Abstract
Simple Summary
Notwithstanding its disfiguring nature, orbital exenteration (OE) has been employed as a surgical intervention for craniofacial lesions, especially in cases of advanced or recurrent tumors. There is a dearth of large studies investigating the clinical and survival outcomes of this procedure. Our study aims to review the literature on the clinical characteristics and outcomes of patients who underwent OE. In the univariable analysis, we found that a positive surgical margin after OE was significantly associated with worse overall survival (OS). Conversely, sex, tumor recurrence, type of OE, and tumor histopathology were not found to exert significant effects on OS.
Abstract
Background: The outcomes of orbital exenteration (OE) in patients with craniofacial lesions (CFLs) remain unclear. The present review summarizes the available literature on the clinical outcomes of OE, including surgical outcomes and overall survival (OS). Methods: Relevant articles were retrieved from Medline, Scopus, and Cochrane according to PRISMA guidelines. A systematic review and meta-analysis were conducted on the clinical characteristics, management, and outcomes. Results: A total of 33 articles containing 957 patients who underwent OE for CFLs were included (weighted mean age: 64.3 years [95% CI: 59.9–68.7]; 58.3% were male). The most common lesion was squamous cell carcinoma (31.8%), and the most common symptom was disturbed vision/reduced visual acuity (22.5%). Of the patients, 302 (31.6%) had total OE, 248 (26.0%) had extended OE, and 87 (9.0%) had subtotal OE. Free flaps (33.3%), endosseous implants (22.8%), and split-thickness skin grafts (17.2%) were the most used reconstructive methods. Sino-orbital or sino-nasal fistula (22.6%), flap or graft failure (16.9%), and hyperostosis (13%) were the most reported complications. Regarding tumor recurrences, 38.6% were local, 32.3% were distant, and 6.7% were regional. The perineural invasion rate was 17.4%, while the lymphovascular invasion rate was 5.0%. Over a weighted mean follow-up period of 23.6 months (95% CI: 13.8–33.4), a weighted overall mortality rate of 39% (95% CI: 28–50%) was observed. The 5-year OS rate was 50% (median: 61 months [95% CI: 46–83]). The OS multivariable analysis did not show any significant findings. Conclusions: Although OE is a disfiguring procedure with devastating outcomes, it is a viable option for carefully selected patients with advanced CFLs. A patient-tailored approach based on tumor pathology, extension, and overall patient condition is warranted.
Keywords: orbital exenteration, craniofacial, carcinoma, survival, outcomes, systematic review, meta-analysis
1. Introduction
Orbital exenteration (OE) is an invasive surgical procedure intended to remove the orbit contents in cases of lesions invading the orbital cavity and periocular structures [1,2,3]. Since its description in the 16th century by Bartisch [4,5], it has been performed for the surgical management of a variety of conditions, including malignancies, infections, trauma, and inflammatory diseases [3,6,7,8]. The most common indications of OE are craniofacial cancers, including squamous cell carcinoma (SCC) and basal cell carcinoma (BCC) [2,8,9,10]. OE can result in a wide range of complications, most commonly sino-orbital fistulas, graft or flap failure, and surgical site infection or non-healing wounds [4,10,11,12,13,14,15].
Different surgical techniques for OE have emerged over the past decades, including ablative surgery, total OE, subtotal or eyelid-sparing OE, and extended OE [16]. While ablative surgery focuses on eliminating tumor tissue with negative margins, total OE removes all orbit content, subtotal OE spares some orbital tissue, and extended OE removes adjacent structures beyond the orbit. Additional surgeries, such as lymph node dissection, may be performed simultaneously [1,16,17,18]. Following OE, several reconstructive techniques are usually considered to cover the evacuated orbit and surrounding region as needed. These techniques vary from spontaneous granulation to skin grafts, regional flaps, and free flaps [6,8,9,19]. In addition, cosmetic rehabilitation, in the form of a prosthesis or other socket-reconstructive methods, may be discussed with patients to ameliorate the damaging impact of OE on their quality of life, including self-image and social functioning [4,20,21,22,23].
In this study, we aimed to address the scattered data on the clinical and survival outcomes of patients with craniofacial lesions (CFLs) managed with OE. The existing literature primarily consists of case reports and series. By conducting a comprehensive systematic review of the literature, we sought to provide a comprehensive summary of the findings and bridge the knowledge gaps regarding the role of OE in the management of CFLs, as well as its associated clinical and survival outcomes.
2. Materials and Methods
2.1. Literature Search
A systematic review and meta-analysis, registered with PROSPERO (ID: CRD42023429608), were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [24]. The databases of Medline, Scopus, and Cochrane were comprehensively searched from their inception until July 2022. A search strategy, employing both medical subject headings (MeSH) and terms and keywords, was implemented using the Boolean operators OR and AND. The search terms utilized were “Orbital OR Orbit” AND “Exenteration.” The retrieved papers were imported into Mendeley (Version 2.80.1, Mendeley Ltd., July 2022, London, UK), and any duplicate records were eliminated.
2.2. Study Selection
Inclusion and exclusion criteria were defined to ensure study selection consistency. Studies were included if they (1) were retrospective or prospective studies of adult patients (≥18 years) who underwent OE procedure for CFLs and (2) reported data on clinical features, procedural details, and treatment outcomes. Studies were excluded if they (1) were meta-analyses, reviews, editorials, letters, books, or case reports, (2) contained insufficient clinical data (i.e., lacking patient demographics or management details and outcomes), (3) included 5 or less patients, (4) presented inseparable data of pediatric and adult patients or inseparable data of OE and other procedures, or (5) were written in a foreign language.
The titles and abstracts of all extracted papers were independently evaluated by two authors (KB and JQ), using the predetermined inclusion and exclusion criteria. Then, studies that met the inclusion criteria underwent further independent assessment through a full-text review by the same two authors. In cases of discrepancies, a third author (OBA) was consulted to resolve them. Additionally, the references of the included articles were screened to identify any additional relevant studies.
2.3. Data Extraction
Data from the studies that met the inclusion criteria were extracted by one author (JQ) and independently verified by another author (OBA) to ensure accuracy. The extracted variables included the author’s name, publication date, level of evidence, sample size, sex, symptoms at presentation, histological and clinical characteristics, management approaches and treatment modalities, complications, recurrence rates, and survival outcomes. Missing data were either not reported by the authors or were indistinguishable from other data.
2.4. Data Synthesis and Quality Assessment
The primary outcomes of interest were the overall survival (OS) and survival predictive factors. The secondary objectives were summarizing the characteristics of the CFLs (e.g., tumor histopathology) that required OE procedure, the management course including the type and extent of OE, and local and distant recurrence. The OS was reported according to the follow-up and survival protocol of the original papers. The level of evidence of each article was assessed using the 2011 Oxford Centre for Evidence-Based Medicine guidelines, and all articles were classified as level IV evidence [25]. Two authors (JQ and ASH) independently evaluated the risk of bias for each article using the Joanna Briggs Institute checklists [26]. The assessment revealed that all the included papers had a low risk of bias (Supplementary Table S1). Throughout this article, we followed the extent of resection classification for OE as proposed by Frezzotti et al. [27]. They categorized OE as subtotal, total, and radical, ranging from I to VI. Subtotal OE contains types I, II, and III, all of which spare the eyelid skin. Type I spares palpebral and bulbar conjunctiva, type II spares palpebral conjunctiva only, and type III is limited to sparing the deeper muscle layer. The second category, total OE, is composed of type IV only, where the eyelid skin is resected. The third category, radical, also called extended OE, includes types V and VI. In type V, the orbit cavity bones are resected, and in type VI, the resection is extended to adjacent structures. Tumor recurrence was classified as local, regional, and distant, just as they were reported in the included articles.
