Abstract
Simple Summary
This multicenter cohort study is the first to compare the clinical outcomes between the Atezolizumab-plus-bevacizumab (Ate/Bev) and transarterial-chemoembolization-plus-radiotherapy (TACE + RT) therapies in hepatocellular carcinoma (HCC) patients with portal vein tumor thrombosis (PVTT) who had no metastasis. Through detailed analyses, our study revealed that the Ate/Bev treatment provided superior one-year survival compared to the TACE + RT treatment. The superior outcome of the Ate/Bev therapy was constantly observed in patients with an extensive HCC burden. Meanwhile, patients with unilobar disease demonstrated comparable outcomes between the two treatment groups. Finally, in the propensity score-matching analysis, both one-year survival and progression-free survival rates were higher in the Ate/Bev treatment group. These results suggest that Ate/Bev treatment should be considered as the primary treatment option for HCC patients with PVTT. With respect to TACE + RT, this could also be considered as an alternative treatment option alongside Ate/Bev therapy in patients with unilobar intrahepatic HCC.
Abstract
This study aimed to compare the treatment outcomes of atezolizumab-plus-bevacizumab (Ate/Bev) therapy with those of transarterial chemoembolization plus radiotherapy (TACE + RT) in hepatocellular carcinoma (HCC) patients with portal vein tumor thrombosis (PVTT) and without metastasis. Between June 2016 and October 2022, we consecutively enrolled 855 HCC patients with PVTT. After excluding 758 patients, 97 patients (n = 37 in the Ate/Bev group; n = 60 in the TACE + RT group) were analyzed. The two groups showed no significant differences in baseline characteristics and had similar objective response and disease control rates. However, the Ate/Bev group showed a significantly higher one-year survival rate (p = 0.041) compared to the TACE + RT group, which was constantly displayed in patients with extensive HCC burden. Meanwhile, the clinical outcomes were comparable between the two groups in patients with unilobar intrahepatic HCC. In Cox-regression analysis, Ate/Bev treatment emerged as a significant factor for better one-year survival (p = 0.049). Finally, in propensity-score matching, the Ate/Bev group demonstrated a better one-year survival (p = 0.02) and PFS (p = 0.01) than the TACE + RT group. In conclusion, Ate/Bev treatment demonstrated superior clinical outcomes compared to TACE + RT treatment in HCC patients with PVTT. Meanwhile, in patients with unilobar intrahepatic HCC, TACE + RT could also be considered as an alternative treatment option alongside Ate/Bev therapy.
Keywords: hepatocellular carcinoma, portal vein thrombosis, survival, progression free survival, response, immune checkpoint inhibitors, transarterial chemoembolization, radiotherapy
1. Introduction
Liver cancer is the seventh most common cancer worldwide, and hepatocellular carcinoma (HCC) is the most dominant type of liver cancer, accounting for the fourth most common cause of cancer mortality [1,2]. Although surveillance for HCC has been conducted using ultrasonography and serum alpha-fetoprotein, more than half of patients with HCC are at an intermediate stage or advanced stage [2]. For patients with advanced-stage HCC, systemic therapy is the mainstream treatment, and sorafenib, a multi-kinase inhibitor, has been widely used as the first-line therapy over the past decade [3].
Recently, immune checkpoint inhibitors (ICIs) have revolutionized the treatment strategy for advanced hepatocellular carcinoma. Particularly, atezolizumab-plus-bevacizumab (Ate/Bev) therapy provides better treatment outcomes compared to sorafenib [4,5]. The IMbrave150 study demonstrated a one-year survival rate of 67.2% with Ate/Bev compared to 54.6% with sorafenib [4]. Therefore, Ate/Bev therapy is recommended as the first-line therapy for advanced HCC, including for patients with portal vein tumor thrombosis (PVTT) [6,7,8,9].
Meanwhile, for HCC patients with macrovascular invasion, including PVTT, transarterial chemoembolization plus radiotherapy (TACE + RT) has also demonstrated better survival than sorafenib in a randomized clinical trial (RCT) [10]. The RCT showed that the TACE + RT group had a significantly longer progression-free survival (PFS) and overall survival compared to the sorafenib group (55.0 vs. 43.0 weeks, respectively; p = 0.04) [10]. Based on these results, the guidelines from the 2022 Korean HCC guideline have placed TACE + RT (with a grade B for the quality of evidence) as a recommended treatment option for HCC patients with PVTT. This recommendation stands alongside Ate/Bev therapy, which holds a grade A for the quality of evidence [11].
However, there have been limited data on comparing the efficacy and safety between Ate/Bev and TACE + RT in HCC patients with PVTT. This raises the question of which treatment provides better outcomes for HCC patients with PVTT. To address this question, we aimed to compare the treatment outcomes of Ate/Bev and TACE + RT in HCC patients with PVTT.
