Abstract
Background:
There have been conflicting reports on the incidence of invasive pneumococcal diseases (IPD) after implementation of vaccination with 7- and 13-valent pneumococcal conjugate (PCV7 and PCV13) vaccinations. Some studies from different geographic locations observed a rebound increase in IPD incidence caused by non-PCV13 serotypes.
Objective:
To estimate the incidence of IPD in pre-PCV13 (PCV7 era, 2002–2010) and post-PCV13 (2011–2018) time periods.
Methods:
We conducted a population-based, cohort study of all IPD cases in Olmsted County, Minnesota, USA, using the Rochester Epidemiology Project from January 1, 2002, to December 31, 2018.
Results:
Overall, 187 cases of IPD were identified. The incidence of IPD significantly decreased from 11.1 (95% CI 9.1, 13.2) to 5.6 (95% CI 4.3, 6.9) per 100,000 person-years when the pre-and post-PCV13 periods (2002–2010 vs. 2011–2018) were compared. Sixty percent (112/187) of IPD cases had previously received at least one dose of pneumococcal vaccine. Among the IPD cases in the post-PCV13 period, there was an increase in non-PCV13 serotypes, mainly 11A: from 1.0% (1/105) to 6.2% (4/64) and 33F: from 2.9% (3/105) to 15.6% (10/64). While PCV13/non-PCV7 serotypes declined from 38.1% (40/105) to 15.6% (10/64). At 30 days after an IPD diagnosis, the survival rate was 88.8% (95% CI 84.4, 93.4).
Conclusions:
A marked decline in IPD incidence occurred during the post-PCV13 era. Due to an observed rise in non-PCV13 serotypes, coupled with multiple factors that impact the epidemiology of IPD, ongoing surveillance of IPD, particularly due to non-PCV13 serotypes, is warranted.
Introduction
Invasive pneumococcal disease (IPD) includes a variety of clinical syndromes caused by Streptococcus pneumoniae invasion of sterile body sites. The more common clinical syndromes are bloodstream infection, bacteremic pneumonia, and meningitis 1. IPD can be complicated by significant morbidity, mortality, with a substantial economic burden 2. While there are no contemporary data addressing the cost of IPD care in infants and children, especially in the conjugate vaccine era, an estimated cost of IPD care in adults in the United States from 2004 to 2040 was expected to increase by $2.5 billion annually 3.
Asymptomatic nasopharyngeal colonization of S. pneumoniae plays a critical role in the pathogenesis of IPD. Vaccination, however, reduces colonization of vaccine serotypes of S. pneumoniae, thus, decreasing the transmission from asymptomatic carriers to individuals in a high-risk population 4. A 23-valent pneumococcal polysaccharide vaccine (PPSV23) was first introduced in the United State in 1983 for high-risk group aged two years and older. However, its launch did not impact the highest IPD incidence in infant and children aged less than two years. Hence, a conjugate vaccine was developed for childhood vaccination 5, 6. Implementing a 7-valent pneumococcal conjugate vaccine (PCV7) for infants and children up to 59-months of age in February 2000 impacted the incidence of IPD, which showed a significant decline 7, 8. The PCV7 was available in Minnesota after the Advisory Committee on Immunization Practices (ACIP) recommendation in October 2000. Children aged 2–23 months should receive three doses of PCV7 at two months interval with the fourth dose at age 12–15 months. High-risk children aged 24–59 months should receive a total of three doses at two months interval 9.
Our previous population-based study focusing on the incidence of IPD from 1995–2007 in adults greater than 50 years and demonstrated an increase in the non-PCV7 serotypes. The incidence of IPD rose from 9.2 to 32.8 per 100,000 person-years when comparing 2002–2004 to 2005–2007 10. In March 2010, a 13-valent pneumococcal conjugate vaccine (PCV13) was introduced as a replacement for PCV7 for infants and children using the same vaccination schedule. The ACIP recommended PCV13 in immunocompromised adults in 2012 followed by all adults aged 65 or older in 2014 11, 12. The coverage of PCV vaccination for children aged 24–35 months in Minnesota from 2010 to 2018 was around 75–82%. Based on findings from the Center of Diseases Control and Prevention Emerging Infections Program Network: Active Bacterial Core Surveillance (ABCs) Report, the incidence of IPD cases has gradually declined each year since introduction of PCV13 13. The ABCs’ database demonstrated a longitudinal trend of IPD cases in select counties and states in the United States. However, this observation may not represent the actual incidence of a population of interest due to limited geographical surveillance 14. Additionally, it did not include demographic and other features of clinical syndromes, and IPD serotypes. Therefore, an updated epidemiologic investigation using comprehensive population-based data is warranted. We conducted a population-based investigation to profile the incidence trend and clinical characteristics of IPD cases in Olmsted County, Minnesota from 2002 to 2018.
