of repeated environmental genotypes found in the same location in hospitals I and II and the number of genotypes common to two different locations. This comparative analysis indicated that a similar percentage of repeated genotypes was found in different locations of the same hospital and confirmed the absence of one or a few genotypes at a single location in a hospital. In addition, the percentage of common genotypes was similar between hospitals I and II and inside each hospital, suggesting that at least some of the strains typed originated from the overall environment, in this case, the Paris atmosphere. This was confirmed by the low numbers of common genotypes found when the genotypes of the population of the strains in hospitals I and II were compared to the genotypes of a population of strains isolated outside Paris. Interestingly, the five common genotypes in this comparison were isolated in Lille, which is 100 miles from Paris (data not shown).
Analysis of clinical isolates.
Isolates from 73 patients in the four hospitals were analyzed. Multiple isolates (two to six) were available from 27 of these 73 patients (Table 3). Only 11 of the 27 patients were infected with a single genotype, suggesting that the majority of the patients studied simultaneously harbored at least two strains.
TABLE 3.
Analysis of A. fumigatus isolates and genotypes from patients
| Hospital | Isolates
|
Patients
|
|||||
|---|---|---|---|---|---|---|---|
| No. of isolates | No. of genotypes | No. of common genotypes | No. of patients | No. of patients with multiple isolates | No. of patients with multiple isolates with the same genotype | No. of patients infected with a genotype common to another patient or the environmenta | |
| I | 30 | 23 | 5 | 16 | 10 | 4 | 5 |
| II | 8 | 3 | 2 | 4 | 1 | 1 | 3 |
| III | 31 | 24 | 5 | 22 | 8 | 4 | 6 |
| IV | 46 | 30 | 5 | 31 | 8 | 2 | 16 |
| Total | 115 | 80 | 17 | 73 | 27 | 11 | 30 |
Genotypes recovered from at least two patients or a patient and the environment of the hospital where the patient was hospitalized.
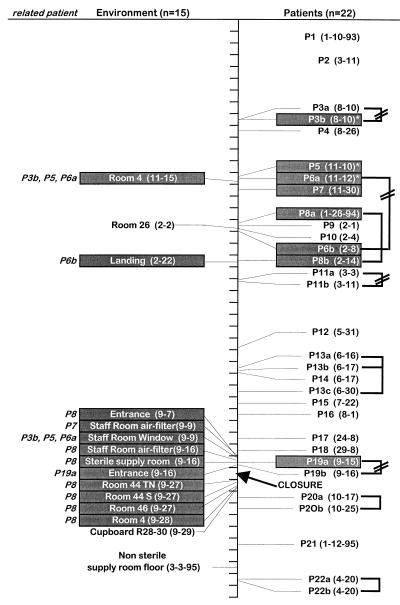
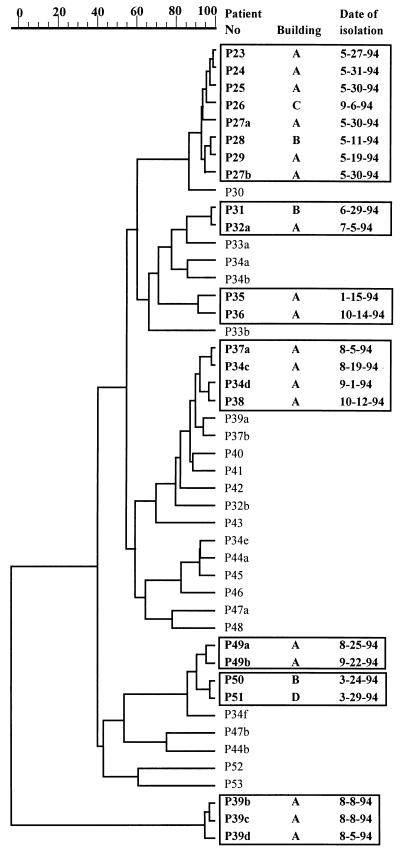
In hospital I, 2 of 16 patients were infected with the same strain (two isolates at a 1-year interval). Seven isolates from four patients in hospital I, corresponding to four genotypes, displayed hybridization patterns identical to those of the environmental isolates, irrespective of the sector of the hospital in which these patients were hospitalized. In hospital II, the same genotype was isolated from two patients (of four patients surveyed). An isolate from one patient in hospital II was found to be identical to an environmental strain found at two different locations in the hospital. Figure 6 shows that, with the exception of 3 patients (patients P3, P5, and P6) infected with the same strain, all 19 other patients with IA studied in the hematology unit of hospital III were infected with a strain of a different genotype. However, when the 15 available environmental isolates were compared with the patient strains, 12 of 15 isolates (corresponding to five distinct genotypes) were found to be identical to eight isolates recovered from six distinct patients. Among the 46 