2. Materials and Methods
2.1. Patients
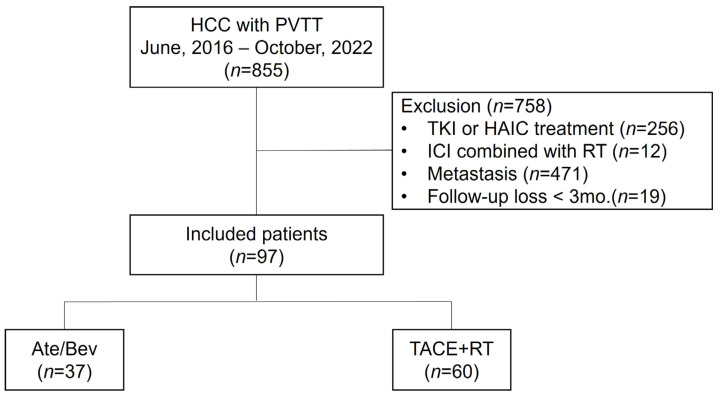
In this retrospective, multicenter study conducted across eight university hospitals that are part of The Catholic University of Korea, HCC patients with PVTT (n = 855), who were treated between June 2016 and October 2022, were consecutively screened for eligibility. Among them, 758 patients were excluded for the following reasons: patients treated with tyrosine kinase inhibitors or hepatic artery infusion therapy (n = 256), patients treated with ICI and RT simultaneously (n = 12), patients with distant metastasis (n = 471), and patients lost to follow-up before three months (n = 19). Finally, 97 patients treated with Ate/Bev (n = 37) or TACE + RT (n = 60) were included and analyzed (Figure 1). HCC was diagnosed based on histologic and/or radiologic findings according to international guidelines [12,13]. The presence of PVTT was confirmed using multiphase contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI), and the grade of PVTT was classified according to the Liver Cancer Study Group of Japan [12,13,14]. This study was conducted following the Declaration of Helsinki and was approved by the Institutional Review Board of the Catholic University of Korea (XC23RIDI0050).
Figure 1.
Study flow chart. HCC—hepatocellular carcinoma; VP4 PVTT—major portal vein tumor thrombus; TKIs—tyrosine kinase inhibitors; HAIC—hepatic artery infusion chemotherapy; ICIs—immune checkpoint inhibitors; RT—radiotherapy.
2.2. Treatment Regimens
Ate/Bev therapy was administered intravenously at a dose of 1200 mg of atezolizumab plus 15 mg/kg of body weight of bevacizumab every 3 weeks and continued until the presence of disease progression or intolerable toxicity [4]. For TACE treatment, 2 mg/kg of cisplatin or doxorubicin (50 mg) was infused after selection of the feeding artery for HCC. Subsequently, the feeding arteries were embolized using a mixture of 5–10 mL of cisplatin and iodized oil (Lipiodol Ultra-Fluide; Laboratoire Guerbet, Aulnay-Sous-Bois, France), followed by a gelatin sponge (Gelfoam; Upjohn, Hastings, MI, USA) in patients with appropriate portal blood flow. However, for patients with severely impaired portal blood flow, the embolization was not omitted. TACE treatment was repeated every 6–8 weeks according to the patient’s status [10]. After the first TACE, external beam RT was performed on the PVTT with a planned total dose of 50 Gy in 5 fractions.
2.3. Assessment of Outcomes
Given the recent widespread use of Ate/Bev treatment in Korea, we chose one-year survival as the primary outcome. This was defined as the time interval from the initiation of treatment to either death or the last follow-up of up to one year. Secondary outcomes included the one-year PFS, objective response rate (ORR), and disease control rate (DCR). Treatment responses were assessed by multiphase liver CT or MRI every 6 to 9 weeks according to Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) [15]. Measurements of the tumor and responses to the treatments were independently reviewed by two expert radiologists (D.J. Shim and D. Kim) without knowledge of the treatment methods and clinical outcomes. PFS was defined as the length of time from the first treatment time to progression according to RECIST 1.1, death, or the last follow-up. Treatment-related adverse events (AEs) were evaluated using the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE v5.0) [16].
2.4. Statistical Analysis
Baseline characteristics of the included patients are represented as a mean ± standard deviation or median (interquartile range) for quantitative variables and as counts (%) for categorical variables, as appropriate. Comparisons between groups were analyzed using the Student’s t-test or Mann–Whitney U test for continuous variables and the chi-square test or Fisher’s exact test for categorical variables, as appropriate. Kaplan–Meier analysis was used to estimate the one-year survival and one-year PFS. To identify the risk factors for one-year survival, Cox-regression analysis was performed. Furthermore, to mitigate selection bias and potential confounders by equating baseline variables between the Ate/Bev and TACE + RT groups, we employed a 2:1 nearest-neighbor propensity score matching (PSM) method. Two-sided p-values < 0.05 were considered significant. All statistical analyses were conducted using R software (version 4.3.0; http://carn.r-project.org accessed on 21 April 2023).
