Abstract
A PCR–reverse cross-blot hybridization assay procedure that is able to rapidly identify 13 species of clinically relevant mycobacteria was evaluated for routine use in the identification of acid-fast isolates growing in BACTEC 460 TB (12B and 13A) and BACTEC 9000 MB (Myco/F) liquid media. Eight of the probes used were already described by Kox et al. (L. F. F. Kox et al., J. Clin. Microbiol. 33:3225–3233, 1995). In addition, we used six other probes specific for M. chelonae, M. malmoense or M. szulgai, M. genavense, M. gordonae, M. terrae, and M. marinum/M. ulcerans that we designed ourselves. This procedure allowed us to identify 459 mycobacterial species directly from broth cultures of 5,466 clinical samples collected over 1 year and processed with the radiometric or nonradiometric BACTEC system. Our results were in agreement with those obtained by conventional identification methods and also with those obtained by mycolic acid analysis by high-performance liquid chromatography. This assay seems to be a reliable procedure for the routine identification of mycobacteria, providing an accurate identification of mycobacterial isolates more rapidly than conventional tests, with remarkable implications for an efficacious specific antimycobacterial therapy.
During the last 10 years, the number of new cases of infections due to Mycobacterium tuberculosis has increased (25, 32). Factors contributing to the resurgence of tuberculosis include the human immunodeficiency virus epidemic, the immigration of people from countries with a high incidence of tuberculosis, and an increase in the medically underserved population. Moreover, various diseases caused by mycobacteria other than M. tuberculosis such as M. avium and M. intracellulare are now commonly associated with severe immunosuppression (9, 15, 31). Other opportunistic mycobacterial infections associated with human immunodeficiency virus infection are caused by M. kansasii, M. xenopi, M. fortuitum, M. scrofulaceum, M. malmoense, and M. genavense (9, 14). Consequently, rapid methods for the identification of clinically relevant mycobacterial species are welcome, since the therapeutic treatments for mycobacterial diseases can differ depending on the species responsible for the infection (30).
Current identification of mycobacterial species is based on traditional biochemical tests; these methods are time-consuming, because most of the mycobacterial species need at least 4 weeks of culture on conventional media before sufficient growth and biomass are obtained to permit identification.
An important development for the rapid isolation of mycobacteria from clinical samples was the introduction of liquid medium for primary cultures, which increased the rates of isolation of mycobacteria (19, 33). In addition, methods based on lipid composition analysis by gas-liquid chromatography (28) and high-performance liquid chromatography (HPLC) (5) and on the use of species-specific DNA or RNA probes (12, 23) have been developed for the identification of mycobacteria to the species level. On the other hand, methods based on gas-liquid chromatography and HPLC require expensive laboratory equipment, and the use of the DNA or RNA probes allows the identification of a limited number of mycobacterial species.
Recently, several molecular biology-based methods have been developed for the accurate and rapid identification of mycobacteria from clinical isolates and/or clinical samples. Most of these tests are based on PCR amplification of sequences specific only for M. tuberculosis (6, 7, 20), while others also use the target the sequences coding for 16S rRNA (1, 16, 29) or hsp65 (10, 27) for the identification of nontuberculous mycobacteria.
In this work we describe the successful routine application of a molecular biology-based method based on PCR amplification of 16S rRNA gene sequences and a subsequent reverse cross-hybridization assay with species-specific probes for the direct identification of mycobacteria grown in primary cultures in liquid medium found to be positive with the BACTEC 460 TB and BACTEC 9000 MB systems. Of the 14 probes used, 8 have already been reported (21), whereas 6 have been originally designed.
MATERIALS AND METHODS
Reference strains.
Reference mycobacterial strains (M. tuberculosis H37 Rv TMC 102, ATCC 27294, Erdman strain TMC 107, and ATCC 35801; M. avium ATCC 25291; M. chelonae ATCC 35752; M. fortuitum ATCC 6841; M. intracellulare ATCC 13950; M. kansasii ATCC 12478; M. marinum ATCC 927; M. xenopi ATCC 19250; M. smegmatis ATCC 607; M. terrae ATCC 15755; as well as M. genavense, M. malmoense, and M. gordonae [clinical isolates]) were tested in this study.