clinical isolates collected in 1994 from 31 patients in hospital IV, 30 different genotypes were identified (Fig. 7). In five cases, the same strain was isolated from several patients. Interestingly, in May 1994 the same strain was recovered from six different patients hospitalized in two different wards. This strain was isolated again in September 1994 from another patient in a different building. For the four other genotypes, identical isolates from different patients were collected at different times of the year. Although only five environmental isolates from hospital IV were typed, one of these isolates was found to be identical to a strain collected from two patients (data not shown).
FIG. 6.
Temporal distribution of environmental and clinical isolates in the bone marrow transplant unit of hospital III showing the identities (isolates indicated by the shaded boxes) between isolates collected from patients (P numbers) and from the environment. The patients infected with isolates identical to the environmental isolates are indicated in italics at the left of the environment column. Isolates from the same patients belonging to different genotypes are indicated by a broken line. Only one genotype has been repeatedly isolated from three patients (marked with asterisks).
FIG. 7.
Dendrogram (unweighted pair group with arithmetic averages) of the clinical isolates analyzed in hospital IV. The date of isolation and the different buildings (marked with different letters) where the patient (P) was hospitalized are indicated. Identities between isolates are framed. The scale at the top represents percent identity.
In conclusion, pairs of isolates with identical genotypes collected from 2 patients or from a patient and the patient’s environment in the same hospital were found for 30 of 73 patients studied. These genotypes accounted for 17 of the 80 clinical genotypes (21%) studied, whereas only 21 (4.8%) common genotypes were encountered among 433 unique environmental genotypes encountered in hospitals I and II (chi-square value of 26.6, indicating a highly significant difference, with P being <0.01 for the two groups of common genotypes). Comparison of these data suggested that, at least for these 30 patients, infection was nosocomial.
DISCUSSION
This report is the first extensive molecular biology-based epidemiological study of A. fumigatus and aspergillosis in the hospital environment. Such a study was made possible by the unique specificity of the molecular fingerprinting system used (16, 32). In a concurrent study in which A. fumigatus isolates collected from cystic fibrosis patients were typed, 140 isolates collected from the same patient over a period of 2 years had the same genotype, and no change in the 140 hybridization patterns, even for the DNA fragment in the high-molecular-weight range, was seen (31). Similarly, no change in the restriction fragment length polymorphism patterns was observed when a collection of strains was kept freeze-dried or regularly transferred in the laboratory for more than 10 years (16) or when a strain was repeatedly passaged by animal inoculation (data not shown). These typing data give confidence to the epidemiological results of this study.