2.5. Statistical Analysis
The statistical analyses in this study were conducted using Stata software (StataCorp. 2023. Stata Statistical Software: Release 17. College Station, TX, USA: StataCorp. LLC.). Continuous variables were summarized by reporting weighted means (effect size of means) and 95% confidence intervals (CIs). Categorical variables, on the other hand, were summarized by reporting frequencies and percentages or weighted proportions (effect size of proportions) and 95% CIs. Missing observations in any variables were excluded from the analysis. The survival data were presented as median survival in months, accompanied by 95% CIs, and 5-year and 10-year survival rates, which were visualized using Kaplan–Meier curves [28]. The log-rank test was employed to assess the null hypothesis of no difference in survival across the categories of each categorical variable. To examine potential factors influencing survival, both univariable and multivariable analyses were conducted using the Cox proportional hazards model [29]. Only variables that were statistically significant in the univariable analysis were included in the multivariable analysis. To assess the proportional hazards assumption, Schoenfeld’s global test was employed to estimate time-varying covariance in the multivariable analysis. The results indicated that the assumption was met. Two-tailed p-value < 0.05 was considered statistically significant for all analyses conducted. Data from all included studies were pooled, and a meta-analysis was performed using the random effects model. To summarize pooled proportions, a meta-analysis of proportion was conducted, while a meta-analysis of means was employed to summarize pooled means. Subgroup analysis was conducted to assess statistical differences among various groups. Heterogeneity was assessed using the χ2 test and the Higgins I2 test [30]. Publication bias was evaluated using funnel plots and Egger’s test, with a p-value less than 0.05 indicating the presence of bias [31]. We confirmed that all studies demonstrated visual symmetry, and none of the tested endpoints revealed publication bias (Supplementary Figure S1).
3. Results
3.1. Study Selection
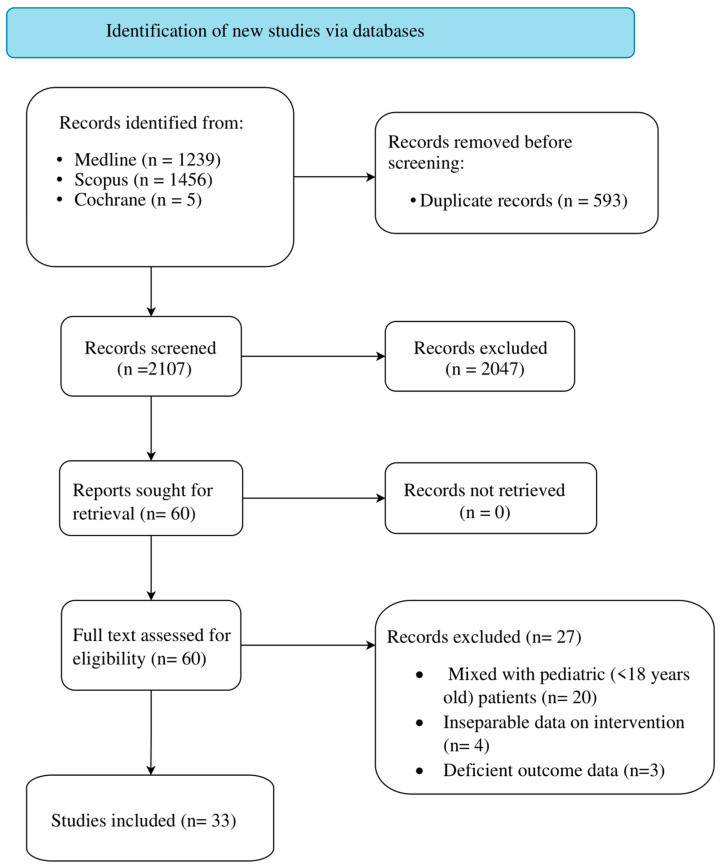
The initial literature search of databases yielded a total of 2700 articles (Figure 1). After removing duplicate records, the number of articles was reduced to 2107. Out of these, 2047 studies were excluded based on the screening of their titles and abstracts. Sixty papers were selected for retrieval and were assessed for inclusion through a full text review. Among the assessed articles, 27 did not meet our inclusion criteria and were subsequently excluded. Therefore, 33 articles, which were categorized as level IV evidence, were included in the analysis (Supplementary Table S2) [2,3,4,6,8,9,10,11,12,13,14,15,17,18,19,23,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
Figure 1.
PRISMA 2020 Flow Diagram.
3.2. Demographics and Clinical Characteristics of the Cohort
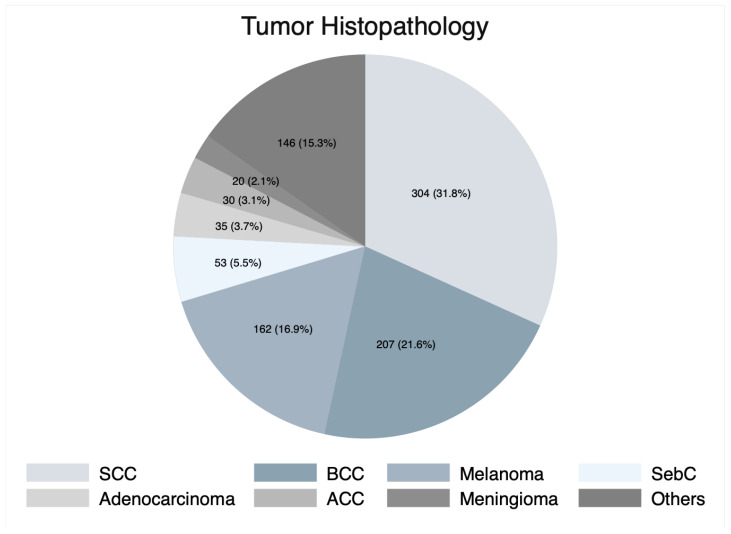
In our cohort, a total of 957 patients who underwent OE were included, among which 558 (58.3%) were male. The weighted mean patient age was 64.3 years (95% CI: 59.9–68.7 years). The most frequently involved anatomical structures were the eyelid (16.2%), orbit (14.4%), and conjunctiva (12.5%) (Table 1). SCC was the most common histopathological diagnosis (n = 304, 31.8%), followed by BCC (n = 207, 21.6%), and melanoma (n = 162, 16.9%) (Figure 2). The most common signs and symptoms at presentation were disturbed vision (reduced visual acuity) (22.5%), facial mass (17.7%), conjunctival injection (14.7%), eye pain (13.0%), and lesion discharge (12.0%) (Table 1).
Table 1.
Involved structures and clinical presentation.
| Variable | n (%) |
|---|---|
| Involved structures | n = 989 |
| Eyelids | 160 (16.2%) |
| Orbit | 142 (14.4%) |
| Conjunctiva | 124 (12.5%) |
| Canthus | 79 (8.0%) |
| Sinuses | 59 (6.0%) |
| Face | 41 (4.1%) |
| Lacrimal gland | 37 (3.7%) |
| Choroid | 23 (2.3%) |
| Nose | 22 (2.2%) |
| Unspecified peri-oculus | 19 (2.0%) |
| Periorbital skin | 15 (1.5%) |
| Eye | 14 (1.4%) |
| Cheek | 10 (1.0%) |
| Brain | 9 (0.9%) |
| Supraorbital/brow | 8 (0.8%) |
| Lacrimal sac | 7 (0.7%) |
| Ethmoid bone | 6 (0.6%) |
| Lacrimal duct | 4 (0.4%) |
| Maxilla | 3 (0.3%) |
| Temporal skin | 3 (0.3%) |
| Extraocular muscles | 2 (0.2%) |
| Temple | 2 (0.2%) |
| Alveoli | 1 (0.1%) |
| Nasopharynx | 1 (0.1%) |
| Ophthalmic nerve | 1 (0.1%) |
| Optic nerve | 1 (0.1%) |
| Parotid | 1 (0.1%) |
| Periorbital fat | 1 (0.1%) |
| Others | 194 (19.6%) |
| Presenting signs and symptoms | n = 113 * |
| Disturbed vision | 47 (22.5%) |
| Facial mass | 37 (17.7%) |
| Conjunctival injection | 31 (14.7%) |
| Eye pain | 27 (13.0%) |
| Lesion discharge | 25 (12.0%) |
| Epiphora | 18 (8.6%) |
| Diplopia | 16 (7.7%) |
| Orbital discomfort | 8 (3.8%) |
Data are reported as frequencies and percentages. * The total number of patients with symptoms does not sum up to the number of symptoms as many patients had multiple symptoms.