Specimens.
A total of 5,466 clinical specimens (including sputum, urine, stool, bronchoalveolar lavage, gastric and cerebrospinal fluid, blood, and tissue biopsy specimens) were submitted for cultures for mycobacteria. These specimens were processed and cultured by well-known protocols (8, 17, 33).
Detection of growth.
Growth on solid medium was detected by visual observation of colonies, while in radiometric BACTEC medium, growth was detected with the BACTEC 460 TB instrument and a growth index (GI) of 30 or more was considered positive. Growth in BACTEC 9000 MB was detected with the BACTEC 9000 MB instrument, based on the development of fluorescence. All BACTEC-positive cultures were confirmed to be positive by making smears with samples from the broth and staining for acid-fast bacteria; moreover, they were subcultured onto sheep blood agar plates to detect possible contamination with non-acid-fast bacteria. All positive broth cultures found to be smear positive for acid-fast bacilli were processed for DNA extraction.
DNA extraction.
Prior to DNA extraction, the acid-fast bacteria present in each positive bottle were inactivated by heating them at 80°C for 30 min. Subsequently, 0.5 ml of each sample was transferred to 1.5-ml screw-cap microcentrifuge tube, the tube was centrifuged at 12,000 × g for 5 min, and the pellet was resuspended in 100 μl of distilled water and subjected to three cycles of boiling and freezing (5 min at 100°C, 5 min at −20°C) (13). Then, an equal volume of chloroform was added and the samples were vortexed and centrifuged at 12,000 × g for 10 min. The aqueous phase containing the extracted DNA was used for amplification or was transferred to a clean microcentrifuge tube and stored at −20°C until it was used.
Isolation of DNA from the reference strains was performed as described previously (1). The isolated DNA was suspended in TE (0.01 M Tris-HCl, 0002 M EDTA [pH 8]) at 10 ng/μl and stored at 4°C.
PCR.
The PCRs were performed in a Gene Amp PCR System 2400 (Perkin-Elmer Cetus, Norwalk, Conn.) under the conditions previously described by Kox et al. (21). Briefly, a total of 20 μl of the DNA-containing supernatant was added to PCR mixture containing 50 mM KCl, 10 mM Tris HCl (pH 8.3), 1.5 mM MgCl2, 0.01% (wt/vol) gelatin, deoxynucleoside triphosphates (dATP, dGTP, dCTP, and dUTP) at a concentration of 200 μM (each), primers pMyc14bio and pMyc7 at a concentration of 200 nM (each), and 1 U of Taq DNA polymerase (Boehringer Mannheim, Mannheim, Germany) per 50-μl reaction volume. The presence of amplified DNA was visualized by agarose gel electrophoresis (2% in TAE buffer [Tris acetate, 0.05 M {pH 8}, EDTA 0.01 M] for 1 h and 30 min at 70 V at room temperature) and staining with ethidium bromide.
Reverse cross-blot hybridization assay.