Fingerprinting of the strains of A. fumigatus typed in this study indicated the high degree of genetic diversity of the A. fumigatus population. The percent coverage, i.e., 1 − (number of strains observed once/total number of isolates) × 100, which estimates the proportion of the population represented by the strains occurring in the individual samples (18, 19, 29), was 39% in hospital I and 46% in hospital II. These data indicate that the chances that the next isolate collected will belong to a strain different from the strains that have already been typed are 61 and 54 in 100 for these two hospitals, respectively. This result is in agreement with the huge genetic diversity found in a previous study with strains of A. fumigatus of unrelated geographical origin (12). In a hospital environment, without assuming anything about the population of strains, the expected frequency of strains (F) was determined by S/r(r + 1), where S is the total number of strains observed and r is the observed frequency of the different strains (19, 29). The calculated and observed values of F for hospital I match reasonably well. However, the values observed for hospital II for the strains collected 45 and 25 times are much higher than the expected calculated values. This result suggests the occurrence of a particular but unknown event which would have been responsible for the release of these two preponderant strains. When the total 24-month period of survey of hospital I is analyzed by successive 6-month periods, the percentage of unique genotypes does not decrease between the first 6 months and the last 6 months of the survey. The absence of increase in the percent coverage over time suggests that the sample typed represents only a small portion of the population of strains present in the hospital. The size of the strain population n can be determined by the formula n = 4p (1 − p)/i2, where p is the frequency of the event (here, the percentage of repeated genotypes) with a confidence interval equal to ±i at a 5% risk (37). By supposing that all strains are equally represented in the hospital and by using an i value of 2% for the precision of the sampling data, the population of strains in the hospital would be composed of about 2,400 isolates accounting for 1,400 different genotypes, indicating that we typed less than 20% of the total population.
All of the clinical isolates typed were not isolated from sterile sites or biopsy specimens. Under these conditions, a strain isolated from a patient could either be infecting the patient or contaminating the airways. Indeed, sputum or even bronchoalveolar lavage specimen cultures have sometimes been reported in the past to be insensitive for use in the diagnosis of IA since specimens from patients without IA may be positive by culture (14, 24, 28). However, a careful analysis of data from all studies quantifying the occurrence of positive cultures of respiratory tract specimens from patients with IA and control patients (1, 3, 14, 20, 23, 24, 28, 30, 42) showed that positive cultures were always found for a significantly higher number of infected patients than noninfected patients. These data suggest that A. fumigatus isolates from cultures of sputum or nonbiopsy bronchoscopic specimens from patients with aspergillosis would represent the infecting strains rather than contaminating strains. Even though we suppose that all the isolates from patients are the infecting strains, a nosocomial origin of the infection could be suggested for only 40% of the patients analyzed in the present study as shown by the identity between isolates from patients and those from the environment of the same hospital. The absence of identity between genotypes found for isolates from 60% of the patients and the environmental strains does not exclude the possibility of a nosocomial infection. It may only indicate that due to the high degree of genetic diversity of the A. fumigatus population, the environmental population typed reflects a limited portion of the fungal population actually breathed by the patients. However, the portage of a propagule load obtained by the patient before his or her admission to the hospital cannot be excluded (33). The occurrence of the same A. fumigatus population in the air of all wards of the same hospital, air which is consequently breathed by all patients treated in the hospital, explains why patients hospitalized in different wards can be infected with the same strain (which was seen in hospitals I and IV). Alternatively, the extreme diversity of the airborne spores explains why patients in an IA outbreak are usually infected with different strains. If IA occurs 3 months (15, 34, 41) after bone marrow transplantation, it can be calculated from our results that every patient inhales about 5,000 different genotypes. The probability that two patients will be infected with the same strain is consequently low. Assessment of a nosocomial infection requires the molecular typing of several isolates from each patient as well as a large number of isolates from the environment to be able to identify the contaminating environmental strain.
ACKNOWLEDGMENTS
This work was supported by grants AOM 95221 Programme Hospitalier de Recherche Clinique and INSERM-CNAMTS grant 3AM051.
We thank J. R. Taylor and D. Geiser for English and scientific corrections and R. Summerbell for the useful suggestion that we use Good’s hypothesis to analyze our data.