Methods
Study design and population
This study is a population-based, cohort study using the Rochester Epidemiology Project (REP) to identify all incident cases of IPD among residents of Olmsted County, Minnesota, USA, from January 1, 2002, to December 31, 2018. Patients who were initially diagnosed with IPD prior to 2002 or declined research authorization were excluded. Baseline demographics, comorbidities, clinical diagnoses, pneumococcal vaccination records, and microbiology data were collected. In addition, vital status, date of death, and date of most recent clinical contact through January 2021 were ascertained. All medical records were individually reviewed to confirm the accuracy of data. Both the Mayo Clinic and Olmsted Medical Center Institutional Review Boards approved the study.
The REP is a well-maintained medical record linkage infrastructure for patients residing in Olmsted County, Minnesota. It contains electronic indexes of demographic data, diagnostic codes, surgical procedure codes, drug prescriptions, and death records from providers affiliated with the Mayo Clinic, Olmsted Medical Center, and its branch offices, three affiliated hospitals, and additional local providers 15, 16. As compared with the US census estimates for July 2014, the REP captures approximately 99.9% of Olmsted County’s population 15, 16. Based on census information, age, sex, and racial/ethnic characteristics of people living in Olmsted County are comparable to the population of Minnesota and the Midwestern United States, but the population is less ethnically diverse than the rest of the United States. As of 2010, 86% of the Olmsted County population was white as compared to approximately 72% of that in the United States.
Case definitions, susceptibility testing and serotype
IPD was defined by clinical syndromes associated with a laboratory-confirmed isolation of S. pneumoniae from sterile body sites, including blood, cerebrospinal fluid (CSF), peritoneal fluid, pleural fluid, and synovial fluid. Agar dilution (Mayo Clinic) and broth microdilution (Olmsted Medical Center) methods were used for susceptibility testing of S. pneumoniae isolates. In vitro penicillin susceptibility determinations were based on 2008 revised breakpoints for S. pneumoniae from the Clinical and Laboratory Standards Institute 17. For a non-meningitis syndrome, breakpoints for penicillin minimum inhibitory concentrations (MIC) were ≤ 2 μg/mL for susceptible; 4 μg/mL for intermediate; and ≥ 8 μg/mL for resistant. For meningitis, ≤ 0.06 μg/mL and ≥ 0.12 μg/mL were susceptible and resistant, respectively.
All S. pneumoniae isolates were sent to the Minnesota Department of Health for serotyping. Multiplex polymerase chain reaction and Quellung methods were used. The pneumococcal serotype were categorized into two main groups, vaccine, and non-vaccine serotypes, as described in previous studies 10, 18. Vaccine serotypes included 1) PCV7 serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F); 2) PCV13 serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C,19A, 19F, and 23A); 3) PPSV23 serotypes (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F). Since some serotypes overlapped among groups, we used the terms “PCV13/non-PCV7 (1, 3, 6A, 7F, and 19A)” and “PPSV23/non-PCV13 (8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F)” for clarity. Non-vaccine serotypes included 6C, 7C, 15A, 16F, 35B, 35F, 21, 23A, 23B, 24A, 31, or 34.
Objective
The study’s primary objective was to estimate the incidence of IPD in a defined population, which was stratified into pre-PCV13 (2002–2010) and post-PCV13 (2011–2018) time periods. This was based on widespread vaccination with PCV13 after its approval in early 2010 12. In addition, the prevalence of IPD serotypes was compared between the time periods.
Statistical analysis
Data were analyzed using SAS version 9.4 statistical software (SAS Institute, NC; Cary, NC). Age- and sex-specific incidence rates in Olmsted County during both periods (2002–2010, 2011–2018) were calculated; the numerator was the number of persons with an incident IPD diagnosis, and the denominator was obtained from the REP census using persons of all ages 19. Rates were age- and sex-adjusted to the total population of the United States in 2010. The 95% confidence intervals (CI) for incidence rates were calculated assuming a Poisson error distribution. Incidence rates were compared between the pre- and post-PCV13 periods by fitting generalized linear regression models assuming a Poisson error structure. Observations used for the regression analysis were incidence counts, which were offset by the natural logarithm of the number of person-years. In addition, among those with IPD, the occurrence of specific pneumococcal serotypes was compared between the time periods using the chi-square test. All calculated p-values were two-sided, and p-values less than 0.05 were considered statistically significant.
Overall survival following the date of the IPD diagnosis was calculated using the Kaplan-Meier method. Expected survival was derived using Hakulinen’s method 20 based on the age and sex specific mortality rates in Minnesota and compared with the observed survival using a one-sample logrank test.
Results
Baseline demographics
A total of 187 cases of IPD diagnosis were identified in Olmsted County from 2002 to 2018. Of them, 164 (88%) were white and 97 (52%) were male. At the time of diagnosis, mean age was 58.8 years (median 65.2 and range from 0.6 to 99.6 years). The median Charlson Comorbidity index among cases was 4 [Interquartile range (IQR) 1–9]. Detailed baseline demographics and comorbidities by era of IPD diagnosis are included in Table 1.