The amplicons that were obtained were analyzed as described by Kox et al. (21) by the reverse cross-blot hybridization assay with specific probes. pMyc5a, pTub1, pAvi3, pInt5, pKan1, pXen1, pFor1, and pSme1 were the probes described by Kox et al. (21) and corresponded to 16S rRNA gene sequences specific for Mycobacterium spp., M. tuberculosis complex, M. avium, M. intracellulare, M. kansasii, M. xenopi, M. fortuitum, and M. smegmatis, respectively. The other probes, specific for M. malmoense or M. szulgai (5′-CCCCAAGGCATGCGCCTCGG-3′), M. genavense (5′-CACCAAAAAACATGCGTTCCG-3′), M. gordonae (5′-CATGTGTTCTGTGGTCCTATTC-3′), M. terrae (5′-ACCACAGAACATGCATCCCA-3′), M. chelonae (5′-CCACTCACCATGAAGTGTGTGGT-3′), and M. marinum or M. ulcerans (5′- ACCACAGGACATGAATCCCGTG-3′), were also designed to hybridize to the 16S rRNA gene region so that their melting temperatures were comparable. The specificities of these sequences were evaluated by comparing them with the 16S rRNA sequences specific for the most important mycobacterial species by using DNASIS for Windows software (Hitachi Software Engineering, San Bruno, Calif.). Moreover, these additional probes are similar but not identical to those described by Kox et al. (22). The oligonucleotide probes were subjected to the tailing reactions with dTTP (21) to permit the efficient capture of the PCR products in the reverse cross-blot hybridization assay. Then, they were blotted on top of a positively charged nylon membrane (Boehringer Mannheim) in the cross-blotter apparatus (Accutran cross; ACC 100/0; Schleicher & Schuell, Dassel, Germany). Two membrane panels were prepared as described above; the first included probes for Mycobacterium spp. and those specific for M. tuberculosis complex, M. avium, M. intracellulare, M. fortuitum, M. gordonae, and M. xenopi, and the second included probes specific for Mycobacterium spp., M. chelonae, M. genavense, M. kansasii, M. malmoense or M. szulgai, M. marinum or M. ulcerans, M. smegmatis, and M. terrae. The PCR products were denatured by heating (100°C for 10 min) and were added, in the cross-blotter apparatus, to the slots of the membrane in hybridization solution (5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 1% blocking reagent [Boehringer Mannheim], 0.1% N-lauroylsarcosine, 0.02% sodium dodecyl sulfate [SDS]) at 60°C, and the hybridized PCR products were detected by incubation with streptavidin-alkaline phosphatase and a color substrate, according to the manufacturer’s instructions (Boehringer Mannheim).
Identification by biochemical tests and DNA probes.
Aliquots of BACTEC 460 TB and BACTEC 9000 MB liquid cultures containing acid-fast cells were subcultured onto Middlebrook 7H10 agar plates and Löwenstein-Jensen slants. The acid-fast isolates were identified by standard methods for the determination of the species of the mycobacterial isolates (17). In addition, commercial DNA probes were used directly from the BACTEC broth cultures (GI, ≥100) for the rapid identification of M. tuberculosis, M. avium, M. intracellulare, and M. gordonae, according to the manufacturer’s instructions (AccuProbe, Gen-Probe, Inc., San Diego, Calif.).
HPLC.
Mycolic acid analysis of mycobacteria was performed with a Hewlett-Packard 1100 HPLC system (Hewlett-Packard, Waldbronn, Germany). The saponification of mycobacterial cells and derivatization of the mycolic acids to p-bromophenacyl esters were performed by the conditions described by other investigators (4). They were separated by using a reverse-phase C18 ultrasphere-XL cartridge column (Beckman Instruments, Inc., Fullerton, Calif.) with a particle size of 3 μm by using the conditions described previously (11).
The mycolic acid patterns resulting from the clinical isolates were identified by the Sherlock system (MIDI, Inc., Newark, Del.).
RESULTS
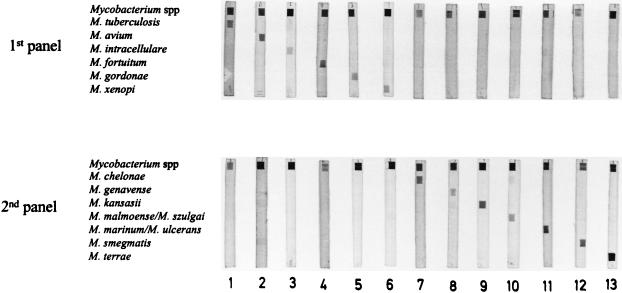
In order to validate the PCR and reverse cross-blot hybridization assay for identification purposes, 13 mycobacterial species including reference strains and clinical isolates were tested. As Fig. 1 shows, the resulting PCR products specifically hybridized only with the genus-specific probe and the corresponding species-specific probe, thus confirming the validity of this method. Similar results were obtained by other investigators (21). The number of probes tested included those for M. chelonae, M. genavense, M. malmoense, M. terrae, M. marinum, and M. gordonae, all species with increasing clinical relevance (2, 3, 9). These probes were specifically designed to increase the number of identifiable mycobacteria.
