Abstract
Simple Summary
Benign and intermediate bone tumours are often treated by intralesional curettage together with local adjuvants in order to the reduce risk of local relapse. However, the role of different adjuvants used is still discussed controversially. In the present systematic literature review, altogether 3316 cases of benign/intermediate bone tumours were summarised with regards to the use of local adjuvants, as well as their respective impact on local recurrence. Overall, 32 different combinations of local adjuvants were identified. Although some tumour entities may benefit from the addition of a local adjuvant, the main treatment step remains thorough curettage of the lesion.
Abstract
Local adjuvants are used upon intralesional resection of benign/intermediate bone tumours, aiming at reducing the local recurrence (LR) rate. However, it is under debate whether, when and which local adjuvants should be used. This PRISMA-guideline based systematic review aimed to analyse studies reporting on the role of adjuvants in benign/intermediate bone tumours. All original articles published between January 1995 and April 2020 were potentially eligible. Of 344 studies identified, 58 met the final inclusion criteria and were further analysed. Articles were screened for adjuvant and tumour type, follow-up period, surgical treatment, and development of LR. Differences in LR rates were analysed using chi-squared tests. Altogether, 3316 cases (10 different tumour entities) were analysed. Overall, 32 different therapeutic approaches were identified. The most common were curettage combined with high-speed burr (n = 774; 23.3%) and high-speed burr only (n = 620; 18.7%). The LR rate for studies with a minimum follow-up of 24 months (n = 30; 51.7%) was 12.5% (185/1483), with the highest rate found in GCT (16.7%; 144/861). In comparison to a combination of curettage, any adjuvant and PMMA, the sole application of curettage and high-speed burr (p = 0.015) reduced the LR rate in GCT. The overall complication rate was 9.6% (263/2732), which was most commonly attributable to postoperative fracture (n = 68) and osteoarthritis of an adjacent joint during follow-up (n = 62). A variety of adjuvants treatment options are reported in the literature. However, the most important step remains to be thorough curettage, ideally combined with high-speed burring.
Keywords: local adjuvant, benign bone tumour, intralesional resection, local recurrence, treatment
1. Introduction
Benign and locally aggressive tumours and tumour-like lesions summarise a heterogeneous group of lesions preferably found in the bones of children, adolescents and young adults [1]. They are most frequently treated by intralesional curettage [2], sometimes combined with high-speed burring [3], as well as the adjunct of adjuvant treatments. The latter aim at further reducing the local recurrence (LR) rate. Phenol is used as an adjuvant as it leads to chemical coagulation of protein-rich substances and thus necrosis of remnant tumour cells [4].
Cryotherapy with liquid nitrogen can likewise destroy remnant tumour cells not removed during curettage but is also associated with complications such as delayed bone healing, wound-healing deficits, and fractures [5,6]. Ethanol is used to wash out phenol, but it also acts as a local adjuvant by exerting cytotoxic effects, yet to a lesser extent than phenol [7]. Further adjuvants used following bone tumour curettage include liquid zinc chloride [8], hydrogen peroxide [9], and argon-plasma laser [10].
Polymethylmethacrylate (PMMA) is not only considered an adjuvant for having thermal properties during polymerisation thus eventually leading to necrosis of remnant tumour cells, but it is also a defect filler [11]. Furthermore, its radio-opaque nature enables early detection of potential LRs [12,13]. However, there is an ongoing debate regarding whether, when and which adjuvants should be applied for different benign and locally aggressive tumour entities, given their differing aggressiveness, side effects and potential to locally recur [14].
The present systematic literature review aimed at analysing treatment combinations with adjuvants beyond surgical therapy (i.e., curettage and/or high-speed burr) of benign and locally aggressive tumours as well as tumour-like lesions. Furthermore, the frequency of local recurrences and complications associated with the different adjuvant treatments were investigated.
2. Materials and Methods
A systematic literature review in PubMed, adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, was performed. All English, Spanish or German original articles published from January 1995 to April 2020 and dealing with the application of adjuvants in benign and semi-malignant tumours of bone were potentially eligible.
The following search terms were used: adjuvant treatment AND curettage AND benign bone tumour; adjuvant treatment AND curettage AND intermediate bone tumour; adjuvants AND curettage AND benign bone tumour; adjuvants AND curettage AND intermediate bone tumour; curettage AND adjuvant treatment AND benign bone tumour; curettage AND adjuvant treatment AND intermediate bone tumour (Supplementary Table S1).
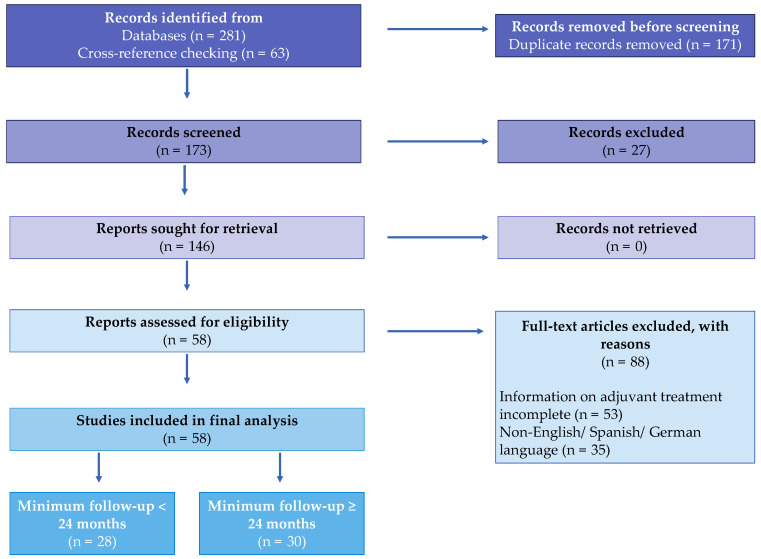
Of the initially 281 studies identified through the defined search terms and 63 additional articles selected through cross-reference checking, 171 duplicates were removed. The remaining 173 articles were subsequently screened. In the next step, 27 articles were excluded for not meeting the inclusion criteria based on the title and/or abstract, leaving 146 studies to be assessed for eligibility. Of these, 35 were excluded for not being in the English, German, or Spanish language, or for lacking complete information on the adjuvant treatment applied (n = 53). Therefore, 58 studies could be included in the final analysis, of which 30 reported on patients with a minimum follow-up ≥ 24 months, and 28 reported on patients with a minimum follow-up less than 24 months (Figure 1). Screening and initial checks for the eligibility of the studies were performed by one author (V.R.), and re-checking of these potential studies was performed by a second author (M.A.S.).
Figure 1.
PRISMA flow chart showing selection of studies included in the systematic review.
The following information was ascertained for each study included: study type, title, author, year of publication, journal, time of follow-up (mean; minimum, maximum), histological diagnosis, number of patients/tumours treated, type of surgical therapy (curettage, high-speed burr), type of adjuvant used (phenol, liquid nitrogen [cryotherapy], ethanol, electrocauterization, argon-plasma coagulation, hydrogen peroxide [H2O2], PMMA, sodium hyponitrite [Na2N2O2], liquid zinc chloride), type of defect filling (autograft, allograft, synthetic device), number of cases with complications, type of complications, treatment groups affected by complications, and number of cases with local recurrence. Notably, PMMA was considered an active adjuvant (due to its thermic properties) rather than a defect filling material.