Table 1:
Baseline characteristic of all IPD cases, by era of IPD diagnosis.
| Characteristic | Pre-PCV13 (2002–2010, N=115) | Post-PCV13 (2011–2018, N=72) | Total (N=187) |
|---|---|---|---|
|
| |||
| Sex | |||
| Female | 56 (48.7%) | 34 (47.2%) | 90 (48.1%) |
| Male | 59 (51.3%) | 38 (52.8%) | 97 (51.9%) |
| Age at IPD diagnosis (years) | |||
| Mean (SD) | 56.3 (26.6) | 62.8 (27.1) | 58.8 (26.9) |
| Median (IQR) | 62.2 (46.9, 76.2) | 70.4 (49.5, 84.0) | 65.2 (46.9, 79.9) |
| Range | (0.6–97.7) | (1.0–99.6) | (0.6–99.6) |
| Race | |||
| White | 99 (86.1%) | 65 (90.3%) | 164 (87.7%) |
| Black or African American | 6 (5.2%) | 3 (4.2%) | 9 (4.8%) |
| Asian | 2 (1.7%) | 1 (1.4%) | 3 (1.6%) |
| American Indian/Alaskan Native | 1 (0.9%) | 0 (0.0%) | 1 (0.5%) |
| Other | 4 (3.5%) | 3 (4.2%) | 7 (3.7%) |
| Unknown/Not disclosed | 3 (2.6%) | 0 (0.0%) | 3 (1.6%) |
| Ethnicity | |||
| Hispanic or Latino | 5 (4.3%) | 3 (4.2%) | 8 (4.3%) |
| Not Hispanic or Latino | 86 (74.8%) | 67 (93.1%) | 153 (81.8%) |
| Unknown/Not disclosed | 24 (20.9%) | 2 (2.8%) | 26 (13.9%) |
| Comorbidities | |||
| Weighted CCI, median (IQR) | 3 (1, 9) | 6 (2, 10.5) | 4 (1, 9) |
| Myocardial infarction | 23 (20.0%) | 10 (13.9%) | 33 (17.6%) |
| Congestive heart failure | 39 (33.9%) | 31 (43.1%) | 70 (37.4%) |
| Peripheral vascular disease | 23 (20.0%) | 33 (45.8%) | 56 (29.9%) |
| Cerebrovascular disease | 30 (26.1%) | 18 (25.0%) | 48 (25.7%) |
| Dementia | 10 (8.7%) | 12 (16.7%) | 22 (11.8%) |
| Chronic pulmonary disease | 69 (60.0%) | 44 (61.1%) | 113 (60.4%) |
| Ulcer | 17 (14.8%) | 6 (8.3%) | 23 (12.3%) |
| Mild liver disease | 17 (14.8%) | 20 (27.8%) | 37 (19.8%) |
| Diabetes mellitus | 25 (21.7%) | 27 (37.5%) | 52 (27.8%) |
| Diabetes with organ damage | 12 (10.4%) | 15 (20.8%) | 27 (14.4%) |
| Hemiplegia | 8 (7.0%) | 6 (8.3%) | 14 (7.5%) |
| Moderate/severe renal disease | 32 (27.8%) | 20 (27.8%) | 52 (27.8%) |
| Moderate/severe liver disease | 2 (1.7%) | 6 (8.3%) | 8 (4.3%) |
| HIV or AIDS | 0 (0.0%) | 2 (2.8%) | 2 (1.1%) |
| Rheumatologic disease | 10 (8.7%) | 11 (15.3%) | 21 (11.2%) |
| Metastatic solid tumor | 16 (13.9%) | 11 (15.3%) | 27 (14.4%) |
| Other cancer | 43 (37.4%) | 31 (43.1%) | 74 (39.6%) |
| Body mass index (kg/m2) b | N=98 | N=63 | N=161 |
| < 18.5 | 6 (6.1%) | 4 (6.3%) | 10 (6.2%) |
| 18.5- <25 | 29 (29.6%) | 20 (31.7%) | 49 (30.4%) |
| 25 - <30 | 19 (19.4%) | 13 (20.6%) | 32 (19.9%) |
| 30+ | 33 (33.7%) | 24 (38.1%) | 57 (35.4%) |
| Not documented | 11 (11.2%) | 2 (3.2%) | 13 (8.1%) |
| Mean (SD) | 28.3 (8.5) | 28.6 (8.0) | 28.4 (8.3) |
Abbreviation: AIDS, acquired immunodeficiency disease syndrome; CCI, Charlson comorbidity index; HIV, human immunodeficiency virus; IQR, interquartile range; IPD, invasive pneumococcal disease; N, number; SD, standard deviation
Among patients diagnosed with IPD at age 18 years or greater
Incidence rate
For the pre-PCV13 period, the overall age- and sex-adjusted incidence rate was 11.1 (95% CI 9.1, 13.2) per 100,000 person-years, with 10.1 (95% CI 7.4, 12.7) in females and 12.2 (95% CI 9.1, 15.4) in males. When stratified by age, the highest incidence was observed in those greater than 65 years of age at 40.9 (95% CI 30.5, 53.6) per 100,000 person-years, while the age group of 5–18 years had the lowest incidence at 2.7 (95% CI 1.0, 6.0) per 100,000 person-years. The overall age- and sex-adjusted incidence rate significantly decreased to 5.6 (95% CI 4.3, 6.9) per 100,000 person-years in the post-PCV13 period, with 4.9 (95% CI 3.2, 6.5) in females and 6.5 (95% CI 4.4, 8.6) in males. When stratified by age groups, a significant decrease in the IPD incidence rate was observed in the 19–65 years group (Table 2).