Figure 2.
Pie chart of tumor histopathology. SCC, squamous cell carcinoma; BCC, basal cell carcinoma; SebC, sebaceous cell carcinoma; ACC, adenoid cystic carcinoma.
3.3. Patient Management
Around one-third of the patients (n = 302, 31.6%) underwent total OE, and the other two-thirds underwent extended (n = 248, 26.0%), subtotal (n = 87, 9.0%), or unspecified (n = 320, 33.4%) OE (Table 2). The term “unspecified OE” is used to describe OE cases where the type and extent (total, subtotal, or extended) were not clearly specified in the original articles. A group of patients (n = 146) required additional surgical interventions, such as maxillectomy (39.0%), lymph node resection (24.7%), ethmoidectomy (11.6%), and craniotomy (9.6%). A total of 168 (17.6%) patients experienced complications after OE. The most common complications included sino-orbital or sino-nasal fistulas (22.6%), flap or graft failure (16.9%), hyperostosis (13.0%), wound dehiscence or non-healing (10.7%), and cerebrospinal fluid leak (8.5%) (Table 2).
Table 2.
Orbital exenteration and complications.
| Variable | n (%) |
|---|---|
| Orbital exenteration extent of resection | n = 957 |
| Total | 302 (31.6%) |
| Extended | 248 (26.0%) |
| Subtotal | 87 (9.0%) |
| Unspecified | 320 (33.4%) |
| Additional surgical interventions | n = 146 |
| Maxillectomy | 57 (39.0%) |
| Lymph node resection | 36 (24.7%) |
| Ethmoidectomy | 17 (11.6%) |
| Craniotomy | 14 (9.6%) |
| Parotidectomy | 5 (3.4%) |
| Craniofacial resection | 4 (2.7%) |
| Enucleation | 4 (2.7%) |
| Rhinectomy | 3 (2.1%) |
| Tracheostomy | 3 (2.1%) |
| Posterior orbit sparing | 2 (1.4%) |
| Temporal bone resection | 1 (0.7%) |
| Complications | n = 168 * |
| Sino-orbital/nasal fistula | 40 (22.6%) |
| Flap/graft/implant failure (loss/necrosis/atrophy) | 30 (16.9%) |
| Hyperostosis | 23 (13.0%) |
| Wound dehiscence/non-healing | 19 (10.7%) |
| CSF leak | 15 (8.5%) |
| Surgical site infection | 12 (6.7%) |
| Brain invasion | 7 (4.0%) |
| Hematoma | 6 (3.3%) |
| Socket hemorrhage | 6 (3.3%) |
| Socket draining | 3 (1.7%) |
| Transient facial weakness | 3 (1.7%) |
| Myocardial infarction | 2 (1.1%) |
| Stroke | 2 (1.1%) |
| Pulmonary infection | 1 (0.6%) |
| Delirium tremens | 1 (0.6%) |
| Eyelid fistula | 1 (0.6%) |
| Meningitis | 1 (0.6%) |
| Orbital abscess | 1 (0.6%) |
| Osteitis | 1 (0.6%) |
| Permanent facial weakness | 1 (0.6%) |
| Pulmonary edema | 1 (0.6%) |
| Trigeminal neuralgia | 1 (0.6%) |
Data are reported as frequencies and percentages. CSF, cerebrospinal fluid. * The total number of patients with complications does not sum up to the number of complications as many patients had multiple and complications.
Following OE, various reconstructive techniques were utilized (Table 3). The most commonly employed reconstructive techniques were free flap (33.3%), endosseous implants (22.8%), and split-thickness skin grafting (17.2%). The anterolateral thigh flap was the most used reconstructive material (26.6%), followed by the musculocutaneous flap (21.6%), and the radial forearm flap (14.5%).
Table 3.
Reconstructive techniques and materials.
| Variable | n (%) |
|---|---|
| Reconstructive technique | n = 820 |
| Free flap | 273 (33.3%) |
| Endosseous implant | 187 (22.8%) |
| Split-thickness skin graft | 141 (17.2%) |
| Full-thickness skin graft | 65 (8.0%) |
| Facial prosthesis | 56 (6.8%) |
| Spontaneous granulation | 54 (6.6%) |
| Orbital implant | 24 (2.9%) |
| Surgical closure of the orbit | 10 (1.2%) |
| Titanium plates | 10 (1.2%) |
| Reconstructive material | n = 587 |
| Anterolateral thigh flap | 156 (26.6%) |
| Musculocutaneous flap | 127 (21.6%) |
| Radial forearm flap | 85 (14.5%) |
| Forehead flap | 39 (6.6%) |
| Temporalis myofascial flap | 39 (6.6%) |
| Lid flap | 23 (3.9%) |
| Rectus abdominis flap | 21 (3.6%) |
| Dermis-fat graft | 19 (3.2%) |
| Latissimus dorsi flap | 11 (1.9%) |
| Temporalis muscle flap | 11 (1.9%) |
| Cheek rotation flap | 10 (1.7%) |
| Facio-cervico-pectoral flap | 9 (1.5%) |
| Pericranial flap | 7 (1.2%) |
| Abdomen/sub-mammary skin | 6 (1.0%) |
| Mustarde flap | 4 (0.6%) |
| Cervicofacial flap | 3 (0.5%) |
| Fibula osteocutaneous flap | 2 (0.3%) |
| Lateral arm flap | 2 (0.3%) |
| Scalp flap | 2 (0.3%) |
| Buccal mucosa graft | 1 (0.2%) |
| Deep inferior epigastric perforators flap | 1 (0.2%) |
| Fasciocutaneous flap | 1 (0.2%) |
| Galea frontalis flap | 1 (0.2%) |
| Gastrocnemius flap | 1 (0.2%) |
| Gracilis flap | 1 (0.2%) |
| Parotid/left cheek skin | 1 (0.2%) |
| Pectoralis major flap | 1 (0.2%) |
| Temporoparietal fascia flap | 1 (0.2%) |
| Thoracodorsal artery perforator flap | 1 (0.2%) |
| Vastus lateralis flap | 1 (0.2%) |
Data are reported as frequencies and percentages.
Of the total 957 patients, 414 (43.3%) received one or more radiation therapy treatments. Specifically, 266 patients (62.7%) underwent adjuvant radiotherapy, while 106 (25.0%) and 52 (12.3%) underwent primary and neoadjuvant radiotherapies, respectively. Chemotherapy was used in 47 patients as neoadjuvant (n = 16, 31.4%) or adjuvant (n = 35, 68.0%) therapies. The study revealed that only 29 patients received both chemotherapy and radiotherapy before or after OE (Table 4).
Table 4.
Non-surgical treatment: chemotherapy and radiotherapy.
| Variable | n (%) |
|---|---|
| Chemotherapy | n = 47 † |
| Neoadjuvant | 16 (31.4%) |
| Adjuvant | 35 (68.6%) |
| Radiotherapy | n = 414 † |
| Primary a | 106 (25.0%) |
| Neoadjuvant | 52 (12.3%) |
| Adjuvant | 266 (62.7%) |
| Patients had both chemotherapy and radiotherapy. | n = 29 |
Data are reported as frequencies and percentages. a Primary refers to prior radiotherapy before orbital exenteration. † The total number of patients who received chemotherapy or radiotherapy does not sum up to the number of patients in adjuvant or neoadjuvant treatment groups as many patients had both treatments.