In case of incomplete case-based information on treatment combinations and/or recurrence rates applied within individual studies, only those patients with full information available were included in the systematic review. As some studies did report on more than one tumour in a single patient, tumour cases rather than individual patients were counted.
Statistical Analysis
Descriptive and explorative analyses were performed. To allow for evaluation of local recurrence beyond 2 years following surgery, separate analyses for studies reporting on follow-up less than (n = 28) or beyond 24 months (n = 30) were performed. For the largest 4 tumour entities (giant cell tumour of bone [GCT], aneurysmal bone cyst [ABC], atypical cartilaginous tumour [ACT], chondroblastoma), LR rate was assessed depending on adjuvant treatments applied (for studies with a minimum follow-up ≥ 24 months only) and estimated using a chi-squared or Fisher’s exact test. Curettage combined with high-speed burr was defined as the control group for the individual treatments. For better comparison, treatment groups were summarised as follows: curettage only, curettage + burr, curettage + adjuvant, curettage + PMMA, curettage + burr + adjuvant, curettage + adjuvant + PMMA, curettage + burr + PMMA, curettage + burr + adjuvant + PMMA. As not all studies provided information on complications, relative frequencies of complications were calculated based on the patient number with information on complications available (n = 2732). A p-value of < 0.05 was considered statistically significant. All statistical analyses were carried out using Stata Version 16.1 (StataCorp, College Station, TX, USA).
3. Results
A total of 3316 cases with 10 different tumours and tumour-like lesions were reported in the 58 studies [7,8,9,10,12,13,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]. The most common entities were GCT in 2235 cases (67.4%), ACT in 333 cases (10.0%), ABC in 262 cases (7.9%), enchondroma in 235 cases (7.1%), and chondroblastoma in 219 cases (6.6%; Table 1).
Table 1.
Number of cases separated by tumour entities and minimum follow-up less than or more than 24 months.
| Tumour Entity | Total Case Number (n = 3316) | Minimum Follow-Up ≥ 24 Months (n = 1483) | Minimum Follow-Up < 24 Months (n = 1833) |
|---|---|---|---|
| GCT | 2235 | 861 | 1374 |
| Chondroblastoma | 219 | 201 | 18 |
| ABC | 262 | 165 | 97 |
| ACT | 333 | 167 | 166 |
| EC | 235 | 57 | 178 |
| Osteoblastoma | 7 | 7 | 0 |
| Fibrous dysplasia | 14 | 14 | 0 |
| Non-ossifying fibroma | 5 | 5 | 0 |
| Osteoid osteoma | 5 | 5 | 0 |
| Chondromyxoid fibroma | 1 | 1 | 0 |
Legend: GCT—giant cell tumour of bone; ABC—aneurysmal bone cyst; ACT—atypical cartilaginous tumour; EC—enchondroma.
After excluding studies reporting on follow-up less than 24 months, 1483 cases in 30 studies remained in the analysis [8,9,12,15,16,17,18,20,21,27,33,35,37,40,41,42,43,46,48,50,52,53,54,55,57,58,61,63,64,65], with GCT again contributing to the majority of cases (n = 861; 58.1%; Table 1).
3.1. Treatment
Altogether, 32 different therapeutic approaches were identified in the 58 studies (Supplementary Table S2). The most common treatment was curettage combined with high-speed burr (n = 774; 23.3%), followed by curettage only (n = 620; 18.7%), and curettage combined with PMMA (n = 243; 7.3%). Defect filling (apart from PMMA) was carried out in 1390 of 3316 cases identified. The vast majority had received bone transplants (n = 1357; 97.6%), and bioactive materials and synthetic bone transplants had been used in 29 (2.1%) and 4 cases (0.3%), respectively. In detail, defect filling devices involved an auto- or allograft (as not further specified by studies; n = 615), a bone transplant (without further specification; n = 408), an autograft (n = 153), an allograft (n = 125), a spongious bone transplant (n = 56), bioactive materials (n = 29), synthetic bone transplants (n = 3), or hydroxyapatite (n = 1).
3.1.1. GCT
Altogether, 22 different treatment combinations were identified for GCT (n = 2235). Curettage combined with high-speed burr was the most frequent one (n = 665; 29.8%), followed by curettage only (n = 272; 12.2%) and curettage combined with PMMA (n = 229; 10.2%).
3.1.2. ACT
Fourteen different treatment combinations were reported for ACT. Of the 333 ACT cases, 93 were treated with a combination of curettage, phenol and PMMA (n = 93; 27.9%). Furthermore, 85 cases had received curettage, phenol and ethanol (25.5%), and 56 cases received curettage only (16.8%).
3.1.3. ABC
Ten different treatments were identified for ABC (n = 262). A combination of curettage, high-speed burr and cryotherapy was the most common (n = 98; 37.4%). A further 41 cases had undergone curettage and high-speed burr only (15.6%), and 31 cases had undergone a combination of curettage, high-speed burr, ethanol and electrocauterization (11.8%).
3.1.4. Chondroblastoma
The 219 chondroblastoma cases had been treated with 11 differing combinations. The most frequent ones were curettage combined with high-speed burr (n = 97; 44.3%) and curettage only (n = 47; 21.5%).
3.1.5. Enchondroma
Four treatment combinations were identified for the 235 enchondromas. The vast majority had undergone curettage only (n = 189; 80.4%). Sixteen cases had been treated with a combination of curettage, high-speed burr, H2O2 and PMMA (6.8%), and another 16 cases had been treated with curettage, high-speed burr, cryotherapy and PMMA (6.8%). The remaining 14 cases had undergone curettage, cryotherapy and sodium hyponitrite treatment (6.0%).
3.1.6. Rare Tumours/Tumour-like Lesions
Of the seven osteoblastomas identified, three had been treated with curettage, high-speed burr and phenol, two with curettage only, one with curettage, high-speed burr and cryotherapy, and one with curettage and PMMA. Curettage only had been used in all fibrous dysplasia (n = 14), osteoid osteoma (n = 5), NOF (n = 5), and chondromyxoid fibroma (n = 1) cases.
3.2. Local Recurrences
Overall, 602 LRs were reported in 3316 cases, amounting to an overall recurrence rate of 18.2%. Of 1483 cases with a minimum follow-up of 24 months, 185 developed a local recurrence (12.5%). Thereafter, all LR-related analyses were carried out for cases with a minimum follow-up of 24 months.
Split by the four most frequent tumour entities, LR rates amounted to 16.7% for GCT (144/861), 9.0% for chondroblastoma (18/201), 8.5% for ABC (14/165), and 1.2% for ACT (2/167).
The remaining five LRs had been reported in fibrous dysplasia (2/14), osteoid osteoma (2/5), and NOF (1/5).
For GCT, LR rate could be significantly reduced by a combination of curettage and high-speed burr in comparison to curettage, adjuvant and PMMA (28.9%; p = 0.015). LR rates for the remaining treatment combinations were not significantly different in comparison to curettage and high-speed burr (all p > 0.05; Table 2).
Table 2.