Table 2:
Incidence rate (per 100,000 person-years) of IPD stratified by years and age groups.
| Pre-PCV13, 2002–2010 | Post-PCV13, 2011–2018 | p-value compared the two time periods | |||
|---|---|---|---|---|---|
|
| |||||
| Number of cases | Rate (95% CI) | Number of cases | Rate (95% CI) | ||
|
| |||||
| Overall, rates are age- and sex-adjusted b | 115 | 11.1 (9.1, 13.2) | 72 | 5.6 (4.3, 6.9) | <.001 |
| By gender, rates are age-adjusted b | |||||
| Female | 56 | 10.1 (7.4, 12.7) | 34 | 4.9 (3.2, 6.5) | .01 |
| Male | 59 | 12.2 (9.1, 15.4) | 38 | 6.5 (4.4, 8.6) | .01 |
| By age group (years) | |||||
| <5 | 11 | 12.3 (6.2, 22.1) | 6 | 6.6 (2.4, 14.3) | .21 |
| 5–18 | 6 | 2.7 (1.0, 6.0) | 3 | 1.3 (0.3, 3.8) | .30 |
| 19–50 | 20 | 3.7 (2.3, 5.7) | 9 | 1.7 (0.8, 3.2) | .04 |
| 51–64 | 26 | 15.9 (10.4, 23.3) | 12 | 5.5 (2.8, 9.5) | .01 |
| 65+ | 52 | 40.9 (30.5, 53.6) | 42 | 24.6 (17.7, 33.2) | .10 |
Abbreviation: CI, confidence interval; IPD, invasive pneumococcal disease; PCV-13, 13-valent pneumococcal conjugate vaccine
Adjusted to the population structure of the U.S. total population in 2010.
Pneumococcal isolates and susceptibility
Of 187 cases, 167 (89.3%) had S. pneumoniae exclusively isolated from blood cultures. Six additional cases had positive cultures from blood with another clinical specimen, including 3 (1.6%) CSF and 1 (0.5%) each for abdominal fluid, synovial fluid, and pleural fluid. Among cases with negative blood cultures, 6 (3.2%) had pneumococci isolated from pleural fluid, 4 (2.1%) from CSF, and 4 (2.2%) synovial fluid. Penicillin susceptibility data were available for 177 (94.6%) isolates; 162 (86.6%), 11 (5.9%) and 4 (2.1%) were fully susceptible, intermediate susceptible and resistant, respectively.
Pneumococcal serotypes
Pneumococcal serotypes were available in 169 (90.4%) cases. Serotypes 4, 6B, 9V, or 19F (part of PCV7, PCV13 and PPSV23 serotypes) were isolated from 13 (7.7%) patients. All of them were isolated during the pre-PCV13 period. The overall age- and sex- adjusted incidence of IPD with PCV13/non-PCV7 serotypes (as defined in the Methods section) had decreased from 3.7 (95% CI 2.6, 4.9) per 100,000 person-years in the pre-PCV13 period to 0.8 (95% CI 0.3, 1.2) in the post-PCV13 period (p<.001). The occurrence of PCV13/non-PCV7 serotypes among the cases declined from 38.1% (40/105) in the pre-PCV13 period to 15.6% (10/64) in the post-PCV13 period (p=.002). There was not a statistically significant difference in the overall age- and sex- adjusted incidence of IPD with PPSV23/non-PCV13 serotypes with rates of 2.4 (95% CI 1.5, 3.4) and 2.7 (95% CI 1.8, 3.6) per 100,000 person-years in the pre-PCV13 and post-PCV13 periods, respectively (p=.39). However, among the IPD cases, the occurrence of PPSV23/non-PCV13 serotypes had increased from 24.8% (26/105) to 54.7% (35/64) (p<.001). Within this category, the proportion of patients with serotype 11A increased from 1.0% (1/105) to 6.2% (4/64) and the proportion of patients with serotype 33F increased from 2.9% (3/105) to 15.6% (10/64). The overall age- and sex- adjusted incidence of IPD with non-vaccine serotypes was not significantly different between the two periods with rates of 2.5 (95% CI 1.5, 3.5) and 1.5 (95% CI 0.8, 2.2) per 100,000 person-years in the pre-PCV13 and post-PCV13 periods, respectively (p=.18). Non-vaccine serotypes were isolated from 24.8% (26/105) and 29.7% (19/64) in the pre-PCV13 and post-PCV13 periods, respectively. The number of cases and age- and sex- adjusted incidence rates for these serotype categories by calender period are presented in Supplemental Table 1, along with the types of non-vaccine serotypes.
