Abstract
Simple Summary
Recurrent and metastatic head and neck cancer has limited treatment options and survival time is measured in months. Toll-like receptor agonists have been shown to improve tumor immune responses in preclinical studies and several clinical trials have now been performed. We performed a meta-analysis of existing clinical trials for recurrent and metastatic head and neck cancer and found there was no treatment benefit of these agents. While they do not appear to cause more adverse events, additional clinical trials may need to focus on new agents or drug combinations.
Abstract
Background: Recurrent and metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) has poor survival rates. Immunotherapy is the standard of care for R/M HNSCC, but objective responses occur in a minority of patients. Toll-like receptor (TLR) agonists promote antitumor immune responses and have been explored in clinical trials. Methods: A search for clinical trials using TLR agonists in HNSCC was performed under PRISMA guidelines. Data on patient characteristics, safety, and efficacy were collected and analyzed. Results: Three phase 1b trials with 40 patients and three phase 2 trials with 352 patients studying TLR8 and TLR9 agonists in combination with other treatment regimens for HNSCC were included. In phase 2 trials, there was no significant change in the objective response rate (RR = 1.13, CI 0.80–1.60) or association with increased grade 3+ adverse events (RR = 0.91, CI 0.76–1.11) associated with TLR agonist use. Conclusion: TLR agonists do not appear to provide additional clinical benefits or increase adverse events in the treatment of HNSCC. Given these results across multiple clinical trials and drug regimens, it is unlikely that additional trials of TLR agonists will demonstrate clinical benefits in HNSCC.
Keywords: immunotherapy, Toll-like receptor, head and neck cancer, meta-analysis, systematic review
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) arises from the upper aerodigestive tract and is the sixth most common malignancy worldwide [1]. HNSCC is treated with either surgical resection, radiation, chemotherapy, or a combination of these modalities, depending on its stage and location. This includes platinum-based chemotherapy in combination with cetuximab, especially in cases where a tumor is inoperable [2]. Based on studies using the SEER database, the 5-year survival for oral cavity and pharynx cancer was 68% and 61% for laryngeal cancer from 2012 to 2018 [3,4]. These survival rates have remained largely flat over the last several decades, despite improvements in surgical techniques, radiation delivery, and systemic therapies.
Over the last decade, immune checkpoint inhibitors (ICI) have emerged as an important treatment modality for HNSCC. These drugs inhibit the interaction between tumor and immune cells via the PD-1 and CTLA-4 receptors, thereby unmasking immunogenicity that leads to the increased immune cell infiltration of tumors. The Keynote-048 trial demonstrated that ICIs alone improve overall survival for recurrent and metastatic (R/M) HNSCC compared to cetuximab, establishing this regimen as the current standard of care for this treatment group [5]. Additionally, the combination of pembrolizumab with cetuximab has been potent [6]. Additional studies using ICIs in neoadjuvant and adjuvant settings are ongoing and likely to change treatment paradigms in the future. However, despite these successes, only about 20% of patients with HNSCC currently benefit from ICIs [7]. Considerable research is underway to identify pathways that may augment this response rate.
Toll-like receptors (TLRs) are key innate immune activating receptors capable of recognizing a set of conserved pathogens or damage-associated molecular patterns (PAMPs and DAMPs). TLRs can be expressed on the cell surface (TLR1, 2, 4, 5, 6) or on endosomal membranes (TLR3, 7, 8, and 9) [8]. In the context of cancer, antigen presenting cells, cancer cells, and other stromal cells can express TLRs and trigger inflammatory responses upon ligand binding [8]. TLR agonists are being increasingly evaluated as a therapeutic approach for cancer, as the engagement of TLRs can stimulate a pro-inflammatory cascade that could ultimately lead to greater tumor immune infiltration and the subsequent clearance of cancer cells or the potentiation of other immunotherapies. Here, we perform a meta-analysis of clinical trials of TLR agonists in HNSCC, including motolimod (TLR8 agonist), SD-101 (TLR9 agonist), IMO-2055 (TLR9 agonist), and EMD 1201081 (TLR9 agonist). For phase 1 trials we focus on evaluating the safety profile of the therapeutics, and for phase 2 trials we evaluate the efficacy of these agonists.
2. Materials and Methods
This systematic review was performed in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [9]. The search term “Toll like receptors AND head and neck cancer” was used as a query to search Pubmed, Embase, and the Cochrane Library. References were also queried within selected articles. Inclusion criteria were clinical trials studying injections of TLR agonists, adult (>18 years of age) patients with HNSCC, at least 3 patients present in the study, adverse event reporting, clinical outcome reporting, and English language articles. The search was independently performed by two authors (SM and AF). This study was registered with the Open Science Framework (OSF) in accordance with PRISMA guidelines (https://osf.io/t8539).
Statistical analysis was performed in the R statistical software (version 4.2.1) environment. The Metafor statistical package was used for meta-analysis [10]. Relative risk ratios for objective responses and adverse events were generated, comparing studies with either placebo or low-dose TLR agonists to high-dose TLR agonists. A random effects model was used to generate an estimated average risk ratio for all studies. Relevant clinicodemographic variables were also extracted from the articles for presentation.