3.4. Clinical and Survival Outcomes
Out of the 957 patients included in the review, data on tumor recurrence were available in 210 (22.0%) patients. Among these, 38.6% (n = 109) had local recurrence, while 32.3% (n = 91) and 6.7% (n = 19) experienced distant and regional recurrence, respectively. In addition, CFLs invaded perineural and lymphovascular regions in 17.4% and 5.0% of the patients, respectively. Notably, some patients experienced multiple intracranial and extracranial tumor recurrences (Table 5).
Table 5.
Recurrence and clinical outcome.
| Variable | n (%)/n (95% CI) |
|---|---|
| Recurrence | n = 210 * |
| Local | 109 (38.6%) |
| Distant | 91 (32.3%) |
| Regional | 19 (6.7%) |
| Perineural invasion | 49 (17.4%) |
| Lymphovascular invasion | 14 (5.0%) |
| Surgical margins | n = 444 |
| Positive | 136 (30.6%) |
| Negative | 308 (69.4%) |
| Status | n = 587 |
| Alive | 372 (63.4%) |
| Dead | 215 (36.6%) |
| Death cause | n = 195 |
| Disease complication | 144 (73.8%) |
| Unrelated cause | 51 (26.2%) |
| Overall median survival time, months | 61 (95% CI: 46–83) |
| Overall survival rates | |
| 5-year | 50.0% |
| 10-year | 24.0% |
| Weighted mean follow-up time, months | 23.6 (95% CI: 13.8–33.4) |
Data are reported as weighted mean and 95% confidence interval (CI) or frequencies and percentages. * The total number of patients with recurrence does not sum up to the number of patients with each type of recurrence as many patients had multiple types of recurrences.
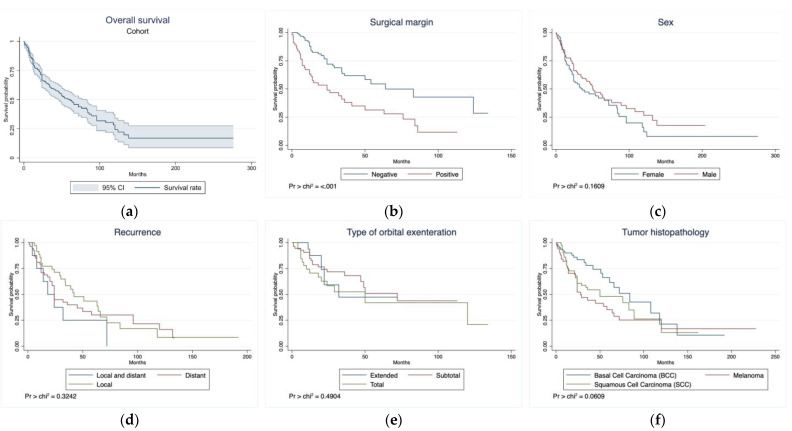
During a weighted mean follow-up time of 23.6 months (95% CI: 13.8–33.4 months), 215 (36.6%) patients died, and the cause of death was reported in only 195 patients. Among these, 144 (73.8%) patients died due to disease-related complications (Table 5). The 5-year OS rate was 50.0%, and the median OS time was 61 months (95% CI: 46–83 months; Table 5; Figure 3a). The presence of positive surgical margins was associated with a lower survival time (median: 24 months, 95% CI: 12–41 months) compared with negative surgical margins (median: 64 months, 95% CI: 36–NA months, p < 0.001; Figure 3b). Sex, tumor recurrence, type of the OE, and tumor histopathology did not show any statistically significant survival effect (Figure 3c–f).
Figure 3.
Kaplan–Meier curves of the (a) overall survival of the entire cohort and overall survival based on (b) surgical margins, (c) sex, (d) recurrence, (e) type of orbital exenteration, and (f) tumor histopathology. Pr > chi2 denotes the p value of log-rank test.
Multivariable analysis of OS using the Cox proportional hazards model did not result in any significant survival predictors (Table 6).
Table 6.
Overall survival using Cox proportional hazards model of patient and treatment characteristics.
| Predictor Variables | Overall Survival | |||
|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | |||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age | 1.01 (1.00–1.02) | 0.04 * | 1.03 (1.00–1.06) | 0.09 |
| Male (vs. female) | 0.78 (0.54–1.11) | 0.17 | NA | NA |
| SCC (vs. BCC) | 1.58 (0.92–2.72) | 0.10 | NA | NA |
| Melanoma (vs. BCC) | 1.90 (1.09–3.32) | 0.02 * | 2.88 (0.70–11.9) | 0.14 |
| Total (vs. Subtotal) OE | 1.25 (0.50–3.10) | 0.63 | NA | NA |
| Positive (vs. negative) margin | 2.39 (1.48–3.86) | <0.001 * | 2.04 (0.88–4.70) | 0.10 |
Variables mentioned in parenthesis were used as “bases” when running the Cox proportional hazards model. * Association is significant at the 0.05 level (two-tailed). Bold text denotes statistical significance. HR, hazard ratio; CI, confidence interval; SCC, squamous cell carcinoma; BCC, basal cell carcinoma; OE, orbital exenteration; NA, not applicable.
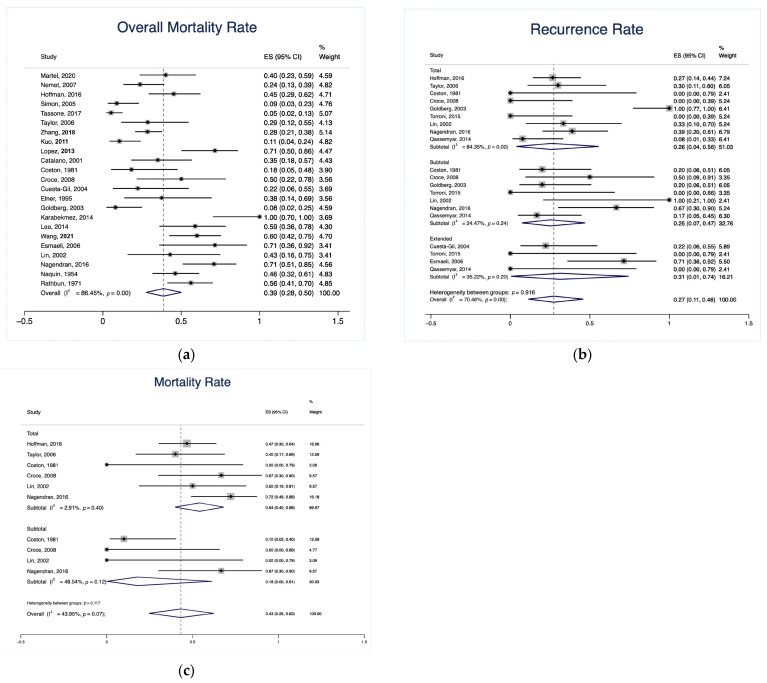
The total cohort exhibited a weighted overall mortality rate of 39.0% (95% CI: 28.0–50.0%; Figure 4a). In the subgroup meta-analysis, the extended OE group had an insignificantly higher rate of tumor recurrence (31.0% [95% CI: 1.0–74.0%]) compared with the total OE group (26.0% [95% CI: 4.0–56.0%]) and the subtotal OE group (25.0% [95% CI: 7.0–47.0%], p = 0.92; Figure 4b). The total OE group had an insignificantly higher mortality rate (54.0% [95% CI: 40.0–68.0%]) compared with the subtotal OE group (18.0% [95% CI: 0.0–61.0%], p = 0.12; Figure 4c).
Figure 4.
Forest plot of (a) overall mortality rate of the cohort, (b) tumor recurrence rate based on the type of OE, and (c) mortality rate based on the type of OE. OE, orbital exenteration [2,3,4,6,8,9,10,11,12,14,17,18,19,34,35,37,40,41,42,43,44,45,46,47,48].