Impact of adjuvant treatments on local recurrence rate for studies with a minimum follow-up of 24 months, split by the 4 most common tumour entities.
| Giant Cell Tumour (GCT) of Bone | ||||
| Total | No LR | LR | p -value | |
| Curettage + burr | 263 | 219 | 44 | N/A |
| Curettage | 12 | 8 | 4 | 0.233 * |
| Curettage + adjuvant | 95 | 83 | 12 | 0.346 |
| Curettage + adjuvant + PMMA | 83 | 59 | 24 | 0.015 |
| Curettage + burr + adjuvant | 164 | 133 | 31 | 0.602 |
| Curettage + burr + adjuvant + PMMA | 223 | 198 | 25 | 0.082 |
| Curettage + burr + PMMA | 22 | 18 | 4 | 0.773 * |
| Curettage + PMMA | 1 | 1 | 0 | n.c. |
| Aneurysmal Bone Cyst (ABC) | ||||
| Total | No LR | LR | p -value | |
| Curettage + burr | 36 | 34 | 2 | N/A |
| Curettage | 20 | 19 | 1 | 1.000 * |
| Curettage + burr + adjuvant | 101 | 91 | 10 | 0.428 |
| Curettage + burr + adjuvant + PMMA | 2 | 2 | 0 | 1.000 * |
| Curettage + PMMA | 6 | 5 | 1 | 0.378 * |
| Atypical Cartilaginous Tumour (ACT) | ||||
| Total | No LR | LR | p -value | |
| Curettage + burr | 5 | 0 | 0 | N/A |
| Curettage | 7 | 6 | 1 | 1.000 * |
| Curettage + adjuvant | 23 | 23 | 0 | n.c. |
| Curettage + adjuvant + PMMA | 93 | 0 | 0 | n.c. |
| Curettage + burr + adjuvant | 16 | 15 | 1 | 1.000 * |
| Curettage + burr + adjuvant + PMMA | 21 | 21 | 0 | n.c. |
| Curettage + burr + PMMA | 1 | 1 | 0 | n.c. |
| Curettage + PMMA | 1 | 0 | 0 | n.c. |
| Chondroblastoma | ||||
| Total | No LR | LR | p -value | |
| Curettage + burr | 97 | 89 | 8 | N/A |
| Curettage | 34 | 30 | 4 | 0.508 * |
| Curettage + adjuvant + PMMA | 2 | 0 | 2 | 0.009 * |
| Curettage + burr + adjuvant | 34 | 32 | 2 | 1.000 * |
| Curettage + burr + PMMA | 28 | 28 | 0 | 0.116 |
| Curettage + PMMA | 6 | 4 | 2 | 0.103 * |
Legend: * Fisher’s exact test; N/A—not applicable; n.c.—not calculated. p-values in bold highlight statistically significant results.
No significant difference in LR rate could be found for any treatment combination compared to curettage and high-speed burr for the treatment of ABC (all p > 0.05; Table 3).
Table 3.
Type of complications reported in individual studies, together with frequencies and treatment groups affected.
| Type of Complication | Count (n; % of 263) | Affected Treatment Group |
|---|---|---|
| Postoperative fracture | 68 (25.9%) | Curettage + burr (n = 24) Curettage + burr + argon beam + H2O2 + PMMA (n = 6) Curettage only (n = 5) Curettage + burr + PMMA (n = 5) Curettage + burr + H2O2 + PMMA (n = 4) Curettage + burr + cryotherapy + PMMA (n = 3) Curettage + burr + cryotherapy (n = 2) Curettage + burr + phenol (n = 2) Curettage + phenol + ethanol (n = 2) Curettage + cryotherapy + sodium hyponitrite (n = 2) Curettage + cryotherapy (n = 1) Curettage + ethanol (n = 1) Not defined (n = 11) |
| Osteoarthritis of adjacent joint | 62 (23.6%) | Curettage + burr (n = 19) Curettage + burr + PMMA (n = 12) Curettage + burr + argon beam + H2O2 + PMMA (n = 8) Curettage + phenol + ethanol + PMMA (n = 6) Curettage + PMMA (n = 4) Curettage + burr + cryotherapy + PMMA (n = 4) Curettage + burr + cryotherapy (n = 3) Curettage only (n = 2) Curettage + liquid zinc chloride (n = 2) Curettage + burr + phenol + PMMA (n = 1) Curettage + burr + phenol + electrocauterization + PMMA (n = 1) |
| Persisting pain | 30 (11.4%) | Curettage only (n = 21) |
| Curettage + PMMA (n = 7) | ||
| Curettage + burr (n = 2) | ||
| Deep wound infection | 16 (6.1%) | Curettage + burr + cryotherapy (n = 3) Curettage only (n = 2) Curettage + cryotherapy (n = 2) Curettage + burr + PMMA (n = 2) Curettage + burr (n = 1) Curettage + liquid zinc chloride (n = 1) Curettage + burr + phenol (n = 1) Curettage + cryotherapy + sodium hyponitrite (n = 1) Curettage + phenol + ethanol + PMMA (n = 1) Curettage + burr + cryotherapy + PMMA (n = 1) Not defined (n = 1) |
| Nerve injury | 14 (5.3%) | Curettage + cryotherapy (n = 4) |
| Curettage + burr + cryotherapy (n = 4) | ||
| Curettage only (n = 2) | ||
| Curettage + burr (n = 1) | ||
| Curettage + PMMA (n = 1) | ||
| Curettage + burr + PMMA (n = 1) | ||
| Curettage + burr + phenol (n = 1) | ||
| Superficial wound infection | 12 (4.6%) | Curettage + burr (n = 3) |
| Curettage only (n = 2) | ||
| Curettage + ethanol (n = 1) | ||
| Curettage + burr + cryotherapy (n = 1) | ||
| Curettage + burr + phenol (n = 1) | ||
| Curettage + phenol + ethanol (n = 1) | ||
| Curettage + burr + electrocauterization + PMMA (n = 1) | ||
| Not defined (n = 2) | ||
| Restricted mobility | 10 (3.8%) | Curettage + burr (n = 5) |
| Curettage + PMMA (n = 2) | ||
| Curettage only (n = 1) | ||
| Curettage + burr + phenol (n = 1) | ||
| Not defined (n = 1) | ||
| Physeal arrest | 9 (3.4%) | Curettage only (n = 2) |
| Curettage + burr (n = 2) | ||
| Curettage + burr + phenol (n = 2) | ||
| Curettage + PMMA (n = 1) | ||
| Curettage + burr + cryotherapy (n = 1) | ||
| Not defined (n = 1) | ||
| Joint collapse | 6 (2.3%) | Curettage + burr (n = 3) |
| Curettage + cryotherapy (n = 3) | ||
| Limb deformity | 5 (1.9%) | Curettage + burr (n = 2) |
| Curettage + PMMA (n = 2) | ||
| Not defined (n = 1) | ||
| Non-union | 5 (1.9%) | Curettage + burr (n = 3) |
| Curettage + phenol + ethanol + PMMA (n = 2) | ||
| Implant irritation | 4 (1.5%) | Curettage + burr (n = 2) |
| Curettage + burr + H2O2 + PMMA (n = 2) | ||
| Skin necrosis | 3 (1.1%) | Curettage only (n = 1) |
| Curettage + cryotherapy (n = 1) | ||
| Not defined (n = 1) | ||
| Crack of affected bone | 2 (0.8%) | Not defined (n = 2) |
| Delayed wound healing | 2 (0.8%) | Curettage only (n = 2) |
| Abnormal banded signal around PMMA on MRI | 2 (0.8%) | Curettage + PMMA (n = 2) |
| Intraoperative fracture | 2 (0.8%) | Curettage + burr (n = 1) |
| Curettage + phenol (n = 1) | ||
| PMMA excavation | 2 (0.8%) | Curettage + PMMA (n = 1) |
| Curettage + phenol + PMMA (n = 1) | ||
| Cartilage defect | 1 (<0.5%) | Curettage + burr + cryotherapy (n = 1) |
| Deep vein thrombosis | 1 (<0.5%) | Curettage only (n = 1) |
| Lung embolism | 1 (<0.5%) | Curettage only (n = 1) |
| Unknown | 1 (<0.5%) | Curettage + burr + phenol (n = 1) |
| Periarticular ossification | 1 (<0.5%) | Curettage + burr (n = 1) |
| Bone graft reabsorption | 1 (<0.5%) | Curettage + burr + phenol (n = 1) |
| Secondary sarcoma | 1 (<0.5%) | Curettage + PMMA (n = 1) |
| Skin blisters | 1 (<0.5%) | Curettage + burr + cryotherapy (n = 1) |
| Venous gas embolism | 1 (<0.5%) | Curettage + cryotherapy + sodium hyponitrite (n = 1) |
Likewise, for ACT, none of the treatment combinations significantly altered the LR rate in comparison to the defined “standard treatment” of curettage and high-speed burr (all p > 0.05 or p-value not calculated; Table 2).