FIG. 1.
Analysis by reverse cross-blot hybridization of PCR products derived from 13 mycobacterial species. Lane 1, M. tuberculosis H37 Rv TMC 102; lane 2, M. avium ATCC 25291; lane 3, M. intracellulare ATCC 13950; lane 4, M. fortuitum ATCC 6841; lane 5, M. gordonae (clinical specimen); lane 6, M. xenopi ATCC 19250; lane 7, M. chelonae ATCC 35752; lane 8, M. genavense (clinical specimen); lane 9, M. kansasii ATCC 12478; lane 10, M. malmoense (clinical specimen); lane 11, M. marinum ATCC 927; lane 12, M. smegmatis ATCC 607; lane 13, M. terrae ATCC 15755.
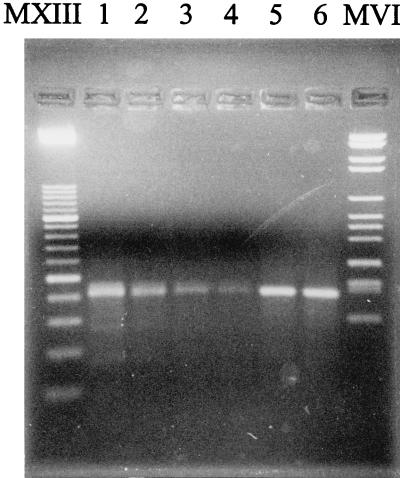
A total of 459 broth cultures detected as positive by the BACTEC 460 TB (GI, ≥30) and BACTEC 9000 MB instruments were microscopy positive for acid-fast bacteria and were subjected to PCR and the reverse cross-blot hybridization assay. All were found to be PCR positive, and their amplicons gave specific hybridization results; thus, the specificity of the assay was estimated to be 100%. We observed that the amount of amplicon obtained from the broth cultures correlated approximately with the BACTEC GI (Fig. 2). However, the different amounts of PCR products did not influence the intensity of the hybridization signal, which was always easily readable.
FIG. 2.
Results of PCR performed with samples from BACTEC cultures with different GI values. MXIII, Marker XIII (Boehringer Mannheim); lane 1, GI of 100; lane 2, GI of 70; lane 3, GI of 50; lane 4, GI of 30; lane 5, GI of 190; lane 6, GI of 320; MVI, Marker VI (Boehringer Mannheim).
Usually, we tested the amplicons with the first panel of probes. Subsequently, the samples found to be positive only with the genus-specific probe were challenged with the second panel of oligonucleotide probes.
The results obtained for the species that we identified were as follows: 172 M. tuberculosis (37.4%), 144 M. xenopi (31.4%), 85 M. avium (18.6%), 43 M. gordonae (9.4%), 4 M. fortuitum (0.8%), 3 M. marinum (0.6%), 2 M. kansasii (0.4%), and 2 M. chelonae (0.4%) isolates and 1 isolate (0.2%) each of M. intracellulare, M. malmoense, M. terrae, and M. genavense.
In the meantime, all acid-fast microorganisms derived from the positive BACTEC liquid cultures were subjected to identification by biochemical tests and also with commercial DNA probes. Moreover, all the isolates were submitted to mycolic acid analysis by HPLC. We observed perfect agreement among all techniques used, but the times to detection were different, thus highlighting the validity of the PCR–reverse cross-blot hybridization assay.
DISCUSSION
The increasing frequency of isolation of mycobacterial species other than M. tuberculosis as a consequence of the consistent numbers of immunocompromised patients led us to investigate the routine use of a PCR-based molecular biology-based method in order to rapidly identify several mycobacterial species growing in BACTEC 460 TB and BACTEC 9000 MB liquid media.