Pneumococcal vaccines
A total of 112 (59.9%) patients received at least one dose of pneumococcal vaccine prior to an IPD diagnosis. Among the 102 cases with identified pneumococcal serotypes who had received a prior relevant vaccine, 3 (2.9%) patients had received either PCV7 or PCV13 and developed IPD from PCV7 or PCV13 serotypes. Forty-six (45.1%) patients had received PPSV23 only and developed IPD from PPSV23 serotypes. Six (5.9%) cases had received either PCV7 or PCV13 along with PPSV23 and still developed IPD from vaccine serotype. Non-vaccine serotypes were recovered from 36 (35.3%) patients who received at least one dose of the pneumococcal vaccine. There were 11 (10.8%) patients who developed IPD from a different vaccine serotype (i.e., received the PPSV23 vaccine and the serotype was PCV7 or PCV13 or received the PCV7 or PCV13 vaccine and the serotype was PPSV23). A detailed summary of serotypes and vaccination status were included in Supplemental Table 2.
Mortality
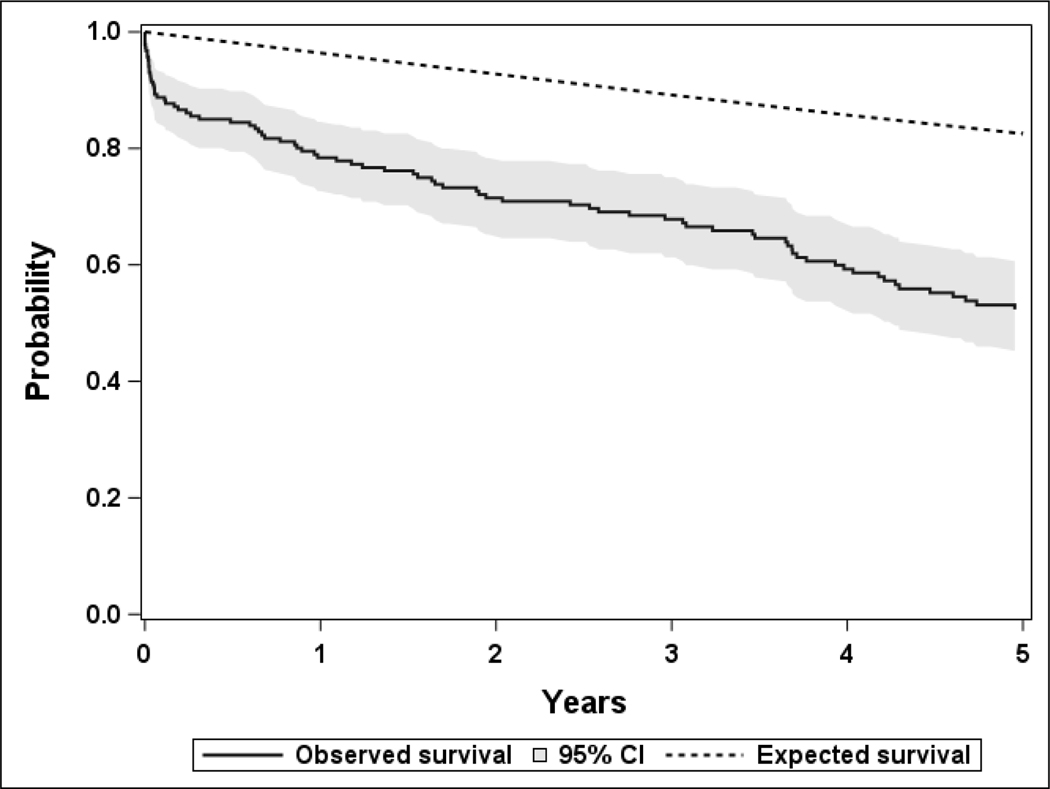
As of January 2021, 108 deaths have been documented among the 187 patients. Among 79 patients not known to be deceased, the median duration of follow-up between IPD diagnosis and last clinical visit was 5.8 years [IQR, 2.6–12.9 years]. Twenty-one patients died within 30 days of an IPD diagnosis yielding a 30-day survival rate of 88.8% (95% CI 84.4, 93.4) overall, with 86.7% (95% CI 79.9, 94.0) for females and 90.7% (95% CI 85.1, 96.7) for males. When categorized by age, the 30-day survival rates were 94.6% (95% CI 90.1, 99.3) and 83.0% (95% CI 75.7, 90.9) for patients diagnosed with IPD at <65 years of age and 65 years of age or older, respectively. A total of 40 patients died within 1 year of an IPD diagnosis yielding a 1-year survival rate of 78.4% (95% CI 72.7, 84.6) overall, with 78.6% (95% CI 70.5, 87.6) for females and 78.2% (95% CI 70.3, 86.9) for males. The 1-year survival rates were 91.3% (95% CI 85.7, 97.2) and 66.0% (95% CI 57.0, 76.3) for patients diagnosed with IPD at <65 years of age and 65 years of age or older, respectively. The observed survival of patients in this cohort was significantly less than what would be expected based on age- and sex-specific mortality rates in Minnesota (p<.001, Figure 1).