3. Results
3.1. Baseline Characteristics
The mean age of participants (N = 97) was 59.1 years, and 87 patients (89.7%) were male. The majority of patients (n = 73, 76.0%) had hepatitis B infection. The median alpha-fetoprotein (AFP) level was 240.3 ng/mL, and the mean tumor size was 8.2 cm. Approximately half of the included patients (n = 45, 46.4%) had grade-4 PVTT (VP4) at the time of treatment, and there were no significant differences in baseline characteristics between the Ate/Bev and TACE + RT groups (Table 1).
Table 1.
Baseline characteristics of entire population before and after propensity score matching.
| Variables | Total (n = 97) |
General Model | Propensity Score Matching Model | ||||
|---|---|---|---|---|---|---|---|
| Ate/Bev Group | TACE + RT Group | p-Value | Ate/Bev Group | TACE + RT Group | p-Value | ||
| (n = 37) | (n = 60) | (n = 27) | (n = 32) | ||||
| Age, years | 59.1 ± 10.7 | 60.7 ± 12.9 | 58.1 ± 9.0 | 0.283 | 58.1 ± 12.0 | 59.6 ± 9.1 | 0.578 |
| Male | 87 (89.7%) | 35 (94.6%) | 52 (86.7%) | 0.366 | 25 (92.6%) | 30 (93.8%) | 1.000 |
| Cause (n, %) | 0.074 | 0.954 | |||||
| HBV | 73 (76.0%) | 24 (64.9%) | 49 (81.7%) | 20 (74.1%) | 25 (78.1%) | ||
| Others | 23 (24.2%) | 13 (35.1%) | 11 (18.3%) | 7 (25.9%) | 7 (21.9%) | ||
| Treatment-naïve (n, %) | 67 (69.1%) | 22 (59.5%) | 45 (75.0%) | 0.167 | 16 (59.3%) | 25 (78.1%) | 0.199 |
| AST, IU/mL | 70.5 ± 46.1 | 64.1 ± 35.6 | 74.5 ± 51.5 | 0.245 | 68.5 ± 38.2 | 78.5 ± 56.6 | 0.425 |
| ALT, IU/mL | 42.2 ± 31.0 | 35.0 ± 23.7 | 46.5 ± 33.6 | 0.051 | 38.6 ± 26.6 | 49.2 ± 41.3 | 0.240 |
| T.bil, mg/dL | 1.0 ± 0.6 | 0.9 ± 0.7 | 1.1 ± 0.5 | 0.205 | 1.0 ± 0.7 | 1.1 ± 0.5 | 0.945 |
| Alb, g/dL | 3.7 ± 0.6 | 3.8 ± 0.4 | 3.7 ± 0.7 | 0.158 | 3.9 ± 0.4 | 3.9 ± 0.4 | 0.855 |
| PLT, 103/μL | 151.7 ± 64.2 | 162.6 ± 66.0 | 145.0 ± 62.6 | 0.190 | 169.0 ± 69.2 | 151.9 ± 66.6 | 0.339 |
| INR. | 1.1 [1.0; 1.2] | 1.1 [1.0; 1.1] | 1.1 [1.0; 1.2] | 0.293 | 1.1 [1.0; 1.2] | 1.1 [1.0; 1.2] | 0.976 |
| Cr, mg/dL | 0.8 [0.7; 0.9] | 0.7 [0.7; 0.9] | 0.8 [0.6; 0.9] | 0.442 | 0.7 [0.7; 0.9] | 0.9 [0.6; 1.0] | 0.488 |
| CP class A | 84 (86.6%) | 34 (91.9%) | 50 (83.3%) | 0.371 | 24 (88.9%) | 27 (84.4%) | 0.902 |
| AFP, ng/mL | 240.3 [23.9; 1917.6] | 484.4 [40.0; 3175.0] | 189.2 [20.4; 1408.4] | 0.277 | 237.0 [29.9; 2730.0] | 274.6 [27.1; 1408.4] | 0.632 |
| PIVKA, mAU/mL | 1723.0 [161.0; 6670.0] | 2877.0 [230.0; 7521.0] | 1344.0 [140.9; 5870.5] | 0.653 | 2877.0 [245.4; 7753.3] | 3436.3 [208.8; 7065.6] | 0.849 |
| Tumor Size, cm | 8.2 ± 4.6 | 7.6 ± 4.8 | 8.5 ± 4.5 | 0.354 | 7.8 ± 5.0 | 8.8 ± 4.9 | 0.448 |
| Multiple intrahepatic HCC (≥2 nodule) | 68 (70.1%) | 27 (73.0%) | 41 (68.3%) | 0.798 | 19 (70.4%) | 23 (71.9%) | 1.000 |
| Bilobar intrahepatic HCC | 34 (35.1%) | 14 (37.8%) | 20 (33.3%) | 0.816 | 10 (37.0%) | 12 (37.5%) | 1.000 |
| VP4 PVTT | 45 (46.4%) | 15 (40.5%) | 30 (50.0%) | 0.485 | 13 (48.1%) | 15 (46.9%) | 1.000 |
Ate/Bev—atezolizumab plus bevacizumab; TACE + RT—transarterial chemoembolization plus radiotherapy; AST—aspartate aminotransaminase; ALT—alanine aminotransaminase; T.bil—total bilirubin; Alb—albumin; PLT—platelet; INR—international normalized ratio; Cr, creatinine; CP—Child–Pugh; AFP—alpha-fetoprotein; PIVKA—Protein induced by vitamin K absence-II; HCC—hepatocellular carcinoma; VP4 PVTT—major portal vein tumor thrombus.