The BACTEC systems are both efficient for the detection of acid-fast bacteria, allowing the growth of the mycobacteria in a few days. However, the markedly reduced time of the assay (33) should be accomplished by an equally advantageous rapid identification method. At present, the most commonly used identification procedures are based on biochemical characteristics, but not only are they time-consuming but their results are also difficult to interpret, even by experienced personnel. PCR-based methods could overcome these difficulties. For this reason, we chose a method based on the analysis of the PCR products after amplification from the 16S rRNA gene in a reverse cross-blot hybridization assay (21). Technically, this method is simple. A PCR product can be hybridized with different probes simultaneously, and the results obtained are easily readable and not subjected to misinterpretation. Moreover, we enhanced the validity of the method by introducing oligonucleotide probes specific for clinically relevant species (9), such as M. genavense, M. malmoense, and M. marinum. In addition, we evaluated the routine use of this method with broth cultures found to be positive with the BACTEC instruments. The validity of the PCR–reverse cross-blot hybridization analyses was confirmed by comparison of the results with those obtained by the usual identification methods, such as biochemical tests, tests with commercial DNA probes, and HPLC analysis. The comparison showed perfect agreement among the different techniques and confirmed the validity of this method. This PCR–reverse cross-blot hybridization assay demonstrated several advantages not only with respect to conventional techniques based on the morphologic and biochemical features of mycobacteria but also when compared with HPLC analysis. In fact, the latter method requires expensive laboratory equipment and experienced personnel, factors that limit its application for the routine identification of mycobacterial species. Moreover, the commercially available automated systems for HPLC pattern recognition (e.g., the Sherlock system [MIDI]) are unable to distinguish M. avium from M. intracellulare and M. scrofulaceum or M. xenopi from M. celatum.
The benefits of the PCR–reverse cross-blot hybridization method are also remarkable when the method is compared with other molecular biology-based techniques. Recently, the use of commercial nonradioactive probes (AccuProbe; Gen-Probe) directed against 16S rRNA have contributed to simplification of the procedure and shortening of the time necessary for the identification of slowly growing mycobacteria such as M. avium, M. intracellulare, M. gordonae, and M. tuberculosis (12, 23). Yet, this method is unable to identify other clinically relevant species, such as M. xenopi, a species among the opportunistic mycobacteria that is frequently isolated in Europe and that represents one of the most common agents of pulmonary infection due to nontuberculous mycobacteria (24). We conclude that the limited number of identifiable species makes the commercial DNA probes insufficient for use in the routine identification of all clinical mycobacterial isolates. Moreover, as noted previously (19, 26), they are frequently insensitive for the identification of acid-fast isolates in liquid media due to the inadequate cell mass produced. In our experience, the BACTEC GI needed to perform an analysis with DNA probes was greater than that required by our molecular biology-based method.
The advantages of the PCR–reverse cross-blot hybridization method are appreciable compared to those of the other recent and innovative molecular biology-based procedures, such as those that analyze the PCR products by sequencing analysis (16, 18) or restriction enzyme procedures (29). Even though it is effective, the latter method presents some problems with regard to the interpretation of the results. In fact, the method of Telenti et al. (27), which is able to identify the majority of mycobacteria, was proposed for use in the routine identification of clinical mycobacterial isolates (26), but it needed a computer analysis of the restriction patterns to clearly evidence the small differences among the bands. In contrast, the results obtained by our method are easy to read, since after the hybridization between the biotinylated PCR product and the specific oligonucleotide probe, a single colored precipitate on the strip corresponds to a unique species.
In conclusion, our results suggest that PCR–reverse cross-blot hybridization, because of its ability to identify a wide variety of clinically relevant mycobacteria including the potentially pathogenic environmental species, can be adopted by laboratories with PCR facilities for the rapid detection of the acid-fast isolates growing in BACTEC 460 TB and 9000 MB system media.
ACKNOWLEDGMENTS
This work was supported by the National Tuberculosis Project (Istituto Superiore di Sanitá-Ministero della Sanitá), contract 96/D/T10.
We thank Saveria Pastore for critical reading of the manuscript.