3. Results
3.1. Study Selection
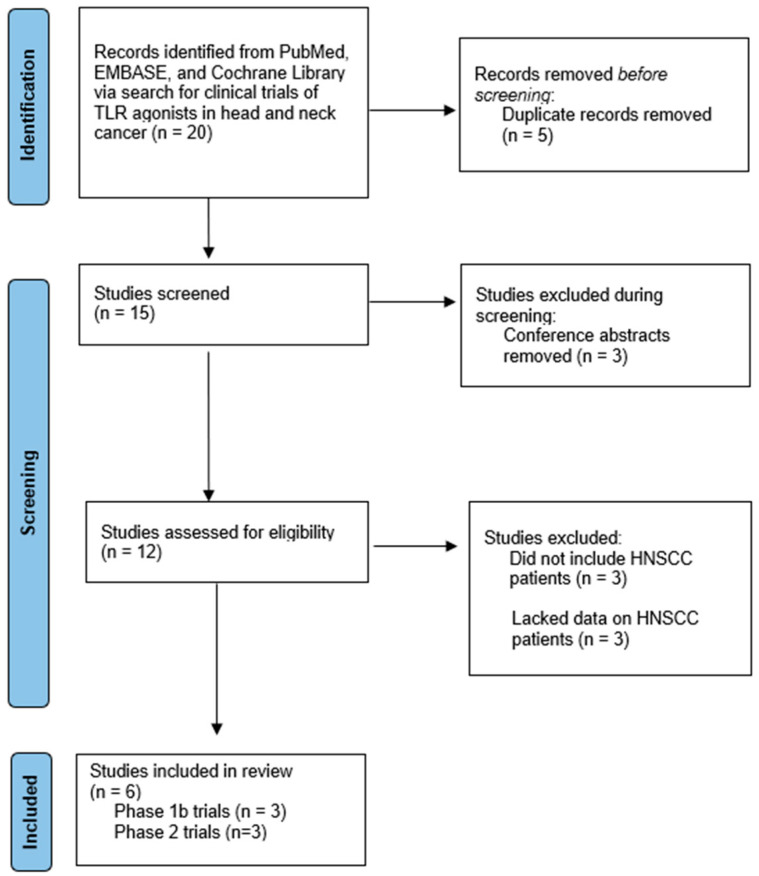
From the initial 334 hits, we identified 20 candidate studies. Five studies were excluded because they were duplicates listed in multiple databases surveyed. Three studies were excluded because they were conference abstracts that were not yet peer reviewed. Three studies were excluded because they did not include HNSCC patients. Finally, three studies were excluded because they had HNSCC patients but failed to provide enough information about these patients’ baseline characteristics, the safety and tolerability of treatment, and/or the efficacy of the treatment. This left us with six final studies that were included in our analysis. Three studies were phase 1b trials of either motolimod or IMO-2055. The other three studies were phase 2 trials of motolimod, SD-101, or EMD 1201081. For these six studies, we collected data on patient characteristics, the safety of TLR agonists and adverse events related to treatment, and the efficacy of therapy. Figure 1 outlines the selection process of studies included in this systematic review [11].
Figure 1.
PRISMA flow diagram of study screening and inclusion.
3.2. Phase 1b Trials
We evaluated the safety of TLR agonists in HNSCC as reported in three phase 1b clinical trials. Two trials evaluated motolimod and one trial evaluated IMO-2055 [12,13,14]. All three trials evaluated these TLR agonists in combination with other agents, including cetuximab. Table 1 reports the baseline patient characteristics of the cohorts enrolled in these trials. The three trials enrolled 13–14 patients each. There were notable differences between these studies. Importantly, Shayan et al. studied patients with untreated HNSCC in a neoadjuvant systemic therapy trial prior to surgery, while the other two papers studied R/M HNSCC. There were also differences in tumor subsite distribution between the two motolimod studies, with Chow et al. having a greater oropharyngeal cancer representation and Shayan et al. having a larger proportion of oral cavity tumors.
Table 1.
Baseline cohort characteristics for phase 1b trials.
| Study | Chow et al. 2017 [12] | Shayan et al. 2018 [13] | Machiels et al. 2013 [14] * |
|---|---|---|---|
| Agent | Motolimod | Motolimod | IMO-2055 |
| Treatment | Motolimod + cetuximab | Motolimod + cetuximab | IMO-2055 + 5-fluorouracil, cisplatin, and cetuximab |
| Patient Population | R/M HNSCC | Untreated HNSCC | R/M HNSCC |
| Number of Patients | 13 | 14 | 13 |
| Median Age | 62 | 61 | 59 |
| ECOG | |||
| 0 (%) | 15 | - | 62 |
| 1 (%) | 70 | - | 38 |
| ≥2 (%) | 15 | - | 0 |
| Sex | |||
| Male (%) | 77 | 64 | 92 |
| Female (%) | 23 | 36 | 8 |
| Tumor Site | |||
| Oral cavity (%) | 15 | 71 | 38 |
| Oropharynx (%) | 46 | 7 | 38 |
| Larynx (%) | 23 | 14 | 8 |
| Hypopharynx (%) | 8 | 7 | 15 |
| Other (%) | 8 | 0 | 0 |
| HPV Status | |||
| Positive (%) | 23 | - | - |
| Negative (%) | 8 | - | - |
| Unknown (%) | 70 | - | - |
| Prior Treatment | |||
| Chemotherapy (%) | 77 | - | 69 |
| Radiation (%) | 92 | - | 100 |
| Surgery (%) | 70 | - | 77 |
| Cetuximab (%) | 77 | - | - |
| Recurrence Type | |||
| Locoregional (%) | 8 | - | 31 |
| Distant Metastasis (%) | 46 | - | 69 |
| Both (%) | 46 | - | 0 |
* Trial terminated early for safety concerns.