3.2. One-Year Survival and PFS in the Entire Population
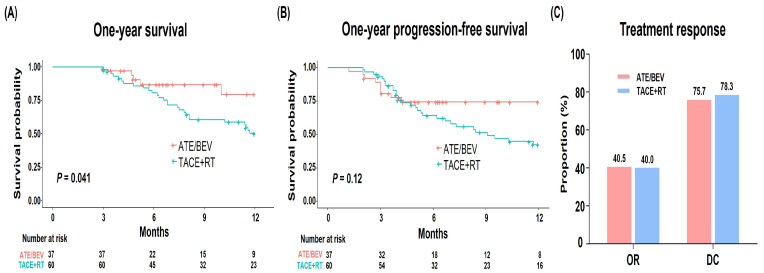
During the one-year follow-up, a total of 32 patients (33.0%), consisting of 5 patients from the Ate/Bev group and 27 patients from the TACE/RT group, died due to hepatic failure or HCC progression. The survival rate at one year was significantly higher in the Ate/Bev group compared to the TACE + RT group (79.7% vs. 50.3%, respectively; p = 0.041; Figure 2A). The one-year PFS was marginally higher in the Ate/Bev group (74.4% vs. 42.4%, respectively; p = 0.12; Figure 2B). Moreover, patients in the Ate/Bev group, who demonstrated a robust response at approximately 3 months, generally sustained this response throughout the first year.
Figure 2.
(A) One-year survival, (B) progression-free survival, and (C) treatment response in the entire cohort.
3.3. Treatment Responses and AEs
During treatment, the Ate/Bev and TACE + RT showed a similar ORR (n = 15, 40.5% vs. n = 24, 40.0%, respectively; p = 1.000) and DCR (n = 28, 75.7% vs. n = 47, 78.3%, respectively; p = 0.957) (Figure 2C). Of the 97 patients, 16 patients (16.5%) experienced AEs, without significant differences between the Ate/Bev and TACE + RT groups (18.9% vs. 15.0%, respectively; p = 0.823). This lack of statistical difference persisted when we compared AEs grade-wise. Specifically, in the Ate/Bev group, the two most common AEs were proteinuria (n = 3; grade 1) and variceal bleeding (n = 2; grade 3). No immune-related AEs were observed during Ate/Bev therapy. In the TACE + RT group, variceal bleeding (n = 5; grade 3) was the most frequent AE (Table 2). Additionally, two patients in the TACE + RT group experienced HCC rupture (grade 4).
Table 2.
Adverse events (AEs).
| Adverse Events (AEs) | Total | Ate/Bev | TACE + RT | p-Value |
|---|---|---|---|---|
| (n = 97) | (n = 37) | (n = 60) | ||
| AE yes | 16 (16.5%) | 7 (18.9%) | 9 (15.0%) | 0.823 |
| AE types according to grades | ||||
| Grade 1–2 | 0.068 | |||
| - Proteinuria | 3 (3.1%) | 3 (8.1%) | 0 (0.0%) | |
| - Others | 2 (2.1%) | 1 (2.7%) | 1 (1.7%) | |
| Grade 3–4 | 0.524 | |||
| - Varix bleeding | 7 (7.2%) | 2 (5.4%) | 5 (8.3%) | |
| - Hepatic encephalopathy | 2 (2.1%) | 1 (2.7%) | 1 (1.7%) | |
| - HCC rupture | 2 (2.1%) | 0 (0.0%) | 2 (3.3%) |
3.4. Subgroup Analysis
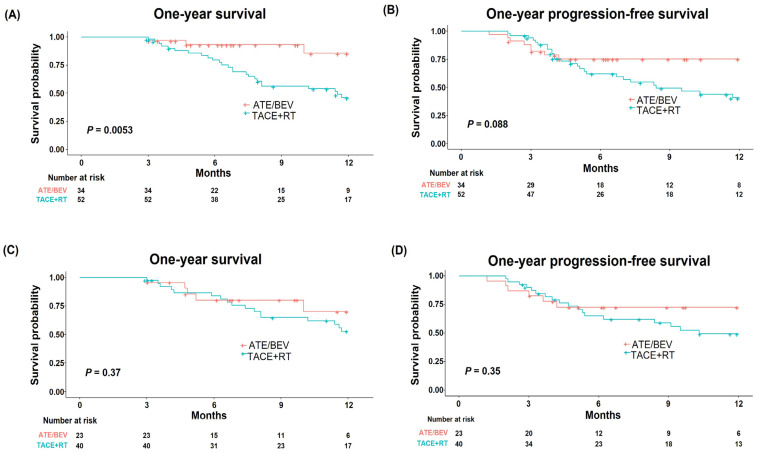
Because most patients had extensive HCC burden (n = 86, 88.7%), we further evaluated the efficacy of both treatments in those patients. Extensive HCC burden was defined as HCC patients with multiple tumors, a tumor size of ≥7 cm, or VP4 PVTT. Due to the poor prognosis associated with VP4 PVTT compared to VP1–3 PVTT, patients with VP4 were included in the extensive-HCC-burden category [14,17]. In patients with extensive HCC burden, the Ate/Bev group displayed a higher one-year survival rate compared to the TACE + RT group (85.7% vs. 46.2%, respectively; p = 0.005; Figure 3A). The one-year PFS was marginally higher in the Ate/Bev group compared to the TACE + RT group (75.4% vs. 40.8%, respectively; p = 0.088; Figure 3B). A superior one-year survival rate and marginally higher one-year PFS in the Ate/Bev group compared to the TACE + RT group were also demonstrated in patients with multiple intrahepatic HCC (n = 68, 70.1%; Supplementary Figure S1A,B).