4. Discussion
OE serves as a surgical treatment for advanced or recurrent CFLs. While the existing literature covers OE extensively, comprehensive studies concerning survival outcomes post-procedure are sparse. Univariable analysis showed a correlation between positive surgical margins post-OE and diminished OS. Sex, tumor recurrence, type of OE, and tumor histopathology did not significantly affect OS.
4.1. Demographics and Clinical Characteristics of the Cohort
We found that the majority of patients (58.3%) were male, with a weighted mean age of 64.3 years (95% CI: 59.9–68.7 years), consistent with other studies on CFLs [49,50,51,52]. OE was primarily indicated for lesions invading the orbit and adjacent structures. Thus, the eyelid (16.2%), orbit (14.4%), and conjunctiva (12.5%) were frequently affected in our study. SCC was the predominant histopathological indication for OE (31.8%), followed by BCC (21.6%), and melanoma (16.9%). Similar to our results, several other reports indicated a higher incidence of SCC affecting the craniofacial skull base structures [9,10,14,15,53].
Various symptoms were reported by patients who subsequently became eligible for OE. Among these, disturbed vision was the most prevalent symptom (22.5%) in the present study, which is reasonable given the anatomical location of CFLs [54].
4.2. Management Paradigm and Complications
We found that a third (31.6%) of patients underwent total OE, while 26.0% and 9.0% had extended OE and subtotal OE, respectively, demonstrating the variability in resection extents required by different patient conditions. The extent of resection directly correlates with disfigurement severity, impacting patients’ self-perception and life quality [55].
In the subgroup meta-analysis, our findings indicated that the types of OE, extended, total, and subtotal, for CFLs had no significant differences in terms of recurrence and mortality rates. These results are in line with other studies that have demonstrated no significant effect of the OE type on survival outcomes [56]. Further research should focus on identifying the optimal OE type to balance improved survival rates and the minimization of complications.
Multiple surgical procedures—such as maxillectomy (39.0%), lymph node resection (24.7%), ethmoidectomy (11.6%), and craniotomy (9.6%)—were necessary for some patients, underscoring the invasiveness of OE and CFLs. Similarly, many studies in the literature have evinced the need for adjunctive surgeries to eradicate lesions involving adjacent structures, particularly the lymph nodes, cranial and facial bones [1,57,58]. These findings highlight the complexity and invasiveness of CFLs, emphasizing the importance of adopting a multidisciplinary approach to manage this high-risk group of patients.
Following OE, various reconstructive techniques were used, including free flap (33.3%), endosseous implants (22.8%), and split-thickness skin grafting (17.2%). The most frequently used reconstructive material was the anterolateral thigh flap (26.6%), followed by the musculocutaneous flap (21.6%), and the radial forearm flap (14.5%). Several studies have proposed that free flaps and skin grafts can be employed individually or in combination to reconstruct the socket post-OE [2,33,49]. In terms of specific techniques, split-thickness skin grafting is favored due to its lower complication rates, while free flaps are typically reserved for more complex and extended OE, though not exclusively so [2,11,17,59]. A long-term study conducted by Baum et al. [15] has found that endosseous implants had a survival rate of 88.0% and were recommended for rehabilitation after OE. These findings underscore the importance of carefully selecting the appropriate reconstructive material based on the nature of the patient’s CFLs, the complexity and extent of exenteration, as well as the potential advantages offered by the material under consideration.
In our study, 168 patients (17.6%) experienced complications. The most frequent ones were sino-orbital or sino-nasal fistulas (22.6%), flap or graft failure (16.9%), and hyperostosis (13.0%). Several studies have documented complications following OE, encompassing sino-orbital fistulas, graft or flap failure, and surgical site infection or non-healing [5,7,53,60,61]. These complications can be ascribed to the anatomical site of the surgery, the nature of the reconstructive material employed, and the overall health status of the patient. One potential solution to mitigate surgical complications is to ensure the correct approach is taken with optimal visualization. This can lead to improved nasal symptoms and a reduction in related headaches, particularly in cases where a combined nasal approach is utilized [62,63].
Our study observed that a considerable portion of patients received radiotherapy (43.3%) and chemotherapy (4.9%), administered as primary, neoadjuvant, or adjuvant therapy. While the small size of the studies included in our review made it challenging to ascertain the potential benefit of adjuvant radiotherapy, numerous studies in the skull base literature have highlighted its effectiveness in enhancing local tumor control and diminishing tumor recurrence rates [64,65]. However, it is important to consider the potential complications of chemotherapy and radiotherapy and weigh the risks and benefits carefully. It is particularly noteworthy that radiotherapy has been significantly associated with the development of naso-orbital fistulas [11].
4.3. Clinical and Survival Outcomes
Over the course of the weighted mean follow-up period of 23.6 months, we observed a weighted mortality rate of 39.0% (95% CI: 28.0–50.0%). The majority of these deaths (73.8%) were due to disease-related complications. Our mortality rate is consistent with rates reported by other studies that have investigated similar pathologies. Rahman et al. [66] described a cohort of patients who underwent OE, with 43.8% being diagnosed with BCC. They reported an overall mortality rate of 38.0% over a 12-year period and concluded that after a 3-year period, a BCC patient’s risk of mortality increased by 30.4% compared to non-BCC patients. Similarly, Aryasit et al. [49] investigated a cohort where 35.9% of patients had SCC invading the orbit and reported a comparable mortality rate of 56.4%. Additionally, our review revealed a 5-year OS rate of 50.0%, with a median OS time of 61 months (95% CI: 46–83 months). These findings accord with earlier skull base studies of similar pathologies that were managed with various surgical approaches, which have demonstrated a 5-year OS rate ranging from 54.0% to 64.0% [56,67,68,69]. These results further substantiate our hypothesis that the unfavorable outcomes of OE are primarily due to the invasive nature of the included pathologies.
We found a local recurrence rate of 38.6%, a distant recurrence rate of 32.3%, and a regional recurrence rate of 6.7%, with tumors invading perineural and lymphovascular regions present in 17.4% and 5.0% of the patients, respectively. Similarly, many studies of craniofacial tumors in the literature corroborate these findings and reported local recurrence rates ranging from 8.5% to 36.0% [32,67]. However, our multivariable Cox proportional hazards model did not identify any significant predictors of OS, including tumor recurrence.
In the univariable analysis, we observed that the presence of positive surgical margins was associated with a shorter survival time (median: 24 months, 95% CI: 12–41 months) compared with negative surgical margins (median: 64 months, 95% CI: 36–NA months, p < 0.001). However, the association between the status of surgical margins and survival remains a topic of debate. While some studies have supported our findings and illustrated that positive surgical margins are associated with reduced survival, other studies failed to demonstrate a correlation between survival and tumor margin status [17,50,70,71,72]. One hypothesis is that micrometastases might already be present in cases of locally advanced tumors.
4.4. Limitations
The retrospective nature of our analysis poses limitations on our results. The articles included in our study were subject to significant selection biases and exhibited substantial heterogeneity in the methodology and the assessment of clinical outcomes. Additionally, the small sample sizes of most of the included articles reduced the statistical power of multiple endpoints.
5. Conclusions
OE is typically reserved for cases of advanced or recurrent CFLs. Despite the associated high rates of complications, recurrence, and mortality, our study suggests that it could be a valid option, provided that the management plan is meticulously tailored for each patient. To improve outcomes, rigorous postoperative monitoring and long-term follow-up are crucial. Such measures could potentially mitigate the devastating outcomes and poor prognosis associated with OE. Considering the profound psychological and social impacts on a patient’s self-image and quality of life, comprehensive rehabilitation and meticulous reconstruction following OE are strongly recommended.