Curettage combined with adjuvant and PMMA in chondroblastoma performed worse in terms of LR rate (100%) as compared with curettage and high-speed burr (8.2%; p = 0.009). All other treatment combinations reached similar LR rates to curettage and high-speed burr only (all p > 0.05; Table 2).
3.3. Complications
Nine of fifty-eight studies (involving 584 patients) did not provide information on any complications [13,19,28,35,36,39,44,47,62]. A further nine studies reported 0 complications in their respective cohorts (altogether 436 patients) [17,21,23,24,32,43,49,54,63]. In the remaining 40 studies (involving 2296 patients), at least one complication had been observed [7,8,9,10,12,15,16,18,20,25,26,27,29,30,31,33,34,37,38,40,41,42,45,46,48,50,51,52,53,55,57,58,59,60,61,64,65,66,67,68]. Thus, 263 complications occurred in 2732 patients with information on adverse events available, amounting to an overall complication rate of 9.6%. The most common complications were postoperative fractures (n = 68/2732, 2.5%), osteoarthritis of the adjacent joint during follow-up (n = 62/2732; 2.3%), persisting pain (n = 30/2732; 1.1%), and nerve injuries (n = 14/2732; 0.5%). A detailed description of complications, frequencies and affected treatment group is visible in Table 3.
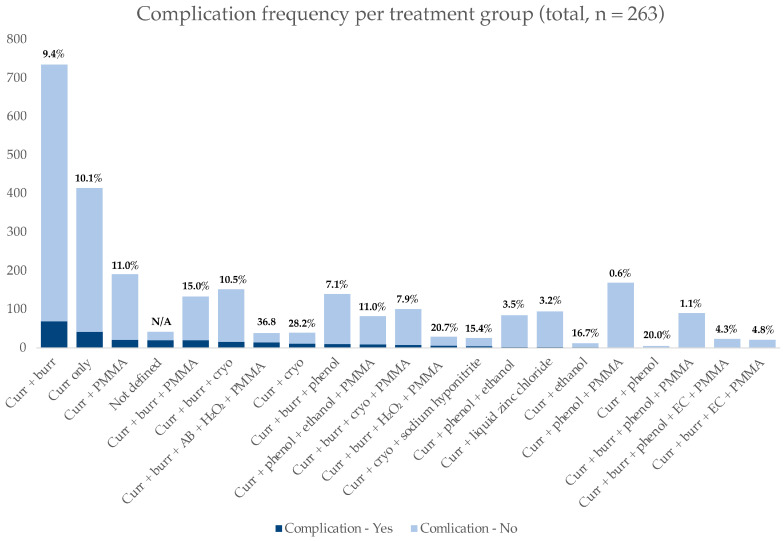
The highest complication rate was present in the cohort of patients treated with curettage + high-speed burr + argon beam + H2O2 + PMMA (14/38; 36.8%), followed by curettage + cryotherapy (11/28; 28.2%), curettage + high-speed burr + H2O2 + PMMA (6/29; 20.7%), curettage + phenol (1/5; 20.0%), curettage + ethanol (2/12; 16.7%), and curettage + cryotherapy + sodium hyponitrite (4/26; 15.4%). Figure 2 provides detailed information on complications per treatment group together with relative percentages.
Figure 2.
Frequency of complications as reported by treatment group. Relative percentages of complication frequencies per treatment group are provided. Legend: Curr—curettage; AB—argon beam; cryo—cryotherapy; EC—electrocauterisation.
4. Discussion
According to the present systematic review, 32 different treatment combinations have been described in the literature for curettage of benign and locally aggressive tumours of bone, as well as tumour-like lesions. Most lesions (with GCT of bone, followed by ACT and ABC being the most common entities) were treated with curettage and high-speed burr, or curettage only. Overall LR and complication rates amounted to 18.2% (12.5% in studies with a minimum follow-up of 24 months) and 9.6%.
GCT of bone presented with the highest LR rate, which appeared to be reduced by the addition of phenol and PMMA in comparison to curettage and high-speed burr alone. On the other hand, chondroblastoma LR rates were reduced by the addition of high-speed burr rather than any further adjuvant.
Intralesional curettage of benign bone tumours and tumour-like lesions aims at reducing the rate of LR to a minimum. This goal is achieved by meticulous curettage (i.e., macroscopic removal) of the tumour either alone or in combination with high-speed burr to also target the lesion’s border by mechanical disruption [61,65,69]. However, differing tumour biology also results in varying risk of recurrence that must be taken into consideration upon surgery and in the decision to use adjuvants and which to use.
Unsurprisingly, GCT of bone had the highest local recurrence rate, amounting to 16.7% for studies with a minimum follow-up of 24 months. Interestingly, the sole use of curettage and high-speed burr appeared to significantly reduce the LR rate in comparison to curettage, use of an adjuvant, and PMMA. This contradicts observations made by Gava et al. [70] in a systematic review mainly focussing on defect filling rather than the different adjuvant treatments, which discovered that the LR rate tends to be reduced in GCT upon use of an adjuvant. On the other hand, the systematic review and meta-analysis by Algawahmed et al. reached the conclusion that the use of adjuvants does not significantly alter the LR rate in GCT [14].
In chondroblastoma, the addition of high-speed burr to curettage of the lesion seems to be of greater importance in terms of LR-rate reduction than any adjuvant applied, yet the low number of cases reporting on treatment combinations omitting high-speed burring has to be considered when interpreting these results [16,57].