Figure 3.
One-year survival and progression-free survival in patients with (A,B) extensive HCC burden and (C,D) unilobar intrahepatic HCC. Extensive HCC burden was defined as HCC patients with multiple tumors, a tumor size of ≥7 cm, or VP4 PVTT. HCC—hepatocellular carcinoma; VP4 PVTT—major portal vein tumor thrombus.
To assess the potential efficacy of TACE in patients with unilobar intrahepatic HCC, we analyzed treatment outcomes in this patient group (n = 63, 64.9%). The one-year survival was similar between the Ate/Bev and TACE + RT groups (70.2% vs. 52.8%, respectively; p = 0.37; Figure 3C). The Ate/Bev group also showed a one-year PFS comparable to that of the TACE + RT group (p = 0.36, Figure 3D). Comparable results were consistently observed in patients with unilobar intrahepatic HCC without VP4 PVTT (Supplementary Figure S1C,D), suggesting the possibility of TACE + RT as an alternative treatment option in these patient groups.
3.5. Factors Associated with One-Year Survival and PSM Analysis
Given the overall superior efficacy of the Ate/Bev group compared to the TACE + RT group, we sought to reaffirm the effectiveness of Ate/Bev treatment using Cox-regression analysis and PSM. In Cox-regression analysis, the Ate/Bev treatment was documented as the only significant risk factor for one-year survival (hazard ratio, 0.38; 95% confidence interval, 0.15–1.00; p = 0.049; Table 3).
Table 3.
Cox-regression analysis for one-year survival.
| Characteristics | HR | 95% CI | p-Value |
|---|---|---|---|
| Age ≥ 60 | 1.75 | 0.86, 3.54 | 0.12 |
| Male | 2.15 | 0.51, 9.03 | 0.3 |
| Ate/Bev Tx. | 0.38 | 0.15, 1.00 | 0.049 |
| AST ≥ 60, IU/mL | 1.47 | 0.73, 2.96 | 0.3 |
| ALT ≥ 40, IU/mL | 1.16 | 0.58, 2.31 | 0.7 |
| T.bil ≥ 1.0, mg/dL | 1.97 | 0.98, 3.96 | 0.058 |
| Alb ≤ 3.5, g/dL | 1.13 | 0.46, 2.75 | 0.8 |
| INR ≥ 1.1 | 1.00 | 0.50, 2.00 | 1.0 |
| AFP ≥ 200, ng/mL | 0.93 | 0.47, 1.87 | 0.8 |
| PIVKA ≥ 40, mAU/mL | 0.89 | 0.31, 2.55 | 0.8 |
| Size ≥ 8.0, cm | 1.42 | 0.71, 2.85 | 0.3 |
| Multiple intrahepatic HCC | 0.63 | 0.31, 1.28 | 0.2 |
| Bilobar intrahepatic HCC | 0.98 | 0.46, 2.07 | 1.0 |
| VP4 PVTT | 1.33 | 0.66, 2.66 | 0.4 |
HR—hazard ratio; CI—confidence interval; Ate/Bev Tx.; atezolizumab plus bevacizumab treatment; AST—aspartate transaminase, T.bil—total bilirubin; Alb—albumin; INR—international normalized ratio; AFP—alpha-fetoprotein; PIVKA—protein induced by vitamin K absence-II; HCC—hepatocellular carcinoma; VP4 PVTT—major portal vein tumor thrombus.