Figure 1 -.
The observed survival after IPD diagnosis of 187 patients between 2002 and 2018 compared to the expected survival based on age- and sex-specific mortality rates in Minnesota (p<.001).
Discussion
Our study examined incidence rates of IPD in Olmsted County during 2002–2010 (pre-PCV13 period) and 2011–2018 (post-PCV13 period). The incidence rate had significantly decreased from 11.1 per 100,000 person-year in the pre-PCV13 period to 5.6 per 100,000 person-year in the post-PCV13 period. As compared to the pre-PCV13 period, the number of IPD cases due to PCV13/non-PCV7 serotypes had remarkably decreased while PPSV23/non-PCV13 serotypes had slightly increased. This demonstrated additional benefits of PCV13 to that of PCV7 in the prior era. The large majority of IPD cases in our population had bloodstream infections, with only a small number of cases presented with meningitis. Notably, the penicillin susceptibility pattern was comparable to that described in previous studies 10, 21. In addition, our 30-day mortality of 11% was lower than that (15%−20%) described in previous studies 22–24. This observation could be due to differences in time points, population age, comorbidities, socioeconomic status, and access to care.
Based on recent ABCs data, an estimated incidence rate of IPD in the United States was 9.2 per 100,000 person-years in 2019 25. The low incidence rate in our population compared to surveillance data from select states and counties within could be explained by a difference in baseline demographics, surveillance limitation, and geographical variation of serotype distributions 14, 26, 27. When focusing on the incidence rate after implementation of PCV13, a significant reduction has been consistently demonstrated in several studies. One US population-based study showed that the overall IPD incidence declined by 64% from July 2012 to June 2013, primarily due to a reduction in PCV13/non-PCV7 serotypes 14. A similar trend of sustained reduction was observed in other studies from different countries 28–31. Interestingly, a recent population-based study in France illustrated an initial decrease in cases from 2010 to 2014, followed by an incidence increase after 2015 32. The authors postulated that non-PCV13 serotypes were responsible for the more recent upward trend. This incidence rebound, however, was not detected in our study.
An inverse trend of specific serotypes, PCV13/non-PCV7 reduction, and minimal PPSV23/non-PCV13 expansion observed in our study could be explained by a serotype replacement effect. The serotype replacement is caused by an increase in asymptomatic carriers of non-vaccine serotypes leading to an uptick in IPD cases 33, 34; a PCV7-driven serotype replacement was seen in our prior study 10. A similar phenomenon was observed with PCV13 serotypes in several studies from Europe 32, 35–37. Surprisingly, the serotype replacement effect has not been prominent in the United States after PCV13 implementation 13, 38, 39 and factors responsible for this have yet to be defined. Some hypotheses include differences in surveillance technique, transmission dynamics, risk factors, and pathogen evolution 38.
Our study demonstrated that more PPSV23/non-PCV13 IPD cases in the post-PCV13 period were driven by serotype 11A and 33F. This finding was recently described in the global pneumococcal sequence clusters whole-genome sequencing study 39. The authors found that common serotypes in the post-PCV13 era included serotypes 5, 12F, 15B/C, 19A, 33F, and 35B/D, but the most common cause of IPD in the United States was serotype 33F. A substantial increase in other PPSV23/non-PCV13 or non-vaccine serotypes was not observed in our investigation. When vaccination status is considered, the proportion of individuals who developed IPD from vaccine serotypes despite receiving vaccine were not different between pre- and post-PCV13 period. We noted the number of IPD patients who developed PCV serotypes after PCV vaccine was lower than that of patients who developed non-PCV/PPSV23 or non-vaccine serotype related IPD.
Our study provides important insights regarding the contemporary incidence and characteristics of IPD, serotypes, clinical presentation, and mortality. Our study has several strengths. First, the temporal incidence rate of IPD was calculated from an extensive database that captured more than 99% of all outpatient and inpatient medical records for the Olmsted County population over the 17 years of the study period. Therefore, we can ascertain that our longitudinal data are valid, accurate, and complete. Second, clinical data, serotypes, and vaccine records were robust, with minimal missing data. Third, our findings have aligned with data of the ABCs report. The generalizability to an external population is therefore possible.