Supplementary Materials
The following are available online at: https://www.mdpi.com/article/10.3390/cancers15174285/s1, Figure S1: Funnel plot of (A) overall mortality rate of the cohort, (B) tumor recurrence rate based on the type of OE, and (C) mortality rate based on the type of OE. OE, orbital exenteration. Table S1: Risk of bias assessments for included studies. Table S2: Overview of clinical characteristics and outcomes of included studies.
Author Contributions
Conceptualization, O.B.-A. and J.Q.; methodology, J.Q.; software, J.Q.; validation, O.B.-A. and A.S.H.; formal analysis, J.Q.; investigation, J.Q., A.S.H. and K.B.; resources, A.S.H., K.B., P.P., T.H., A.S., M.S., A.F.K., H.A.-A.-S., K.Y., A.A.C.-G. and T.Y.E.A.; visualization, J.Q.; data curation, J.Q.; writing—original draft preparation, J.Q.; writing—review and editing, O.B.-A., A.S.H., P.P., H.A.-A.-S., K.Y., A.A.C.-G. and T.Y.E.A.; supervision, O.B.-A. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sokoya M., Cohn J.E., Kohlert S., Lee T., Kadakia S., Ducic Y. Orbital Reconstruction: Considerations in Orbital Exenteration. Semin. Plast. Surg. 2019;33:103–105. doi: 10.1055/S-0039-1685209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Z., Ho S., Yin V., Varas G., Rajak S., Dolman P.J., McNab A., Heathcote J.G., Valenzuela A. Multicentred International Review of Orbital Exenteration and Reconstruction in Oculoplastic and Orbit Practice. Br. J. Ophthalmol. 2018;102:654–658. doi: 10.1136/bjophthalmol-2017-310681. [DOI] [PubMed] [Google Scholar]
- 3.Simon G.J.B., Schwarcz R.M., Douglas R., Fiaschetti D., McCann J.D., Goldberg R.A. Orbital Exenteration: One Size Does Not Fit All. Am. J. Ophthalmol. 2005;139:11–17. doi: 10.1016/j.ajo.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg R.A., Kim J.W., Shorr N. Orbital Exenteration: Results of an Individualized Approach. Ophthalmic Plast. Reconstr. Surg. 2003;19:229–236. doi: 10.1097/01.IOP.0000066699.53489.88. [DOI] [PubMed] [Google Scholar]
- 5.Kesting M.R., Koerdt S., Rommel N., Mücke T., Wolff K.D., Nobis C.P., Ringel F., Frohwitter G. Classification of Orbital Exenteration and Reconstruction. J. Craniomaxillofac Surg. 2017;45:467–473. doi: 10.1016/j.jcms.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Martel A., Oberic A., Moulin A., Zografos L., Bellini L., Almairac F., Hamedani M. Orbital Exenteration and Conjunctival Melanoma: A 14-Year Study at the Jules Gonin Eye Hospital. Eye. 2020;34:1897–1902. doi: 10.1038/s41433-020-0767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ali M.J., Pujari A., Dave T.V., Kaliki S., Naik M.N. Clinicopathological Profile of Orbital Exenteration: 14 Years of Experience from a Tertiary Eye Care Center in South India. Int. Ophthalmol. 2016;36:253–258. doi: 10.1007/s10792-015-0111-5. [DOI] [PubMed] [Google Scholar]
- 8.Nemet A.Y., Martin P., Benger R., Kourt G., Sharma V., Ghabrial R., Danks J. Orbital Exenteration: A 15-Year Study of 38 Cases. Ophthalmic Plast. Reconstr. Surg. 2007;23:468–472. doi: 10.1097/IOP.0b013e318158e994. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman G.R., Jefferson N.D., Reid C.B.A., Eisenberg R.L. Orbital Exenteration to Manage Infiltrative Sinonasal, Orbital Adnexal, and Cutaneous Malignancies Provides Acceptable Survival Outcomes: An Institutional Review, Literature Review, and Meta-Analysis. J. Oral. Maxillofac. Surg. 2016;74:631–643. doi: 10.1016/j.joms.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Kuo C.H., Gao K., Clifford A., Shannon K., Clark J. Orbital Exenterations: An 18-Year Experience from a Single Head and Neck Unit. ANZ J. Surg. 2011;81:326–330. doi: 10.1111/j.1445-2197.2010.05592.x. [DOI] [PubMed] [Google Scholar]
- 11.Tassone P., Gill K.S., Hsu D., Nyquist G., Krein H., Bilyk J.R., Murchison A.P., Evans J.J., Heffelfinger R.N., Curry J.M. Naso- or Orbitocutaneous Fistulas after Free Flap Reconstruction of Orbital Exenteration Defects: Retrospective Study, Systematic Review, and Meta-Analysis. J. Neurol. Surg. B Skull Base. 2017;78:337–345. doi: 10.1055/S-0037-1600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor A., Roberts F., Kemp E.G. Orbital Exenteration--a Retrospective Study over an 11 Year Period Analyzing All Cases from a Single Unit. Orbit. 2006;25:185–193. doi: 10.1080/01676830600575584. [DOI] [PubMed] [Google Scholar]
- 13.Gill K.S., Hsu D., Tassone P., Pluta J., Nyquist G., Krein H., Bilyk J., Murchison A.P., Iloreta A., Evans J.J., et al. Postoperative Cerebrospinal Fluid Leak after Microvascular Reconstruction of Craniofacial Defects with Orbital Exenteration. Laryngoscope. 2017;127:835–841. doi: 10.1002/lary.26137. [DOI] [PubMed] [Google Scholar]
- 14.López F., Suárez C., Carnero S., Martín C., Camporro D., Llorente J.L. Free Flaps in Orbital Exenteration: A Safe and Effective Method for Reconstruction. Eur. Arch. Otorhinolaryngol. 2013;270:1947–1952. doi: 10.1007/s00405-012-2308-9. [DOI] [PubMed] [Google Scholar]
- 15.Baum S., Klein M., Mohr C., Weischer T. Long-Term Results of Endosseous Implants as Retention Elements of Orbital Epitheses, Reconstruction Techniques, and Aftercare after Radical Tumor Resection. Int. J. Oral. Maxillofac. Implants. 2019;34:745–751. doi: 10.11607/jomi.6988. [DOI] [PubMed] [Google Scholar]
- 16.Martel A., Baillif S., Nahon-Esteve S., Gastaud L., Bertolotto C., Lassalle S., Lagier J., Hamedani M., Poissonnet G. Orbital Exenteration: An Updated Review with Perspectives. Surv. Ophthalmol. 2021;66:856–876. doi: 10.1016/j.survophthal.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Nagendran S.T., Lee N.G., Fay A., Lefebvre D.R., Sutula F.C., Freitag S.K. Orbital Exenteration: The 10-Year Massachusetts Eye and Ear Infirmary Experience. Orbit. 2016;35:199–206. doi: 10.1080/01676830.2016.1176210. [DOI] [PubMed] [Google Scholar]
- 18.Wang W.Y., Liao S.L., Wei Y.H. Orbital Exenteration: A 20-Year Experience from a Tertiary Center in Taiwan. J. Formos. Med. Assoc. 2021;120:1493–1499. doi: 10.1016/j.jfma.2020.10.030. [DOI] [PubMed] [Google Scholar]