The cumulative analysis of adjuvant treatments for ACTs of the extremities, formerly known as chondrosarcoma G1, did not allow identification of any adjuvant that would significantly reduce LR rate. This was mainly caused by the overall low LR rate reported (1.2%; 2/167) that was even lower than the one identified for their benign counterpart enchondroma (3.5%; 2/57). This fact appears surprising at first, given that ACTs are considered more aggressive than EC, with higher LR rates [71]. Yet, the numbers should be interpreted with caution, given that the diagnostic criteria to distinguish between enchondroma and ACT are yet to be defined and may have varied between studies and time periods [72,73].
ABCs were historically considered tumour-like lesions emerging due to increased intraosseous venous pressure. However, the detection of a ubiquitin-specific protease (USP6) gene translocation within some ABCs has moved them towards the group of “true” neoplasms [74]. According to this systematic review, no specific adjuvant seems to significantly alter LR rate in comparison to curettage and high-speed burr alone, which is also in line with conclusions reached by other authors [58,69]. Notably, percutaneous techniques with sclerotherapy are becoming more common in the treatment of ABCs, with the main advantage being low morbidity and the possibility to perform repeated procedures [75].
Interestingly, a study reporting on curettage of NOF as a tumour-like lesion (alongside other benign bone tumours) was likewise identified in this systematic review, although NOFs are nowadays considered as “leave-me-alone” lesions [46].
Finally, potential side effects of adjuvants reported in the literature and/or still applied in clinical practice must be taken into consideration. The overall complication rate herein observed amounted to 9.6%, with postoperative fractures and osteoarthritis of the adjacent joint during follow-up being the most common. For example, phenol is known for its toxic properties when being inhaled, ingested or in contact with the skin [68]. The latter leads to bleaching and irritation, whereas signs of phenol intoxication following ingestion include nausea, pain, and diarrhoea [76]. Ultimately, intoxication may lead to renal and hepatic damage, CNS depression, pulmonary oedema and circulatory as well as respiratory failure [68,76]. Therefore, phenol is nowadays only rarely used as an adjuvant, and has even been prohibited in some countries for safety reasons [66]. For other adjuvants such as PMMA, no such toxic side effects have been reported, either for patients or surgeons, rendering them a safe and effective agent. Interestingly, the treatment combination most commonly associated with complications was curettage combined with high-speed burr, argon beam, H2O2 and PMMA (36.8% complication rate), followed by curettage and cryotherapy (28.2% complication rate). The latter rate is not surprising, given the fact that the application of cryotherapy has been reported to lead to local tissue necrosis resulting in early (e.g., skin necrosis) or late (e.g., postoperative fracture) complications [5,77,78]. However, one has to be cautious with the provided numbers, as not every study reported on complications, and some did not provide sufficient information to allow delineation regarding which treatment group the respective adverse event had occurred.
Some limitations have to be considered when interpreting the results of the present study. The heterogeneity of studies included (10 different tumour entities, 32 various treatment combinations) impeded a large and uniform analysis, specifically regarding the impact of adjuvant-combinations on LR rate. Also, detailed descriptions of treatment combinations used by the authors and the resulting LR rates for individual patients were not uniformly available; therefore, on some occasions not all patients reported within an individual study could be included. This has to be considered as another limitation of the study, which eventually distorted the influence of specific adjuvants on cumulative LR rate. Another limitation is the fact that nearly half of the studies had to be excluded when calculating the LR rate, given that the minimum follow-up of 24 months had not been reached. Furthermore, as already outlined above, not every study provided sufficient information on the occurrence of potential complications, or allowed for delineation regarding which treatment group had ultimately been affected by the respective complication. Thus, the given complication numbers have to be interpreted bearing these limiting factors in mind.
5. Conclusions
In conclusion, the present systematic review retrieved a myriad of treatment combinations used upon curettage of benign and locally aggressive bone tumours as well as tumour-like lesions. Despite these available treatment options, the overall most important treatment to lower the rate of recurrence appears to be meticulous surgical curettage, either alone or preferably in combination with high-speed burring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15174258/s1, Table S1: PubMed search terms, Table S2: Treatment combination types.
Author Contributions
Conceptualization, M.A.S. and A.L.; methodology, V.R. and M.A.S.; validation, A.L. and M.A.S.; formal analysis, V.R. and M.A.S.; data curation, V.R. and M.A.S.; writing—original draft preparation, M.A.S.; writing—review and editing, A.L. and V.R.; supervision, A.L. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Vlychou M., Athanasou N. Radiological and pathological diagnosis of paediatric bone tumours and tumour-like lesions. Pathology. 2008;40:196–216. doi: 10.1080/00313020701813784. [DOI] [PubMed] [Google Scholar]
- 2.Chigira M., Watanabe H., Arita S., Noda K., Shimizu T., Shinozaki T., Nagase M. Remodeling of large bone defects in the treatment of space-occupying lesions. Curettage without bone graft for treating benign bone tumors. Arch. Orthop. Trauma Surg. 1992;111:61–65. doi: 10.1007/BF00443468. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs C.P., Jr., Hefele M.C., Peabody T.D., Montag A.G., Aithal V., Simon M.A. Aneurysmal bone cyst of the extremities. Factors related to local recurrence after curettage with a high-speed burr. J. Bone Jt. Surg. Am. 1999;81:1671–1678. doi: 10.2106/00004623-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Mittag F., Leichtle C., Kieckbusch I., Wolburg H., Rudert M., Kluba T., Leichtle U. Cytotoxic effect and tissue penetration of phenol for adjuvant treatment of giant cell tumours. Oncol. Lett. 2013;5:1595–1598. doi: 10.3892/ol.2013.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobs P.A., Clemency R.E., Jr. The closed cryosurgical treatment of giant cell tumor. Clin. Orthop. Relat. Res. 1985;192:149–158. doi: 10.1097/00003086-198501000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Marcove R.C., Weis L.D., Vaghaiwalla M.R., Pearson R. Cryosurgery in the treatment of giant cell tumors of bone: A report of 52 consecutive cases. Clin. Orthop. Relat. Res. 1978;134:275–289. doi: 10.1097/00003086-197807000-00044. [DOI] [PubMed] [Google Scholar]