There are several limitations of our work. First, the overall number of IPD cases was relatively small, especially post-PCV13. This limited our ability to draw conclusions about serotypes’ distribution between pre- and post-PCV13 periods. Additionally, we could not confirm vaccine effectiveness amongst the cases. Second, some meningitis cases did not have positive CSF cultures, which potentially impacted penicillin susceptibility interpretation. However, we overcame this by manually reviewing all possible meningitis cases to ensure the accuracy of penicillin susceptibility interpretation. Third, the population included an unusually high percentage of health care professionals and their families. Therefore, health care access may be more easily obtainable than that for the general population. Given the high percentage of Caucasians in Olmsted County, this may limit the generalizability of our study. However, studies comparing various chronic diseases in Olmsted County with those in other communities in the United States indicate that data from this population can be extrapolated to a large part of this country’s 16. Finally, the use of a retrospective study design is subject to several biases, including reviewer bias.
Conclusion
We demonstrated an approximate ~50% decline in the incidence rate of IPD during the post-PCV13 period in Olmsted County, Minnesota. In contrast, a minimal uptick in IPD cases caused by PPSV23/non-PCV13 serotypes was seen, but the overall number of cases for this group was small. The complexities of the epidemiology of IPD dictate that ongoing surveillance is warranted.
Supplementary Material
Acknowledgement
This study used the resources of the Rochester Epidemiology Project (REP) medical records-linkage system, which is supported by the National Institute on Aging (NIA; AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users. The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health (NIH) or the Mayo Clinic.
Funding
This research was made possible through support from Division of Allergic Disease, Small Grant Project - the Mayo Clinic Center for Clinical and Translational Science grant support (UL1TR000135).
Abbreviations
- ABC
Active Bacterial Core Surveillance
- ACIP
Advisory Committee on Immunization Practices
- CI
confidence intervals
- CSF
cerebrospinal fluid
- IPD
invasive pneumococcal disease
- MIC
minimum inhibitory concentrations
- PCV7
7-valent pneumococcal conjugate vaccine
- PCV13
13-valent pneumococcal conjugate vaccine
- PPSV23
23-valent pneumococcal polysaccharide vaccine
- REP
Rochester Epidemiology Project
- US
United States
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fitzgerald D, Waterer GW. Invasive Pneumococcal and Meningococcal Disease. Infectious disease clinics of North America. 2019;33:1125–1141. [DOI] [PubMed] [Google Scholar]
- 2.Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20 Suppl 5:45–51. [DOI] [PubMed] [Google Scholar]
- 3.Zhang D, Petigara T, Yang X. Clinical and economic burden of pneumococcal disease in US adults aged 19–64 years with chronic or immunocompromising diseases: an observational database study. BMC infectious diseases. 2018;18:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Usuf E, Bottomley C, Bojang E, et al. Persistence of Nasopharyngeal Pneumococcal Vaccine Serotypes and Increase of Nonvaccine Serotypes Among Vaccinated Infants and Their Mothers 5 Years After Introduction of Pneumococcal Conjugate Vaccine 13 in The Gambia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019;68:1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austrian R. A brief history of pneumococcal vaccines. Drugs Aging. 1999;15 Suppl 1:1–10. [DOI] [PubMed] [Google Scholar]
- 6.Rodgers GL, Whitney CG, Klugman KP. Triumph of Pneumococcal Conjugate Vaccines: Overcoming a Common Foe. The Journal of infectious diseases. 2021;224:S352–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yildirim I, Shea KM, Pelton SI. Pneumococcal Disease in the Era of Pneumococcal Conjugate Vaccine. Infectious disease clinics of North America. 2015;29:679–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuehn B. Pneumococcal Vaccine Recommendation. Jama. 2020;323:112–112. [DOI] [PubMed] [Google Scholar]
- 9.Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49:1–35. [PubMed] [Google Scholar]
- 10.Tsigrelis C, Tleyjeh IM, Lahr BD, Nyre LM, Virk A, Baddour LM. Trends in invasive pneumococcal disease among older adults in Olmsted County, Minnesota. The Journal of infection. 2009;59:188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63:822–825. [PMC free article] [PubMed] [Google Scholar]
- 12.Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR Morb Mortal Wkly Rep. 2012;61:394–395. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2019; Trends by Serotype Group. Vol 20212021. [Google Scholar]