REFERENCES
- 1.Aisner J, Murillo J, Schimpff S C, Steere A C. Invasive aspergillosis in acute leukemia: correlation with nose cultures and antibiotic use. Ann Intern Med. 1979;90:4–9. doi: 10.7326/0003-4819-90-1-4. [DOI] [PubMed] [Google Scholar]
- 2.Aisner J, Schimpff S C, Bennett J E, Young V M, Wiernik P H. Aspergillus infections in cancer patients: association with fire proofing materials in a new hospital. JAMA. 1976;235:411–412. [PubMed] [Google Scholar]
- 3.Albelda S M, Talbot G H, Gerson S L, Miller W T, Cassileth P A. Role of fiberoptic bronchoscopy in the diagnosis of invasive pulmonary aspergillosis in patients with acute leukemia. Am J Med. 1984;76:1027–1034. doi: 10.1016/0002-9343(84)90853-2. [DOI] [PubMed] [Google Scholar]
- 4.Arbeit R D, Maslow J N, Mulligan M E. Polymerase chain reaction-mediated genotyping in microbial epidemiology. Clin Infect Dis. 1994;18:1018–1019. doi: 10.1093/clinids/18.6.1017. [DOI] [PubMed] [Google Scholar]
- 5.Arnow P M, Andersen R L, Mainous P D, Smith E J. Pulmonary aspergillosis during hospital renovation. Am Rev Respir Dis. 1978;118:49–53. doi: 10.1164/arrd.1978.118.1.49. [DOI] [PubMed] [Google Scholar]
- 6.Arnow P M, Sadigh M, Costas C, Weil D, Chudy R. Endemic and epidemic aspergillosis associated with in-hospital replication of Aspergillus organisms. J Infect Dis. 1991;164:998–1002. doi: 10.1093/infdis/164.5.998. [DOI] [PubMed] [Google Scholar]
- 7.Barnes R A, Rogers T R. Control of an outbreak of nosocomial aspergillosis by laminar air-flow isolation. J Hosp Infect. 1989;14:89–94. doi: 10.1016/0195-6701(89)90110-2. [DOI] [PubMed] [Google Scholar]
- 8.Belkacemi L, Hopwood V, Barton R C, Evans E G V. Typage moléculaire d’Aspergillus fumigatus par la technique de random amplified polymorphic DNA (RAPD) J Mycol Med. 1996;6:197–198. [Google Scholar]
- 9.Bodey G, Bueltmann B, Duguid W, Gibbs D, Hanak H, Hotchi M, Mall G, Martino P, Meunier F, Milliken S, Naoe S, Okudaira M, Scevola D, Van’t Wout J. Fungal infections in cancer patients: an international autopsy survey. Eur J Clin Microbiol Infect Dis. 1992;11:99–109. doi: 10.1007/BF01967060. [DOI] [PubMed] [Google Scholar]
- 10.Bodey G P, Vartivarian S. Aspergillosis. Eur J Clin Microbiol Infect Dis. 1989;8:413–437. doi: 10.1007/BF01964057. [DOI] [PubMed] [Google Scholar]
- 11.Buffington J, Reporter R, Lasker B A, McNeil M M, Lanson J M, Ross L A, Mascola L, Jarvis W R. Investigation of an epidemic of invasive aspergillosis: utility of molecular typing with the use of random amplified polymorphic DNA probes. Pediatr Infect Dis J. 1994;13:386–393. [PubMed] [Google Scholar]
- 12.Debeaupuis J P, Sarfati J, Chazalet V, Latgé J P. Genetic diversity among clinical and environmental isolates of Aspergillus fumigatus. Infect Immun. 1997;65:3080–3085. doi: 10.1128/iai.65.8.3080-3085.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denning D W. Invasive aspergillosis in immunocompromised patients. Curr Opin Infect Dis. 1994;7:456–462. [Google Scholar]
- 14.Fisher B D, Armstrong D, Yu B, Gold J W M. Invasive aspergillosis: progress in early diagnosis and treatment. Am J Med. 1981;71:571–577. doi: 10.1016/0002-9343(81)90208-4. [DOI] [PubMed] [Google Scholar]
- 15.Gerson S T, Talbot G H, Hurwitz S, Strom B L, Lusk E J, Cassileth P A. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345–351. doi: 10.7326/0003-4819-100-3-345. [DOI] [PubMed] [Google Scholar]