- 7.Jones K.B., DeYoung B.R., Morcuende J.A., Buckwalter J.A. Ethanol as a local adjuvant for giant cell tumor of bone. Iowa Orthop. J. 2006;26:69–76. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhen W., Yaotian H., Songjian L., Ge L., Qingliang W. Giant-cell tumour of bone. The long-term results of treatment by curettage and bone graft. J. Bone Jt. Surg. Br. 2004;86:212–216. doi: 10.1302/0301-620X.86B2.14362. [DOI] [PubMed] [Google Scholar]
- 9.Shemesh S.S., Pretell-Mazzini J., Quartin P.a.J., Rutenberg T.F., Conway S.A. Surgical treatment of low-grade chondrosarcoma involving the appendicular skeleton: Long-term functional and oncological outcomes. Arch. Orthop. Trauma Surg. 2019;139:1659–1666. doi: 10.1007/s00402-019-03184-w. [DOI] [PubMed] [Google Scholar]
- 10.Cummings J.E., Smith R.A., Heck R.K., Jr. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: A preliminary study. Clin. Orthop. Relat. Res. 2010;468:231–237. doi: 10.1007/s11999-009-0914-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bini S.A., Gill K., Johnston J.O. Giant cell tumor of bone. Curettage and cement reconstruction. Clin. Orthop. Relat. Res. 1995;321:245–250. [PubMed] [Google Scholar]
- 12.Wada T., Kaya M., Nagoya S., Kawaguchi S., Isu K., Yamashita T., Yamawaki S., Ishii S. Complications associated with bone cementing for the treatment of giant cell tumors of bone. J. Orthop. Sci. 2002;7:194–198. doi: 10.1007/s007760200033. [DOI] [PubMed] [Google Scholar]
- 13.Balke M., Schremper L., Gebert C., Ahrens H., Streitbuerger A., Koehler G., Hardes J., Gosheger G. Giant cell tumor of bone: Treatment and outcome of 214 cases. J. Cancer Res. Clin. Oncol. 2008;134:969–978. doi: 10.1007/s00432-008-0370-x. [DOI] [PubMed] [Google Scholar]
- 14.Algawahmed H., Turcotte R., Farrokhyar F., Ghert M. High-Speed Burring with and without the Use of Surgical Adjuvants in the Intralesional Management of Giant Cell Tumor of Bone: A Systematic Review and Meta-Analysis. Sarcoma. 2010;2010:586090. doi: 10.1155/2010/586090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angelini A., Hassani M., Mavrogenis A.F., Trovarelli G., Romagnoli C., Berizzi A., Ruggieri P. Chondroblastoma in adult age. Eur. J. Orthop. Surg. Traumatol. 2017;27:843–849. doi: 10.1007/s00590-017-1996-7. [DOI] [PubMed] [Google Scholar]
- 16.Ebeid W.A., Hasan B.Z., Badr I.T., Mesregah M.K. Functional and Oncological Outcome After Treatment of Chondroblastoma With Intralesional Curettage. J. Pediatr. Orthop. 2019;39:e312–e317. doi: 10.1097/BPO.0000000000001293. [DOI] [PubMed] [Google Scholar]
- 17.El-Moatasem E.-H.M., Abdel-Rahman M., Eid M.A. Extended curettage and adjuvant therapy for benign tumors of the talus. Foot. 2015;25:79–83. doi: 10.1016/j.foot.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Farfalli G.L., Albergo J.I., Piuzzi N.S., Ayerza M.A., Muscolo D.L., Ritacco L.E., Aponte-Tinao L.A. Is Navigation-guided En Bloc Resection Advantageous Compared With Intralesional Curettage for Locally Aggressive Bone Tumors? Clin. Orthop. Relat. Res. 2018;476:511–517. doi: 10.1007/s11999.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Özer D., Arıkan Y., Gür V., Gök C., Akman Y.E. Chondroblastoma: An evaluation of the recurrences and functional outcomes following treatment. Acta Orthop. Et Traumatol. Turc. 2018;52:415–418. doi: 10.1016/j.aott.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farouk H.A., Saladin M., Senna W.A., Ebeid W. All-endoscopic management of benign bone lesions; a case series of 26 cases with minimum of 2 years follow-up. Sicot. J. 2018;4:50. doi: 10.1051/sicotj/2018041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim Y.W., Tan M.H. Treatment of benign giant cell tumours of bone in Singapore. Ann. Acad. Med. Singap. 2005;34:235–237. [PubMed] [Google Scholar]
- 22.Omlor G.W., Lohnherr V., Hetto P., Gantz S., Fellenberg J., Merle C., Guehring T., Lehner B. Surgical therapy of benign and low-grade malignant intramedullary chondroid lesions of the distal femur: Intralesional resection and bone cement filling with or without osteosynthesis. Strat. Trauma Limb Reconstr. 2018;13:163–170. doi: 10.1007/s11751-018-0321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbeitsgemeinschaft K., Becker W.T., Dohle J., Bernd L., Braun A., Cserhati M., Enderle A., Hovy L., Matejovsky Z., Szendroi M., et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J. Bone Jt. Surg. Am. 2008;90:1060–1067. doi: 10.2106/JBJS.D.02771. [DOI] [PubMed] [Google Scholar]
- 24.Dabak N., Gocer H., Cirakli A. Advantages of Pressurized-Spray Cryosurgery in Giant Cell Tumors of the Bone. Balk. Med J. 2016;33:496–503. doi: 10.5152/balkanmedj.2016.150473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Z.-H., Yin J.-Q., Xie X.-B., Zou C.-Y., Huang G., Wang J., Shen J.-N. Local control of giant cell tumors of the long bone after aggressive curettage with and without bone cement. BMC Musculoskelet. Disord. 2014;15:330. doi: 10.1186/1471-2474-15-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malek F., Krueger P., Hatmi Z.N., Malayeri A.A., Faezipour H., O’Donnell R.J. Local control of long bone giant cell tumour using curettage, burring and bone grafting without adjuvant therapy. Int. Orthop. 2006;30:495–498. doi: 10.1007/s00264-006-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mashhour M.A., Abdel Rahman M. Lower recurrence rate in chondroblastoma using extended curettage and cryosurgery. Int. Orthop. 2014;38:1019–1024. doi: 10.1007/s00264-013-2178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohaidat Z.M., Al-Jamal H.Z., Bany-Khalaf A.M., Radaideh A.M., Audat Z.A. Giant cell tumor of bone: Unusual features of a rare tumor. Rare Tumors. 2019;11:2036361319878894. doi: 10.1177/2036361319878894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohler D.G., Chiu R., McCall D.A., Avedian R.S. Curettage and cryosurgery for low-grade cartilage tumors is associated with low recurrence and high function. Clin. Orthop. Relat. Res. 2010;468:2765–2773. doi: 10.1007/s11999-010-1445-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasaki H., Nagano S., Shimada H., Yokouchi M., Setoguchi T., Ishidou Y., Kunigou O., Maehara K., Komiya S. Diagnosing and discriminating between primary and secondary aneurysmal bone cysts. Oncol. Lett. 2017;13:2290–2296. doi: 10.3892/ol.2017.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreuder H.B., Pruszczynski M., Veth R.P., Lemmens J.A.M. Treatment of benign and low-grade malignant intramedullary chondroid tumours with curettage and cryosurgery. Eur. J. Surg. Oncol. (EJSO) 1998;24:120–126. doi: 10.1016/S0748-7983(98)91459-7. [DOI] [PubMed] [Google Scholar]