Additionally, studies in R/M HNSCC varied in their reporting of prior lines of treatment before trial enrollment. Chow et al. reported that 54% of patients had received one prior chemotherapy treatment, while 23% had received two or more prior chemotherapies. Machiels et al. noted that 100% of patients enrolled received prior curative treatment, but did not define how many lines of treatment patients received. Furthermore, Chow et al.’s paper showed that 92% of patients had distant metastases, while only 69% of patients in Machiels et al.’s study had distant metastases.
Table 2 summarizes key information about the safety of the TLR agonists based on adverse events (AEs) reported in each study. Motolimod was associated with one grade 3 or higher AE in Chow et al.’s study. Shayan et al.’s study did not report any grade 4 or 5 AEs associated with motolimod. In Machiels et al., 92% of patients receiving IMO-2055 experienced a grade 3+ AE, with one fatal AE and 31% of patients being discontinued from the study due to AEs. Given the significant toxicities associated with IMO-2055, the trial was terminated early. Overall, IMO-2055 was associated with a much poorer safety profile compared to motolimod amongst phase 1b trials.
Table 2.
Adverse events in ≥20% of patients in phase 1b trials.
| Study | Chow et al. 2017 [12] | Shayan et al. 2018 [13] | Machiels et al. 2013 [14] * |
|---|---|---|---|
| Agent | Motolimod | Motolimod | IMO-2055 |
| Treatment | Motolimod + cetuximab | Motolimod + cetuximab | IMO-2055 + 5-fluorouracil, cisplatin, and cetuximab |
| Number of Patients | 13 | 14 | 13 |
| Dosage | Motolimod: 2.5 mg/m2, 3.0 mg/m2, or 3.5 mg/m2 Cetuximab: 250 mg/m2 |
Motolimod: 2.5 mg/m2 Cetuximab: 400 mg/m2 loading then 250 mg/m2 |
IMO-2055: 0.16 mg/kg or 0.32 mg/kg Cetuximab: 400 mg/m2 loading then 250 mg/m2 Cisplatin: 100 mg/m2/day 5-fluorouracil: 1000 mg/m2/day |
| Flu-Like Symptoms (%) | 92 | 36 | - |
| Injection Site Reaction (%) | 92 | 79 | 54 |
| Fatigue (%) | 85 | 21 | 39 |
| Rash (%) | 38 | 79 | 39 |
| Grade 3+ AEs (%) | 8 | - * | 92 |
| Fatal AEs (%) | 0 | 0 | 8 |
| Discontinued Due to AEs (%) | 0 | 0 | 31 |
* No grade 4 or 5 AEs reported.
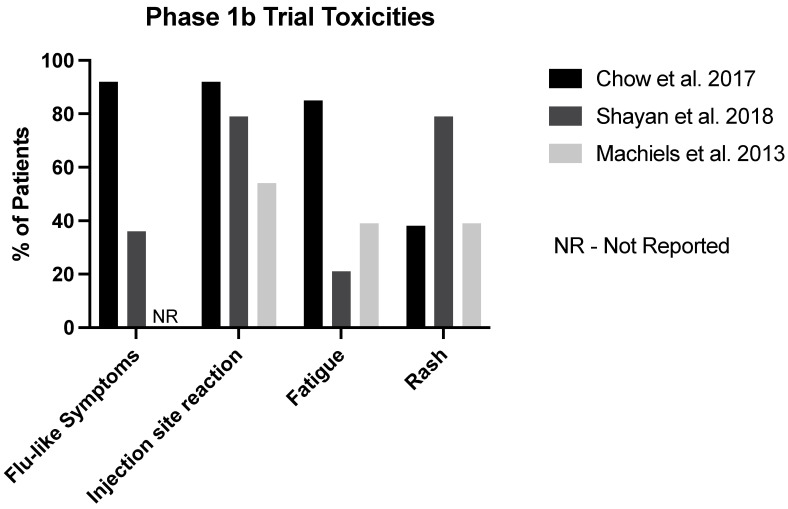
Chow et al.’s study of motolimod and Machiels et al.’s study of IMO-2055 reported some efficacy data. For IMO-2055, 23% of patients reported a partial response and 0% had a complete response. For Chow et al.’s motolimod study, 15% of patients had a partial response and 0% had a complete response. Due to it being a neodjuvant trial, Shayan et al.’s motolimod study did not report efficacy data (Figure 2).
Figure 2.
Summary of common toxicities noted in phase 1b trials [12,13,14].
3.3. Phase 2 Trials
Three phase 2 trials evaluating SD-101 (Cohen et al.), motolimod (Ferris et al.), and EMD 1201081 (Ruzsa et al.) in patients with R/M HNSCC were included in our analysis [15,16,17]. Table 3 outlines the baseline patient characteristics of these studies in patients. Cohen et al. included comparisons between low dose (2 mg/lesion) and high dose (8 mg/lesion) groups, while Ferris et al. and Ruzsa et al. reported comparisons to a placebo group that did not receive a TLR agonist. In Cohen et al.’s study, 24% of patients did not receive any prior systemic therapy, while 35% of patients in Ferris et al.’s study did not. In Ruzsa et al.’s study, all but one patient had prior chemotherapy. Only 37% of patients in Ruzsa et al.’s study had metastases, while 92% had them prior to enrollment for Cohen et al.’s study. Ferris et al.’s study did not report how many patients had metastatic cancer.