- 14.Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. The Lancet. Infectious diseases. 2015;15:301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ, 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocca WA, Grossardt BR, Brue SM, et al. Data Resource Profile: Expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol. 2018;47:368–368j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinstein MP, Klugman KP, Jones RN. Rationale for revised penicillin susceptibility breakpoints versus Streptococcus pneumoniae: coping with antimicrobial susceptibility in an era of resistance. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;48:1596–1600. [DOI] [PubMed] [Google Scholar]
- 18.van Werkhoven CH, Huijts SM. Vaccines to Prevent Pneumococcal Community-Acquired Pneumonia. Clin Chest Med. 2018;39:733–752. [DOI] [PubMed] [Google Scholar]
- 19.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Therneau T, Offord J. Expected survival based on hazard rates (Update). Mayo Clinic—Section of Biostatistics. Technical Report Series: Citeseer; 1999. [Google Scholar]
- 21.Jenkins SG, Brown SD, Farrell DJ. Trends in antibacterial resistance among Streptococcus pneumoniae isolated in the USA: update from PROTEKT US Years 1–4. Ann Clin Microbiol Antimicrob. 2008;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demirdal T, Sen P, Emir B. Predictors of mortality in invasive pneumococcal disease: a meta-analysis. Expert Rev Anti Infect Ther. 2021;19:927–944. [DOI] [PubMed] [Google Scholar]
- 23.Morton JB, Morrill HJ, LaPlante KL, Caffrey AR. Predictors of Mortality Among U.S. Veterans With Streptococcus Pneumoniae Infections. Am J Prev Med. 2017;52:769–777. [DOI] [PubMed] [Google Scholar]
- 24.Yu VL, Chiou CC, Feldman C, et al. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2003;37:230–237. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2019. Vol 20212021. [Google Scholar]
- 26.Hausdorff WP, Siber G, Paradiso PR. Geographical differences in invasive pneumococcal disease rates and serotype frequency in young children. Lancet (London, England). 2001;357:950–952. [DOI] [PubMed] [Google Scholar]
- 27.Rosen JB, Thomas AR, Lexau CA, et al. Geographic variation in invasive pneumococcal disease following pneumococcal conjugate vaccine introduction in the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53:137–143. [DOI] [PubMed] [Google Scholar]
- 28.Harboe ZB, Dalby T, Weinberger DM, et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59:1066–1073. [DOI] [PubMed] [Google Scholar]
- 29.Jayasinghe S, Menzies R, Chiu C, et al. Long-term Impact of a “3 + 0” Schedule for 7-and 13-Valent Pneumococcal Conjugate Vaccines on Invasive Pneumococcal Disease in Australia, 2002–2014. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;64:175–183. [DOI] [PubMed] [Google Scholar]
- 30.De Wals P, Lefebvre B, Deceuninck G, Longtin J. Incidence of invasive pneumococcal disease before and during an era of use of three different pneumococcal conjugate vaccines in Quebec. Vaccine. 2018;36:421–426. [DOI] [PubMed] [Google Scholar]
- 31.Berezin EN, Jarovsky D, Cardoso MRA, Mantese OC. Invasive pneumococcal disease among hospitalized children in Brazil before and after the introduction of a pneumococcal conjugate vaccine. Vaccine. 2020;38:1740–1745. [DOI] [PubMed] [Google Scholar]
- 32.Ouldali N, Varon, Levy, et al. Invasive pneumococcal disease incidence in children and adults in France during the pneumococcal conjugate vaccine era: an interrupted time-series analysis of data from a 17-year national prospective surveillance study. The Lancet. Infectious diseases. 2021;21:137–147. [DOI] [PubMed] [Google Scholar]
- 33.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet (London, England). 2011;378:1962–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho P-L, Chiu SS, Law PY, Chan EL, Lai EL, Chow K-H. Increase in the nasopharyngeal carriage of non-vaccine serogroup 15 Streptococcus pneumoniae after introduction of children pneumococcal conjugate vaccination in Hong Kong. Diagnostic microbiology and infectious disease. 2015;81:145–148. [DOI] [PubMed] [Google Scholar]
- 35.Weinberger R, von Kries R, van der Linden M, Rieck T, Siedler A, Falkenhorst G. Invasive pneumococcal disease in children under 16 years of age: incomplete rebound in incidence after the maximum effect of PCV13 in 2012/13 in Germany. Vaccine. 2018;36:572–577. [DOI] [PubMed] [Google Scholar]
- 36.Ladhani SN, Collins S, Djennad A, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. The Lancet Infectious Diseases. 2018;18:441–451. [DOI] [PubMed] [Google Scholar]
- 37.Tin Tin Htar M, Christopoulou D, Schmitt HJ. Pneumococcal serotype evolution in Western Europe. BMC infectious diseases. 2015;15:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewnard JA, Hanage WP. Making sense of differences in pneumococcal serotype replacement. The Lancet Infectious Diseases. 2019;19:e213–e220. [DOI] [PubMed] [Google Scholar]
- 39.Lo SW, Gladstone RA, van Tonder AJ, et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: an international whole-genome sequencing study. The Lancet. Infectious diseases. 2019;19:759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.