- 32.Solooki S., Keikha Y., Vosoughi A.R. Can ethanol be used as an adjuvant to extended curettage in order to reduce the recurrence rate of aneurysmal bone cyst? Rev. Bras. Ortop. 2017;52:349–353. doi: 10.1016/j.rbo.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Geest I.C.M., van Noort M.P., Schreuder H.W.B., Pruszczynski M., de Rooy J.W.J., Veth R.P.H. The cryosurgical treatment of chondroblastoma of bone: Long-term oncologic and functional results. J. Surg. Oncol. 2007;96:230–234. doi: 10.1002/jso.20804. [DOI] [PubMed] [Google Scholar]
- 34.Hirn M., de Silva U., Sidharthan S., Grimer R.J., Abudu A., Tillman R.M., Carter S.R. Bone defects following curettage do not necessarily need augmentation. Acta Orthop. 2009;80:4–8. doi: 10.1080/17453670902804505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klenke F.M., Wenger D.E., Inwards C.Y., Rose P.S., Sim F.H. Giant cell tumor of bone: Risk factors for recurrence. Clin. Orthop. Relat. Res. 2011;469:591–599. doi: 10.1007/s11999-010-1501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masui F., Ushigome S., Kamitani K., Asanuma K., Fujii K. Chondroblastoma: A study of 11 cases. Eur. J. Surg. Oncol. (EJSO) 2002;28:869–874. doi: 10.1053/ejso.2002.1276. [DOI] [PubMed] [Google Scholar]
- 37.Rahman M.A., El Masry A.M., Azmy S.I. Review of 16 cases of aneurysmal bone cyst in the proximal femur treated by extended curettage and cryosurgery with reconstruction using autogenous nonvascularized fibula graft. J. Orthop. Surg. 2018;26:2309499018783905. doi: 10.1177/2309499018783905. [DOI] [PubMed] [Google Scholar]
- 38.Tunn P.U., Schlag P.M. Giant cell tumor of bone. An evaluation of 87 patients. Z Orthop Ihre Grenzgeb. 2003;141:690–698. doi: 10.1055/s-2003-812400. [DOI] [PubMed] [Google Scholar]
- 39.Brown M.T., Gikas P.D., Bhamra J.S., Skinner J.A., Aston W.J.S., Pollock R.C., Saifuddin A., Briggs T.W.R. How safe is curettage of low-grade cartilaginous neoplasms diagnosed by imaging with or without pre-operative needle biopsy? Bone Jt. J. 2014;96-B:1098–1105. doi: 10.1302/0301-620X.96B8.32056. [DOI] [PubMed] [Google Scholar]
- 40.Dierselhuis E.F., Gerbers J.G., Ploegmakers J.J., Stevens M., Suurmeijer A.J., Jutte P.C. Local Treatment with Adjuvant Therapy for Central Atypical Cartilaginous Tumors in the Long Bones: Analysis of Outcome and Complications in One Hundred and Eight Patients with a Minimum Follow-up of Two Years. J. Bone Jt. Surg. 2016;98:303–313. doi: 10.2106/JBJS.O.00472. [DOI] [PubMed] [Google Scholar]
- 41.Peeters S., Van der Geest I., de Rooy J., Veth R., Schreuder H. Aneurysmal bone cyst: The role of cryosurgery as local adjuvant treatment. J. Surg. Oncol. 2009;100:719–724. doi: 10.1002/jso.21410. [DOI] [PubMed] [Google Scholar]
- 42.Moon M.-S., Kim S.-S., Moon J.-L., Kim S.-S., Moon H. Treating Giant Cell Tumours with Curettage, Electrocautery, Burring, Phenol Irrigation, and Cementation. J. Orthop. Surg. 2013;21:209–212. doi: 10.1177/230949901302100219. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y.-C., Wu P.-K., Chen C.-F., Chen W.-M. Intralesional curettage of central low-grade chondrosarcoma: A midterm follow-up study. J. Chin. Med. Assoc. 2017;80:178–182. doi: 10.1016/j.jcma.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Dürr H.R., Maier M., Jansson V., Baur A., Refior H.J. Phenol as an adjuvant for local control in the treatment ofgiant cell tumour of the bone. Eur. J. Surg. Oncol. (EJSO) 1999;25:610–618. doi: 10.1053/ejso.1999.0716. [DOI] [PubMed] [Google Scholar]
- 45.Gaston C.L., Bhumbra R., Watanuki M., Abudu A.T., Carter S.R., Jeys L.M., Tillman R.M., Grimer R.J. Does the addition of cement improve the rate of local recurrence after curettage of giant cell tumours in bone? J. Bone Jt. Surg. 2011;93-B:1665–1669. doi: 10.1302/0301-620X.93B12.27663. [DOI] [PubMed] [Google Scholar]
- 46.Horstmann P.F., Hettwer W.H., Petersen M.M. Treatment of benign and borderline bone tumors with combined curettage and bone defect reconstruction. J. Orthop. Surg. 2018;26:2309499018774929. doi: 10.1177/2309499018774929. [DOI] [PubMed] [Google Scholar]
- 47.Lausten G.S., Jensen P.K., Schiødt T., Lund B. Local recurrences in giant cell tumour of bone. Int. Orthop. 1996;20:172–176. doi: 10.1007/s002640050057. [DOI] [PubMed] [Google Scholar]
- 48.Lin W.-H., Lan T.-Y., Chen C.-Y., Wu K., Yang R.-S. Similar Local Control between Phenol- and Ethanol-treated Giant Cell Tumors of Bone. Clin. Orthop. Relat. Res. 2011;469:3200–3208. doi: 10.1007/s11999-011-1962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morii T., Mochizuki K., Tajima T., Satomi K. Treatment outcome of enchondroma by simple curettage without augmentation. J. Orthop. Sci. 2010;15:112–117. doi: 10.1007/s00776-009-1419-7. [DOI] [PubMed] [Google Scholar]
- 50.Suneja R., Grimer R.J., Belthur M., Jeys L., Carter S.R., Tillman R.M., Davies A.M. Chondroblastoma of bone. J. Bone Jt. Surg. 2005;87:974–978. doi: 10.1302/0301-620X.87B7.16009. [DOI] [PubMed] [Google Scholar]
- 51.Sheth D.S., Healey J.H., Sobel M., Lane J.M., Marcove R.C. Giant cell tumor of the distal radius. J. Hand Surg. 1995;20:432–440. doi: 10.1016/S0363-5023(05)80102-9. [DOI] [PubMed] [Google Scholar]
- 52.Shih H.-N., Hsu R.W.-W., Sim F.H. Excision Curettage and Allografting of Giant Cell Tumor. World J. Surg. 1998;22:432–437. doi: 10.1007/s002689900411. [DOI] [PubMed] [Google Scholar]
- 53.Su Y.-P., Chen W.-M., Chen T.-H. Giant-cell tumors of bone: An analysis of 87 cases. Int. Orthop. 2004;28:239–243. doi: 10.1007/s00264-004-0564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trieb K., Bitzan P., Lang S., Dominkus M., Kotz R. Recurrence of curetted and bone-grafted giant-cell tumours with and without adjuvant phenol therapy. Eur. J. Surg. Oncol. (EJSO) 2001;27:200–202. doi: 10.1053/ejso.2000.1086. [DOI] [PubMed] [Google Scholar]
- 55.van der Heijden L., van der Geest I.C.M., Schreuder H.W.B., van de Sande M.A.J., Dijkstra P.D.S. Liquid Nitrogen or Phenolization for Giant Cell Tumor of Bone?: A Comparative Cohort Study of Various Standard Treatments at Two Tertiary Referral Centers. JBJS. 2014;96:e35. doi: 10.2106/JBJS.M.00516. [DOI] [PubMed] [Google Scholar]