Table 3.
Baseline cohort characteristics for phase 2 trials of R/M HNSCC.
| Trial Reference | Cohen et al. 2022 [15] | Ferris et al. 2018 [16] | Ruzsa et al. 2014 [17] | |||
|---|---|---|---|---|---|---|
| Agent | SD-101 | Motolimod | EMD 1201081 | |||
| Treatment Group | SD-101 8 mg + pembrolizumab | SD-101 2 mg + pembrolizumab | EXTREME regimen + motolimod | EXTREME regimen + placebo | EMD 1201081 + cetuximab | Cetuximab only |
| Number of Patients | 23 | 28 | 100 | 95 | 53 | 53 |
| Median Age | 65 | 63 | 58 | 60 | 58 | 57 |
| ECOG | ||||||
| 0 (%) | 26 | 18 | 38 | 39 | 23 | 23 |
| 1 (%) | 74 | 82 | 62 | 61 | 77 | 77 |
| ≥2 (%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Sex | ||||||
| Male (%) | 91 | 68 | 85 | 85 | 85 | 85 |
| Female (%) | 9 | 32 | 15 | 15 | 15 | 15 |
| Tumor Site | ||||||
| Oral cavity (%) | 57 | 46 | 27 | 27 | - | - |
| Oropharynx (%) | 9 | 32 | 40 | 45 | - | - |
| Larynx (%) | 17 | 11 | 22 | 21 | - | - |
| Hypopharynx (%) | 0 | 7 | 4 | 5 | - | - |
| Other (%) | 17 | 4 | 7 | 1 | - | - |
| HPV Status | ||||||
| Positive (%) | 26 | 36 | 60 * | 65 * | - | - |
| Negative (%) | 26 | 39 | 33 * | 28 * | - | - |
| Unknown (%) | 48 | 25 | 8 * | 7 * | - | - |
| Prior Treatment | ||||||
| Chemotherapy (%) | - | - | 63 | 58 | 98 | 100 |
| Radiation (%) | 87 | 75 | 79 | 85 | 85 | 77 |
| Surgery (%) | 96 | 86 | 56 | 56 | 38 | 53 |
| Cetuximab (%) | - | - | 10 | 21 | ||
| Recurrence Type | ||||||
| Locoregional (%) | 9 | 7 | - | - | 70 | 57 |
| Distant Metastasis (%) | 61 | 57 | - | - | 30 | 43 |
| Both (%) | 30 | 36 | - | - | - | - |
* HPV status reported for oropharyngeal tumors only.
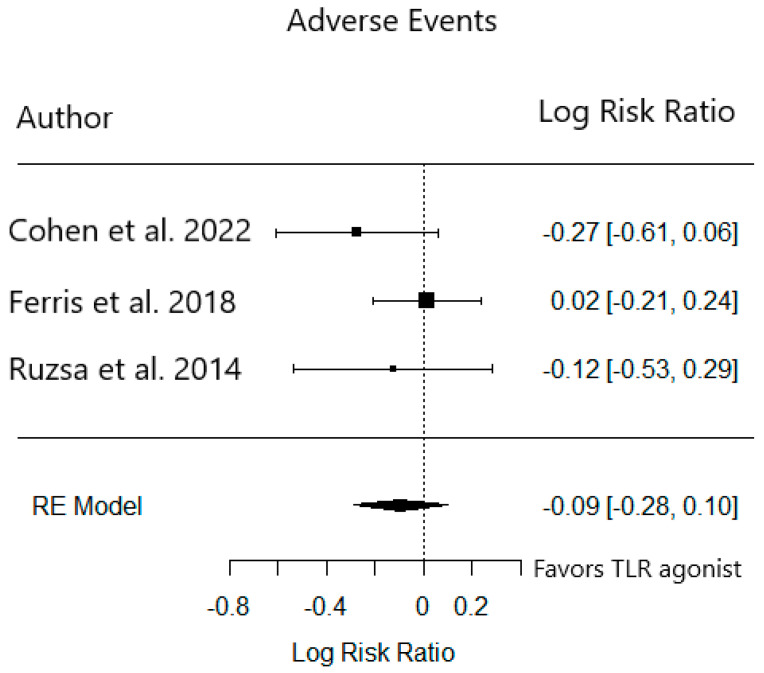
In Cohen et al.’s SD-101 study, more grade 3+ AEs occurred in the cohort receiving the 8 mg dose (34.8%) compared to the 2 mg dose (14.8%). In Ferris et al.’s motolimod study, similar rates of AEs were reported and fatal AEs occurred in both the treatment and placebo groups. Finally, in Ruzsa et al.’s EMD 1201081 study, patients were discontinued due to AEs in both treatment and placebo cohorts (Table 4). We pooled these studies to evaluate the AE rates in the placebo or low-dose group (n = 176) compared to the treatment group (n = 176). There was no significant difference in the relative risk of grade 3+ AEs between these groups (RR 0.91, 95% CI 0.76–1.11) (Figure 3).
Table 4.