- 19.Qassemyar A., Aljudaibi N., Wavreille O., Mortier L., Martinot-Duquennoy V., Guerreschi P. Orbital Exenteration and Periorbital Skin Cancers. J. Oral. Maxillofac. Surg. 2014;72:811–816. doi: 10.1016/j.joms.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Greig A.V.H., Jones S., Haylock C., Joshi N., McLellan G., Clarke P., Kirkpatrick W.N.A. Reconstruction of the Exenterated Orbit with Osseointegrated Implants. J. Plast. Reconstr. Aesthet. Surg. 2010;63:1656–1665. doi: 10.1016/j.bjps.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Van Den Elzen M.E.P., Versnel S.L., Hovius S.E.R., Passchier J., Duivenvoorden H.J., Mathijssen I.M.J. Adults with Congenital or Acquired Facial Disfigurement: Impact of Appearance on Social Functioning. J. Craniomaxillofac Surg. 2012;40:777–782. doi: 10.1016/j.jcms.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Ariani N., Visser A., van Oort R.P., Kusdhany L., Rahardjo T.B.W., Krom B.P., van der Mei H.C., Vissink A. Current State of Craniofacial Prosthetic Rehabilitation. Int. J. Prosthodont. 2013;26:57–67. doi: 10.11607/ijp.3220. [DOI] [PubMed] [Google Scholar]
- 23.Nassab R.S., Thomas S.S., Murray D. Orbital Exenteration for Advanced Periorbital Skin Cancers: 20 Years Experience. J. Plast. Reconstr. Aesthet. Surg. 2007;60:1103–1109. doi: 10.1016/j.bjps.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.OCEBM Levels of Evidence—Centre for Evidence-Based Medicine (CEBM), University of Oxford. [(accessed on 16 July 2023)]. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
- 26.Munn Z., Barker T.H., Moola S., Tufanaru C., Stern C., McArthur A., Stephenson M., Aromataris E. Methodological Quality of Case Series Studies: An Introduction to the JBI Critical Appraisal Tool. JBI Evid. Synth. 2020;18:2127–2133. doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed] [Google Scholar]
- 27.Frezzotti R., Bonanni R., Nuti A., Polito E. Radical Orbital Resections. Adv. Ophthalmic Plast. Reconstr. Surg. 1992;9:175–192. [PubMed] [Google Scholar]
- 28.Rich J.T., Neely J.G., Paniello R.C., Voelker C.C.J., Nussenbaum B., Wang E.W. A Practical guide to understanding kaplan-meier curves. Otolaryngol. Head. Neck Surg. 2010;143:331–336. doi: 10.1016/j.otohns.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deo S.V., Deo V., Sundaram V. Survival Analysis—Part 2: Cox Proportional Hazards Model. Indian. J. Thorac. Cardiovasc. Surg. 2021;37:229–233. doi: 10.1007/s12055-020-01108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins J.P.T., Thompson S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.Sterne J.A.C., Sutton A.J., Ioannidis J.P.A., Terrin N., Jones D.R., Lau J., Carpenter J., Rücker G., Harbord R.M., Schmid C.H., et al. Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 32.Cumming B., Sideris A., Holmes T.R., Jacobson I., Havas T. Orbital Exenteration: Tumour Diversity and Survival in a Tertiary Referral Centre. Aust. J. Otolaryngol. 2019;2:23. doi: 10.21037/ajo.2019.09.03. [DOI] [Google Scholar]
- 33.Gerring R.C., Ott C.T., Curry J.M., Sargi Z.B., Wester S.T. Orbital Exenteration for Advanced Periorbital Non-Melanoma Skin Cancer: Prognostic Factors and Survival. Eye. 2017;31:379–388. doi: 10.1038/eye.2016.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catalano P.J., Laidlaw D., Sen C. Globe Sparing Orbital Exenteration. Otolaryngol. Head. Neck Surg. 2001;125:379–384. doi: 10.1067/mhn.2001.118247. [DOI] [PubMed] [Google Scholar]
- 35.Croce A., Moretti A., D’Agostino L., Zingariello P. Orbital Exenteration in Elderly Patients: Personal Experience. Acta Otorhinolaryngol. Ital. 2008;28:193. [PMC free article] [PubMed] [Google Scholar]
- 36.Elkhamary S.M., Galindo-Ferreiro A., Akaishi P., Muiños-Diaz Y., Cechetti S.P., Cintra M.B., Cruz A.A.V. Hyperostosis Following Orbital Exenteration. Ophthalmic Plast. Reconstr. Surg. 2017;33:241–243. doi: 10.1097/IOP.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 37.Lee P.S., Sedrak P., Guha-Thakurta N., Chang E.I., Ginsberg L.E., Esmaeli B., Debnam J.M. Imaging Findings of Recurrent Tumors after Orbital Exenteration and Free Flap Reconstruction. Ophthalmic Plast. Reconstr. Surg. 2014;30:315–321. doi: 10.1097/IOP.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 38.Maheshwari R. Review of Orbital Exenteration from an Eye Care Centre in Western India. Orbit. 2010;29:35–38. doi: 10.3109/01676830903234020. [DOI] [PubMed] [Google Scholar]
- 39.Ogun G.O., Ogun O.A., Bekibele C.O., Akang E.E. Intraepithelial and Invasive Squamous Neoplasms of the Conjunctiva in Ibadan, Nigeria: A Clinicopathological Study of 46 Cases. Int. Ophthalmol. 2009;29:401–409. doi: 10.1007/s10792-008-9257-8. [DOI] [PubMed] [Google Scholar]
- 40.Torroni A., Cervelli D., Gasparini G., Grussu F., Moro A., Marianetti T.M., Foresta E., Azzuni C., Pelo S. Anterior Retrograde Approach to the Myofascial Temporalis Muscle for Orbital Reconstruction: Series of 9 Consecutive Cases. Ann. Plast. Surg. 2015;74:37–42. doi: 10.1097/SAP.0b013e31828bb582. [DOI] [PubMed] [Google Scholar]
- 41.Esmaeli B., Golio D., Kies M., DeMonte F. Surgical Management of Locally Advanced Adenoid Cystic Carcinoma of the Lacrimal Gland. Ophthalmic Plast. Reconstr. Surg. 2006;22:366–370. doi: 10.1097/01.iop.0000232164.00208.b4. [DOI] [PubMed] [Google Scholar]
- 42.Rathbun J.E., Beard C., Quickert M.H. Evaluation of 48 Cases of Orbital Exenteration. Am. J. Ophthalmol. 1971;72:191–199. doi: 10.1016/0002-9394(71)91613-8. [DOI] [PubMed] [Google Scholar]
- 43.Coston T.O., Small R.G. Orbital Exenteration--Simplified. Trans. Am. Ophthalmol. Soc. 1981;79:136–152. [PMC free article] [PubMed] [Google Scholar]
- 44.Lin H.-F., Lui C.-C., Hsu H.-C., Lin S.-A. Orbital Exenteration for Secondary Orbital Tumors: A Series of Seven Cases. Chang. Gung Med. J. 2002;25:599–605. [PubMed] [Google Scholar]
- 45.Naquin H.A. Exenteration of the Orbit. AMA Arch. Ophthalmol. 1954;51:850–862. doi: 10.1001/archopht.1954.00920040860011. [DOI] [PubMed] [Google Scholar]
- 46.Elner V.M., Burnstine M.A., Goodman M.L., Dortzbach R.K. Inverted Papillomas That Invade the Orbit. Arch. Ophthalmol. 1995;113:1178–1183. doi: 10.1001/archopht.1995.01100090104030. [DOI] [PubMed] [Google Scholar]
- 47.Karabekmez F.E., Selimoglu M.N., Duymaz A., Karamese M.S., Keskin M., Savaci N. Management of Neglected Periorbital Squamous Cell Carcinoma Requiring Orbital Exenteration. J. Craniofac Surg. 2014;25:729–734. doi: 10.1097/SCS.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 48.Cuesta-Gil M., Concejo C., Acero J., Navarro-Vila C., Ochandiano S. Repair of Large Orbito-Cutaneous Defects by Combining Two Classical Flaps. J. Cranio-Maxillofac. Surg. 2004;32:21–27. doi: 10.1016/j.jcms.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Aryasit O., Preechawai P., Hirunpat C., Horatanaruang O., Singha P. Factors Related to Survival Outcomes Following Orbital Exenteration: A Retrospective, Comparative, Case Series. BMC Ophthalmol. 2018;18:186. doi: 10.1186/s12886-018-0850-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fleming J.C., Morley I., Malik M., Orfaniotis G., Daniel C., Townley W.A., Jeannon J.P. Orbital Exenteration and Reconstruction in a Tertiary UK Institution: A 5-Year Experience. Orbit. 2021;40:306–315. doi: 10.1080/01676830.2020.1775262. [DOI] [PubMed] [Google Scholar]