REFERENCES
- 1.Avaniss-Aghajani E, Jones K, Holtzman A, Aronson T, Glover N, Boian M, Froman S, Brunk C F. Molecular technique for rapid identification of mycobacteria. J Clin Microbiol. 1996;34:98–102. doi: 10.1128/jcm.34.1.98-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berman S M, Kim R C, Haghighat D, Mulligan M E, Fierer J, Wyle F C. Mycobacterium genavense infection presenting as a solitary brain mass in a patient with AIDS: case report and review. Clin Infect Dis. 1994;19:1152–1154. doi: 10.1093/clinids/19.6.1152. [DOI] [PubMed] [Google Scholar]
- 3.Böttger E C. Mycobacterium genavense: an emerging pathogen. Eur J Clin Microbiol Infect Dis. 1994;13:932–936. doi: 10.1007/BF02111494. [DOI] [PubMed] [Google Scholar]
- 4.Butler W R, Kilburn J O. Identification of major slowly growing pathogenic mycobacteria and Mycobacterium gordonae by high-performance liquid chromatography of their mycolic acids. J Clin Microbiol. 1988;26:50–53. doi: 10.1128/jcm.26.1.50-53.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler W R, Jost K C, Jr, Kilburn J O. Identification of mycobacteria by high-performance liquid chromatography. J Clin Microbiol. 1991;29:2468–2472. doi: 10.1128/jcm.29.11.2468-2472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarridge J E, III, Shawar R M, Shinnick T M, Plikaytis B B. Large-scale use of polymerase chain reaction for detection of Mycobacterium tuberculosis in a routine mycobacteriology laboratory. J Clin Microbiol. 1993;31:2049–2056. doi: 10.1128/jcm.31.8.2049-2056.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenach K D, Sifford M D, Cave M D, Bates J H, Crawford J T. Detection of Mycobacterium tuberculosis in sputum samples using a polymerase chain reaction. Am Rev Respir Dis. 1991;144:1160–1163. doi: 10.1164/ajrccm/144.5.1160. [DOI] [PubMed] [Google Scholar]
- 8.Fadda G, Roe S L. Recovery and susceptibility testing of Mycobacterium tuberculosis from extrapulmonary specimens by the BACTEC radiometric method. J Clin Microbiol. 1984;19:720–721. doi: 10.1128/jcm.19.5.720-721.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falkinam J O., III Epidemiology of infection by nontuberculous mycobacteria. Clin Microbiol Rev. 1996;9:177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiss E H, Chehab F F, Brooks G F. DNA amplification and reverse dot blot hybridization for detection and identification of mycobacteria to the species level in the clinical laboratory. J Clin Microbiol. 1992;30:1220–1224. doi: 10.1128/jcm.30.5.1220-1224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glickman S E, Kilburn J O, Butler W R, Ramos L S. Rapid identification of mycolic acid patterns of mycobacteria by high-performance liquid chromatography using pattern recognition software and a Mycobacterium library. J Clin Microbiol. 1994;32:740–745. doi: 10.1128/jcm.32.3.740-745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto M, Oka S, Okuzimi K, Kimura S, Shimada K. Evaluation of acridinium-ester-labeled DNA probes for identification of Mycobacterium tuberculosis and Mycobacterium avium-Mycobacterium intracellulare complex in culture. J Clin Microbiol. 1991;29:2473–2476. doi: 10.1128/jcm.29.11.2473-2476.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heym B, Honore N, Truffot-Pernot C, Banerjee A, Schurra C, Jacobs W R, Jr, van Embden J D A, Grosset J H, Cole S T. Implications of multidrug resistance for the future of short course chemotherapy of tuberculosis: a molecular study. Lancet. 1994;344:293–298. doi: 10.1016/s0140-6736(94)91338-2. [DOI] [PubMed] [Google Scholar]
- 14.Hoffner S E. Pulmonary infections caused by less frequently encountered slow-growing environmental mycobacteria. Eur J Clin Microbiol Infect Dis. 1994;13:937–941. doi: 10.1007/BF02111495. [DOI] [PubMed] [Google Scholar]