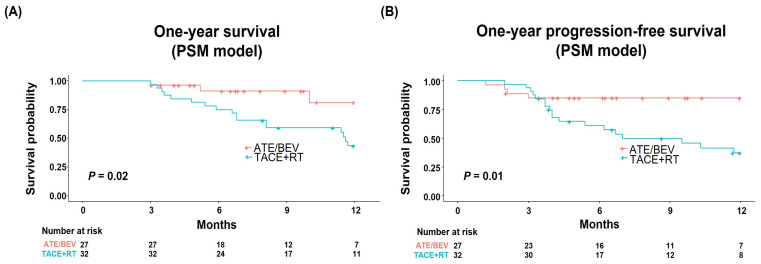
Finally, we compared the treatment outcomes of the two groups after PSM to further diminish any selection bias and possible confounders. The baseline characteristics were well balanced between the Ate/Bev (n = 27) and TACE + RT (n = 32) groups after PSM (Table 1). In the PSM model, the Ate/Bev group significantly outperformed the TACE + RT group in terms of both one-year survival (p = 0.02; Figure 4A) and one-year PFS (p = 0.01; Figure 4B), reaffirming the superior efficacy of the Ate/Bev treatment compared to TACE + RT.
Figure 4.
(A) One-year survival and (B) progression-free survival after propensity score matching.
4. Discussion
This multicenter cohort study is the first to compare the clinical outcomes of Ate/Bev and TACE + RT therapies in patients with PVTT who had no metastasis. Through detailed analyses, our study revealed that Ate/Bev treatment displayed better one-year survival compared to TACE + RT treatment. Furthermore, in the PSM analysis, both one-year survival and PFS rates were higher in the Ate/Bev treatment group. Thus, these results suggest that Ate/Bev treatment may be advantageous for the treatment of patients with PVTT.
Although Ate/Bev treatment has become a first-line therapy in advanced HCC cases, there are several other treatment options for patients with PVTT, including TACE + RT and hepatic artery infusion chemotherapy [11,18,19]. In addition, transarterial radioembolization presents an intriguing option, offering internal radiation without inducing vessel occlusion [20]. Among these treatments, as the TACE + RT has demonstrated superior outcomes compared to sorafenib [10], our study results elucidate the optimal choice for treating patients with PVTT by comparing Ate/Bev and TACE + RT. Indeed, the superior effectiveness of Ate/Bev therapy in our study reinforces the use of Ate/Bev treatment as the standard treatment for advanced HCC. In alignment with findings from the study by Richard Finn et al. [4], patients in the Ate/Bev group who responded well early on appeared to maintain this response without progression throughout the study period, potentially contributing to the superior outcomes of Ate/Bev therapy. Moreover, our results for one-year survival and PFS appear slightly superior to those reported in previous clinical trials [4,17]. This difference might be due to the distinct patient populations between the studies. Specifically, our study focused on HCC patients with PVTT who had no distant metastasis, and we included patients both with and without VP4 PVTT. These characteristics may have contributed to the more favorable outcomes observed in our cohort. Through this comparison, our findings underscore the potential advantages of Ate/Bev therapy over TACE + RT in treating HCC patients with PVTT.
Along with our novel results, suggesting the superior effectiveness of the Ate/Bev group, we also performed subgroup analyses to identify the preferred groups for each treatment. The superior outcome of the Ate/Bev therapy was constantly observed in patients with extensive HCC burden in our study. These results might be attributable to difficulties in embolizing feeding vessels during TACE in patients with VP4 PVTT and to the diminished efficacy of TACE in patients with a tumor size of ≥7 cm [21,22]. Meanwhile, patients with unilobar disease demonstrated comparable outcomes between the two groups. Given that TACE is technically more feasible for treating unilobar disease compared to bilobar disease, particularly in patients without VP4 PVTT [11,22], it seems that in addition to the Ate/Bev treatment, TACE + RT treatment could be considered as an alternative treatment option for these patient groups.
When treating HCC patients with PVTT, it is crucial to be mindful of potential AEs. In our analysis, we did not observe significant differences in the occurrence of AEs between the Ate/Bev and TACE + RT groups. However, grade 3 or 4 AEs, such as variceal bleeding or HCC rupture, were somewhat more prevalent in the TACE + RT group, indicating a potentially higher risk of severe toxicities with this approach. These observations bolster the safety profile of Ate/Bev therapy for HCC patients with PVTT. Meanwhile, when administering Ate/Be therapy, one must remain vigilant about the potential risk of reactivation of pre-existing autoimmune disease [20], despite the absence of patients with such diseases in our cohort.