- 16.Girardin H, Latgé J P, Srikantha T, Morrow B, Soll D R. Development of DNA probes for fingerprinting Aspergillus fumigatus. J Clin Microbiol. 1993;31:1547–1554. doi: 10.1128/jcm.31.6.1547-1554.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girardin H, Sarfati J, Traoré F, Dupouy-Camet J, Derouin F, Latgé J P. Molecular epidemiology of nosocomial invasive aspergillosis. J Clin Microbiol. 1994;32:684–690. doi: 10.1128/jcm.32.3.684-690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gochenor S E. Fungi of a Long Island oak-birch forest. II. Population dynamics and hydrolase patterns for the soil Penicillia. Mycologia. 1984;76:218–231. [Google Scholar]
- 19.Good I J. The population frequencies of species and the estimation of population parameters. Biometrika. 1953;40:237–264. [Google Scholar]
- 20.Horvath J, Dummer S. The use of respiratory-tract cultures in the diagnosis of invasive pulmonary aspergillosis. Am J Med. 1996;100:171–178. doi: 10.1016/s0002-9343(97)89455-7. [DOI] [PubMed] [Google Scholar]
- 21.Iwen P C, Davis J C, Reed E C, Winfield B A, Hinrichs S H. Airborne fungal spore monitoring in a protective environment during hospital construction, and correlation with an outbreak of invasive aspergillosis. Infect Control Hosp Epidemiol. 1994;15:303–306. doi: 10.1086/646916. [DOI] [PubMed] [Google Scholar]
- 22.Iwen P C, Reed E C, Armitage J O, Bierman P J, Kessinger A, Vose J M, Arneson M A, Winfield B A, Woods G L. Nosocomial invasive aspergillosis in lymphoma patients treated with bone marrow or peripheral stem cell transplants. Infect Control Hosp Epidemiol. 1993;14:131–139. doi: 10.1086/646698. [DOI] [PubMed] [Google Scholar]
- 23.Jeffery G M, Beard M E J, Ikram R B, Chua J, Allen J R, Heaton D C, Hart D N J, Schousboe M I. Intranasal amphotericin-B reduces the frequency of invasive aspergillosis in neutropenic patients. Am J Med. 1991;90:685–692. [PubMed] [Google Scholar]
- 24.Kahn F W, Jones J M, England D M. The role of bronchoalveolar lavage in the diagnosis of invasive aspergillosis. Am J Clin Pathol. 1986;86:518–523. doi: 10.1093/ajcp/86.4.518. [DOI] [PubMed] [Google Scholar]
- 25.Leenders A, Van Belkum A, Janssen S, De Marie S, Kluytmans J, Wielenga J, Löwenberg B, Verbrugh H. Molecular epidemiology of apparent outbreak of invasive aspergillosis in an hematology ward. J Clin Microbiol. 1996;34:345–351. doi: 10.1128/jcm.34.2.345-351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin D M, Lehmann P F, Hamory B H, Padhye A A, Durry E, Pinner R W, Lasker B A. Comparison of three typing methods for clinical and environmental isolates of Aspergillus fumigatus. J Clin Microbiol. 1995;33:1596–1601. doi: 10.1128/jcm.33.6.1596-1601.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loudon K W, Burnie J P, Coke A P, Matthews R C. Application of polymerase chain reaction to fingerprinting Aspergillus fumigatus by random amplification of polymorphic DNA. J Clin Microbiol. 1993;31:1117–1122. doi: 10.1128/jcm.31.5.1117-1121.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer R D, Young L S, Armstrong D, Yu B. Aspergillosis complicating neoplastic disease. Am J Med. 1973;54:6–15. doi: 10.1016/0002-9343(73)90077-6. [DOI] [PubMed] [Google Scholar]