Adverse events in patients in phase 2 trials.
| Trial Reference | Cohen et al. 2022 [15] | Ferris et al. 2018 [16] | Ruzsa et al. 2014 [17] | |||
|---|---|---|---|---|---|---|
| Agent | SD-101 | Motolimod | EMD 1201081 | |||
| Treatment Group | SD-101 8 mg + pembrolizumab | SD-101 2 mg + pembrolizumab | EXTREME regimen + motolimod | EXTREME regimen + placebo | EMD 1201081 + cetuximab | Cetuximab only |
| Number of Patients | 23 | 27 | 86 | 86 | 54 | 53 |
| Dosage | SD-101 8 mg in 1 lesion + pembrolizumab | SD-101 2 mg in 1–4 lesions + pembrolizumab | Motolimod 3 mg/m2 + cisplatin 100 mg/m2 + fluorouracil 1000 mg/m2 + cetuximab 400 mg/m2 loading then 250 mg/m2 | Placebo + cisplatin 100 mg/m2 + fluorouracil 1000 mg/m2 + cetuximab 400 mg/m2 loading then 250 mg/m2 | EMD 1201081 0.32 mg/kg + cetuximab 400 mg/m2 loading then 250 mg/m2 | Cetuximab 400 mg/m2 loading then 250 mg/m2 |
| Chills | 44 | 11 | 37 | 6 | - | - |
| Pyrexia | 26 | 22 | 43 | 12 | 19 | 6 |
| Injection Site Reaction | 17 * | 4 * | 39 | 0 | 20 | 0 |
| Fatigue | 74 | 56 | 43 | 45 | 15 | 23 |
| Rash | - | - | 19 | 27 | 30 | 32 |
| Grade 3+ AEs (%) | 35 | 15 | 39 | 40 | 56 | 51 |
| Fatal AEs (%) | 0 | 0 | 5 | 8 | 0 | 0 |
| Discontinued Due to AEs (%) | - | - | - | - | 19 | 15 |
* Injection site reaction not directly reported, so injection site erythema used as a proxy.
Figure 3.
Relative risk of grade 3+ adverse events in phase 2 trials [15,16,17].
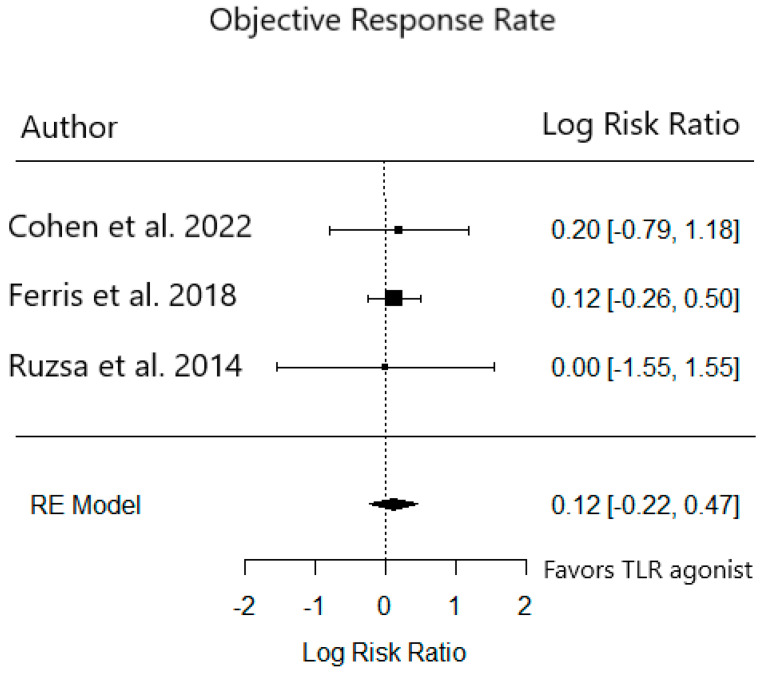
Efficacy data were reported by all phase 2 trials (Table 5). Objective response rates (ORR) in the treatment groups in Ferris et al.’s motolimod study and Ruzsa et al.’s EMD 1201081 study were not significantly different from the placebo group. The median PFS in the 2 mg cohort of SD-101 in Cohen et al.’s study was 2.5 months, vs. 2.3 months in the 8 mg cohort. PFS in the treatment vs. the placebo group for Ferris et al.’s motolimod study was 6.1 vs. 5.9 months. Finally, PFS in the treatment vs. the placebo group for Ruzsa et al.’s EMD 1201081 study was 1.5 vs. 1.9 months. We pooled the ORR in these studies to compare the relative risk of response in the placebo or low-dose group (n = 176) to the treatment group (n = 176) There was no significant difference in ORR between these groups (RR 0.93 95% CI 0.60–1.44) (Figure 4). Lastly, funnel plots of grade 3+ AEs and ORR between groups in these studies did not show any evidence of publication bias in these phase 2 trials (data not shown).
Table 5.