Our study highlights the efficacy of Ate/Bev treatment, even in patients with PVTT. Atezolizumab, which inhibits PD-L1 on tumor cells or tumor-infiltrating immune cells, recovers the function of effector CD8+ T cells [23,24,25]. Moreover, bevacizumab shows synergistic effects by vessel normalization and a reduction in the immunomodulatory effect of VEGF on immune cells [26,27,28]. Considering the therapeutic mechanism of RT, which induces DNA damage and the remodeling of the tumor microenvironment, future studies using a combination of RT and Ate/Bev are necessary to improve the prognosis of patients with PVTT [29,30].
Our study has several limitations. First, there is the retrospective design of the present study. Second, this study analyzed a relatively small number of patients and had a short follow-up period. Because the Ate/Bev treatment was only covered by the Korean National Health Insurance starting from May 2022, its use has only recently become more widespread, resulting in a relatively short follow-up duration in our study. The one-year survival and PFS duration might seem short, but it is reasonable to use them to gauge the efficacy of both treatments, given the unfavorable prognosis in HCC patients with PVTT. Notably, this is the first study to compare the outcomes of the two treatments in HCC patients with PVTT. Despite the limited number of patients included, this was a multicenter study that reflected real-world data. Through detailed analyses, including PSM, our study provides insights into the superior efficacy of Ate/Bev therapy compared to TACE + RT therapy in treating HCC patients with PVTT.
5. Conclusions
In conclusion, our study suggests that Ate/Bev treatment yields superior clinical outcomes compared to TACE + RT in HCC patients with PVTT. In the context of current Korean HCC guidelines, which recommend both treatment options, our results could influence future updates by advocating for Ate/Bev as the primary treatment option for this patient population. For clinicians following different guidelines or practices, our findings offer evidence for considering Ate/Bev as a front-line therapy for HCC with PVTT. With respect to TACE + RT, it could also serve as an alternative treatment strategy alongside Ate/Bev therapy in patients with unilobar intrahepatic HCC. Given these observations, larger studies are warranted to further validate our findings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15174423/s1, Figure S1: One-year survival and progression-free survival in patients with (A,B) multiple intrahepatic HCC, and (C,D) unilobar intrahepatic HCC without VP4 PVTT. HCC, hepatocellular carcinoma; VP4 PVTT, major portal vein tumor thrombus.
Author Contributions
S.K.L. and J.H.K. (Jung Hyun Kwon) are responsible for the study concept and design. S.K.L., S.W.L., H.L.L., D.S.S., S.W.N., H.Y., J.M.Y., H.Y.K., A.L., H.N., S.-H.K. and C.W.K. collected the data. S.K.L., S.H.B., J.W.H., P.S.S., J.W.J., J.Y.C., J.H.K. (Ji Hoon Kim), M.J.S., U.I.C., D.J.S., D.K., M.K. and S.K.Y. analyzed and interpreted the data. S.K.L. and J.H.K. (Jung Hyun Kwon) wrote the manuscript. J.H.K. (Jung Hyun Kwon) supervised the study. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted following the Declaration of Helsinki and was approved by the Institutional Review Board of the Catholic University of Korea (XC23RIDI0050).
Informed Consent Statement
Patient consent was waived due to the retrospective study design.
Data Availability Statement
Data are not available due to ethical issues.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (No. 2021R1I1A1A01050954; S.K.L.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73((Suppl. S1)):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrero J.A., Kudo M., Venook A.P., Ye S.L., Bronowicki J.P., Chen X.P., Dagher L., Furuse J., Geschwind J.H., de Guevara L.L., et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J. Hepatol. 2016;65:1140–1147. doi: 10.1016/j.jhep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 5.Cheon J., Yoo C., Hong J.Y., Kim H.S., Lee D.W., Lee M.A., Kim J.W., Kim I., Oh S.B., Hwang J.E., et al. Efficacy and safety of atezolizumab plus bevacizumab in Korean patients with advanced hepatocellular carcinoma. Liver Int. 2022;42:674–681. doi: 10.1111/liv.15102. [DOI] [PubMed] [Google Scholar]
- 6.Su G.L., Altayar O., O’Shea R., Shah R., Estfan B., Wenzell C., Sultan S., Falck-Ytter Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology. 2022;162:920–934. doi: 10.1053/j.gastro.2021.12.276. [DOI] [PubMed] [Google Scholar]