- 29.Moore W E C, Holdeman L V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974;27:961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nalesnik M A, Myerowitz R L, Jenkins R, Lenkey J, Herbert D. Significance of Aspergillus species isolated from respiratory secretions in the diagnosis of invasive pulmonary aspergillosis. J Clin Microbiol. 1980;11:370–376. doi: 10.1128/jcm.11.4.370-376.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neuvéglise C, Sarfati J, Debeaupuis J P, Vu-Thien H, Just J, Tournier G, Latgé J P. Longitudinal study of Aspergillus fumigatus strains isolated from cystic fibrosis patients. Eur J Clin Microbiol Infect Dis. 1997;16:747–750. doi: 10.1007/BF01709257. [DOI] [PubMed] [Google Scholar]
- 32.Neuvéglise C, Sarfati J, Latgé J P, Paris S. Afut1, a retrotransposon-like element from Aspergillus fumigatus. Nucleic Acids Res. 1996;24:1428–1434. doi: 10.1093/nar/24.8.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patterson J E, Zidouh A, Miniter P, Andriole V T, Patterson T F. Hospital epidemiologic surveillance for invasive aspergillosis: patient demographics and the utility of antigen detection. Infect Control Hosp Epidemiol. 1997;18:104–108. doi: 10.1086/647563. [DOI] [PubMed] [Google Scholar]
- 34.Ribaud P, Esperou-Bourdeau H, Devergie A, Gluckman E. Aspergillose invasive et allogreffe de moelle. Pathol Biol. 1994;43:652–655. [PubMed] [Google Scholar]
- 35.Rodriguez E, De Meeus T, Mallié M, Renaud F, Symoens F, Mondon P, Piens M A, Lebeau B, Viviani M A, Grillot R, Nolard N, Chapuis F, Tortorano A M, Bastide J M. Multicentric epidemiological study of Aspergillus fumigatus isolates by multilocus enzyme electrophoresis. J Clin Microbiol. 1996;34:2559–2568. doi: 10.1128/jcm.34.10.2559-2568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogers T R. Epidemiology and control of nosocomial fungal infections. Curr Opin Infect Dis. 1995;8:287–290. [Google Scholar]
- 37.Schwartz D. Méthodes statistiques à l’usage des médecins et des biologistes. Statistique en biologie et en médecine. Flammarion, Paris, France: Médecine-Sciences; 1963. [Google Scholar]
- 38.Sherertz R J, Belani A, Kramer B S, Elfenbein G J, Weiner R S, Sullivan M L, Thomas R G, Samsa G P. Impact of air filtration on nosocomial Aspergillus infection. Unique risk of bone marrow transplant recipients. Am J Med. 1987;83:709–718. doi: 10.1016/0002-9343(87)90902-8. [DOI] [PubMed] [Google Scholar]
- 39.Wald A, Leisenring W, Van Burik J A, Bowden R A. Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–1466. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 40.Walsh T J, Dixon D M. Nosocomial aspergillosis: environmental microbiology, hospital epidemiology, diagnosis and treatment. Eur J Epidemiol. 1989;5:131–142. doi: 10.1007/BF00156818. [DOI] [PubMed] [Google Scholar]
- 41.Wingard J R, Beals S U, Santos G W, Merz W G, Saral R. Aspergillus infections after bone marrow transplant. Bone Marrow Transplant. 1987;2:175–181. [PubMed] [Google Scholar]
- 42.Yu V L, Muder R R, Poorsattar A. Significance of isolation of Aspergillus from respiratory tract in the diagnosis of invasive pulmonary aspergillosis. Results from three-year prospective study. Am J Med. 1986;81:249–254. doi: 10.1016/0002-9343(86)90259-7. [DOI] [PubMed] [Google Scholar]