Efficacy data for phase 2 trials of R/M HNSCC.
| Trial Reference | Cohen et al. 2022 [15] | Ferris et al. 2018 [16] | Ruzsa et al. 2014 [17] | |||
|---|---|---|---|---|---|---|
| Agent | SD-101 | Motolimod | EMD 1201081 | |||
| Treatment Group | SD-101 8 mg + pembrolizumab | SD-101 2 mg + pembrolizumab | EXTREME regimen + motolimod | EXTREME regimen + placebo | EMD 1201081 + cetuximab | Cetuximab only |
| Number of Patients | 28 | 23 | 100 | 95 | 53 | 53 |
| Median Age | 63 | 65 | 58 | 60 | 58 | 57 |
| ORR (%) | 21 | 26 | 38 | 34 | 6 | 6 |
| CR (%) | 7 | 0 | 2 | 5 | 0 | 0 |
| PR (%) | 14 | 26 | 36 | 28 | 6 | 6 |
| SD (%) | 25 | 22 | 22 | 24 | 32 | 38 |
| PD (%) | 36 | 39 | 9 | 8 | 40 | 34 |
| Median PFS | 2.5 | 2.3 | 6.1 | 5.9 | 1.5 | 1.9 |
| Median OS | Not reached | 9 | 13.5 | 11.3 | 6.3 | - |
Figure 4.
Relative risk of objective response to TLR agonist in phase 2 trials [15,16,17].
4. Discussion
In this meta-analysis, we evaluate the safety and efficacy of TLR agonists reported in various clinical trials of patients with HNSCC. We pooled 40 patients in phase 1b and 176 patients in phase 2 trials receiving TLR agonists in combination with other systemic therapies to identify both adverse event rates and treatment efficacy. Based on currently available studies, it does not appear that the addition of TLR agonists to standard-of-care regimens for R/M HNSCC provides treatment benefits or higher rates of grade 3+ AEs.
While TLR agonists have demonstrated efficacy in preclinical studies [18,19,20,21], this meta-analysis demonstrates that they may not have a role for the management of R/M HNSCC. There are several possible reasons for the lack of efficacy across three phase 2 trials. Importantly, the majority of patients with R/M HNSCC had previously undergone radiation therapy, which can act as a double-edged sword to promote systemic tumor antigen presentation to immune effectors, but can also be partly immunosuppressive in a post-treatment setting [22,23]. The extent to which this interplay occurs likely varies between patients. In one of the phase 2 trials included in this study, Cohen et al. performed transcriptomic analysis of tumors pre- and post-injection and showed significantly enhanced CD8+ T cell and NK cell gene expressions within tumors of responders, but no difference in non-responders. Additionally, there was no difference in response rates for patients with high and low PD-L1 expression in this study. However, whether this response relates to the TLR agonist or pembrolizumab given concurrently is not known, since neither were tested separately. But, based on the ineffectiveness of TLR agonists in other studies, is likely to relate to ICIs that are now the standard of care for R/M HNSCC patients.
Shayan et al. was unique among the studies included in this meta-analysis in that TLR agonist was given to treatment-naïve patients in a neoadjuvant setting in combination with cetuximab. This study also evaluated pre- and post-treatment tumor samples for tumor immune infiltration and found that motolimod plus cetuximab was associated with a decreased induction of Tregs and enhanced CD8+ T cell infiltration of tumors following treatment. While this treatment-naïve group offers a better model for TLR-driven immune infiltration, this study was again limited by the lack of a control group as Cetuximab is associated with similar effects on the tumor-immune microenvironment [24]. Despite limitations of these studies, the mechanisms of TLR-agonist stimulation in clinical trials are unlikely to have relevance if they lack a treatment benefit.
The rate of grade 3+ AEs ranged from 8% to 92% in each cohort, with Machiels et al.’s study of IMO-2055 notably being terminated early due to safety concerns. Fatal AEs were uncommon but were reported in Machiels et al.’s IMO-2055 study and Ferris et al.’s motolimod study. Since these patients were receiving cetuximab, ICI, and other systemic therapies that have high rates of grade 3+ AEs, the contribution of TLR agonists to overall AE reporting is unclear. Importantly, pooling AE rates in phase 2 trials did not show an increased risk of AEs in the TLR agonist group. Looking at AEs reported for these TLR agonists in other trials, in a trial of SD-101 in melanoma, 27% of patients had a grade 3–4 AE related to SD-101, 41% had a serious adverse event, and no patient had dose-limiting toxicities or death [25]. A phase 2 trial of motolimod in ovarian cancer also did not identify severe toxicities leading to treatment discontinuation, but did identify a serious adverse event in 40.8% of patients in both the placebo and motolimod groups [26]. In a trial of IMO-2055 in non-small cell lung cancer, 34% had a grade 3+ AE and 11% had serious AEs related to IMO-2055 [27]. These studies, in combination with the data presented in this trial, suggest that TLR agonists do not add a significant risk for severe AEs beyond toxicities associated with standard-of-care therapies.
There have been numerous clinical trials of TLR agonists in cancers beyond HNSCC [28]. SD-101 is being tested in combination with anti-PD-1 and/or additional agents in pancreatic, prostate, breast, uveal melanoma, and hepatic or other solid tumors [28]. TLR7/8 agonist NKTR-262 increased CD11c dendritic cell recruitment to tumors in melanoma, but a trial of NKTR-262 in combination with bempegaldesleukin with or without nivolumab was terminated. BDB001 and CV8102 are other TLR7/8 agonists being investigated in solid tumors and melanoma, respectively [29]. Additionally, lefitolimod and cavrotolimod are TLR9 agonists being evaluated in solid tumors, while tilsotolimod and vidutolimod/CMP-001 are TLR9 agonists being tested in melanomas [29]. As additional trial data are published for TLR agonists in HNSCC, evidence that demonstrates clinical benefits in conjunction with other treatments may surface that may warrant a re-examination of previously reported clinical trials.