- 7.Foerster F., Gairing S.J., Ilyas S.I., Galle P.R. Emerging immunotherapy for HCC: A guide for hepatologists. Hepatology. 2022;75:1604–1626. doi: 10.1002/hep.32447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valery M., Cervantes B., Samaha R., Gelli M., Smolenschi C., Fuerea A., Tselikas L., Klotz-Prieux C., Hollebecque A., Boige V., et al. Immunotherapy and Hepatocellular Cancer: Where Are We Now? Cancers. 2022;14:4523. doi: 10.3390/cancers14184523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reig M., Forner A., Rimola J., Ferrer-Fàbrega J., Burrel M., Garcia-Criado Á., Kelley R.K., Galle P.R., Mazzaferro V., Salem R., et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon S.M., Ryoo B.Y., Lee S.J., Kim J.H., Shin J.H., An J.H., Lee H.C., Lim Y.S. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma with Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018;4:661–669. doi: 10.1001/jamaoncol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin. Mol. Hepatol. 2022;28:583–705. doi: 10.3350/cmh.2022.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heimbach J.K., Kulik L.M., Finn R.S., Sirlin C.B., Abecassis M.M., Roberts L.R., Zhu A.X., Murad M.H., Marrero J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 13.Galle P.R., Forner A., Llovet J.M., Mazzaferro V., Piscaglia F., Raoul J.-L., Schirmacher P., Vilgrain V. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Khan A.R., Wei X., Xu X. Portal Vein Tumor Thrombosis and Hepatocellular Carcinoma—The Changing Tides. J. Hepatocell. Carcinoma. 2021;8:1089–1115. doi: 10.2147/JHC.S318070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Common Terminology Criteria for Adverse Events (CTCAE) Version 5. [(accessed on 27 November 2017)]; Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
- 17.Cheng A.L., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Lim H.Y., Kudo M., Breder V., Merle P., et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022;76:862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 18.Goh M.J., Sinn D.H., Kim J.M., Lee M.W., Hyun D.H., Yu J.I., Hong J.Y., Choi M.S. Clinical practice guideline and real-life practice in hepatocellular carcinoma: A Korean perspective. Clin. Mol. Hepatol. 2023;29:197–205. doi: 10.3350/cmh.2022.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo L., Wei X., Feng S., Zhai J., Guo W., Shi J., Lau W.Y., Meng Y., Cheng S. Radiotherapy prior to or after transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus: A randomized controlled trial. Hepatol. Int. 2022;16:1368–1378. doi: 10.1007/s12072-022-10423-7. [DOI] [PubMed] [Google Scholar]
- 20.Facciorusso A., Serviddio G., Muscatiello N. Transarterial radioembolization vs chemoembolization for hepatocarcinoma patients: A systematic review and meta-analysis. World J. Hepatol. 2016;8:770–778. doi: 10.4254/wjh.v8.i18.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang Y., Jeong S.W., Young Jang J., Jae Kim Y. Recent Updates of Transarterial Chemoembolilzation in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2020;21:8165. doi: 10.3390/ijms21218165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raoul J.L., Forner A., Bolondi L., Cheung T.T., Kloeckner R., de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi: 10.1016/j.ctrv.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.K., Choi J.Y., Jung E.S., Kwon J.H., Jang J.W., Bae S.H., Yoon S.K. An Immunological Perspective on the Mechanism of Drug Induced Liver Injury: Focused on Drugs for Treatment of Hepatocellular Carcinoma and Liver Transplantation. Int. J. Mol. Sci. 2023;24:5002. doi: 10.3390/ijms24055002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu X., Lu Y., Qin S. Atezolizumab and bevacizumab for hepatocellular carcinoma: Mechanism, pharmacokinetics and future treatment strategies. Future Oncol. 2021;17:2243–2256. doi: 10.2217/fon-2020-1290. [DOI] [PubMed] [Google Scholar]
- 25.Rajapakse J., Khatiwada S., Akon A.C., Yu K.L., Shen S., Zekry A. Unveiling the complex relationship between gut microbiota and liver cancer: Opportunities for novel therapeutic interventions. Gut Microbes. 2023;15:2240031. doi: 10.1080/19490976.2023.2240031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S.K., Lee S.W., Jang J.W., Bae S.H., Choi J.Y., Yoon S.K. Immunological Markers, Prognostic Factors and Challenges Following Curative Treatments for Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021;22:10271. doi: 10.3390/ijms221910271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y., Hu H., Yuan X., Fan X., Zhang C. Advances in Immune Checkpoint Inhibitors for Advanced Hepatocellular Carcinoma. Front. Immunol. 2022;13:896752. doi: 10.3389/fimmu.2022.896752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donne R., Lujambio A. The liver cancer immune microenvironment: Therapeutic implications for hepatocellular carcinoma. Hepatology. 2023;77:1773–1796. doi: 10.1002/hep.32740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z., Zhang Y., Hong W., Wang B., Chen Y., Yang P., Zhou J., Fan J., Zeng Z., Du S. Gut microbiota modulate radiotherapy-associated antitumor immune responses against hepatocellular carcinoma Via STING signaling. Gut Microbes. 2022;14:2119055. doi: 10.1080/19490976.2022.2119055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K., Yu H.M., Xiang Y.J., Cheng Y.Q., Ni Q.Z., Guo W.X., Shi J., Feng S., Zhai J., Cheng S.Q. Efficacy and safety of radiotherapy combined with atezolizumab plus bevacizumab in treating hepatocellular carcinoma with portal vein tumour thrombus: A study protocol. BMJ Open. 2022;12:e064688. doi: 10.1136/bmjopen-2022-064688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not available due to ethical issues.