- 15.Horsburgh C R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 16.Hughes M S, Skuce R A, Beck L A, Neil S D. Identification of mycobacteria from animals by restriction enzyme analysis and direct DNA cycle sequencing of polymerase chain reaction-amplified 16S rRNA gene sequences. J Clin Microbiol. 1993;31:3216–3222. doi: 10.1128/jcm.31.12.3216-3222.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kent P T, Kubika G P. Public health mycobacteriology. A guide for the level III laboratory. Atlanta, Ga: Centers for Disease Control; 1985. [Google Scholar]
- 18.Kirschner P, Springer B, Vogel U, Meier A, Wrede A, Kiekenbeck M, Bange F C, Böttger E C. Genotypic identification of mycobacteria by nucleic acid sequence determination: report of a 2-year experience in a clinical laboratory. J Clin Microbiol. 1993;31:2882–2889. doi: 10.1128/jcm.31.11.2882-2889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knolte F S, Metchock B. Mycobacterium. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: American Society for Microbiology; 1995. pp. 400–437. [Google Scholar]
- 20.Kox L F F, Rhienthong D, Medo Miranda A, Udomsantisuk N, Ellis K, Van leeuwen J, van Heusden S, Kuijper S, Kolk A H J. A more reliable PCR for detection of Mycobacterium tuberculosis in clinical samples. J Clin Microbiol. 1994;32:672–678. doi: 10.1128/jcm.32.3.672-678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kox L F F, van Leeuwen J, Knijper S, Jansen H M, Kolk A H J. PCR assay based on DNA coding for 16S rRNA for detection and identification of mycobacteria in clinical samples. J Clin Microbiol. 1995;33:3225–3233. doi: 10.1128/jcm.33.12.3225-3233.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kox L F, Jansen H M, Knijper S, Kolk A H. Multiplex PCR assay for immediate identification of the infecting species in patients with mycobacterial disease. J Clin Microbiol. 1997;35:1492–1498. doi: 10.1128/jcm.35.6.1492-1498.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebrun L, Espinasse F, Poveda J D, Vincent-Levy-Frebault V. Evaluation of nonradioactive DNA probes for identification of mycobacteria. J Clin Microbiol. 1992;30:2476–2478. doi: 10.1128/jcm.30.9.2476-2478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picardeau M, Vincent V. Development of a species-specific probe for Mycobacterium xenopi. Res Microbiol. 1995;146:237–243. doi: 10.1016/0923-2508(96)80279-8. [DOI] [PubMed] [Google Scholar]
- 25.Raviglione M C, Snider D E, Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 26.Taylor T B, Patterson C, Hale Y, Safranek W W. Routine use of PCR-restriction fragment length polymorphism analysis for identification of mycobacteria growing in liquid media. J Clin Microbiol. 1997;35:79–85. doi: 10.1128/jcm.35.1.79-85.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Telenti A, Marches F, Bald M, Badly F, Böttger E, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tisdall P A, Roberts G D, Anhalt J P. Identification of clinical isolates of mycobacteria with gas-liquid chromatography alone. J Clin Microbiol. 1979;10:506–514. doi: 10.1128/jcm.10.4.506-514.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaneechoutte M, de Beenhouwer H, Clayes G, Verschraegen G, De Rouck A, Paepe N, Elaichouni A, Portaels F. Identification of Mycobacterium species by using amplified ribosomal DNA restriction analysis. J Clin Microbiol. 1993;31:2061–2065. doi: 10.1128/jcm.31.8.2061-2065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace R J, Jr, O’Brien R, Glassroth J, Raleigh J, Dutt A. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am Rev Respir Dis. 1990;142:940–953. doi: 10.1164/ajrccm/142.4.940. [DOI] [PubMed] [Google Scholar]
- 31.Wolinsky E. Mycobacterial diseases other than tuberculosis. Clin Infect Dis. 1992;15:1–12. doi: 10.1093/clinids/15.1.1. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Global program on AIDS. The HIV/AIDS pandemic: 1993 overview. WHO/EPA/CNP/EVA/93.1. Geneva, Switzerland: World Health Organization; 1993. [Google Scholar]
- 33.Zanetti S, Ardito F, Sechi L, Sanguinetti M, Molicotti P, Delogu G, Pinna M P, Nacci A, Fadda G. Evaluation of nonradiometric system (Bactec 9000 MB) for detection of mycobacteria in human clinical samples. J Clin Microbiol. 1997;35:2072–2075. doi: 10.1128/jcm.35.8.2072-2075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]