It is important to note limitations of this study in evaluating future clinical trials for TLR agonists. Notably, only 176 patients from phase 2 trials were included and treatment modalities varied between trials. This is consistent with current clinical practice, where systemic therapy regimens are not consistent among patients with R/M HNSCC due to differences in performance status and comorbidities that may limit a patient’s ability to receive platinum-based chemotherapy or cetuximab. Now that the Keynote-048 study has shown that pembrolizumab alone is efficacious in this patient group, a clinical trial design similar to Cohen et al. but comparing ICIs alone to ICIs with TLR agonists seems best powered to definitively ascertain their clinical benefit. If TLR agonists are not found to have a benefit in well-powered clinical trials, studying other in situ vaccines, like oncolytic viruses, may also determine whether injection-based strategies aimed at unlocking intratumoral immune responses are worth pursuing.
5. Conclusions
In this systematic review, we evaluate the safety and efficacy of TLR agonists reported in various phase 1b and phase 2 trials of HNSCC. Overall, TLR agonists are generally tolerable and not associated with fatal or therapy-terminating toxicities. However, TLR agonists in phase 2 trials typically did not show significant clinical benefits. TLR agonists thus do not appear to be highly appealing as a future therapeutic avenue for immunotherapy in HNSCC.
Author Contributions
Conceptualization, S.M. and A.F.; methodology, S.M. and A.F.; formal analysis, S.M., M.C., F.B., V.D., J.B.S. and A.F.; writing—original draft preparation, S.M., M.C. and A.F.; writing—review and editing, S.M., M.C., F.B., V.D., J.B.S. and A.F.; All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Johnson D.E., Burtness B., Leemans C.R., Lui V.W.Y., Bauman J.E., Grandis J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vermorken J.B., Mesia R., Rivera F., Remenar E., Kawecki A., Rottey S., Erfan J., Zabolotnyy D., Kienzer H.-R., Cupissol D., et al. Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. N. Engl. J. Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 3.Cancer of the Larynx—Cancer Stat Facts. [(accessed on 10 April 2023)]; SEER. Available online: https://seer.cancer.gov/statfacts/html/laryn.html.
- 4.Cancer of the Oral Cavity and Pharynx—Cancer Stat Facts. [(accessed on 10 April 2023)]; SEER. Available online: https://seer.cancer.gov/statfacts/html/oralcav.html.
- 5.Burtness B., Harrington K.J., Greil R., Soulières D., Tahara M., de Castro G., Jr., Psyrri A., Basté N., Neupane P., Bratland A., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 6.Sacco A.G., Chen R., Worden F.P., Wong D.J.L., Adkins D., Swiecicki P., Chai-Ho W., Oppelt P., Ghosh D., Bykowski J., et al. Pembrolizumab plus Cetuximab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: An Open-Label, Multi-Arm, Non-Randomised, Multicentre, Phase 2 Trial. Lancet Oncol. 2021;22:883–892. doi: 10.1016/S1470-2045(21)00136-4. [DOI] [PubMed] [Google Scholar]
- 7.Shibata H., Saito S., Uppaluri R. Immunotherapy for Head and Neck Cancer: A Paradigm Shift From Induction Chemotherapy to Neoadjuvant Immunotherapy. [(accessed on 15 March 2022)];Front. Oncol. 2021 11:727433. doi: 10.3389/fonc.2021.727433. Available online: https://www.frontiersin.org/article/10.3389/fonc.2021.727433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urban-Wojciuk Z., Khan M.M., Oyler B.L., Fåhraeus R., Marek-Trzonkowska N., Nita-Lazar A., Hupp T.R., Goodlett D.R. The Role of TLRs in Anti-cancer Immunity and Tumor Rejection. Front. Immunol. 2019;10:2388. doi: 10.3389/fimmu.2019.02388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow L.Q., Morishima C., Eaton K.D., Baik C.S., Goulart B.H., Anderson L.N., Manjarrez K.L., Dietsch G.N., Bryan J.K., Hershberg R.M., et al. Phase Ib Trial of the Toll-like Receptor 8 Agonist, Motolimod (VTX-2337), Combined with Cetuximab in Patients with Recurrent or Metastatic SCCHN. Clin. Cancer Res. 2017;23:2442–2450. doi: 10.1158/1078-0432.CCR-16-1934. [DOI] [PubMed] [Google Scholar]
- 13.Shayan G., Kansy B.A., Gibson S.P., Srivastava R.M., Bryan J.K., Bauman J.E., Ohr J., Kim S., Duvvuri U., Clump D.A., et al. Phase Ib Study of Immune Biomarker Modulation with Neoadjuvant Cetuximab and TLR8 stimulation in Head and Neck Cancer to Overcome Suppressive Myeloid Signals. Clin. Cancer Res. 2018;24:62–72. doi: 10.1158/1078-0432.CCR-17-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machiels J.-P., Kaminsky M.-C., Keller U., Brümmendorf T.H., Goddemeier T., Forssmann U., Delord J.-P. Phase Ib trial of the Toll-like receptor 9 agonist IMO-2055 in combination with 5-fluorouracil, cisplatin, and cetuximab as first-line palliative treatment in patients with recurrent/metastatic squamous cell carcinoma of the head and neck. Investig. New Drugs. 2013;31:1207–1216. doi: 10.1007/s10637-013-9933-z. [DOI] [PubMed] [Google Scholar]