- 56.Verdegaal S.H.M., Brouwers H.F.G., van Zwet E.W., Hogendoorn P.C.W., Taminiau A.H.M. Low-Grade Chondrosarcoma of Long Bones Treated with Intralesional Curettage Followed by Application of Phenol, Ethanol, and Bone-Grafting. JBJS. 2012;94:1201–1207. doi: 10.2106/JBJS.J.01498. [DOI] [PubMed] [Google Scholar]
- 57.Wallace M.T., Henshaw R.M. Results of cement versus bone graft reconstruction after intralesional curettage of bone tumors in the skeletally immature patient. J. Pediatr. Orthop. 2014;34:92–100. doi: 10.1097/BPO.0b013e31829b2f61. [DOI] [PubMed] [Google Scholar]
- 58.Wang E.H.M., Marfori M.L., Serrano M.V.T., Rubio D.A. Is curettage and high-speed burring sufficient treatment for aneurysmal bone cysts? Clin. Orthop. Relat. Res. 2014;472:3483–3488. doi: 10.1007/s11999-014-3809-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou X., Zhao B., Keshav P., Chen X., Gao W., Yan H. The management and surgical intervention timing of enchondromas: A 10-year experience. Medicine. 2017;96:e6678. doi: 10.1097/MD.0000000000006678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benevenia J., Rivero S.M., Moore J., Ippolito J.A., Siegerman D.A., Beebe K.S., Patterson F.R. Supplemental Bone Grafting in Giant Cell Tumor of the Extremity Reduces Nononcologic Complications. Clin. Orthop. Relat. Res. 2017;475:776–783. doi: 10.1007/s11999-016-4755-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blackley H.R., Wunder J.S., Davis A.M., White L.M., Kandel R., Bell R.S. Treatment of Giant-Cell Tumors of Long Bones with Curettage and Bone-Grafting*. J. Bone Jt. Surg. 1999;81:811–820. doi: 10.2106/00004623-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Kivioja A.H., Blomqvist C., Hietaniemi K., Trovik C., Walloe A., Bauer H.C.F., Jorgensen P.H., Bergh P., Follerås G. Cement is recommended in intralesional surgery of giant cell tumors: A Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008;79:86–93. doi: 10.1080/17453670710014815. [DOI] [PubMed] [Google Scholar]
- 63.Lackman R.D., Hosalkar H.S., Ogilvie C.M., Torbert J.T., Fox E.J. Intralesional Curettage for Grades II and III Giant Cell Tumors of Bone. Clin. Orthop. Relat. Res. 2005;438:123–127. doi: 10.1097/01.blo.0000180051.27961.c3. [DOI] [PubMed] [Google Scholar]
- 64.Mermerkaya M.U., Bekmez S., Karaaslan F., Danisman M., Kosemehmetoglu K., Gedikoglu G., Ayvaz M., Tokgozoglu A.M. Intralesional curettage and cementation for low-grade chondrosarcoma of long bones: Retrospective study and literature review. World J. Surg. Oncol. 2014;12:336. doi: 10.1186/1477-7819-12-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prosser G.H., Baloch K.G., Tillman R.M., Carter S.R., Grimer R.J. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin. Orthop. Relat. Res. 2005:211–218. doi: 10.1097/01.blo.0000160024.06739.ff. [DOI] [PubMed] [Google Scholar]
- 66.Omlor G.W., Lange J., Streit M., Gantz S., Merle C., Germann T., Mechtersheimer G., Fellenberg J., Lehner B. Retrospective analysis of 51 intralesionally treated cases with progressed giant cell tumor of the bone: Local adjuvant use of hydrogen peroxide reduces the risk for tumor recurrence. World J. Surg. Oncol. 2019;17:73. doi: 10.1186/s12957-019-1613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schreuder H.W.B., Veth R.P.H., Pruszczynski M., Lemmens J.A.M., Koops H.S., Molenaar W.M. Aneurysmal Bone Cysts Treated By Curettage, Cryotherapy And Bone Grafting. J. Bone Jt. Surg. 1997;79:20–25. doi: 10.1302/0301-620X.79B1.0790020. [DOI] [PubMed] [Google Scholar]
- 68.Verdegaal S.H., Hartigh J.D., Hogendoorn P.C., Brouwers H.F., Taminiau A.H. Phenol levels during intralesional curettage and local adjuvant treatment of benign and low-grade malignant bone tumours. Clin. Sarcoma Res. 2012;2:10. doi: 10.1186/2045-3329-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park H.Y., Yang S.K., Sheppard W.L., Hegde V., Zoller S.D., Nelson S.D., Federman N., Bernthal N.M. Current management of aneurysmal bone cysts. Curr. Rev. Musculoskelet. Med. 2016;9:435–444. doi: 10.1007/s12178-016-9371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gava N.F., Engel E.E. Treatment alternatives and clinical outcomes of bone filling after benign tumour curettage. A systematic review. Orthop. Traumatol. Surg. Res. 2022;108:102966. doi: 10.1016/j.otsr.2021.102966. [DOI] [PubMed] [Google Scholar]
- 71.Andreou D., Gilg M.M., Gosheger G., Werner M., Hardes J., Pink D., Leithner A., Tunn P.-U., Streitbürger A. Metastatic Potential of Grade I Chondrosarcoma of Bone: Results of a Multi-institutional Study. Ann. Surg. Oncol. 2015;23:120–125. doi: 10.1245/s10434-015-4852-1. [DOI] [PubMed] [Google Scholar]
- 72.Ferrer-Santacreu E.M., Ortiz-Cruz E.J., Díaz-Almirón M., Kreilinger J.J.P. Enchondroma versus Chondrosarcoma in Long Bones of Appendicular Skeleton: Clinical and Radiological Criteria—A Follow-Up. J. Oncol. 2016;2016:8262079. doi: 10.1155/2016/8262079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crim J., Schmidt R., Layfield L., Hanrahan C., Manaster B.J. Can imaging criteria distinguish enchondroma from grade 1 chondrosarcoma? Eur. J. Radiol. 2015;84:2222–2230. doi: 10.1016/j.ejrad.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 74.Ye Y., Pringle L.M., Lau A.W., Riquelme D.N., Wang H., Jiang T., Lev D., Welman A., Blobel G.A., Oliveira A.M., et al. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-κB. Oncogene. 2010;29:3619–3629. doi: 10.1038/onc.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Varshney M.K., Rastogi S., Khan S.A., Trikha V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin. Orthop. Relat. Res. 2010;468:1649–1659. doi: 10.1007/s11999-009-1144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bentur Y., Shoshani O., Tabak A., Binnun A., Ramon Y., Ulman Y., Berger Y., Nachlieli T., Peled Y.J. Prolonged Elimination Half-Life of Phenol After Dermal Exposure. J. Toxicol. Clin. Toxicol. 1998;36:707–711. doi: 10.3109/15563659809162619. [DOI] [PubMed] [Google Scholar]
- 77.Veth R., Schreuder B., van Beem H., Pruszczynski M., de Rooy J. Cryosurgery in aggressive, benign, and low-grade malignant bone tumours. Lancet Oncol. 2005;6:25–34. doi: 10.1016/S1470-2045(05)70023-1. [DOI] [PubMed] [Google Scholar]
- 78.Malawer M.M., Bickels J., Meller I., Buch R.G., Henshaw R.M., Kollender Y. Cryosurgery in the treatment of giant cell tumor. A long-term followup study. Clin. Orthop. Relat. Res. 1999;359:176–188. doi: 10.1097/00003086-199902000-00019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.