- 51.Kato J.M., da Fonseca F.L., Matayoshi S. Survival Following Orbital Exenteration at a Tertiary Brazilian Hospital. Rev. Col. Bras. Cir. 2016;43:042–047. doi: 10.1590/0100-69912016001009. [DOI] [PubMed] [Google Scholar]
- 52.Langlois B., Jacomet P.V., Putterman M., Morax S., Galatoire O. Evaluation of Reconstructive Techniques after Orbital Exenteration in 56 Cases. J. Fr. Ophtalmol. 2012;35:667–677. doi: 10.1016/j.jfo.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 53.Cinar C., Arslan H., Bingol U.A., Aydin Y., Cetinkale O. The New Anatomical Classification System for Orbital Exenteration Defect. J. Craniofac Surg. 2017;28:1687–1693. doi: 10.1097/SCS.0000000000003746. [DOI] [PubMed] [Google Scholar]
- 54.Dallan I., Picariello M., Fiacchini G. Conservative Management of Orbital Involvement in Malignant Tumors: Is the Paradigm Evolving? A Critical Review. Curr. Opin. Otolaryngol. Head. Neck Surg. 2022;30:125–129. doi: 10.1097/MOO.0000000000000791. [DOI] [PubMed] [Google Scholar]
- 55.Mukoyama N., Nishio N., Kimura H., Kishi S., Tokura T., Kimura H., Hiramatsu M., Maruo T., Tsuzuki H., Fujii M., et al. Prospective Evaluation of Health-Related Quality of Life in Patients Undergoing Anterolateral Craniofacial Resection with Orbital Exenteration. J. Neurol. Surg. B Skull Base. 2020;81:585–593. doi: 10.1055/s-0039-1694010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baum S.H., Oeverhaus M., Saxe F., Mohr C. Modified Types of Orbital Exenteration, Survival, and Reconstruction. Graefes Arch. Clin. Exp. Ophthalmol. 2020;258:2305–2312. doi: 10.1007/s00417-020-04812-7. [DOI] [PubMed] [Google Scholar]
- 57.Fleming J.C., Linder J.S., Karcioglu Z.A. Orbital Hyperostosis Following Exenteration. Ophthalmic Plast. Reconstr. Surg. 2008;24:378–382. doi: 10.1097/IOP.0b013e3181832661. [DOI] [PubMed] [Google Scholar]
- 58.Sugawara T., Aoyagi M., Ogishima T., Kawano Y., Tamaki M., Yano T., Tsunoda A., Ohno K., Maehara T., Kishimoto S. Extended Orbital Exenteration for Sinonasal Malignancy with Orbital Apex Extension: Surgical Technique and Clinical Analysis. J. Neurosurg. 2015;123:52–58. doi: 10.3171/2014.9.JNS141256. [DOI] [PubMed] [Google Scholar]
- 59.Badhey A., Haidar Y., Genden E. Orbital Reconstruction: Soft Tissue Microvascular Reconstruction of Orbital Exenteration Defects. Semin. Plast. Surg. 2019;33:125–131. doi: 10.1055/S-0039-1685480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Al-Hity A., Gregory M.E., Kemp E.G. The Intraoperative Use of Polydioxanone Foil to Reduce the Risk of Sino-Orbital Fistula Formation in Orbital Exenteration. Orbit. 2018;37:140–144. doi: 10.1080/01676830.2017.1383463. [DOI] [PubMed] [Google Scholar]
- 61.Kiratli H., Koç İ. Orbital Exenteration: Institutional Review of Evolving Trends in Indications and Rehabilitation Techniques. Orbit. 2018;37:179–186. doi: 10.1080/01676830.2017.1383466. [DOI] [PubMed] [Google Scholar]
- 62.Cocuzza S., Maniaci A., Di Luca M., Mantia I.L., Grillo C., Spinato G., Motta G., Testa D., Ferlito S. Long-Term Results of Nasal Surgery: Comparison of Mini-Invasive Turbinoplasty. J. Biol. Regul. Homeost. Agents. 2020;34:1203–1208. doi: 10.23812/19-522-L-4. [DOI] [PubMed] [Google Scholar]
- 63.Maniaci A., Merlino F., Cocuzza S., Iannella G., Vicini C., Cammaroto G., Lechien J.R., Calvo-Henriquez C., La Mantia I. Endoscopic Surgical Treatment for Rhinogenic Contact Point Headache: Systematic Review and Meta-Analysis. Eur. Arch. Otorhinolaryngol. 2021;278:1743–1753. doi: 10.1007/s00405-021-06724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skinner H.D., Garden A.S., Rosenthal D.I., Ang K.K., Morrison W.H., Esmaeli B., Pinnix C.C., Frank S.J. Outcomes of Malignant Tumors of the Lacrimal Apparatus: The University of Texas MD Anderson Cancer Center Experience. Cancer. 2011;117:2801–2810. doi: 10.1002/cncr.25813. [DOI] [PubMed] [Google Scholar]
- 65.Hsu A., Frank S.J., Ballo M.T., Garden A.S., Morrison W.H., Rosenthal D.I., Hatef E., Esmaeli B. Postoperative Adjuvant External-Beam Radiation Therapy for Cancers of the Eyelid and Conjunctiva. Ophthalmic Plast. Reconstr. Surg. 2008;24:444–449. doi: 10.1097/IOP.0b013e31818be098. [DOI] [PubMed] [Google Scholar]
- 66.Rahman I., Maino A., Cook A.E., Leatherbarrow B. Mortality Following Exenteration for Malignant Tumours of the Orbit. Br. J. Ophthalmol. 2005;89:1445–1448. doi: 10.1136/bjo.2005.072892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roche P., Timon C. Orbital Exenteration in Periorbital Malignancies. Surgeon. 2012;10:189–193. doi: 10.1016/j.surge.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Patel S.G., Singh B., Polluri A., Bridger P.G., Cantu G., Cheesman A.D., DeSa G.M., Donald P., Fliss D., Gullane P., et al. Craniofacial Surgery for Malignant Skull Base Tumors: Report of an International Collaborative Study. Cancer. 2003;98:1179–1187. doi: 10.1002/cncr.11630. [DOI] [PubMed] [Google Scholar]
- 69.Wong J.C.L., Thampy R., Cook A. Life Expectancy Following Orbital Exenteration. Br. J. Ophthalmol. 2015;99:1–4. doi: 10.1136/bjophthalmol-2013-304436. [DOI] [PubMed] [Google Scholar]
- 70.Traylor J.I., Christiano L.D., Esmaeli B., Hanasono M.M., Yu P., Suki D., Zhang W., Raza S.M., Hanna E.Y., DeMonte F. Outcomes of Orbital Exenteration for Craniofacial Lesions. Cancer. 2021;127:2465–2475. doi: 10.1002/cncr.33526. [DOI] [PubMed] [Google Scholar]
- 71.Sullivan C.B., Andresen N.S., Kendell N., Al-Qurayshi Z., Pagedar N.A. Survival Outcomes for Advanced Cutaneous Squamous Cell Carcinoma of the Head and Neck. Ann. Otol. Rhinol. Laryngol. 2019;128:949–955. doi: 10.1177/0003489419848786. [DOI] [PubMed] [Google Scholar]
- 72.Mouriaux F., Martinot V., Pellerin P., Patenotre P., Rouland J.F., Constantinides G. Survival after Malignant Tumors of the Orbit and Periorbit Treated by Exenteration. Acta Ophthalmol. Scand. 1999;77:326–330. doi: 10.1034/j.1600-0420.1999.770316.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.