- 15.Cohen E.E., Nabell L., Wong D.J., Day T., Daniels G.A., Milhem M., Deva S., Jameson M., Guntinas-Lichius O., Almubarak M., et al. Intralesional SD-101 in Combination with Pembrolizumab in Anti-PD-1 Treatment-Naïve Head and Neck Squamous Cell Carcinoma: Results from a Multicenter, Phase II Trial. Clin. Cancer Res. 2022;28:1157–1166. doi: 10.1158/1078-0432.CCR-21-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferris R.L., Saba N.F., Gitlitz B.J., Haddad R., Sukari A., Neupane P., Morris J.C., Misiukiewicz K., Bauman J.E., Fenton M., et al. Effect of Adding Motolimod to Standard Combination Chemotherapy and Cetuximab Treatment of Patients With Squamous Cell Carcinoma of the Head and Neck. JAMA Oncol. 2018;4:1583–1588. doi: 10.1001/jamaoncol.2018.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruzsa A., Sen M., Evans M., Lee L.W., Hideghety K., Rottey S., Klimak P., Holeckova P., Fayette J., Csoszi T., et al. Phase 2, open-label, 1:1 randomized controlled trial exploring the efficacy of EMD 1201081 in combination with cetuximab in second-line cetuximab-naïve patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) Investig. New Drugs. 2014;32:1278–1284. doi: 10.1007/s10637-014-0117-2. [DOI] [PubMed] [Google Scholar]
- 18.Owen A.M., Fults J.B., Patil N.K., Hernandez A., Bohannon J.K. TLR Agonists as Mediators of Trained Immunity: Mechanistic Insight and Immunotherapeutic Potential to Combat Infection. [(accessed on 28 April 2023)];Front. Immunol. 2021 11:622614. doi: 10.3389/fimmu.2020.622614. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2020.622614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams S. Toll-like receptor agonists in cancer therapy. Immunotherapy. 2009;1:949–964. doi: 10.2217/imt.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaczanowska S., Joseph A.M., Davila E. TLR agonists: Our best frenemy in cancer immunotherapy. J. Leukoc. Biol. 2013;93:847–863. doi: 10.1189/jlb.1012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farooq M., Batool M., Kim M.S., Choi S. Toll-Like Receptors as a Therapeutic Target in the Era of Immunotherapies. [(accessed on 28 April 2023)];Front. Cell Dev. Biol. 2021 9:756315. doi: 10.3389/fcell.2021.756315. Available online: https://www.frontiersin.org/articles/10.3389/fcell.2021.756315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur P., Asea A. Radiation-induced effects and the immune system in cancer. [(accessed on 28 April 2023)];Front. Oncol. 2012 2:191. doi: 10.3389/fonc.2012.00191. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2012.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carvalho H.d.A., Villar R.C. Radiotherapy and immune response: The systemic effects of a local treatment. Clinics. 2018;73((Suppl. 1)):e557s. doi: 10.6061/clinics/2018/e557s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith J.D., Ludwig M.L., Bhangale A.D., Brummel C., Swiecicki P.L., Worden F.P., Chinn S.B., Stucken C.L., Rosko A.J., Prince M.E.P., et al. Tumor immune microenvironment alterations using induction cetuximab in a phase II trial of deintensified therapy for p16-positive oropharynx cancer. Head Neck. 2023;45:1281–1287. doi: 10.1002/hed.27344. [DOI] [PubMed] [Google Scholar]
- 25.Ribas A., Medina T., Kummar S., Amin A., Kalbasi A., Drabick J.J., Barve M., Daniels G.A., Wong D.J., Schmidt E.V., et al. SD-101 in Combination with Pembrolizumab in Advanced Melanoma: Results of a Phase Ib, Multicenter Study. Cancer Discov. 2018;8:1250–1257. doi: 10.1158/2159-8290.CD-18-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monk B.J., Brady M.F., Aghajanian C., Lankes H.A., Rizack T., Leach J., Fowler J.M., Higgins R., Hanjani P., Morgan M., et al. A phase 2, randomized, double-blind, placebo- controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: A Gynecologic Oncology Group partners study. Ann. Oncol. 2017;28:996–1004. doi: 10.1093/annonc/mdx049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith D.A., Conkling P., Richards D.A., Nemunaitis J.J., Boyd T.E., Mita A.C., de La Bourdonnaye G., Wages D., Bexon A.S. Antitumor activity and safety of combination therapy with the Toll-like receptor 9 agonist IMO-2055, erlotinib, and bevacizumab in advanced or metastatic non-small cell lung cancer patients who have progressed following chemotherapy. Cancer Immunol. Immunother. 2014;63:787–796. doi: 10.1007/s00262-014-1547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anwar M.A., Shah M., Kim J., Choi S. Recent clinical trends in Toll-like receptor targeting therapeutics. Med. Res. Rev. 2019;39:1053–1090. doi: 10.1002/med.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rolfo C., Giovannetti E., Martinez P., McCue S., Naing A. Applications and clinical trial landscape using Toll-like receptor agonists to reduce the toll of cancer. npj Precis. Oncol. 2023;7:26. doi: 10.1038/s41698-023-00364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]